Abstract
Spanning functions from the simplest reflex arc to complex cognitive processes, neural circuits have diverse functional roles. In the cerebral cortex, functional domains such as visual processing, attention, memory, and cognitive control rely on the development of distinct yet interconnected sets of anatomically distributed cortical and subcortical regions. The developmental organization of these circuits is a remarkably complex process that is influenced by genetic predispositions, environmental events, and neuroplastic responses to experiential demand that modulates connectivity and communication among neurons, within individual brain regions and circuits, and across neural pathways. Recent advances in neuroimaging and computational neurobiology, together with traditional investigational approaches such as histological studies and cellular and molecular biology, have been invaluable in improving our understanding of these developmental processes in humans in both health and illness. To contextualize the developmental origins of a wide array of neuropsychiatric illnesses, this review describes the development and maturation of neural circuits from the first synapse through critical periods of vulnerability and opportunity to the emergent capacity for cognitive and behavioral regulation, and finally the dynamic interplay across levels of circuit organization and developmental epochs.
Keywords: neural circuits, brain development, neuroimaging, development, children, adolescents
INTRODUCTION
Neural circuits are arguably the primary supracellular mediators of the brain's diverse functional capacities. A circuit typically refers to a set of interconnected components that together subserve a specific function. A neural circuit in the brain may be a cluster of neurons that receives electrochemical information that the circuit modifies and transmits to other circuits for further modification. Alternatively, a neural circuit may comprise a network of interconnected brain regions that together integrate vast amounts of information and perform more complicated cognitive and regulatory functions. Clearly, these distributed neural circuits are neither present at birth nor are they invariant through life. In fact, the development of the brain's circuitry requires the coordination of an extraordinarily complex set of neurodevelopmental events.
The structure and function of neural circuits perpetually changes and evolves from the time of first contact between nerve cells. The interplay of inherent genetic programs with a wide range of environmental exposures and experiences determines the birth, death, and cellular characteristics of neurons, as well as the formation and reformation of their axons, dendrites, and synapses. Neural circuits consequently have diverse configurations and functional attributes within finite set of genetically and environmentally constrained set of possible designs and functions, while largely unfolding along a predictable developmental timeline.
The flexibility and diversity of developmental outcomes create a dialectic of adaptation with vulnerability during development from the level of individual circuits to the maturing person. The opportunities for adaptive change and the periods of critical vulnerability during the development of neural circuits are themselves dynamic, temporally specific, and sensitive to experience and environmental exposures. As cognitions, emotions, and behaviors in health and illness are increasingly viewed in terms of the functioning of neural circuits, defining typical trajectories for the maturation of those circuits provides a crucial background against which genetic alterations and the effects of adverse experience on brain maturation can be compared and contrasted to identify the developmental origins of neuropsychiatric illnesses.
Understanding the processes of development and organization of the functional properties of neural circuits requires access to defined circuits with known and measurable properties at different points in development. Ethical, methodological, and practical considerations understandably constrain the study of the nascent human nervous system and the neural circuits it contains (Huisman et al, 2002; Huttenlocher and Dabholkar, 1997; Levitt, 2003). Neurodevelopmental data in human beings and nonhuman primates, consisting mainly of postmortem and neuroimaging studies, are both limited and inherently constrained (Fogliarini et al, 2005; Huttenlocher, 1979, 1990; Huttenlocher and Dabholkar, 1997; Levitt, 2003). The remarkable evolutionary conservation of neurodevelopmental events and their timing across species, however, help to validate the extension of knowledge about prenatal brain development from animals to human beings (Bystron et al, 2008; Finlay and Darlington, 1995; Katz, 2007; Levitt, 2003; Lund and Lewis, 1993; Marin-Padilla, 1988). In this review, we integrate established and emerging knowledge of development to describe the normal maturation of neural circuits. We also provide specific examples of neuropsychiatric disorders that are commonly seen by child psychiatrists and pediatric neurologists to illustrate how knowledge of normal circuit development can inform the study and treatment of developmental psychopathologies.
THE EARLIEST NEURAL CIRCUITS
Early Gestational Events Establish a Framework for the Genesis of Neural Circuits
The neuro-ontogenic process in humans begins at gestational age (GA) weeks 2–3 with the folding and fusion of ectoderm to form the neural tube (Ladher and Schoenwolf, 2005) (Figure 1). At week 4 of gestation, the rostral portion of the neural tube forms three vesicles that are destined to give rise to the forebrain, the midbrain, and the hindbrain (Jessell and Sanes, 2000; Rash and Grove, 2006b; Rhinn et al, 2006; Stern, 2001). The rostral-most prosencephalic (forebrain) vesicle then forms two vesicles that are destined to become the telencephalon (cerebral cortex) and the diencephalon (thalamus, hypothalamus, and other structures). This is followed by a complex, dynamic, sequential, and yet temporally overlapping series of cellular events that are genetically determined, epigenetically directed, and environmentally influenced. By GA weeks 5–6, neuroblasts, or neuronal precursors, are proliferating rapidly within the ventricular zone (germinal matrix) that lines the cerebral ventricles (Bystron et al, 2008; Ghashghaei et al, 2007; Hatten, 1993; Jessell and Sanes, 2000; Kornack and Rakic, 1995; Levitt, 2003; McManus et al, 2004; Molliver et al, 1973; Monk et al, 2001; Pencea et al, 2001; Rakic, 1978, 1982, 1988, 1995, 2003; Rash and Grove, 2006a).
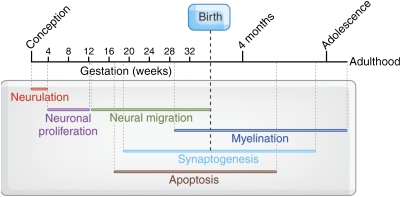
Figure 1.
Timeline of major events in brain development. This diagram represents brain development beginning with neurolation, and proceeding with neuronal migration, synaptogenesis, pruning, myelination, and cortical thinning. Reproduced with permission and modified from Giedd (1999) (Copyright 1999) American Psychiatric Association.
The laminar structure of the cerebral cortex is encoded early in development. By GA week 8, neuroblasts begin to differentiate into either specific neuronal cell types or macrogila, depending on their location within a complex topographic matrix of molecular gradients in the ventricular zone layer (Figure 2). Postmitotic cells migrate out of this layer to form cortical laminae in an ‘inside–out' manner in which deeper cortical layers are formed before more superficial ones (Hatten, 1993; Kornack and Rakic, 1995; Rakic, 1978, 1988, 1995). Most postmitotic neurons travel along radial glial cells that serve as guides on the path of neurons to their final destination (Rakic, 1972; Rakic et al, 1994b). Radial glia themselves may also give rise to neurons in the developing cortex (Liu and Rao, 2004; Malatesta et al, 2000; Miller, 2002; Miyata et al, 2001; Noctor et al, 2001). Migration depends on a complex set of molecular interactions between neurons and the scaffolding glia (Chao et al, 2009; Gressens, 2000; Hatten, 1999). Another smaller group of neurons originates from the primordia of the basal ganglia nuclei (the medial and lateral ganglionic eminences) and migrates tangentially (ie parallel to the outer cortical surface) to destinations in the developing cerebral cortex and thalamus, giving rise to all of the GABAergic neurons in the mature brain (McManus et al, 2004; Monk et al, 2001; O'Rourke et al, 1992; Van Eden et al, 1989). Neuronal migration peaks between GA weeks 12 and 20 and is largely complete by GA weeks 26–29 (de Graaf-Peters and Hadders-Algra, 2006; Gupta et al, 2005) (Figure 2).
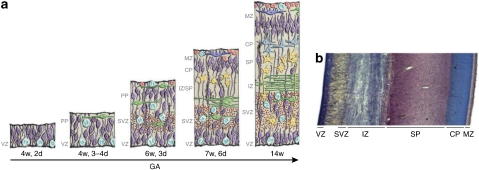
Figure 2.
The embryonic layers of the developing human neocortex. (a) Schematic illustration of the development of the layers in the human neocortex. (b) Histochemical section of the human fetal brain at GA 16 weeks stained with cresyl violet to show cortical lamination. (a) Reproduced with permission from Bystron et al (2008) (Copyright 2008) Nature Publishing Group; (b) reproduced with permission from Kostović et al (2002) (Copyright 2002) Oxford University Press.
Errors in neuronal migration can have profound neurodevelopmental consequences. Lissencephaly, or ‘smooth brain,' for example, is a disorder of neuronal migration that disrupts the normal patterning of gyri and sulci. Its functional consequences range from mental retardation to death in infancy (Olson and Walsh, 2002). The various causes of lissencephaly include mutations in genes encoding cytoskeletal proteins, components of the basal lamina, glycosyltransferases, and components of the reelin signaling pathway. Disturbances in neuronal migration can also produce foci of ectopic cortical tissue in the white matter. These gray matter foci contain both GABAergic and glutamatergic cells that can produce seizure disorders (Gomez et al, 1999; Uhlmann et al, 2002).
Early Synapses in the Developing Brain
As neurons complete their migration, they extend axons and dendrites to appropriate synaptic partners. Scaffolding cells and molecular gradients are important in the assembly of these synaptic connections. Also inherent to both the construction and maturation synapses within neural circuits are their continuous refinement and modification. The synaptic connections among neurons early in development are often transient stepping-stones toward the more stable connections that characterize more mature circuits.
The earliest synaptic connections are formed at approximately GA week 5 by neurons located in the first recognizable cortical layer known as the preplate (alternatively termed the primordial plexiform layer) (Marin-Padilla, 1971; Raedler and Raedler, 1978; Rickmann et al, 1977; Stewart and Pearlman, 1987; Supèr et al, 1998; Wood et al, 1992). On their way to the preplate, and later to the subplate, axons of dorsal thalamic neurons are guided by molecular interactions with a population of tangentially migrating neurons termed as ‘corridor' cells, which, similar to radial glial cells, are a class of scaffolding cells (Lopez-Bendito et al, 2006). The neurons in the preplate serve as initial synaptic targets for neuronal projections from the developing thalamus and brainstem. Preplate cells are aptly termed as pioneer neurons because they form temporary connections with presynaptic cells, serving as placeholder targets until the appropriate postsynaptic neurons are in place and ready to form more mature connections (Chao et al, 2009). Neurons within the preplate form a primitive, yet functionally active, early cortical circuit (Bayer and Altman, 1990; Kostovic and Molliver, 1974; Kostovic and Rakic, 1990; Marin-Padilla, 1971, 1978; Mrzljak et al, 1990).
The axonal glycoprotein, neural cell adhesion molecule L1, mediates the interaction between the cytoskeleton and extracellular matrix and, therefore, has an important function in neuronal migration and differentiation (Sonderegger and Rathjen, 1992; Thelen et al, 2002). Mutations in the gene encoding this protein produce X-linked mental retardation syndrome CRASH (corpus callosum agenesis, retardation, aphasia, spastic paraplegia, hydrocephalus) (Jouet et al, 1994; Rosenthal et al, 1992). Alcohol exposure impairs the function of cell adhesion molecule L1, which then may contribute to the features of fetal alcohol syndrome (Ramanathan et al, 1996; Tang et al, 2006).
