Abstract
Serotonergic and noradrenergic pathways are the main targets of antidepressants. Their differential effects on emotion processing-related brain activation are, however, to be further characterized. We aimed at elucidating the neural sites of action of an acute differential serotonergic and noradrenergic influence on an emotion-processing task, which was earlier shown to be associated with depressiveness. In a single-blind pseudo-randomized crossover study, 21 healthy subjects (16 subjects finally included in the analysis) participated to ingest a single dose at three time points of either 40 mg citalopram, a selective serotonin-reuptake inhibitor, 8 mg reboxetine, a selective noradrenaline-reuptake inhibitor, or placebo 2–3 h before functional magnetic resonance imaging (fMRI). During fMRI, subjects performed a task comprising the anticipation and perception of pictures of either ‘known' (positive, negative, neutral) or ‘unknown' valence (randomly 50% positive or negative). In direct comparison with citalopram and with placebo, reboxetine increased brain activity in the medial thalamus. Citalopram modulated certain prefrontal and insular areas more prominently. Other frontal and parieto-occipital areas were modulated by both drugs. In conclusion, the functional network involved in emotional information processing could be modulated by the acute application of selective noradrenergic and serotonergic drugs revealing a noradrenergic effect in thalamic and frontal areas, and a prefrontal and insular focus of serotonergic modulation. These findings could have implications for future selection criteria concerning personalized antidepressant medication in depression.
Keywords: emotion processing, citalopram, reboxetine, functional imaging, depression, treatment
INTRODUCTION
The term ‘personalized medication' has been introduced in the scientific discussion in the context of pharmacogenetics (Holsboer, 2008) to identify subgroups of patients for which response to a specific drug could be predicted by genetic markers. A similar concept is the search for biological markers concerning functional, biochemical, or anatomical differences potentially acting as treatment response predictors or also for psychiatric diagnostics based on neurobiology (Gottesman and Gould, 2003; Flint and Munafo, 2007). For the treatment of depressive disorders, the choice of the individually effective antidepressant medication remains a main problem. For over 50 years, the available antidepressant drugs primarily target monoaminergic pathways, either through the serotonergic or the noradrenergic system, or both (Schildkraut, 1965; Hirschfeld, 2000; Schloss and Henn, 2004). However, until today no neurobiological criteria have been identified to decide which type of antidepressant for an individual patient would improve his or her depression (Bruder et al, 2008; Mayberg, 2003).
Knowing the specific influence of noradrenergic and serotonergic antidepressants on emotion-processing-related brain activation, and identifying patterns of brain activation indicating an association with a more serotonergic or noradrenergic dysfunction in the depressed patient, could provide predictors for treatment response to select that antidepressant leading to a faster remission. This would save time and resources, which otherwise are spent trying different antidepressants.
Earlier studies have identified a network of regions involved in depressive information processing including the amygdalar complex, thalamic, insular, cingulate, and prefrontal cortical regions (eg reviews: Phillips et al, 2003; Drevets, 2001; Clark et al, 2009; Gotlib and Hamilton, 2008). Cognitive processes in depression are characterized by a negative or ‘pessimistic' bias toward the future (as well as toward ‘the self [and] the personal world'), as outlined by Beck in the cognitive triad (Beck, 2005, 1967). We developed a task addressing ‘pessimism' on the neural level, with ‘pessimism' being regarded as the expectation of a negative outcome when anticipating an event of unknown, ie either pleasant or unpleasant emotional valence. Using functional magnetic resonance imaging (fMRI), this model has shown a pattern of brain activation in thalamic, midbrain, insular, cingulate, and prefrontal cortical regions during the anticipation of ambiguously cued events. The more depressed the healthy subjects and the depressed patients were, the more this pattern resembled the pattern during the anticipation of negatively cued stimuli (Herwig et al, 2007c, 2009). As this neurocognitive model of depressive information processing has shown sensitivity for depressiveness in healthy subjects and patients with major depressive disorder, it was combined in this study with a neuropharmacological approach to differentiate and identify brain regions involved in depressive emotion processing that are susceptible to modulation by monoaminergic antidepressants. We used an acute serotonergic and noradrenergic challenge during the emotional anticipation paradigm. Single doses of either citalopram, the most selective serotonin-reuptake inhibitor (SSRI, Hyttel, 1982; Joubert et al, 2000), or reboxetine as most selective noradrenergic-reuptake inhibitor (Kent, 2000; Tatsumi et al, 1997) were expected to enhance the serotonergic or noradrenergic transmission compared with placebo in those regions innervated by serotonergic or noradrenergic neurons and activated by the task. Until now, these regions are poorly characterized. In particular, there is no direct comparison of the modulating effects of these neurotransmitters in the brain during functional challenges. Noradrenergic and serotonergic innervations are widespread and strongly intertwined. However, the striatal region has strong serotonergic innervations and is nearly free of noradrenergic innervations (Logan et al, 2007; Kung et al, 2004), whereas the medial thalamic region is known for noradrenergic binding (Smith et al, 2006). Thus, we considered respective modulations. The results of this study, together with earlier findings of functional changes in depression, are intended to reveal potential regions of interest as indicators for future differential treatment of depressed patients.
MATERIALS AND METHODS
Subjects
Twenty-one healthy subjects (mean age (±SD) 28.14±5.8 years, all right handed, 14 female) were recruited through direct address and email-newsletter advertisement. After complete description of the study, they gave written informed consent to take part. The study was approved by the local ethics committee. Each subject was intended to undergo three fMRI scans. Exclusion criteria were any history of neurological or psychiatric illness, assessed by a semistructured interview, significant head injury, pregnancy, actual medication (other than oral contraceptives), excessive consummation of alcohol, cigarettes and caffeine, consummation of illegal drugs, participation in another study with intake of pharmaceuticals and contraindications against MRI examination. Before inclusion, subjects completed psychometric assessment of state depression (self-rating depression scale SDS (Zung, 1965), German version, cutoff score 50/100), trait anxiety (State Trait Anxiety Inventory STAI (Spielberger et al, 1970), German version (Laux et al, 1981), cutoff score 44/80), and personality traits (Eysenck Personality Inventory EPI, neuroticism/extraversion (Eysenck and Eysenck, 1964), German version (Eggert, 1974)). To confirm right-handedness, participants completed a questionnaire of handedness (Annett, 1970, German version) before the first scan. For demographic and psychometric data of the included subjects consider Supplementary Table S1.
One subject had to be excluded because of pathological findings (gray-matter T2 hyperintensities without earlier clinical symptoms), one subject aborted the study and withdrew consent, and three subjects had to be excluded because of repetitive excessive head movements during scanning (>3 mm in at least one direction) or to technical problems in at least two of the three scans such that only one scan remained at most which was not sufficient for the intra-subject comparisons. Of the remaining 16 subjects with at least two analyzable scans, in three subjects each one scan could not be completed because of technical reasons (error of the MR scanner). In total, we obtained 45 datasets that were included in the analysis as outlined graphically in the supplementary material (Supplementary Table S2).
