Abstract
Fractalkine/CX3CL1 is a neuron-associated chemokine, which modulates microglia-induced neurotoxicity activating the specific and unique receptor CX3CR1. CX3CL1/CX3CR1 interaction modulates the release of cytokines from microglia, reducing the level of tumor necrosis factor-α, interleukin-1-β, and nitric oxide and induces the production of neurotrophic substances, both in vivo and in vitro. We have recently shown that blocking adenosine A1 receptors (A1R) with the specific antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) abolishes CX3CL1-mediated rescue of neuronal excitotoxic death and that CX3CL1 induces the release of adenosine from microglia. In this study, we show that the presence of extracellular adenosine is mandatory for the neurotrophic effect of CX3CL1 as reducing adenosine levels in hippocampal cultures, by adenosine deaminase treatment, strongly impairs CX3CL1-mediated neuroprotection. Furthermore, we confirm the predominant role of microglia in mediating the neuronal effects of CX3CL1, because the selective depletion of microglia from hippocampal cultures treated with clodronate-filled liposomes causes the complete loss of effect of CX3CL1. We also show that hippocampal neurons obtained from A1R−/− mice are not protected by CX3CL1 whereas A2AR−/− neurons are. The requirement of functional A1R for neuroprotection is not unique for CX3CL1 as A1R−/− hippocampal neurons are not rescued from Glu-induced cell death by other neurotrophins such as brain-derived neurotrophic factor and erythropoietin, which are fully active on wt neurons.
Keywords: neuroprotection, fractalkine, excitotoxicity, A1R, microglia, clodronate
INTRODUCTION
The chemokine family comprises more than 40 members in four different subfamilies, whose expression in the nervous system has been correlated with different pathological conditions (Tran and Miller, 2003). CX3CL1, also called fractalkine, is the unique member of the CX3C (or δ) family, and, together with CXCL16 are the only two transmembrane chemokines described until now. It is converted to a soluble form on cleavage from the plasma membrane through the action of metalloproteinases, like a disintegrin and metalloproteinase domains (ADAM) 10 and ADAM17 on leukocytes (Hundhausen et al, 2003) or cathepsin S in the spinal cord (Clark et al, 2007). CX3CL1 is constitutively expressed in the nervous system, but levels in the brain can be modulated under diverse pathological conditions (Pan et al, 1997; Hughes et al, 2002; Kastenbauer et al, 2003; Sunnemark et al, 2005; Huang et al, 2006). The presence and the stimulation (Zujovic et al, 2000, 2001; Mizuno et al, 2003; Cardona et al, 2006; Lyons et al, 2009) of the CX3CL1 receptor CX3CR1 has been correlated with a reduced release of interleukin-1-β (IL-1-β) and tumor necrosis factor-α (TNF-α) from microglial cells and a lower rate of neuronal degeneration in different experimental models of neuropathologies such as experimental autoimmune encephalomyelitis, 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine hydrochloride striatal injection, lipopolysaccharide administration, and superoxide dismutase (SOD1) mutation (Huang et al, 2006; Cardona et al, 2006). These data attest to a role of the pair CX3CL1/CX3CR1 in reducing neuronal degeneration on several types of brain injury. Exceptions are however reported: in experimental transient brain ischemia the absence of CX3CL1 or CX3CR1 is correlated with reduced IL-1-β and TNF-α production and a better outcome for neurons (Soriano et al, 2002; Dénes et al, 2008).
The neurotrophic activity of exogenously administered CX3CL1 has been related to the simultaneous production of protective factors from microglial cells and in particular with the activation of adenosine receptors (ARs, Lauro et al, 2008). Adenosine is a cellular metabolite whose intracellular and extracellular levels can be rapidly modulated by variation of cellular metabolic state (see Fredholm, 2007). Under physiological conditions, in the brain, ATP can be released by neurons and glial cells (Pascual et al, 2005; Burnstock, 2007). The released ATP is rapidly degraded to ADP, AMP, and adenosine by the sequential activity of extracellular nucleotidase (Zimmermann, 1996). Pathological stimuli, which led to the imbalance of membrane potential, like energy failure because of a reduced tissue perfusion, prolonged activation of glutamate (Glu) receptors (Manzoni et al, 1994), transient oxygen and glucose deprivation (Lloyd et al, 1993), or prolonged electric activity like that observed during seizures (Cunha et al, 1996) have all been associated with an increased release of adenosine in the extracellular space, in some cases becauses of altered activity of adenosine kinase (Boison, 2006).
Adenosine is a pleiotropic agent, which, in the nervous system, exerts a wide range of effects (see Fredholm et al, 2005): it has a general inhibitory pre-synaptic activity on glutamatergic transmission (Dolphin and Archer, 1983), modulates the response to noxious stimuli (de Mendonça et al, 2000), regulates pain sensation (Sawynok and Liu, 2003), and has been implicated in pre-conditioning (reviewed in Fredholm, 2007). Adenosine effects are mediated through four G protein coupled receptors (GPCRs). It has long been recognized that adenosine is a modulator and that therefore signaling through its receptors occurs together with signaling through other GPCRs such as metabotropic Glu receptors (mGluRs), dopamine, purine, and cannabinoid receptors, (Agnati et al, 2003), as well as tyrosine kinase receptors such as the fibroblast growth factor receptors (FGFRs, Flajolet et al, 2008). For example, ARs enhance the effect of other substances, such as FGF (Flajolet et al, 2008) ATP (Gerwins and Fredholm, 1992; Färber et al, 2008), brain-derived neurotrophic factor (BDNF) (Diógenes et al, 2007; Tebano et al, 2008), and GDNF (Gomes et al, 2009).
