Abstract
Psychiatric neurosurgery, specifically stereotactic ablation, has continued since the 1940s, mainly at a few centers in Europe and the US. Since the late 1990s, the resurgence of interest in this field has been remarkable; reports of both lesion procedures and the newer technique of deep brain stimulation (DBS) have increased rapidly. In early 2009, the US FDA granted limited humanitarian approval for DBS for otherwise intractable obsessive-compulsive disorder (OCD), the first such approval for a psychiatric illness. Several factors explain the emergence of DBS and continued small-scale use of refined lesion procedures. DBS and stereotactic ablation have been successful and widely used for movement disorders. There remains an unmet clinical need: current drug and behavioral treatments offer limited benefit to some seriously ill people. Understandings of the neurocircuitry underlying psychopathology and the response to treatment, while still works in progress, are much enhanced. Here, we review modern lesion procedures and DBS for OCD in the context of neurocircuitry. A key issue is that clinical benefit can be obtained after surgeries targeting different brain structures. This fits well with anatomical models, in which circuits connecting orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), basal ganglia, and thalamus are central to OCD pathophysiology and treatment response. As in movement disorders, dedicated interdisciplinary teams, here led by psychiatrists, are required to implement these procedures and maintain care for patients so treated. Available data, although limited, support the promise of stereotactic ablation or DBS in carefully selected patients. Benefit in such cases appears not to be confined to obsessions and compulsions, but includes changes in affective state. Caution is imperative, and key issues in long-term management of psychiatric neurosurgery patients deserve focused attention. DBS and contemporary ablation also present different patterns of potential benefits and burdens. Translational research to elucidate how targeting specific nodes in putative OCD circuitry might lead to therapeutic gains is accelerating in tandem with clinical use.
Keywords: biological psychiatry, neurosurgery, obsessive-compulsive disorder, ablation, deep brain stimulation, gamma knife
INTRODUCTION
Although neurosurgery for psychiatric illness in the form of stereotactic ablation has continued since the 1940s, it has reemerged as a focus of tremendous interest in the last decade. There are several key reasons for this. The conception of major psychiatric illnesses as disorders of the brain is well established. There is a much greater focus on the neurocircuitry involved in the symptoms of these illnesses and, in particular, in the anatomy underlying therapeutic change. There has been refinement in both stereotactic ablation and in the newer technique of deep brain stimulation (DBS), introduced in its modern form in the 1980s and now a standard of care for movement disorders. Most importantly, there is recognition that despite conscientious use of our best empirically based behavioral and medication treatments, some patients continue to suffer severe symptoms and marked impairment. Although the enthusiasm with which psychiatric neurosurgery is sometimes greeted hearkens back to that in the beginning of the era of freehand ‘psychosurgery,' when prefrontal lobotomy saw wide, indiscriminate use, the parallels are quite limited. Those crude operations had some therapeutic effects, but were accompanied by unacceptable and even tragic adverse effects. Techniques, procedures, and practices have evolved steadily since then. Contemporary ablation reproducibly places lesions in specific brain targets, guided by magnetic resonance imaging (MRI), stereotactic instruments, and specialized software. Radiosurgery, a newer method dating from the 1970s, obviates the need for craniotomy. DBS does require craniotomy to implant stimulating electrodes in specific brain targets, but is intentionally nonablative, and permits flexible and largely reversible modulation of brain function. For any of these procedures, the criteria for patient selection are strict, and the process of case review to determine appropriate candidacy has been formalized. Currently, neurosurgery is predominantly reserved for patients with severe, incapacitating obsessive-compulsive disorder (OCD) or major depression who have failed to benefit after exhaustive conventional treatment. At leading centers, surgery does not occur unless a multidisciplinary committee approves it for a given patient, whose informed consent must be clear and documented. Considerable clinical data supports the effectiveness and safety of modern neurosurgery. Psychiatric neurosurgery has consistently yielded substantial improvement in symptoms and functioning in ∼35–70% of cases, with morbidity and mortality drastically lower than earlier procedures. Major centers continue to gather information prospectively. Controlled trials are underway using gamma ventral capsulotomy for OCD, and DBS for OCD or for major depression. This review focuses on surgery for OCD.
The scientific context of psychiatric neurosurgery is developing rapidly. Although the notion that cortico-limbic systems were important in disordered behavior influenced the early lesion procedures, it largely was developed empirically. Although sometimes criticized for this reason, as for any clinical therapy the relevant issues are safety and efficacy, and not correction of pathophysiological processes that are not yet fully understood. However, in addition to the promise of modern lesion procedures and DBS as clinical treatments, they permit testing of hypotheses derived from the results of lesions or from human neuroimaging. Psychiatric neurosurgery is thus developing in the current era of translational research. Transfer of data from clinical results to cross-species anatomical, neuroimaging, and physiological studies of neural networks involved promise to illuminate mechanisms of therapeutic action. This should lead to improvements in current ablative procedures and DBS. Beyond that, other invasive device-based techniques, including electrical stimulation via electrode grids on the cortical surface or DBS that can respond selectively to patterns of brain activity putatively associated with symptoms (‘responsive' or ‘closed loop' stimulation) may be informed by such work. Such methods are early in development and will not be discussed further here. Likewise, to the extent that there are a variety of less invasive methods evolving for therapeutic neuromodulation, including transcranial magnetic stimulation (TMS), such modalities are reviewed elsewhere in this volume by George and Aston-Jones.
Obsessive-compulsive disorder is typically a chronic disorder and affects 2–3% of the population. It is characterized by persistent, intrusive thoughts and impulses (obsessions), and repetitive intentional behaviors (compulsions). These symptoms persist despite individuals' attempts to resist them and are accompanied by marked and often overwhelming anxiety. It is estimated that over 20% of OCD patients may be refractory to available psychological and pharmacological treatments (Husted and Shapira, 2004). The pathogenesis of OCD remains unknown. In contrast, the pathophysiology of OCD, although incompletely understood, appears from converging lines of evidence to involve abnormal functioning in medial and orbital frontal–basal ganglia–thalamic circuits. As discussed in detail below, stereotactic neurosurgical lesions in the anterior limb of the internal capsule, the anterior cingulate, and/or the subcaudate region, all of which affect this circuit, appear beneficial in the treatment of highly refractory OCD. The same seems to be true of DBS within this circuitry.
Only a relatively small subgroup of OCD patients would be considered potential candidates for surgery. These individuals are severely and chronically impaired by the illness, and fail to improve adequately after aggressive use of behavioral and medication treatments. Although many more patients have undergone ablation than DBS thus far (Goodman and Insel, 2009; Greenberg et al, 2003), the use of stimulation is becoming more widely disseminated. We discuss the results of both kinds of surgical treatment in the context of anatomical models of OCD pathophysiology, which have been in development since the 1980s. Several different anatomical targets have been used for either technique with apparent success, a key point for refining anatomical models of OCD.
CLINICAL FEATURES OF OCD
The disorder's defining symptoms are obsessions (unwanted, recurrent intrusive thoughts that cause anxiety) and compulsions (repetitive behaviors that the patient feels driven to perform, often in response to an obsession) (APA, 1994). Onset commonly occurs before adulthood (Rasmussen and Eisen, 1992). In contrast to schizophrenia, major depression, or bipolar disorder, OCD can appear in full-blown form even before puberty (Grados and Riddle, 2008). In family studies, onset of obsessions or compulsions before age 18 is highly predictive of OCD in relatives (Nestadt et al, 2000). Illnesses appearing during the course of OCD include, most commonly, affective illness, anxiety disorders, and putative ‘OCD spectrum' conditions. The latter prominently include tic disorders (Koran, 1999). OCD tends to be chronic (Pinto et al, 2006), an important feature in consideration of surgeries that can have irreversible effects (intended or unintended, see below). Potential surgical candidates would come from clinical settings, since aggressive treatment trials are required before evaluation for surgery. In a naturalistic clinical sample (Mancebo et al, 2008), 91% of OCD patients met lifetime criteria for at least one other DSM-IV axis I diagnosis. Over a third (37%) of the sample had severe OCD symptoms (total Yale-Brown Obsessive Compulsive Scale (YBOCS) severity ranged from a score of 24–31). Six percent had extreme OCD symptoms (YBOCS severity 32–40, the scale maximum). The most common co-occurring disorders were major depression, social phobia, alcohol use disorder, panic, and specific phobia. Three-fourths of candidates (74%) met lifetime mood disorder criteria, 52% had a lifetime anxiety disorder diagnosis, and 25% had a lifetime substance use disorder. Over a third (38%) were unable to work due to psychopathology, and 14% of the sample was receiving disability benefits due to OCD (Mancebo et al, 2008). The latter finding was not surprising, as OCD has been ranked as a major cause of disability in industrialized countries (Murray and Lopez, 1997). Quality of life can be severely affected (Eisen et al, 2006). Despite the relatively large percentage of patients who are severely affected, chronically ill, and highly refractory to treatment, only a subset would be candidates for surgery. That subgroup has been little studied, either in terms of phenomenology or neurocircuitry. Some comorbidities, such as substance abuse and severe personality disorders, have historically been strong relative contraindications for neurosurgery. Other issues affecting the consideration of surgery include psychosocial stability, proximity to the treatment center, and resources adequate for long-term follow-up. These factors are particularly important for DBS, in which stimulation requires ongoing adjustment and monitoring, and devices must be surgically replaced, a process that incurs significant costs that are often not fully covered by health insurance. Insurance coverage for ablative procedures is also an issue, although after the procedures, follow-up care is generally conventional medication and behavioral treatment.
TREATMENTS
Conventional treatments for OCD are well established (Bandelow et al, 2008). Cognitive-behavior therapy, including exposure and ritual prevention and medications, particularly serotonin reuptake inhibitors (SRIs), are first-line treatments. Meta-analytic studies have consistently found that both classes of treatment are effective (Eddy et al, 2004; Kobak et al, 1998). Improvement after medication is usually partial. Consistent with this, a 35% reduction in YBOCS severity scores is a typical response criterion in pharmacological trials. The same criterion is usually applied in surgical studies (below). In a naturalistic clinical study, over one-third of participants receiving recommended doses of SRIs did not perceive substantial long-term benefit from pharmacotherapy (Mancebo et al, 2006). Interestingly, improvement in OCD symptoms may not correspond closely to improvement in quality of life after treatment (Tenney et al, 2003). This issue bears particular scrutiny with regard to psychiatric neurosurgery.
Symptom reduction reported after a course of behavior therapy is usually greater than that after SRI monotherapy (Foa et al, 2005). However, substantial proportions of patients may refuse behavior therapy or drop out before completing a course. Concurrent axis I disorders or personality disorder traits can influence behavior therapy completion and outcomes (Steketee et al, 2001). Enhancing the effectiveness of behavior therapy pharmacologically, for example with -cycloserine, a partial agonist at the N-methyl--aspartate glutamatergic receptor, is an intriguing strategy that has generated considerable interest (Wilhelm et al, 2008), although it remains experimental. A key issue when surgery is considered, for both medication and behavioral treatments, is to systematically determine whether trials have been adequate and exhaustive (Gabriels et al, 2003) on the basis of the best available information on treatment of refractory patients (Husted and Shapira, 2004). Residential treatment using specialized programs for highly refractory patients (Stewart et al, 2005) should be considered before surgery.
POTENTIAL RELEVANCE OF OCD SUBTYPES TO NEUROSURGERY
Ablation or DBS might preferentially affect some clinical features of OCD patients, but have little to no effect on others. Obsessions and compulsions themselves are heterogeneous. A large body of work using factor analysis has delineated dimensional structures for the OCD symptoms measured by the YBOCS Symptom Checklist (Goodman et al, 1989). Collectively, between three and six dimensions have been identified (Baer, 1994; Bloch et al, 2008; Leckman et al, 2001; Pinto et al, 2008). Most relevant here are intriguing findings that different symptom dimensions might have at least partly separable neural correlates (Mataix-Cols et al, 2004; Phillips et al, 2000; Rauch et al, 1998; van den Heuvel et al, 2009). Similarly, neuroimaging findings in compulsive hoarding may differ from those in OCD patients without hoarding symptoms (Saxena et al, 2004). In addition, the form of OCD symptoms varies as well as the content. Compulsive urges can be tied to obsessions about a feared consequence, as in the familiar examples of compulsive washing to prevent ‘contamination' or in checking rituals to prevent harm. However, individuals with OCD often report compulsions that must be repeated until a feeling that something is ‘just right' is attained. Such compulsions are motivated by a sense of ‘incompleteness,' not pathological fear (Rasmussen and Eisen, 1994) (Coles et al, 2005; Summerfeldt, 2004).
