Abstract
The current review systematically documents the role of γ-amino-butyric acid (GABA) in different aspects of fear memory—acquisition and consolidation, reconsolidation, and extinction, and attempts to resolve apparent contradictions in the data in order to identify the function of GABAA receptors in fear memory. First, numerous studies have shown that pre- and post-training administration of drugs that facilitate GABAergic transmission disrupt the initial formation of fear memories, indicating a role for GABAA receptors, possibly within the amygdala and hippocampus, in the acquisition and consolidation of fear memories. Similarly, recent evidence indicates that these drugs are also detrimental to the restorage of fear memories after their reactivation. This suggests a role for GABAA receptors in the reconsolidation of fear memories, although the precise neural circuits are yet to be identified. Finally, research regarding the role of GABA in extinction has shown that GABAergic transmission is also disruptive to the formation of newly acquired extinction memories. We argue that contradictions to these patterns are the result of variations in (a) the location of drug infusion, (b) the dosage of the drug and/or (c) the time point of drug administration. The question of whether these GABA-induced memory deficits reflect deficits in retrieval is discussed. Overall, the evidence implies that the processes mediating memory stability consequent to initial fear learning, memory reactivation, and extinction training are dependent on a common mechanism of reduced GABAergic neurotransmission.
Keywords: GABA, fear, memory, extinction, consolidation, reconsolidation
A substantial body of research over the past 30 years has been directed toward understanding the psychological processes involved in learned fear and identifying their neural mechanisms. Several lines of evidence have led to the view that this system is inhibited by excitation of γ-amino-butyric acid (GABA) receptors. For instance, systemic administration of drugs (eg, benzodiazepines, BZs) that facilitate excitation of these receptors alleviates symptoms of anxiety in people, and their infusion into the amygdala reduces learned fear responses in non-human animals.
The purpose of this review is to systematically examine the role of GABA in different aspects of fear memory—namely, acquisition and consolidation, reconsolidation, and extinction. The literature relating to these issues is extensive, and the findings have sometimes been contradictory. Consequently, a number of key questions have been raised regarding the function of GABA in memory storage. First, does GABA have a consistent role in these different aspects of fear memory? Specifically, do GABA agonists consistently impair memory across conditioning, reactivation, and extinction training paradigms (see Figure 1)? Second, is GABA selectively involved in the acquisition of fear memories, or is it also implicated in post-training memory consolidation? Finally, is GABA implicated in memory retrieval or expression processes? This review attempts to answer these questions by providing a systematic analysis of previous work in order to locate trends and to provide possible explanations for unusual and ambiguous results. It is our primary view that the processes mediating memory persistence after initial fear learning, reactivation, and extinction are dependent on a common mechanism of reduced GABAergic inhibitory neurotransmission.
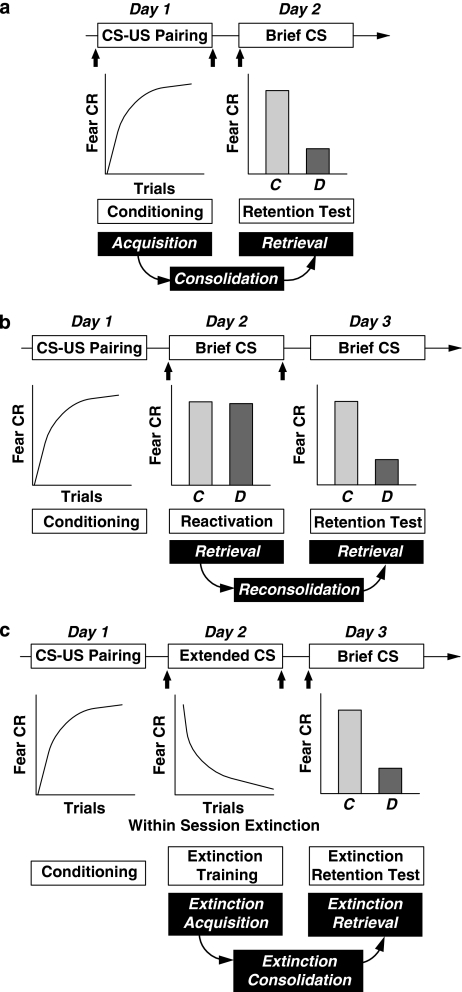
Figure 1.
Schematics for the phenomena of acquisition (a), reconsolidation (b) and extinction (c). The first line in each schematic is procedural, with the arrows indicating usual points of drug administration in different published studies. The second line indicates what happens behaviorally, in particular the extent of fear responding by the control, C, and animals receiving the disruptive agent, D. In this case, the disruptive agent is the GABAA receptor agonist. The third line is the common paradigm nomenclature for that part of the procedure. The fourth line indicates the key assumed memory processes important to that particular phenomenon; preceding processes are assumed (eg, for reconsolidation and extinction, the initial acquisition process is assumed; for extinction, it may be that some reconsolidation occurs during the early phase of extinction training). It should be noted that the process of ‘retrieval' indicates access to the memory representation; additional processes that may then interfere with or facilitate CR expression are not explicitly indicated (nor are basic sensory processes that are assumed to be operative during acquisition). CR=conditioned response; CS=conditioned stimulus; US=unconditioned stimulus; brief CS=a limited number or duration of CS presentations; extended CS=many or long duration presentation of CS.
In the field of memory, the terminology for procedures and assumed processes and concepts are often confused (Roediger et al, 2007). On the basis of previous research and theory on acquisition, reconsolidation, and extinction phenomena, Figure 1 provides a schematic outline of the procedural aspects of conditioned stimulus (CS) presentation and typical drug administration timing, expected conditioned response (CR) patterns, paradigmatic terminology, and assumed key memory processes. In this review, we will first give a brief summary of the effects of GABA on acquisition, reconsolidation, and extinction, followed by an outline of the pharmacology of GABAA receptors. This will be followed by a detailed consideration of the role of GABAA receptors in each of these three phenomena. For historical reasons, most research has been conducted on acquisition, followed by extinction, and finally reconsolidation, and so this is the order in which the phenomena are discussed. For the extinction and reconsolidation sections, the relevant studies are summarized in Tables; however, for the acquisition and consolidation section, this was not possible given the vast number of studies. In each section, only seminal studies are described to illustrate particular points.
OUTLINE
What has been repeatedly found in previous research is that GABA is disruptive to the acquisition, reconsolidation, and extinction of fear memories. Although there are some contradictory findings, upon close examination, it is evident that the inconsistencies could be due to variations in procedural factors, such as the specific type of drug used, the dosage, the brain region and time point of drug infusion, or the type of memory paradigm that was used. This will become clearer in our detailed discussion of these memory phenomena in the subsequent sections.
When GABAA receptors are administered before fear conditioning, memory at test is typically disrupted. These memory impairments have been attributed to a disruption in the initial acquisition of the fear memory. Alternative accounts have been proposed, such as state-dependent learning, or the formation of a context-specific inhibitory association. Evidence for these alternative accounts is either minimal or mixed. Moreover, it has been shown that GABAA receptor antagonists administered before fear conditioning facilitate subsequent fear memory, further indicating that GABAergic transmission is disruptive to the acquisition of fear memories.
Numerous studies have also shown that post-training administration of GABAA receptor agonists disrupt, whereas GABAA receptor antagonists facilitate subsequent fear memory retention, indicating that GABAergic transmission is also detrimental to the consolidation of fear memories. Mixed findings have emerged, particularly with respect to the type of ligands used. Specifically, BZs (a type of GABAA receptor agonist) do not always disrupt retention when administered post-training. However, we propose that these null effects might be due to the specific site of drug infusion—that is, BZs are capable of disrupting retention if administered centrally, as opposed to systemically.
The disruptive effects of GABA have also been demonstrated when GABAA receptor agonists are administered after a brief re-exposure to the CS (ie, reactivation). Similarly, post-reactivation administration of GABAA antagonists have been shown to facilitate memory retention. These studies suggest that GABAergic transmission is also detrimental to the reconsolidation of fear memories after retrieval. Some studies have either observed no effects or temporary memory deficits; however, these findings could be attributable to the specific region of drug infusion, the type of drug, and the duration of CS re-exposure.
There has been a wealth of research indicating that GABAA receptor agonists administered after extinction training disrupt extinction memory. Specifically, animals continue to display fear responding, indicating that GABA is disruptive to the acquisition and/or consolidation of extinction memories. Some studies have observed results inconsistent with this account, although we argue that these contradictory findings are attributable to the precise brain region of drug infusion, and the dosage of the drug. In terms of post-extinction-training drug administration, GABAA receptor agonists disrupt, whereas GABAA receptor antagonists facilitate extinction retention, indicating that GABAergic transmission also impedes the consolidation of extinction memories. There is some evidence that GABA can actually facilitate extinction; that is, animals show reduced fear responding at test. However, we propose that this is limited to pre-test administration of GABAA receptor agonists. This facilitating effect could be because the agonist is inhibiting fear responding, or because it is reducing anxiety.
PHARMACOLOGY OF GABAA RECEPTORS
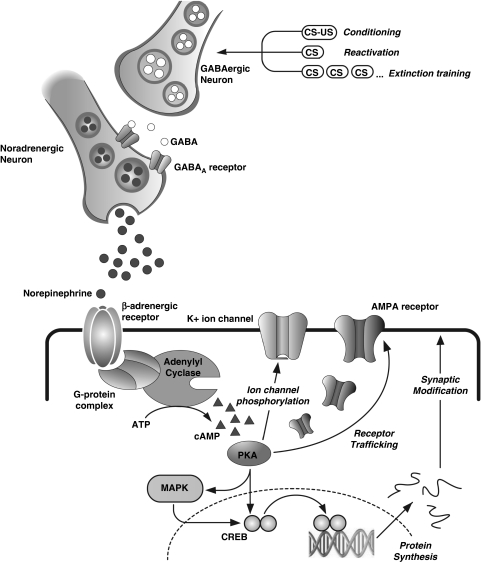
GABA is the main inhibitory neurotransmitter in the mammalian central nervous system (CNS) (Brioni et al, 1989; Castellano et al, 1989). It produces its actions by binding to either the GABAA or GABAB receptor, with GABAA receptors being more abundant within the brain (Pirker et al, 2000). GABAA receptors operate as gated chloride ion channels (see Figure 2). Binding of GABA to this receptor triggers opening of the channel, causing influx of negatively charged chloride ions into the neuron, leading to reduced excitatory neurotransmission. The mammalian GABAA receptor is comprised of seven classes of subunits, each having multiple variants (α1–α6, β1–β3, γ1–γ3, ρ1–ρ3, δ, ɛ, θ) (Pirker et al, 2000; Rudolph et al, 2001). Most functional GABAA receptors are made up of two α-subunits, two β-subunits, and one γ-subunit or alternatively two α-subunits, one β-subunit, and two γ-subunits, which together comprise the central ion channel (see Figure 2; Esmaeili et al, 2009; Haefely, 1989; Mehta and Ticku, 1999; Pirker et al, 2000; Savic et al, 2005). Specifically, the most predominant GABAA receptors in the mammalian CNS have an α1β2γ2 combination, as first demonstrated by Fritschy et al (1992).
Figure 2.
Hypothetical schematic model of the GABAA receptor channel, made of two α-, two β- and a single γ-subunit (ie, a 2 : 2 : 1 stoichiometry). Also displayed are the common GABAA receptor ligands described in this review, and their respective binding sites if known. Note that the GABA binding site is located at the junction between the alpha and beta subunits. Agonists such as muscimol, and antagonists such as bicuculline bind to this site. Neurosteroids may also bind to interfacial residues between the α- and β-subunits. Neurosteroids may additionally exert modulatory effects at α-subunit transmembrane domains. The benzodiazepine binding site is located at the interface of the α- and γ- subunits. Antagonists such as flumazenil and inverse agonists such as FG7142 also bind to this site. The precise binding site of barbiturates has not been identified. Non-competitive antagonists such as picrotoxin bind to distinct non-competitive sites located at the chloride ion channel.
There are several different ligands that bind to GABAA receptors, many of which have distinct binding sites. Included in the GABAA receptor agonists are full agonists such as GABA and muscimol, which bind to and activate the GABAA receptor complex at the GABA binding site, located at the interface of α- and β-subunits (Mehta and Ticku, 1999; see Figure 2, Table 1). The consequence is an opening of chloride channels, leading to an influx of chloride ions and increased neuronal inhibition (Johnston, 1996). Other GABAA receptor agonists include BZs (eg, midazolam) that bind to a separate binding site localized at the interface of the γ- and α-subunits (Mehta and Ticku, 1999). Barbiturates (eg, pentobarbital) and neurosteroids (eg, allopregnanolone) are other types of agonists that bind to the GABAA complex at a distinct site from both GABA and BZs (Amin and Weiss, 1993; Mehta and Ticku, 1999). Both compounds potentiate GABAergic responses at low concentrations, but may activate the receptor directly at higher doses (Mehta and Ticku, 1999). GABAA receptor partial agonists such as 5-(4-piperidyl)isoxazol-3-ol show similar effects to the full agonists, but have a reduced efficacy of binding to and activating the GABAA receptor complex (Johnston, 1996).