The Rise and Fall of the Subplate: from Synapses to Early Neural Circuits
The subplate is a transient though important embryonic cortical layer that forms within the preplate (Allendoerfer and Shatz, 1994; Bystron et al, 2008). The subplate is thicker than all other cortical layers between GA weeks 18 and 22, when it is up to five times thicker than the cortical plate. During this period, the subplate is rich with synapses and contains evidence of a laminar organization. The differential timing of developmental events across brain regions is evident in the subplate, in which somatosensory regions develop earlier than visual regions (Kostovic and Rakic, 1990). Neurons in these portions of the subplate receive preliminary afferent inputs from the visual and somatosensory thalamus, cholinergic afferents from the basal forebrain, and monoaminergic afferents from the brainstem (Allendoerfer and Shatz, 1994; de Graaf-Peters and Hadders-Algra, 2006; Ghosh et al, 1990; Ghosh and Shatz, 1992; Kostovic and Rakic, 1984, 1990; McConnell et al, 1989; Supèr et al, 1998).
Subplate neurons have the molecular components necessary for functional GABAergic transmission (Meinecke and Rakic, 1992), which, unlike in the mature nervous system, is excitatory during much of early development (Ben Ari et al, 1997). Electrophysiological recordings have shown that although they are electrically silent at rest, subplate neurons are capable of propagating neural signals across monoaminergic, cholinergic, and glutamatergic synapses (Friauf et al, 1990; Isaac et al, 1997). Thus, the architectural framework and functional capacities of the major neurotransmitter systems are established early in gestation, even though their function in early cortical development is at present poorly understood in humans. Exposure to drugs of abuse or medications that alter signaling, metabolism, or other elements of neurotransmitter physiology may affect the development of neural circuits and neurotransmitter systems in profound or more subtle, but still significant ways.
As in the preplate, synaptic connections in the subplate serve as placeholders ahead of later, more enduring connections for thalamocortical neurons. Thus, subcortical afferents to the subplate may transiently connect to their future postsynaptic targets in layer IV of the cortex through intermediary subplate neurons (Ghosh et al, 1990; Ghosh and Shatz, 1992), which themselves project to the layers of the future cerebral cortex, the cortical plate, and marginal zone (Allendoerfer and Shatz, 1994; Kanold, 2004). After pausing in the subplate, subcortical afferents make more permanent connections within the cortical plate through a process of synaptic refinement that begins slowly around GA week 20, reaches its peak between GA weeks 24–28, and continues into the perinatal period. This refinement of synaptic connections causes the dissolution of the subplate, which can be observed in fetal MRI scans (Huisman et al, 2002; Kostović et al, 2002). After 28 weeks of gestation, the declining subplate largely contains neurons that are destined for association areas and commissural pathways, which are among the last of cortical regions and pathways to develop (Kostovic and Rakic, 1990).
DEVELOPMENT OF NEURAL CIRCUITS WITHIN THE CORTICAL PLATE IN THE PERINATAL PERIOD
Overview
As postmitotic cells at GA weeks 7–8 migrate from the ventricular zone into the preplate, they form another embryonic cellular layer known as the cortical plate (Bystron et al, 2008; Rickmann and Wolff, 1981; Supèr et al, 1998) (Figure 2). As it forms within the preplate, the cortical plate splits the preplate into two layers: the subplate below and the marginal zone above. The cortical plate will produce laminae II–VI of the mature cerebral cortex, whereas the marginal zone will ultimately form cortical layer I.
The dissolution of the subplate and the concurrent maturation of the cortical plate signals a transition in the development of cortical circuits. Transient and intermediate connections now yield to more enduring patterns of neural connections that hold greater potential for generating goal-directed activity. As it develops, the cortical plate increasingly acquires the organizational features of the mature cortex. Lamination is present first in the primary sensory and motor cortices as early as GA week 25. By GA week 32, the developing cortex has a full adult complement of distinct vertical lamina (Kostovic et al, 1995) containing afferents of all the major neurotransmitter systems (Levitt, 2003) and a diversity of differentiated glia and neuronal cell types (Lund and Lewis, 1993), including excitatory glutamatergic spiny pyramidal neurons and GABAergic nonpyramidal interneurons (DeFelipe and Farinas, 1992; Nieuwenhuys, 1994).
Cortical Lamination and Local Neural Circuits
Similar to the mature cortex, the developing cortical plate consists of 3–6 cellular layers. Depending on the specific cortical region, cortical layer I is formed between GA weeks 24 and 34, and layers III and IV appear between GA weeks 32 and 34 (Kostovic et al, 1995). Each layer contains a distinct array of cells types, the morphology, and laminar location of which dictate the pattern of local and distant projections that each cell may send or receive (Figure 3).
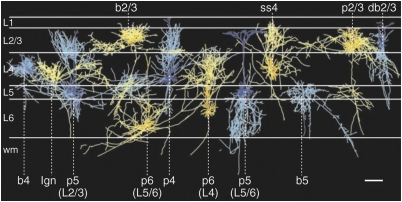
Figure 3.
The diversity of neurons within cortical laminae. Reconstructed coronal view of the different cell types that are represented in mature cortical layers I–VI. Cells were colored for ease of viewing, as follows: axons, bright blue or bright yellow; dendrites; dark blue or dark yellow. L, cortical layer; wm, white matter; b, basket cells; db, double bouquet cell; p, pyramidal cells; ss, spiny stellate cells. Scale bar, 300 μm. Reproduced with permission from Binzegger et al (2004) (Copyright 2004) Society for Neuroscience.
Cells in adjacent vertical lamina are organized into functional radial ontogenetic columns, each of which consists of many smaller minicolumns with their longest axes arranged perpendicular to the cortical surface (Hubel and Wiesel, 1962; Mountcastle et al, 1957). Pyramidal neurons are clonally derived from radial glial cells and can reside in a number of layers within the same minicolumn (Yu et al, 2009). As they mature, beginning approximately in the late third trimester, these clonally derived cells preferentially form functional excitatory synaptic connections with one another, rather than with adjacent cells that are not derived from the same progenitor (Yu et al, 2009). Short-range connections bind cells of nearby minicolumns horizontally and cells of differing laminae within each column vertically (Mountcastle, 1997).
Distinctions among cortical laminae are based on the cell types and connections that predominate in each layer. The canonical pattern of laminar connections that forms cortical microcircuits has been best defined in the visual cortex (Figure 4). Layer VI contains pyramidal cells with rich dendritic arbors that project excitatory axons to the thalamus (Gilbert, 1983; Marin-Padilla, 1970). This layer also sends and receives excitatory and inhibitory input from more superficial cortical layers within each minicolumn. It contains local inhibitory and excitatory interneurons, and it receives input from subcortical and other cortical layers. The presence and extent of cellular differentiation in layer IV reflects the evolutionary origin and complexity of cortical tissue across the cortical surface. Isocortical (also termed homotypic or eulaminate) regions contain a granular layer IV and are typically involved in higher-order processing. Peri-allocortical (or transitional) regions have a less developed layer IV, whereas allocortical (or heterotypic) regions lack layer IV and are typically limbic cortices. Of all cortical laminae, layer V contains the largest pyramidal cells, which send excitatory projections to the basal ganglia, brainstem, and spinal cord. Similar to layer VI and to more superficial layers, layer V also receives and sends other excitatory and inhibitory projections.
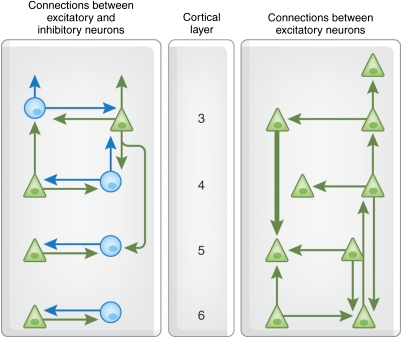
Figure 4.
Schematic of the connections between layers 3 and 6 neurons of the cerebral cortex in adults. This diagram represents a summary of data from paired intracellular recordings and dye filling of connected neurons from the visual cortex of adult rats and cats. Triangles, excitatory neurons; circles, inhibitory interneurons. Clearly, many more types of interlaminar connections remain to be described in detail. Reproduced with permission from (Thomson and Bannister, 2003) (Copyright 2003) Oxford University Press.
Excitatory neurons in layer IV, such as pyramidal and spiny stellate cells, are the primary targets for thalamic inputs (Kostovic and Goldman-Rakic, 1983). Neurons in layer III receive convergent inputs from inhibitory interneurons that originate within this layer and in deeper layers. Layer III pyramidal cells project to ipsilateral and contralateral cortical regions (Mrzljak et al, 1988). Similarly, layer II receives diverse inputs but contains smaller pyramidal neurons that send projection to less distant cortical regions. Finally, the connectivity and computational functions of the most superficial lamina, layer I, are also the least understood. Within layer I, axons originating from other cortical regions terminate on apical dendrites of pyramidal cells lying in deeper laminae.
Although the cellular composition and patterns of connections of these cortical laminae are more heterogeneous, complex, and overlapping than the following schematic description would suggest (Douglas and Martin, 2004; Gilbert, 1983; Thomson and Bannister, 2003), the neural circuits formed by connections to, from, and within each lamina of the visual cortex serves as a model region for studying the development of microcircuits in other cortical regions (Figure 4). In the much-simplified archetypal cortical microcircuit, for example, thalamic afferents project to layer IV, from which excitatory neurons project to pyramidal neurons in layers III and II. Excitatory pyramidal cells in layer III project to pyramidal cells in layer V and to layer II. Pyramidal cells in layer V project to pyramidal cells in layers III and IV. Finally, layer VI pyramidal cells project to layer IV.
In general, laminar outputs also follow a characteristic pattern. Layers II and III extend cortical afferents, with layer II projecting more locally and layer III extending more distant projections. Layers V and VI project to subcortical regions, with layer VI projecting primarily to the thalamus and layer V projecting to the brainstem, midbrain, and basal ganglia. Within each microcircuit, inhibitory interneurons regulate this basic flow of information from one excitatory neuron to the next. Excitatory cells in most layers project onto inhibitory interneurons in other layers. In turn, inhibitory interneurons may project onto excitatory neurons or onto other inhibitory interneurons. Thus, the complex connections of the developing cortex are both nested and overlapping, and the features that will endure as they are fine-tuned throughout all of perinatal and postnatal life.
Progressive and Regressive Forces Shape Neural Circuits
The dynamics of synaptogenesis in the cortex of the developing fetus have been reasonably well characterized (Becker et al, 1984; Bourgeois, 1997; Bourgeois et al, 1994; Huttenlocher et al, 1982a; Michel and Garey, 1984; Mrzljak et al, 1988, 1992, 1990; Rakic et al, 1986). Synaptic density within the cortical plate grows at a rate of about 4% per week until approximately GA weeks 26–28, which is the time of peak transfer of afferent synaptic connections from the subplate to the cortical plate (Zecevic, 1998). Dendritic arborization and synaptogenesis accelerate in the third trimester to produce a thickening of the developing cortex (Huttenlocher and Dabholkar, 1997), which coincides with the appearance of cortical gyri and sulci on fetal MRI scans (Garel et al, 2001; Huisman et al, 2002). GA week 34 marks entry into the peak period of synaptogenesis, during which almost 40 000 new synapses are formed every second, a process that continues well into early postnatal life.