Patients vary in other ways that can be described categorically, such as current and lifetime comorbidity (see above and Nestadt et al, 2003) and patterns of psychopathology in their relatives (Nestadt et al, 2001). Affective features of illness are worth special attention in OCD neurosurgery for several reasons. Major depression is the most frequent comorbid condition in OCD, and the earlier the OCD onset, the more likely depression is to emerge (Hong et al, 2004). Over decades, reports have consistently indicated that depression and OCD improve after the same lesion procedures, including anterior cingulotomy, subcaudate tractotomy, anterior capsulotomy, and limbic leucotomy (for review see Greenberg et al, 2003). The same seems to be true after DBS of the ventral anterior limb of the internal capsule/ventral striatum (VC/VS), after which an otherwise intractable depression has improved (Malone et al, 2009). However, some co-occurring disorders might worsen after surgery, as in the case of a patient whose OCD had an excellent response to anterior thermocapsulotomy (described below), which was largely negated by the worsening of kleptomanic and bulimic symptoms, which had existed before surgery (Albucher et al, 1999). OCD sufferers also vary in co-occurrence of categorical personality disorders and in how they vary along personality dimensions (Samuels et al, 2000). These are the factors that may influence resilience in the face of severe chronic illness, likelihood of treatment response (Corchs et al, 2008), and, in terms of DBS particularly, in the propensity to conscientiously adhere to the treatment regimens that must include close follow-up over the long term.
The major relevance of OCD subtypes for neurosurgery is likely to be in response prediction. Current psychiatric neurosurgical procedures, although generally well tolerated, are not innocuous. Patients least likely to benefit should be identified so that they can be spared from the risks of surgery and potential later complications. It should be emphasized, although how useful phenomenological data will prove to be in this regard is unknown. Clinical observations in a long-term, open-label study suggests that responses of the VC/VS to DBS might be greater in patients in whom OCD symptoms are associated with a feared consequence than when they are primarily motivated by incompleteness (Greenberg et al, 2006).
NEUROCIRCUITRY MODELS OF OCD
Neuroimaging studies support the central role of frontal-basal ganglia–thalamic circuits in the pathophysiology of OCD. Converging lines of evidence have pointed to the abnormalities in this circuit, specifically involving orbital frontal cortex (OFC) and anterior cingulate cortex (ACC), as well as the striatum and medial thalamus.
Structural Neuroimaging
Structural neuroimaging studies of OCD have found subtle differences in OFC, striatal, and thalamic volumes in subjects with OCD vs controls (Jenike et al, 1996; Robinson et al, 1995). Psychotropic drug-naive pediatric patients had more gray matter in regions comprising cortical–striatal–thalamic—cortical (CSTC) circuits, consistent with functional neuroimaging reporting hypermetabolism and increased regional cerebral blood flow in striatal, anterior cingulate, and orbital frontal regions in the resting state (Szeszko et al, 2008). A preliminary voxel-based morphometry study found significantly greater gray matter density within the hypothesized circuitry in pediatric OCD patients compared with healthy controls or their unaffected siblings (Gilbert et al, 2008). Moreover, magnetic resonance spectroscopy (MRS) studies have provided complementary evidence of neurochemical abnormalities in this circuitry. Using MRS, investigators have found reductions in N-acetyl aspartate (a purported marker of healthy neurons within the striatum and medial thalamus in OCD (for example, see Bartha et al, 1998; Ebert et al, 1997)).
Connectivity
Anatomical connections within the circuitry implicated in OCD may also be abnormal. A diffusion tensor MRI (DTI) study comparing individuals with OCD and healthy subjects found increased fractional anisotropy (a measure of white matter structure) in the cingulum bundle bilaterally and in the left anterior limb of the internal capsule compared with healthy controls. This was interpreted as evidence of abnormal connections between nodes within putative OCD circuitry linked by these tracts (Cannistraro et al, 2007) and as consistent with observations that ablating or modulating these white matter pathways (as in anterior cingluotomy, anterior capsulotomy, or DBS on the VC/VS) is associated with therapeutic improvement.
Functional Neuroimaging
Functional neuroimaging studies have consistently documented hyperactivity at rest in CSTC circuits when comparing OCD subjects with controls. Further, this regional hyperactivity is accentuated during provocation of the OCD symptomatic state vs control states (McGuire et al, 1994; Rauch et al, 1994). Conversely, several studies have consistently found reduction in activity in these same regions after successful treatment of OCD, regardless of the mode of treatment, including pharmacological (for example see Baxter et al, 1992; Swedo et al, 1992), behavioral (for example see Baxter et al, 1992; Schwartz et al, 1996), and neurosurgical (Mindus et al, 1986) therapies. Interestingly, there is also imaging evidence to suggest that at pretreatment, regional activity within OFC predicts subsequent response to treatment with medication or behavior therapy (for example see Brody et al, 1998; Rauch, 2003; Saxena et al, 1999; Swedo et al, 1992). Another structure central to putative OCD circuitry, the basal ganglia, has been studied using PET imaging combined with a task developed specifically to probe this region, implicit sequence learning. Although OCD patients and healthy volunteers learnt the serial reaction time task equally well, patterns of concomitant brain activation were different. In controls, there was bilateral inferior striatal activation, whereas OCD patients did not recruit inferior striatum, but instead showed bilateral medial temporal activation, which is normally associated with explicit, or conscious, processing (Rauch et al, 1997). Although a subsequent study using fMRI failed to find overall group differences in striatal recruitment between OCD patients and controls, it replicated abnormal recruitment of medial temporal (hippocampal) region activation during implicit sequence learning. OFC activity during the task was also greater in patients vs controls. The authors (Rauch et al, 2007) suggested that the failure to replicate the striatal abnormality found in the earlier study might be attributable to differences among OCD symptom dimensions.
Integrating Anatomical and Neurochemical Models
Work integrating these perspectives is less advanced than studies of either the neurocircuitry or neuropharmacology of OCD alone. In part this is because individual research groups have tended to focus primarily on one aspect or the other, and not necessarily on relationships between them.
Nevertheless, anatomical hypotheses derived from neuroimaging (Dougherty et al, 2004), clinical pharmacology of OCD, and basic neuropharmacology of the implicated circuitry (Blier et al, 2006; Greenberg et al, 1997; Westenberg et al, 2007) provide some key starting points. As already discussed, human neuroimaging data suggest that abnormalities in OFC/ACC–basal ganglia–thalamic circuitry are central to the pathophysiology of OCD and responses to treatment. In particular, the magnitude of OFC activity is proportional to symptom severity and pretreatment activity within this same region predicts subsequent medication response. In this regard, there is more evidence that anti-OCD medications act through serotonin (5-HT) systems (Benkelfat et al, 1989; Greenberg et al, 1998) than that 5-HT dysfunction is necessarily central to OCD pathogenesis. However, recent work suggests that variation in serotonergic genes, for example, those expected to result in less synaptic serotonin, might contribute to OCD in at least some cases (Ozaki et al, 2003; Hu et al, 2006; Wendland et al, 2008). Interestingly, a very early statement of a ‘serotonin hypothesis' of OCD (Yaryura-Tobias et al, 1977) also noted how such putative neurochemical abnormalities likely occurred in the context of brain anatomical networks that work early in DBS implicated in emotion and motivation more generally.
Nodes within the putative OCD circuitry have been proposed to be sites of action for pharmacotherapy. That OFC activity is associated with OCD symptoms is well established. That it represents a substrate for pharmacotherapy also resonates with the results from animal studies; chronic administration of selective serotonergic reuptake inhibitors in rodents leads to serotonergic receptor changes in OFC over the same time course that antiobsessional effects are observed in humans (Mansari et al, 1995). Another rodent study suggested that activation of normosensitive postsynaptic 5-HT2-like receptors may mediate the effect of enhanced 5-HT release in the OFC after SRI treatment (El Mansari and Blier, 2005). Moreover, elevated glutamatergic transmission from OFC/ACC to striatum has been inferred from MRS measurements of an elevated glutamate index (Glx peak) within the striatum that is correlated with OCD symptom severity, and returns toward normal with successful treatment (Rosenberg et al, 2000). The interest in using imaging phenotypes for genetic studies is rapidly growing. For example, association was identified between a polymorphism of the ionotropic, N-methyl--aspartate 2B (GRIN2B) glutamate receptor and decreased glutamate (Glx) in the ACC, a region consistently implicated in OCD (Arnold et al, 2009).
There is also evidence implicating dopaminergic systems in OCD. Addition of dopamine receptor antagonists to SRI treatment may result in therapeutic benefit in patients poorly responsive to SRI monotherapy (Denys et al, 2004), suggesting that modulation of dopaminergic mechanisms may have therapeutic effects. More direct evidence for a possible role of dopamine systems in OCD pathophysiology comes from neuroimaging findings of elevated dopamine transporter density in the caudate nucleus and putamen in psychotropic medication-naïve OCD patients (van der Wee et al, 2004). Moreover, genetic research supports a potential role for catechol-O-methyl-transferase and D2 gene variants in at least a subgroup of male OCD sufferers with early symptom onset (Denys et al, 2006).
Integrating Cognitive Findings With Anatomical Models
We also note recent attempts to integrate anatomical models with cognitive models of OCD. This work is also at a relatively early stage, but is also expanding rapidly, in part because of the promise of using cognitive functions to derive endophenotypes of brain information processing abnormalities in OCD in genetic and other studies. For example, as discussed above, implicit sequence learning has been found to be abnormal in OCD using cognitive probes and/or functional neuroimaging (Kathmann et al, 2005), further implicating fronto-striatal dysfunction in the illness. Other emerging foci of interest as putative cognitive endophenotypes in OCD are indices of inhibitory functions and behavioral flexibility (Bannon et al, 2002; Chamberlain et al, 2005, 2007). In terms of neurocircuitry, abnormally reduced activation of several cortical regions, including lateral OFC, was found on functional MRI during reversal learning in both OCD patients and clinically unaffected close relatives (Chamberlain et al, 2008). A recent proposal of how anatomical and psychological models of OCD might be integrated provides a heuristic for further work in this highly important area (Huey et al, 2008).
In summary, as will be discussed further below, multiple lines of evidence, including multimodal human imaging data, pharmacological studies, and research with cognitive probes supports neurocircuitry models of OCD. These models are, in turn, consistent with the empirically developed targets for modern ablative neurosurgical therapies and DBS (see Rauch, 2003 for review).
SURGERY FOR OCD: HISTORY
Lobotomy emerged before the era of empirical psychiatry, when therapeutic nihilism for severe disorders was pervasive. The ethical and regulatory context for research and clinical treatment, as we know them today, essentially did not exist. Beginning in the 1930s, reports of successful outcomes after the procedure, which from the beginning included patients with severe obsessional symptoms, were greeted with much publicity (NewYorkTimes, 1937). As has been well documented (Pressman, 1998; Valenstein, 1986), lobotomy's use accelerated at a tremendous rate in the mid-20th century, until effective therapeutic alternatives appeared and its heavy adverse effect burden was recognized (El-Hai, 2005). There had been skepticism from the beginning (Fins, 2003), but it was only decades later, when wide use of lobotomy had already faded in favor of a dramatically more limited use of stereotactic procedures, that opposition rose to the national level in the US and elsewhere (National Commission, 1977). Interestingly, early proponents, even Walter Freeman, who eventually developed an evangelist's zeal for lobotomy, had actually begun by emphasizing the role of careful patient selection and the need for systematic, multidisciplinary study of surgical outcomes (Freeman et al, 1942).