Table 1. Various Forms of GABAA Receptor Ligands, their Pharmacological Action and Physiological Effects.
| Family of compounds | Subtype | Pharmacological action | Physiological effects | Examples of ligands |
|---|---|---|---|---|
| GABAA receptor agonists | Full agonists | Full agonists bind to and activate the GABAA receptor complex at the GABA binding site located at the interface of α- and β-subunits (see Figure 2), enhancing inhibitory synaptic transmission by causing chloride ion channels to open. The consequence is typically an inflow of chloride ions to the neuron leading to hyperpolarization (Johnston, 1996). | Undersupply is linked to seizures, tremors, and insomnia (Johnston, 1996). Drugs that increase GABA have anxiolytic, anti-convulsant, and relaxant properties. | GABA, muscimol |
| Benzodiazepines | Benzodiazepines bind to a site distinct from GABA, which is localized at the interface of the γ- and α-subunits. Benzodiazepines are allosteric modulators of the GABAA receptor, meaning that when bound to the receptor, they facilitate GABA transmission. This is achieved in two ways: first, by increasing the ability of GABA to bind and activate the receptor and second, by increasing the likelihood of chloride channel opening in response to GABA binding (Haefely, 1989; Johnston, 1996). | Decreased anxiety, muscle tension and vigilance, increased relaxation, and anti-convulsant effects (Haefely, 1989; Johnston, 1996). | Midazolam Diazepam Flurazepam Clonazepam Chlorodiazepoxide | |
| Barbiturates | At low concentrations, barbiturates facilitate GABAergic inhibitory transmission, and at higher concentrations, activate the receptor directly suggesting two binding sites, which are distinct from GABA, benzodiazepines, and other modulators such as neurosteroids (see Figure 2; Mehta and Ticku, 1999). The precise binding sites have not yet been identified, although the presence of the α-subunit appears to influence the ability of barbiturates to potentiate GABAA receptors. Barbituates may also facilitate inhibitory transmission by blocking AMPA receptors, which prevents the binding of the excitatory neurotransmitter, glutamate, to this receptor (Amin and Weiss, 1993; Johnston, 1996; Mehta and Ticku, 1999; Taverna et al, 1994). | Mild sedation and anesthesia (at low doses; Johnston, 1996). | Pentobarbital | |
| Neurosteroids | Neurosteroids modulate GABAA receptor activity at a binding site distinct from GABA, BZs, and barbiturates (Mehta and Ticku, 1999; Lan and Gee, 1994). At low concentrations, they potentiate GABA currents, and at higher concentrations they activate the receptor directly, indicating the presence of two distinct binding sites (Hosie et al, 2006). Neurosteroids may potentiate GABAergic responses by binding to a site located at the cavity of α-subunit transmembrane domains. Direct receptor activation may take place at interfacial residues between α- and β-subunits (Hosie et al, 2006). This direct activation may be strengthened by neurosteroid occupation of both binding sites. | Anxiolyitc, sedative, and anti-convulsant effects (Lan and Gee, 1994). | Allopregnanolone | |
| GABAA receptor partial agonists | Partial agonists | Partial agonists have similar effects to full agonists; however, they have a reduced efficacy of binding to and activating the GABAA receptor complex (Johnston, 1996). | Comparable effects to full agonists. | 4-PIOL THIP |
| GABAA receptor antagonists | Competitive GABAA receptor antagonists | Competitive antagonists bind to the GABAA receptor complex, particularly acting at GABA recognition sites. They are competitive because they occupy the GABA binding site, preventing GABA from binding to and activating the receptor. Thus, they block the inhibitory effects of GABA (Johnston, 1996). | These agents have convulsant properties. | Bicuculline |
| Non-competitive GABAA receptor antagonists | Non-competitive GABAA receptor antagonists antagonize the inhibitory effects of GABA, but this is not achieved by inhibiting the binding of GABAA agonists and benzodiazepines to the GABAA receptor. Instead, they bind to sites located at the chloride ion channel of GABAA receptors (that is, the picrotoxin binding site), possibly causing the chloride ion channel to close. This effectively prevents the movement of chloride ions into the channel, leading to hyperpolarization and reduced inhibitory transmission (Johnston, 1996). | Produces effects opposite to benzodiazepines and barbiturates, and at high doses can cause convulsions (Carlson, 2004). | Picrotoxin Lindane | |
| Benzodiazepine site antagonists | Antagonists bind to the benzodiazepine site but they do not activate the receptor, but instead block the access of agonists and inverse agonists to this site. Consequently, they may block or reverse the effects of benzodiazepines such as midazolam or FG7142. However, the binding of GABA to the GABAA receptor site is unchanged (Da Cunha et al, 1999; Haefely, 1989). | Counteract the physiological effects of benzodiazepine receptor agonists and inverse agonists (Haefely, 1989). | Flumazenil | |
| Inverse agonists | Full inverse antagonists | Inverse agonists bind to the benzodiazepine site; however their effects are pharmacologically opposite to BZs. That is, they reduce inhibitory GABA transmission by decreasing chloride channel opening, and reducing the affinity for GABA to bind to and activate GABAA receptors (Haefely, 1989; Harris and Westbrook, 1998a). | Physiological effects include increased anxiety, convulsions, and spasms (Haefely, 1989). | DMCM |
| Partial Inverse Agonists | Similar to full inverse agonists, however, they have a reduced efficacy of binding to and activating the BZ site (Haefely, 1989; Harris and Westbrook, 1998a). | Anxiogenic effects at low doses and mild convulsions (File and Pellow, 1988). | FG7142 β-CCM |
Abbreviations: AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; BZ, Benzodiazepine; DMCM, methyl-6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate; GABA, γ-amino butyric acid; THIP, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol; β-CCM, methyl-β-carboline-3-carboxylate; 4-PIOL, 5-(4-piperidyl) isoxazol-3-ol.
Another family of compounds associated with GABAA receptors are the GABAA receptor antagonists. Competitive antagonists such as bicuculline occupy the GABA-binding site, preventing GABA from binding to and activating the receptor. It should be noted that such antagonists may have behavioral effects where there is tonic GABAergic inhibition. Non-competitive antagonists such as picrotoxin antagonize the inhibitory effects of GABA by binding to distinct picrotoxin-binding sites located at the chloride ion channel of GABAA receptors, possibly causing the chloride ion channel to close. This action blocks the movement of chloride ions into the channel, which prevents hyperpolarization and consequently reduces inhibitory transmission (Johnston, 1996). Antagonists such as flumazenil bind to the BZ site blocking the access of agonists and inverse agonists to this binding site. However, these compounds do not prevent the binding of GABA (and other direct agonists or antagonists) to the GABA-binding site. The last family of GABAA receptor ligands are the inverse agonists. Full inverse agonists such as DMCM bind to the BZ site but reduce inhibitory GABA transmission by decreasing the chloride channel opening and reducing the affinity for GABA to bind to and activate GABAA receptors (Johnston, 1996). Partial inverse agonists such as FG7142 are similar to the inverse agonists but have a reduced efficacy of binding to and inducing a functional change in the receptor (Harris and Westbrook, 1998a). For more information on the various forms of GABAA receptor ligands, including the pharmacological action and physiological effects, see Table 1.
GABA and the Acquisition and Consolidation of Fear Memories
In Pavlovian fear conditioning, a neutral CS is paired with an aversive unconditioned stimulus (US), such as a footshock. According to Shumyatsky et al (2002), the CS can either be unimodal or multimodal. A unimodal CS refers to a discrete cue affecting a single sensory modality such as a tone, a light, or an odor. Alternatively, a multimodal cue can affect multiple sensory modalities and is composed of a configuration of cues, such as a context. After acquiring the association between the CS and US, the animal typically demonstrates conditioned fear responses (CR), such as freezing, to the previously neutral CS.
The next sections will discuss the role of GABA in the acquisition and consolidation of newly acquired fear memories; processes that are assumed to occur in the conditioning paradigm (see Figure 1). Acquisition refers to the processes mediating the initial learning of the CS–US association within the actual conditioning session (formation of the short-term memory representation). In contrast, consolidation refers to the progressive, time-dependent stabilization process that transforms fragile short-term memories into relatively permanent or persistent long-term memories (Abel and Lattal, 2001; Myers and Davis, 2007). GABA administered before conditioning will most likely affect the initial acquisition of learned fear, although consolidation processes may also be affected. In contrast, GABAergic drugs applied after conditioning should exclusively affect consolidation.
Pre-Training Manipulations
Pre-training administration of GABAergic drugs usually leads to decreased CR during the retention test (see Figure 1). This is a robust finding in the literature, and suggests that GABAergic transmission usually inhibits initial acquisition of fear memories, and possibly their consolidation as well. This raises the question of the adaptive value of GABAergic memory disruption. The answer may be that it is maladaptive to remember everything that happens to us (eg, it may be maladaptive to remember every detail of the different environments we encounter everyday). GABA allows us to adaptively forget (Kim et al, 2006).
The study of pre-training GABA manipulations has primarily focused on drugs that modulate BZ action. Pre-training administration of BZs has been shown to disrupt retention of inhibitory avoidance (IA; Dickinson-Anson and McGaugh, 1997; Jensen et al, 1979; Pain et al, 2002) as well as contextual fear memory (Harris and Westbrook, 1998b). Furthermore, Dickinson-Anson and McGaugh (1993) demonstrated that pre-training infusions of MDZ into the amygdala disrupted retention of IA, thus suggesting that BZs modulate memory processes through their effects within the amygdala. Such amnesic effects are reversed after co-administration of the BZ site antagonist, flumazenil (Izquierdo et al, 1990a), indicating that the amnesic effects of BZs are specifically mediated by their binding to the BZ site. Consistent with these findings, pre-training injection of the BZ site inverse agonist β-CCM facilitated memory retention in a variety of tasks, such as passive avoidance, contextual memory, and imprinting (see Venault et al, 1986). Collectively, these studies show that increasing GABA transmission impairs, whereas decreasing GABA transmission facilitates fear memory acquisition.
Harris and Westbrook (1998a, 1999, 2001) argued that administration of a BZ (ie, MDZ) before conditioning does not prevent rats from learning the CS–US association, but rather it regulates where and when that learning is subsequently expressed. Specifically, they showed that pre-training administration of MDZ decreased freezing when rats were tested 24 h later, suggesting that MDZ interfered with acquisition of auditory fear conditioning. However, the MDZ-induced deficit in freezing was no longer evident if rats were tested 22 days after conditioning, if they were tested in a chamber that differed from fear conditioning, or if they were injected with the pain-inducing agent formalin before testing. That is, MDZ-treated rats showed comparable levels of freezing to controls.
Harris and Westbrook (1999, 2001) argued that animals conditioned under MDZ are still able to acquire and consolidate the excitatory fear memory (ie, the CS–US association), but at the same time, MDZ causes animals to acquire a context-specific inhibitory (CS-no US) association (Harris and Westbrook, 1998a, 1999). This inhibitory association inhibits the expression of the fear memory, but only within the conditioning context. Here, conditioning context refers to both the physical and internal cues (emotional, hormonal, and neurochemical) that are present during conditioning (Bouton, 1993). Consequently, if the context is changed before testing, animals will not be able to retrieve the inhibitory association. Instead, the excitatory fear memory will be retrieved, causing restoration of fear responding. Essentially, Harris and Westbrook's (1998a, 1999) findings suggest that BZ administration before conditioning does not disrupt acquisition but instead elicits a context-specific retrieval deficit. One possibility is that MDZ strengthens a latent capacity of the conditioning context to inhibit expression of the CS–US association learned during training. Changing the context reduces this inhibition, causing a reinstatement of fear responding. Thus, it is possible that the CR deficits after pre-training administration of GABAA receptor agonists, observed in previous studies, might simply be attributable to a retrieval deficit. Farkash and Cranney (2010) found no contextually conditioned fear recovery in MDZ rats 11 days after initial conditioning; however, until other studies have tested conditioned responding under context shift conditions, we can only tentatively conclude that pre-training BZs interfere with the acquisition/consolidation (rather than retrieval) of newly acquired fear memories.
Post-Training Manipulations
Numerous studies have shown that post-training GABA manipulations lead to reduced fear responding at test, indicating a role for GABAA receptors in memory consolidation. For example, post-training administration of the GABA agonist muscimol disrupts memory for IA whether given systemically or locally into the amygdala, hippocampus, or entorhinal cortex (Ammassari-Teule et al, 1991; Brioni et al, 1989; Carbo Tano et al, 2009; Introini-Collison et al, 1994). Rossato et al (2004) examined the time course of GABA involvement in memory, and showed first, that IA was disrupted when muscimol was infused immediately post-training into the CA1 region of the hippocampus and within the basolateral amygdala (BLA). Second, muscimol produced amnesia when administered into the entorhinal cortex between 30 and 180 min post-training. Finally, when infused into the posterior parietal cortex, muscimol was amnesic if administered between 90 and 180 min post-training. The evidence suggests that memory consolidation requires reducing GABAergic inhibitory transmission in a variety of brain regions, at varying times after the conditioning session, thus ensuring sufficient activation of glutamate receptors to initiate memory consolidation (Rossato et al, 2004).
Post-training administration of GABAergic antagonists appears to facilitate memory consolidation. For example, bicuculline leads to increased fear responding in contextual fear conditioning with crabs (Carbo Tano et al, 2009) and with IA if administered systemically or directly into the hippocampus, entorhinal, or parietal cortex (Introini-Collison et al, 1994; Luft et al, 2004). Castellano and McGaugh (1990) demonstrated that the effects of both muscimol and bicuculline were not due to state dependency, as pre-testing injections did not reverse the effects of these drugs on memory retention. Similarly, using IA, Dickinson-Anson and McGaugh (1993) demonstrated that post-training intra-BLA infusions of bicuculline blocked the retention-impairing effects of systemically administered diazepam. This result indicates that the amnesic effects of diazepam are mediated by GABAA receptors, and that these receptors are involved in the consolidation of conditioned fear memories.
Overall, these results suggest that activation of GABAA receptors immediately after conditioning is detrimental to the storage of fear memories. However, mixed findings have been observed using post-training administration of BZs. A number of studies have shown that although pre-training BZs disrupt fear memory retention, immediate post-training infusions are ineffective (eg, Bustos et al, 2006), thus leading some researchers to conclude that BZs specifically impair the acquisition, but not the consolidation of fear memories (Pereira et al, 1989). The difference between the effects of muscimol and BZs could be due to the pharmacological differences between these two classes of drugs. Specifically, muscimol, unlike BZs, is also a partial agonist at GABAC receptors (Woodward et al, 1993). Nevertheless, several studies have shown that post-training BZs do reduce conditioned responding, indicating modulation of memory consolidation (Gafford et al, 2005; Jensen et al, 1979). Moreover, systemic (Izquierdo et al, 1990b) or intra-BLA (Da Cunha et al, 1999) infusion of the BZ site antagonist flumazenil facilitates retention of IA. Thus, it appears that BZs are capable of modulating fear memory consolidation processes, and this might take place within the amygdala and hippocampus.
These mixed findings with BZs may be due to (a) the behavioral paradigm, (b) the specific kind of BZ used across studies, or (c) the specific location of drug infusion. The behavioral paradigm is an unlikely reason as most of the studies previously mentioned have used IA or contextual fear conditioning, and observed both null effects and memory deficits after BZ administration. The specific kind of BZ may be a valid reason for the inconsistencies, although a variety of BZs have been shown to modulate fear memory when administered post-training (eg, flumazenil, Da Cunha et al, 1999; MDZ, Gafford et al, 2005; flurazepam, Jensen et al, 1979). This leaves the location of drug infusion as the remaining candidate for the contradictory findings. All the studies that have observed null effects after post-training BZs had injected the drug systemically. In contrast, nearly all the studies that have shown BZ-induced memory impairments or enhancements used centralized infusions of BZs into the amygdala (Da Cunha et al, 1999; Izquierdo et al, 1990a) or hippocampus (Gafford et al, 2005). The reason why centralized infusions of BZs after training are more effective in producing memory deficits than are systemic injections is not clear. It could be that the involvement of BZ receptors (in the BLA or hippocampus) in memory consolidation is transient. Consequently, centralized infusions of BZs directly into these brain regions will be fast enough to affect consolidation (Izquierdo et al, 1990a). In contrast, systemic treatments may not reach the brain soon enough to affect this transient, BZ-sensitive period of memory consolidation. Alternatively, it may be that activation of the BZ site in some areas of the brain interferes with mnemonic processes, whereas BZ site activation in other parts of the brain opposes these effects (see Zavitsanou et al, 1999, regarding a similar situation for dopaminergic effects).