Drug Treatment
Each subject received before each of the three scans a usual therapeutic target dose of either 40 mg citalopram (CIT), 8 mg reboxetine (RBX), or placebo (PLC, lactose) in a single-blind pseudo-randomized order, each two tablets in a closed wrap with the instruction of intake about 2.5 h before start of scanning, as peak concentrations of both drugs were observed 2–4 h after oral application (Joubert et al, 2000; Fleishaker, 2000). A minimal washout period of 1 week was implemented between the scans, corresponding to at least five half-lives of both substances. The order of drug administration across sessions was counterbalanced by pseudo-randomization across subjects. Before and after scanning subjects were asked for side effects and concerning their experiences with the experiment in the scanner.
Experimental Design
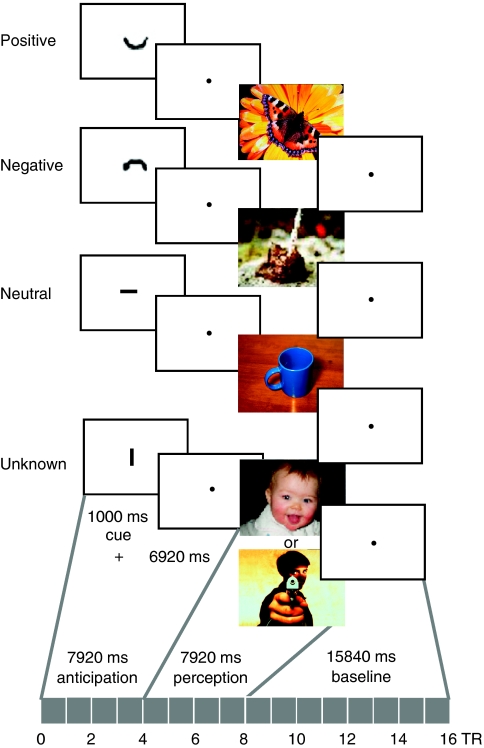
During fMRI scanning, participants performed a task (programmed with Presentation, Neurobehavioral Systems, USA), which consisted of 56 trials with expectation (exp) and perception of emotional pictures (Figure 1, description in Herwig et al, 2007c). Within each trial, subjects were first presented a cue (duration 1000 ms), depicting either a ‘smiling' (‘positive') ‘∪', a ‘non-smiling' (‘negative') ‘∩', or a ‘neutral' symbol ‘−', indicating the emotional valence of the upcoming picture, or a symbol, after which randomly either a pleasant or an unpleasant picture appeared: the ‘unknown' condition ‘∣‘. Notably, the term ‘unknown' as used here refers to the fact that in this condition, the emotional valence of the upcoming picture was unknown, as it was cued ambiguously. The cues were 1/20 of screen height and the pictures filled the screen. The cues were highly abstract, physically comparable, and intuitively understandable; no prominent working memory was activated to establish their meaning. The following anticipation period lasted 6920 ms (blank screen with fixation point; cue plus anticipation: 4 TR). Subsequently, emotional pictures from the International Affective Picture System (IAPS, Lang et al, 2005) were presented for 7920 ms (4 TR). During the following baseline period (15840 ms, 8 TR), the blood oxygen level-dependent (BOLD) signal could wear off before the next trial. The main conditions were ‘known' and ‘unknown' emotional valence. The ‘known' trials consisted of three sub-conditions—positive, negative, neutral—resulting in four different conditions. Each condition—positive (ps), negative (ng), neutral (nt), and unknown (uk, comprising ps : ng=1 : 1)—comprised 14 trials (randomized order). The positive condition provided a balance for the known negative trials and for the equality of the ‘unknown' condition. Participants were instructed to expect the emotional stimuli after the cue, to be aware of the indicated emotional valence, and to watch the following picture. All participants performed a training session before scanning.
Figure 1.
Schematic summary of the paradigm for anticipation and perception of emotional stimuli. Presented are the four conditions with the respective cues indicating the valence of the following picture: ‘∪' before a ‘positive' picture, ‘∩' before a ‘negative' picture, ‘−' before a ‘neutral' picture, ‘ ∣ ' before a picture of ‘unknown' valence that could have been either positive or negative. The cue was presented for 1000 ms, followed by an anticipation period of 6920 ms (cue + anticipation=4 TR, 1 TR=1980 ms). Thereafter the respective picture was shown for 7920 ms (4 TR). Here, the cues are enlarged for presentational reasons.
We used three sets of each 56 stimuli, in which the stimuli were matched between and within each set for equal difference of valence from neutral (IAPS picture rating, Lang et al, 2005), and for complexity and content (Supplementary Table S3). Within all sets, the pictures were chosen in such way that arousal was matched according to the IAPS picture rating as much as possible between pleasant and unpleasant stimuli (detailed discussion in Herwig et al, 2007b), though arousal in the positive pictures was lower in all sets compared with the negative pictures because of inherent characteristics of the IAPS pictures and of positive pictures in general (Lang et al, 2005). The sets did not differ concerning content, complexity, arousal, and pleasure. The sets were randomly assigned to the sessions; no set was shown twice to the same subject to avoid memory effects. Immediately after each scan, the subjects rated the emotional valence (very negative=1, very positive=9) of the presented pictures (presented again as printouts) on a visual analog scale (Supplementary Table S4).
fMRI Acquisition
Imaging was performed with a 3.0 T GE Signa HD Scanner (GE Medical Systems, Milwaukee, 8-channel head coil). Echo-planar imaging was performed for functional MR imaging (repetition time (TR)/echo time (TE) 1980/32 ms, 22 sequential axial slices, whole brain, slice thickness/gap 4.5/0.5 mm, resulting voxel size 3.4 × 3.4 × 5 mm, matrix 64 × 64 pixels, FOV 220 mm). Altogether, 908 volumes were obtained per subject, 16 per run. High-resolution three-dimensional (3D) T1-weighted anatomical volumes were acquired (TR/TE 9.9/2.9 ms; matrix size 256 × 256; 1 × 1 × 1 mm, axial orientation) for coregistration with the functional data. The stimuli were presented through digital goggles (Resonance Technologies, Northridge).
fMRI Data Analysis and Statistics
fMRI data were analyzed using BrainVoyager QX 1.10 (Brain Innovation, Maastricht, The Netherlands). The first four images of each functional scan were rejected to allow for T2* equilibration effects. Preprocessing of the functional scans included motion correction, slice scan-time correction, high frequency temporal filtering, and removal of linear trends. Coregistration of functional and 3D structural measurements was computed by relating T2*-weighted images and the T1-weighted 3D structural measurement, which yields a functional dataset. Structural and functional datasets were transformed into Talairach space (Talairach and Tournoux, 1988), resulting in a voxel size of 3 × 3 × 3 mm, then spatially smoothed with an 8 mm full-width half-maximum Gaussian kernel for subsequent group analysis.
The primary design matrix of each run consisted of a baseline and eight predictors, defined to present the anticipation conditions (negative, pleasant, neutral, unknown) and the analogous presentation conditions. The design matrix was build for analyzing intra-subject comparisons between the three treatment-conditions: CIT, RBX, PLC. These conditions were convoluted with a two-parameter gamma hemodynamic response function (HRF, Glover, 1999) provided by Brainvoyager (time to response peak 5 s, time to undershoot peak 15 s). There are no direct methodical studies concerning potential effects of citalopram and reboxetine on the HRF. Two studies, however, mentioned no changes of the hemodynamic reaction compared with PLC in a visual and a motor task, respectively (reboxetine: Miskowiak et al, 2007; citalopram: Wingen et al, 2008).