In this study, we have further examined the role of adenosine in mediating the neurotrophic activity of CX3CL1. We have explored the role of microglial cells, the release of adenosine, and the receptors responsible for the effect.
MATERIALS AND METHODS
Materials
Transwell inserts were from BD Labware (Franklin Lakes, NJ); recombinant rat CX3CL1 and recombinant human BDNF were from Calbiochem/Merck (Nottingham, UK); adenosine deaminase (ADA) from calf intestinal mucosa, recombinant human erythropoietin (EPO), S-(4-nitrobenzyl)-6-thioinosine (NBTI), α-β-methyleneadenosine 5′-diphosphate sodium salt (AOPCP), and poly--lysine were from Sigma-Aldrich (Milan, Italy); all culture media were from Invitrogen Life Technologies (San Giuliano Milanese, Italy); Cl2MDP (or clodronate) was a gift of Roche Diagnostics GmbH (Mannheim, Germany).
Animals and Cell Lines
Procedures using laboratory animals were in accordance with the international guidelines on the ethical use of animals from the European Communities Council Directive of 24 November 1986 (86/609/EEC). CX3CR1GFP/GFP mice (Jung et al, 2000) were obtained from Jackson Laboratory; A1R−/− (Johansson et al, 2001) and A2AR−/− (Chen et al, 1999) were backcrossed at least 10 times on a C57BL/6 background; CX3CL1−/− (Cook et al, 2001) were kindly provided by Dr Richard M Ransohoff (Cleveland Clinic, OH).
Hippocampal Neuronal Cultures
Primary hippocampal neuronal cultures were prepared from 0–2-day-old (p0–p2) C57BL/6 (wt), A1R−/−, A2AR−/−, CX3CL1−/−, and CX3CR1GFP/GFP mice. Briefly, after careful dissection from diencephalic structures, the meninges were removed and hippocampal tissues were chopped and digested for 15 min at 37°C in 0.025% trypsin and Hank's balanced salt solution (HBSS). Cells were washed twice with HBSS to remove the excess of trypsin, mechanically dissociated in minimal essential medium (MEM) with Earl's Salts and GLUTAMAX supplemented with 10% dialyzed and heat inactivated fetal bovine serum (FBS), 100 μg/ml gentamycin, and 25 mM KCl cells were plated at a density of 2.5 × 105 in the same medium on poly--lysine (100 μg/ml)-coated plastic 24-well dishes. After 1–2 h, the medium was replaced with serum-free Neurobasal/B27 medium. Cells were kept at 37°C in 5% CO2 for 11 days with twice a week medium replacement (1 : 1 ratio). At this time point we have 2.1 × 105±0.05 × 105 alive cells (which corresponds to about 85% of initially plated cells); no significant differences were obtained in the number of alive cells in hippocampal preparations obtained from the brains of wt and genetically modified mice after 11 days in culture. With this method we obtained 60–70% neurons, 30–35% astrocytes, 4–8% microglia, as determined with β-tubulin III, GFAP, and IBA-I staining. For details, see Supplementary Methods. Cells were used for experiments after 11 days.
Microglia Cultures
Cortical mixed glia cultures were obtained from p0–p1 mice. Cerebral cortexes were chopped and digested in 20 U/ml papain for 40 min at 37°C. Cells (5.0 × 105 cells/cm2) were plated on dishes coated with poly--lysine (100 μg/ml) in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin. After 7–9 days cells were shaken for 2 h at 37°C to detach and collect microglial cells. These procedures gave almost pure (no more than 2% contamination) microglial cell populations, as verified by staining with GFAP and IBA-I Abs.
Excitotoxicity Experiments
To induce excitotoxicity, 11-day-old hippocampal cultures were washed and stimulated in modified Locke's buffer (CaCl2 2.3 mM, glucose 5.6 mM, glycine 10 mM, NaCl 154 mM, KCl 5.6 mM, NaHCO3 3.6 mM, Hepes 5 mM pH 7.2) with 100 μm Glu alone or together with 100 nM CX3CL1 or vehicle, for 30 min. After stimulation, cells were washed in Locke's buffer and re-incubated in the conditioned Neurobasal/B27 medium for additional 18 h. When BDNF (100 nM) and EPO (40 U/ml, 10 nM) were used, hippocampal neurons were pre-treated for 7 h with drugs or vehicle; medium was removed and cells were treated as described above with Glu (in the presence or in the absence of BDNF and EPO), washed, and further incubated with the original medium containing BDNF or EPO for additional 18 h, till the end of the experiment. For experiments with conditioned medium, microglial cells (obtained from wt mice) were treated with CX3CL1 for 30 min, washed, and re-incubated in growth medium. Eight hours after CX3CL1 treatment, media were collected and used to stimulate neuronal cultures (obtained from CX3CR1GFP/GFP mice) treated with Glu to induce excitotoxicity. Conditioned media from glia cultures were always diluted 1 : 1 with the original medium of neuronal cultures. For experiments with ADA, hippocampal cells were pre-incubated for 1 h with 1 U/ml ADA, treated with Glu or Glu/CX3CL1 in the presence of ADA, washed, and reincubated in the original conditioned medium for 18 h. For experiments with conditioned media, medium obtained from CX3CL1-stimulated microglia was treated with or without ADA (1 U/ml) for 1 h before administration to neuronal cells treated with Glu. In these protocols, ADA was present till the end of the experiments. To evaluate neuron viability, cells were then treated with detergent-containing buffer (0.05% ethyl hexadecyl dimethylammonium bromide, 0.028% acetic acid, 0.05% Triton X-100, 0.3 mM NaCl, 0.2 mM MgCl2, in PBS pH 7.4) and counted in a hemacytometer as already described (Lauro et al, 2008). Alternatively, cell viability was analyzed by the MTT assay: in detail, 5 mg/ml MTT was added 1 : 10 to the cell medium and incubated for 2 h; the medium was aspired, cells were treated with DMSO, and incubated at 37°C for 10 min. Samples were then analyzed with a microplate reader at 490 and 630 nm to subtract background. In all excitotoxicity experiments, results are expressed as % of cell survival, taking as 100% untreated cells in control conditions. Exactly the same procedures (plated cell number, volumes of reagents) were applied to experiments with cells obtained from mice of different genotypes for comparison of cell viability also under basal conditions.