Regardless of the precise mechanism, these studies collectively demonstrate that GABAergic drugs in general, when administered post, can modulate memory retention. This strongly suggests that GABAA receptors are involved in the consolidation of newly acquired fear memories. However, there are two caveats. First, the effect of GABAergic drugs may vary depending on the precise location of drug infusion. As discussed above, post-training BZs may be more effective at disrupting retention if administered centrally as opposed to systemtically. In addition, GABAA receptor agonists may actually facilitate memory if infused in brain regions such as the prelimbic cortex (PL) (discussed in detail in subsequent sections). Second, given the findings of Harris and Westbrook (1998b, 1999, 2001) previously discussed, it could be proposed that the memory impairments caused by post-training GABA are context-specific retrieval deficits. However, this is unlikely to be the case, because the drug is administered post-acquisition. In conclusion, there is convincing evidence that GABAA receptors are involved in consolidation processes.
Pre-Testing Manipulations
Some researchers have suggested that the memory impairments produced by GABAA receptor agonists are due to state-dependent learning (eg, Nakagawa et al, 1993; Patel et al, 1979). That is, animals fail to show evidence of retention at test because the internal state during retrieval (ie, drug absent) does not match the internal state during initial training or storage (ie, drug present; Castellano and McGaugh, 1990). This implies that administering the drug before testing should reinstate the memory deficit, because the internal state should now correspond to that during initial memory formation. Essentially, proponents of the state-dependent learning perspective argue that GABA-mediated memory deficits are due to retrieval, rather than a storage failure.
Indeed, some evidence suggests that the retention-impairing effects of pre-training GABAA receptor agonists are due to state dependency. For example, pre-training administration of drugs such as chlordiazepoxide (CDP) (Goldberg et al, 1973; Henriksson and Jarbe, 1971; Oishi et al, 1972; Patel et al, 1979; Furukawa et al, 1987), diazepam (Nakagawa et al, 1993), muscimol (Nakagawa et al, 1993), and halazepam (Patel et al, 1979) disrupt retention in both passive and active avoidance memory paradigms. However, injection of the same drugs before testing produces a reinstatement of avoidance responding in the drug-treated animals, although a similar result was not obtained by Farkash and Cranney (2010) when MDZ was injected before testing. Nonetheless, these findings indicate that in some instances, BZ-induced memory deficits are due to state dependency.
Nevertheless, research by Castellano and McGaugh (1989, 1990) suggests that state dependency cannot completely explain the memory-modulating effects of GABAA receptor agonists and antagonists. Specifically, they found that post-training administration of muscimol disrupted, whereas bicuculline and picrotoxin facilitated, retention of IA when animals were tested 24 h later. Importantly, injection of the same drugs before testing did not reverse these effects.
These studies suggest that pre-training administration of GABAA receptor agonists might disrupt retrieval by producing some form of state dependency. In light of these findings, it is possible that the GABA-induced memory deficits discussed in the previous sections may represent retrieval rather than storage failure. In other words, injecting the same drug before testing might have alleviated the retention impairments observed in previous studies. At present, a state-dependent learning account cannot be completely ruled out as an explanation for retention deficits induced by pre-training administration of GABAA receptor agonists. However, if the drugs are injected post-training, we can be more confident that these drugs are specifically affecting post-training memory storage processes (Castellano and McGaugh, 1990). Future studies should include additional tests examining pre-test drug infusions to determine if the memory-modulation produced by GABAergic drugs is due to state-dependency or memory storage processes.
Molecular Manipulations
Heldt and Ressler (2007) attempted to elucidate the role of GABA in fear conditioning. They examined the changes in mRNA levels of GABA-related genes after the acquisition of Pavlovian fear—in particular, they examined changes in mRNA levels of the six GABAA receptor subunits (α1, α2, α3, α5, β1, and γ2), and various GABA-related proteins, such as GABA-transporter 1 (GAT1), GABAA receptor-associated protein (GABARAP), and GABAA clustering protein gephyrin, within the amygdala. They observed that following paired tone-shock exposures, there were significant decreases in the α1, α5, and GAD67 mRNA levels, indicating a reduced number of functional BZ receptors immediately after fear conditioning. Consistent with this, they observed that there was decreased binding of [3H]-flunitrazepam (a BZ) within the amygdala, showing that after fear conditioning there is a reduced quantity of functional BZ receptors. Fewer functioning BZ receptors would reduce GABAergic inhibitory transmission and consequently enhance excitatory neurotransmission within the amygdala. In a similar study, Chhatwal et al (2005) observed that after acquisition of auditory fear conditioning, there was a reduction in mRNA levels of the gephyrin protein in BLA slices. This protein is important for the promotion and stabilization of GABAA clusters, and is thus important for GABAA receptor function. Furthermore, they observed a significant reduction in BZ binding, indicating a reduction in BZ-sensitive GABAA receptors after paired presentations of a CS and US.
In light of these findings, it can be speculated that memory consolidation after initial training requires a downregulation of GABAA receptor binding and inhibitory neurotransmission (Chhatwal et al, 2005; Heldt and Ressler, 2007). According to Chhatwal et al (2005), reduced GABA transmission would disinhibit BLA glutamatergic neurons leading to greater excitatory transmission. This increase in excitation would support the development of amygdala long-term potentiation (LTP), which is argued to be an important mechanism in the formation of fear memories (Maren and Quirk, 2004). Consistent with this evidence, research has shown that GABAergic drugs, such as midazolam, CL218,872, or diazepam, can inhibit LTP in hippocampal slices (Evans and Viola-McCabe, 1996; del Cerro et al, 1992; McNamara et al, 1995). This molecular evidence strongly implicates a negative role for GABAA receptor functioning within the amygdala in the cellular storage of fear memories.
Neural Bases
Amygdala
The acquisition and consolidation of fear memories appears to be critically dependent on the amygdala. Extensive evidence implicates the amygdala in the acquisition of fear conditioning. For example, LA neurons exhibit robust increases in activity (ie, neural plasticity) in response to the CS throughout conditioning (Maren and Quirk, 2004), and pre-training inactivation of the amygdala using muscimol leads to a disruption in long-term fear memory (Muller et al, 1997). Evidence that the amygdala is critical in consolidating fear memories comes from studies using post-training drug infusions and showing decreased fear responding at test (see Figure 1; eg, anisomycin, Cammarota et al, 2004).
Studies have shown that post-training intra-amygdala infusions of GABAA receptor agonists disrupt, whereas antagonists facilitate the retention of fear memories (Brioni et al, 1989; Rossato et al, 2004). Lesions to the amygdala have been shown to block the memory-modulating effects of post-training administrations of muscimol and bicuculline (Ammassari-Teule et al, 1991). In terms of ligands specifically targeting the BZ site, pre-training intra-amygdala infusions of MDZ have been shown to disrupt retention of contextual fear conditioning (Dickinson-Anson and McGaugh, 1993; Harris and Westbrook, 1998b). Moreover, the disruptive effect of pre-training systemic administration of MDZ is blocked by post-training administration of bicuculline into the amygdala (Dickinson-Anson and McGaugh, 1997). Conversely, post-training intra-BLA infusion of the BZ site antagonist flumazenil facilitates retention of IA (Da Cunha et al, 1999; Izquierdo et al, 1990a). Interestingly, infusion of BZ site agonists and antagonists within the central nucleus of the amygdala (CeA) often has no effect on memory retention (eg, Da Cunha et al, 1999; de Souza Silva and Tomaz, 1995). These findings indicate that the amygdala, particularly the BLA, mediates the disruptive effects of GABA on the initial acquisition and consolidation of fear memories (see Carrive, 2000, regarding the possibility that CeA has a tonic inhibitory effect on downstream fear–response centers, and that fear-initiated BLA interaction with the CeA leads to the disinhibition of those structures and thus fear responding).
As described earlier, functioning GABAA receptors are composed of multiple subunits—at least one α-, one β-, and one γ-subunit. Pirker et al (2000) examined the distribution of GABAA receptor subunits in the amygdala. They found prominent labeling of the α1, α2, β1–β3, and γ2 subunits in regions such as the basomedial, basolateral, and lateral amygdala (LA), areas which are critically involved in fear learning (Pirker et al, 2000). Interestingly, recent research has suggested that different subunits are involved in different pharmacological actions in response to GABAergic drugs. Specifically, the α1-subunit has been shown to be specifically responsible for the amnesic effects of BZs (Rudolph et al, 2001). For example, Rudolph et al (1999) examined the effect of pre-training administrations of diazepam on retention of passive avoidance in mice with a point mutation of the α1-subunit (α1-HR mice) and wild-type (WT) controls. Diazepam disrupted memory retention in the WT controls as expected, however, memory retention was intact in the α1-HR mice. Moreover, Savic et al (2005) demonstrated that the α1-selective BZ receptor agonist, zolpidem, disrupted retention of passive avoidance when administered before training. These results suggest that GABAergic drugs disrupt memory consolidation in part by binding to α1-subunit-containing GABAA receptors in the BLA/LA.
Hippocampus
Another important neural circuit involved in fear learning is the hippocampus, particularly when the CS is a context, such as in contextual fear conditioning and IA. Contextual fear conditioning and retention require two processes: (1) the formation of a contextual representation and (2) the contextual representation being associated with shock (von Hertzen and Giese, 2005). It is believed that the hippocampus mediates process (1), whereas the BLA mediates process (2). Consistent with this proposal, studies have shown that the hippocampus is involved in the acquisition and consolidation of contextual fear memories (Anagnostaras et al, 1999; Bast et al, 2001; Kim and Fanselow, 1992; Frankland et al, 2006; Rossato et al, 2004).
In line with these findings, hippocampal GABAA receptors have an important role in modulating fear memory storage. For example, post-training intra-hippocampal infusion of muscimol disrupts, whereas bicuculline facilitates, the retention of IA (Luft et al, 2004; Rossato et al, 2004). More recently, Gafford et al (2005) demonstrated that infusions of MDZ into the hippocampus after training disrupted the retention of contextual fear memory.
Studies examining the composition of GABAA receptor subunits in the hippocampus have shown abundant staining for subunits α1, α2, α4, α5, β1-3, and γ2 (Pirker et al, 2000). Recent evidence has shown that the α5-subunit is critically involved in mediating hippocampal-dependent memory and cognitive tasks (Rudolph and Mohler, 2006). Specifically, mice that possess a partial deficit in α5-containing GABAA receptors in the hippocampus show a marked improvement in trace fear conditioning, which is a hippocampally mediated task. In contrast, they show no improvement in delay fear conditioning, a task which is independent of the hippocampus (Crestani et al, 2002; Yee et al, 2004). These mice also show improved water-maze learning, which is a hippocampal-dependent task (Collinson et al, 2002). Moreover, an inverse agonist specifically targeting α5-containing GABAA receptors has been shown to enhance learning in WT animals (Chambers et al, 2004; Sternfeld et al, 2004). Therefore, GABA could modulate contextual fear memory storage by binding to α5-containing GABA receptors in the hippocampus.
GABA AND EXTINCTION OF FEAR MEMORIES
In extinction, subjects are given prolonged exposure to the CS but in the absence of the associated US (see Figure 1). The fear responses exhibited in the presence of the CS are reduced (ie, extinguished) over the course of these CS-alone exposures (within-session extinction). The stability of this behavioral phenomenon is typically measured 24 h later in an extinction retention test, in which the CS is briefly presented without the US (Myers and Davis, 2002, 2007). One possible mechanism underlying extinction is an erasure or weakening of the association between the CS and the US (eg, Rescorla and Wagner, 1972; see also Myers et al, 2006). However, evidence for the restoration of responding to the CS following: (a) the passage of time since extinction training (spontaneous recovery), (b) a change in physical context at test (renewal), and (c) re-exposure to the US (reinstatement) shows that much, if not all, of the original memory remains intact (Myers and Davis, 2002). Thus, it has been proposed that the primary process in extinction is that of new learning, where animals acquire an inhibitory CS-no US association (extinction memory) that masks or competes with the expression of the original excitatory association (Bouton, 1993; Konorski, 1948; Pavlov, 1960). Evidence suggests that extinguished cues possess the properties of a conditioned inhibitor; namely, they pass both the retardation and summation tests (Calton et al, 1996; Pearce, 1997; Schachtman et al, 2000). Moreover, Bouton (1993) argues that the inhibitory CS–US association formed during extinction training is context-specific. That is, animals will only exhibit extinguished responding (ie, low levels of fear responding) within the extinction-training context. Figure 1 loosely follows Myers and Davis' (2002, 2007) definitions of extinction training as the critical component of the procedure, within-session extinction as the decrement in fear responding that occurs during the extinction training procedure, extinction retention as the decrement in fear responding observed at a later time point, and extinction as the theoretical process.
The following sections outline the role of GABA in the extinction of classically conditioned fear memories. The results across the reviewed studies are mixed and at times, contradictory, indicating a complex role of GABA in fear extinction (see Table 2). Nonetheless, we propose that just as GABA disrupts the acquisition and consolidation of newly acquired fear memories, GABA also interferes with the acquisition and consolidation of fear extinction memories.