CURRENT NEUROSURGERY FOR OCD
Modern ablative procedures are stereotactically guided by MRI, and specialized targeting hardware and software. This has resulted in progressively more accurate placement of generally smaller lesions, either by ‘open' lesion procedures (by craniotomy) or without craniotomy using radiosurgical instruments such as the gamma knife. DBS requires craniotomy to implant stimulating electrodes, but is intended to be nonablative. Damage to brain tissue appears generally confined to about 1.3 mm diameter volume displaced by the brain ‘leads' themselves and the surrounding microenvironment. Hemorrhages on device insertion, however, although relatively rare, can have long-lasting or permanent consequences. Although risk of this intraoperative bleeding has not been well characterized for the targets used for DBS in psychiatric disorders, in other populations, it appears on the order of about 0.5% per implantation, that is, 1% for a typical patient receiving bilateral brain leads (Benabid et al, 2009). A much higher rate of serious intraoperative hemorrhage was found (1 in 17 patients implanted) in a recent study of DBS of the subthalamic nucleus for OCD (Mallet et al, 2008). This may, in part, have been due to the particular surgical target; it is also possible that the fact that none of the staff at the implanting centers were highly experienced in implantation at this target for OCD. Infection represents another significant risk, and may necessitate device removal or cause other sequelae. A high rate of this complication (2 of 17 patients) was noted in the same study of subthalamic DBS for OCD (Mallet et al, 2008). Long-term (decades) presence of the device in brain by itself or coupled with ongoing stimulation has not been identified to put patients at significant risk of tissue damage or other adverse effects on the brain (Benabid et al, 2009), this theoretical risk should be monitored in patients undergoing DBS at new brain targets for new indications over the long term. There is a risk that DBS implantations will not be effective in individual cases because of specific placement of the brain electrodes or device programming factors. For all these and other reasons, psychiatric neurosurgery researchers urge caution in abundance (OCD-DBS Collaborative Group, 2002), with careful attention to interdisciplinary (Greenberg et al, 2006) and ethical (Fins et al, 2006) requirements.
The great appeal of DBS in comparison with lesions is that it permits focal, adjustable, and reversible modulation of the brain. This is an obvious advantage in research applications. Effects of DBS on symptoms can be studied in controlled clinical trials using sham stimulation in within-subjects and parallel group designs. DBS can be coupled with neuroimaging, electrophysiological, and cognitive probes to compare effects of stimulation with no stimulation conditions, in the acute or chronic setting. In clinical care, adjustability means that therapeutic effects may be optimized and adverse effects reduced or eliminated. At the same time, the adjustability of DBS poses a challenge in the clinical setting, especially early in its application to a given target and particular patient population. The ‘parameter space' comprises combinations of stimulation amplitudes, pulse widths, frequencies, and activation of individual electrodes. Parameter setting methods were initially borrowed from DBS for movement disorders, and increasingly from experience in psychiatric illness.
At expert centers offering surgery for OCD, strict criteria for patient selection are observed. Processes for determining appropriate candidacy and assessing the consent process are formalized. Surgical intervention is reserved for patients with severe, incapacitating OCD who have failed an exhaustive array of standard medication and behavioral treatments. Surgery is typically not recommended unless a multidisciplinary committee reaches consensus regarding its appropriateness for a given candidate and the patient renders informed consent. Although systematic data on patients who are referred for surgery or who apply themselves for consideration for surgery to US centers have not yet been reported, a study in Belgium found that 26 of 91 patients whose cases were presented to the centralized Flemish Advisory Board for psychiatric neurosurgery were not accepted for surgery, either because they did not actually meet OCD diagnostic criteria (9 of 91) or somewhat more commonly (in 17 of 91) because it was unclear whether they had received adequate conventional treatments before presenting for consideration (Gabriels et al, 2008).
Using contemporary selection criteria and modern stereotactic surgical techniques, available long-term follow-up data suggest that neurosurgery for OCD yields meaningful improvement in symptoms and functioning in 40–70% of cases. As discussed in more detail below, at present, effectiveness outcomes appear similar for lesion procedures and DBS. There are, however, many more cases in the literature for lesion procedures than for DBS, in which data remain relatively sparse. A notable limitation is that case series or open-label studies dominate the literature for both ablation and DBS (Greenberg et al, 2003, 2008; Nuttin et al, 1999), with a few exceptions for DBS (Abelson et al, 2005; Mallet et al, 2008; Nuttin et al, 2008). In addition, it is not clear whether patient samples selected for ablation or DBS are fully comparable, further complicating attempts to compare outcomes. Morbidity and mortality (see below) are drastically lower for current ablative procedures and DBS than for the earlier procedures, but are not absent.
Major centers providing these treatments continue to gather information prospectively. In addition, four centers with experience in lesion procedures for OCD, DBS for OCD, or both techniques (Butler Hospital/Brown University, Massachusetts General Hospital, the Cleveland Clinic, and the University of Florida) are collaborating in an NIMH-supported multicenter clinical trial of DBS for OCD (ClinicalTrials.gov: NCT00640133A). A recent proposal from NIMH (Goodman and Insel, 2009) suggests that data from such studies and from clinical use of DBS for OCD under the newly granted US FDA Humanitarian Device Exemption approval become part of a national data registry for psychiatric neurosurgery. Such a registry was originally proposed in 1977 (National Commission, 1977), but never implemented.
SURGICAL TECHNIQUES
Although they became increasingly influenced by theories implicating cortico-subcortical systems in disordered behavior, and in OCD specifically, the lesion procedures were originally developed largely empirically. The same is true for the initial uses of DBS in OCD, as the surgical target was at first based on anterior capsulotomy. However, in addition to the promise of modern lesion procedures and DBS as clinical treatments (in which the central issues are safety and effectiveness), they permit testing of hypotheses derived from the results of lesions or from neuroimaging. Psychiatric neurosurgery is now developing in a scientific context in which translation of data between clinical results to cross-species anatomical, neuroimaging, and physiological studies of the neural networks involved promises to illuminate the anatomical and physiological mechanisms of therapeutic action (see Translational Research, below).
STEREOTACTIC ABLATION
Four lesion procedures for OCD remain in use. In each, bilateral lesions are made stereotactically under MRI-guidance. In addition to OCD, all have been used for highly refractory depression. Some have been applied to non-OCD anxiety disorders (for example, anterior capsulotomy), chronic medically intractable pain (anterior cingulotomy), as well as for OCD.
Anterior Capsulotomy
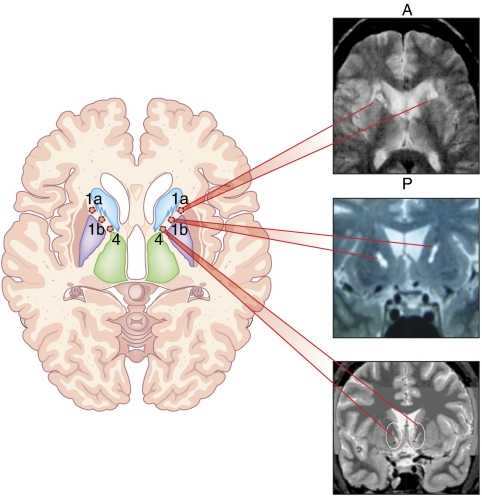
Anterior capsulotomy was first performed by Tailarach and colleagues in France (Figure 1, site 1a). Capsulotomy was further developed by Lars Leksell and colleagues in Sweden (Greenberg et al, 2003). Lesions are placed within the anterior limb of the internal capsule, impinging on the VS immediately inferior to the capsule. The intent is to interrupt fibers of passage between prefrontal cortex and subcortical nuclei including the dorsomedial thalamus. The original anterior capsulotomy procedure is performed using thermocoagulation through burr holes in the skull. For over 15 years, capsulotomy has also been performed using the Leksell Gamma Knife, a radiosurgical instrument that makes craniotomy unnecessary. Unless repeated procedures are used, gamma ventral capsulotomy lesions are substantially smaller than those induced by thermocapsulotomy, remaining within the middle to ventral portion of the anterior capsule. This more recent version of capsulotomy has been called ‘gamma knife ventral capsulotomy.' In contrast to thermocapsulotomy, gamma knife ventral capsulotomy (Figure 1, site 1b) may be performed as an outpatient procedure. The relative benefits and burdens of this radiosurgical approach are the focus of ongoing research, including a controlled study of gamma knife ventral capsulotomy for OCD underway at the University of Sao Paulo, Brazil, the first study of its kind for a lesion procedure in psychiatry.
Figure 1.
Axial schematic of target sites for surgical procedures linked to postoperative MRI images. Site 1a. Anterior capsulotomy (thermocapsulotomy) linked to postoperative axial MRI (A=anterior, P=posterior). Site 1b. Gamma ventral capsulotomy linked to a coronal MRI. Site 4. Ventral capsule/ventral striatum (VC/VS) deep brain stimulation linked to postimplantation coronal CT scan fused with preoperative MRI.
Anterior Cingulotomy
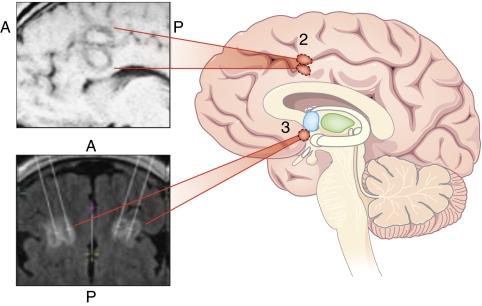
When the first cingulotomies were performed, for intractable pain, it was noticed that patients with comorbid anxiety or depressive conditions had the best results (Figure 2, site 2). Ballantine et al (1987) subsequently showed the safety of anterior cingulotomy and studied its efficacy for a broad range of psychiatric indications. Since 1962, the group at Massachusetts General Hospital has performed over 1000 cingulotomies, and continues to do so today. Two or three ∼1.0 cm3 lesions are made on each side by thermocoagulation through burr holes, under local anesthesia. The target is within ACC (Brodmann areas 24 and 32), at the margin of the cingulum white matter bundle. Since 1991, anterior cingulotomy has been MRI-guided. As making the smallest possible lesion is the goal, depending on their response, patients may return months after the first operation for a second procedure to extend the lesions.
Figure 2.
Saggital schematic of target sites for surgical procedures linked to postoperative MRI images. Site 2. Anterior cingulotomy linked to postoperative saggital MRI (A=anterior, P=posterior). Site 3. Subcaudate tractotomy linked to postoperative axial MRI.
Subcaudate Tractotomy
Subcaudate tractotomy, introduced in 1964, was one of the first attempts to limit adverse effects by restricting lesion size (Figure 2, site 3). By targeting the substantia innominata (just inferior to the head of the caudate nucleus), the goal was to interrupt white matter tracts connecting OFC to subcortical structures. The surgery involved placement of an array of radioactive yttrium-90 seeds at the desired centroid. This yielded lesion volumes of ∼2 cm3.
Limbic Leukotomy
Limbic Leukotomy was introduced by Kelly et al (1973) in the UK. It combines subcaudate tractotomy and anterior cingulotomy (Figure 2, sites 2 and 3). The lesions have typically been made by thermocoagulation or with a cryoprobe. Historically, the precise placement of the lesions was guided by intraoperative stimulation; pronounced autonomic responses were believed to designate the optimal lesion site.
DEEP BRAIN STIMULATION
Deep brain stimulation for psychiatric illness was tried as early as 1948, when JL Pool used stimulation through an electrode in the caudate nucleus in an attempt to treat a woman with depression and anorexia (Pool, 1954). Psychiatric DBS began in earnest 50 years later, when Nuttin et al (1999) reported on a series of otherwise intractable OCD cases stimulated at a site based initially on the anterior capsulotomy target. The procedure is essentially the same as for routine use of DBS for movement disorders. Small-diameter (1.27 mm) brain ‘leads' with multiple (usually four) electrode contacts are implanted into the brain through burr holes in the skull. Craniotomy is done under local anesthesia, and patients are typically sedated but awake during surgery. Lead placement, which is typically bilateral, is guided by multimodal imaging and specialized computerized targeting platforms. In contrast to DBS implantations in Parkinson's disease, microelectrode recording is not routinely used for target identification in OCD cases. Intraoperative ‘macrostimulation' (that is, stimulation through the lead just implanted) is used to determine whether there are adverse effects (Shapira et al, 2006), or physiological (Okun et al, 2004) or behavioral effects of acute stimulation that may suggest that the lead location should be modified. In a separate phase of surgery or on a different day, the neurostimulator is implanted subdermally under general anesthesia (for example, in the upper chest wall). It is connected to the brain leads by extension wires tunneled under the skin.
In contrast to lesions, stimulation, being adjustable, has dynamic effects on neurocircuitry, including the implantation site itself and functionally connected regions. Various combinations of electrodes can be activated, at adjustable polarity, intensity, and frequency; DBS thus permits flexible ‘neuromodulation.' The great clinical advantage of this is that parameters can be optimized for individual patients. The process of optimizing parameters for individual patients, typically performed by a specially trained psychiatrist in the outpatient setting, can be quite time-consuming, and requires attentive, long-term follow-up. In cases in which no beneficial settings can be identified despite extensive efforts, the electrodes can be inactivated, and the devices may be removed. In that event, devices are usually only partly explanted, with the brain electrodes left in place given the not fully clarified but apparently small risk of hemorrhage on removal. In our experience, full device explantation has happened relatively rarely.