The timing of synaptogenesis differs across cortical layers and across developing cortical regions. Following the inside–out sequencing of cortical lamination, synaptogenesis begins earlier in deeper layers than in more superficial ones (Huttenlocher, 1990). Although several studies reported no differences in the rates of synaptogenesis across somatosensory, visual, prefrontal, and limbic cortices (Bourgeois et al, 1994; Rakic et al, 1986; Zecevic, 1998; Zecevic et al, 1989), other studies have found that synaptogenesis begins earlier in primary motor areas and later in anterior regions, such as the prefrontal cortex (PFC) (Huttenlocher, 1984, 1990; Huttenlocher and Dabholkar, 1997).
The cellular and molecular mechanisms that underlie expansion of the developing central nervous system are counterbalanced by the culling of neurons and their processes (Cowan et al, 1984). The number of neurons in the human brain peaks at GA week 28. However as many as half of the neurons produced during neurogenesis die in the process of apoptosis, or naturally occurring cell death, by the end of adolescence (Lossi and Merighi, 2003). The reasons for the excess production of most neuronal types and glia are not fully understood.
Many of the culled neurons are eliminated during two discrete developmental epochs (Gohlke et al, 2006; Lossi and Merighi, 2003; Rakic and Zecevic, 2000). The first wave of apoptosis involves primarily proliferating precursor cells and young postmitotic neuroblasts in the ventricular zone (Blaschke et al, 1996). It begins around GA week 7 and continues throughout the first trimester. This wave is governed by intracellular processes related to cell-cycle regulation (de la Rosa and de Pablo, 2000). The second wave of apoptosis peaks around GA weeks 19–23 and eliminates postmitotic neurons within the cortical plate. In this wave, apoptosis is regulated by synaptic activity, various forms of cell–cell contact, and a broad range of diffusible and membrane-bound trophic factors that are produced by glia and other neurons (Lossi and Merighi, 2003). Cells that send processes to incorrect regions are eventually eliminated because those cells are less likely to fire synchronously with other inputs to the postsynaptic cell, which then fails to release the trophic factors necessary for the survival of presynaptic neurons (Eyre et al, 2001; Goda and Davis, 2003). Thus, coherent neuronal activity is also important for the survival and maintenance of neurons, synapses, and neural circuits.
Glial Cells and Myelination Support Development of Neural Circuits
Microglia, oligodendrocytes, and astrocytes, the glial components of white matter in the central nervous system, participate in a host of important support functions for neurons, including guidance of neuronal migration, regulation of the composition of the extracellular environment, modulation of synaptic connections, and clearance of neurotransmitters. Premyelinating oligodendrocytes populate the developing cortex during gestation. Their subsequent production of myelin enhances the speed and fidelity of the transmission of information encoded in action potentials that propagate along neurons. Between GA weeks 20 and 28, mature myelin is detected first in subcortical regions and later in cortical regions. At GA week 35, it is detected in the precentral and postcentral gyri and optic radiation, and at GA week 40, it is present in the acoustic radiation (Iai et al, 1997). On MRI, some myelinated white matter can be discerned in the brains of preterm infants by GA week 29, but the majority of white matter appears unmyelinated (Hüppi et al, 1998). Between GA weeks 36 and 40, the proportion of total brain volume that contains myelinated white matter increases from 1 to 5% (Hüppi et al, 1998).
Premyelinating oligodendrocytes may be particularly sensitive to perinatal hypoxia or ischemia, which may disrupt white matter tracts in the frontal and temporal lobe to produce cerebral palsy or mental retardation (Back et al, 2001; Ness et al, 2001). Exposure of the developing brain at this time to environmental toxins, drugs of abuse, nutritional deficiencies, and the effects of preterm birth is also thought to disrupt myelination and thereby predispose to poor cognitive, neurodevelopmental, and neuropsychiatric outcomes.
Functional Correlates of Neural Circuit Development in the Perinatal Period
The increasing organizational complexity and activity-dependent remodeling of neuronal connections establish the structural foundation that supports the early functional properties of cortical circuits. Neurons with multiple axonal branches can innervate and influence numerous target cells, whereas complex dendritic trees allow for the synthesis of inputs from other cells that are many and diverse (Jessell and Sanes, 2000). The honing of connectivity among these morphologically and neurochemically diverse neuronal populations generates correspondingly varies computational functions, ranging from a limited input and response to a complex integration of multiple signals, which together assimilate information from different regions of the brain (de Graaf-Peters and Hadders-Algra, 2006; Jessell and Sanes, 2000; Levitt, 2003). Thus, increasing structural organization heralds an increased functional organization of cortical circuits.
The in vivo study of functional activity in the fetal brain is challenging. Yet, electrophysiological measurements in preterm infants, have shown patterns of electrical activity that change in parallel with anatomical development of the cortex between GA weeks 20 and 40 (Graziani et al, 1968; Kostovic et al, 1995; Kurtzberg et al, 1984; Novak et al, 1989; Vaughan and Kurtzberg, 1989). These developmental changes in the EEG signal, seen most prominently over the sensorimotor cortices, may represent increased activity in the neurons of superficial cortical layer III relative to deeper cortical layer IV (Kostovic et al, 1995). As layer IV receives thalamic input, whereas layer III projects to other cortical layers and, therefore, supports higher-order processing, EEG evidence of increased activity in layer III may reflect the functional maturation of sensorimotor cortices. These early maturational events herald the subsequent broader functional development of both local and distributed cortical circuits.
DEVELOPMENT OF NEURAL CIRCUITS IN INFANCY
Overview
The early postnatal period represents a time of dramatic change in brain structure and function. The brain grows to about 70% of its adult size by 1 year of age and to about 80% of adult size by age 2 years (Dekaban, 1978; Knickmeyer et al, 2008). This increase in brain volume during the first year of life is greatest in the cerebellum, followed by subcortical areas and then the cerebral cortex (Knickmeyer et al, 2008), which increases in volume by an impressive 88% in the first year, 15% in the second year, and then more modestly but steadily thereafter (Knickmeyer et al, 2008). Development of gray and white matter, myelination, synaptogenesis, pruning, and synaptic modification establishes the fundamental anatomical organization for the initial functioning of neural circuits in utero. These progressive and regressive developmental processes continue in early postnatal life. Subsequently, local connections within cortical circuits are fine-tuned, and complex longer-range connections among circuits form an increasingly unified and functionally organized neural network.
During early postnatal life, the newborn does not simply experience novel stimuli passively; the infant also elaborates behaviors that powerfully influence its environment (Kandel et al, 2000). Together with inborn genetic factors, novel experiential inputs and behavioral responses act on a still immature brain substrate to stimulate the further development of neural circuits. With time, experience has an increasingly more prominent function in the shaping of neural circuitry.
Early Postnatal Development of Neural Circuits
The newborn brain at 2–4 weeks of age is approximately 36% the size of an adult brain (Knickmeyer et al, 2008). Histological study of newborn brains has shown that granular layer IV is already present in all neocortical areas (Kostovic and Rakic, 1980). Primary cortical areas, including motor, somatosensory, visual, and auditory cortices, can be identified cytoarchitectonically. Association cortices, however, are less clearly delineated at this age.
Although the macroscopic visualization of brain structure using MRI in preterm and term infants cannot inform our knowledge of the cellular events of normal brain development, neuroimaging does provide a rare glimpse into the anatomic features of brain development during late gestation and early postnatal life. Anatomical MRI of preterm infants shows that total brain volume increases nearly three-fold between GA weeks 29 and 41 (Hüppi et al, 1998). During this period, gray matter grows from 35 to 50% of total brain volume. MRI of term infants aged GA 39–48 weeks shows a robust increase in total gray matter volume across this developmental window compared with other brain compartments (Gilmore et al, 2007b). Infants born at term have more gray matter in sensorimotor and visual cortices, as well as in parieto-occipital regions, compared with preterm infants (Peterson et al, 2003). Gray matter growth is pronounced in occipital and parietal cortices in the first weeks of life.
During the early postnatal period, cortico–cortico fibers within the remnants of the subplate connect with their cortical postsynaptic targets (Kostovic and Rakic, 1990; Schwartz et al, 1991). Cells expressing markers that are characteristic of neuroblasts and early neurons have been identified in the subventricular zone of newborns (Weickert et al, 2000), and migrating neurons can still be found in white matter regions (Kostovic and Rakic, 1980). Although cortical neurogenesis and neuronal migration continue through the postnatal period (Bhardwaj et al, 2006; Shankle et al, 1999), more likely is that the expansion of glia and myelination during this period makes a more significant contribution to the dramatic brain growth observed in newborns and toddlers (Dekaban, 1978). Thus, perinatal and early postnatal brain development features the onset of myelination and a striking development of gray matter connections, especially in sensorimotor and visual cortices.
Abnormal brain size in early postnatal life often heralds the later onset of neurodevelopmental disorders. Malnutrition, for example, decreases brain size in infancy and produces a broad range of adverse effects on neural circuit development (Holden, 2007). Conversely, accelerated brain growth in infancy seems to be one early manifestation of a wide range of developmental delays of motor, language, and cognitive functions that includes autism (Courchesne et al, 2001; Hazlett et al, 2005). MRI findings in autistic individuals suggest expansion of both gray and white matter compartments (Hardan et al, 2006; Piven et al, 1995). These findings may indicate the persistence of exuberant synapses and myelin or reduced apoptosis, and abnormal patterns of brain connectivity (Belmonte et al, 2004). However, the precise underlying cellular and molecular determinants of enlarged brain size in autistic children have yet to be defined.
Synaptogenesis and Synaptic and Axonal Pruning in Infancy
Cortical gray matter continues to grow through the first few years of life, although its rate is most robust during the first postnatal year. The trajectory of expansion of gray matter has been shown with MRI (Knickmeyer et al, 2008) and is consistent with postmortem studies showing that the elaboration of dendrites, spines, and synapses continues to accelerate at a near logarithmic pace through the first 350–400 postnatal days (Andersen, 2003; Bourgeois, 1997; Lund and Lewis, 1993; Supèr et al, 1998). The first 2 years of life see the arborization of both pyramidal cells and GABAergic inhibitory interneurons (Mrzljak et al, 1990), as well as the relative expansion of cortical layers II and III, compared with other layers (Huttenlocher and Dabholkar, 1997; Landing et al, 2002; Zecevic et al, 1989).
The time course of synaptogenesis differs across cortical regions. In the primary visual cortex, for example, after a burst of synapse formation between age 3 and 4 months, synaptic density reaches its peak at 140–150% of adult levels between the ages of 4 and 12 months, after which the mean number of synapses per neuron decline (Bourgeois, 1997; Bourgeois and Rakic, 1993; Huttenlocher, 1990; Huttenlocher et al, 1982a, 1982b; Michel and Garey, 1984). Synaptogenesis in the PFC begins about the same time as in visual cortex, but it does not reach its peak period until age 8 months, continuing thereafter through the second year of life (Huttenlocher and Dabholkar, 1997; Kostovic et al, 1995).
The elimination of axons, dendrites, and synapses, and the death of neurons through apoptosis, are important counterpart processes to the elaboration of supernumerary axons, dendrites, and synapses (Cowan et al, 1984). Pruning processes begin in late gestation and become increasingly active postnatally. As with synaptogenesis, the time course for pruning differs across brain regions, with sensory and motor cortices undergoing dramatic fine-tuning after birth, followed by association cortices and the corpus callosum, and later by regions that subserve higher cognitive functions (Levitt, 2003).