Table 2. Summary of Studies Examining the Role of GABA in the Extinction of Conditioned Fear.
| Study | Species | Drug | Time of administration | Route of administration | Proce- dure (task) | Findings | Role of GABA in extinction |
|---|---|---|---|---|---|---|---|
| Akirav (2007) | Rat | Muscimol | Post-extinction training | BLA | CTA | Intra-BLA muscimol disrupted extinction of CTA. The disruption persisted for at least 14 days. DCS reversed this effect. | Disrupts consolidation |
| Akirav et al (2006) | Rat | Muscimol | Pre and Post extinction training | IL, BLA | AFC | Pre-extinction training intra-IL infusion of muscimol facilitated extinction retention. Intra-BLA muscimol following short extinction facilitated extinction. | Facilitated acquisition |
| Berlau and McGaugh (2006) | Rat | Bicuculline | Post-extinction training | BLA | CFC | Bilateral intra-BLA bicuculline infusion enhanced extinction retention. | Disrupts consolidation |
| Muscimol, NE | Post-extinction training | DH | CFC | Muscimol had no effect on extinction, nor did it block the effects of NE (which enhanced extinction). | No effect | ||
| Bouton et al (1990) | Rat | MDZ, CDP | Pre-extinction training | Systemically | CFC | MDZ and CDP impaired long term expression of the extinction memory. However, pre-test CDP injection reinstated extinguished responding. | Disrupts acquisition through state dependency |
| Bustos et al (2009) | Rat | Midazolam (MDZ) | Post-extinction training | Systemic | CFC | MDZ disrupted extinction retention. | Disrupts consolidation |
| Chhatwal et al (2005) | Rat | H3-Flu | Variable times post-extinction training | BLA | LFC | The levels of gephyrin protein and mRNA were significantly increased 6 h following extinction training. Also, at both 2 and 6 h after extinction training there was increased binding of the benzodiazepine, H3-Flu to GABAA receptors. | Important for acquisition and/or expression |
| Cloutier et al (2006) | Male SD rats | Lindane | Pre-exposed to Lindane for 3 days or 5 days/week | Systemic | CFC | Pre-treatment with Lindane significantly impaired extinction, as freezing remained elevated in Lindane-treated rats relative to controls. | Disrupts acquisition |
| Corcoran and Maren (2001) | Male long-Evans rats | Muscimol | Pre-extinction-testing | DH | LFC | Muscimol-treated rats did not show renewal | Facilitated expression |
| Corcoran et al (2005) | Rats | Muscimol | Pre-extinction training and pre-extinction-testing | DH | AFC | Muscimol administered before extinction training, disrupted extinction retention. Muscimol infused before testing, abolished renewal. | Disrupts acquisition and facilitates expression. |
| Delamater and Treit (1988) | Male rats | CDP | Pre-extinction training | Systemic | CTA | CDP disrupted extinction of illness and shock-based taste aversions | Disrupts acquisition |
| Disorbo et al (2009) | Male SD rats | Muscimol | Pre- and post-extinction training | Systemic | CTA | Muscimol administered post, but not pre, produced a resistance to extinction across extinction-training sessions | Disrupts acquisition and consolidation |
| Dubrovina and Zinov'ev (2008) | Normal and stressed Male C57Bl/6J mice | Muscimol Bicuculline Baclofen Faclofen | Before fear acquisition | Systemic | Passive avoidance | Stressed rats exhibited impaired extinction compared to controls. Muscimol impaired extinction in control mice, but had no effect on stressed mice. Baclofen prolonged extinction in control mice, and facilitated it in stressed animals. Bicuculline had no effect. Faclofen delayed extinction in controls, and accelerated extinction in stressed mice. Interpretation of these results is difficult given that drugs were administered before conditioning. | Effects vary depending on emotional state of the animal |
| Goldman (1977) | Rat | CDP | Pre-extinction training | Systemic | AFC | CDP-treated animals failed to exhibit extinguished responding. | Disrupts acquisition |
| Gorman et al (1979) | Rat | Diazepam | Pre-extinction training | Systemic | IA | DZP produced a dose-dependent disruption in extinction retention | Disrupts acquisition |
| Graham (2006) | Rat | Bicuculline | Pre- and post-extinction training | Systemic | LFC | Administration of bicuculline facilitated extinction; however, the effects were unreliable. | Disrupts acquisition and consolidation |
| Harris and Westbrook (1998a) | Rat | FG7142 | Pre-extinction training and pre-re-extinction training | Systemic | CFC | FG7142 disrupted within-session extinction and extinction retention. The disruption was abolished when rats were tested in a different context | Facilitates acquisition and expression |
| Hart et al (2009) | Rat | MDZ (BZ/indirect agonist) | Pre-extinction training and pre-re-extinction training | Systemic and Intra BLA | CFC | MDZ disrupted within-session extinction and extinction retention, but had no effect on re-extinction memory. MDZ disrupted re-extinction when animals were extinguished under the influence of MDZ. | Disrupts acquisition of extinction, not re-extinction |
| Heldt and Ressler (2007) | c57BI/6J Mice | H3-Flu | Post-extinction training | BLA | AFC | Rats exhibited increased expression of GABAA receptor subunits within the amygdala—specifically α1 (LA), α2 (in the CE) and β2 (in the BLA). Rats also exhibited increased levels of gephyrin and GAD67 protein in the BLA. | Facilitates the acquisition and/or expression |
| Hobin et al (2006) | Rat | Muscimol (full agonist) | Pre-extinction testing | Ventral hippocampus | CFC | Muscimol disrupted context-specific fear memory retrieval. Specifically, animals exhibited extinguished responding regardless of the test context. Therefore, animals did not exhibit renewal. | Facilitates expression |
| Ishitobi et al (2009) | Rat | MDZ and Propofol | During conditioning (between CS and US onset) | Systemic | CTA | MDZ and propofol disrupted retention and enhanced extinction of CTA. Results are difficult to interpret since drugs were administered during conditioning, and not extinction-training. | Facilitates acquisition |
| Jacobson et al (2006) | Mice | GABA-B(1a) −/− and GABA-B(1b) −/− mice. | Pre-training | CTA | GABA-B(1b) KO mice failed to acquire CTA. In contrast GABA-B(1a) KO mice, failed to extinguish the aversion. | Facilitates acquisition and expression | |
| Kamano (1972) | Rats | CDP Amobarbital | Pre extinction training | Systemic | IA | Both CDP and Amobarbital disrupted extinction retention. | Disrupts acquisition |
| Kim and Richardson (2007) | Rat (PND 16 and 23) | FG7142 | Pre extinction testing | Systemic | AFC | Pre-testing FG7142 produced a context-specific deficit in the expression of extinction learning in PND23 rats, but not PND-18 rats. | Facilitates expression in older animals |
| Kim and Richardson (2009) | Rat | FG7142 | Pre-extinction testing | Systemic | AFC | Higher freezing was shown in the FG7142 rats compared to vehicle rats, regardless of whether the test context was similar or different to the extinction-training context. | Facilitates expression |
| Lin, Mao, Chen, and Gean (2008) | Rat | WIN55212-2 Bicuculline | Pre-conditioning (once per day for 7 days) and pre-extinction training | Systemic and IL | LFC | In control rat slices of the IL, WIN application reduced GABAergic inhibitory transmission. Extinction was intact among these rats. In contrast, WIN pre-treated rats showed persistent GABAergic inhibitory transmission in response to WIN application. These rats also displayed a resistance to the extinction-enhancing effects of pre-extinction training WIN administration. Furthermore, administration of bicuculline into the IL produced an extinction-like reduction in startle in both control and WIN-treated animals. | Disrupts acquisition |
| Marsciano et al (2002) | Mice | CB1−/− mice and control CB1+/+ mice | Pre-training | LA | AFC | In control CB+/+ mice, but not CB−/− mice, long-term depression (LTD) was induced successfully in LA slices. This was associated with a suppression of GABAA receptor-mediated inhibitory post-synaptic currents. | Disrupts acquisition and/or consolidation |
| McGaugh et al (1990) | Male CD1 mice | Picrotoxin | Post-extinction-training | Systemic | AFC | Picrotoxin facilitated extinction | Disrupts consolidation |
| Nomura and Matsuki (2008) | Male SD rats | Ethanol | Post-extinction | Systemic | CFC | Ethanol had no effect on the retention of extinction. | Not involved in extinction |
| Pereira et al (1989) | Female Wistar rats | Diazepam | Pre-extinction-training | Systemic | Shuttle avoidance | DZP disrupted extinction retention, regardless of whether extinction training occurred 2 or 24 h after conditioning. | Disrupts acquisition |
| Shumyatsky et al (2002) | Mice | Genetically modified mice with a GRPR KO | Pre-extinction training | CFC and AFC | GRPR KO's exhibited greater freezing compared to wild-type controls. Both groups showed reduced freezing over subsequent brief-CS re-exposure sessions. | Facilitates acquisition and/or expression | |
| Taub et al (1977) | Male SD rats | CDP | Pre-extinction training | Systemic | IA | Animals treated with CDP before extinction training disrupted extinction retention. | Disrupts acquisition |
| Yee et al (2004) | Alpha-5(H105R) mutant mice | Genetically modified mice, expressing fewer α5 subunit-containing GABA receptors in the hippocampus | Knock-in-pre-training. | Hippocampus | AFC | During extinction testing, female but not male, mutant mice exhibited greater levels of freezing indicating impaired extinction. However, across additional testing sessions, both male and female mice displayed a resistance to extinction. | Facilitates acquisition and/or expression |
Abbreviations: AFC, auditory fear conditioning; BLA, basolateral amygdala; BZ, Benzodiazepine; CB1+/+, wild-type mouse; CB1−/−, CB1 receptor-deficient mouse; CDP, chlordiazepoxide; CD1, cluster of differentiation 1 a family of glycoproteins; CFC, contextual fear conditioning; CS, conditioned stimulus; CTA, conditioned taste aversion; C57Bl/6J, CE, mouse strain; DH, dorsal hippocampus; DCS, d-cycloserine; DZP, diazepam; GABA, gamma-amino butyric acid; GABA-B(1a), subunit 1a of the GABA-B receptor; GABA-B(1b), subunit 1b of the GABA-6B receptor; GAD67, glutamic acid decarboxylase 67; GRP, gastrin-releasing peptide; GRPR, gastrin-releasing peptide receptor; H3-Flu, H3-flunitrazepam; IA, inhibitory avoidance; IL, Infralimbic cortex; KO, knockout; LA, lateral amygdala; LFC, light fear conditioning; MDZ, midazolam; mRNA, messenger ribonucleic acid; NE, norepinephrine; PND, post-natal day; SD, Sprague–Dawley rat strain; US, unconditioned stimulus; WIN 55,212-2, 3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-yl](1-naphthalenyl)methanone.
Pre-Extinction Training Manipulations
Extinction is regarded as a form of new learning, whereby animals learn that the CS is no longer dangerous within a specific context (Myers and Davis, 2002). As drugs that facilitate GABA transmission have been shown to interfere with the acquisition and consolidation of fear learning, it follows that such drugs administered before extinction training should also block the acquisition of extinction memories (Davis and Myers, 2002). The common finding in the literature across a variety of different aversive paradigms is that increasing GABAergic transmission before extinction training disrupts extinction retention (eg, Delamater and Treit, 1988; Goldman, 1977; Kamano, 1972). For example, Pereira et al (1989) reported that pre-extinction training administration of diazepam led to continued avoidance responding during the extinction retention test. In addition, Hart et al (2009) observed that MDZ administered systemically and directly into the BLA before extinction training interfered with extinction of contextual fear. That is, MDZ-treated rats exhibited greater freezing at test compared with controls. Consistent with this evidence, muscimol infused directly into the dorsal or ventral hippocampus, before extinction of auditory fear, disrupts retention of extinction. That is, muscimol-treated animals continued to exhibit high levels of freezing to the tone irrespective of the context they were tested in (Corcoran et al, 2005; Corcoran and Maren, 2001; Hobin et al, 2006). The authors concluded that muscimol interfered with the context-specificity of extinction such that animals exhibited fear responding regardless of the test context.
This evidence collectively suggests that administration of GABAA receptor agonists before extinction training interferes with extinction retention. This may be due to a disruption of within-session extinction learning (ie, acquisition), or a disruption in post-training consolidation processes. The evidence suggests that the extinction memory deficit could be due to a combination of disrupted acquisition and consolidation. Specifically, some of the above studies have shown that GABA agonists lead to deficits at the extinction retention test, but leave within-session extinction intact (eg, Corcoran et al, 2005; Hart et al, 2009; Hobin et al, 2006), or actually enhance within-session acquisition (eg, Corcoran et al, 2005; Delamater and Treit, 1988). This would indicate that the extinction memory deficit at test is due to a disruption in post-training consolidation processes. In contrast, other studies have shown that GABA agonists actually disrupt within-session extinction, indicating that the extinction memory deficit at test is due to a failure to initially acquire the short-term extinction memory (eg, Akirav et al, 2006; Delamater and Treit, 1988, Hart et al, 2009).
Alternatively, pre-extinction training GABA administration could disrupt extinction retention by producing some form of state dependency (Bouton et al, 1990). That is, the GABA-induced deficits in extinction retention may simply represent retrieval, rather than storage failure. These researchers observed that pre-training administration of the BZ CDP interfered with the extinction of contextual fear as demonstrated by elevated levels of freezing at test. However, the extinguished fear response (ie, reduced freezing) returned when animals were injected with CDP before testing. Thus, the authors concluded that the drug did not disrupt extinction learning, but instead made the expression of extinction state dependent. They proposed that rats extinguished under CDP acquired an extinction memory that was specific to the drug state. Consequently, when rats were tested, they were unable to retrieve the extinction memory because the internal state during testing (ie, drug absent) did not match the internal state during extinction training (ie, drug present). However, injecting CDP before testing reinstated low levels of freezing because rats were returned to the internal state of extinction training, which allowed them to retrieve the extinction memory (Bouton et al, 1990).
What these findings suggest is that administering BZs before extinction training does not actually disrupt the acquisition or consolidation of the extinction memory, but instead, disrupts its retrieval. However, the reinstatement of extinguished responding by pre-test CDP may have been due to an alternative mechanism. Specifically, the reduction in freezing at test may simply have been caused by the anxiolytic effects of this drug, rather than the drug returning the animals to the internal context of extinction training. The dose of CDP that Bouton et al (1990) administered before testing was 10 mg/kg. Studies have shown that similar doses of CDP (ie, 2.5, 3, 5, and 10 mg/kg) can produce anxiolytic effects in a number of behavioral paradigms, such as novelty induced hypophagia (Bechtholt et al, 2008), punished drinking, and punished lever pressing (Sanger et al, 1985). Thus, a strong candidate for an alternative explanation of Bouton et al's (1990) finding of reduced freezing after pre-test CDP administration is decreased anxiety. Other studies have shown that pre-test administration of GABAergic drugs can ‘facilitate the expression of extinction', even if the drug is not administered before extinction training (Corcoran and Maren, 2001, 2005; Hobin et al, 2006), again suggesting anxiolytic rather than state-dependency effects. Additional evidence against the state-dependency account is provided by Hart et al (2009) with the re-extinction paradigm. Ultimately, the findings of Bouton et al (1990) do not readily permit the conclusion that BZ-induced disruption of extinction is simply the result of state dependency, or in other words, a retrieval failure.