At first, 3D statistical parametric maps with separate subject predictors were computed using a fixed effects general linear model for the conjoint contrast (exp ng >exp nt)&(exp uk >exp nt) in each of the treatment conditions (CIT, RBX, PLC) at a statistical level of p<0.001 (uncorrected, considering the conjunction of contrasts). This conjoint contrast reflected both situations considered to be unpleasant, the ‘negative', and the ‘unknown' expectation, of which the concerning brain activations earlier were found to be associated with depressiveness (Herwig et al, 2007c), and that were suggested to resemble unpleasant feelings (eg in depression). The resulting ROIs were used to build masks for the three combined datasets for a comparison of the two conditions within these sets (CIT/PLC, RBX/PLC, CIT/RBX—exemplarily shown in Supplementary Figure 1). Accordingly, in these combined masked datasets, separate beta maps for each subject were computed for the single contrasts exp ng >exp nt and exp uk >exp nt for each of the treatment conditions These maps were combined in a repeated measures ANOVA with the within-subjects factor ‘treatment' (CIT, RBX, PLC). Statistical level at this step was set at p<0.001 in the comparisons of CIT and RBX to PLC, and p<0.01 in the comparison CIT vs RBX. Additional explorative analyses with a more lenient masking, acquired by an addition of the fixed effects masks of the single emotion expectation contrasts vs neutral (exp ng >exp nt, exp uk >exp nt, each at the statistical level of p<0.00001) in each of the treatment conditions, are shown in the supplementary material (Supplementary Table S5). Further analyses of an interaction of medication and emotional expectation are added in the supplementary material as well (Supplementary Table S6).
To analyze the effect of CIT and RBX on ‘pessimistic' information processing, which means that the expectation of unknown cued emotional stimuli evokes a pattern of brain activation resembling the activation during the anticipation of negative, not of positive stimuli, we applied the ‘pessimism contrast' (Herwig et al, 2007c) in each treatment condition: conjunction (exp ng >exp nt) & (exp ng >exp ps) & (exp uk >exp nt) & (exp uk >exp ps). This multiple conjoint contrast was calculated at p<0.05 uncorrected. When computing this ‘pessimism contrast' in the citalopram dataset, we detected only marginal activations, because of the influence of citalopram during the positive expectation, such that we could not compute a reasonable mask for the comparison of the ‘pessimism contrast' to PLC. For this reason, we analyzed the ‘pessimism contrast' only in the comparison of PLC and reboxetine in the combined masked dataset (as described above) with repeated measures ANOVA (p<0.005). In all analyses, we used a common cluster threshold of 5 voxel à 3 × 3 × 3 mm (135 mm3).
To proof the placebo basis of our data and to compare this with earlier reports, we additionally performed an explorative separate analysis of the placebo data concerning the basic contrasts exp ng >exp nt and exp uk >exp nt (for details consider supplementary data). The results were descriptively and qualitatively compared with an earlier dataset (Herwig et al, 2007c). Anatomical regions were identified according to the Talairach and Tournoux system (Talairach and Tournoux, 1988).
We focused on the expectation period as a model for potential depressive expectations toward ambiguous events, and thus the analysis of the presentation period was out of the scope of the study and is not presented here.
RESULTS
Subjective Pharmacological Effects
Subjective reactions to the administered drugs were common, but not pronounced. After reboxetine, subjects reported mostly a sensation of piloerection, impaired visual accommodation, slight xerostomia, nausea and feelings of dysphagia, and rather fatigue than excitement or nervousness; sleep was nearly undisturbed. After citalopram, three subjects reported changes of feeling (intensified feelings) and one experienced acoustical sensations/pseudo-hallucinations (music) during scanning. One subject nearly panicked with citalopram in the scanner (though it was the second time in the scanner), but could be calmed verbally to finish the examination. Problems with sleep were frequent (12/17) after citalopram, mostly problems with falling asleep. Three subjects reported one episode of diarrhea about 4–8 h after intake of citalopram. The reported side effects after placebo were inconsistent, some dizziness, little nausea.
Behavioral Analysis
Single doses of citalopram and reboxetine did not influence the ratings of the emotional valence of the presented pictures after scanning compared with placebo (see Supplementary Table S4).
Effects of Serotonergic Modulation Compared with Placebo
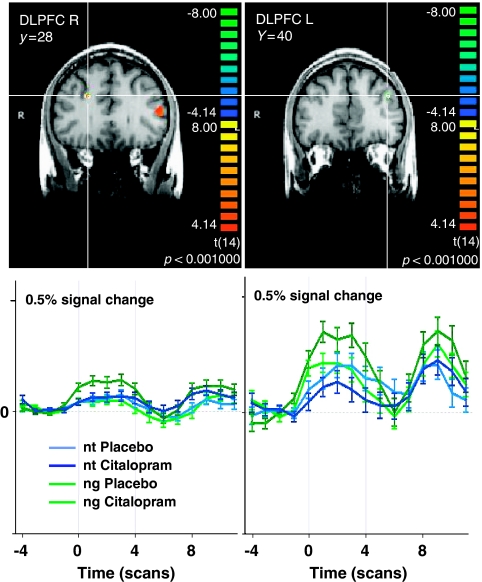
Acute enhancement of serotonergic neurotransmission by citalopram during the anticipation of negative emotional stimuli increased brain activity compared with placebo (Table 1a; Figure 2) in frontal cortical areas as the right middle frontal gyrus (MidFG, Brodmann area (BA) 6/9, part of the dorsolateral prefrontal cortex (DLPFC)), medial prefrontal cortex (MPFC, BA 4/6), and inferior frontal gyrus (IFG, BA 46, within the left ventrolateral prefrontal cortex (VLPFC)). Subcortically, activity was increased in the right dorsal striatal complex, in particular in the caudate body and tail, and in the upper dorsal midbrain. During the anticipation of unknown emotional stimuli (Table 1a), brain activity was increased by citalopram in the same regions of the dorsal striatal complex (caudate body and tail) and additionally in the right inferior parietal lobe (BA 40), but not in frontal regions.