SURGICAL OUTCOMES
Outcomes after Ablation
For all four contemporary ablative procedures, outcome cannot be fairly assessed until at least 6 months up to 2 years postoperatively. Early publications usually reported measures of global improvement (for example, covering the range of outcomes from much improved to worse after surgery (Greenberg et al, in press)). Most reports considered ratings from improved to much improved as indicative of significant improvement. More recent reports used disorder-specific rating (for example, the YBOCS for OCD). Systematic prospective studies of anterior cingulotomy (Dougherty et al, 2002) as well as gamma capsulotomy for refractory OCD have been conducted. Of these modern ablative procedures, it seems that none is superior to capsulotomy. However, direct comparisons of procedures within the same study are rare, with perhaps only a single published example (Kullberg, 1977).
Anterior capsulotomy outcomes
During the 1950s, Leksell performed anterior capsulotomies in 116 patients, with favorable responses in 50% of patients with OCD and 48% of patients with major affective disorders (Herner, 1961). Poorer results were seen with schizophrenia (14%) and non-OCD anxiety disorders (20%). In 1977, Bingley and co-workers reported satisfactory results in 71% of patients with OCD who underwent anterior capsulotomy (Bingley et al, 1977). In a review of 253 cases of anterior capsulotomy for OCD, Waziri (1990) suggested that 67% exhibited significant improvement. A 1994 review of capsulotomy cases reported in the literature found that 64% (137/213) had satisfactory outcomes, with OCD patients receiving the greatest benefit (Mindus et al, 1994). Interpretation of these data is limited, as follow-up information in 149 cases was insufficient to determine the outcome. The need to maximize capture of long-term follow-up data is essential for all surgical procedures, as discussed elsewhere in this article. Overall, short-term side effects of thermocapsulotomy (the original procedure, see below) include headache, confusion, and incontinence. Weight gain, fatigue, memory loss, incontinence, and seizure have been reported as rare but more lasting side effects (Feldman et al, 2001). Anterior capsulotomies were typically performed by thermocoagulation. However, over the last 15 years, an increasing number of capsulotomies have been performed by Gamma Knife radiosurgery, typically involving smaller lesions, which remain in the ventral portion of the anterior capsule. Thus, this newer procedure has been called ‘gamma ventral capsulotomy'. Radiosurgery typically has a short postoperative recovery period, and as noted can be an outpatient procedure. Therapeutic response (35% YBOCS improvement) has been reported at 60% for patients with OCD who underwent gamma ventral capsulotomies. A recent comparison of the two types of capsulotomy revealed lasting improvement (35% YBOCS reduction 4–17 years after surgery) in 48% of OCD patients with no significant difference in benefit between the procedures (Ruck et al, 2008). The data do suggest, however, that there may be less risk, as well as possibly better outcomes, associated with relatively smaller total lesion volumes. Serious adverse effects of the gamma knife radiosurgical procedure include radiation-induced edema and delayed cyst formation (which may or may not be symptomatic). Development of clinically significant edema seems to reflect individual differences in sensitivity to radiation that remain poorly understood. Late cyst formation (>5 years postsurgery) has occurred in 1.6–3.6% of patients after surgery for arteriovenous malformations, and may, in part, be related to the extent of postoperative radiation-induced edema (Pan et al, 2005).
Anterior cingulotomy outcomes
In 1987, Ballantine and co-workers reviewed the long-term results of 198 patients who underwent the procedure for major affective disorder, OCD or anxiety disorder. Significant improvement, determined using a subjective functional/symptomatic rating scale, occurred for patients with severe affective disorders (62%), OCD (56%), and anxiety disorders (79%) (Ballantine et al, 1987). When these data were analyzed using more rigid outcome criteria, only 33% showed substantial benefit from the cingulotomy (Cosgrove, 2000). A 1996 review of 34 patients who had undergone cingulotomies since 1991 reported that, of patients with OCD, 27% were full responders and another 27% were partial responders. Most recently, it should be noted, however, that it may take as long as 3–6 months for the beneficial effects of cingulotomies to emerge (Cosgrove, 2000). Cingulotomies have a relatively low rate of side effects. Of more than 1000 cingulotomies performed at Massachusetts General Hospital, there had been no deaths, no infections, and only two subdural hematomas (Greenberg et al, in press). Short-term postoperative side effects include headache, nausea, or difficulty in urination, which usually resolve within a few days. The most common serious side effect is seizure (rates ranging from 1 to 9%) (Binder and Iskandar, 2000). Owing to the relatively good success, and clearly low morbidity and mortality rates, cingulotomy has been the most widely used psychiatric neurosurgical procedure in North America over the last several decades. A more recent study (Kim et al, 2003) of stereotactic bilateral anterior cingulotomy as a treatment for refractory OCD found a mean YBOCS severity reduction of 36% after 12 months, 6 of the 14 patients met responder criteria (35% YBOCS reduction plus a Clinical Global Impression score of much or very much improved) at 12 months. There was no significant cognitive dysfunction after cingulotomy.
Subcaudate tractotomy outcomes
By 1973, this procedure had been performed in over 650 cases of major depression, OCD, or anxiety with reported success rates around 50% (Knight, 1973). In 1994, Bridges and colleagues reviewed 1300 cases of subcaudate tractotomy treating anxiety, phobic anxiety, OCD, MDD, or bipolar disorder (Bridges et al, 1994). They concluded that 40–60% of patients are benefited by the procedure to the point of living normal or near-normal lives. Suicide rates were reduced from 15% (in a similarly affected control group) to 1%. Short-term side effects include somnolence lasting up to a few days postoperatively, confusion lasting up to 1 month postoperatively, and possibly temporary decreases in certain cognitive functions. The most common major adverse effect is seizure (at least 1.6% of cases), but mild personality changes have been noted (Greenberg et al, in press). Only one surgery-related death was reported. A more recent case report described improvement in a single patient who underwent subcaudate tractotomy using a newer, ‘frameless' stereotactic subcaudate tractotomy procedure (Woerdeman et al, 2006).
Limbic leucotomy outcomes
In an early study, Kelly et al (1973) reported significant improvement (using a 5-point global rating scale) in 89% of patients with OCD, 66% of patients with anxiety, 78% of patients with MDD, and 80% of patients with schizophrenia. In 1993, Hay and colleagues reported a moderate to marked improvement in 38% of their 26 OCD patients treated with limbic leucotomy (Hay et al, 1993). Most recently, Cho et al (2008) reported a marked response (in the top three categories of the CGPS) in 69% of their patients (n=18) with intractable affective disorders who underwent limbic leucotomy. They showed significant improvement in depression (HDRS averages reduced from 42 to 20), anxiety (average Hamilton Anxiety Rating Scale (HARS) scores reduced from 104 to 57), and negative symptoms (Negative Symptom Scale averages dropped from 57 to 33). These results were sustained over a 7-year follow-up period. In addition, Price et al (2001) have reported that limbic leucotomy may benefit patients with severe self-mutilation from repetitive, tic-like behaviors. The most significant complications reported for this procedure are seizure and enduring lethargy. Overall, short-term side effects for limbic leucotomy include headache, confusion, lethargy, perseveration, and lack of sphincter control. Although most of these effects are transient, it is common for confusion to last several days, thus patients often have a longer postoperative hospital stay with this procedure than with either cingulotomy or subcaudate tractotomy (Greenberg et al, in press). A more recent study of limbic leucotomy for OCD reported that mean YBOCS scores decreased from 34 to 3, which is a large decrease compared with that seen in other studies of the effects of neurosurgery in OCD. In total, 10 of these 12 patients had long-term follow-up (for a mean of 45 months), during which they were described as having returned to a previous (improved) level of psychosocial functioning (Kim et al, 2002).
DBS OUTCOMES
For OCD, benefit has been reported after application of DBS to the VC/VS (Greenberg et al, 2008), more anterior in the internal capsule (Abelson et al, 2005), the ventral caudate (Aouizerate et al, 2004), or the subthalamic nucleus (Mallet et al, 2008). The OCD targets have been most influenced by the more focal stereotactic ablation procedures that have continued to be in use since the 1940s, by anatomical considerations derived from neuroimaging, and by direct clinical observations (Mallet et al, 2008). Modification of the VC/VS target (caudally, see Figure 2, site 4) led to improved effectiveness for OCD with lower current drain (Greenberg et al, 2008). Safety profiles have been quite variable in the small-scale reports given above on effects of DBS for OCD. Adverse effects have ranged from transient psychiatric symptom exacerbations (Greenberg et al, 2008) to permanent neurological sequelae (Mallet et al, 2008) or, on longer-term follow-up, suicide in the context of psychosocial stress (Abelson et al, 2005). However, as is also the case for efficacy, only quite tentative conclusions about safety can be drawn given the data available. In terms of mechanisms of therapeutic action, the VC/VS target has also been proposed to be part of longitudinal fiber pathways that are the targets of anterior capsulotomy and the more specific gamma ventral capsulotomy for OCD (Lopes et al, 2009, in press). Moreover, numerous functional neuroimaging studies are consistent with the hypothesis that pathways coursing through the VC/VS are implicated in OCD. Interestingly, in terms of mechanism of action, mood and other depressive symptoms, including anhedonia, improved rapidly after VC/VS stimulation in OCD patients. Moreover, mood symptoms worsened faster than did obsessions and compulsions when DBS was interrupted (Greenberg et al, 2008). Interestingly, stimulation in the same region has notably also produced rapid relief of anhedonia in patients who received DBS for major depression (Schlaepfer et al, 2008).
Important limitations of this rapidly growing, but still small, DBS literature for OCD should be kept in mind: (1) Entry criteria have varied, including in-patient characteristics, which may have prognostic significance, such as predominance of symptom subtypes within the primary categorical diagnosis, extent of treatment resistance, and patterns of co-occurring disorders. These and other clinical features may have important implications for initial response as well as for maintenance of therapeutic gains over the long term. (2) Definitions of therapeutic success vary. ‘Response' has meant different things across studies. For example, a 25% reduction in YBOCS-measured OCD severity was defined as a response for a multicenter study of subthalamic nucleus stimulation for OCD (Mallet et al, 2008), whereas a 35% YBOCS decrease was the threshold in studies of the VC/VS (Greenberg et al, 2008). (3) Targets may be reported as intended (‘planned') target, postoperative (image-determined) targets, or both. To facilitate improved clinical outcomes and translational research accuracy, analyses of locations of active electrode contacts and parameters associated with the best outcomes are extremely important. (4) Data on concomitant treatments may be collected variably. Patients who will present for DBS in either clinical trials or for clinical use will, by definition, have undergone an exhaustive series of medication and behavioral treatments. Many will likely be on complex, multi-drug regimens during baseline evaluations as well as during postsurgical follow-up. Behavioral treatments may be ongoing, have been abandoned, or may be renewed after surgery. This is important as one hypothesis of DBS's (and ablation's) mechanism of action is that surgery may facilitate behavior therapy (Greenberg et al, 2006). (5) Adverse event data collection and reporting have varied. This has sometimes been done apparently using clinical assessments only, or semi-structured or structured instruments. In addition to its critical safety role, adverse event reporting can provide important data on the neurocircuitry affected by DBS (Okun et al, 2004; Shapira et al, 2006). Without careful attention to all of these factors, as the field moves forward, judgments about relative effectiveness of DBS at any given target, or between DBS and ablative procedures, may be misleading. This will reduce the value of these unique data in refining anatomical models of mechanisms of action of neurosurgery for OCD, and in turn, affect their contribution to advancing models of OCD neurocircuitry.
NEUROCIRCUITRY OF OCD AND SURGICAL TARGETS
In OCD, interruption of reciprocal projections between OFC and thalamus would theoretically decrease reverberating (amplified) activity in the OFC–caudate–pallidal–thalamic circuit, possibly leading to reduced OCD symptoms. However, studies comparing postsurgical with presurgical regional cerebral metabolism must take into account the potential confounds of symptomatic improvement in OCD, which characteristically yield a profile of attenuated hyperactivity throughout the circuit. Improvement in co-occurring mood and anxiety symptoms (defined either categorically or dimensionally) after surgery may also affect activity on neuroimaging measures.