Two studies have provided evidence that is inconsistent with the account that GABA interferes with the formation of extinction memories. First, Harris and Westbrook (1998a) examined the effects of FG7142, an inverse agonist at the BZ site which decreases the likelihood of GABA binding to the GABAA receptor, leading to reduced GABAergic neurotransmission. They found that pre-training and pre-testing administration of FG7142 disrupted extinction of auditory fear conditioning. In other words, compared with controls, FG7142-treated rats exhibited higher freezing to the CS when tested in the extinction-training context. Furthermore, this disruption was specific to the extinction context, as vehicle and FG7142 rats demonstrated similar levels of freezing when tested in a different context. This led the authors to suggest that FG7142 produced a context-specific disruption in the acquisition and expression of extinction. The authors concluded that fear extinction involves the acquisition of a context-specific inhibitory association that is controlled by the context of extinction training, and that GABA binding to GABAA receptors mediates this context-specific inhibition of fear expression (Harris and Westbrook, 1998a). This finding indicates that decreasing GABA transmission before extinction training impairs extinction retention. Given the evidence previously discussed, we would expect that decreasing GABA transmission should facilitate extinction retention. How can we account for this unexpected result? First, the injection of FG7142 was systemic, so it is possible that the disruptive effects of FG7142 were due to the drug acting within a specific brain region leading to disruption of the extinction process. Second, the dose of FG7142 used in this study was 10 mg/ml, which is relatively high. Research has shown that a 10 mg/kg dose of FG7142 can actually impair, rather than facilitate fear memory formation (File and Pellow, 1988). Therefore, at present, the findings of Harris and Westbrook (1998a, 1998b) do not necessarily contradict the claim that pre-training GABAergic drugs disrupt extinction retention.
A study by Akirav et al (2006) also yielded findings that seem to contradict the idea that pre-training GABAergic drugs disrupt extinction learning. These authors demonstrated that administrations of muscimol into the infralimbic region (IL) of the mPFC before an extinction training session facilitated extinction of auditory fear conditioning. This evidence indicates that increasing GABAergic tone before extinction training facilitates, rather than impairs, the retention of extinction. This finding contradicts the dominant model of fear learning and extinction (Maren and Quirk, 2004; Pare et al, 2004).
The dominant model of fear learning states that following classical conditioning, the CS excites neurons in the BLA, causing activation of neurons in the central amygdala, leading to conditioned responding (Quirk et al, 2003; Pare et al, 2004). It is proposed that following extinction training, the mPFC, particularly the IL is critical for inhibiting amygdala responses to the CS, and thereby preventing conditioned responding (Milad et al, 2004, 2007; Milad and Quirk, 2002; Quirk et al, 2003; Rosenkranz et al, 2003; Quirk and Mueller, 2008). Furthermore, several findings suggest that the consolidation of extinction involves complex molecular cascades taking place in the IL (Burgos-Robles et al, 2007; Hugues et al, 2004; Mueller et al, 2008; Santini et al, 2004; Sierra-Mercado et al, 2006). Given these results, it is unusual that Akirav et al (2006) observed that pre-extinction training infusions of muscimol into the IL actually facilitated extinction retention.
One possible explanation is that the infusion of muscimol may have spread to the adjacent PL. Research is beginning to suggest that this region is critically involved in the expression rather than inhibition of fear responding (Burgos-Robles et al, 2009; Knapska and Maren, 2008; Schiller and Johansen, 2009). The findings of Burgos-Robles et al (2009) suggest that neural activity in the PL drives fear responding (possibly through projections to the basal amygdala; Schiller and Johansen, 2009) and modulates extinction (Burgos-Robles et al, 2009). Schiller and Johansen (2009) proposed that a failure to extinguish fear memories may result from excessive PL activity (during conditioning, extinction training and/or testing) leading to more fear responding at test. Following this logic, decreasing neural activity in the PL should be associated with decreases in fear responding. If muscimol did in fact spread to the PL in the Akirav et al (2006) study, neural activity would have decreased in this region during extinction training. Because the PL is believed to drive the expression of conditioned fear responding, inactivation of this region would reduce freezing at test, which was what Akirav et al (2006) observed. Indeed, Akirav et al (2006) acknowledge that their muscimol infusion probably spread to neighboring regions of the PFC. These results are important because they demonstrate that the effects of GABA on extinction retention may vary depending on the precise location of drug infusion.
In summary, most of the evidence indicates that increasing GABAergic transmission before extinction training disrupts the retention of extinction, which is consistent with our main proposal. This effect may be due to GABA disrupting within-session extinction learning, post-training consolidation processes, or by inducing state dependency, although the state dependency account is less compelling. One should also keep in mind, based on the results of Akirav et al (2006), that although pre-training GABA administration normally impairs extinction, different effects may occur depending on the precise location of drug infusion.
Post-Extinction Training Manipulations
In this section, we outline studies examining the role of post-extinction training application of GABAergic drugs. Because the drug is administered after extinction training, the consolidation of extinction memories will most likely be affected (see Figure 1), and state-dependent explanations for memory deficits are less relevant.
Disorbo et al (2009) initially trained animals to acquire an aversion to saccharin, which was paired with lithium chloride (LiCl). Across 5 days of acquisition, animals showed a significant reduction in consumption indicating that animals acquired CTA. Animals then received extinction, whereby saccharin was presented without LiCl for 19 consecutive days. Experimental animals received muscimol injections either before (pre-muscimol group) or after (post-muscimol group) each extinction session, whereas control rats received saline. The results showed that, only for rats that received post-extinction-training muscimol (post-muscimol group), extinction was disrupted. Specifically, these rats continued to avoid the saccharin flavour, indicating impaired extinction retention. Interestingly, the pre-muscimol group demonstrated intact extinguished responding, which suggests that muscimol was not simply inhibiting flavour consumption in the post-muscimol group, but was instead interfering with memory storage processes. The authors concluded that the disruptive effects of muscimol were due to impaired consolidation of the SAC-no illness extinction memory. Collectively, these results, along with the other studies summarized in Table 2, provide strong evidence that GABAergic transmission is detrimental to the consolidation of extinction memories.
If GABAA receptor agonists impair extinction consolidation, then antagonists such as picrotoxin and bicuculline should facilitate extinction. Consistent with this, McGaugh et al (1990) demonstrated that administration of picrotoxin immediately after extinction of auditory fear conditioning facilitated extinction retention—that is, picrotoxin-treated rats exhibited fewer avoidance responses compared with control rats. Similarly, Berlau and McGaugh (2006) observed that post-extinction training infusions of bicuculline directly into the BLA facilitated the extinction of contextual fear conditioning. The authors concluded that bicuculline enhanced the consolidation of extinction learning.
Still, some studies have shown that administering GABAergic drugs immediately after extinction training has no effect on subsequent extinction retention (Berlau and McGaugh, 2006; Makkar et al, 2010; Nomura and Matsuki, 2008). Even more interesting, Akirav et al (2006) showed that infusions of muscimol into the BLA after short extinction led to a significant reduction in freezing at test. In other words, increasing GABA transmission after extinction training improved extinction retention. This finding appears to contradict the claim that GABA disrupts the consolidation of extinction memories. However, upon close examination of the methodology used by Akirav et al (2006), it is likely that this enhancement of extinction is really a disruption in reconsolidation (a process described in the next session). The short extinction session, which the authors used more closely, resembles a short reactivation session (see Figure 1). Specifically, the session consisted of five CS exposures, which may be too little to produce extinction. This is shown by the fact that animals were not exhibiting a significant decline in fear responding across the CS exposures. Thus, the decreased freezing at test is most likely disrupted reconsolidation of the original memory trace, rather than facilitated consolidation of the extinction memory trace. In light of these methodological issues, the results of Akirav et al (2006) do not contradict the claim that increasing GABAergic transmission after extinction training interferes with the consolidation of extinction memories.
An important question is how administering GABAergic drugs before or immediately after extinction training interferes with extinction memory. According to Davis and Myers (2002), as extinction represents a form of new learning, there are structural and functional changes that occur in neurons. This extinction-related plasticity consists of strengthening connections between the sensory pathways transmitting information about the CS, and a group of GABAergic neurons critical for inhibiting fear responding when animals are later tested. The consolidation of this neural plasticity would require excitation rather than inhibition of target cells (ie, membrane depolarization, activation of NMDA receptors to initiate calcium entry), possibly through the activity of excitatory neurotransmitters such as glutamate (Davis and Myers, 2002). Therefore, GABA agonists administered during this critical period of plasticity (ie, consolidation) will most likely inhibit these neural processes, producing a disruption in extinction retention. Conversely, GABA antagonists would decrease GABAergic inhibition, thereby facilitating extinction consolidation (Yee et al, 2004; see Figure 3). In summary, the formation of fear extinction memories is dependent on reduced GABAergic transmission after extinction training. Indeed, the reviewed evidence is consistent with this neural account.
Figure 3.
Yee et al's (2004) schematic representation of the complex GABAergic circuits involved in the acquisition, consolidation, and expression of extinction, as well as the interaction between GABA and excitatory neurotransmitters, particularly glutamate binding to NMDA receptors, in the storage of extinction memories. The nodes illustrated in this diagram (circular nodes denoting glutamatergic units, and octagonal nodes denoting GABAergic units) represent assemblies or networks of neurons. The left hand side of the diagram demonstrates the formation of an association between a conditioned stimulus (CS) such as a tone, and an unconditioned stimulus (US) such as a shock. This excitatory learning is hypothesized to involve NMDA-mediated synaptic plasticity. GABAergic transmission may be involved in suppressing excitatory activity, thereby impairing the consolidation of fear memories. GABAergic antagonists and inverse agonists, which reduce GABAergic activity, would reduce such tonic inhibition, thus facilitating the acquisition and storage of the CS–US fear memory. The neural pathway of extinction is displayed on the right hand side. This involves the formation of links between nodes carrying information about the CS to GABAergic units, which leads to reduced expression of the conditioned response (CR) following CS presentation. In addition, representations related to detection of the absence of the US (as in extinction training) and sensory inputs carrying information about the extinction context are also hypothesized to connect to these GABAergic units which are involved in reducing CR output. These connections to the GABAergic units are strengthened by NMDA-dependent mechanisms during extinction learning, and these connections are themselves under the modulation of additional GABAergic interneurons. Therefore, the presence of GABAergic drugs during acquisition or consolidation phases of extinction would inhibit the excitatory neural processes (involving glutamate and NMDA receptors), which are critically involved in storing the extinction memory. In addition, it is evident that after extinction training, the expression of extinguished responding at test requires the activation of GABAergic units to suppress conditioned fear responding (bottom, right). The administration of GABAergic drugs during this phase of extinction would enhance inhibitory transmission, facilitating the expression of conditioned responding (eg, low levels of freezing, startle, heart rate). Copyright (2010) Wiley. Figure used with permission from Yee et al (2004), GABAA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear, Eur J Neurosci, John Wiley and Sons.
Effects of GABA Administered Pre-Extinction Retention Test
Administration of GABAergic drugs before extinction retention testing will most likely modulate the retrieval of the extinction memory, or the expression of fear. The evidence strongly indicates that increasing GABAergic transmission leads to decreased fear responding. For example, pre-test administration of muscimol directly into the dorsal or ventral hippocampus led to lower levels of fear responding (regardless of test context) than did administration of vehicle (Corcoran et al, 2005; Hobin et al, 2006). According to Corcoran et al (2005), muscimol infusion before testing removed the context-specificity of extinction, causing animals to exhibit extinguished responding (ie, low levels of freezing) in all contexts. Consistent with this evidence, decreasing GABAergic transmission with the inverse agonist FG7142 before extinction testing produces a recovery of fear responding to a previously extinguished tone or context, suggesting a failure to inhibit fear responding (Kim and Richardson, 2007, 2009; Harris and Westbrook, 1998a).
Collectively, the evidence indicates that increased GABAergic transmission is required for animals to display low levels of fear responding during the extinction retention test. Why might this be the case? As previously mentioned, extinction represents the active inhibition of fear responding. The mPFC inhibits the expression of fear responding during extinction by suppressing amygdala neural firing in response to a CS (Maren and Quirk, 2004; Milad and Quirk, 2002; Milad et al, 2007; Rosenkranz et al, 2003; Quirk et al, 2003; Quirk and Mueller, 2008). Therefore, if extinction represents the inhibition of fear responding, the application of GABAergic drugs before testing could facilitate extinguished responding by increasing inhibitory neurotransmission (Yee et al, 2004; see Figure 3). The evidence is consistent with this inhibitory account. Another plausible reason why administration of GABAergic drugs before extinction testing leads to less fear responding is because these agents are simply decreasing anxiety, and consequently rats display less fear responding. Similarly, infusing inverse agonists before extinction testing would increase anxiety, and therefore enhance fear responding. It is clear that further research is needed to disambiguate the anxiolytic and inhibition accounts regarding the effects of pre-test GABA on extinction expression, possibly by examining drugs targeting specific subtypes of the GABAA receptor. Studies have shown that mice with a point mutation in the α2-subunit of the GABAA receptor fail to show reduced anxiety in a light–dark choice test (Low et al, 2000; Rudolph et al, 2001). This indicates that the α2-subunit is specifically involved in anxiety reduction. If the anxiety-reduction account is correct, then injecting drugs into normal mice that specifically activate α2-containing GABAA receptors before testing should enhance extinction expression. Alternatively, in order to test the inhibition account, future studies could use mice with a point mutation of the α2-subunit. The point mutation ensures that these animals are not able to show anxiolytic effects in response to drug administration, but also ensures that GABAergic inhibitory neurotransmission is still functional (Rudolph et al, 2001). If these animals still display low levels of freezing at test in response to pre-test administration of GABAA receptor agonists (eg, BZs), then this reduction in fear responding cannot be due to anxiolytic effects as the α2 subunit is not functional. Rather, the low level of freezing is most likely due to enhanced GABA-mediated inhibition of fear responding.
Genetic and Molecular Manipulations
This section focuses on research that has used genetically modified animals to study GABA as well as studies that have examined changes in the expression of GABAergic molecules after extinction training. First, Yee et al (2004) studied the effect of mutant α5(H105R) mice on extinction of auditory fear conditioning. These mice express fewer GABAA receptors containing the α5-subunit. A reduction in α5-containing GABAA receptors would equate to reduced GABAergic inhibitory transmission. The results demonstrated that both male and female mutant mice, compared with WT mice, did not exhibit a decline in conditioned responding across testing sessions, consistent with a disruption in extinction. Consistent with these findings, Jacobson et al (2006) showed that GABA-B(1b) knockout (KO) mice, that is, mice lacking functional GABA-B1b receptors, exhibited a disruption in extinction of CTA memory. In another study, Shumyatsky et al (2002) examined mice with a gastrin-releasing peptide receptor (GRPR) KO on extinction of fear. Gastrin-releasing peptide (GRP) binds to the GRPR and increases GABAergic inhibitory transmission. GRP is highly expressed within the LA, and its receptor (GRPR) is expressed selectively in inhibitory interneurons within the LA. Mice that possess this KO have impaired GABAergic transmission. The authors observed that GRPR KO mice exhibited greater conditioned freezing in cue and contextual fear conditioning retention tests compared with WT control mice, suggesting enhanced acquisition and/or consolidation of the fear memory. Across subsequent test sessions, which occurred at 2, 7, and 15 weeks later, the KO mice continued to exhibit higher levels of freezing compared with the WT mice. However, both groups of mice showed a decrease in freezing. Because no interaction was reported (just main effects for mouse type and session), it cannot be said that there was resistance or facilitation of extinction. Indeed, without the appropriate control groups (with no interim exposures) it is not possible to counter an alternative explanation in terms of forgetting. Moreover, because the CS (cue or context) re-exposures were not of long duration (2 and 3 min, respectively), it is unclear whether extinction or reconsolidation processes would have dominated (see next section on reconsolidation).