Table 1. ANOVA of the Treatment Conditions on the Conditions exp ng>exp nt and exp uk>exp nt Each in the Combined Masked Datasets.
| Brodmann area | tal x | tal y | tal z | n (voxel) | t max | p max | |
|---|---|---|---|---|---|---|---|
| (a) CIT > PLC | |||||||
| exp ng>exp nt | |||||||
| Med FG R/MPFC | 4 | 3 | 0 | 58 | 1240 | 6.499 | 0.000014 |
| SFG R/MPFC | 6 | 3 | 11 | 55 | 487 | 6.172 | 0.000024 |
| Middle FG/DLPFC R | 6 | 27 33 | −10 −8 | 32 35 | 332 | 5.865 | 0.00004 |
| Middle FG R /DLPFC | 9 | 21 | 29 | 28 | 112 | 5.575 | 0.0007 |
| VLPFC/IFG L | 46 | −49 | 29 | 10 | 308 | 8.178 | 0.000001 |
| Striatum/caudate tail R | 18 | −28 | 24 | 301 | 8.168 | 0.000001 | |
| Striatum/caudate body R | 8 | −7 | 22 | 241 | 7.253 | 0.000004 | |
| Dorsal midbrain L | −3 | −34 | −1 | 550 | 6.137 | 0.000026 | |
| exp uk > exp nt | |||||||
| Striatum/caudate tail R | 18 | −28 | 24 | 301 | 8.817 | 0.000000 | |
| Striatum/caudate body R | 9 | −8 | 22 | 164 | 6.896 | 0.000007 | |
| Inferior parietal lobule R | 40 | 54 | −49 | 39 | 259 | 6.773 | 0.000009 |
| (b) RBX >PLC | |||||||
| exp ng>exp nt | |||||||
| MPFC/SFG L | 6 | −6 | 8 | 65 | 1200 | 4.986 | 0.00032 |
| DLPFC/middle FG L | 46 | −42 | 36 | 19 | 711 | 6.850 | 0.00002 |
| MPFC/Med FG L | 6/8 | 0 | 26 | 37 | 3526 | 6.130 | 0.00005 |
| Post. cingulate L | 23 | −3 | −19 | 24 | 379 | 7.752 | 0.000005 |
| Supramarginal gyrus/STG R | 39/22 | 60 | −40 | 19 | 824 | 5.655 | 0.00011 |
| Ventrolateral thalamus R | 18 | −16 | 1 | 143 | 5.628 | 0.00011 | |
| Mediodorsal thalamus L | −3 | −13 | 4 | 1210 | 7.221 | 0.00001 | |
| Midbrain, SN L | −3 | −16 | −14 | 4319 | 7.381 | 0.00001 | |
| exp uk>exp nt | |||||||
| DLPFC/middle FG L | 46 | −42 | 36 | 19 | 809 | 8.439 | 0.000002 |
| Post. cingulate L | 23 | −3 | −19 | 28 | 1505 | 10.601 | 1.9002 e−07 |
| Post. cingulate L | 23 | −6 | −43 | 25 | 174 | 5.345 | 0.000175 |
| Supramarginal gyrus R/STG R | 39/22 | 63 | −40 | 19 | 174 | 5.246 | 0.00021 |
| Parieto-occipital sulcus R | 19/7 | 21 | −62 | 31 | 145 | 5.416 | 0.00016 |
| Inferior-occipital (lingual) gyrus L | 18 | −6 | −85 | −10 | 174 | 6.278 | 0.00004 |
| (c) CIT >RBX | |||||||
| exp ng>exp nt | |||||||
| MPFC/SFG R | 6 | 10 | 20 | 58 | 100 | 3.900 | 0.00182 |
| Middle FG/DLPFC R | 6 | 27 | −5 | 31 | 1402 | 8.606 | 0.000001 |
| Precentral gyrus L | 6 | −33 | −16 | 56 | 177 | 3.701 | 0.00266 |
| VLPFC/ant ins/IFG L | 47 | −33 | 17 | −11 | 95 | 4.063 | 0.00134 |
| Striatum/caudate body R | 18 | −22 | 29 | 342 | 6.256 | 0.00003 | |
| exp uk>exp nt: | No regions | ||||||
| (d) RBX >CIT | |||||||
| exp ng>exp nt | |||||||
| Mediodorsal thalamus L (FIG) | −3 | −16 | 1 | 938 | 5.344 | 0.00013 | |
| exp uk>exp nt | |||||||
| Cingulate L | 31 | −12 | −40 | 25 | 457 | 4.444 | 0.0007 |
| FFG L | 37 | −36 | −46 | −11 | 253 | 3.939 | 0.0017 |
| Mediodorsal thalamus R (FIG) | 3 | −13 | 1 | 354 | 3.695 | 0.0027 | |
| Midbrain/SN L | −6 | −18 | −14 | 267 | 4.897 | 0.0003 | |
| Extended amygdala L | −21 | −4 | −5 | 487 | 5.427 | 0.0001 | |
| Precuneus L | 7 | −3 | 52 | 46 | 2681 | 5.202 | 0.0002 |
| Inferior occipital gyrus L | 18 | −36 | −82 | −15 | 1448 | 5.400 | 0.0001 |
ant, anterior; CIT, citalopram; DLPFC, dorsolateral prefrontal gyrus; FFG, fusiform gyrus; IFG, inferior frontal gyrus; ins, insula; L, left; med FG, medial frontal gyrus; MPFC, medial prefrontal cortex; ng, negative; nt, neutral; PLC, placebo; post, posterior; R, right; RBX, reboxetine; SFG, superior frontal gyrus; SN, substantia nigra; STG, superior temporal gyrus; tal, Talairach coordinates; uk, unknown; VLPFC, ventrolateral prefrontal gyrus; &, conjunction (AND).
Note: p<0.001 for CIT>PLC and RBX>PLC, p<0.01 for RBX/CIT.
Figure 2.
Serotonergic modulation of brain activation during the anticipation of negative (ng) vs neutral (nt) pictures. Shown are significant brain regions in the comparison Citalopram >Placebo in bilateral DLPFC in coronal brain sections with below the respective averaged event-related time courses of BOLD response. Anticipation period between the gray bars are shown as in all figures, thereafter the perception of the respective picture. Significance level of the random effects analysis p<0.001, color bars representing t-values. R, right; L, left; y, Talairach coordinate indicating the position of the coronal section.
In both analyses, there were no regions with decreased activity after citalopram compared with PLC.
With more lenient masking, additional regions with increased activity because of pretreatment with citalopram in the right insula (BA 13) and in the region of the right bed nucleus of stria terminalis and the adjacent extended amygdalar region were detected in the anticipation of negative emotional stimuli (Supplementary Table S5a). This additional analysis revealed no further relevant activations during the anticipation of unknown emotional stimuli.
The above-mentioned ‘pessimism contrast' (conjunction exp ng >exp nt & exp ng >exp ps & exp uk >exp nt & exp uk >exp ps) revealed no regions in the comparison citalopram vs placebo at the statistical level p<0.005.
The explorative analysis of the interaction between all emotion expectation contrasts and the medication (citalopram/placebo) resulted qualitatively in no further activations (Supplementary Table S6a).
Effects of Noradrenergic Modulation Compared with Placebo
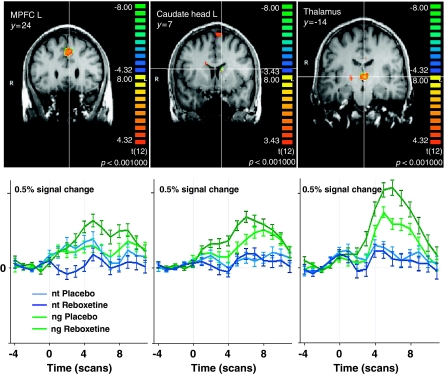
Acute enhancement of noradrenergic neurotransmission by reboxetine increased brain activity compared with placebo during the anticipation of negative emotional stimuli (Table 1b; Figure 3) in frontal regions such as the medial frontal gyrus (BA 6/8) and the left superior frontal gyrus (SFG, BA 6) within the MPFC and the left MidFG (BA 46) as part of the left DLPFC, additionally in right temporal (BA 39/22) and posterior cingulate cortical regions (BA 23). Subcortically, we found increased activations in the medial thalamus bilateral comprising the mediodorsal nuclei, in a region in the left ventrolateral thalamus and in the left medial midbrain, fitting the left substantia nigra.