Capsulotomy, subcaudate tractotomy, limbic leucotomy, and DBS at the VC/VS and related targets, all target reciprocal excitatory midline thalamic/orbital and medial cortex links, as well as orbital and medial projections to the VS. These three procedures also affect connections between midline thalamus and the subgenual and pregenual cingulate, and may have actions overlapping those of cingulotomy, especially because the cingulate has multiple reciprocal links with OFC (for reviews see Rauch, 2003; Zald and Kim, 2001). Thus, disruption of pathological OFC–caudate or reciprocal OFC–thalamic communications could underlie therapeutic effects of anterior capsulotomy. Consistent with this view, acute DBS produced increased regional cerebral blood flow, as measured by O15-PET, in OCD patients within the hypothesized circuitry, including OFC, thalamus, subgenual cingulate, striatum, and globus pallidus, when active stimulation at the most ventral contact was compared with control conditions (Rauch et al, 2006). Note that the increase in perfusion observed could be due to a number of different changes at the cellular and microcircuit level, including, for example, facilitation of inhibitory processes.
In terms of chronic changes after surgery, functional imaging in a small cohort of patients with severe anxiety disorders showed reductions in activity within orbitomedial frontal cortex after anterior capsulotomy (Mindus et al, 1986). A small study of chronic DBS near the thermocapsulotomy target found reduced FDG-PET metabolism in the orbital and medial cortex in two out of three patients (Abelson et al, 2005). DBS at the more posterior VC/VS site was also associated with prefrontal metabolic activity on FDG-PET, especially in the subgenual ACC. Interestingly, preoperative resting metabolic activity in the subgenual ACC predicted the reduction in YBOCS OCD severity after chronic stimulation (Van Laere et al, 2006). Note that in the case of limbic leucotomy, lesions similar to those of anterior cingulotomy and those of subcaudate tractotomy are combined. Hence, this multi-site operation would presumably combine the benefits (as well as potential adverse effects) of those two procedures. A single case study of limbic leucotomy has demonstrated reduced activity within the caudate nuclei in a patient with OCD and Tourette Syndrome (TS), when comparing postsurgical to presurgical regional cerebral oxygen metabolism (Sawle et al, 1993). Both OCD and TS symptoms improved in this patient after limbic leucotomy.
In capsulotomy, especially the original thermocapsulotomy procedure, which produces larger lesions than gamma ventral capsulotomy, the ventral portion of the lesion may compromise adjacent territories of the striatum as well as interrupting OFC/subgenual ACC–thalamic connections. This can occur if the lesions interrupt fronto-striatal projections, if the lesions themselves impinge on the striatum, or if infiltration of edema surrounding the lesions encroaches on the striatum itself or fronto-striatal projections. Again, for OCD disruption of pathological CSTC circuitry at the level of OFC–caudate or reciprocal OFC–thalamic communications could underlie the therapeutic effects of anterior capsulotomy. Interestingly, an MRI study of anterior capsulotomy for OCD indicated that appropriate placement of lesions within the right anterior capsule was critical to subsequent therapeutic response (Lippitz et al, 1999). Furthermore, functional imaging data from a small cohort of patients with severe anxiety disorders undergoing anterior capsulotomy showed reductions in activity within orbital and medial frontal cortex from presurgical to postsurgical scans (Mindus et al, 1986). Most recently, a pilot study using voxel-based MRI morphometry found that the volume of gray matter in the right inferior frontal gyrus had increased after gamma ventral capsulotomy (Cecconi et al, 2008).
In anterior cingulotomy, the lesions are within dorsal ACC and typically impinge on the cingulum bundle. Thus, in addition to reducing cortical mass and activity within dorsal ACC, these lesions will likely modify cingulo–striatal projections, and possibly disinhibit pregenual ACC. Given the constituents of the cingulum bundle, it is also possible that its disruption in cingulotomy could influence reciprocal connections between the ACC and several other structures, including OFC, amygdala, hippocampus, and posterior cingulate cortex (Mufson and Pandya, 1984). In fact, comparisons of presurgical with postsurgical MRI data indicate that, 6–12 months after cingulotomy, volume appeared reduced within the caudate nucleus and posterior cingulate cortex (Rauch et al, 2000, 2001b). Given the prevailing neurocircuitry model of OCD, any of these changes after surgery could relate to therapeutic improvement. Posterior cingulate cortex, which is not typically central in OCD circuitry models, is well positioned to modulate activity within the OFC–caudate frontal–basal ganglia–-thalamic circuit (Cohen et al, 1992; Kemp and Powell, 1970; Vogt and Pandya, 1987; Zald and Kim, 1996). Interestingly, a functional neuroimaging study of OCD demonstrated that presurgical activity within posterior cingulate cortex correlated with subsequent response after anterior cingulotomy (Rauch et al, 2001a). In addition, lesions of the cingulum might interrupt ascending influences of the amygdala on dorsal brain structures.
Metabolic and neurochemical changes in regions functionally connected to lesion or DBS targets can also be studied before and after surgery, and also by comparing different DBS parameter sets with each other and with no stimulation control conditions. Such experimental paradigms can also be implemented to take advantage of cognitive tasks to probe nodes of the neurocircuitry during imaging acquisitions. As abnormalities in dopaminergic function have been reported in OCD (Denys et al, 2004, 2006; van der Wee et al, 2004), imaging this neurochemical system in the context of neurosurgery may be of considerable interest. Specific examples include using dopamine transporter ligands to probe transporter or receptor levels or displacement. Here, it is of interest that dopamine transporter ligand binding was elevated in unmedicated OCD patients in striatal nodes of the network of interest (van der Wee et al, 2004). Dopamine and serotonin probe ligands could be combined, as in a study that found that 5-HT(2A) receptor availability was reduced in frontal polar, dorsolateral, medial frontal, parietal, and temporal associative cortex of OCD patients. It was intriguing that 5-HT(2A) ligand binding in orbitofrontal and dorsolateral frontal cortex correlated with clinical severity (Perani et al, 2008; Reimold et al, 2007). In addition, that same study found a concomitant reduction in D2 ligand ([11C]raclopride) uptake in the striatum, and especially in VS. The results were consistent with a possible decrease in activity in a serotonergic subsystem together with elevated synaptic dopamine in the psychotropic-naïve patients studied, who were also free of comorbid disorders. Recently another interesting approach has been shown to be feasible, that is the study of radiotracer displacement during probe tasks compared with control conditions. Such tasks could be designed to probe anatomically selective parts of the neurocircuitry of interest. For example, Lappin et al (2009) found that a sequential motor learning paradigm and a spatial planning task were both associated with increased displacement of [11C]raclopride in the striatum compared with a control (rest) condition. This change in ligand dynamics, an indirect measure of synaptic dopamine, was seen in both sensorimotor and associative, but not limbic striatum. These and other behavioral challenges could be used, for example, before and after DBS to elucidate functional changes in neurotransmitter systems localized within specific regions of the neural networks of interest.
Thus, ligand-based imaging (or MRS imaging of glutamate systems as described above) before and after DBS or stereotactic ablation may reveal changes in dopamine, serotonin, glutamate, or other neurotransmitter systems. However, a potential confound for molecular imaging methodologies is that patients undergoing neurosurgery will virtually by definition be receiving complex, multi-drug treatment regimens that may be difficult to change on clinical grounds and which can complicate interpretation of radioligand experiments. Moreover, the subpopulation of OCD patients who might be considered surgical candidates may also differ from the more general population of OCD patients in terms of clinical phenotypes, patterns of co-occurring categorical disorders or personality dimensions, or on any of the indices of neurotransmitter function that radioligand studies can assess. Given such limitations, converging evidence using multiple methods in patients and in parallel basic research will be essential to develop a coherent model of changes associated with DBS that are most important for therapeutic change.
TRANSLATIONAL RESEARCH AND PSYCHIATRIC NEUROSURGERY
The effects of ablation or DBS on the neurocircuitry of interest in OCD remain poorly understood. The mechanisms of action of ablation at the surgical target itself are conceptually simpler than those of DBS. Even in movement disorders, in which DBS has been used clinically in over 55 000 patients worldwide since the mid-1980s and research on mechanisms of DBS action is more advanced, how stimulation produces therapeutic benefits remains incompletely delineated (Montgomery and Baker, 2000; Vitek, 2002). Stimulation can modulate firing rates, normalize irregular burst firing patterns, and reduce low-frequency oscillations associated with a Parkinsonian state (Chang et al, 2007). Such effects could result from propagation of stimulation orthodromically or antidromically along fibers in the target region. Computational modeling has found a role in advancing conceptions of the neural networks via which DBS may act. In one computational model, antidromic propagation occurred across a range of stimulation frequencies and axon segment geometries, but was strongly dependent on the geometry of the node of Ranvier at the axonal bifurcation (Grill et al, 2007). DBS-induced antidromic activation may thus lead to widespread activation or inhibition of targets remote from the electrode, effects which should be considered when interpreting results of studies using functional imaging or evoked potentials to investigate mechanisms of action of DBS. In general, stimulation most probably affects the likelihood that particular kinds of information will flow through the circuits of interest. For DBS as well as lesions, an analogy with ‘biased competition' theories of information processing regulating attention may be apt (Beck and Kastner, 2009).
Wherever translational research leads, an improved understanding of anatomical networks through which DBS acts, and of its physiological actions themselves, should help clinical researchers achieve several important and interrelated goals. As discussed above, the role of a systematic and comprehensive data registry in advancing this goal is essential (Goodman and Insel, 2009).
Response Prediction
Finding predictors that are meaningful on an individual level, based on endophenotypes derived from neuroimaging or cognitive measures, might be possible. Potential presurgical metabolic predictors of responses to neurosurgery include FDG uptake in the posterior cingulate for the procedure of anterior cingulotomy, and in the subgenual cingulate for DBS of the VC/VS (Dougherty et al, 2003; Rauch et al, 2001a; Van Laere et al, 2006). Combining such measures with clinical phenotypes that prove to be predictive of response based on larger clinical studies, such as the current NIMH-sponsored controlled trial of DBS for OCD (ClinicalTrials.gov: NCT00640133A), might improve the prediction further. This is an important issue, as the risks, costs, and burdens of either refined ablation or DBS are nontrivial. Individuals unlikely to enjoy a meaningfully positive response should be spared those potential burdens. However, the predictive power of clinical, neuroimaging, or other endophenotypic features, alone or in combination, will need to be substantial in order for the prediction to have clinical value. Some clinical predictors of SRI response, for example, are by definition absent in patients who would be judged as possible candidates for surgery. These clinical features include absence of previous therapies, and moderate YBOCS OCD severity (a score of 22 or less), which were found on stepwise multivariate analysis to predict better OCD outcomes after 12 weeks of paroxetine or venlafaxine treatment by Denys et al (2003). That same study found low Hamilton Depression Rating Scale scores (6–15), which are quite unlikely in patients presenting for surgery as a whole, to predict better outcomes after SRIs. Furthermore, it might be difficult to collect data on some other patient characteristics that have been found to be predictors in medication trials. This is because features that have been found to be negative predictors of response to SRI treatment, personality disorders, for example (Denys and de Geus, 2005), may be strong relative contraindications to surgery. Hence, innovative approaches to response prediction in patients undergoing neurosurgery will be needed. That could include, as mentioned elsewhere in this review, the establishment of comprehensive data registries for psychiatric neurosurgery outcomes within and across diagnoses, the application and/or development of endophenotypic biomarkers, and a systematic review of predictors of responses to conventional treatments for OCD and commonly co-occuring disorders, including major depression.
Parameter Setting
Initially, selection of DBS parameters for use in OCD was based on those used in movement disorders. Subsequent experience has allowed stimulator programming to be based somewhat more on clinical observations gained over years of work with OCD patients receiving chronic DBS (Greenberg et al, 2008; Nuttin et al, 2008). Translating findings from anatomical research may allow more refined targeting and device design. Similarly, physiological findings may help narrow the ‘parameter space' so that the most efficient DBS parameters may be used. This will, in theory, lead to an enhanced ability to maximize efficacy, minimize current drain, and also to potentially minimize the side effects by better targeting of stimulation-induced electric fields so that they include as few nonessential structures as possible.
Translation to Other Device-Based Treatments
Similarly, improvements in electrode design and in the ‘design' (shapes) of the electrical pulses themselves should be influenced by translational research. Such insights will likely influence the development of neuromodulation techniques beyond the scope of this review, including vagus nerve or transcranial magnetic stimulation, and cortical surface stimulation.
