Abstract
Atomoxetine and reboxetine are commonly used as selective norepinephrine reuptake inhibitors (NRIs) for the treatment of attention-deficit/hyperactivity disorder and depression, respectively. Furthermore, recent studies have suggested that NRIs may be useful for the treatment of several other psychiatric disorders. However, the molecular mechanisms underlying the various effects of NRIs have not yet been sufficiently clarified. G-protein-activated inwardly rectifying K+ (GIRK or Kir3) channels have an important function in regulating neuronal excitability and heart rate, and GIRK channel modulation has been suggested to be a potential treatment for several neuropsychiatric disorders and cardiac arrhythmias. In this study, we investigated the effects of atomoxetine and reboxetine on GIRK channels using the Xenopus oocyte expression assay. In oocytes injected with mRNA for GIRK1/GIRK2, GIRK2, or GIRK1/GIRK4 subunits, extracellular application of atomoxetine or reboxetine reversibly reduced GIRK currents. The inhibitory effects were concentration-dependent, but voltage-independent, and time-independent during each voltage pulse. However, Kir1.1 and Kir2.1 channels were insensitive to atomoxetine and reboxetine. Atomoxetine and reboxetine also inhibited GIRK currents induced by activation of cloned A1 adenosine receptors or by intracellularly applied GTPγS, a nonhydrolyzable GTP analogue. Furthermore, the GIRK currents induced by ethanol were concentration-dependently inhibited by extracellularly applied atomoxetine but not by intracellularly applied atomoxetine. The present results suggest that atomoxetine and reboxetine inhibit brain- and cardiac-type GIRK channels, revealing a novel characteristic of clinically used NRIs. GIRK channel inhibition may contribute to some of the therapeutic effects of NRIs and adverse side effects related to nervous system and heart function.
Keywords: atomoxetine, reboxetine, selective norepinephrine reuptake inhibitor, GIRK channel, ethanol, Xenopus oocyte
INTRODUCTION
Atomoxetine (originally named tomoxetine) and reboxetine are commonly used as selective norepinephrine reuptake inhibitors (NRIs) for the treatment of attention-deficit/hyperactivity disorder and depression, respectively (Hajós et al, 2004; Garland and Kirkpatrick 2004; Simpson and Plosker, 2004; Supplementary Figure S1). Their clinical efficacy is hypothesized to be linked mainly with potent inhibition of presynaptic norepinephrine transporters (Wong et al, 2000; Hajós et al, 2004; Simpson and Plosker, 2004). Furthermore, recent studies have suggested that the drugs are potentially useful for the treatment of several other psychiatric conditions, including anxiety disorders, eating disorders, substance use disorders, and narcolepsy (Kadhe et al, 2003; Hajós et al, 2004; Szerman et al, 2005; McElroy et al, 2007; Geller et al, 2007; Wilens et al, 2008). However, the molecular mechanisms underlying the various effects of NRIs have not yet been sufficiently clarified.
G-protein-activated inwardly rectifying K+ (GIRK) channels(also known as Kir3 channels) are members of a major subfamily of inwardly rectifying K+ (Kir) channels that include seven subfamilies (Reimann and Ashcroft, 1999). Four GIRK channel subunits have been identified in mammals (Kubo et al, 1993b; Krapivinsky et al, 1995; Lesage et al, 1995). Neuronal GIRK channels are predominantly heteromultimers composed of GIRK1 and GIRK2 subunits in most brain regions or homomultimers composed of GIRK2 subunits in the substantia nigra (Lesage et al, 1995; Karschin et al, 1996; Liao et al, 1996; Inanobe et al, 1999), whereas atrial GIRK channels are heteromultimers composed of GIRK1 and GIRK4 subunits (Krapivinsky et al, 1995). The channels are activated by various Gi-protein-coupled receptors, such as M2 muscarinic, α2 adrenergic, D2 dopaminergic, opioid, nociceptin/orphanin FQ, CB1 cannabinoid, and A1 adenosine receptors, through the direct action of G-protein βγ subunits (North, 1989; Dascal, 1997; Kobayashi and Ikeda, 2006). Additionally, ethanol activates GIRK channels independently of G-protein-coupled signaling pathways (Kobayashi et al, 1999; Lewohl et al, 1999). GIRK channels have an important function in regulating neuronal excitability, synaptic transmission, and heart rate (North, 1989; Lüscher et al, 1997; Signorini et al, 1997; Kuzhikandathil and Oxford, 2002; Kovoor et al, 2001). Furthermore, recent studies have suggested that GIRK channel modulation has the potential for treating several neuropsychiatric disorders and cardiac arrhythmias (Hashimoto et al, 2006; Kobayashi and Ikeda 2006; Cruz et al, 2008). Therefore, GIRK channel modulators may affect various brain and cardiac functions. In this study, the effects of atomoxetine and reboxetine on GIRK channels were examined using the Xenopus oocyte expression assay.
MATERIALS AND METHODS
Preparation of Specific mRNAs
Plasmids containing the entire coding sequences for the mouse GIRK1, GIRK2, and GIRK4 channel subunits and the Xenopus A1 adenosine receptor (A1R) were obtained previously (Kobayashi et al, 1995, 1999, 2000, 2002). cDNAs for rat Kir1.1 in pSPORT (Ho et al, 1993) and mouse Kir2.1 in pcDNA1 (Kubo et al, 1993a) were generously provided by Dr Steven C Hebert (Yale University) and Dr Lily Y Jan (University of California, San Francisco), respectively. These plasmids were linearized by digestion with the appropriate enzymes as described previously (Ho et al, 1993; Kubo et al, 1993a; Kobayashi et al, 2000). The specific mRNAs were synthesized in vitro using the mMESSAGE mMACHINE in vitro transcription kit (Ambion, Austin, TX, USA).