Figure 3.
Noradrenergic modulation of brain activation during the anticipation of negative (ng) vs neutral (nt) pictures. Shown are significant brain regions in the comparison Reboxetine >Placebo in coronal brain sections with below the respective averaged event-related time courses of BOLD response. Significance level of the random effects analysis p<0.001. MPFC, medial prefrontal cortex; L, left; y, Talairach coordinate indicating the position of the coronal section.
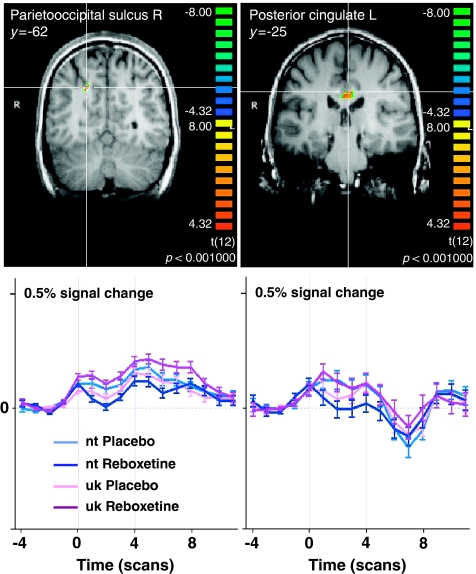
During the anticipation of unknown stimuli (Table 1b; Figure 4), noradrenergic modulation increased brain activity in the left MidFG (BA 46) as part of the left DLPFC, in a right temporal (BA 39/22), and two posterior cingulate cortical regions (both BA 23). In the occipital lobe, there were increased activations in the left inferior occipital gyrus (BA 18) and the right parieto-occipital sulcus (BA 19/7).
Figure 4.
Noradrenergic modulation of brain activation during the anticipation of unknown cued (uk) vs neutral (nt) pictures. Shown are significant brain regions in the comparison Reboxetine >Placebo in coronal brainsections with below the respective averaged event-related time courses of BOLD response. Significance level of the random effects analysis p<0.001. R, right; L, left; y, Talairach coordinate indicating the position of the coronal section.
The analysis of the effect of reboxetine in the mentioned ‘pessimism contrast' (conjunction exp ng >exp nt & exp ng >exp ps & exp uk >exp nt & exp uk >exp ps, Table 2) revealed increased activities compared with PLC in frontal regions as the right SFG (BA 6) within the MPFC, the right IFG (BA 9/44) and the left MidFG (BA 8) within the DLPFC, right precentral gyrus (BA 6/40), in right-sided temporal regions such as the right superior temporal gyrus (BA 22/39) and the dorsal right middle temporal gyrus (BA21/37), and in dorsal cortical regions such as left posterior cingulate cortex (BA 31/23) and bilateral precuneus (BA 7). Subcortically, reboxetine enhanced brain activity compared with PLC in the bilateral mediodorsal thalamus and in a region in the left dorsal midbrain, including the inferior colliculi.
Table 2. ANOVA of the Treatment Condition RBX/PLC on the Conditions exp ng>exp nt and exp uk>exp nt in the Combined Masked Datasets of the Conjoined ‘Pessimism' Contrast.
| Brodmann- area | tal x | tal y | tal z | n (voxel) | t max | p max | |
|---|---|---|---|---|---|---|---|
| (a) exp ng > exp nt | |||||||
| MPFC/SFG R | 6 | 18 | 29 | 53 | 176 | 4.620 | 0.00059 |
| DLPFC/middle FG L | 8 | −45 | 8 | 40 | 1178 | 5.338 | 0.00018 |
| DLPFC/IFG R | 9/44 | 42 | 8 | 22 | 177 | 4.659 | 0.00055 |
| Precentral gyrus R | 6/40 | 61 | −13 | 22 | 786 | 5.388 | 0.00016 |
| Posterior cingulate L | 31 | −3 | −37 | 35 | 302 | 4.347 | 0.00095 |
| STG/supramarginal gyrus R | 22/39 | 60 | −40 | 19 | 783 | 5.655 | 0.00011 |
| Mediodorsal thalamus | 3 | −13 | 2 | 491 | 5.489 | 0.00014 | |
| Midbrain/SN L | −6 | −28 | −23 | 910 | 6.142 | 0.00005 | |
| (b) exp uk > exp nt | |||||||
| MPFC/SFG R | 6 | 17 | 29 | 52 | 300 | 4.337 | 0.00097 |
| Posterior cingulate L/precuneus | 31/23 | −3 | −37 | 35 | 1066 | 5.639 | 0.00011 |
| STG/supramarginal gyrus R | 22/39 | 63 | −40 | 19 | 503 | 5.246 | 0.00021 |
| MTG R | 21/37 | 63 | −34 | 0 | 273 | 5.446 | 0.00015 |
| Precuneus R | 7 | 25 | −61 | 28 | 1618 | 5.680 | 0.00010 |
| Precuneus L | 7 | −14 | −61 | 37 | 258 | 5.159 | 0.00024 |
| Midbrain L | −6 | −28 | −14 | 552 | 6.792 | 0.00002 |
DLPFC, dorsolateral prefrontal cortex; exp, expectation phase; FG, frontal gyrus; IFG, inferior frontal gyrus; L, left; MPFC, medial prefrontal cortex; MTG, middle temporal gyrus; ng, negative; nt, neutral; PLC, placebo; ps, positive; R, right; RBX, reboxetine; SFG, superior frontal gyrus; SN, substantia nigra; STG, superior temporal gyrus; tal, Talairach coordinates; uk, unknown; &, conjunction (AND).
Note: p<0.005; pessimism-contrast: exp ng>exp nt&exp ng>exp ps&exp uk>exp nt&exp uk>exp ps.
The explorative use of more lenient masks revealed qualitatively no additional activation (Supplementary Table S5b), the analysis of the interaction between all emotion expectation conditions and the treatment conditions reboxetine and placebo revealed additionally a region in the right amygdalar complex (Supplementary Table S6b).
In all analyses, there were no regions with higher activity after pretreatment with placebo compared with reboxetine.
Direct Comparison of Serotonergic and Noradrenergic Modulation
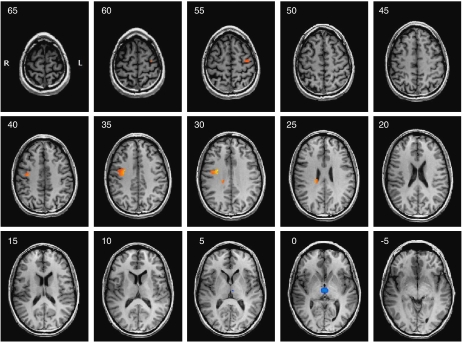
The direct comparison of serotonergic and noradrenergic modulation of brain activity revealed increased activations after pretreatment with citalopram that were limited to the anticipation of negative stimuli (Table 1c; Figure 5): citalopram increased neural responses within frontal regions such as the right SFG (BA 6) within the MPFC, the right MidFG (BA 6) as part of the right DLPFC, and the left precentral gyrus (BA 6) within the left DLPFC as well as the left IFG (BA 47) within the left VLPFC. Subcortically, the right striatal complex (caudate) was more active after pretreatment with citalopram.