Across these genetic studies, the predominant finding is that when GABA transmission is impaired, extinction is also disrupted. In other words, most of the studies seem to suggest that GABA actually facilitates the formation of extinction memories. These results appear to be in direct opposition to the pharmacological studies, in which the predominant finding is that increasing GABAergic transmission is detrimental to the acquisition and consolidation of extinction memory. How can we account for this apparent contradiction? A likely explanation is that these animals are acquiring a very strong fear memory due to the impairment in GABA transmission. In other words, the disruption in GABA receptor functioning in these animals would have reduced inhibitory transmission, presumably leading to an over-consolidated fear memory that was resistant to extinction. Indeed, as shown in the results of Shumyatsky et al (2002), mice with a KO of the GRPR showed higher levels of freezing relative to WT mice after fear conditioning, supporting the over-consolidation account. An alternative (and not mutually exclusive) explanation is that the impaired GABA functioning in the KO mice disrupted the ability for these animals to inhibit their fear responding during the extinction retention test. As outlined in the previous section, normal GABA functioning is critical for the inhibition of fear responding during extinction testing. Essentially, these genetic KO studies do not necessarily disprove the claim that GABA is detrimental to the formation of extinction memories. However, they also do not provide definitive answers as to the precise role of GABA in the various phases of extinction (see Figure 1). This is because, first, KO mutations may produce compensatory changes in neuronal functioning during development, making it difficult to identify the precise role the genetic manipulation has on behavior during testing (Rudolph et al, 2001). Future genetic studies could use knock-in point mutations to modify GABAA receptor functioning, as this method does not appear to elicit significant changes in the brain development.
Second, a major problem with genetic studies in general is that the genetic modification typically exists before initial fear conditioning. Consequently, it is difficult to specify whether the extinction deficit (ie, elevated fear responding) at test is because the KO has affected acquisition or consolidation of the initial fear memory, or acquisition, consolidation or expression of the extinction memory. Examination of within-session extinction may allow researchers to distinguish between alternative accounts. For example, if the genetically modified animal shows an initially higher level of fear responding than controls, this suggests that the manipulation enhanced the acquisition and/or consolidation of the initial fear memory. If the KO animals fail to show a decrement in fear responding during extinction training, this indicates that the manipulation disrupted initial acquisition of the extinction memory. Finally, if the genetically modified animals display a comparable initial level and decrement in freezing relative to controls, but then display elevated freezing at test, this indicates that the genetic manipulation has interfered with either the consolidation or retrieval of the extinction memory.
The idea that GABAergic transmission is important for the expression of extinguished responding is supported by molecular studies. First, Chhatwal et al (2005) demonstrated in BLA slices from rats, increased levels of gephyrin protein and gephyrin protein mRNA 6 h after extinction training. These molecules are important for stabilizing GABAA receptors in order to make them functional. Furthermore, they also observed increased levels of BZ binding, and also elevated levels of GABAA receptor subunits within amygdala neurons, several hours after fear extinction training. This suggests that the inhibition of fear after extinction training is dependent upon a delayed increase in GABA activity within the amygdala. Consistent with these findings, Heldt and Ressler (2007) found that after extinction training, rats exhibited increased expression of GABAA receptor subunits within the amygdala—specifically α1 (LA), α2 (in the CE), and β2 (in the BLA). These subunits are critical for the formation of functional, BZ-specific GABAA receptors (Rudolph et al, 2001; Pirker et al, 2000; Sieghart, 1995). Furthermore, rats exhibited increased levels of gephyrin and GAD67 protein in the BLA. GAD67 is a marker of GABAergic activity (Heldt and Ressler, 2007). These molecular studies demonstrate that several hours after the start of extinction training there is upregulation of molecules and receptors that facilitate GABA transmission. This suggests that the expression of extinguished responding after extinction training is dependent upon functional GABAergic transmission.
CONCLUSION
The role of GABA in extinction has been extensively investigated, and the findings have been diverse, inconsistent, and quite often contradictory. However, upon closer examination of the evidence as well as the methodological procedures used in different studies, it is clear that GABA produces consistent effects that are dependent on the time point of drug administration, the dosage of drug used, as well as the brain region of drug infusion.
Administration of GABAergic drugs either before or immediately after extinction training blocks response inhibition, which is consistent with our position that GABA disrupts the acquisition and consolidation of extinction memories. Those studies that have observed findings inconsistent with this claim have either used elevated doses of drug (Harris and Westbrook, 1998a), or infused the drug into specific brain regions that may have directly inhibited fear expression (Akirav et al, 2006). Thus, it appears that the long-term storage of extinction memories, such as new fear memories, is dependent upon reduced GABAergic neurotransmission.
In contrast, when GABAergic drugs are infused before testing, conditioned responding is less than those for vehicle infusion, suggesting facilitation of response inhibition circuitry. Conversely, when GABAergic transmission is downregulated before testing (either by infusion of a GABA antagonist, or GABA KO), fear responding is increased, suggesting interference with response inhibition mechanism, rather than with any learning processes (Yee et al, 2004; see Figure 3).
In summary, the evidence suggests that GABA has opposing effects on extinction depending on when the drug is administered. GABAergic drugs have an impairing effect on extinction when administered shortly before or after extinction training, which we argue is due to interference with extinction memory consolidation processes. In contrast, GABAergic drugs enhance response inhibition when administered before testing, possibly by decreasing anxiety, facilitating retrieval, or more likely by facilitating the inhibition of fear responding.
GABA AND RECONSOLIDATION OF FEAR MEMORIES
The traditional view of how the brain stores new memories is that a consolidation process stabilizes initially fragile memories over time until they become ‘fixed' in the brain (McGaugh, 2000; Nader, 2007). During a critical window of time after the initial encoding of the short-term memory representation, new memories are susceptible to amnesic and memory-enhancing agents. Once consolidated, however, these memories are insensitive to disruption (Cestari et al, 2006; McGaugh, 2000). This latter claim has been challenged by the recent revitalization of the notion of ‘reconsolidation' (Lee et al, 2008; Lewis, 1979; Spear, 1973; Miller and Springer, 1973; Nader, 2007; Sara, 2000). In a typical reconsolidation procedure, rats are initially subjected to fear conditioning, then 24 h later, they undergo a reactivation session, which involves a brief re-exposure to the CS alone (see Figure 1). Immediately after reactivation a manipulation is applied, such as administration of anisomycin, a protein synthesis inhibitor known to disrupt memory consolidation. During a test 24 h later, treated rats show reduced fear responding suggesting that the retrieved memory has become labile again, and requires reconsolidation. Both the amygdala (Nader et al, 2000) and the hippocampus (Debiec et al, 2002) have been implicated in reconsolidation, and Tronson and Taylor (2007) concluded that there are both similarities and differences in the cellular processes underlying consolidation and reconsolidation.
Previously, it was suggested that GABA receptors were functionally implicated in initial memory formation. As discussed, GABA agonists such as muscimol interfered with memory consolidation, while GABA antagonists such as bicuculline enhanced memory consolidation. Although the number of studies exploring the relationship between reconsolidation and GABA has been modest in comparison to the number of studies examining consolidation (see Table 3 for a summary of reconsolidation studies), the results generally point towards an impairment of reconsolidation, which is again consistent with our view that GABAergic transmission is detrimental to the acquisition and storage of memories. To date, only post-reactivation drug administration has been reported (eg, there are no studies reporting pre-reactivation or pre-test administration of GABAergic drugs). For example, Bustos et al (2006) reported that MDZ (indirect GABA agonist), administered immediately after reactivation in a context-conditioning paradigm, led to reduced responding 24 h later (Test 1) relative to rats administered saline. This decreased responding was still evident in a further within-subjects test (Test 2) 10 days later. Furthermore, fear responding was not reinstated following exposure to a reminder shock or an additional (albeit weak) context-shock pairing (see also Kim and Richardson, 2007). Bustos et al (2009) expanded on these results and observed that the disruptive effects of GABA on memory reconsolidation depend on the age of the memory, reactivation length, and dose of the drug. For example, increasing the interval between initial training and reactivation reduces the vulnerability of the memory to disruption by MDZ. Destabilization of these memories requires longer reactivation periods and higher doses of the drug. Extending these findings, Makkar et al (2010) showed that administration of MDZ following reactivation produced a persistent disruption of discrete cue auditory fear memory (see Figure 4). Essentially, these findings provide strong evidence that GABAergic neurotransmission is functionally implicated in the re-storage of fear memories following reactivation.
Table 3. Summary of Studies Examining the Role of GABA in the Reconsolidation of Conditioned Fear.
| Study | Species | Time of administration | Drug | Infusion | Task | Findings | When were animals re-tested with respect to reactivation? | Was the deficit or enhancement temporary? | Role of GABA in reconsolidation |
|---|---|---|---|---|---|---|---|---|---|
| Amaral et al (2007) | Rat | Post-reactivation | Muscimol | Dorsal hippocampus | IA | Muscimol temporarily disrupted IA memory retention. | 2 days later | Yes | Inhibits retrieval |
| Bustos et al (2006) | Rat | Post-reactivation | MDZ | Systemic | CFC | MDZ produced a persistent deficit in memory retention. | 10 days later | No | Disrupts re-storage |
| Bustos et al (2009) | Rat | Post-reactivation | MDZ | Systemic | CFC | MDZ impaired fear memory retention when administered after 3, and 5-min, but not 1-min reactivation periods. Longer reactivation sessions and higher doses of MDZ were required to disrupt remote memory traces. | 10 days later (or more) | No | Disrupts re-storage |
| Carbo Tano et al (2009) | Crab | Post-reactivation | Muscimol | Systemic | CFC | Muscimol disrupted consolidation and reconsolidation of contextual fear memory in a dose-dependent manner. | 1 day later | Not examined | Disrupts re-storage |
| Bicuculline | Systemic | CFC | Bicuculline facilitated consolidation and reconsolidation of contextual fear memory. | 1 day later | Not examined | Disrupts re-storage | |||
| Makkar et al (2010) | Rat | Post-reactivation | MDZ | Systemic and BLA | CFC | MDZ produced a persistent deficit in fear responding. MDZ had no effect when injected into the BLA, or when it was injected following long reactivation (extinction). | 11 days later | No | Disrupts re-storage |
| Nomura and Matsuki (2008) | Rat | Post-reactivation | Ethanol, Picrotoxin | Systemic and intra- amygdala | CFC | Ethanol enhanced consolidation and reconsolidation of fear memory. The effect was reversed with co-administration of picrotoxin. Ethanol had no effect on extinction, nor when it was infused into the BLA. | 3, 14, and 28 days later | Yes | Enhances re-storage |
| Zhang and Cranney (2008) | Rat | Post-reactivation | MDZ | Systemic | CFC | MDZ blocked reconsolidation. This effect was comparable among rats with high and low levels of pre-existing anxiety. | 10 days later | No | Disrupts re-storage |
| Post-reactivation | Bicuculline | Systemic | CFC | Bicuculline temporary facilitated memory retention. | 10 days later | Yes | Modulates retrieval |
Abbreviations: AFC, auditory fear conditioning; BLA, basolateral amygdala; CFC, contextual fear conditioning; IA, inhibitory avoidance; MDZ, midazolam.
Figure 4.
The effect of MDZ on the reconsolidation of auditory fear memory. Mean (±SEM) percentage freezing during tests 1 and 2 for rats administered MDZ (n=8) or saline (n=7). Test 1 took place 24 h after reactivation and test 2 took place 10 days after test 1. MDZ-treated rats display a deficit in freezing that is evident at both test sessions. MDZ=midazolam, SAL=saline (after reactivation).
Further evidence for the disruptive effects of GABA on reconsolidation of fear memories was observed by Carbo Tano et al (2009). They examined the effects of the GABAA agonist muscimol and antagonist bicuculline on the consolidation and reconsolidation of contextual fear memory in the crab Chasmagnathus. Results demonstrated that immediate but not delayed (30 min) post-training muscimol injections produced a dose-dependent disruption of contextual fear memory consolidation. This deficit was reversed if crabs received co-administration of bicuculline, indicating that the effect was mediated by GABAA receptors. Importantly, the investigators showed that administration of muscimol 45 min, but not 60 min, after reactivation significantly reduced fear responding. This disruption by muscimol was specific to re-exposure to the training context, showing that the effects of muscimol were specific to memory reactivation. These results strongly indicate that muscimol explicitly disrupted a reconsolidation process. They next examined the influence of post-training and post-reactivation administration of bicuculline. Results showed that immediate but not delayed (1 h) post-training bicuculline facilitated retention of a weak fear memory, showing that bicuculline specifically enhanced a consolidation process. Similarly, post-reactivation administration of bicuculline enhanced retention of fear memory. The effect occurred when bicuculline was injected 45 min, and 2 h, but not 4 h following reactivation, implying that bicuculline specifically enhanced a reconsolidation process. These results provide very strong evidence for the role of GABAA receptors in both the storage and re-storage of fear memories. Increasing GABAergic transmission with muscimol interferes, whereas decreasing GABAergic transmission with bicuculline facilitates, both consolidation and reconsolidation of contextual fear memories. In summary, then, the majority of the evidence indicates that GABA agonists administered after CS re-exposure leads to less fear responding, suggesting that activation of GABAA receptors interferes with the reconsolidation of fear memories.
Reconsolidation vs Extinction
The studies reviewed above indicate that infusion of a GABA agonist leads to a reduction in fear, suggesting that reconsolidation was impaired. Alternatively, the decline in fear responding might have reflected a facilitation of extinction. This is because the reactivation session is procedurally similar to an extinction training trial, as animals are exposed to the CS without the US (Nader, 2003; see Figure 1). However, as extinction is regarded as a form of new learning, GABA agonists would be expected to disrupt extinction learning, rather than facilitate it (Akirav, 2007; Myers and Davis, 2007). Numerous studies have shown that BZs do in fact impair extinction retention (see Table 2). Furthermore, the MDZ-induced memory deficits observed by Bustos et al (2006, 2009) and Cranney and co-workers (Makkar et al, 2010; Zhang and Cranney, 2008) did not exhibit spontaneous recovery or reinstatement, which are common features of extinguished responding. This evidence indicates that the freezing deficits produced by post-reactivation MDZ represent a disruption of reconsolidation and not a facilitation of extinction.