Transwell Migration Assays
Chemotaxis assay was performed on microglia obtained from mice cortex. Cells were re-suspended in serum-free medium and plated on poly--lysine-treated 12 mm transwells (8 μm pore size polycarbonate; 5 × 105 cells/well). The lower chambers contained CX3CL1 100 nM, prepared in the same medium. The chambers were incubated for 2 h at 37°C in a moist 5% CO2 atmosphere. After incubation, cells were treated with 10% trichloroacetic acid on ice for 10 min and the non-migrating cells, adhering to the upper face of the filters, were scraped off, whereas cells on the lower side were stained with a solution containing 50% isopropanol, 1% formic acid, and 0.5% (w/v) brilliant blue R250 and dried on a glass slide. The number of migrating cells was counted in 20 fields with a × 63 objective.
Depletion of Microglia with Clodronate Liposomes
Mixed hippocampal cultures obtained from CX3CR1GFP/GFP mice were treated for different times with liposomes encapsulating clodronate (Cl2MBP) or as control, with empty liposomes. Clodronate liposomes as well as control liposomes without clodronate were prepared according to the standard method (van Rooijen and Sanders, 1994). The resulting standard suspension of clodronate liposomes is containing 1.2 mg of Clodronate per 1 ml of the suspension. This liposome suspension was diluted 1 : 10 in the growth medium. At different time points, from 5 to 72 h, cell cultures were analyzed with a fluorescence microscope to recognize and count the number of EGFP-labeled microglial cells. Cultures were stained with Hoechst to visualize total cell nuclei.
HPLC Analysis
Eleven-day-old rat hippocampal cultures were pre-treated in Locke's buffer for 10 min with the transporter inhibitor NBTI or with the ectonucleotidase inhibitor AOPCP, and then stimulated 30 min with CX3CL1 100 nM or vehicle, whereas primary microglial cell cultures were only treated in Locke's buffer for 30 min with CX3CL1 or vehicle. After this time, cells were washed and reincubated in their original conditioned medium, in the presence or in the absence of the inhibitors and, after additional 6 h (or 7.5 h for microglia), the media were collected, added with ice-cold acetonitrile, centrifuged for 5 min at 1.440 g, and the resulting supernatants were analyzed by HPLC. Cells remaining in the dish were analyzed for protein content with a BCA assay.
Chromatographic analyses were conducted using a Merck Hitachi HPLC system equipped with programmable autosampler (model L-7250), pump (model L-7100), and diode array detector (model L-7455). Data were stored and processed using appropriate software (D-7000 HPLC System Manager Ver. 3.1; Hitachi). Separation was achieved by using a column Reprosil-Pur C18-AQ (5 μm, 250 mm × 4 mm) with precolumn Reprosil-Pur C18-AQ 5 μm, 5 mm × 4 mm (Dr Maisch, Ammerbruch, Germany). Elution was performed isocratically with a mobile phase consisting of 10 mM potassium phosphate (pH 6) and acetonitrile (90 : 10). The pump flow rate was set at 1.0 ml/min, and the injection volume was 40 μl. Adenosine was monitored by UV diode array detection at 260 nm, and was identified on the basis of its retention time (3.90 min) and spectral data relative to reference standards. All separations were conducted at room temperature. The limit of detection and quantification for adenosine was found to be 18.7 and 187 nM, respectively.
Statistical Data Analysis
For all the experiments shown in the manuscript, significance was evaluated with t-test analysis and differences between groups of data were considered highly significant with P⩽0.01 (**) and significant with P⩽0.05 (*).