Potential to Improve Stereotactic Ablation
As discussed above, the trajectory of psychiatric neurosurgery has involved progressively more selective and smaller lesions, made with progressively better accuracy. The effectiveness of lesion procedures looks, from available data, to be comparable with that of DBS (Greenberg et al, 2003). It is very likely that a better delineation of the neural networks of action of the existing modalities of psychiatric neurosurgery would lead to ablative procedures with improved outcomes compared with the current generation of those techniques. A recent multidisciplinary position statement affirmed that continued research on lesion procedures in psychiatry is both needed and ethically sound (Rabins et al, in press).
Thus, the clinical and scientific promise of modern psychiatric neurosurgery will best be realized if it develops in connection with multidisciplinary translational research. The studies described above provide evidence relevant to delineating the neural network(s) by which DBS may act in OCD. Parallel basic science work is building on insights from anatomy of frontal–basal ganglia–thalamic circuitry, which is described by Haber and Knutson (2009, in press). The circuitry underlying the effects of surgical treatments in OCD is complex and under active investigation using multiple tools, including conventional track tracing methods and 3-D modeling in nonhuman primates, and DTI in humans (Haber et al, 2006; McFarland and Haber, 2002; Petrides and Pandya, 2007; Szeszko et al, 2005; Lehericy et al, 2004). The emphasis on white matter tracts for these invasive therapies raises important issues concerning exactly which white pathways (and structures) are affected at different lesion and stimulation sites. On the basis of primate tracing and modeling studies, we know that different sites will involve a different subset of structures and these different subsets are likely to relate to different clinical outcomes (Figure 3). For example, lesions or stimulation that target cortical white matter tracts vs the internal capsule will affect a subset of both cortico–cortical connections in addition to cortico–subcortical connections. Moreover, it is important to remember that all white matter bundles passing through the site will be affected, and not limited to the adjacent cortical areas. Thus, a site located in the subgenual white matter, for example, will affect OFC fibers traveling to the contralateral hemisphere, in addition to all pathways from the adjacent subgenual cortical area. This site will also involve all subcortical connections derived from the region immediately adjacent to the target. Therefore, a subgenual white matter site will also involve, not only specific cortico–cortical connections but also a subset of fibers traveling to the thalamus, striatum, and brainstem.
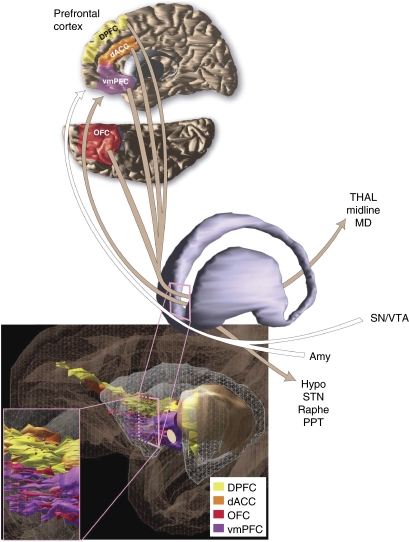
Figure 3.
Schematic representation of reciprocal connections between prefrontal cortex regions and the ventral portion of the anterior limb of the internal capsule and the adjacent ventral striatum (VC/VS). Prefrontal cortex—VC/VS connections are color-coded to delineate pathways linking specific prefrontal regions. Other connections of the VC/VS represented: THAL (mediodorsal and midline nuclei of the thalamus), SN/VTA (substantia nigra and ventral tegmental area), Amy (amygdala), Hypo (hypothalamus), STN (subthalamic nucleus), Raphe (raphe nuclei), PPT (pedunculopontine tegmental nucleus).
In contrast, VC/VS sites are unlikely to involve cortico–cortical connections, but will involve a larger, but still limited subset of cortico–subcortical connections. These include: cortico–thalamic; thalamo–cortical; and cortical brainstem pathways. In addition, there are major fiber bundles that run near the VC. Although not technically considered as internal capsule, nonetheless they may be affected by stimulation depending on the exact location of the electrodes. These include (but are not limited to) cortico–amygdala fibers running through the ventral amygdalofugal pathway, cortico–ventral striatal projections, cortico–hypothalamic fibers, and ascending dopaminergic and serotonergic fibers.
However, cortical fibers in subcortical pathways enter the IC at different AP levels and subsequently change their position as they travel caudally through the capsule. Figure 3 illustrates the organizational complexity and interweaving of prefrontal cortical fiber bundles as they pass through the internal capsule. Moreover, the relative dorsal-ventral, medial-lateral position of specific fiber bundles varies at different rostral-caudal levels. For example, as illustrated in the figure, generally fibers from the dACC and DPFC travel dorsal to those from ventral cortical regions. The VC contains primarily fibers from the OFC and vmPFC. However, these fibers travel in different positions in the internal capsule, including the small bundles that are embedded in the VS. For example, at rostral striatal levels, fibers traveling in the VC include some, but not all fibers from the OFC. In particular, those from caudal and lateral cortical areas that have not yet formed bundles in the capsule. At more caudal levels, at the junction of the internal capsule and anterior commissure, fibers from the caudal OFC join the axons from rostral OFC and those from vmPFC. Moreover, fibers connecting cortex with the amygdala (in the ventral amygdalofugal pathway) sweep ventral and medial to the anterior commissure. Likewise, ascending monoamine fibers pass just ventral to the anterior commissure. Thus, depending on the exact location of the electrode site, these fibers can be involved during stimulation.
In summary, depending on the specific stimulation site, different fiber bundles and therefore different sets of structures will be affected. Tracing studies in nonhuman primates combined with DTI in humans is a powerful tool to first validate human DTI studies, and then delineate the organizational details of fiber connections. These approaches, which have been used for other fiber tracts (Makris et al, 2009), together with careful clinical evaluation, are the key elements to identify DBS targets better tailored for subsets of patient populations.
Work that employs electrophysiological methods in animals has already helped generate hypotheses about how DBS-like stimulation may affect the circuitry of interest (McCracken and Grace, 2007, 2009). And, whereas developing animal models of OCD has been quite a difficult problem, coupling such models with electrical stimulation of the circuitry implicated in OCD has recently shown promise in identifying neurocircuitry which, when modulated, seems to influence behaviors relevant to OCD (van Kuyck et al, 2003, 2008). As each of these lines of research advances, translational interchange between them should also become more fruitful as hypotheses are refined and tested across these levels of analysis. Such translational work in progress will be expected to point toward behavioral processes (including those shared across categorical diagnoses), neurocircuitry, and physiological and pharmacological mechanisms involved in a number of neuropsychiatric conditions.
CONCLUSION
Contemporary neurosurgical treatments represent an important option for appropriately selected patients with severe and otherwise intractable OCD. Although the effectiveness and safety of these procedures has improved, active research in this area promises further advances. In particular, it is foreseeable that neuroimaging techniques may soon be used to aid in patient selection by predicting treatment response or (more speculatively) to individually tailor surgical interventions. Moreover, DBS offers the prospect of an adjustable and reversible method for effectively modifying brain function to relieve suffering and improve the quality of life in these severe and treatment-refractory cases. It is also conceivable that neurosurgical treatments might help guide development of novel pharmacological therapies based on advances in understanding the physiological roles of neurochemical systems in defined locations within relevant circuitry. Effects of surgical treatments may shed light on dimensions of illness that cross categorical diagnoses, and the mechanisms of therapeutic change. Finally, there is the intriguing possibility that neurosurgery might in fact enhance the benefits of subsequent behavioral therapies, which proved to be ineffective before surgery. How surgical interventions could accomplish that could become the subject of research meaningfully bridging the behavioral and neurobiological levels of analysis, with potential implications for treatment development.
One of the impressive advances in studying the biology of OCD has been associated with the implementation of functional neuroimaging in this intriguing disorder. These studies, which established a role for frontal–basal ganglia–thalamic circuits, have been key in contributing to the deepening of our understanding of OCD. Moreover, the well-defined circuitry could serve not only to refine treatment (neurosurgery, DBS, etc.) but also as a tool for characterizing endophenotypes. It could help to explore the relationship of OCD with other disorders (that is, other anxiety disorders on one hand, and behavioral addictions on the other hand). Finally, OCD circuitry models are being used to understand the effects of neurosurgical interventions for severely affected OCD patients who fail to respond to conventional behavioral and medication treatments. The models permit hypothesis testing that may help to refine existing neurosurgical approaches (lesion procedures or DBS) as well as other potential brain circuit-based interventions for OCD (such as TMS, which remains little explored in the illness).
FUTURE DIRECTIONS
Clinical studies of stereotactic ablation and DBS will, in the near term, focus on collection of controlled data in larger samples than those that have typically been studied to date. Very long-term follow-up of patient outcomes in multiple domains will be essential to assess the degree of enduring benefit and to identify risks and burdens of these invasive procedures. There is a recognized need to create data registries combining patient characteristics, surgical and stimulation targeting information from neuroimaging, stimulation parameters, and outcomes. This will be important not only to evaluate potential predictors of response but also to refine these interventions through translational research. Measuring patient characteristics both before and after surgery systematically using common end points across types of psychiatric neurosurgery will be important in this effort and will require enhanced collaboration across centers doing this work. This applies across categorical diagnoses as well as across expert clinical centers, as benefit after either focal ablation or DBS seems not to be confined to obsessions and compulsions, but includes, for example, changes along affective dimensions more generally. Neuroimaging work will apply enhanced structural and functional methods (including diffusion tensor imaging) to better delineate the circuitry of interest in patients and in nonhuman primate and other animal models. Relevant methods will also include electrophysiology, existing and evolving methods for tract tracing, and molecular imaging. Similarly, parallel behavioral models in humans and animals should help elucidate how targeting specific nodes within the neurocircuitry of interest affects behavioral endophenotypes that represent pathways toward therapeutic change.
Acknowledgments
The authors thank Cynthia Read for editorial and graphics assistance. We also thank Dr PA Woerdeman for permission to use an image from his doctoral thesis (Frameless image-guided neurosurgery in motion, University of Utrecht, 2008, www.dpp-utrecht.nl) depicting frameless stereotactic subcaudate tractotomy, which is reproduced here within Figure 2.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Disclosure
Dr Greenberg has received research funding, honoraria and consultant fees from Medtronic, Inc. a leading manufacturer of DBS devices. Dr Rauch has received honoraria and/or consultation fees from Neurogen, Sepracor, Novartis, and Medtronic, Inc. He has also participated in research funded by Medtronic, Inc., Cyberonics, Cephalon, and Northstar. A comprehensive disclosure for SLR is supplied as Supplementary material online. Dr Haber has received honoraria fees from Lilly and consultation fees from Medtronic, Inc.