Electrophysiological Analysis
Adult female Xenopus laevis frogs (Copacetic, Soma, Aomori, Japan) were anesthetized by immersion in water containing 0.15% tricaine (Sigma-Aldrich, St Louis, MO, USA). A small incision was made on the abdomen to remove several ovarian lobes from the frogs, which were humanely killed after the final collection. All procedures for the care and treatment of animals were carried out in accordance with National Institutes of Health guidelines and were approved by our Institutional Animal Care and Use Committee. Xenopus oocytes (Stages V and VI) were manually isolated from the ovary and maintained in Barth's solution (Kobayashi et al, 2002). Oocytes were injected with mRNA for GIRK1/GIRK2 or GIRK1/GIRK4 combinations (each 0.15 ng), GIRK2 (1 ng), Kir1.1 (2 ng), Kir2.1 (0.3 ng), or A1R (5 ng). The oocytes were incubated at 19°C in Barth's solution and manually defolliculated after treatment with 0.8 mg ml−1 collagenase (Wako Pure Chemical Industries, Osaka, Japan) for 1 h. Whole-cell currents of the oocytes were recorded 3–8 days after injection with a conventional two-electrode voltage clamp (Kobayashi et al, 1999; Ikeda et al, 2003). All recordings were carried out at room temperature (19°C) to avoid damage to Xenopus oocytes and the effects of temperature (Fraser and Djamgoz, 1992; Weber, 1999). The membrane potential was held at −70 mV unless otherwise specified. Microelectrodes were filled with 3 M KCl. The oocytes were placed in a 0.05 ml narrow chamber and continuously superfused with a high-potassium (hK) solution (96 mM KCl, 2 mM NaCl, 1 mM MgCl2, 1.5 mM CaCl2, and 5 mM HEPES, pH 7.4 with KOH) or a K+-free high-sodium (ND98) solution (98 mM NaCl, 1 mM MgCl2, 1.5 mM CaCl2, and 5 mM HEPES, pH 7.4 with NaOH) at a flow rate of 2.5 ml/min. In the hK solution, the K+ equilibrium potential was close to 0 mV, and the inward K+ current flow through the Kir channels was observed at negative holding potentials as shown earlier (Ho et al, 1993; Kubo et al, 1993a; Lesage et al, 1995; Kobayashi et al, 2006). Additionally, to examine the effects of the NRIs on outward K+ currents, a perfusion solution containing 4 mM K+ (K4 solution) was made by substituting NaCl with KCl in the ND98 solution. To examine the effects of the drugs on GIRK channels activated by G-protein activation, 13.8 nl of 100 mM Li4-guanosine-5′-O-(3-thiotriphosphate) (GTPγS; Sigma-Aldrich), a nonhydrolyzable G-protein activator, dissolved in distilled water was injected into an oocyte using a nanoliter injector (World Precision Instruments, Sarasota, FL, USA) as described earlier (Kovoor et al, 1995). Furthermore, to examine the effects of intracellular atomoxetine, 23 nl of 10 mM atomoxetine dissolved in distilled water was injected into an oocyte using a nanoliter injector (Kobayashi et al, 2003), and the oocyte currents were then continuously recorded for ∼30–40 min. As the volume of the Xenopus oocytes used was ∼1 μl, the intracellular concentration of atomoxetine was presumed to be ∼225 μM. For analysis of concentration–response relationships, data were fitted to the following logistic equation: drug inhibition=max/1+(EC50/[drug])nH, with max being the maximal inhibition attainable, EC50 being the concentration of a drug that produces 50% of the maximal current response for that drug, [drug] being the concentration of an NRI and nH being the Hill coefficient, using KaleidaGraph (Synergy Software, Reading, PA, USA). The concentrations required to reduce control currents, by 25 and 50% (IC25 and IC50, respectively), were calculated from the concentration–response relationships.
Statistical Analysis
Data are expressed as mean±SEM, and n is the number of oocytes tested. Statistical analysis of the differences between groups was performed using Student's t-test, paired t-test, one-way analysis of variance (ANOVA), or two-way ANOVA followed by Tukey–Kramer post hoc test. Values of P<0.05 were considered statistically significant.
Compounds
Tomoxetine hydrochloride (recently renamed atomoxetine hydrochloride) and reboxetine mesylate were purchased from Tocris Cookson (Bristol, UK) and dissolved in dimethyl sulfoxide (DMSO) or distilled water. The stock solution of each compound was stored at −30°C until use. Ethanol was purchased from Wako Pure Chemical Industries. Each compound was added to the perfusion solution in appropriate amounts immediately before the experiments.
RESULTS
Inhibition of GIRK Channels by Atomoxetine and Reboxetine
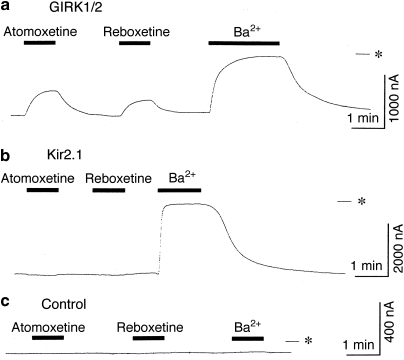
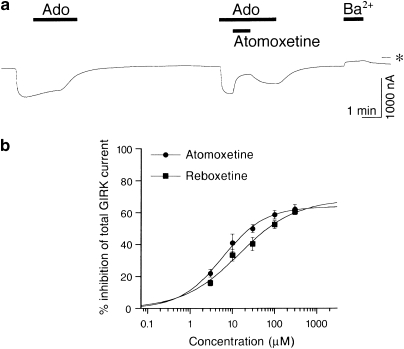
In Xenopus oocytes injected with GIRK1 and GIRK2 mRNAs, basal GIRK currents, which depend on free G-protein βγ subunits present in the oocytes because of the inherent activity of G-proteins (Dascal, 1997), were observed at a holding potential of −70 mV in an hK solution containing 96 mM K+ (Figure 1a). Extracellular application of 30 μM atomoxetine or reboxetine reversibly reduced the inward currents through the expressed GIRK channels (Figure 1a). The current responses to an additional 100 μM atomoxetine during the application of 3 mM Ba2+, which blocks Kir channels, were not significant (reduction of inward currents by 5.5±5.0 nA; <1% inhibition of the Ba2+-sensitive current components; n=4). The 3 mM Ba2+-sensitive current components (910.5±65.7 nA, n=14) are considered to correspond to the magnitude of GIRK currents in oocytes expressing GIRK channels (Kobayashi et al, 1999). Atomoxetine and reboxetine produced no significant response in a K+-free ND98 perfusion solution containing 98 mM Na+ instead of the hK solution (n=4; data not shown), suggesting that the NRI-sensitive current components show K+ selectivity. Additionally, application of DMSO or distilled water, the solvent vehicle, at the highest concentration (0.3%) induced no significant current response in the hK or ND98 solutions (n=5; data not shown). However, in oocytes injected with mRNA for Kir1.1, an ATP-regulated Kir channel (Ho et al, 1993), or Kir2.1, a constitutively active Kir channel (Kubo et al, 1993a), extracellular application of 300 μM atomoxetine or reboxetine had no significant effects on the inward currents through the channels in the hK solution (<3% change of the Ba2+-sensitive current components; 583.3±59.7 nA for Kir1.1, n=4; 1306.7±179.8 nA for Kir2.1, n=4; Figure 1b). In uninjected oocytes, 300 μM atomoxetine and reboxetine as well as 3 mM Ba2+ caused no significant response (3.8±2.9, 0±0, and 6.8±0.7 nA, respectively; n=4, 4, and 7, respectively; Figure 1c) compared with oocytes injected with GIRK mRNA, suggesting no significant effects of atomoxetine, reboxetine, or Ba2+ on intrinsic oocyte channels. Furthermore, in oocytes injected with GIRK1 and GIRK2 mRNAs, outward currents observed at a holding potential of −30 mV in a K4 solution containing 4 mM K+ were reversibly reduced by 30 μM atomoxetine (n=4), 30 μM reboxetine (n=4), or 3 mM Ba2+ (the Ba2+-sensitive current components, 85.2±32.8 nA, n=8; Supplementary Figure S2), whereas in uninjected oocytes, the NRIs at 100 μM and 3 mM Ba2+ caused no significant response (3.0±0.9 nA for atomoxetine, 0±0 nA for reboxetine, and 7.6±1.3 nA for Ba2+; n=4, 4, and 8, respectively). The results suggest that the NRIs also inhibited outward GIRK currents. Similarly, in oocytes injected with either GIRK1 and GIRK4 mRNAs or GIRK2 mRNA, atomoxetine and reboxetine inhibited basal GIRK currents under the same conditions (3 mM Ba2+-sensitive current components for GIRK1/4, 1027.5±112.6 nA, n=10; 3 mM Ba2+-sensitive current components for GIRK2, 757.0±51.5 nA, n=12; Figure 2). The results suggest that atomoxetine and reboxetine inhibited GIRK channels, but not Kir1.1 and Kir2.1 channels.