Figure 5.
Overview of the serotonergic (red) and noradrenergic (blue) activations during the anticipation of negatively vs neutrally cued pictures. Contrast Citalopram >Reboxetine. Shown are continuous transversal brain sections with a distance of 5 mm ranging from z=65 to z=−5. Significance level of the random effects group comparison p<0.01. R, right; L, left.
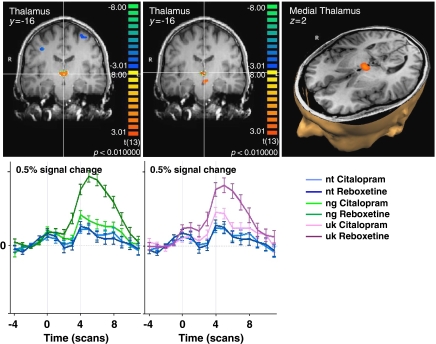
Reboxetine increased brain activity compared with citalopram (Table 1d) during the anticipation of negative stimuli in the bilateral medial thalamus (Figure 6). During the anticipation of unknown stimuli reboxetine compared with citalopram influenced the mediodorsal thalamus, a region in the left ventromedial midbrain, fitting the left substantia nigra, and a region apart from the left lateral globus pallidus, fitting the extended amygdalar complex. Cortically, reboxetine increased activity compared with citalopram during the anticipation of unknown stimuli in the posterior cingulate cortex (BA 31) and left occipital regions such as the left inferior occipital gyrus (BA 18), the left fusiform gyrus (BA 37), and the left precuneus (BA 7).
Figure 6.
Direct comparison of noradrenergic and serotonergic modulation of brain activation during the anticipation of negatively (ng) and unknown cued (uk) vs neutral (nt) pictures in the medial thalamus. Shown is the significant brain region in the comparison Reboxetine >Citalopram in coronal and transversal brain sections with below the respective averaged event-related time courses of BOLD response. Significance level of the random effects analysis p<0.01. y, z, Talairach coordinate indicating the position of the sections.
An explorative analysis with the more lenient masking (Supplementary Table S5c + d) and the analysis of the interaction between all emotion expectation contrasts and the medication citalopram/reboxetine (Supplementary Table S6c) revealed qualitatively no additional results.
Analysis of Placebo Condition
The explorative separate analysis of the placebo condition concerning the basic contrasts exp ng >exp nt and/or exp uk >exp nt (for details consider supplementary material and Supplementary Figure S2) revealed activations in bilateral dorsolateral and ventrolateral prefrontal regions, in bilateral insula, in the midbrain and the medial thalamus, in the region of the left bed nucleus of the stria teminalis as extended amygdala region, and in temporo-occipital and parietal regions. All these areas were also activated in an earlier dataset using the same paradigm, however, with different scanning parameters, in native subjects (Herwig et al, 2007c), according to a qualitative descriptive comparison. Differences to that dataset particularly concerned less or lacking cingulate and distinct amygdalar activations. All areas reported above to be active with serotonergic or noradrenergic modulation were qualitatively essentially comparable in the present placebo condition and in the earlier sample.
DISCUSSION
The aim of this study was to elucidate and differentiate the influence of acute noradrenergic and serotonergic modulation onto brain activations during depressiveness-associated emotional information processing. Our main findings provide evidence for a selective focus of noradrenergic effects on the medial thalamic region and a more prominent dorso- and ventrolateral prefrontal focus of serotonergic effects. Medial and dorsolateral prefrontal regions were modulated by both citalopram and reboxetine, but with a preponderance of serotonergic effects. Other regions, such as the cingulate, known to be activated during the anticipation of emotional stimuli and involved in depressive information processing, were not found to be modulated differentially in our approach compared with placebo.
Primarily, the placebo sample can be regarded as a suitable basis for the comparison with the medication conditions, as it shows comparable activations with an earlier report (Herwig et al, 2007c), particularly concerning the regions reported here to be modulated in the medication conditions. The chosen substances, citalopram and reboxetine, act as considerably selective reuptake inhibitors of serotonin and noradrenaline and have shown to be effective in the treatment of depressive disorders (for a comprehensive review on citalopram: Joubert et al, 2000; on reboxetine: Kent, 2000). In the treatment of depression, the antidepressant effects of all classical substances develop in the first place after repeated application and a duration of days to a few weeks (Katz et al, 2004). Considered biological correlates of this delay of effect of selective reuptake inhibitors are considered to be adaptive changes in the balance of the transmitter systems with initial down-regulation or desensitization of inhibitory autoreceptors. Consecutively, a remodeling takes place at the synaptic and intracellular level, resulting in adaptive neuroplastic changes with increased neuroneogenesis (Nemeroff and Owens, 2002; Berton and Nestler, 2006; Schloss and Henn, 2004). These changes occur at noradrenergic and serotonergic synapses, thus representing the sites of action of the reuptake transporters and accordingly of the reuptake-inhibiting drugs. The effects of single dose application of these antidepressant drugs occur at the same places. The sites of action of antidepressants within functional systems of the brain are so far not clearly characterized. Such knowledge could be valuable for the neurobiological differentiation of subtypes of patients with depressive disorders toward a more serotonergic or noradrenergic dysfunction, leading to a neurobiologically indicated treatment with a more effective response to rather noradrenergic or serotonergic acting antidepressants.
Therefore, we combined a pharmacological with a functional approach. We specifically enhanced the neurotransmission of noradrenaline and serotonin by acutely and selectively blocking the reuptake of the respective transmitters during an emotional task to identify the sites of action of both drugs by differences of brain activity compared with placebo. Considering priming or facilitating actions of these antidepressants, the modulation of brain activity should depend on the activation of the respective regions because of the task: the greater the activation by the task, the greater the influence of the reuptake inhibitor.
The noradrenergic and serotonergic modulated brain regions fit with known localizations of noradrenaline transporters (Kung et al, 2004; Logan et al, 2007; Schou et al, 2005) and serotonin transporters (Varnas et al, 2004; Laruelle et al, 1988), although because of the widespread distribution of serotonergic and noradrenergic neurons and sites of innervation, this confirmation is rather a weak one concerning specificity, increasing the relevance of the additional functional approach.