RESULTS
Microglia Depletion with Clodronate Liposomes Impairs the Neuroprotective Activity of CX3CL1
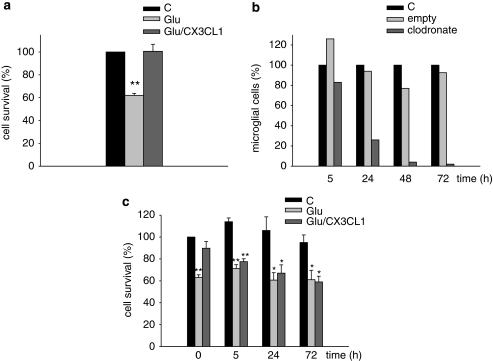
To study the neuroprotective role of CX3CL1, we used an injury model involving glutamate (Glu)-induced excitotoxicity in hippocampal cultures obtained from p0–p2 mice. On treatment with Glu (100 μM, 30 min), we consistently obtained about 40–50% of cell death in comparison with untreated control cultures. This corresponded to about 70% of total neuronal loss on Glu treatment, as assessed by immunofluorescence analysis with β-tubulin III staining (data not shown). We confirm, in this manuscript, that CX3CL1 protects hippocampal neurons from Glu-induced excitotoxicity (Figure 1a) similarly to what already shown in neuronal preparations, which contain different ratios of neurons:astrocytes:microglia (Limatola et al, 2005; Lauro et al, 2008). Given that CX3CR1 are predominantly expressed in microglial cells, it is likely that microglial cells mediate the neurotrophic effect of CX3CL1. To substantiate this, hippocampal neuronal cultures were treated with clodronate liposomes to specifically kill microglia (van Rooijen et al, 1996; Marín-Teva et al, 2004). We first performed a kinetic analysis on hippocampal cultures obtained from CX3CR1GFP/GFP mice, where microglial cells are labeled by EGFP (Jung et al, 2000). Data reported in Figure 1b indicate that the number of EGFP-labeled microglial cells selectively decreases with time in hippocampal cultures treated with clodronate-filled liposomes, whereas it is not affected in cultures treated with empty liposomes. At the same time points chosen for microglia cell viability, liposome-treated hippocampal cultures were analyzed for CX3CL1 responsiveness in terms of protection from Glu-induced toxicity. To this end, cells were treated with empty or clodronate-filled liposomes, washed, stimulated with Glu, and analyzed for viability 18 h later. Results in Figure 1c show that, after 72 h of treatment with clodronate liposomes (when microglial cells in culture have almost completely disappeared see Figure 1b), CX3CL1 is not able to reduce Glu-induced toxicity. Unexpectedly, the same effect is observed as early as 5 h of clodronate liposome treatment (Figure 1c), when most of microglial cells are still present, suggesting that microglia are strongly affected well before they are eliminated and in such a way that response to CX3CL1 is strongly impaired at this time point. Note that, in basal conditions (C), clodronate-filled liposomes did not significantly modify total cell survival in culture, suggesting that neither neurons nor astrocytes, which together account for more than 95% of total cell population (see Materials and methods), are significantly affected by these treatments. Neuronal cell treatment with empty liposomes did not affect CX3CL1-induced neuroprotection (Supplementary Figure S1).
Figure 1.
Effect of microglia depletion with clodronate liposomes on CX3CL1-mediated neurotrophic activity. (a) Hippocampal cultures were treated with Glu (100 μM, 30 min) or vehicle in the presence or in the absence of CX3CL1 (100 nM) and analyzed for viability 18 h later as described in the text. Results are expressed as % of cell survival in treated (Glu and Glu/CX3CL1) vs untreated (C) cells (taken as 100%) and are the mean±SE of 12 duplicate experiments. (b) Hippocampal cultures from CX3CR1GFP/GFP mice were treated with empty or clodronate-filled liposomes for the indicated times and analyzed for the presence of EGFP-positive cells under fluorescence microscopy. Results are expressed as percentage of EGFP-positive cells (microglia) in the liposome-treated cultures vs control (C) untreated cells, and represent the mean of two duplicate independent experiments. (c) Alternatively hippocampal cultures, treated with clodronate-filled liposomes, were analyzed for Glu-induced excitotoxicity in the presence or in the absence of CX3CL1. Results are expressed as percentage of cell survival, taking 100% as untreated cells at time 0. Data represent the mean±SE of four duplicate experiments. For each time point, statistical significance was analyzed in treated (Glu and Glu/CX3CL1) vs untreated (C) samples. *P⩽0.05; **P⩽0.01.
Hippocampal Neurons from CX3CL1−/− Mice Are not More Vulnerable to Glu Injury in Comparison with wt Neurons
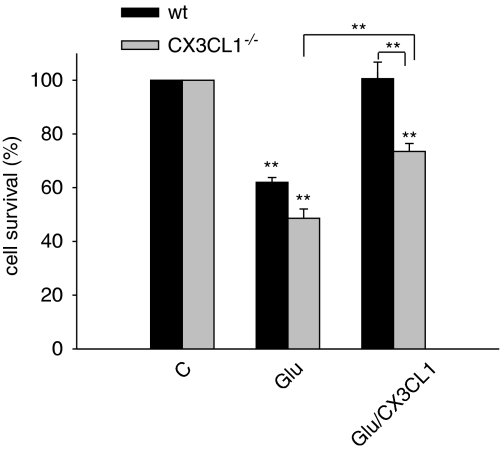
To investigate the role of endogenous CX3CL1 as neuroprotective agent on Glu-induced excitotoxicity, hippocampal cultures were obtained from CX3CL1−/− mice, treated with different Glu concentrations (from 1 μM to 1 mM), and analyzed for cell viability. No significant differences in neuron death were observed between wt and CX3CL1−/− mice at all tested Glu concentrations (Supplementary Figure S2). This suggests that endogenous levels of CX3CL1, neither before nor after Glu treatment (Chapman et al, 2000; Erichsen et al, 2003; Limatola et al, 2005), are sufficient to protect neurons by excitotoxicity under our in vitro conditions. To analyze whether the effect of the administration of the soluble form of CX3CL1 could be different in wt vs CX3CL1−/− mice, evidencing a possible cooperative role of the endogenous CX3CL1, excitotoxicity experiments were performed as shown in Figure 2. Data obtained indicate that in the absence of endogenous (membrane bound and shed forms) CX3CL1, exogenous administration of soluble CX3CL1 is still able to reduce Glu-induced cell death (CX3CL1−/− mice: Glu 48.6±3.5% vs Glu/CX3CL1 73.5±3.0% P⩽0.001) albeit at lower levels. No differences in cell viability were observed, in the absence of Glu, between wt and CX3CL1−/− cultures (data shown in the legend of Figure 2).
Figure 2.
Endogenous levels of CX3CL1 are not sufficient to protect neurons by excitotoxicity. Eleven-day-old hippocampal cultures obtained from wt or CX3CL1−/− mice were treated with Glu (100 μM, 30 min) or Glu/CX3CL1 and analyzed for viability 18 h later. Results are expressed as % of cell survival taking as 100% the control (C), untreated cells, for each mouse strain and are the mean±SE of five duplicate experiments. Statistical significance is analyzed between treated and untreated cells for each mouse strain, unless differently indicated. The number of cells in wt and CX3CL1−/− mice was not significantly different in untreated samples (41±4.3 and 45±2.4, respectively, per microscopic field, × 10). *P⩽0.05; **P⩽0.01.