Figure 1.
Inhibitory effects of atomoxetine and reboxetine on GIRK channels expressed in Xenopus oocytes. (a) In an oocyte injected with GIRK1 and GIRK2 mRNAs, current responses to 10 μM atomoxetine, 10 μM reboxetine, and 3 mM Ba2+ are shown. (b) In an oocyte injected with Kir2.1 mRNA, current responses to 100 μM atomoxetine, 100 μM reboxetine, and 3 mM Ba2+ are shown. (c) In an uninjected oocyte, no significant current responses to 300 μM atomoxetine, 300 μM reboxetine, or 3 mM Ba2+ are shown. Current responses were measured at a membrane potential of −70 mV in an hK solution containing 96 mM K+. Asterisks show the zero current level. Horizontal bars indicate the duration of application.
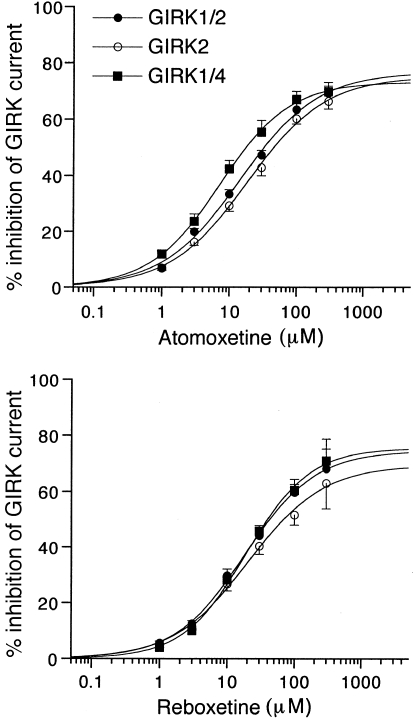
Figure 2.
Concentration–response relationships for the inhibitory effects of atomoxetine and reboxetine on GIRK1/2, GIRK2, and GIRK1/4 channels. The magnitudes of inhibition of GIRK currents by the drugs were compared with the 3 mM Ba2+-sensitive current components in oocytes expressing GIRK1/2, GIRK2, and GIRK1/4 channels (910.5±65.7 nA, n=14; 757.0±51.5 nA, n=12; and 1027.5±112.6 nA, n=10, respectively). Each point and error bar represents the mean±SEM of the percentage responses.
Characteristics of Inhibition of GIRK Channels by Atomoxetine and Reboxetine
The concentration–response relationships of the inhibitory effects of atomoxetine and reboxetine on GIRK1/2, GIRK2, and GIRK1/4 channels were investigated. Figure 2 shows that inhibition of these types of GIRK channels by atomoxetine and reboxetine was concentration-dependent. Table 1 shows the EC50 and nH values obtained from the concentration–response relationships and the percentage inhibition of the GIRK currents by the NRIs at the highest concentrations tested. Additionally, because the drugs could not completely block these types of GIRK channels, even at the highest concentrations tested, the IC25 and IC50 values were also calculated to further compare the effects of the drugs (Table 1). The inhibition of GIRK1/4 channels by atomoxetine was more effective at 10 and 30 μM than inhibition of GIRK2 channels (P<0.05, Tukey–Kramer post hoc test), although the effects of atomoxetine at the highest concentration on three types of channels were similar (P>0.05, Tukey–Kramer post hoc test; Figure 2a; Table 1). In contrast, the inhibitory effects of reboxetine on these types of channels were statistically similar (P>0.05 at each concentration, Tukey–Kramer post hoc test), although the inhibition of GIRK2 channels by 100 and 300 μM reboxetine was slightly less effective than inhibition of the other channel types (Figure 2b). Furthermore, inhibition of GIRK1/4 channels by 10 μM atomoxetine was more effective than 10 μM reboxetine (P<0.05, Tukey–Kramer post hoc test), whereas the effects of atomoxetine on GIRK1/2 and GIRK2 channels were similar to reboxetine (P>0.05 at each concentration, Tukey–Kramer post hoc test).
Table 1. Inhibitory Effects of Atomoxetine and Reboxetine on GIRK Channels.
|
Atomoxetine |
Reboxetine |
|||||
|---|---|---|---|---|---|---|
| GIRK1/2 | GIRK2 | GIRK1/4 | GIRK1/2 | GIRK2 | GIRK1/4 | |
| EC50 (μM) | 10.9±1.3 | 12.4±1.5 | 6.5±0.4 | 13.7±1.3 | 15.5±2.1 | 19.4±1.7 |
| IC25 (μM) | 5.4±0.4 | 6.1±0.5 | 2.9±0.2 | 7.8±1.1 | 8.8±1.4 | 9.0±0.4 |
| IC50 (μM) | 33.3±4.9 | 52.2±10.2 | 14.3±1.6 | 48.2±11.1 | 64.0±18.3 | 41.0±4.9 |
| % Max | 69.3±2.5 | 67.0±1.3 | 73.1±0.8 | 68.3±10.6 | 63.1±9.1 | 71.1±4.2 |
| (n) | (8) | (6) | (5) | (6) | (6) | (5) |
| nH | 0.96±0.09 | 0.89±0.09 | 0.88±0.07 | 0.94±0.03 | 0.91±0.04 | 0.93±0.04 |
Mean±SEM of the concentration of a drug that produces 50% of the maximal effect (EC50) and the concentrations required to reduce basal GIRK currents by 25 and 50% (IC25 and IC50, respectively) are shown in μM. The values of % max indicate the mean±SEM percentage inhibition of basal GIRK currents by a drug at the highest concentrations tested (300 μM). The number of Xenopus oocytes tested (n) is indicated in parentheses. The nH values indicate the mean±SEM of Hill coefficients.