Only a handful of recent studies have examined acute effects of serotonergic- or noradrenergic-reuptake inhibitors with functional MRI: McKie et al, 2005 infused citalopram and observed changes in brain activity compared with baseline during and after infusion in cortical (frontal, temporal, cingular, occipital) and subcortical (caudate, parahippocampal gyrus/amygdala, thalamus; deactivation in the pons) regions. In a study of Del-Ben et al, 2005, subjects performed three tasks addressing behavioral inhibition, reinforcement processing, and covert emotional face recognition after infusion of citalopram. Citalopram caused changes in similar cortical (frontal, temporal, occipital) and subcortical (dorsal thalamic, amygdalar) regions. In a comparable approach, Anderson et al, 2007 found after infusion of citalopram increased activity in frontal, insular, and occipital regions as well as in the dorsal thalamus during the recognition of disgusted faces. A fourth study concentrated on the changes in amygdalar activity during infusion of citalopram with an emotional face processing task (Bigos et al, 2008). Applying escitalopram, the s-enantiomer of citalopram, during a sustained attention task, Wingen et al, 2008 found increased activity only in right temporal regions, but decreased brain activity in thalamic, frontal, and premotor areas. A recent study (Murphy et al, 2009) applied 20 mg citalopram 3 h before fMRI using the masked and unmasked emotional faces, revealing a reduced activity of the right amygdala after unmasked fearful faces. This reduction of amygdalar activity, although contradictory to the above-mentioned earlier findings in human beings (McKie et al, 2005; Del-Ben et al, 2005; Bigos et al, 2008) and in animals (Burghardt et al, 2007, 2004; Forster et al, 2006), is not in a direct conflict with our results of a stronger modulation of the amygdala by noradrenergic reuptake inhibition.
Untill now, three studies used acutely applied reboxetine. During a categorization task with self-referential emotional words during fMRI, reboxetine induced changes in a fronto-parietal cortical network (Miskowiak et al, 2007). The other studies focused on the amygdala during the perception of emotional facial movies (Onur et al, 2009, Kukolja et al, 2008), the former reporting additionally increased activations in frontal, cingular, and occipital brain regions.
Further studies administered (es)citalopram (Kumar et al, 2008; Rose et al, 2006) and reboxetine (Norbury et al, 2008, Harmer et al, 2004) for 3 or 7 days, which may be at the very beginning of an antidepressive effect (Katz et al, 2004), and at which time regulatory effects are to be supposed.
In this study, serotonergic modulation during the anticipation of negative and unknown emotional stimuli affected prefrontal regions (right MidFG, SFG, DLPFC, left IFG), the dorsal striatum, and the midbrain. These regions have been shown to be involved in the anticipation of negative or unpleasant stimuli in many studies (eg, Abler et al, 2007; Chua et al, 1999; Nitschke et al, 2006; Ploghaus et al, 1999; Herwig et al, 2007a,2007c; Jensen et al, 2003) as well as in the anticipation of stimuli after an undecided cue (‘unknown' condition, Herwig et al, 2007c; Critchley et al, 2001). Most of these studies showed additional activations in the (extended) amygdalar region and in the cingulate cortex, both here not clearly modulated by serotonin. In our study, citalopram modulated both the processing of negative and positive emotional information. Though not primarily addressed in our study, citalopram modulated brain activations during the anticipation of positive stimuli in such a way that it provides a possible explanation why our analysis of the combined ‘pessimism contrast', including the comparison between the unknown and the negative condition with the positive anticipation period, revealed no differences. This points to increased activations because of serotonergic modulation in the same regions activated by the negative and unknown anticipation, which was not the case with placebo or with reboxetine. Accordingly, serotonin modulates regions involved in several cognitive–emotional functions such as response inhibition, motivational learning, valence appraisal, as well as varying effects on anxiety (acute application: increased anxiety, chronic SSRI: reduced anxiety), and mood (review: Cools et al, 2008).
Earlier studies have identified noradrenergic modulation to act functionally more specific on attention and arousal (Berridge, 2008; Aston-Jones et al, 1999), additionally with possible implications in anxiety and fear processing (Onur et al, 2009). In particular, the mediodorsal nucleus of the thalamus, which was in this study strongly activated by reboxetine, is known to be part of the ascending reticular activating system, the rostral continuation of the reticular formation (Van der Werf et al, 2002). Medial thalamic regions further receive input from viscero-sensitive and pain mediating brainstem areas and are considered to form a relay within the visceroceptive pathway toward, for example, insular regions (Vogt, 2005; Craig, 2002). Our data of modulating influences of noradrenergic enhancement during the anticipation of negative and unknown stimuli in prefrontal, cingulate, temporal, parieto-occipital, and thalamic regions fit with the anatomical and neurochemical distribution of noradrenergic innervation in the brain with a lack of noradrenergic innervation in the striatum including the caudate (eg, Kung et al, 2004; Wilson et al, 2003; Schou et al, 2005; Varnas et al, 2004; Houle et al, 2000).
Concerning the post-scanning rating of the emotional pictures, we found no significant influence of the medication. Earlier studies found influences of the acute administration of citalopram (eg, Harmer et al, 2003; Bhagwagar et al, 2004) and reboxetine (De Martino et al, 2008; Miskowiak et al, 2007) on some emotional paradigms; others, however, found no significant behavioral effects of acute doses of citalopram (Del-Ben et al, 2005; Wingen et al, 2008) and reboxetine (O'Carroll and Papps, 2003) in emotional tasks.
From a systemic view, antidepressant medication was recently proposed to affect primarily basic emotion processing regions as the amygdala, rebalancing the dysfunctional interrelation to the prefrontal cortex in a bottom-up-mode (DeRubeis et al, 2008). Our data expand this model by adding several prefrontal regions as well as thalamic and striatal areas as primary places of action of antidepressant substances. Thereby, citalopram may be suggested to act more on regions mediating behavior planning and executive control within the DLPFC (Fuster, 2000) and cognitive–emotional integration in the VLPFC (Mayberg, 2003). Reboxetine can be suggested to modulate viscero-sensitive afferences and arousal functions in medial thalamic regions (Vogt, 2005; Berridge, 2008; Aston-Jones et al, 1999). All these functional domains can be considered to be affected in depression (DeRubeis et al, 2008), as shown in our specific task (Herwig et al, 2009). However, until now such findings neither provide a functional differentiation into subtypes of depression nor treatment response prediction.
With respect to the aim of improving therapy strategies for depression and of developing a personalized treatment approach in mind, future patients could be examined by fMRI with a task similar to ours to identify dysfunctional brain regions in the domain of emotion processing on the single subject level. According to this pattern, those antidepressants modulating the affected areas could be selected, which increases the activity in the respective dysfunctionally hyperactive region a dysfunctional signal in the sense of a pathological attractor for the information processing in the respective functional module. The modulatory antidepressant may thus promote neuroplastic adaptations representing the therapeutic effect. On the basis of our findings, hyperactivation in medial thalamic regions, for instance, might be better modulated with a noradrenaline-prone antidepressant. The principles of this approach may be a basis for personalized treatment based on neurobiological findings in psychiatric disorders.
Reflecting possible limitations of our study, we consider the relatively low number of analyzable subjects and scans with the analysis of 13–15 subjects corresponding to 26–30 scans in each comparison. However, the repeated-measures cross-over design of the study provided sufficient statistical power. Another specificity of our study is the lack of direct behavioral control or measure during the scan. We dispensed intentionally of any such direct behavioral measure, as the preparation and execution of an answer in any form would have been a distraction from the task and could have interfered with the addressed brain activities because of action preparation and cognitive evaluation. Participants confirmed their attention to the task in the interview after scanning. This was further verified by controlling individual brain activation in visual areas. Furthermore, the study was performed with healthy volunteers. The validity of the results for patients with depressive disorder still has to be shown.