Role and Origin of Extracellular Adenosine
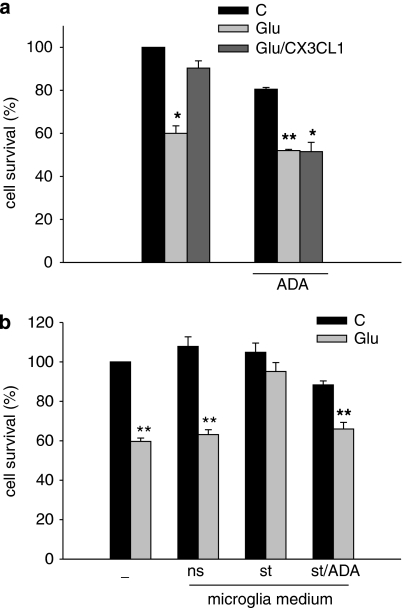
We have previously shown that CX3CL1 induces the release of adenosine from the murine microglial cell line BV2 and from mixed hippocampal cultures (Lauro et al, 2008). However, as immortalized cell lines may differ from primary cells, we wanted to investigate (i) whether primary microglia also release adenosine on CX3CL1 treatment and (ii) whether the reduction of extracellular adenosine levels, by treating cultured cells with ADA (the enzyme that degrades adenosine to inosine), is sufficient to prevent CX3CL1 neuroprotection against Glu-induced excitotoxicity. Supplementary Figure S3 shows that CX3CL1 treatment of primary cultures of murine microglia induces adenosine release, as previously shown with BV2 cells (Lauro et al, 2008). Results in Figure 3a show that ADA treatment (1 U/ml, 1 h, 37°C) of Glu-treated hippocampal cultures completely abolished CX3CL1-mediated neuroprotection. Interestingly, ADA treatment per se already results in some cell toxicity (19.5±0.9% reduction of cell viability), suggesting that basal adenosine levels contribute to keep cells healthy.
Figure 3.
ADA treatment abolishes the neuroprotective effect of CX3CL1. (a) Eleven-day-old hippocampal cultures were pre-incubated or not with ADA (1 U/ml) for 1 h and then co-stimulated with Glu or Glu/CX3CL1. Results represent the mean±SE of five independent duplicate experiments and are expressed as percentage of cell survival taking as 100% untreated cells in the absence of ADA. For each experimental condition, statistical significance was analyzed in treated (Glu and Glu/CX3CL1) vs untreated (C) samples. *P⩽0.05; **P⩽0.01. (b) Glu-injured CX3CR1GFP/GFP hippocampal neurons were treated with the medium conditioned by primary microglia not stimulated (ns) or stimulated with CX3CL1 (st). Alternatively, the conditioned (st) medium was collected, incubated for 1 h with ADA (1 U/ml; st/ADA) and given to CX3CR1GFP/GFP hippocampal neurons treated with Glu (100 μM) as described in ‘Materials and methods' section. Cell survival was analyzed after 18 h. Results represent the mean±SE of three independent duplicate experiments, and are expressed as percentage of cell survival taking, as 100%, untreated cells in control condition. For each experimental condition, statistical significance is analyzed between treated (Glu) and untreated (C) cells. *P⩽0.05; **P⩽0.01.
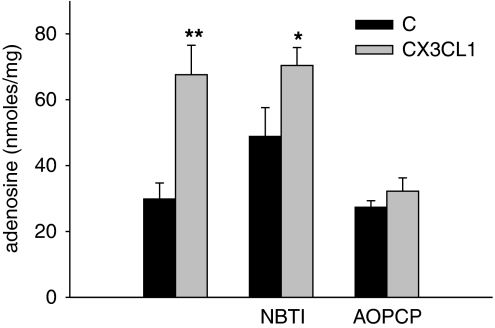
We next used the medium conditioned by CX3CL1-stimulated (st) primary wt microglia (at the same time point shown in Supplementary Figure S3), to reduce Glu-induced cell death of CX3CR1GFP/GFP neurons (confirming previous data with the microglia cell line BV2, Lauro et al, 2008); in the absence of CX3CL1 (not stimulated cells, ns), this medium is not able to prevent Glu-induced cell death (Figure 3b). When st medium was pre-treated with ADA (1 U/ml, 1 h, 37°C) and then given to CX3CR1GFP/GFP hippocampal neurons, the neuroprotective properties were completely lost (Figure 3b). Extracellular adenosine, which accumulates on CX3CL1 stimulation of hippocampal cultures (Lauro et al, 2008; Figure 4), likely derived from released ATP because in the presence of the specific ectonucleotidase inhibitor, AOPCP (1 μM, 10 min pre-incubation), the level of extracellular adenosine was not increased by CX3CL1 treatment (Figure 4). When higher levels of AOPCP were used (5 μM), the same results were obtained on CX3CL1 treatment (data not shown). However, in those conditions, the basal extracellular levels of adenosine were reduced at the limit of method detection. In contrast, the presence of the equilibrative transporter inhibitor NBTI did not significantly alter extracellular adenosine accumulation on CX3CL1 treatment (Figure 4).
Figure 4.