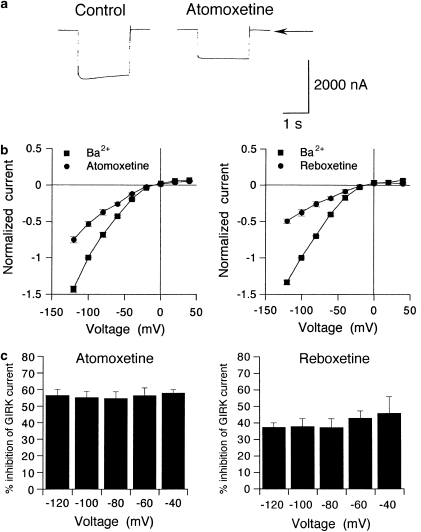
Instantaneous GIRK1/2 currents elicited by the voltage step to −100 mV from a holding potential of 0 mV were diminished in the presence of 30 μM atomoxetine applied for 3 min (Figure 3a). The percentage inhibition of the steady-state GIRK current at the end of the voltage step by atomoxetine was not significantly different from that of the instantaneous current (P>0.05, paired t-test; n=9 at −40, −60, −80, −100, and −120 mV, respectively). For reboxetine, similar results were observed (n=7). These results suggest that the channels were inhibited by atomoxetine and reboxetine primarily at the holding potential of 0 mV and time-independently during each voltage pulse. Similar to the 3 mM Ba2+-sensitive current components corresponding to the magnitudes of basal GIRK currents, the magnitudes of currents reduced by 30 μM atomoxetine in oocytes expressing GIRK1/2 channels increased with negative membrane potentials, and the current–voltage relationships showed strong inward rectification (n=9; Figure 3b), indicating a characteristic of GIRK currents. The percentage inhibition of GIRK1/2 currents by 30 μM atomoxetine showed no significant difference across voltages between −120 and −40 mV (no significant atomoxetine effect × membrane potential effect interaction, P>0.1, one-way ANOVA; P>0.1 across voltages, Tukey–Kramer post hoc test; Figure 3c). For reboxetine, similar results were observed (n=7; Figure 3b and c). The results suggest that the inhibition of GIRK channels by atomoxetine and reboxetine was voltage-independent. Furthermore, similar results were obtained in oocytes expressing GIRK1/4 channels (n=5 for atomoxetine and n=4 for reboxetine; data not shown). Therefore, atomoxetine and reboxetine may have similar actions as GIRK channel inhibitors.
Figure 3.
Characteristics of the inhibitory effects of atomoxetine and reboxetine on GIRK currents. (a) Representative GIRK1/2 currents elicited by a voltage step to −100 mV for 2 s from a holding potential of 0 mV in the presence or absence of 30 μM atomoxetine applied for 3 min. Current responses were recorded in an hK solution containing 96 mM K+. Arrow indicates the zero current level. (b) Current–voltage relationships of the magnitudes of the current component sensitive to 3 mM Ba2+ and the magnitudes of currents reduced by 30 μM atomoxetine (left) or 30 μM reboxetine (right) in oocytes expressing GIRK1/2 channels. Current responses to a drug were normalized to the 3 mM Ba2+-sensitive current component measured at a membrane potential of −100 mV (1219.7±79.2 nA, n=14). (c) Percentage inhibition of GIRK1/2 channels by atomoxetine or reboxetine over the voltage range of −120 to −40 mV. The magnitudes of inhibition of GIRK currents by 30 μM atomoxetine (left, n=8) or 30 μM reboxetine (right, n=6) at the end of the voltage pulses were compared with the 3 mM Ba2+-sensitive current components. All values are expressed as mean±SEM.
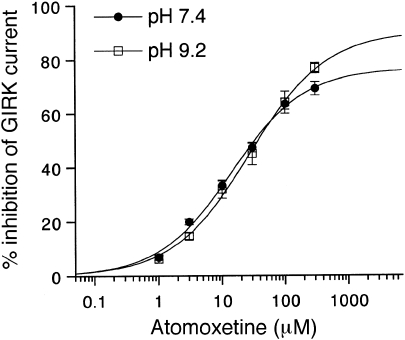
Atomoxetine possesses a secondary amine group with a pKa value of 9.23 (Eli Lilly and Company Data Sheet; Supplementary Figure S1). At physiological pH or below, atomoxetine exists mainly in a protonated form, ∼98.5% at pH 7.4, and the proportion of the uncharged form increases with an increase in pH. We examined whether changes in extracellular pH would affect GIRK channel inhibition by atomoxetine. However, in oocytes expressing GIRK1/2 channels, the percentage inhibition of GIRK channels by atomoxetine at the same concentrations was not significantly affected by extracellular pH 7.4 and 9.2 (no significant pH × atomoxetine interaction, P>0.5, two-way ANOVA; P>0.1 at each concentration, Tukey–Kramer post hoc test; Figure 4). The results indicate that a marked increase in the proportion of the uncharged form may not significantly affect all of the effects on GIRK channels, suggesting that GIRK channel inhibition may be mediated by both forms of atomoxetine with similar effectiveness.
Figure 4.
Concentration–response relationships for inhibition of GIRK channels by atomoxetine at different pH values. The magnitudes of inhibition of GIRK currents by atomoxetine were compared with the 3 mM Ba2+-sensitive current components in oocytes expressing GIRK1/2 channels (1021.5±100.8 nA, pH 7.4, n=8; 852.4±141.4 nA, pH 9.2, n=6). Current responses were measured at a membrane potential of −70 mV in an hK solution containing 96 mM K+. Each point and error bar represents the mean±SEM of the percentage responses.