Supplementary Material
References
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Albucher RC, Curtis GC, Pitts K. Neurosurgery for obsessive-compulsive disorder: problems with comorbidity. Am J Psychiatry. 1999;156:495–496. doi: 10.1176/ajp.156.3.495a. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- APA 1994Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington, DC [Google Scholar]
- Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer L. Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry. 1994;55 (Suppl:18–23. [PubMed] [Google Scholar]
- Ballantine HT, Jr, Bouckoms AJ, Thomas EK, Giriunas IE. Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry. 1987;22:807–819. doi: 10.1016/0006-3223(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Zohar J, Hollander E, Kasper S, Moller HJ, Zohar J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders—first revision. World J Biol Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Res. 2002;110:165–174. doi: 10.1016/s0165-1781(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RWJ, Carr TJ, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. 1992Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder Arch Gen Psychiatry 49681–689.An important early publication using neuroimaging to delineate neurocircuitry putatively involved in OCD pathophysiology. [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res. 2009;49:1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P.2009Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease Lancet Neurol 867–81.The most comprehensive series of cases describing outcomes and adverse effects in DBS for movement disorders. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Murphy DL, Zohar J, Hill JL, Grover G, Insel TR. Clomipramine in obsessive-compulsive disorder. Further evidence for a serotonergic mechanism of action. Arch Gen Psychiatry. 1989;46:23–28. doi: 10.1001/archpsyc.1989.01810010025004. [DOI] [PubMed] [Google Scholar]
- Binder DK, Iskandar BJ.2000Modern neurosurgery for psychiatric disorders Neurosurgery 479–21.discussion 21–23. [DOI] [PubMed] [Google Scholar]
- Bingley T, Leksell L, Meyerson BA, Rylander G.1977Long term results of stereotactic capsulotomy in chronic obsessive-compulsive neurosisIn: Sweet WH, Obrador Alcalde S, Martín-Rodríguez JG (eds).Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy. Proceedings of the Fourth World Congress of Psychiatric Surgery; 7–10 September 1975, Madrid, SpainUniversity Park Press: Baltimore; 287–299. [Google Scholar]
- Blier P, Habib R, Flament MF. Pharmacotherapies in the management of obsessive-compulsive disorder. Can J Psychiatry. 2006;51:417–430. doi: 10.1177/070674370605100703. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008;165:1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges PK, Bartlett JR, Hale AS, Poynton AM, Malizia AL, Hodgkiss AD.1994Psychosurgery: stereotactic subcaudate tractomy. An indispensable treatment Br J Psychiatry 165599–611.discussion 612–593. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Schwartz JM, Stoessel PW, Maidment K, Phelps ME, et al. 1998FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive-compulsive disorder Psychiatry Res 841–6.An important example of response prediction using PET in OCD. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, et al. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety. 2007;24:440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Cecconi JP, Lopes AC, Duran FL, Santos LC, Hoexter MQ, Gentil AF, et al. Gamma ventral capsulotomy for treatment of resistant obsessive-compulsive disorder: a structural MRI pilot prospective study. Neurosci Lett. 2008;447:138–142. doi: 10.1016/j.neulet.2008.09.061. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ.2005The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers Neurosci Biobehav Rev 29399–419.A heuristically valuable study of putative OCD endophenotypes. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson's disease. Neurosci Biobehav Rev. 2007;31:643–657. doi: 10.1016/j.neubiorev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Cho DY, Lee WY, Chen CC. Limbic leukotomy for intractable major affective disorders: a 7-year follow-up study using nine comprehensive psychiatric test evaluations. J Clin Neurosci. 2008;15:138–142. doi: 10.1016/j.jocn.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Gross M, Nordahl TE, Semple WE, Oren DA, Rosenthal N. Preliminary data on the metabolic brain pattern of patients with winter seasonal affective disorder. Arch Gen Psychiatry. 1992;49:545–552. doi: 10.1001/archpsyc.1992.01820070039006. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG, Frost RO, Steketee G. Not just right experiences and obsessive-compulsive features: experimental and self-monitoring perspectives. Behav Res Ther. 2005;43:153–167. doi: 10.1016/j.brat.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Corchs F, Corregiari F, Ferrao YA, Takakura T, Mathis ME, Lopes AC, et al. Personality traits and treatment outcome in obsessive-compulsive disorder. Rev Bras Psiquiatr. 2008;30:246–250. doi: 10.1590/s1516-44462008000300012. [DOI] [PubMed] [Google Scholar]
- Cosgrove GR. Surgery for psychiatric disorders. CNS Spectr. 2000;5:43–52. doi: 10.1017/s1092852900007665. [DOI] [PubMed] [Google Scholar]
- Denys D, Burger H, van Megen H, de Geus F, Westenberg H. A score for predicting response to pharmacotherapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2003;18:315–322. doi: 10.1097/00004850-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Denys D, de Geus F. Predictors of pharmacotherapy response in anxiety disorders. Curr Psychiatry Rep. 2005;7:252–257. doi: 10.1007/s11920-005-0078-4. [DOI] [PubMed] [Google Scholar]
- Denys D, Van Nieuwerburgh F, Deforce D, Westenberg H. Association between the dopamine D2 receptor TaqI A2 allele and low activity COMT allele with obsessive-compulsive disorder in males. Eur Neuropsychopharmacol. 2006;16:446–450. doi: 10.1016/j.euroneuro.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Denys D, Zohar J, Westenberg HG.2004The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence J Clin Psychiatry 65(Suppl 1411–17.An influential statement of potential involvement of dopaminergic mechanisms in OCD. [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, et al. 2002Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder Am J Psychiatry 159269–275.A key study of cingulotomy for OCD using modern diagnoses and assessment methods. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Jenike MA. Pharmacotherapy for obsessive-compulsive disorder. J Clin Psychol. 2004;60:1195–1202. doi: 10.1002/jclp.20083. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–1017. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- Ebert D, Speck O, Konig A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Res. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Dutra L, Bradley R, Westen D. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24:1011–1030. doi: 10.1016/j.cpr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Mancebo MA, Pinto A, Coles ME, Pagano ME, Stout R, et al. 2006Impact of obsessive-compulsive disorder on quality of life Compr Psychiatry 47270–275.An early systematic study of outcomes beyond symptom severity in OCD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Blier P. Responsiveness of 5-HT(1A) and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry Neurosci. 2005;30:268–274. [PMC free article] [PubMed] [Google Scholar]
- El-Hai J. The Lobotomist: A Maverick Medical Genius and His Tragic Quest to Rid the World of Mental Illness. J. Wiley: Hoboken, N.J.; 2005. [Google Scholar]
- Feldman RP, Alterman RL, Goodrich JT. Contemporary psychosurgery and a look to the future. J Neurosurg. 2001;95:944–956. doi: 10.3171/jns.2001.95.6.0944. [DOI] [PubMed] [Google Scholar]
- Fins JJ.2003From psychosurgery to neuromodulation and palliation: history's lessons for the ethical conduct and regulation of neuropsychiatric research Neurosurg Clin North Am 14303–319.ix–x. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Rezai AR, Greenberg BD.2006Psychosurgery: avoiding an ethical redux while advancing a therapeutic future Neurosurgery 59713–716.An updated statement of ethical recommendation for the field of psychiatric neurosurgery from authors of the original DBS for OCD Collaborative Group statement on this issue. [DOI] [PubMed] [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. 2005Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder Am J Psychiatry 162151–161.A landmark study comparing behavioral and medication treatments. [DOI] [PubMed] [Google Scholar]
- Freeman W, Watts JW, Hunt T.1942Psychosurgery; Intelligence, Emotion And Social Behavior Following Prefrontal Lobotomy For Mental Disorders Charles C. Thomas: Springfield, Ill; Early systematic research in several outcome domains from Freeman and colleagues. Freeman later became an ‘evangalist' for lobotomy, an enduring caution to the field. [Google Scholar]
- Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107:275–282. [PubMed] [Google Scholar]
- Gabriels L, Nuttin B, Cosyns P.2008Applicants for stereotactic neurosurgery for psychiatric disorders: role of the Flemish advisory board Acta Psychiatr Scand 117381–389.A very important study of appropriate and inappropriate referrals for psychiatric neurosurgery. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Keshavan MS, Diwadkar V, Nutche J, Macmaster F, Easter PC, et al. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: a preliminary voxel-based morphometry study. Neurosci Lett. 2008;435:45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Insel TR.2009Deep brain stimulation in psychiatry: concentrating on the road ahead Biol Psychiatry 65263–266.The most recent statement considering science, treatment, and policy in this are from the NIMH. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grados MA, Riddle MA. Two times four is four: OCD dimensions, classes, and categories. J Am Acad Child Adolesc Psychiatry. 2008;47:731–733. doi: 10.1097/CHI.0b013e318173f/20. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Altemus M, Murphy DL. The role of neurotransmitters and neuropeptides in obsessive-compulsive disorder. Int Rev Psychiatry. 1997;9:31–34. [Google Scholar]
- Greenberg BD, Benjamin J, Martin JD, Keuler D, Huang SJ, Altemus M, et al. Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl) 1998;140:434–444. doi: 10.1007/s002130050787. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Dougherty DD, Rauch SL.in pressNeurosurgical treatments: lesion procedures and deep brain stimulationIn: Sadock BJ, Sadock VA, Ruiz P (eds).Kaplan and Sadock's Comprehensive Textbook of Psychiatry9th edn.Lippincott, Williams & Wilkins: Philadelphia; 3314–3322. [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, et al. 2008Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience Molecular PsychiatryE-pub ahead of print. The most comprehensive presentation of technique and results of DBS at the VC/VS target for OCD. [DOI] [PMC free article] [PubMed]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Nuttin B, Rezai AR. Education and neuromodulation for psychiatric disorders: a perspective for practitioners. Neurosurgery. 2006;59:717–719. doi: 10.1227/01.NEU.0000243349.97248.AA. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Price LH, Rauch SL, Jenike MA, Malone D, Friehs G, et al. 2003Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues Neurosurg Clin North Am 14199–212.A comprehensive review of lesion procedures for OCD or depression. [DOI] [PubMed] [Google Scholar]
- Grill WM, Cantrell MB, Robertson MS. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J Comput Neurosci. 2007;24:81–93. doi: 10.1007/s10827-007-0043-9. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical inputs, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Knutson B. The reward circuit and risky behavior (a macroview) Neuropsychopharmacol. 2009, in press.
- Hay P, Sachdev P, Cumming S, Smith JS, Lee T, Kitchener P, et al. Treatment of obsessive-compulsive disorder by psychosurgery. Acta Psychiat Scand. 1993;87:197–207. doi: 10.1111/j.1600-0447.1993.tb03356.x. [DOI] [PubMed] [Google Scholar]
- Herner T.1961Treatment of mental disorders with frontal stereotactic thermo-lesions: a follow-up of 116 cases Acta Psychiatr Scand 58(suppl 361–140.29441 [Google Scholar]
- Hong JP, Samuels J, Bienvenu OJ, III, Cannistraro P, Grados M, Riddle MA, et al. Clinical correlates of recurrent major depression in obsessive-compulsive disorder. Depress Anxiety. 2004;20:86–91. doi: 10.1002/da.20024. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Zahn R, Krueger F, Moll J, Kapogiannis D, Wassermann EM, et al. A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2008;20:390–408. doi: 10.1176/appi.neuropsych.20.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted DS, Shapira NA. A review of the treatment for refractory obsessive-compulsive disorder: from medicine to deep brain stimulation. CNS Spectr. 2004;9:833–847. doi: 10.1017/s109285290000225x. [DOI] [PubMed] [Google Scholar]
- Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Rupertseder C, Hauke W, Zaudig M. Implicit sequence learning in obsessive-compulsive disorder: further support for the fronto-striatal dysfunction model. Biol Psychiatry. 2005;58:239–244. doi: 10.1016/j.biopsych.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Kelly D, Richardson A, Mitchell-Heggs N, Greenup J, Chen C, Hafner RJ. Stereotactic limbic leucotomy: a preliminary report on forty patients. Br J Psychiatry. 1973;123:141–148. doi: 10.1192/bjp.123.2.141. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The cortico-striate projections in the monkey. Brain. 1970;93:525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chang JW, Koo MS, Kim JW, Suh HS, Park IH, et al. Anterior cingulotomy for refractory obsessive-compulsive disorder. Acta Psychiatr Scand. 2003;107:283–290. doi: 10.1034/j.1600-0447.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Kim MC, Lee TK, Choi CR. Review of long-term results of stereotactic psychosurgery. Neurol Med Chir (Tokyo) 2002;42:365–371. doi: 10.2176/nmc.42.365. [DOI] [PubMed] [Google Scholar]
- Knight G. Further observations from an experience of 660 cases of stereotactic tractotomy. Postgrad Med J. 1973;49:845–854. doi: 10.1136/pgmj.49.578.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak KA, Greist JH, Jefferson JW, Katzelnick DJ, Henk HJ. Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Psychopharmacology (Berl) 1998;136:205–216. doi: 10.1007/s002130050558. [DOI] [PubMed] [Google Scholar]
- Koran LM. Obsessive-Compulsive and Related Disorders in Adults: a Comprehensive Clinical Guide. Cambridge Univ. Press: Cambridge; 1999. [Google Scholar]
- Kullberg G.1977Differences in effects of capsulotomy and cingulotomyIn: Sweet WH, Obrador WS, Martín-Rodríguez JG (eds).Neurosurgical Treatment in Psychiatry, Pain and Epilepsy University Park Press: Baltimore; 208–301. [Google Scholar]
- Lappin JM, Reeves SJ, Mehta MA, Egerton A, Coulson M, Grasby PM. Dopamine release in the human striatum: motor and cognitive tasks revisited. J Cereb Blood Flow Metab. 2009;29:554–564. doi: 10.1038/jcbfm.2008.146. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Alsobrook JP, Pauls DL.2001Symptom dimensions in obsessive-compulsive disorder: toward quantitative phenotypes Am J Med Genet 10528–30.An early and influential statement about differences in OCD symptom dimensions that has inspired clinical and neuroimaging research. [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C.1999Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere Neurosurgery 44452–458.discussion 458–460. [DOI] [PubMed] [Google Scholar]
- Lopes AC, Greenberg BD, Noren G, Canteras MM, Busatto G, Mathis M, et al. 2009, in pressTreatment of resistant obsessive-compulsive disorder with ventral capsular/ventral striatal gamma capsulotomy: a pilot prospective study J Neuropsychiatry Clin Neurosci The first controlled trial of an ablative procedure in psychiatry, underway as of this writing, is described here. [DOI] [PubMed]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19:777–785. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. 2008Subthalamic nucleus stimulation in severe obsessive-compulsive disorder N Engl J Med 3592121–2134.The first trial of DBS of the STN for OCD. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. 2009Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression Biol Psychiatry 65267–275.The first trial of DBS of the VC/VS for intractable depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancebo MC, Eisen JL, Pinto A, Greenberg BD, Dyck IR, Rasmussen SA. The Brown longitudinal obsessive compulsive study: treatments received and patient impressions of improvement. J Clin Psychiatry. 2006;67:1713–1720. doi: 10.4088/jcp.v67n1107. [DOI] [PubMed] [Google Scholar]
- Mancebo MC, Greenberg B, Grant JE, Pinto A, Eisen JL, Dyck I, et al. Correlates of occupational disability in a clinical sample of obsessive-compulsive disorder. Compr Psychiatry. 2008;49:43–50. doi: 10.1016/j.comppsych.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansari ME, Bouchard C, Blier P.1995Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors Neuropsychopharmacol 13117–127.An early study integrating anatomical and pharmacological perspectives within putative OCD neurocircuitry in the rodent. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA.2009Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo J Neurosci 295354–5363.Two groundbreaking studies of potential physiological mechanisms of action of stimulation analogous to DBS at the VC/VS in the rodent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994;164:459–468. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- Mindus P, Bergstrom K, Thuomas KA, Hindmarsh T. Magnetic resonance imaging of stereotactic radiosurgical lesions in the internal capsule. Acta Radiol Suppl. 1986;369:614–617. [PubMed] [Google Scholar]
- Mindus P, Ericson K, Greitz T. Regional cerebral glucose metabolism in anxiety disorders studied with positron emission tomography before and after psychosurgical intervention. A preliminary report. Acta Radiol Suppl. 1986;369:444–448. [PubMed] [Google Scholar]
- Mindus P, Rasmussen SA, Lindquist C. Neurosurgical treatment for refractory obsessive-compulsive disorder: implications for understanding frontal lobe function. J Neuropsychiatry Clin Neurosci. 1994;6:467–477. doi: 10.1176/jnp.6.4.467. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr, Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- National Commission . Psychosurgery: Report and Recommendations. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research: [Bethesda, Md.], United States; 1977. [Google Scholar]
- Nestadt G, Addington A, Samuels J, Liang KY, Bienvenu OJ, Riddle M, et al. The identification of OCD-related subgroups based on comorbidity. Biol Psychiatry. 2003;53:914–920. doi: 10.1016/s0006-3223(02)01677-3. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, III, Liang KY, LaBuda M, et al. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle MA, Liang KY, Bienvenu OJ, Hoehn-Saric R, et al. The relationship between obsessive-compulsive disorder and anxiety and affective disorders: results from the Johns Hopkins OCD Family Study. Psychol Med. 2001;31:481–487. doi: 10.1017/s0033291701003579. [DOI] [PubMed] [Google Scholar]
- New York Times 1937Surgery used on the soul-sick; relief of obsessions is reported New York TimesPage 1. Both the enthusiasm and skepticism about surgery in psychiatry in an earlier age are exemplified here.