In conclusion, the study presents a useful way of differentiating neurochemical subsystems involved in the processing of emotional information by combining functional imaging during an emotional task with an acute and specific pharmacological enhancement of serotonergic and noradrenergic neurotransmission in healthy subjects. This differentiation revealed common influences of both neurotransmitters (with a slight overweight of serotonergic effects) in medial and dorsolateral prefrontal regions during the anticipation of negatively and ‘unknown' (ambiguously) cued emotional stimuli. The bilateral medial thalamic region was selectively affected by noradrenergic modulation, unlike medial and dorsolateral prefrontal regions, which were primarily modulated by serotonergic enhancement. This combined method of pharmaco-fMRI points to regions, which in future could possibly be used to detect endophenotypes of depressive syndromes in patients responding primarily to serotonergic- or noradrenergic-acting antidepressants. Next steps in the development of a neurobiologically based administration of differential therapy in depression will be the application of these findings to depressed patients with a prospective view concerning the response to more noradrenergic or serotonergic acting antidepressants.
Acknowledgments
This work was funded by the Swiss National Foundation SNF No. 3200B0-112631) granted to Uwe Herwig. We thank Dr Th Loenneker and Dr J Lichtensteiger for technical help with MRI-scanning and organization, Dr F Esposito from Brainvoyager for help with the analysis, and Dr C Obermann for helpful comments including language editing on this paper.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
DISCLOSURE
The authors declare no conflict of interest, except for income received from the primary employer.
Supplementary Material
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;6:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Del-Ben CM, McKie S, Richardson P, Williams SR, Elliott R, et al. Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport. 2007;13:1351–1355. doi: 10.1097/WNR.0b013e3282742115. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;3:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;9:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;9:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. Harper & Row: New York; 1967. [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;1:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;2:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am J Psychiatry. 2004;1:166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;13:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry. 2008;12:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;10:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;12:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;6 (Pt 1:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;1:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;8:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;2:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- De Martino B, Strange BA, Dolan RJ. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology (Berl) 2008;1:127–136. doi: 10.1007/s00213-007-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;9:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy vs medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;10:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;2:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Eggert D. Eysenck-Persönlichkeits-Inventar EPI. Verlag für Psychologie, Dr CJ Hogrefe: Göttingen; 1974. [Google Scholar]
- Eysenck HJ, Eysenck SB. Manual of the Eysenck Personality Inventory. University of London Press: London; 1964. [Google Scholar]
- Fleishaker JC. Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin Pharmacokinet. 2000;6:413–427. doi: 10.2165/00003088-200039060-00003. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;2:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;2:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Exp Brain Res. 2000;1:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;4:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: current status and unresolved issues. Curr Dir Psychol Sci. 2008;2:159–163. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;4:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;1:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive vs negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;7:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli—an fMRI study. Psychiatry Res. 2007a;1:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, Brühl A, Kottlow M, Schreiter-Gasser U, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007b;2:652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Herwig U, Brühl AB, Kaffenberger T, Baumgartner T, Boeker H, Jäncke L. Neural correlates of ‘pessimistic' attitude in depression. Psychol Med. 2009;1:1–12. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Baumgartner T, Jäncke L. Neural correlates of a ‘pessimistic' attitude when anticipating events of unknown emotional valence. Neuroimage. 2007c;2:848–858. doi: 10.1016/j.neuroimage.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61 (Suppl 6:4–6. [PubMed] [Google Scholar]
- Holsboer F. How can we realize the promise of personalized antidepressant medicines. Nat Rev Neurosci. 2008;8:638–646. doi: 10.1038/nrn2453. [DOI] [PubMed] [Google Scholar]
- Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;11:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Citalopram—pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry. 1982;3:277–295. doi: 10.1016/s0278-5846(82)80179-6. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;6:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Joubert AF, Sanchez C, Larsen F. Citalopram. Hum Psychopharmacol. 2000;6:439–451. doi: 10.1002/1099-1077(200008)15:6<439::AID-HUP222>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, et al. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology. 2004;3:566–579. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;9207:911–918. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Fink GR, et al. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;48:12868–12876. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;8:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Kung M-P, Choi S-R, Hou C, Zhuang Z-P, Foulon C, Kung HF. Selective binding of 2-[125I]iodo-nisoxetine to norepinephrine transporters in the brain. Nucl Med Biol. 2004;5:533–541. doi: 10.1016/j.nucmedbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-6. Center for Research in Psychophysiology, University of Florida: Gainesville, FL; 2005. [Google Scholar]
- Laruelle M, Vanisberg MA, Maloteaux JM. Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry. 1988;3:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar (Testmappe mit Handanweisung, Fragebogen STAI-G Form X1 und Fragebogen STAI-G Form X2) Beltz: Weinheim; 1981. [Google Scholar]
- Logan J, Wang G-j, Telang F, Fowler JS, Alexoff D, Zabroski J, et al. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nucl Med Biol. 2007;6:667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, et al. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 2005;4:680–686. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ. Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage. 2007;3:904–911. doi: 10.1016/j.neuroimage.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;6:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002;5 (Suppl:1068–1070. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;1:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry. 2008;11:1011–1020. doi: 10.1038/sj.mp.4002091. [DOI] [PubMed] [Google Scholar]
- O'Carroll RE, Papps BP. Decision making in humans: the effect of manipulating the central noradrenergic system. J Neurol Neurosurg Psychiatry. 2003;3:376–378. doi: 10.1136/jnnp.74.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C, et al. Noradrenergic enhancement of amygdala responses to fear. Soc Cogn Affect Neurosci. 2009;2:119–126. doi: 10.1093/scan/nsn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;5:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;5422:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology (Berl) 2006;3:339–347. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;5:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schloss P, Henn FA. New insights into the mechanisms of antidepressant therapy. Pharmacol Ther. 2004;1:47–60. doi: 10.1016/j.pharmthera.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schou M, Halldin C, Pike VW, Mozley PD, Dobson D, Innis RB, et al. Post-mortem human brain autoradiography of the norepinephrine transporter using (S,S)-[18F]FMeNER-D2. Eur Neuropsychopharmacol. 2005;5:517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;2:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety Inventory, Manual for the State-Trait-Anxiety Inventory. Consulting Psychologist Press: Palo Alto, CA; 1970. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Thieme Medical: New York; 1988. [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;2-3:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;2–3:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;3:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;7:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Patrick Johnson D, Mozley D, Hussey D, Ginovart N, Nobrega J, et al. Synthesis and in vivo evaluation of novel radiotracers for the in vivo imaging of the norepinephrine transporter. Nucl Med Biol. 2003;2:85–92. doi: 10.1016/s0969-8051(02)00420-1. [DOI] [PubMed] [Google Scholar]
- Wingen M, Kuypers KP, van de Ven V, Formisano E, Ramaekers JG. Sustained attention and serotonin: a pharmaco-fMRI study. Hum Psychopharmacol. 2008;3:221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.