Synaptic Plasticity and Neural Circuit Development in Postnatal Life
Similar to pruning, the remodeling of existing synapses has a key function in the reorganization and fine-tuning of neural circuits. Synaptic plasticity refers to a set of mechanisms that mediate the activity-dependent strengthening or weakening of neuronal connections at the level of the synapse (Citri and Malenka, 2008). The strength and pattern of activity at a given synapse produce transient or enduring depression or potentiation of communication between neurons (Martin et al, 2000; Morris, 2006; Squire et al, 2004). Thus, long-term potentiation and long-term depression are active processes that depend on electrical activity. In Fragile X syndrome, the most common genetic cause of mental retardation, silencing of the Fmr1 gene (Devys et al, 1992) seems to disrupt the balance between long-term potentiation and depression (Bagni and Greenough, 2005; Huber et al, 2002). The broad effects of impairments in activity-dependent modification of synapses in Fragile X underscores the critical importance of the molecular and cellular mechanisms that underlie synaptic plasticity, for normal postnatal brain development and healthy cognitive outcomes.
The reorganization of thalamocortical projections through pruning and synaptic remodeling is also critical for the function of a number of sensory systems. For example, in the development of binocular vision, the correct differentiation of columns in the visual cortex depends on long-term potentiation, long-term depression, and pruning, all of which are regulated by patterns of neuronal activity originating in both retinas (Berardi et al, 2000; Ehrlich and Malinow, 2004; Huttenlocher et al, 1982b; Takahashi et al, 2003). Consistent with a Hebbian-based model of learning, competition between coordinated activities originating in the retina produces the stepwise selection of input from one eye for each neuron. Iterative, coordinated activity stabilizes synaptic connections from one eye, whereas synapses with contralateral afferents that transmit weaker and less organized patterns of activity become less active and are eliminated (Hooks and Chen, 2006).
As neuronal activity moderates the important influences of experience and environment that act to shape the development of neural circuits, alterations in visual sensory input and thus a disordered pattern of neuronal activity during this developmental period produce relatively irreparable disturbances in vision (Huberman, 2007). For example, failure to correct congenital cataracts in infants by 6 months of age or strabismus by 7 years of age produces irreversible impairments in the visual system (Levi and Li, 2009; Maurer et al, 1999). Thus, certain epochs in the maturation of neural circuits for vision appear to constitute critical periods of developmental vulnerability, times when experiential input is necessary for the normal development of specific neural circuits and their functional capacities, and without which the potential for development of those functional capacities is lost forever (Morishita and Hensch, 2008).
Myelination and Neural Circuit Development
Soon after birth, glial cells increase dramatically in size and number, and the pace of myelination continues its prenatal trajectory. Myelination proceeds rapidly during the first year of life before continuing at a slower but steady pace thereafter (Barnea-Goraly et al, 2005; Gao et al, 2009). Similar to synaptogenesis and pruning, myelination has a specific time course that differs across different cortical regions and at variable rates within specific functional circuits. Postmortem and anatomical MRI studies show that myelination advances in a posterior-to-anterior direction, after the general pattern of maturation of neural circuits (Brody et al, 1987; Girard et al, 1991; Kinney et al, 1988; Paus et al, 2001). Sensory pathways myelinate first, followed by motor pathways, and then association areas. Myelination reaches the furthest portions of the frontal lobes between 7 and 11 months of age (Barkovich et al, 1988). Within a given functional circuit, subcortical structures myelinate before cortical regions.
Myelination as viewed using anatomical MRI may lag behind ultrastructural evidence of myelination, particularly before 3 years of age (Matsuzawa et al, 2001; Paus et al, 2001). Diffusion tensor imaging (DTI) measures the restrictions on water diffusion along nerve fiber bundles, which is dominated by the increasing concentrations of myelin during white matter development, and can detect earlier evidence of myelination (Bansal et al, 2007). DTI findings have shown a time course of white matter maturation across the brain that is generally consistent with the findings from other experimental modalities (Berman et al, 2005; Gao et al, 2009; Gilmore et al, 2007a; Hermoye et al, 2006; McGraw et al, 2002; Mukherjee et al, 2001; Partridge et al, 2005; Zhai et al, 2003) (Figure 5). Similar to DTI, developmental proton magnetic resonance spectroscopy (MRS) findings support observations made using other experimental approaches that show an initial rapid elaboration of neuronal processes and synaptogenesis in the first 2 years of life, followed by a subsequent plateau (Horska et al, 2002; Kreis et al, 1993; Toft et al, 1994) that differs in timing across cortical regions (Cady et al, 1996; Hanaoka et al, 1998; van der Knaap et al, 1990).
Figure 5.
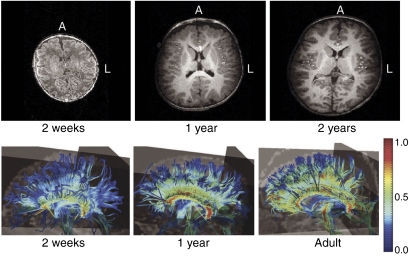
Brain myelination across development. Top panels: T1-weighted axial MRI images acquired longitudinally from one child, showing age-related increase in brain size and white matter intensity. Bottom panels: DTI images of white matter tractography in a cross-sectional comparison showing of age-related increase in the organization of corpus callosum white matter. Each panel represents one subject. Color scale represents fractional anisotropy; higher values correlate with greater organization of fibers tracks. Panels are labeled with age of participants at the time of scan. A, anterior; L left. Reproduced with permission from Gilmore et al (2006) (Copyright 2006) American Psychiatric Association.
Functional Imaging Studies of the Infant Brain: Metabolic Activity During Infancy Measured with PET
As they involve radionucleotide exposure and a relatively invasive technique, positron emission tomography (PET) imaging studies of healthy pediatric populations are unusual. PET imaging of a small clinical population with a near normal neurological examination (abnormalities included fetal distress, seizures, congenital heart disease, and Sturge–Webber syndrome) has shown that subcortical regions and the sensorimotor cortex are the most metabolically active brain regions in neonates younger than 5 weeks of age (Chugani, 1994; Chugani et al, 1987). By 3 months of age, metabolic activity increases in parietal, temporal, and dorsolateral occipital cortices. By 6–8 months of age, frontal regions begin to increase in metabolic activity (Chugani, 1994; Chugani et al, 1987). Similar to other neuroimaging modalities, PET shows that a pattern of change in metabolic activity is consistent with the aforementioned spatial variation in time courses for myelination and synaptogenesis.
Development of Functional Connectivity in Infancy
A development in functional imaging involves the use of measurements of brain activity acquired at rest to identify functionally relevant patterns of activity that represent intrinsic spontaneous brain activity (Binder et al, 1999; Biswal et al, 1995; Hampson et al, 2002; Mazoyer et al, 2001). Sets of regions that share temporally correlated activity at rest are believed to constitute functional networks (Beckmann et al, 2005; Damoiseaux et al, 2006; Deluca et al, 2006; Fox et al, 2005, 2006; Fox and Raichle, 2007; Greicius et al, 2003; Kelly et al, 2008; Raichle et al, 2001). These techniques have shown a number of distinct functional networks in neonates at GA 39–44 weeks of age, including visual, sensorimotor, and auditory processing networks, as well as a prefrontal network (Fransson et al, 2007). In addition, functionally correlated medial and lateral parietal areas may represent a rudimentary default-mode network, a system that when mature consists of precuneus, midline prefrontal, and lateral parietal regions and that has been linked to abstract and autobiographical self-focused processing (Gusnard et al, 2001; Uddin et al, 2007). This putative presence of a default-mode network is consistent with ERP findings, suggesting that interhemispheric connectivity comes on line earlier than longer-range anteroposterior connections (Bell and Fox, 1992). Functional connectivity methods also suggest that sensory networks may undergo rapid development in the first year of life, ahead of similar developmental changes in the visual network that are more prominent across the second year of life (Lin et al, 2008).
Behavioral Correlates of Neural Circuit Development in Infancy
Although the development of a broad range of distinct functional capacities such as motor, sensory, and cognitive functions has been widely described, methodological limitations in the study of brain function in infancy has constrained our understanding of the development of specific neural circuits that subserve these capacities. Neonates exhibit complex, self-generated, but disorganized movements that do not seem goal oriented (de Vries et al, 1985; Prechtl and Hopkins, 1986). Eye saccades at this age are elicited reflexively and are directed by environmental stimuli rather than through endogenous control (Atkinson, 1984), consistent with maturation of somatosensory and motor areas ahead of the visual and association cortices. The behavioral changes that mark the third postnatal month, including inhibitory control over reflexive behaviors and saccades, as well as goal-directed behaviors such as target-directed head–eye coordination and reaching to grasp, may reflect the cortical remodeling and myelination of association areas occurring at that time (Goodkin, 1980). The appearance of hand-to-hand transfer around 6 months of age further corresponds temporally with the increasing anatomical and functional coordination among sensory, motor, and association circuits (Rothbart et al, 1990).
Vulnerability of the Developing Neural Circuit During Infancy
The brain is sensitive to harmful exposures such as toxins, drugs, nutritional deficiencies, infection, medical illness, and environmental stressors throughout the life cycle. As certain neurodevelopmental processes occur within relatively narrow temporal windows, however, the maturing brain during fetal life and infancy may be particularly susceptible to adverse influences. For instance, early separation from caregivers, abuse, neglect, or social deprivation in infancy or early childhood can produce enduring behavioral and neurocognitive deficits (Carpenter and Stacks, 2009; Kreppner et al, 2007). Conversely, early interventions designed to remove or attenuate the effects of these exposures during specific developmental epoch may avert negative sequelae (Tarabulsy et al, 2008; Welsh et al, 2007). Human studies based on animal models have shown that an interaction of early life experiences (such as stress, socialization, and maternal care) with genetic variation in the promoter for the serotonin transporter influences the phenotypic expression of behavioral traits and psychopathology (Caspi et al, 2003; Uher and Mcguffin, 2008). Similarly, animal studies have shown that maternal care influences behavior and future parenting of offspring through epigenetic modification of the chromatin at specific genetic loci (Francis et al, 1999). Nonetheless, critical or sensitive developmental periods of vulnerability to the development of behavioral traits and psychopathology have not been as well defined as they have been for the development of sensory and motor systems.
NEURAL CIRCUIT DEVELOPMENT IN CHILDHOOD AND ADOLESCENCE
Overview
Although brain size grows only from 80 to 90% of its adult size between the ages of 2 and 5 years (Dekaban, 1978), myelination and synaptic remodeling are particularly active during this so-called ‘plateau' phase of development. Our understanding of the cellular changes during this phase of development lags behind the knowledge of the brain's computational capacities from behavioral studies in children (Diamond, 1995). Similarly, a growing body of longitudinal neuroimaging data describes age-related changes in morphological features and functional organization of the brain throughout childhood and adolescence, although the precise correlates of these observations at the cellular and molecular level are largely speculative.
Neural Circuit Development During Early and Middle Childhood
Histological studies suggest that synaptogenesis continues after infancy, but at a slower pace, as cortical gray matter continues to increase in volume through the ages of 4 or 5 years (Levitt, 2003). As in other developmental epochs, the dynamics of synaptic remodeling differs across cortical regions. Synaptic density peaks first in primary sensory areas, followed by the association areas and then the PFC, cortices that subserve higher cognitive functions. Similarly, local cortical connections appear before more distant ones. Thickening of cortical layer II, which makes short range cortical connections with other cortical regions, precedes thickening of layer III, which makes longer-range connections with other cortical regions, between ages 15 and 72 months (Landing et al, 2002). In the PFC, the absolute number of dendritic spines on layer IIIc pyramidal neurons, a major source of associative and commissural projections, peaks at 29 month and declines after 6 years of age (Kostovic et al, 1995).