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B.1999Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder Lancet 3541526.The first modern use of DBS in psychiatry. [DOI] [PubMed] [Google Scholar]
- Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2008;62 (6 Suppl 3:966–977. doi: 10.1227/01.neu.0000333764.20575.d6. [DOI] [PubMed] [Google Scholar]
- OCD-DBS Collaborative Group Deep brain stimulation for psychiatric disorders. Neurosurgery. 2002;51:519. [PubMed] [Google Scholar]
- Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR, et al. What's in a ‘smile?' Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L. Late cyst formation following gamma knife surgery of arteriovenous malformations. J Neurosurg. 2005;102 (Suppl:124–127. doi: 10.3171/jns.2005.102.s_supplement.0124. [DOI] [PubMed] [Google Scholar]
- Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, et al. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Marks IM, Senior C, Lythgoe D, O'Dwyer AM, Meehan O, et al. A differential neural response in obsessive-compulsive disorder patients with washing compared with checking symptoms to disgust. Psychol Med. 2000;30:1037–1050. doi: 10.1017/s0033291799002652. [DOI] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, III, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Mancebo MC, Eisen JL, Pagano ME, Rasmussen SA. The Brown Longitudinal Obsessive Compulsive Study: clinical features and symptoms of the sample at intake. J Clin Psychiatry. 2006;67:703–711. doi: 10.4088/jcp.v67n0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool JL. Psychosurgery in older people. J Am Geriatr Soc. 1954;7:456–466. doi: 10.1111/j.1532-5415.1954.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Pressman JD. Last Resort: Psychosurgery and the Limits of Medicine. Cambridge University Press: Cambridge, UK; New York, NY; 1998. [Google Scholar]
- Price BH, Baral I, Cosgrove GR, Rauch SL, Nierenberg AA, Jenike MA, et al. Improvement in severe self-mutilation following limbic leucotomy: a series of 5 consecutive cases. J Clin Psychiatry. 2001;62:925–932. doi: 10.4088/jcp.v62n1202. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Appleby BS, Brandt J, DeLong MR, Dunn LB, Gabriëls L, et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior and thought. Arch Gen Psychiatry. in press. [DOI] [PMC free article] [PubMed]
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:743–758. [PubMed] [Google Scholar]
- Rasmussen SA, Eisen JL.1994The epidemiology and differential diagnosis of obsessive compulsive disorder J Clin Psychiatry 55(Suppl5–10.discussion 11–14. [PubMed] [Google Scholar]
- Rauch SL.2003Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders Neurosurg Clin N Am 14213–223.vii–viii. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Cosgrove GR, Cassem EH, Alpert NM, Price BH, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry. 2001a;50:659–667. doi: 10.1016/s0006-3223(01)01188-x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. 2006A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder J Neurosurg 104558–565.These two studies exemplify the potential utility of neuroimaging in response prediction and in probing neural networks modulated by DBS, respectively. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Dougherty DD, Shin LM, Albert N, Manzo P, Leahy L, et al. Neural correlates of factor-analyzed OCD symptom dimensions: a PET study. CNS Spectr. 1998;3:37–43. [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, et al. Volume reduction in caudate nucleus following stereotactic lesions of anterior cingulate cortex in humans: a morphometric magnetic resonance imaging study. J Neurosurgery. 2000;93:1019–1025. doi: 10.3171/jns.2000.93.6.1019. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Makris N, Cosgrove GR, Kim H, Cassem EH, Price BH, et al. A magnetic resonance imaging study of regional cortical volumes following stereotactic anterior cingulotomy. CNS Spectrums. 2001b;6:214–222. doi: 10.1017/s1092852900008592. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, et al. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. J Neuropsychiatry Clin Neurosci. 1997;9:568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Zimmer A, Batra A, Knobel A, Solbach C, et al. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity—a [11C]DASB PET study. J Neural Transm. 2007;114:1603–1609. doi: 10.1007/s00702-007-0785-6. [DOI] [PubMed] [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Ruck C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, et al. 2008Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients Arch Gen Psychiatry 65914–921.A cautionary description of the potentially high burden of adverse effects after ablations of relatively large tissue volumes and/or repeated lesion procedures. [DOI] [PubMed] [Google Scholar]
- Samuels J, Nestadt G, Bienvenu OJ, Costa PT, Jr, Riddle MA, Liang KY, et al. Personality disorders and normal personality dimensions in obsessive-compulsive disorder. Br J Psychiatry. 2000;177:457–462. doi: 10.1192/bjp.177.5.457. [DOI] [PubMed] [Google Scholar]
- Sawle GV, Lees AJ, Hymas NF, Brooks DJ, Frackowiak RS. The metabolic effects of limbic leucotomy in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 1993;56:1016–1019. doi: 10.1136/jnnp.56.9.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, et al. 1999Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder Neuropsychopharmacology 21683–693.A seminal report of changes within putative OCD neurocircuitry after serotonin transporter inhibitors and of the potential predictive value of regional brain metabolism. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038–1048. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. 2008Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression Neuropsychopharmacology 33368–377.A key report emphasizing a focus on potential behavioral endophenotypes of response to DBS in depression. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr, Martin KM, Phelps ME.1996Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder Arch Gen Psychiatry 53109–113.An influential paper delineating changes in regional brain metabolism after successful behavior therapy for OCD. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, et al. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee G, Chambless DL, Tran GQ. Effects of axis I and II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agoraphobia. Compr Psychiatry. 2001;42:76–86. doi: 10.1053/comp.2001.19746. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Stack DE, Farrell C, Pauls DL, Jenike MA. Effectiveness of intensive residential treatment (IRT) for severe, refractory obsessive-compulsive disorder. J Psychiatr Res. 2005;39:603–609. doi: 10.1016/j.jpsychires.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Summerfeldt LJ. Understanding and treating incompleteness in obsessive-compulsive disorder. J Clin Psychol. 2004;60:1155–1168. doi: 10.1002/jclp.20080. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, et al. Cerebral glucose metabolism in childhood onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry. 1992;49:690–694. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, et al. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: an optimized voxel-based morphometry study. Am J Psychiatry. 2008;165:1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- Tenney NH, Denys DA, van Megen HJ, Glas G, Westenberg HG. Effect of a pharmacological intervention on quality of life in patients with obsessive-compulsive disorder. Int Clin Psychopharmacol. 2003;18:29–33. doi: 10.1097/00004850-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Valenstein ES. Great and Desperate Cures: The Rise and Decline of Psychosurgery and Other Radical Treatments for Mental Illness. Basic Books: New York; 1986. [Google Scholar]
- van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132 (Pt 4:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- van der Wee NJ, Stevens H, Hardeman JA, Mandl RC, Denys DA, van Megen HJ, et al. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]{beta}-CIT SPECT. Am J Psychiatry. 2004;161:2201–2206. doi: 10.1176/appi.ajp.161.12.2201. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Brak K, Das J, Rizopoulos D, Nuttin B. Comparative study of the effects of electrical stimulation in the nucleus accumbens, the mediodorsal thalamic nucleus and the bed nucleus of the stria terminalis in rats with schedule-induced polydipsia. Brain Res. 2008;1201:93–99. doi: 10.1016/j.brainres.2008.01.043. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Demeulemeester H, Feys H, De Weerdt W, Dewil M, Tousseyn T, et al. Effects of electrical stimulation or lesion in nucleus accumbens on the behaviour of rats in a T-maze after administration of 8-OH-DPAT or vehicle. Behav Brain Res. 2003;140:165–173. doi: 10.1016/s0166-4328(02)00295-4. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen SA, Greenberg BD, et al. 2006Metabolic imaging of anterior capsular stimulation in refractory obsessive compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum J Nuc Med 47740–747.A study finding that subgenual cortex activity before surgery might have predictive value in patients undergoing DBS for OCD. [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17 (Suppl 3:S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey; II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Waziri R.1990Psychosurgery for anxiety and obsessive-compulsive disordersIn: Burrows, Noyes, Roth (eds).Handbook of Anxiety: The Treatment of Anxiety Elsevier: Amsterdam; 519–535. [Google Scholar]
- Westenberg HG, Fineberg NA, Denys D. Neurobiology of obsessive-compulsive disorder: serotonin and beyond. CNS Spectr. 2007;12 (2 Suppl 3:14–27. [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. 2008Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder Am J Psychiatry 165335–341.quiz 409. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- Woerdeman PA, Willems PW, Noordmans HJ, Berkelbach van der Sprenkel JW, van Rijen PC.2006Frameless stereotactic subcaudate tractotomy for intractable obsessive-compulsive disorder Acta Neurochir (Wien) 148633–637.discussion 637. [DOI] [PubMed] [Google Scholar]
- Yaryura-Tobias JA, Bebirian RJ, Neziroglu F, Bhagavan HN. Obsessive compulsive disorders as a serotonergic defect. Res Commun Psychol, Psychiat and Beha. 1977;2:279–286. [Google Scholar]
- Zald DH, Kim S-W. Anatomy and function of the orbital frontal cortex, I: Anatomy, neurocircuitry, and obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1996;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW.2001The orbitofrontal cortexIn: Salloway SP, Malloy PF, Cummings JL (eds).The Frontal Lobes and Neuropsychiatric Illness APA Press: Washington; 33–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.