Adenosine produced by CX3CL1-stimulated hippocampal cultures is reduced by ectonucleotidases inhibition with AOPCP. Hippocampal neurons were treated with 100 nM CX3CL1 or vehicle for 30 min, in the presence or in the absence of NBTI (1 μM) and AOPCP (1 μM), washed, and re-incubated in growth medium. After 6 h medium was analyzed by HPLC for adenosine content. Results are expressed as nmol of adenosine produced for mg of cellular proteins and significance is analyzed, for each condition, between CX3CL1-treated vs corresponding control samples. Data are the mean±SE from five independent quadruplicate experiments.
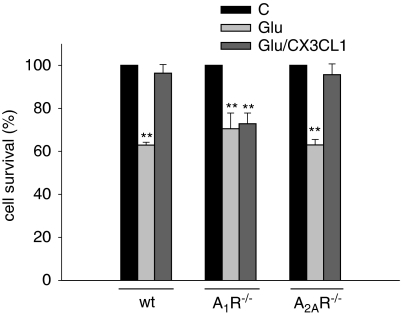
Hippocampal Neurons Obtained from A1R−/− Mice Are not Protected by CX3CL1 Against Glu Excitotoxicity
We recently showed that the protective effect of CX3CL1 against Glu-induced hippocampal neuron injury could be eliminated by the A1R antagonist DPCPX (Lauro et al, 2008). Although selective, this antagonist is not absolutely selective and, to prove the involvement of A1R in the neurotrophic activity of CX3CL1, hippocampal cultures obtained from A1R−/− mice were treated with Glu (100 μM, 30 min) to induce excitotoxicity in the presence or in the absence of CX3CL1. We showed that, in contrast with data obtained in wt mice, A1R−/− cultures were not protected from Glu-induced cell death by CX3CL1 treatment (100 nM, Figure 5) thus providing further evidence that A1Rs are required for the neuroprotective effect of the chemokine (Lauro et al, 2008). To exclude that the lack of neuroprotective effects of CX3CL1 on A1R−/− neurons was due to an impairment of CX3CR1 functional properties on these specific genetically modified mice, experiments were addressed to investigate CX3CL1-induced chemotaxis on microglial cells obtained from A1R−/− mice. Data reported in Supplementary Figure S4 show that A1R−/− microglia responds to CX3CL1 similarly to wt microglia in terms of transwell migration. Similarly, A1R−/− and wt cultured hippocampal neurons responded to CX3CL1 treatment with comparable levels of ERK phosphorylation (data not shown). These data indicate that there is no gross functional impairment of CX3CR1 pathway when A1R are absent. Considering the physical and functional interaction described for A1R/A2AR pair (Ciruela et al, 2008), we wanted to analyze the possible involvement of A2AR in the neurotrophic effect of CX3CL1 using A2AR−/− mice. Data, reported in Figure 5, show the selective involvement of A1R, with no participation of A2AR in CX3CL1-mediated neuroprotection from Glu-excitotoxicity. Furthermore, the number of cells that survived in the controls (C) was not significantly different between wt, A1R−/−, and A2AR−/− mice (respectively, 41±4.3; 41±2.9; 42±1.1 cells per microscopic field ( × 10)).
Figure 5.
Excitotoxic cell death of hippocampal neurons is not inhibited by CX3CL1 in A1R−/− mice. Hippocampal neurons obtained from wt, A1R−/−, or A2AR−/− mice were cultured for 11 days and then stimulated with Glu (100 μM, 30 min) in the presence or in the absence of CX3CL1 (100 nM). Cell death was analyzed after 18 h. Results represent the mean±SE of four independent duplicate experiments and are expressed as % of cell survival taking as 100% the control (C), untreated cells, for each mouse strain. Statistical significance is analyzed between treated and untreated cells for each mouse strain. *P⩽0.05; **P⩽0.01. The number of untreated cells in the control (C) is not significantly different between wt, A1R−/−, and A2AR−/− mice (see data in the text).
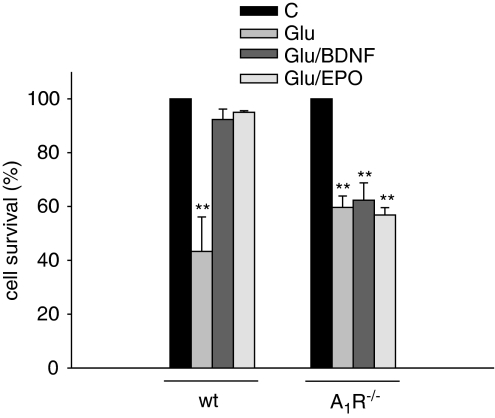
Glu-Injured Hippocampal A1R−/− Cultures Are not Rescued by Other Neurotrophins
To investigate whether A1R presence was a specific requirement for the neurotrophic activity of CX3CL1 or whether it was shared by other neurotrophins, hippocampal A1R−/− cultures were treated with Glu to induce excitotoxicity in the presence of BDNF (100 nM) or EPO (40 U/ml). Data shown in Figure 6 indicate that, under these conditions, both these substances protect wt neurons but fail to preserve A1R−/− neurons, thus suggesting that the presence of functional A1R on neuronal cells is permissive for the activity of different neurotrophic factors. These results further underline that the elimination of the neuroprotective effect of the chemokine cannot be simply explained by a specific loss of ARs and indicate that the mediation of neuroprotective effect by adenosine acting at A1Rs is quite a general phenomenon.
Figure 6.
The neuroprotective effect of BDNF and EPO is abolished in A1R−/− mice. Hippocampal neurons obtained from wt or A1R−/− mice were cultured for 11 days and then stimulated with Glu (100 μM, 30 min) in the presence or in the absence of BDNF (100 nM) or EPO (10 nM). Results represent the mean±SE of four independent duplicate experiments and are expressed as in Figure 5.