Effects of Atomoxetine and Reboxetine on GIRK Channels Activated by a G-Protein-Coupled Receptor or GTPγS
We examined the effects of atomoxetine and reboxetine on GIRK channels activated by a G-protein-coupled receptor. In oocytes co-expressing GIRK1/2 channels and A1Rs (Kobayashi et al, 2002), 100 nM adenosine significantly induced inward GIRK currents (1000.7±76.9 nA, n=10; Figure 5a), and 300 μM atomoxetine or reboxetine alone consistently inhibited basal GIRK currents (3 mM Ba2+-sensitive current components, 157.2±31.3 nA, n=10). The current responses to 100 nM adenosine were reduced by the addition of atomoxetine or reboxetine (n=5 for each NRI; Figure 5a). These results suggest that atomoxetine and reboxetine inhibited total GIRK currents through the GIRK channels activated by the A1R and the basally active GIRK channels. The percentage inhibition of total GIRK currents by atomoxetine or reboxetine (IC25=4.5±1.6 and 8.6±1.7 μM; IC50=42.7±12.3 and 55.1±16.4 μM; nH=0.93±0.04 and 0.79±0.13; n=5, respectively; Figure 5b) was not significantly different from that of basal GIRK currents in oocytes injected with GIRK1 and GIRK2 mRNAs (P>0.05, IC25 and IC50 values for each NRI, Student's t-test; P>0.05 at each concentration, Tukey–Kramer post hoc test), suggesting that the effects of the NRIs on A1R-activated GIRK channels were similar to those on GIRK channels activated by basally free G-protein βγ subunits present in oocytes.
Figure 5.
Effects of atomoxetine and reboxetine on GIRK channels activated by a G-protein-coupled receptor. (a) Current responses to 100 nM adenosine (Ado), 30 μM atomoxetine during application of 100 nM Ado, and 3 mM Ba2+ in an oocyte co-injected with mRNAs for GIRK1 and GIRK2 channels and the A1 adenosine receptor (A1R) are shown. Bars show the duration of application. Asterisk indicates the zero current level. (b) Concentration–response relationships for the inhibitory effects of atomoxetine and reboxetine on total GIRK currents composed of Ado-induced GIRK currents and basal GIRK currents. Each point and error bar represents the mean±SEM of the percentage responses. Current responses were measured at a membrane potential of −70 mV in an hK solution containing 96 mM K+.
GIRK channels are activated by various G-protein-coupled receptors through the direct action of G-protein βγ subunits released from the heterotrimeric G-protein complex (Dascal, 1997; Kobayashi and Ikeda, 2006). The effects of the NRIs on GIRK channels activated by G-protein-coupled signaling mechanisms were further examined using GTPγS, a nonhydrolyzable GTP analogue that maintains G-proteins in an activated state. Injection of GTPγS into Xenopus oocytes injected with GIRK1 and GIRK2 mRNAs increased inward currents with time and reached a steady-state level (938.9±119.2 nA, n=18) as reported earlier (Kovoor et al, 1995). The increased inward currents were completely blocked by 3 mM Ba2+, whereas GTPγS injection into uninjected oocytes had no significant effect on current responses to 3 mM Ba2+ (3.9±2.1 nA, n=9). Increased GIRK currents composed of basal GIRK currents and GTPγS-induced GIRK currents were inhibited by atomoxetine or reboxetine (IC50=29.0±6.2 and 52.3±10.1 μM; nH=1.28±0.04 and 1.14±0.06; n=6 and 12, respectively). The percentage inhibition of total GIRK currents by atomoxetine or reboxetine was not significantly different from that of basal GIRK currents in GTPγS-untreated oocytes injected with GIRK1 and GIRK2 mRNAs (P>0.05, IC50 value for each NRI, Student's t-test; P>0.05 at each concentration, Tukey–Kramer post hoc test), suggesting that the effects of the NRIs on basally active GIRK channels and GIRK channels activated by G-protein activation induced by GTPγS were similar.
Atomoxetine Inhibits Ethanol-Induced GIRK Currents
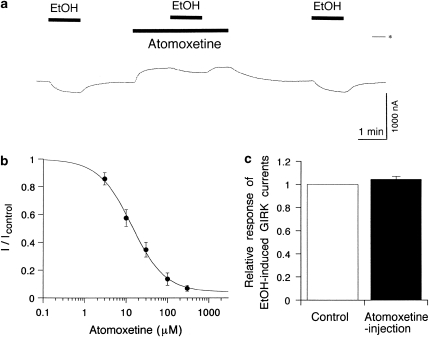
GIRK channels are also activated by ethanol independent of G-protein signaling pathways (Kobayashi et al, 1999). Atomoxetine was shown to reduce cumulative heavy drinking days in the treatment of psychiatric patients with comorbid alcohol use disorders (Wilens et al, 2008). Therefore, we also examined the effects of atomoxetine on GIRK channel activation induced by ethanol. The effects of atomoxetine were evaluated by measuring the amplitude of the ethanol-induced current response during extracellular application of atomoxetine at different concentrations. In oocytes injected with GIRK1 and GIRK2 mRNAs, the GIRK currents induced by 100 mM ethanol (420.0±32.5 nA, n=5) were reversibly attenuated in the presence of atomoxetine (IC25=5.8±1.1 μM; IC50=15.4±3.1 μM; nH=1.22±0.22; n=5; Figure 6a and b). However, 100 mM ethanol-induced GIRK currents were not significantly affected by intracellularly applied atomoxetine (104.3±2.8% of untreated control current, paired t-test, P>0.1, n=5; Figure 6c). Moreover, in oocytes expressing GIRK channels, the basal currents were not significantly affected by intracellularly applied atomoxetine (103.0±2.2% of untreated control current, paired t-test, P>0.1, n=5). The results indicate that intracellular atomoxetine could not inhibit GIRK channels. In contrast, GIRK channel inhibition induced by extracellularly applied atomoxetine, which is mainly protonated at pH 7.4, was reversible with washout (Figures 1a and 6a). As the protonated form may not readily permeate the cell membrane, extracellularly applied atomoxetine may exist mainly on the extracellular side. Altogether, extracellular atomoxetine may inhibit GIRK channels activated by ethanol.
Figure 6.
Effects of atomoxetine on ethanol-induced GIRK currents. (a) Current responses to 100 mM ethanol (EtOH), 100 mM EtOH in the presence of 30 μM atomoxetine, and 100 mM EtOH in an oocyte injected with GIRK1 and GIRK2 mRNAs. Asterisk indicates the zero current level. Bars show the duration of application. (b) Concentration-dependent inhibition of EtOH-induced GIRK currents by atomoxetine. Icontrol is the amplitude of GIRK currents induced by 100 mM EtOH (420.0±32.5 nA, n=5), and I is the current amplitude in the presence of atomoxetine. (c) Lack of effect of intracellular atomoxetine on 100 mM EtOH-induced GIRK currents. The amplitude of EtOH-induced GIRK currents after atomoxetine injection (black bar) was compared with the amplitude of EtOH-induced GIRK currents before the injection (control, white bar) in the same oocyte expressing GIRK channels (n=5). Current responses were measured at a membrane potential of −70 mV in an hK solution containing 96 mM K+. All values are expressed as mean±SEM.