Nonetheless, there are manipulations that can facilitate extinction, but also prevent phenomena such as spontaneous recovery, renewal, and reinstatement, eg, the extinction of a compound composed of two separately extinguished CSs (Rescorla, 2006), or post-extinction training administration of DCS (Ledgerwood et al, 2004). Thus, a lack of spontaneous recovery or reinstatement is not sufficient evidence to discriminate between disrupted reconsolidation and facilitated extinction. Furthermore, as discussed in the previous section, downregulation of the GABAergic system may facilitate extinction in some circumstances (Akirav et al, 2006; Chhatwal et al, 2005; Harris and Westbrook, 1998a; Heldt and Ressler, 2007). Thus, there is still the possibility that the post-reactivation freezing deficits observed by Bustos et al (2006) and Zhang and Cranney (2008) was actually a facilitation of extinction.
Bustos et al (2009) provided very strong evidence that the memory deficit shown by post-reactivation administration of GABAergic drugs (ie, MDZ) is not a facilitation of extinction, but rather a disruption of reconsolidation. First, MDZ administration after a brief (3 or 5-min) re-exposure to the context led to a persistent deficit in fear responding, indicating impaired reconsolidation. To test whether the freezing deficit was merely facilitated extinction, animals were injected with MDZ after an extended re-exposure to the context (ie, extinction training). At test, control rats exhibited low levels of freezing comparable with the last 5 min of extinction training, indicating intact retention of the extinction memory. However, MDZ-treated rats showed greater freezing relative to controls, suggesting that MDZ disrupted extinction (see also Hart et al, 2009). The authors concluded that the reduction in freezing produced by MDZ administration after a brief reactivation is not due to facilitated consolidation of extinction, but a disruption in reconsolidation. These findings further support our position that activation of GABAA receptors is detrimental to the storage of reactivated fear memories and newly acquired extinction memories. Specifically, when CS re-exposure is brief and reconsolidation is the dominant memory process, GABAergic manipulations block the re-storage of the original memory trace, leading to a decrement in fear responding at test (see Figure 1). However, if CS re-exposure is lengthened and the extinction memory is the dominant trace, GABAergic drugs interfere with the consolidation of the extinction memory, leading to elevated freezing at test.
Storage vs Retrieval
Another major theoretical issue is whether the GABA-induced reconsolidation blockade represents a failure to re-store the memory, or a temporary inability to retrieve the memory. Several studies have shown that the memory deficits elicited by post-reactivation MDZ lasted for at approximately 2 weeks after initial conditioning (Bustos et al, 2006, 2009; Makkar et al, 2010; Zhang and Cranney, 2008). Furthermore, this memory deficit was not reinstated after exposure to a reminder shock, or an additional context-shock pairing (Bustos et al, 2006). This evidence implies that post-reactivation administration of GABAergic drugs disrupt the re-storage, and not the retrieval of fear memories.
These long-lasting memory deficits conflict with the research conducted by Harris and Westbrook (1998b, 1999, 2001), examining the effects of MDZ on the initial acquisition of fear conditioning. As discussed earlier, pre-training MDZ decreased freezing when animals were tested; however, this deficit was no longer evident when rats (a) were tested 22 days after conditioning, (b) were tested in a chamber that differed from fear conditioning, or (c) were injected with formalin before testing. They argued that BZs do not disrupt memory storage but simply induce a context-specific inhibition of fear responding (context referring to both the physical features of the environment as well as the internal cues—hormonal, emotional, and neurochemical, which are present during conditioning). Consequently, changing the context before testing would reinstate fear responding. Makkar et al (2010) directly tested this hypothesis with the reconsolidation paradigm. Animals underwent context pre-exposure, conditioning, and memory reactivation followed by MDZ administration. On test day, animals were injected with either saline or epinephrine 15 min before testing in order to shift the internal context of the animal. This is a similar manipulation to the formalin injected before testing in the Harris and Westbrook (1998b) study. Importantly, the results showed that the MDZ-induced freezing deficit persisted despite the pre-test injection of epinephrine. Makkar et al (2010) concluded that MDZ blocked reconsolidation, rather than inducing a context-specific retrieval deficit. However, they acknowledged the possibility that the MDZ-induced retrieval-deficit observed by Harris and Westbrook (1998b) might be specific to the acquisition, and not the reconsolidation of fear memories (Makkar et al, 2010).
In contrast to the above-mentioned findings, Amaral et al (2007) showed that infusion of muscimol into the dorsal hippocampus of rats disrupted retention of IA memory when tested 24 h, but not 48 h after reactivation (Amaral et al, 2007). These results suggest that GABA had temporarily inhibited the retrieval, rather than the reconsolidation of reactivated memories. This discrepancy might be attributable to procedural differences among the studies. First, different GABAergic drugs were used: Those studies that observed long-lasting deficits used MDZ, whereas Amaral et al (2007) infused muscimol. Despite the fact that both drugs are GABAA receptor agonists, they each have distinct binding sites (see Figure 2). It is possible that BZs, specifically, produce long-lasting memory deficits. Second, different memory tasks were used: Amaral et al (2007) used IA, whereas the previous studies examined contextual fear conditioning. Although both tasks require animals to form an association between the context and shock, conditioned responding is measured differently in each task. Finally, the location of drug administration differed in each study: Amaral et al (2007) infused muscimol directly into the hippocampus, whereas in the MDZ studies it was injected systemically. Numerous laboratories have shown that intra-hippocampal infusion of protein synthesis inhibitors and other amnesic drugs after reactivation either have no disruptive effect or only produce a temporary deficit in IA memory (Cammarota et al, 2004; Power et al, 2006; Prado-Alcala et al, 2006; Taubenfeld et al, 2001). This indicates that the hippocampus might not be involved in reconsolidation of IA (Milekic et al, 2007), and may explain why Amaral et al (2007) observed only a transient deficit relative to the enduring deficits shown by Bustos et al (2006, 2009) and Zhang and Cranney (2008). In light of these findings, it can be concluded that post-reactivation injections of GABAA receptor agonists may disrupt the re-storage of fear memories; however, this is dependent on the type of memory paradigm, the type of ligand, and the precise location of drug infusion.
Studies examining the role of GABA in reconsolidation have shown that application of GABA agonists immediately after memory reactivation impairs the retention of fear memories (Amaral et al, 2007; Bustos et al, 2006, 2009; Makkar et al, 2010; Zhang and Cranney, 2008) and application of GABA antagonists facilitate fear memory retention (Carbo Tano et al, 2009; Bustos et al, 2009). These findings are in agreement with the acquisition, consolidation, and extinction evidence discussed in this review, that GABAA agonists disrupt memorial processes.
Neural Bases
Amygdala
As in consolidation, the amygdala is also important for the reconsolidation of fear memories. Studies have demonstrated that post-reactivation infusions of anisomycin into the BLA disrupt retention of auditory fear memories when animals are tested 24 h later (Duvarci and Nader, 2004; Duvarci et al, 2006; Nader et al, 2000; Parsons et al, 2006). This suggests that reactivated memories require protein synthesis-dependent reconsolidation within the amygdala in order to be re-stored.
Fear memory reconsolidation also requires the activation of various receptor pathways within the BLA. Post-reactivation blockade of NMDA receptors (using MK-801; Lee et al, 2006; Suzuki et al, 2004), β-adrenergic receptors (β-ARs) (using propanolol; Debiec and LeDoux, 2004), and GC receptors (using RU486; Jin et al, 2007) within the BLA disrupt long-term fear memory retention. Interestingly, Lee et al (2006) demonstrated that intra-BLA infusion of the partial NMDA agonist DCS before reactivation, facilitated auditory fear memory. This evidence suggests that receptor activity within the BLA is critically involved in the re-storage of reactivated fear memories.
Given these studies, it is possible that GABAA receptor agonists disrupt reconsolidation by binding to GABAA receptors in the amygdala, particularly those containing the α1-subunit. However, Makkar et al (2010) found that intra-BLA infusions of MDZ had no effect on contextual fear memory retention when injected immediately after reactivation. Interpretation of this finding requires caution due to the small sample used in the study. Moreover, this finding does not rule out the possibility that GABAA receptors within the BLA are involved in reconsolidation with other fear memory tasks such as discrete cue conditioning and IA. Future research is clearly needed.
Hippocampus
The hippocampus has also been shown to be important for fear memory reconsolidation, similar to initial consolidation. Studies have demonstrated a requirement for protein synthesis (Debiec et al, 2002; Frankland et al, 2006), mRNA synthesis (Da Silva et al, 2008), transcription factors (nuclear factor κB—Lubin and Sweatt, 2007; ZIF268—Duvarci et al, 2005), and immediate early gene activation (zif268—Hall et al, 2001; c-Fos, junB—Strekalova et al, 2003; SGK3—von Hertzen and Giese, 2005) within the hippocampus for both consolidation and reconsolidation of contextual fear memories.
Recent evidence suggests that hippocampal GABAA receptors may be involved in fear memory reconsolidation. As previously described, Amaral et al (2007) showed that post-reactivation infusion of muscimol into the hippocampus disrupted retention of IA memory. However, this deficit was only temporary. This indicates that intra-hippocampal activation of GABAA receptors after reactivation disrupted retrieval, rather than storage of the fear memory. It is important to note that this is the only study examining the role of hippocampal GABAA receptors in the reconsolidation of fear memories, and further research is warranted. The temporary retrieval deficit observed by Amaral et al (2007) might have been due to the specific task they used—IA. Increasing evidence suggests that the hippocampus might not be involved in the reconsolidation of IA (Cammarota et al, 2004; Milekic et al, 2007; Power et al, 2006; Prado-Alcala et al, 2006; Taubenfeld et al, 2001). Further studies examining other hippocampally mediated tasks, such as contextual fear conditioning or trace fear conditioning, are required to determine whether hippocampal GABAA receptors are involved in the reconsolidation of fear memories.
The evidence discussed indicates that post-reactivation administration of GABAA receptor agonists interferes with fear memory retention. This suggests that memory re-stabilization after retrieval is dependent on reduced GABAergic transmission. However, as demonstrated, scarce research has examined the neural and molecular pathways mediating the effects of GABA on memory re-storage after reactivation.
GABA AND MEMORY ACQUISITION, CONSOLIDATION, RECONSOLIDATION, AND EXTINCTION: A COMMON NEUROMOLECULAR PATHWAY?
The evidence discussed in this review strongly implies that memory storage after conditioning, reactivation, and extinction training (see Figure 1) is dependent on a common mechanism of reduced GABAergic inhibitory neurotransmission. The formation of memory after initial conditioning seems to require the downregulation of GABAergic transmission in neural circuits such as the amygdala, and in the hippocampus if the CS is a context. A reduction in GABAergic inhibitory transmission would allow for the activation of various intracellular cascades that are essential for the stabilization and re-stabilization of the fear memory (Bustos et al, 2006; Luft et al, 2004). Research by McGaugh and co-workers (Hatfield et al, 1999; Introini-Collison et al, 1994; Roozendaal et al, 1999) has indicated that reduced GABA transmission facilitates memory storage by increasing the release of norepinephrine (NE) and enhancing activation of β-ARs within the amygdala.
In support of this hypothesis, Introini-Collison et al (1994) demonstrated that the retention-impairing effects of muscimol were reversed by simultaneous administration of NE. In contrast, the retention-enhancing effects of bicuculline (a drug that decreases GABA transmission) were blocked by simultaneous injection of the β-AR antagonist clenbuterol (a drug that decreases NE activation of β-ARs). Consistent with these findings, Hatfield et al (1999) showed that systemic administration of muscimol (which impairs memory retention) decreased the levels of NE within the amygdala. Conversely, systemic injections of picrotoxin (which typically facilitates retention) increased the levels of NE within the amygdala.
Collectively, these findings seem to suggest that following initial training there is a downregulation of GABAergic transmission in the amygdala and hippocampus. This triggers the release of NE within the amygdala. NE could then mediate memory storage by binding to and activating β-ARs within the amygdala and hippocampus and other brain regions involved in the consolidation of the memory trace (such as the caudate, or cerebral cortex; Hatfield et al, 1999; Mueller and Cahill, 2010; Roozendaal et al, 1999). Indeed, studies have shown that post-training intra-BLA infusion of NE enhances, whereas post-training blockade of β-ARs disrupts the consolidation of memory in both fear conditioning (Liang et al, 1986, 1990; Miranda et al, 2003) and object recognition paradigms (Roozendaal et al, 2008). Stimulation of β-ARs by NE activates adenylyl cyclase, which initiates cAMP formation (Seeds and Gilman, 1971). cAMP then activates PKA, which leads to two outcomes that facilitate memory storage: first, PKA enhances the excitability of neurons by phosphorylation of ion channels and receptors and AMPA receptor trafficking (Kandel, 2001; Hu et al, 2007); second, PKA, together with MAPK, trigger the phosphorylation of transcription factors such as CREB, which initiates gene transcription and synthesis of proteins (Graham, 1990; Kandel, 2001; Eisenberg et al, 2003). The proteins are then used for the synaptic modifications necessary for stabilizing the memory trace (see Figure 5).
Figure 5.
Hypothesized GABAergic molecular pathway of acquisition/consolidation, reconsolidation and extinction consolidation. After each critical procedural event (conditioning, reactivation, and extinction training, respectively; see Figure 1), there is a downregulation of GABAergic molecules within synapses, possibly in different areas of the amygdala, hippocampus and PFC, depending on the phenomenon. This leads to disinhibition of noradrenergic neurons, leading to elevated release of norepinephrine (NE). NE then binds to and activates β-adrenergic receptors. This triggers the intracellular activation of the enzyme adenylyl cyclase (by G-proteins), which initiates the formation of cyclic AMP (cAMP). cAMP then triggers upregulation of Protein Kinase A (PKA). PKA enhances cell excitability by the phosphorylating of receptors and ion channels (such as K+ channels), causing them to close. PKA also facilitates AMPA receptor trafficking. In addition, PKA together with MAPK stimulates the phosphorylation of various transcription factors such as CREB, which are involved in gene transcription and the activation of immediate early genes (IEGs). The products of gene transcription are used for protein synthesis. The proteins are then used for the synaptic modifications necessary for stabilization of the new memory, reactivated memory or the extinction trace. Abbreviations: AMPA=α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; ATP=adenosine triphosphate; cAMP=cyclic AMP; CREB=cAMP response element-binding protein; CS=conditioned stimulus; GABA=γ-amino butyric acid; MAPK=mitogen-activated protein kinase; PKA=Protein kinase A; US=unconditioned stimulus.