DISCUSSION
In this study, we show that microglial cells are required for the neuroprotective activity of CX3CL1, that they release adenosine that activates A1R, and that A1R presence is necessary for the neurotrophic activity of CX3CL1 on Glu-injured neurons. We also describe for the first time that the expression of A1R is necessary for the neurotrophic activity against excitotoxicity of other neurotrophins, such as BDNF and EPO. These findings will be discussed in turn.
The role of adenosine in neuroprotection is very well established: experimental evidence indicates that activation of A1R or inhibition of A2AR improves neuronal recovery on brain injury (Cunha, 2005), whereas the role played by A3R and A2BR in neuroprotection is less clear cut (Michel et al, 1999; Fedorova et al, 2003; Chen et al, 2006; Pugliese et al, 2007). In the present experimental conditions, we did not observe any clear effect of eliminating A2A receptors. It has been reported that, in the brain, the level of adenosine and A1R strongly increases on trauma-like brain ischemia (Pearson et al, 2006), where adenosine is mainly released by astrocytes (Martín et al, 2007) and participates in the protective effect of ischemic pre-conditioning (Heurteaux et al, 1995) or on repetitive seizures, where the rapid modulation of adenosine kinase has been reported (reviewed by Boison, 2006). Even the basal level of extracellular adenosine, and the corresponding tonic activation of ARs, can be responsible of the modulatory activity on synaptic transmission (see Fredholm et al, 2005). Furthermore, AR activation is necessary, as co-receptor requirement, either to permit or to enhance neuronal and glial response to purines (ATP, Gerwins and Fredholm, 1992; Färber et al, 2008), neuropeptides (VIP, Cunha-Reis et al, 2007; CGRP, Sebastião et al, 2000; GDNF, Gomes et al, 2009), cytokines (IL-6, Biber et al, 2008), growth and trophic factors (FGF, Flajolet et al, 2008; BDNF, Diógenes et al, 2004), and chemokines (CX3CL1, Lauro et al, 2008). Data reported in this study show that adenosine, in addition to its well-known direct neuroprotective effect on neurons (see above) and indirect protective effects through CCL2, IL-6, and S-100b release by astrocytes (Schwaninger et al, 1997; Ciccarelli et al, 1999; Wittendorp et al, 2004), appears to enable the neurotrophic activity of different neurotrophins to occur, thereby extending the repertoire of actions for adenosine in brain homeostatic control.
We showed earlier that A1R are probably involved in the neurotrophic activity of CX3CL1 as it was blocked by a relatively selective antagonist (Lauro et al, 2008). We now strongly support this conclusion in experiments where the protective effect is absent in A1R−/− mice. It might be argued that this is due to some functional impairment of CX3CR1 activation in A1R−/− mice. However, another effect of CX3CL1, namely direct induced by receptor stimulation, such as microglia migration, is similarly activated by CX3CL1 both in wt and A1R−/− mice. Furthermore, the neuroprotection induced by other agents was also reduced. Together these observations make it very unlikely that the reason why mice that lack A1Rs are not protected by CX3CL1 is that they are unable to respond to the chemokine.
We report that lack of endogenous CX3CL1 (in CX3CL1−/− mice) does not change hippocampal neuron response to Glu but reduces the protective effects induced by exogenous CX3CL1, suggesting a protective effect of the endogenous protein. We also show that this is not because of some major adaptive response to the targeted deletion of CX3CL1.
In a previous study, we showed that CX3CL1 induces adenosine release from hippocampal cultures and from a murine microglia cell line (Lauro et al, 2008). As the mechanisms underlying adenosine release might vary between different cell types of brain parenchyma, being mostly because of equilibrative transporters in neurons and to extracellularly released ATP, subsequently hydrolyzed by ectonucleotidases (Parkinson et al, 2005), in glia cells, we were interested in defining the potential mechanisms implicated in adenosine release by CX3CL1 in hippocampal mixed cultures. Our evidence that only the specific inhibitor of ectonucleotidases is able to strongly reduce CX3CL1-mediated adenosine release, whereas the inhibitor of equilibrative transport was not, could suggest a predominant involvement of glial cell-dependent nucleotide release in this process. The conclusion that glial cells are particularly important is also corroborated by our observations that the simultaneous treatment of hippocampal cultures with ADA and CX3CL1 completely abolished CX3CL1-mediated neurotrophic effect, and that the same result is obtained when ADA treatment is performed on medium collected from CX3CL1-stimulated primary microglia, 1 h before administration to Glu-treated hippocampal cultures.
It is proposed that microglia has a prominent role in mediating the neuroprotective effects of CX3CL1 (Mizuno et al, 2003; Huang et al, 2006; Cardona et al, 2006) and we have recently shown that CX3CL1-stimulated microglia releases neuroprotective substances that reduce Glu-induced cell death (Lauro et al, 2008). However CX3CL1 does not protect against all types of neuronal damage because in transient brain ischemia (Soriano et al, 2002; Dénes et al, 2008) and in a rat model of Parkinson's disease, intra-striatal CX3CL1 injection induced both microglia-dependent depletion of dopaminergic cells and motor dysfunction (Shan et al, 2009). In this study, we show that the selective ablation of microglia from hippocampal cultures, using clodronate-encapsulating liposomes, has the effect to fully abolish the neuroprotective activity of CX3CL1 toward excitotoxic death of hippocampal neurons, confirming that these cells represent the first target, which primes the functional effects of CX3CL1. It is interesting to note how a few percentage of microglial cells (such as that present in our hippocampal neuronal preparation) can massively influence neuronal response to CX3CL1. This could explain the reported neuroprotective effect of CX3CL1 in almost pure neuronal cultures (Limatola et al, 2005).