DISCUSSION
In this study, we showed that atomoxetine and structurally related reboxetine, clinically used selective NRIs, inhibited brain-type GIRK1/2 and GIRK2 channels and cardiac-type GIRK1/4 channels expressed in Xenopus oocytes. However, Kir1.1 and Kir2.1 channels in other Kir channel subfamilies were insensitive to both NRIs. The inhibitory effects on GIRK channels were concentration-dependent, but voltage-independent, and time-independent during each voltage pulse. The present results suggest that the site of action on the channels may be extracellular. In contrast, blockade of GIRK channels by extracellular Ba2+ and Cs+, which occlude the pore of the open channel, shows a concentration-dependence, a voltage-dependence, and a time-dependence with a comparatively small effect on the instantaneous current but a marked inhibition of the steady-state current at the end of the voltage pulses (Lesage et al, 1995). These observations suggest that atomoxetine and reboxetine may cause an allosteric conformational change in GIRK channels even before the voltage pulses, rather than simple occlusion of the open channel. Interestingly, GIRK channels are also inhibited by the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Kobayashi et al, 2003; Takahashi et al, 2006), despite a great difference in the pharmacological profiles for monoamine transporters between the two NRIs and fluoxetine. The chemical structures of atomoxetine and reboxetine are related to fluoxetine (Boot et al, 2005; Supplementary Figure S1), suggesting that the common moiety of the structures may play a key role in interacting with GIRK channels. Additionally, the Xenopus oocyte expression assay with a conventional two-electrode voltage clamp is generally conducted using defolliculated oocytes, which are still covered over the plasma membrane with the vitelline membrane, at room temperature (Fraser and Djamgoz, 1992; Weber, 1999; Ikeda et al, 2003). Further studies using mammalian cells, including neurons and cardiac myocytes, at physiological temperature may be useful for advancing our understanding of the effects of NRIs on GIRK channels.
Atomoxetine is predominantly metabolized by the genetically polymorphic cytochrome P450 2D6 (CYP2D6) pathway, and its pharmacokinetics and metabolism are characterized by two distinct activities of CYP2D6: active or poor metabolic capability (Witcher et al, 2003; Simpson and Plosker, 2004). The maximum plasma concentrations during treatment with atomoxetine at therapeutic doses ranged from ∼0.7–4.8 μM in CYP2D6 active metabolizers (Witcher et al, 2003), whereas those in CYP2D6 poor metabolizers (∼7% of the Caucasian population) were six-fold higher than those in CYP2D6 active metabolizers (Simpson and Plosker, 2004). Additionally, co-administration of the SSRI paroxetine, a potent inhibitor of CYP2D6, increased the plasma concentrations of atomoxetine by 3.5-fold, with a pharmacokinetic profile similar to CYP2D6 poor metabolizers (Belle et al, 2002), suggesting a significant increase in atomoxetine concentrations with concomitant treatment with CYP2D6 inhibitors. The maximum plasma concentrations of reboxetine at therapeutic doses in depressed patients ranged from 0.5 to 2.1 μM (Poggesi et al, 2000). Additionally, increases in doses of the NRIs are associated with increases in plasma concentrations (Öhman et al, 2001; Witcher et al, 2003), and the concentration in a fatal case of atomoxetine overdose was reported to be up to 32.5 μM (Garside et al, 2006). Recent studies using radiolabeled NRI ligands have indicated that NRIs are extensively distributed in most tissues (Kiyono et al, 2004, 2008; Kanegawa et al, 2006). Indeed, brain and heart levels of NRIs were ∼4.7- to 6.5-fold and 9- to 12-fold higher for atomoxetine (Kiyono et al, 2004) and ∼15- to 16-fold and 21- to 32-fold higher for reboxetine than corresponding blood levels, respectively (Kanegawa et al, 2006; Kiyono et al, 2008). Therefore, brain and heart concentrations during treatment with therapeutic doses of atomoxetine and reboxetine, as well as after overdose, overlap with their effective concentrations in inhibiting brain- and cardiac-type GIRK channels (Figure 2). GIRK channels in the brain and heart may be inhibited by atomoxetine and reboxetine, particularly with the use of atomoxetine with CYP2D6 poor metabolizers or co-administration of CYP2D6 inhibitors. Inhibition of GIRK channels causes a depolarization of membrane potential, resulting in an increase in cell excitability (Kuzhikandathil and Oxford, 2002). GIRK channels have an important function in regulating neuronal excitability, synaptic transmission, and heart rate (Lüscher et al, 1997; Kovoor et al, 2001). Therefore, even partial inhibition of GIRK channels by atomoxetine and reboxetine may affect certain brain and heart functions.
Interestingly, GIRK2 knockout mice exhibit reduced anxiety-related behavior (Blednov et al, 2001). In clinical studies, reboxetine and atomoxetine were effective in the treatment of panic disorder and comorbid anxiety disorder, respectively (Versiani et al, 2002; Geller et al, 2007), suggesting their anxiolytic properties. Although their therapeutic effects are generally thought to be primarily attributable to inhibition of norepinephrine reuptake in the brain (Hajós et al, 2004; Simpson and Plosker, 2004), inhibition of GIRK channels may also contribute to improvement of anxiety symptoms.
GIRK2 knockout mice exhibit spontaneous seizures and are more susceptible to seizures induced by pentylenetetrazol than wild-type mice (Signorini et al, 1997). In animal studies using atomoxetine or reboxetine, convulsions were observed only at extremely high doses (Wong et al, 2000; Wernicke et al, 2007). The incidence of seizures during treatment with NRIs has been reportedly rare (Montgomery, 2005; Wernicke et al, 2007). Brain levels of the drugs in overdose cases may be considerably higher than levels during treatment at therapeutic doses (Poggesi et al, 2000; Kiyono et al, 2004, 2008; Garside et al, 2006; Kanegawa et al, 2006), suggesting that potent inhibition of neuronal GIRK channels by atomoxetine and reboxetine after overdose may contribute to increased seizure activity. However, the NRIs simultaneously increase extracellular levels of norepinephrine in the brain (Hajós et al, 2004; Simpson and Plosker, 2004), and norepinephrine has anticonvulsant effects (Ahern et al, 2006). The enhancement of norepinephrine by NRIs may be involved in the rare incidence of seizures. Although atomoxetine and reboxetine are generally well tolerated and have a benign side effect profile (Hajós et al, 2004; Simpson and Plosker, 2004), the inhibitory effects on GIRK channels may be partly related to the occurrence of other neurological side effects, such as insomnia and dizziness.