The net steady state in the number and density of cortical synapses that characterizes the plateau phase of development is thought to reflect the counterbalancing effects of increased rates of synaptic pruning and a slowing of synapse formation (Levitt, 2003). Cross-sectional PET imaging studies report that brain glucose metabolism becomes qualitatively similar to adult patterns by the end of the first year of life (Chugani, 1994; Chugani and Phelps, 1986; Chugani et al, 1987). However, overall brain metabolism rises to twice that of adult levels by 4–5 years of age and remains high until 9–10 years of age. Increased brain metabolic activity likely reflects the exuberant growth of neurons and glia during late infancy and early childhood. The sustained high levels of metabolic activity during the plateau period may reflect the energy demands of myelination and synaptic remodeling, and pruning.
Myelination of Neural Circuits During Childhood and Adolescence
Neuroimaging studies have contributed considerably to our understanding of the maturational changes in gray and white matter during normal brain development. White matter volumes continue to increase through childhood, adolescence, and adulthood (Bartzokis et al, 2003; Benes et al, 1994; Courchesne et al, 2000; Giedd et al, 1999; Matsuzawa et al, 2001; Paus et al, 2001; Sowell et al, 2003a). The rate of increase in white matter volumes is greatest during infancy. Growth in this compartment continues at a slower albeit steady rate through childhood, adolescence, and adulthood, and peaks in the middle of the fifth decade of life (Matsuzawa et al, 2001; Paus et al, 2001). DTI studies show that rates of increase in measures of white matter organization are greatest before age 10 years (Ashtari et al, 2007; Barnea-Goraly et al, 2005; Ben Bashat et al, 2005; Giorgio et al, 2008; Huang et al, 2006; Klingberg et al, 1999; Schmithorst et al, 2002, 2005; Snook et al, 2005). White matter organization proceeds at different rates among white matter regions and individual white matter tracts, and continues well into adulthood.
Cortical Developmental Trajectories in Late Childhood and Adolescence: Anatomic Imaging Findings
Consistent with animal, postmortem, and histological data, large cross-sectional and longitudinal anatomical MRI studies of the brain suggest that volumes of cortical gray matter begin to decline in late childhood or adolescence (Figure 6) (Giedd et al, 1999, 1996a, 1996b; Gogtay et al, 2004; Jernigan et al, 1991; Lenroot et al, 2007; O'Donnell et al, 2005; Pfefferbaum et al, 1994; Shaw et al, 2008; Sowell et al, 2003a, 2004, 2007; Thompson et al, 2005). Cortical thinning proceeds in a ‘back-to-front' direction, and occurs first in sensorimotor areas, followed by association areas, and lastly by higher-ordered cortical areas such as superior PFC and posterior parietal cortex (see http://www.loni.ucla.edu/~thompson/DEVEL/dynamic.html for time-lapse representations of cortical thinning).
Figure 6.
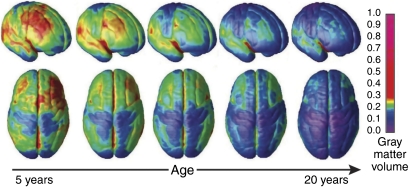
Gray matter maturation across development. Right lateral and top views of the brain showing the dynamic sequence of temporal changes in gray matter volume over the cortical surface. The images represent modeled data from 52 anatomical MRI scans from 13 individuals 4–21 years of age, each scanned four times at approximately 2-year intervals. Color scale represents gray matter units of volume. Reproduced with permission from Gogtay et al (2004) (Copyright 2004) National Academy of Sciences, USA.
Local age-related increases in cortical thickness have also been reported. The thicknesses of perisylvian cortices, in inferior parietal and posterior temporal areas and particularly in the left hemisphere, seem to increase between childhood and adulthood (Sowell et al, 1999, 2001, 2003a). This regional thickening is followed in the fifth decade of life by a thinning that coincides in time with the period of slowed thinning of more dorsal cortical regions. Concurrent with changes in cortical thickness, regional growth that is believed to represent increases in white matter volumes is observed in much of the PFC, rostral anterior cingulate cortex, and temporal poles (Sowell et al, 2003a). Age-related changes in volumes of subcortical gray matter have also been reported, with observed decreases in basal ganglia gray matter (particularly the caudate nucleus) and increases in medial temporal lobe structures, such as the amygdala and hippocampus.
These developmental changes in cortical thickness and brain volumes are consistent with our understanding of the maturational timing of cognitive functions such as attention, working memory, cognitive control, and response inhibition. Further analyses of longitudinal anatomical MRI findings have uncovered a number of functional correlates of cortical maturation. One report suggests that the rates of changes in cortical thickness in regions that subserve higher-order cognitive functions (such as ventrolateral prefrontal, superior prefrontal, and posterior parietal cortices) correlate with measures of intelligence (Shaw et al, 2006). Other reports suggest that cortical thinning in motor regions correlate with fine motor skills and that cortical thickening in left hemispheric language areas may correlate with phonological processing skills (Lu et al, 2007; Sowell et al, 2004). Although cortical thinning is viewed as a reliable marker of maturation, the specific cognitive correlates of these anatomical changes remain unclear.
In childhood-onset schizophrenia, cortical maturation follows a generally normal pattern of thinning, but it proceeds at a faster pace and is spatially more extensive than in typically developing youth (Thompson et al, 2001). Excessive thinning is first detected in parietal cortices, which subserve visuospatial and associative processing, before spreading forward to temporal and prefrontal cortices. The extent of gray matter loss seems to correlate with the severity of psychotic symptoms. These decreases in cortical tissue are thought to involve decreased dendritic arborization and reduced numbers of cells, including glia and neurovasculature (Selemon and Goldman-Rakic, 1999), but they are most commonly viewed as the consequence of excessive synaptic pruning or abnormal in synaptic plasticity (Feinberg, 1982; Liang et al, 2006; Micheloyannis et al, 2006; Rapoport and Gogtay, 2008). Conversely, longitudinal anatomical MRI findings in attention deficit/hyperactivity disorder (ADHD) reveal delays in the time course of cortical maturational, with peak cortical thickness attained later than in typically developing children, particularly in anterior temporal and prefrontal cortices (Shaw et al, 2007).
Putative Mechanisms for Change in Gray Matter Thickness
The cellular underpinnings of changes in cortical thickness have yet to be directly identified. Multiple lines of evidence, however, have generated the consensus hypothesis that cortical thinning may represent two concurrent processes: pruning and myelination. Together, these are believed to refine connectivity within local and distributed networks and to enhance the efficiency and fidelity of signal transmission. Ergo, thinning of association cortices is increasingly regarded as a marker of cortical maturation in healthy children.
Consistent with this belief, animal and postmortem human studies have shown age-related reductions in synaptic density between ages 2 and 16 years (Bourgeois and Rakic, 1993; Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997; Rakic et al, 1986). In the mouse, neuropil, or axonal and dendritic processes, constitutes approximately 60% of cortical volume (Bourgeois and Rakic, 1993), and pruning of these processes may represent the source of cortical thinning detected using MRI (Paus et al, 2008). The hypothesis that cortical thinning represents pruning of synapses, axons, and dendrites is also supported by the evidence that overall brain metabolism, which derives largely from resting neuronal membrane potentials (Mata et al, 1980; Nudo and Masterton, 1986), begins to decline at ages 9–10 years to reach adult levels by age 16–18 years (Chugani, 1994; Chugani and Phelps, 1986; Chugani et al, 1987). The pruning of synaptic processes may, therefore, link cortical thinning with age-related reductions in brain metabolism (Mata et al, 1980; Nudo and Masterton, 1986). The myelination of intracortical axons and the increase in white matter relative to gray matter volumes has also been suggested to affect the quantification of gray matter in MRI data (Paus, 2005; Paus et al, 2008; Sowell et al, 2003a; Yakovlev and Lecours, 1967). This possibility is supported by the findings that regional growth in brain size, which is believed to represent increases in white matter volumes, occurs in conjunction with cortical thinning (Sowell et al, 2003a).
Although genetic factors strongly contribute to the development and maturation of neural circuits, a large body of evidence shows that the fine-tuning of neural circuits is also under environmental influences. Human studies suggest that activity-dependent elimination of excitatory connections is mainly responsible for the 40% reduction of synapses during adolescence (Huttenlocher, 1984; Rakic et al, 1994a). In contrast, levels of local GABAergic inhibitory interneurons remain fairly stable from childhood through adulthood (Changeux and Danchin, 1976; Innocenti, 1981). The relationship between cortical thinning, myelination, and experience-dependent remodeling of neural circuits is growing clearer, but much remains to be done to map the macro- and micro-features of these processes as well as to understand their functional correlates.
Changes in the Organization of Neural Circuits Across Development: Evidence from Imaging Measures of Functional Connectivity
Correlational analyses of fMRI data acquired at rest have recently been applied to the study of developmental changes in functional connectivity among the brain regions that make up functional networks in older children and adolescents. These studies propose two types of age-related changes functional connectivity: decreases in local connectivity among anatomically adjacent but functionally distinct brain regions as they are integrated into their respective networks, and increases in long-range connectivity among nodes that comprise each network (Fair et al, 2007; Kelly et al, 2008). They also support the other lines of evidence suggesting that neural circuits subserving attentional processes mature ahead of those supporting socioemotional functioning.
THE DEVELOPMENT OF NEURAL CIRCUITS SUBSERVING EXECUTIVE FUNCTIONS
Overview
As children and adolescents mature, they navigate an increasingly complex social environment with ever-greater autonomy (Csikszentmihalyi et al, 1977). To succeed in these new life tasks, they must hone and integrate numerous cognitive and behavioral processes, each of which seems to be subserved by a specific neural circuit. These higher-order cognitive processes, collectively termed ‘executive functions,' include allocation of attention, working memory, and self-regulatory control. Although the behavioral manifestations of these processes have been widely studied, little is known about the development of their corresponding neural circuits, and even less is known about the ways in which brain maturation supports the coordination and integration of these distinct cognitive domains. Neuroimaging studies of children and adolescents have enhanced our ability to correlate the maturation of the complex cognitive processes that constitute executive functions with in vivo measures of brain structure and function.
Development of Neural Circuits for Working Memory
Working memory refers to the capacity to maintain, attend to, and update information that is currently relevant and available ‘online' for conscious evaluation and manipulation (Baddele, 1986). In the mature brain, working memory largely depends on an intact dorsolateral PFC (DLPFC) (D'Esposito, 2007; Goldman-Rakic, 1994; Jonides et al, 1993; McCarthy et al, 1994; Owen et al, 1990; Petrides et al, 1993; Wager and Smith, 2003). Working memory also engages attentional processes that are based in a lateral and superior frontoparietal network that include superior frontal sulcus, ventrolateral PFC (VLPFC), intraparietal sulcus, and supramarginal gyrus (Champod and Petrides, 2007; Corbetta et al, 2008; Corbetta and Shulman, 2002; Posner and Petersen, 1990).
