One of the most significant challenges in neuroscience for the twenty-first century is to understand the molecular and cellular basis of neuropsychiatric disorders. Despite extensive research, the last several decades failed to yield clearly validated new drug targets or therapeutic mechanisms for major psychiatric disorders (Hyman, 2008). One important limitation is the lack of pathogenically and physiologically reliable animal and cellular model systems of psychiatric disorders. This challenging situation is now changing through the advances that followed Shinya Yamanaka and his colleagues' successful generation of pluripotent stem cells in 2006, so called ‘induced' pluripotent stem (iPS) cells, from somatic cells by retroviral transduction of four transcription factors (ie, Oct4, Sox2, Klf4, and c-Myc) (Takahashi and Yamanaka, 2006). Furthermore, 1 year later, several groups showed that human iPS cells could be generated from human tissues by similar methods, paving the way to generate disease- and patient-specific iPS cells (Takahashi et al, 2007; Yu et al, 2007; Park et al, 2008). Until these advances, the biological dogma was that the developmental process is unidirectional in that a totipotent zygote becomes more and more restricted until terminally differentiated tissues are generated. These new breakthrough studies showed that epigenetic reprogramming by defined factors could reverse this process.
iPS cell technology is a tantalizing new method of generating genetically matched pluripotent stem cells in vitro. Unlike conventional methods that generate embryonic stem cells from zygotic embryos, it does not require killing of embryos, thus avoiding the associated ethical dilemma. These iPS cells provide useful tools to study the molecular and cellular mechanisms of development and differentiation, particularly early human development. Furthermore, iPS cells derived from patients could be extremely useful for investigating the physiological, cellular, and molecular mechanisms underlying the disease process, which may lead to ideal platforms for drug screening. This new approach may be especially important to advances in psychiatry because (1) the underlying pathogenic mechanisms of mental disorders are largely unknown, (2) cellular models for disease mechanism study are extremely limited, and (3) rational treatments are lacking in most cases.
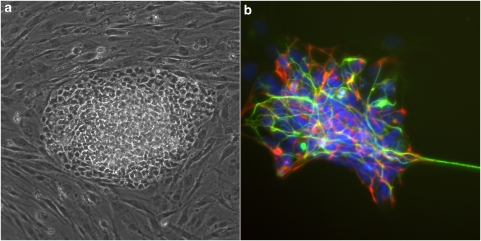
Several major obstacles must be overcome for iPS cell technology to make a full impact in psychiatric disease research. First, most iPS cells have been derived by retroviral or lentiviral introduction of reprogramming factor-encoding genes, resulting in multiple chromosomal disruptions, any of which may cause genetic dysfunction and/or tumor formation. This could be particularly problematic in psychiatric diseases that may have relatively subtle pathogenic mechanisms. Secondly, reprogramming transgenes (in particular, c-Myc and Klf4) are closely associated with oncogenesis, raising the possibility that iPS cells generated by these factors may be prone to tumor formation. Currently, most iPS cells contain the reprogramming transgenes in their chromosomes although their expression is mostly silenced. Reactivation and/or residual leaky expression of these transgenes may trigger altered cell physiology and/or abnormal differentiation properties. To address these issues, recent studies used non-integrating vectors (eg, adenovirus or episomal vectors) or excisable vectors (eg, piggyBac transposon and Cre-recombinase excisable viruses (Yamanaka, 2009)). As these DNA vector-based manipulations are not completely free from potential chromosomal disruption, we recently developed a new method to generate human iPS cells by direct protein delivery without the use of virus or DNA transfection (Kim et al, 2009). These transgene- and DNA-free iPS cells (Figure 1) could be useful not only for disease mechanism study, but also for future customized cell therapy of neuropsychiatric disorders.
Figure 1.
Human iPS cells derived from human newborn fibroblasts by direct protein delivery. (a) These protein-induced human iPS colony shows a typical human ES cell morphology. (b) They can differentiate into all three germ layers including neuroectodermal lineage cells such as nestin-positive neural precursors (red) and Tuj1-positive neurons (green). Nuclei were stained with DAPI (blue).
Footnotes
DISCLOSURE
The author has no conflicts of interest related to the material presented in this paper. This work was partially supported by National Institutes of Health Grants MH048866 and DC006501.
References
- Hyman SE. A glimmer of light for neuropsychiatric disorders. Nature. 2008;455:890–893. doi: 10.1038/nature07454. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]