In the heart, GIRK channels cause slowing of heart rate in response to activation of M2 muscarinic receptors through acetylcholine release from the stimulated vagus nerve (Kubo et al, 1993b; Krapivinsky et al, 1995). GIRK1 or GIRK4 knockout mice exhibit slightly elevated resting heart rates (Bettahi et al, 2002). Atomoxetine and reboxetine are associated with modest increases in heart rate (Hajós et al, 2004; Simpson and Plosker, 2004) and tachycardia in cases of toxicity (LoVecchio and Kashani, 2006). The binding affinities of atomoxetine and reboxetine for the muscarinic receptor are in the low micromolar range (Cusack et al, 1994; Wong et al, 2000; Hajós et al, 2004). Inhibition of norepinephrine reuptake enhances sympathetic nerve activity (Keller et al, 2004). The present results indicate that atomoxetine and reboxetine inhibit cardiac-type GIRK1/4 channels at clinically relevant heart concentrations. Altogether, an increase in heart rate during treatment with the drugs may be related to not only enhancement of sympathetic nerve activity and antagonism of the muscarinic receptor but also inhibition of atrial GIRK channels. Additionally, QT interval prolongation in two cases with atomoxetine overdose was reported (Barker et al, 2004; Sawant and Daviss, 2004). Recently, atomoxetine at micromolar concentrations was shown to inhibit cloned human ether-a-go-go-related gene (hERG) channels underlying rapidly activating delayed rectifier K+ currents using the Xenopus oocyte expression assay (Scherer et al, 2009). Inhibition of delayed rectifier K+ currents induces QT prolongation (Scherer et al, 2009), and QT prolongation after atomoxetine overdose may be related to inhibition of hERG channels but not GIRK channels among cardiac K+ channels. Furthermore, GIRK4 knockout mice are resistant to atrial fibrillation caused by vagal stimulation without showing any changes in atrioventricular nodal function and ventricular arrhythmias (Kovoor et al, 2001). Tertiapine, a selective GIRK blocker in the heart, terminates atrial fibrillation, the most common arrhythmia (Hashimoto et al, 2006). Atomoxetine and reboxetine may therefore have an advantage in treating psychiatric patients with comorbid atrial fibrillation.
Atomoxetine was shown to reduce cumulative heavy drinking days in the treatment of psychiatric patients with comorbid alcohol use disorders (Wilens et al, 2008). Interestingly, GIRK2 knockout mice show reduced ethanol-induced conditioned taste aversion and conditioned place preference and are less sensitive than wild-type mice to some of the acute effects of ethanol, including anxiolysis, habituated locomotor stimulation, and acute handling-induced convulsions (Hill et al, 2003). In the present study, atomoxetine inhibited ethanol-induced GIRK1/2 currents, suggesting that it may suppress some GIRK-related effects of ethanol. Furthermore, GIRK knockout mice also show reduced cocaine self-administration (Morgan et al, 2003) and attenuation of the morphine withdrawal syndrome (Cruz et al, 2008). In the nervous system, GIRK channels are activated by μ-opioid and CB1 cannabinoid receptors (North, 1989; Dascal, 1997; Kobayashi and Ikeda, 2006). Reboxetine and atomoxetine have also been shown to be useful in the treatment of cocaine dependence and marijuana users, respectively (Tirado et al, 2008; Szerman et al, 2005). Inhibition of GIRK channels by atomoxetine and reboxetine may have a role in the treatment of drug addiction.
Acknowledgments
We are grateful to Dr Kansaku Baba for his cooperation and Mr Kazuo Kobayashi (Niigata University) for his assistance. We also thank Dr Steven C Hebert and Dr Lily Y Jan for generously providing the Kir1.1 cDNA and Kir2.1 cDNA, respectively. This work was supported by research grants from the Ministry of Education, Science, Sports, and Culture of Japan and from the Ministry of Health, Labour, and Welfare of Japan.
The authors declare that over the past 3 years Kazutaka Ikeda has received research grants or expenses that are not related to this study from Fujifilm Corporation, the Mitsubishi Foundation, the Naito Foundation, and the Smoking Science Foundation, and a lecture fee from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin. The authors declare that, except for income received from their primary employer and the aforementioned disclosures, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahern TH, Javors MA, Eagles DA, Martillotti J, Mitchell HA, Liles LC, et al. The effects of chronic norepinephrine transporter inactivation on seizure susceptibility in mice. Neuropsychopharmacology. 2006;31:730–738. doi: 10.1038/sj.npp.1300847. [DOI] [PubMed] [Google Scholar]
- Barker MJ, Benitez JG, Ternullo S, Juhl GA. Acute oxcarbazepine and atomoxetine overdose with quetiapine. Vet Hum Toxicol. 2004;46:130–132. [PubMed] [Google Scholar]
- Belle DJ, Ernest CS, Sauer JM, Smith BP, Thomasson HR, Witcher JW. Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics. J Clin Pharmacol. 2002;42:1219–1227. doi: 10.1177/009127002762491307. [DOI] [PubMed] [Google Scholar]
- Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem. 2002;277:48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- Boot J, Cases M, Clark BP, Findlay J, Gallagher PT, Hayhurst L, et al. Discovery and structure-activity relationships of novel selective norepinephrine and dual serotonin/norepinephrine reuptake inhibitors. Bioorg Med Chem Lett. 2005;15:699–703. doi: 10.1016/j.bmcl.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, et al. Absence and rescue of morphine withdrawal in KIR/Kir3 knock-out mice. J Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology. 1994;114:559–565. doi: 10.1007/BF02244985. [DOI] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cell Signal. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Djamgoz MBA.1992Xenopus oocytes: endogenous electrophysiological characteristicsIn: Osborne NN (ed).Current Aspects of the NeurosciencesVol 4. Macmillan: London; 267–315. [Google Scholar]
- Garland M, Kirkpatrick P. Atomoxetine hydrochloride. Nat Rev Drug Discovery. 2004;3:385–386. doi: 10.1038/nrd1387. [DOI] [PubMed] [Google Scholar]
- Garside D, Ropero-Miller JD, Riemer EC. Postmortem tissue distribution of atomoxetine following fatal and nonfatal doses: three case reports. J Forensic Sci. 2006;51:179–182. doi: 10.1111/j.1556-4029.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1119–1127. doi: 10.1097/chi.0b013e3180ca8385. [DOI] [PubMed] [Google Scholar]
- Hajós M, Fleishaker JC, Filipiak-Reisner JK, Brown MT, Wong EHF. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev. 2004;10:23–44. doi: 10.1111/j.1527-3458.2004.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Yamashita T, Tsuruzoe N. Tertiapine, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol Res. 2006;54:136–141. doi: 10.1016/j.phrs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology. 2003;169:108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, et al. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Yoshii M, Sora I, Kobayashi T. Opioid receptor coupling to GIRK channels: in vitro studies using a Xenopus oocyte expression system and in vivo studies on weaver mutant mice. Methods Mol Med. 2003;84:53–64. doi: 10.1385/1-59259-379-8:53. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S, et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhe NG, Chillar AJ, Deshmukh YA. Reboxetine: a novel antidepressant. J Postgrad Med. 2003;49:373–375. [PubMed] [Google Scholar]