We hypothesized that soluble factors released by microglia, such as adenosine, could also activate astrocytes to release neurotrophic substances (Schwaninger et al, 1997; Ciccarelli et al, 1999; Wittendorp et al, 2004), which contribute to neuroprotection.
In conclusion, these data support the notion that CX3CL1 has neurotrophic activity on hippocampal neurons through its activity on microglia, which release soluble factors, among which adenosine. CX3CL1-mediated neuroprotection is only possible in the presence of functional A1R, whose activity is also required for the neuroprotective effect of other neuroactive factors, such as BDNF and EPO, thus showing that A1R co-activation is necessary as permissive signaling, which might reinforces or consent to the accomplishment of survival responses. The relevance of these conclusions remains to be confirmed in more physiological systems, such as neurons obtained from mature brains, or by in vivo studies.
Acknowledgments
This work was granted by Ministero Università & Ricerca scientifica (PRIN to CL), by Fondazione Cenci Bolognetti (to CL), by Ministero Salute (Ricerca finalizzata to CL and FE), and by Swedish Science Research Council (to BBF). We thank Dr Knut Biber for discussion.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Agnati LF, Ferrè S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Biber K, Pinto-Duarte A, Wittendorp MC, Dolga AM, Fernandes CC, Von Frijtag Drabbe Künzel J, et al. Interleukin-6 upregulates neuronal adenosine A1 receptors: implications for neuromodulation and neuroprotection. Neuropsychopharmacol. 2008;33:2237–2250. doi: 10.1038/sj.npp.1301612. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D'Alimonte I, D'Onofrio M, et al. Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia. 1999;27:275–281. [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueňo J, Canals M, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2008;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, et al. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol. 2001;21:3159–3165. doi: 10.1128/MCB.21.9.3159-3165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Vizi ES, Ribeiro JA, Sebastião AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Fontinha BM, Ribeiro JA, Sebastião AM. Tonic adenosine A1 and A2A receptor activation is required for the excitatory action of VIP on synaptic transmission in the CA1 area of the hippocampus. Neuropharmacol. 2007;52:313–320. doi: 10.1016/j.neuropharm.2006.08.003. [DOI] [PubMed] [Google Scholar]
- de Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all. Brain Res Brain Res Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Dénes A, Ferenczi S, Halász J, Környei Z, Kovács K. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Diógenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastião AM. Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus. 2007;17:577–585. doi: 10.1002/hipo.20294. [DOI] [PubMed] [Google Scholar]
- Diógenes MJ, Fernandes CC, Sebastião AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC, Archer ER. An adenosine agonist inhibits and a cyclic AMP analogue enhances the release of glutamate but not GABA from slices of rat dentate gyrus. Neurosci Lett. 1983;43:49–54. doi: 10.1016/0304-3940(83)90127-1. [DOI] [PubMed] [Google Scholar]
- Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, et al. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- Färber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, et al. The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia. 2008;56:331–341. doi: 10.1002/glia.20606. [DOI] [PubMed] [Google Scholar]
- Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/a:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor I, et al. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Diff. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Gerwins P, Fredholm BB. ATP and its metabolite adenosine act synergisticlly to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- Gomes CA, Simões PF, Canas PM, Quiroz C, Sebastião AM, Ferré S, et al. GDNF control of the glutamatergic cortico-striatal pathway requires tonic activation of adenosine A receptors. J Neurochem. 2009;108:1208–1219. doi: 10.1111/j.1471-4159.2009.05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenbauer S, Koedel U, Wick M, Kieseier BC, Hartung HP, Pfister HW. CSF and serum levels of soluble fractalkine (CX3CL1) in inflammatory diseases of the nervous system. J Neuroimmunol. 2003;137:210–217. doi: 10.1016/s0165-5728(03)00085-7. [DOI] [PubMed] [Google Scholar]
- Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, et al. Activity of adenosine receptors type 1 is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- Limatola C, Lauro C, Catalano M, Ciotti MT, Bertollini C, Di Angelantonio S, et al. Chemokine CX3CL1 protects rat hippocampal neurons against glutamate-mediated excitotoxicity. J Neuroimmunol. 2005;166:19–28. doi: 10.1016/j.jneuroim.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindström K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, Smith A, et al. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attenuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Michel PP, Marien M, Ruberg M, Colpaert F, Agid Y. Adenosine prevents the death of mesencephalic dopaminergic neurons by a mechanism that involves astrocytes. J Neurochem. 1999;72:2074–2082. doi: 10.1046/j.1471-4159.1999.0722074.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res. 2005;27:153–160. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pearson T, Damian K, Lynas RE, Frenguelli BG. Sustained elevation of extracellular adenosine and activation of A(1) receptors underlie the post-ischaemic inhibition of neuronal function in rat hippocampus in vitro. J Neurochem. 2006;97:1357–1368. doi: 10.1111/j.1471-4159.2006.03823.x. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, et al. Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem Pharmacol. 2007;74:768–779. doi: 10.1016/j.bcp.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Macedo MP, Ribeiro JA. Tonic activation of A(2A) adenosine receptors unmasks, and of A(1( receptors prevents, a facilitatory action of calcitonin gene-related peptide in the rat hippocampus. Br J Pharmacol. 2000;129:374–380. doi: 10.1038/sj.bjp.0703048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, Yan-Hong T, et al. 2009NEW evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson's disease Neurobiol AgingE-pub ahead of print. [DOI] [PubMed]
- Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallström E, Lassmann H, Olsson T, et al. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation. 2005;2:17–31. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Potenza RL, Grò C, Pepponi R, Armida M, et al. Adenosine A2A receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem. 2008;104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- Wittendorp MC, Boddeke HWGM, Biber K. Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia. 2004;46:410–418. doi: 10.1002/glia.20016. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/s0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.