Rudimentary working memory capabilities have been observed in infants as young as 6 months of age, but a clear capacity for working memory does not develop until later in infancy (Brody, 1981; Diamond, 1990; Millar and Watson, 1979). In Piaget's A-not-B task (Piaget, 1954), an infant first observes a desired object, such as a shiny toy, that is placed in one of two wells, which are both covered. The infant is then allowed to uncover one well and, if discovering the desired object, retrieve it. The trial is then repeated with the object hidden in the same well, and after several successful trials, the object is then hidden in the second well. By 7–8 months of age, infants begin to successfully perform this task if allowed to retrieve the object immediately, but not after a delay as short as a few seconds (Diamond, 1990). By 9 months of age infants can perform this task after a delay. Their capacity to hold simple information online continues to improve in subsequent months (Diamond, 1990). The basic capacity for working memory is solidly in place by middle childhood (Tsujimoto et al, 2004). Working memory in children is easily overwhelmed in tasks requiring manipulation of information in memory or in the presence of distracters (Davidson et al, 2006; Hitch, 2002), whereas adolescents are able to perform more difficult working memory tasks (Davidson et al, 2006; Demetriou et al, 2002; Luciana and Nelson, 1998; Luna et al, 2004; Scherf et al, 2006; Swanson, 1999).
fMRI studies show that during the performance of simple tasks of working memory for several classes of information, older children activate frontoparietal regions (Casey et al, 1995; Crone et al, 2006; Durston et al, 2006; Geier et al, 2009; Klingberg et al, 2002; Konrad et al, 2005; Kwon et al, 2002; Luna et al, 2001; Scherf et al, 2006; Thomas et al, 1999). Thus, the neural circuits for working memory are already established by this age. However, older children fail to fully and appropriately recruit the frontoparietal network during the performance of more challenging tasks of working memory, such as those requiring online manipulation of information (Crone et al, 2006; Konrad et al, 2005; Olesen et al, 2007) or maintaining attention on the task by ignoring distracters (Olesen et al, 2007). Instead, compared with adolescents, children recruit ventromedial regions, such as caudate nucleus, and insula during performance of more complex working memory tasks (Casey et al, 1995; Crone et al, 2006; Durston et al, 2006; Geier et al, 2009; Klingberg et al, 2002; Konrad et al, 2005; Kwon et al, 2002; Luna et al, 2001; Scherf et al, 2006; Thomas et al, 1999). In contrast, compared with adults, adolescents seem to recruit spatially more diffuse portions of frontal and parietal cortices when engaging working memory processes (Durston et al, 2006; Konrad et al, 2005; Scherf et al, 2006). Maturation of the neural circuits that supports working memory processes is, therefore, characterized by a fuller and more consistent recruitment of frontoparietal regions with increasing task difficulty between childhood and adolescence, which is followed by the spatial refinement of these cortical regions between adolescence and adulthood.
Development of Neural Circuits that Subserve Self-regulation
Children frequently must select among competing choices, such as doing their homework and playing with friends, or between having a tasty snack and saving room for dinner. In making these choices, a child must reconcile the conflict between the two competing options available in the context of earlier established expectations and rules, and ultimately must inhibit automatic impulses for immediate gratification in the service of a choice that is less automatic but that is better aligned with the values of the child, family, and society. Self-regulation, therefore, requires development and maintenance of long-term goals and rules that guide and motivate behavior, as well as selecting behaviors that best reconcile these internal imperatives in accord with ever-changing internal and external contingencies (Dubin et al, 2010).
Subsumed under the broad construct of self-regulation, cognitive control refers to a set of mental processes that are responsible for the execution, guiding, and monitoring these desired behaviors, while inhibiting inappropriate or disadvantageous responses (Dubin et al, 2010). Other cognitive processes such as attentional regulation, conflict and error monitoring, and response inhibition are engaged in the service of cognitive control (Bunge et al, 2002; Casey et al, 1997; Dubin et al, 2010; Rubia et al, 2000). The capacity for cognitive control, or an early prototype of it, emerges in infancy. In Piaget's A-not-B task, for example, repeatedly hiding the object in the first two wells and allowing to retrieve it establish a prepotent response to look for the object in this first well (Piaget, 1954). The ability to retrieve the object correctly after it is hidden in the second well, which emerges at 7–8 months of age, relies in part, on response inhibition, or refraining from performing the prepotent behavior of reaching into the first well (Diamond, 1990; Piaget, 1954).
Experimental paradigms such as the Stroop, Simon, Flanker, Go/No-Go, and Stop-Signal tasks require suppression of a more automatic behavior to perform a less automatic one. Attentional regulation, response inhibition, and conflict and error monitoring are cognitive processes that are engaged in the service of cognitive control and successful task performance. Performance on all of these tasks improves steadily throughout development, but does not approach adult levels until at least late childhood or early adolescence (Bunge et al, 2002; Casey et al, 1997; Davidson et al, 2006; Luna et al, 2004; Rubia et al, 2000). As with working memory, the self-regulatory capacity of children can be overwhelmed easily by increasing tasks demands.
In adults, self-regulation relies on broad cortical areas such as supplementary motor area, frontal eye fields, anterior cingulate cortex, DLPFC, VLPFC/lateral orbitofrontal cortex, as well as temporal, and parietal regions all of which have connections with striatum in the subcortex (Leung et al, 2000; Peterson et al, 2002; Peterson et al, 1999). Children and adults engage these frontostriatal circuits when performing tasks that require self-regulatory control (Adleman et al, 2002; Bunge et al, 2002; Casey et al, 1997; Luna et al, 2001; Peterson et al, 1999; Rubia et al, 2000). Findings from fMRI studies suggest that an improving behavioral capacity for cognitive control with advancing age is associated with increasing activation of frontal and striatal circuits (Figure 7) (Adleman et al, 2002; Bunge et al, 2002; Casey et al, 1997; Luna et al, 2001; Marsh et al, 2006; Rubia et al, 2000; Tamm et al, 2002). Evidence suggests that conflict monitoring involves the anterior cingulate cortex, regulation of attention involves DLPFC and parietal cortex, suppression of interference involves VLPFC, and response inhibition involves parietal regions. In tasks of cognitive control, parietal activation continues to increase into adolescence, whereas prefrontal activation continues to increase into adulthood (Adleman et al, 2002; Marsh et al, 2006; Rubia et al, 2006).
Figure 7.
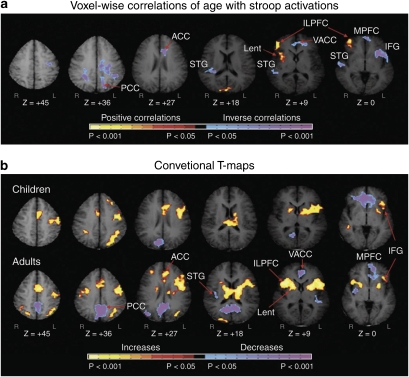
Development of neural circuits for cognitive control. Images represent brain activation in a cross-sectional developmental fMRI of the Stroop task. (a) Voxel-wise correlations of age with Stroop activations are represented in transaxial slices positioned superiorly to inferiorly (left to right). (b) Group composite t-maps for the fMRI signal change associated with the naming of colors in incongruent compared with congruent stimuli for children and adults. Increases in signal during the incongruent relative to congruent are coded in yellow, and decreases are coded in purple or blue. Right frontostriatal (ILPFC and Lent) increases in activity associated with incongruent stimuli came on line progressively with age. Thus, increasing activity in frontostriatal circuits with age supports the developmental improvements in cognitive control in healthy individuals. PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; VACC, ventral anterior cingulate cortex; STG, superior temporal gyrus; Lnuc, lenticular nucleus; LPFC, lateral prefrontal cortex; MPFC, mesial prefrontal cortex; IFG, inferior frontal gyrus (Marsh et al, 2006) (Copyright 2006) John Wiley and Sons.
Both self-regulatory and reward systems participate in the resolution of conflicts between the intrinsic hedonic value of incentives and their value within a broader framework that includes internalized values and other societal influences, personal goals, and potential risks among other consequences. In adults, frontoparietal and frontostriatal networks, including dorsal striatum, nucleus accumbens (NAcc), amygdala, OFC, lateral and superior PFC regions, and posterior parietal cortex, compose the neural circuits subserving reward (Geier and Luna, 2009; O'doherty, 2004). Developmental fMRI studies of reward processing suggested that adolescents differ from adults in the balance between top–down mechanisms of attention and cognitive control based primarily in the PFC and bottom–up motivational and emotional responses to situations of risk and reward based in the NAcc and amygdala (Casey et al, 2008; Ernst and Fudge, 2009; Ernst et al, 2005; Galvan et al, 2006; Spear, 2000). These findings are consistent with the empirically supported view that compared with adults, adolescents are more motivated by rewards, are less averse to risks, and are more easily influenced by peers (Arnett, 1992; Steinberg, 2007).
Age-related increases in activity of frontoparietal and frontostriatal circuits occur concurrently with improvements in working memory and cognitive control. The capacity to manage increasingly complex tasks of self-regulation, especially in the setting of motivational or emotional conflicts, largely emerges in adolescence, a period during which cortical gray matter thinning is seen in frontoparietal regions (Gogtay et al, 2004; Sowell et al, 2004). Similarly, performance on tasks of executive functioning improves along with DTI measures of maturation in frontostriatal and frontoparietal fiber tracts (Barnea-Goraly et al, 2005; Olesen et al, 2003). These lines of evidence suggest that developmental changes in the structure and function of frontoparietal and frontostriatal circuits may be related to remodeling of synaptic connections and greater connectivity between network nodes, which may allow increased engagement and more efficient functioning of these circuits in ever more challenging situations that rely on executive functioning.
Development of Neural Circuits for Executive Functions in Pediatric Psychiatric Disorders
Mental illnesses that emerge in childhood and adolescence are increasingly conceptualized as disorders within neural circuits support various domains of cognitive functioning. Therefore, a thorough understanding of the normal development of brain circuits is critically important for identifying the causes and improving the treatments of psychiatric disorders in children and adolescents. For example, obsessive compulsive disorder (OCD), ADHD, and Tourette's syndrome (TS) are pediatric psychiatric disorders that often co-occur and that are characterized by abnormalities in the functioning of frontostriatal circuits impair self-regulation (Peterson et al, 2001). Children with OCD, for example, perform poorly on tasks of inhibitory control, and these deficits correlate with clinical symptoms (Maia et al, 2008). Anatomical and functional neuroimaging studies suggest that abnormalities in orbitofrontal cortex, striatum, and anterior cingulate cortex disrupt the functioning of frontostriatal circuits (Marsh et al, 2009). Deficits in self-regulation are thought to lead to a diminished capacity to direct attentional resources away from obsessive thoughts and to inhibit the compulsion to carry out rituals (Chamberlain et al, 2005). Increased activation of orbitofrontal and anterior cingulate cortices during tasks of inhibitory control may serve as the loci of dysfunction or may represent compensatory responses for subcortical loci of dysfunction within frontostriatal circuits.
Extensive evidence suggests that persons who have TS are deficient in their ability to activate frontostriatal circuits and to generate a compensatory hyperplasia of frontal cortices that together impair their capacity to inhibit excess activity in sensorimotor circuits. This reduced inhibitory capacity then limits their ability to suppress tics (Marsh et al, 2009). In contrast, children with ADHD have impaired inhibitory control based within dorsal frontostriatal circuits as well as disturbances in motivational and reward processes based within ventral frontostriatal circuits, which together may conspire to produce dysfunction in reward learning, delay tolerance, and goal-oriented activity that characterize this disorder (Sonuga-Barke, 2005; Sowell et al, 2003b). Therefore, differential disturbances in frontostriatal circuits seem to differentially mediate the different psychiatric disorders in children and adolescents.