- Kanegawa N, Kiyono Y, Kimura H, Sugita T, Kajiyama S, Kawashima H, et al. Synthesis and evaluation of radioiodinated (S,S)-2-(α-(2-iodophenoxy)benzyl)morpholine for imaging brain norepinephrine transporter. Eur J Nucl Med Mol Imaging. 2006;33:639–647. doi: 10.1007/s00259-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dißmann E, Stuhmer W, Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NR, Diedrich A, Appalsamy M, Tuntrkool S, Lonce S, Finney C, et al. Norepinephrine transporter-deficient mice exhibit excessive tachycardia and elevated blood pressure with wakefulness and activity. Circulation. 2004;110:1191–1196. doi: 10.1161/01.CIR.0000141804.90845.E6. [DOI] [PubMed] [Google Scholar]
- Kiyono Y, Kanegawa N, Kawashima H, Kitamura Y, Iida Y, Saji H. Evaluation of radioiodinated (R)-N-methyl-3-(2-iodophenoxy)-3-phenylpropanamine as a ligand for brain norepinephrine transporter imaging. Nucl Med Biol. 2004;31:147–153. doi: 10.1016/j.nucmedbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kiyono Y, Sugita T, Ueda M, Kawashima H, Kanegawa N, Kuge Y, et al. Evaluation of radioiodinated (2S,αS)-2-(α-(2-iodophenoxy)benzyl)morpholine as a radioligand for imaging of norepinephrine transporter in the heart. Nucl Med Biol. 2008;35:213–218. doi: 10.1016/j.nucmedbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12:4513–4523. doi: 10.2174/138161206779010468. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun. 1995;208:1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, et al. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kumanishi T. Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K+ (GIRK) channels expressed in Xenopus oocytes. Br J Pharmacol. 2000;129:1716–1722. doi: 10.1038/sj.bjp.0703224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kumanishi T. Functional characterization of an endogenous Xenopus oocyte adenosine receptor. Br J Pharmacol. 2002;135:313–322. doi: 10.1038/sj.bjp.0704475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac) Br J Pharmacol. 2003;138:1119–1128. doi: 10.1038/sj.bjp.0705172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci. 2006;102:278–287. doi: 10.1254/jphs.fp0060708. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Henry DJ, Chavkin C. Agonist-induced desensitization of the mu opioid receptor-coupled potassium channel (GIRK1) J Biol Chem. 1995;270:589–595. doi: 10.1074/jbc.270.2.589. [DOI] [PubMed] [Google Scholar]
- Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, et al. Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol. 2001;37:2136–2143. doi: 10.1016/s0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993a;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993b;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2002;62:119–126. doi: 10.1124/mol.62.1.119. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, et al. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVecchio F, Kashani J. Isolated atomoxetine (STRATTERATM) ingestions commonly result in toxicity. J Emerg Med. 2006;31:267–268. doi: 10.1016/j.jemermed.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova A, Kotwal R, Welge JA, Nelson EB, Lake KA, et al. Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry. 2007;68:390–398. doi: 10.4088/jcp.v68n0306. [DOI] [PubMed] [Google Scholar]
- Montgomery SA. Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract. 2005;59:1435–1440. doi: 10.1111/j.1368-5031.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- North RA. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman D, Norlander B, Peterson C, Bengtsson F. Bioanalysis of racemic reboxetine and its desethylated metabolite in a therapeutic drug monitoring setting using solid phase extraction and HPLC. Ther Drug Monit. 2001;23:27–34. doi: 10.1097/00007691-200102000-00006. [DOI] [PubMed] [Google Scholar]
- Poggesi I, Pellizzoni C, Fleishaker JC. Pharmacokinetics of reboxetine in elderly patients with depressive disorders. Int J Clin Pharmacol Ther. 2000;38:254–259. doi: 10.5414/cpp38254. [DOI] [PubMed] [Google Scholar]
- Reimann F, Ashcroft FM. Inwardly rectifying potassium channels. Curr Opin Cell Biol. 1999;11:503–508. doi: 10.1016/S0955-0674(99)80073-8. [DOI] [PubMed] [Google Scholar]
- Sawant S, Daviss SR. Seizures and prolonged QTc with atomoxetine overdose. Am J Psychiatry. 2004;161:757. doi: 10.1176/appi.ajp.161.4.757. [DOI] [PubMed] [Google Scholar]
- Scherer D, Hassel D, Bloehs R, Zitron E, von Löwenstern K, Seyler C, et al. Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. Br J Pharmacol. 2009;156:226–236. doi: 10.1111/j.1476-5381.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D, Plosker GL. Atomoxetine a review of its use in adults with attention deficit hyperactivity disorder. Drugs. 2004;64:205–222. doi: 10.2165/00003495-200464020-00005. [DOI] [PubMed] [Google Scholar]
- Szerman N, Peris L, Mesías B, Colis P, Rosa J, Prieto A, et al. Reboxetine for the treatment of patients with cocaine dependence disorder. Hum Psychopharmacol Clin Exp. 2005;20:189–192. doi: 10.1002/hup.677. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kobayashi T, Ozaki M, Takamatsu Y, Ogai Y, Ohta M, et al. G protein-activated inwardly rectifying K+ channel inhibition and rescue of weaver mouse motor functions by antidepressants. Neurosci Res. 2006;54:104–111. doi: 10.1016/j.neures.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP. Atomoxetine for treatment of marijuana dependence: a report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94:254–257. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A, et al. Reboxetine, a selective norepinephrine reuptake inhibitor, is an effective and well-tolerated treatment for panic disorder. J Clin Psychiatry. 2002;63:31–37. doi: 10.4088/jcp.v63n0107. [DOI] [PubMed] [Google Scholar]
- Weber WM. Ion currents of Xenopus laevis oocytes: state of the art. Biochim Biophys Acta. 1999;1421:213–233. doi: 10.1016/s0005-2736(99)00135-2. [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Holdridge KC, Jin L, Edison T, Zhang S, Bangs ME, et al. Seizure risk in patients with attention-deficit-hyperactivity disorder treated with atomoxetine. Dev Med Child Neurol. 2007;49:498–502. doi: 10.1111/j.1469-8749.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Weiss MD, Michelson D, Ramsey JL, Moore RJ, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008;96:145–154. doi: 10.1016/j.drugalcdep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Witcher JW, Long A, Smith B, Sauer JM, Heilgenstein J, Wilens T, et al. Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2003;13:53–64. doi: 10.1089/104454603321666199. [DOI] [PubMed] [Google Scholar]
- Wong EHF, Sonders MS, Amara SG, Tinholt PM, Piercey MFP, Hoffmann WP, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.