CONCLUSIONS AND FUTURE DIRECTIONS
Evolving experimental approaches have afforded us an increasingly nuanced view of the development of the brain's circuits as a protracted and even life-long series of interrelated, and partially overlapping, iterative biological processes that follow their own time courses across differing brain regions. The underlying cellular processes are supported by intricate molecular events that are determined by complex interactions of genetic programs with environmental exposures and experience that also drive the dynamic remodeling of circuits throughout the life span. The proper timing and execution of each of these processes is critically important in establishing the optimal functional capability of neural circuits and can, therefore, also serve as a locus of vulnerability for the effects of genetic aberrations and adverse environmental exposures.
The body of knowledge supporting our understanding of normal neural circuits development in human beings, however, is still highly imperfect, as it is derived largely from cross-sectional studies of animal, primarily rodents. These reports supplement a relatively small number of studies in human and nonhuman primates that compare measures of brain structure and function across narrow developmental windows. Thus, although studies in rodents and invertebrates have provided invaluable insights into the genetic, molecular, and cellular events that guide and regulate the development of neural circuits, extrapolating these findings to human beings is hazardous and only partially remedied by scant, but important data from nonhuman primates (Liu et al, 2008). Similarly, the all too common use of data on neural circuit structure and function that are derived at one developmental period to infer prior or subsequent developmental events has fundamental limitations. Therefore, our understanding of the cellular and molecular events that subserve neural circuit maturation during childhood and adolescence in human beings remains provisional and incomplete.
Neuroimaging techniques allow for the noninvasive in vivo study of brain structure, composition, and function, and these methods have made invaluable contributions to our understanding of the development of neural circuits. Although the histological correlates of some imaging measures have been defined, the microscopic underpinnings and biological significance of many other measures have yet to be determined. Also, the majority of pediatric imaging studies to date have made longitudinal or developmental inferences from cross-sectional data, a practice that can be problematic at best for reasons that include bias associated with differential sampling across age groups, masking of within-individual change by variability across individuals, and difficulty in identifying complex developmental trajectories (Kraemer et al, 2000). In part to address these difficulties with imputing developmental trajectories from cross-sectional data, the past decade has witnessed the emergence of several longitudinal anatomical imaging studies of brain development in children and adolescents. These studies have allowed direct examination of the trajectories of postnatal human brain development and, presumably, more valid inferences about their functional correlates.
The concerted and integrated application of established and emerging experimental techniques may help to address some of the limitations in current state of knowledge of neural circuit development. Multimodal in vivo imaging studies of large healthy samples followed over time will facilitate not only the direct and unbiased examination of developmental brain changes, but also the correlation of biological information across imaging modalities, which will include information on brain structure using anatomical MRI, axonal connectivity using DTI, brain function and perfusion using fMRI, and neuronal density and metabolism using MRS. Moreover, application of state-of-the-art cellular and molecular biological techniques to the postmortem study of brains from typically developing children will yield novel insights into the histological and ultrastructural features of neural circuit development. Combining these studies of postmortem tissue with multimodal imaging techniques of the same tissue samples will permit direct investigation of the cellular and molecular correlates of the postmortem imaging data that will dramatically improve our insight into the cellular underpinnings and functional consequences of the developmental changes identified in in vivo imaging data. Combining these molecular biological techniques with longitudinal imaging studies of brain development in nonhuman primates will provide information that is much more relevant than cross-sectional studies of rodent brains have been to improving our understanding of human brain development.
Study of the normal developmental trajectories of brain circuits provides invaluable insights to the genesis and biological underpinnings of developmentally based psychopathologies. Identifying the pathways of normal brain development provides the Archimedean point from which to interpret and understand the aberrant pathways of brain development that produce disease. Conversely, identifying developmental trajectories of the brain that produce disease informs our knowledge of normal brain development by throwing into relief the developmental pathways that are most sensitive to perturbation and that are, therefore, most likely to be of vital importance to building a healthy brain. Together, these lines of investigation should enhance our understanding of the pathogenesis of mental illness. That improved understanding should, in turn, help to guide the design and development of interventions that either correct deviant developmental trajectories or that optimize relatively normal ones, to ensure that our children realize their greatest developmental potentials as they mature into adolescents and young adults.
Acknowledgments
This work was funded in part by NIMH grants MH-K0274677 and T32 MH16434-27.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Atkinson J. Human visual development over the first 6 months of life. A review and a hypothesis. Hum Neurobiol. 1984;3:61–74. [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddele AD. Working Memory. Oxford University Press: New York; 1986. [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bansal R, Chung Y, Dong Z, Duan Y, Gerber A, Hao X, et al. 2007Neuroimaging methods in the study of childhood psychiatric disordersIn: Martin A, Volkmar FR, Lewis M (eds).Lewiss Child and Adolescent Psychiatry: A Comprehensive Textbook4th edn.Lippincott, Williams & Wilkins: Baltimore, MD; 214–234. [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Becker LE, Armstrong DL, Chan F, Wood MM. Dendritic development in human occipital cortical neurons. Brain Res. 1984;315:117–124. doi: 10.1016/0165-3806(84)90083-x. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Deluca M, Devlin J, Smith S. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Dev. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABA A, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois'. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21:503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas R, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Brody LR. Visual short-term cued recall memory in infancy. Child Dev. 1981;52:242–250. [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, et al. Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med. 1996;36:878–886. doi: 10.1002/mrm.1910360610. [DOI] [PubMed] [Google Scholar]
- Carpenter GL, Stacks AM. Developmental effects of exposure to intimate partner violence in early childhood: a review of the literature. Children Youth Serv Rev. 2009;31:831–839. [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci USA. 2007;104:14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Chao D, Ma L, Shen K. Transient cell–cell interactions in neural circuit formation. Nat Rev Neurosci. 2009;10:262–271. doi: 10.1038/nrn2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT.1994Development of regional brain glucose metabolism in relation to behavior and plasticityIn: Dawson G, Fischer KW (eds).Human Behavior and the Developing Brain Guilford: New York; 153–175. [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka R. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O'Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Crone E, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Youth Adolesc. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J, Rombouts S, Barkhof F, Scheltens P, Stam C, Smith S, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when. Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II. Quantitative aspects. Early Hum Dev. 1985;12:99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Deluca M, Beckmann C, Destefano N, Matthews P, Smith S. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: efficiency, working memory, and thinking. Monogr Soc Res Child Dev. 2002;67:1–156. [PubMed] [Google Scholar]
- Devys D, Biancalana V, Rousseau F, Boue J, Mandel JL, Oberle I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. Am J Med Genet. 1992;43:208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Diamond A.1990The development and neural bases of memory functions as indexed by the AB and delayed response tasks in human infants and infant monkeys Ann NY Acad Sci 608267–309.discussion 309–317. [DOI] [PubMed] [Google Scholar]
- Diamond A.1995Guidelines for the study of brain behavior relations during developmentIn: Harvey L, Eisenberg H (eds).Frontal Lobe Dysfunction Oxford University Press: New York; 339–378. [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Maia TV, Peterson BS.2010Cognitive control in the service of self-regulationIn: Koob GF, Le Moal M, Thompson RF (eds).The Encyclopedia of Behavioral Neuroscience Elsevier: Amsterdam, Netherlands [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge J. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson E, Jazbec S, Mcclure E, Monk C, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Fair D, Dosenbach N, Church JA, Cohen A, Brahmbhatt S, Miezin F, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence. J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Levrier O, Figarella-Branger D, et al. Assessment of cortical maturation with prenatal MRI. Part I: Normal cortical maturation. Eur Radiol. 2005;15:1671–1685. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- Fox M, Corbetta M, Snyder AZ, Vincent JL, Raichle M. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, et al. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury JF, et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol. 2001;22:184–189. [PMC free article] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;89:1–10. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet. Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255:1441–1443. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- Giedd J. Brain development, IX: human brain growth. Am J Psychiatry. 1999;156:4. doi: 10.1176/ajp.156.1.4. [DOI] [PubMed] [Google Scholar]
- Giedd J, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Corouge I, Vetsa YS, Smith JK, Kang C, et al. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR Am J Neuroradiol. 2007a;28:1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Gerig G. Fetal and neonatal brain development. Am J Psychiatry. 2006;163:2046. doi: 10.1176/ajp.2006.163.12.2046. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007b;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Girard N, Raybaud C, du Lac P. MRI study of brain myelination. J Neuroradiol. 1991;18:291–307. [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke J, Griffith W, Faustman E. Computational models of neocortical neuronogenesis and programmed cell death in the developing mouse, monkey, and human. Cerebral Cortex. 2006;17:2433–2442. doi: 10.1093/cercor/bhl151. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS.1994The issue of memory in the study of prefrontal functionsIn: Thierry AM (ed).Motor and Cognitive Functions of the Prefrontal Cortex Springer-Verlag: Berlin; 112–122. [Google Scholar]
- Gomez MR, Sampson JR, Whittemore VH.1999Tuberous Sclerosis3rd edn.Oxford University Press: New York [Google Scholar]
- Goodkin F. The development of mature patterns of head-eye coordination in the human infant. Early Hum Dev. 1980;4:373–386. doi: 10.1016/0378-3782(80)90042-0. [DOI] [PubMed] [Google Scholar]
- Graziani LJ, Weitzman ED, Velasco MS. Neurologic maturation and auditory evoked responses in low birth weight infants. Pediatrics. 1968;41:483–494. [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P. Mechanisms and disturbances of neuronal migration. Pediatr Res. 2000;48:725–730. doi: 10.1203/00006450-200012000-00004. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Hasan KM, Trivedi R, Pradhan M, Das V, Parikh NA, et al. Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res. 2005;81:172–178. doi: 10.1002/jnr.20547. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka S, Takashima S, Morooka K. Study of the maturation of the child's brain using 31P-MRS. Pediatr Neurol. 1998;18:305–310. doi: 10.1016/s0887-8994(97)00201-4. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006;163:1290–1292. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME. The role of migration in central nervous system neuronal development. Curr Opin Neurobiol. 1993;3:38–44. doi: 10.1016/0959-4388(93)90033-u. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hitch GJ.2002Developmental changes in working memory: a multicomponent viewIn: Graf P, Ohta N (eds).Lifespan Development of Human Processing MIT Press: Cambridge, Mass; 15–37. [Google Scholar]
- Holden KR. Malnutrition and brain development. Semin Clin Neurol. 2007;6:19–36. [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15:137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards L, et al. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huisman TA, Martin E, Kubik-Huch R, Marincek B. Fetal magnetic resonance imaging of the brain: technical considerations and normal brain development. Eur Radiol. 2002;12:1941–1951. doi: 10.1007/s00330-001-1209-x. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982a;16–17:144–154. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982b;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Iai M, Yamamura T, Takashima S. Early expression of proteolipid protein in human fetal and infantile cerebri. Pediatr Neurol. 1997;17:235–239. doi: 10.1016/s0887-8994(97)00099-4. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Growth and reshaping of axons in the establishment of visual callosal connections. Science. 1981;212:824–827. doi: 10.1126/science.7221566. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical input. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114 (Part 5:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Jouet M, Rosenthal A, Armstrong G, MacFarlane J, Stevenson R, Paterson J, et al. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Sanes JR.2000Sensory experience and the fine-tuning of synaptic connectionsIn: Kandel ER, Schwartz JH, Jessell TM (eds).Principles of Neural Science4th edn.Appleton & Lange: Stamford, Conn; 1115–1130. [Google Scholar]
- Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. Neuroreport. 2004;15:2149–2153. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- Katz PS. Evolution and development of neural circuits in invertebrates. Curr Opin Neurobiol. 2007;17:59–64. doi: 10.1016/j.conb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kelly A, Di Martino A, Uddin L, Shehzad Z, Gee D, Reiss P, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2008;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Goldman-Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol. 1983;219:431–447. doi: 10.1002/cne.902190405. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Petanjek Z, Sitlic G. Ontogenesis of goal-directed behavior: anatomo-functional considerations. Int J Psychophysiol. 1995;19:517–527. doi: 10.1016/0167-8760(94)00081-o. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Molliver ME. A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of the neopallium in the human fetus. Anat Rec. 1974;178:395. [Google Scholar]
- Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we. Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Kreppner JM, Ruttler M, Beckett C, Castle J, Colvert E, Groothues C, et al. Normality and impairment following profound early institutional deprivation: a longitudinal follow-up into early adolescence. Dev Psychol. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Hilpert PL, Kreuzer JA, Vaughan HG., Jr Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birthweight infants. Dev Med Child Neurol. 1984;26:466–475. doi: 10.1111/j.1469-8749.1984.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci USA. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher R, Schoenwolf GC.2005Making a neural tube: neural tube induction and neurrulationIn: Mahendra SR, Jacobson M (eds).Developmental Neurobiology4th edn.Kluwer Academic/Plenum Publishing: New York; 1–20. [Google Scholar]
- Landing BH, Shankle WR, Hara J, Brannock J, Fallon JH. The development of structure and function in the postnatal human cerebral cortex from birth to 72 months: changes in thickness of layers II and III co-relate to the onset of new age-specific behaviors. Pediatr Pathol Mol Med. 2002;21:321–342. doi: 10.1080/02770930290056541. [DOI] [PubMed] [Google Scholar]
- Lenroot R, Gogtay N, Greenstein D, Wells E, Wallace G, Clasen L, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Levi DM, Li RW. Improving the performance of the amblyopic visual system. Philos Trans R Soc Lond B Biol Sci. 2009;364:399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh CH, Styner M, et al. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol. 2008;29:1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Garland M, Duan Y, Stark RI, Xu D, Dong Z, et al. Study of the development of fetal baboon brain using magnetic resonance imaging at 3 Tesla. NeuroImage. 2008;40:148–159. doi: 10.1016/j.neuroimage.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96:279–290. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossi L, Merighi A. In vivo cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. Prog Neurobiol. 2003;69:287–312. doi: 10.1016/s0301-0082(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, et al. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- Maia T, Cooney R, Peterson B. The neural bases of obsessive-compulsive disorder in children and adults. Develop Psychopathol. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res. 1970;23:167–183. doi: 10.1016/0006-8993(70)90037-5. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex neocortex of the Felix domestica. A Golgi study. I. The primordial neocortical organization. Z Anat Entwicklungsgesch. 1971;134:117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M.1988Early ontogenesis of the human cerebral cortexIn: Peters A, Jones EG (eds).Development and Maturation of the Cerebral Cortex Planum: New York; 1–30. [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mata M, Fink DJ, Gainer H, Smith CB, Davidsen L, Savaki H, et al. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980;34:213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, et al. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex. 2001;11:335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Brent HP, Levin AV. Rapid improvement in the acuity of infants after visual input. Science. 1999;286:108–110. doi: 10.1126/science.286.5437.108. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, et al. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol. 2002;179:1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- McManus MF, Nasrallah IM, Gopal PP, Baek WS, Golden JA. Axon mediated interneuron migration. J Neuropathol Exp Neurol. 2004;63:932–941. doi: 10.1093/jnen/63.9.932. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABAA receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Michel AE, Garey LJ. The development of dendritic spines in the human visual cortex. Hum Neurobiol. 1984;3:223–227. [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–66. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Millar WS, Watson JS. The effect of delayed feedback on infant learning reexamined. Child Dev. 1979;50:747–751. [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Kostović I, van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973;50:403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- Monk CS, Webb SJ, Nelson CA. Prenatal neurobiological development: molecular mechanisms and anatomical change. Dev Neuropsychol. 2001;19:211–236. doi: 10.1207/S15326942DN1902_5. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch T. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Davies PW, Berman AL. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957;20:374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol. 1992;316:485–496. doi: 10.1002/cne.903160408. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, van Eden CG, Judas M.1990Neuronal development in human prefrontal cortex in prenatal and postnatal stagesIn: Uylings HBM, van Eden CG, de Bruin JPC, Corne MA, Feenstra MGP (eds).The Prefrontal Cortex: Its Structure, Function and Pathology Elsevier: Amsterdam; 185–222. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 2001;23:203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The neocortex. Anat Embryol. 1994;190:307–337. doi: 10.1007/BF00187291. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG., Jr Cortical responses to speech sounds and their formants in normal infants: maturational sequence and spatiotemporal analysis. Electroencephalogr Clin Neurophysiol. 1989;73:295–305. doi: 10.1016/0013-4694(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- O'doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22:467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd J. Why do many psychiatric disorders emerge during adolescence. Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Peterson B, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GE, Lacadie C. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Pine DS, Cohen P, Brook JS. Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. J Am Acad Child Adolesc Psychiatry. 2001;40:685–695. doi: 10.1097/00004583-200106000-00014. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H. An fMRI Study of Stroop Word-Color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Construction of Reality in the Child. Basic: New York; 1954. [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Prechtl HF, Hopkins B. Developmental transformations of spontaneous movements in early infancy. Early Hum Dev. 1986;14:233–238. doi: 10.1016/0378-3782(86)90184-2. [DOI] [PubMed] [Google Scholar]
- Raedler E, Raedler A. Autoradiographic study of early neurogenesis in rat neocortex. Anat Embryol. 1978;154:267–284. doi: 10.1007/BF00345657. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad Med J. 1978;54 (Suppl 1:25–40. [PubMed] [Google Scholar]
- Rakic P. Early developmental events: cell lineages, acquisition of neuronal positions, and areal and laminar development. Neurosci Res Program Bull. 1982;20:439–451. [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial vs tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci USA. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994a;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rakic P, Cameron RS, Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994b;4:63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–390. doi: 10.1083/jcb.133.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport J, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006a;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006b;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Picker A, Brand M. Global and local mechanisms of forebrain and midbrain patterning. Curr Opin Neurobiol. 2006;16:5–12. doi: 10.1016/j.conb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Chronwall BM, Wolff JR. On the development of non-pyramidal neurons and axons outside the cortical plate: the early marginal zone as a pallial anlage. Anat Embryol. 1977;151:258–307. doi: 10.1007/BF00318931. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Wolff JR. Differentiation of ‘preplate' neurons in the pallium of the rat. Bibliotheca Anat. 1981;19:142–146. [PubMed] [Google Scholar]
- Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat Genet. 1992;2:107–112. doi: 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, Boylan A.1990Regulatory mechanisms in infant developmentIn: Enns JT (ed).The Development of Attention: Research and Theory Elsevier/North-Holland: Amstardam; 47–66. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Rakic P, Goldman-Rakic PS. Early phenotype expression of cortical neurons: evidence that a subclass of migrating neurons have callosal axons. Proc Natl Acad Sci USA. 1991;88:1354–1358. doi: 10.1073/pnas.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Shankle WR, Rafii MS, Landing BH, Fallon JH. Approximate doubling of numbers of neurons in postnatal human cerebral cortex and in 35 specific cytoarchitectural areas from birth to 72 months. Pediatr Dev Pathol. 1999;2:244–259. doi: 10.1007/s100249900120. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch J, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sonderegger P, Rathjen FG. Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J Cell Biol. 1992;119:1387–1394. doi: 10.1083/jcb.119.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sowell E, Peterson B, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003a;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003b;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence new perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16:55–59. [Google Scholar]
- Stern CD. Initial patterning of the central nervous system: how many organizers. Nat Rev Neurosci. 2001;2:92–98. doi: 10.1038/35053563. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Pearlman AL. Fibronectin-like immunoreactivity in the developing cerebral cortex. J Neurosci. 1987;7:3325–3333. doi: 10.1523/JNEUROSCI.07-10-03325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, Soriano E, Uylings HB. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- Swanson HL. What develops in working memory? A life span perspective. Dev Psychol. 1999;35:986–1000. doi: 10.1037//0012-1649.35.4.986. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tang N, He M, O'Riordan MA, Farkas C, Buck K, Lemmon V, et al. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabulsy GM, Pascuzzo K, Moss E, St-Laurent D, Bernier A, Cyr C, et al. Attachment-based intervention for maltreating families. Am J Orthopsychiatry. 2008;78:322–332. doi: 10.1037/a0014070. [DOI] [PubMed] [Google Scholar]
- Thelen K, Kedar V, Panicker AK, Schmid R-S, Midkiff BR, Maness PF. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J Neurosci. 2002;22:4918–4931. doi: 10.1523/JNEUROSCI.22-12-04918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sowell ER, Gogtay N, Giedd JN, Vidal CN, Hayashi KM, et al. Structural MRI and brain development. Int Rev Neurobiol. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Toft PB, Leth H, Lou HC, Pryds O, Henriksen O. Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J Magn Reson Imaging. 1994;4:674–680. doi: 10.1002/jmri.1880040510. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Yamamoto T, Kawaguchi H, Koizumi H, Sawaguchi T. Prefrontal cortical activation associated with working memory in adults and preschool children: an event-related optical topography study. Cereb Cortex. 2004;14:703–712. doi: 10.1093/cercor/bhh030. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uher R, Mcguffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176:509–515. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Mrzljak L, Voorn P, Uylings HB. Prenatal development of GABA-ergic neurons in the neocortex of the rat. J Comp Neurol. 1989;289:213–227. doi: 10.1002/cne.902890204. [DOI] [PubMed] [Google Scholar]
- Vaughan HG, Jr, Kurtzberg D.1989Electrophysiological indices of normal and aberrant cortical maturationIn: Kellaway P, Noebels JL (eds).Developmental Neurophysiology Johns Hopkins University Press: Baltimore, MD; 263–287. [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, et al. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol. 2000;423:359–372. doi: 10.1002/1096-9861(20000731)423:3<359::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Welsh JA, Viana AG, Petrill SA, Mathias MD. Interventions for internationally adopted children and families: a review of the literature. Child Adolesc Social Work J. 2007;24:285–311. [Google Scholar]
- Wood JG, Martin S, Price DJ. Evidence that the earliest generated cells of the murine cerebral cortex form a transient population in the subplate and marginal zone. Dev Brain Res. 1992;66:137–141. doi: 10.1016/0165-3806(92)90150-u. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A.1967The myelogenetic cycles of regional maturation of the brainIn: Minkowski A (ed).Regional Development of the Brain in Early Life Blackwell Scientific Publications: Boston; 3–70. [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;548:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N. Synaptogenesis in layer I of the human cerebral cortex in the first half of gestation. Cereb Cortex. 1998;8:245–252. doi: 10.1093/cercor/8.3.245. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zhai G, Lin W, Wilber KP, Gerig G, Gilmore JH. Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology. 2003;229:673–681. doi: 10.1148/radiol.2293021462. [DOI] [PubMed] [Google Scholar]