Re-exposure to the CS triggers two competing molecular processes: reconsolidation and extinction. According to Eisenberg et al (2003), when reactivation is short, the original memory trace (ie, the CS–US association) returns to a labile state and reconsolidation is initiated. Administration of GABAA receptor agonists disrupt this dominant reconsolidation process, leading to reduced fear responding at test (Bustos et al, 2006, 2009). The findings discussed in this review suggest that the reconsolidation, just as initial consolidation, involves the downregulation of GABAergic neurotransmission. However, very little research has investigated the neural circuits where this may take place, with mixed evidence regarding the amygdala and hippocampus. Nonetheless, we propose that the neurochemical processes mediating reconsolidation of fear memories after brief CS re-exposure proceeds in a manner similar to that following initial training. Specifically, after retrieval, a reduction in GABAergic transmission would take place in specific brain regions, which would then stimulate the release of NE, perhaps within the amygdala (Debiec and LeDoux, 2004). NE would then bind to β-ARs, triggering a similar intracellular cascade of events involving activation of adenylyl cyclise, formation of cAMP, activation of PKA, gene transcription, and the synthesis of proteins. The proteins are then used to re-stabilize the memory trace (see Figure 5).
When CS re-exposure is extended, the formation of a new inhibitory memory (ie, the extinction memory) is triggered within a different neural area (Eisenberg et al, 2003; Lee et al, 2006; Suzuki et al, 2004). At this stage, conditioned responding has begun to extinguish. Administration of the GABAA receptor agonist at this point will disrupt the now dominant extinction process, leading to intact fear responding at test (Akirav, 2007; Berlau and McGaugh, 2006; Bustos et al, 2009). Therefore, the formation of fear extinction memory appears to require a downregulation of GABAergic neurotransmission, consistent with initial consolidation and reconsolidation. Studies outlined in this review indicate that this downregulation takes place in the BLA (Akirav, 2007; Berlau and McGaugh, 2006; Hart et al, 2009), hippocampus (Corcoran et al, 2005; Hobin et al, 2006), and possibly the IL (Akirav et al, 2006). As for consolidation and reconsolidation, we propose that reduced GABA transmission facilitates the storage of extinction memories by increasing the release of NE and activation of β-ARs. In line with this suggestion, Berlau and McGaugh (2006) demonstrated that post-extinction training infusion of bicuculline into the BLA facilitated extinction memory; however, this effect was blocked by simultaneous co-administration of the β-blocker propranolol. Moreover, infusion of NE into the BLA after extinction training was shown to facilitate consolidation of fear extinction memory (Berlau and McGaugh, 2006). In addition, systemic and intra-IL infusions of propranolol impair extinction retention (Ouyang and Thomas, 2005; Mueller et al, 2008). These studies suggest that GABAergic modulation of extinction might depend on noradrenergic mechanisms within the amygdala and possibly the IL. Specifically, after extended re-exposure to the CS (ie, extinction training), there is a downregulation of GABA in neural circuits such as the amygdala, hippocampus, and IL. Once again, this might stimulate the release of NE within these areas which binds to β-ARs, activating a similar intracellular cascade of events (see Figure 5), ultimately leading to the synthesis of proteins which are used to stabilize the new extinction memory trace (Mueller and Cahill, 2009).
The administration of GABAA receptor agonists disrupts memories when administered before and after initial conditioning, re-activation, and extinction training. This indicates that the storage of new, reactivated, and extinction memory traces is dependent on a reduction of GABAergic transmission. Here, it is proposed that, at least for consolidation and extinction, the reduction of GABA transmission facilitates memory storage by increasing NE release and noradrenergic signaling in the amygdala. This triggers an intracellular cascade, leading to the synthesis of proteins, which are used for stabilizing the fear memory trace. In light of this model and the findings discussed in this review, infusion of GABAA receptor agonists such as MDZ might have disrupted consolidation, reconsolidation, or extinction by increasing GABAergic transmission, inhibiting release of NE, and reducing activation of β-ARs within the amygdala, hippocampus and/or IL. This would have prevented the phosphorylation of protein kinases, transcription factors, and IEGs, leading to a reduction in protein synthesis. As a result, storage of the new reactivated or inhibitory (extinction) memory would have been blocked, thereby resulting in a lack of evidence for memory retention at test.
Future studies could investigate the validity of this mechanism through a number of means. For example, if GABAA agonists block consolidation, reconsolidation and/or extinction by inhibiting NE release, simultaneous infusion of NE into brain regions involved in fear memory should reverse the amnesic effects of systemically administered drugs such as midazolam. In contrast, the effects of memory-enhancing drugs such as bicuculline should be blocked by simultaneous administration of drugs that block NE activation of β-ARs (such as propranolol). Another method of investigation, as used by Hatfield et al (1999), involves the use of microdialysis probes implanted into brain regions involved in fear memory. Researchers could examine whether systemic administration of GABAA agonists leads to a decrease in NE levels, or whether GABAA antagonists produces an increase in NE in these brain regions. If the animal displays the respective retention deficit or enhancement, this would provide additional evidence that GABAergic drugs modulate memory by altering the levels of NE within neural circuits such as the amygdala.
Clinical Implications
Throughout this discussion, the evidence has suggested that GABAergic transmission is detrimental to the persistence of fear memories. This may have implications for the treatment of anxiety disorders in humans, particularly those disorders associated with maladaptive and intrusive fear memories such as post-traumatic stress disorder, social phobia, and specific phobia (Day et al, 2004; Durand and Barlow, 2006; Ehlers and Clark, 2000; Hackmann and Holmes, 2004; Hackmann et al, 2000; Rachman, 1991). Specifically, GABA agonists, particularly BZs, such as midazolam or diazepam could be administered immediately after briefly re-exposing patients to fear-related stimuli in order to block the reconsolidation of fear memories, and thereby reduce subsequent anxiety symptoms. Numerous animal studies have shown that the reduced fear responding produced by midazolam does not recover over time, with a shift in the internal state, or following re-exposure to the US (Bustos et al, 2006, 2009; Makkar et al, 2010; Zhang and Cranney, 2008). This suggests that combining BZs with brief cue exposure might be an effective and lasting treatment for anxiety disorders. This is a realistic possibility given that (a) experimental studies have shown that reconsolidation of conditioned fear can be disrupted in humans using the β-adrenoceptor antagonist propranolol (Miller et al, 2004), and (b) midazolam is already being used in clinical settings for its sedative and anxiolytic effects (Pain et al, 2002).
However, there is the possibility that if CS re-exposure is too long, GABAA agonists could disrupt extinction of the fear memory, as demonstrated by Bustos et al (2009). The consequence of disrupting extinction would be a persistence of anxiety symptoms, thereby worsening the problem. Therefore, if BZs are to be used in conjunction with brief cue exposure, clinicians will need to pay close attention to the length of re-exposure to the fear eliciting cue. They will need to ensure that cue exposure is brief, and that reduction in anxiety (ie, within-session extinction) does not occur throughout the exposure session, otherwise the BZ will disrupt the extinction memory, leading to maintenance of fear and anxiety. Further research examining the effect of administering GABAA receptor agonists following variations in the duration of CS re-exposure are required to determine the optimal duration. In addition, the finding that reconsolidation of older fear memories can be disrupted but require longer CS re-exposures and higher drugs dosages (Bustos et al, 2009) introduces additional complications. Specifically, the clinician may need to vary the length of re-exposure and drug dosage based on the age of the memory or the duration of the disorder. This could involve a process of trial-and-error, which may be time-consuming for the therapist and detrimental to the client who is not receiving the immediate treatment they require.
Nonetheless, the finding that MDZ is capable of disrupting the reconsolidation of remote fear memories is promising for the use of BZs and brief cue exposure in treating anxiety disorders. This is because individuals with anxiety disorders often wait many years before seeking treatment for such disorders (Durand and Barlow, 2006; Foa et al, 2000). However, assessing the age of fear memories is a difficult task in the context of human anxiety disorders. For example, in specific phobia, patients often do not remember a specific incident that elicited their fear, claiming to have always been afraid of spiders, or have always been shy (Rachman, 1991). In light of these issues, it is clear that basic research examining the effects of BZs in memory reconsolidation in humans is required in order to determine if combining BZs with brief cue exposure is a potentially viable treatment for human anxiety disorders. In addition, of course, the chemical efficacy of GABA antagonists facilitating long-duration cue exposure (extinction training) also requires further investigation.
CONCLUSION
Throughout this review, we have provided evidence that fear memory formation, reconsolidation, and extinction are dependent upon reduced activation of GABAA receptors in various regions of the brain. Pre- and post-training administration of drugs that increase GABAergic transmission leads to decreased fear responding. This suggests that GABAA receptors, possibly within the amygdala and hippocampus, are involved in the acquisition and consolidation of fear memories. Consistent with these findings, administration of GABAA receptor agonists immediately after a brief CS re-exposure disrupt, whereas GABAA receptor antagonists facilitate subsequent fear responding. This indicates that GABAA receptors are also involved in the reconsolidation of fear memories after retrieval. Finally, increasing GABAergic transmission both before and immediately after extinction training has been shown to block response inhibition. These results indicate that activation of GABAA receptors interferes with the acquisition and consolidation of extinction memories. A number of contradictory results have emerged, although we have presented evidence suggesting that these conflicting results are due to variations in (a) the location of drug infusion, (b) the dosage of the drug and/or (c) the time point of drug administration. Therefore, the evidence presented in this review strongly implies that the processes mediating memory persistence after initial fear learning, memory reactivation, and extinction training are dependent on a common mechanism of reduced GABAergic transmission. The current findings suggest that this downregulation of GABAergic transmission most likely takes place in the amygdala, hippocampus (ie, if the CS is a context), or the mPFC (during extinction training). We propose that the downregulation of GABA modulates memory storage by facilitating the release of NE. NE then binds to β-ARs, initiating an intracellular cascade that culminates in the synthesis of new proteins, which are used for the synaptic changes required to stabilize the new, reactivated, or inhibitory (extinction) memory trace.
The finding that GABA consistently disrupts various forms of memory retention suggests that in the future a viable and lasting treatment for anxiety disorders in humans can be achieved through methods such as combining BZs with brief cue exposure. Furthermore, given the disruptive effect of GABA on memory retention, we can speculate that GABA might have an adaptive role in allowing organisms to forget irrelevant information (Kim et al, 2006).
The authors declare no conflict of interest.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Akirav I. NMDA partial agonist reverses blocking of extinction of aversive memory by GABA (A) agonist in the amygdala. Neuropsychopharmacology. 2007;32:542–550. doi: 10.1038/sj.npp.1301050. [DOI] [PubMed] [Google Scholar]
- Akirav I, Haizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behavioral Brain Res. 2007;180:113–118. doi: 10.1016/j.bbr.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the b subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Ammassari-Teule M, Pavone F, Castellano C, McGaugh JL. Amygdala and dorsal hippocampus lesions block the effects of GABAergic drugs on memory storage. Brain Res. 1991;551:104–109. doi: 10.1016/0006-8993(91)90919-m. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang W, Feldon J. The ventral hippocampus and fear conditioning in rats: Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABAA agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33:2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilisers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Nagahara AH, McGaugh JL. Involvement of the amygdala GABAergic system in the modulation of memory storage. Brain Res. 1989;487:105–112. doi: 10.1016/0006-8993(89)90945-1. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139:831–842. doi: 10.1016/j.neuroscience.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: Decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34:446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- Calton JL, Mitchell KG, Schachtman TR. Conditioned inhibition produced by extinction of a conditioned stimulus. Learning and Motivation. 1996;27:335–361. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. Retrieval does not induce reconsolidation of inhibitory avoidance memory. Learning and Memory. 2004;11:572–578. doi: 10.1101/lm.76804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo Tano M, Molina VA, Maldonado H, Pedreira ME. Memory consolidation and reconsolidation in an invertebrate model: The role of the GABAergic system. Neuroscience. 2009;158:387–401. doi: 10.1016/j.neuroscience.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Carlson NR.2004Physiology of Behavior8th edn.Pearson Education Inc.: Boston [Google Scholar]
- Carrive P. Conditioned fear to environmental context: cardiovascular and behavioural components in the rat. Brain Res. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–179. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Retention enhancement with post-training picrotoxin. Behav Neural Biol. 1989;51:165–170. doi: 10.1016/s0163-1047(89)90797-8. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL. Effects of post training bicuculline and muscimol on retention: lack of state dependency. Behav Neural Biol. 1990;54:156–164. doi: 10.1016/0163-1047(90)91352-c. [DOI] [PubMed] [Google Scholar]
- Cestari V, Costanzi M, Castellano C, Rossi-Arnaud C. A role for ERK2 in reconsolidation of fear memories in mice. Neurobiol Learn Mem. 2006;86:133–143. doi: 10.1016/j.nlm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier S, Forquer MR, Sorg BA. Low level lindane exposure alters extinction of conditioned fear in rats. Toxicology. 2006;217:147–154. doi: 10.1016/j.tox.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission inmice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Scie USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh JL. Microinfusions of flumazenil into the basolateral but not the central nucleus of the amygdala enhance memory consolidation in rats. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- Da Silva WC, Bonini JS, Bevilaqua LRM, Medina JH, Izquierdo I, Cammarota M. Inhibition of mRNA synthesis in the hippocampus impairs consolidation and reconsolidation of spatial memory. Hippocampus. 2008;18:29–39. doi: 10.1002/hipo.20362. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-amino butyric acid in fear extinction: clinical implications for exposure therapy. Soc Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Day S, Holmes EA, Hackmann A. Occurrence of imagery and its link with early memories in agoraphobia. Memory. 2004;12:416–427. doi: 10.1080/09658210444000034. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditoning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Tomaz C. Amnesia after diazepam infusion into basolateral but not central amygdala of Rattus norvegicus. Neuropsycholbiology. 1995;32:31–36. doi: 10.1159/000119209. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Delamater AR, Treit D. Chlordiazepoxide attenuates shock-based and enhances LiCl-based fluid aversions. Learning and Motivation. 1988;19:221–238. [Google Scholar]
- Dickinson-Anson H, McGaugh JL. Midazolam administered into the amygdala impairs retention of an inhibitory avoidance task. Behav Neural Biol. 1993;60:84–87. doi: 10.1016/0163-1047(93)90781-c. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, McGaugh JL. Bicuculline administered into the amygdala after training blocks benzodiazepine-induced amnesia. Brain Res. 1997;752:197–202. doi: 10.1016/s0006-8993(96)01449-7. [DOI] [PubMed] [Google Scholar]
- Disorbo A, Wilson GN, Bacik S, Hoxha Z, Biada JM, Mickley A. Time-dependent retrograde amnesic effects of muscimol on conditioned taste aversion extinction. Pharmacol, Biochem Behav. 2009;92:319–326. doi: 10.1016/j.pbb.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovina NI, Zinov'ev DR. Contribution of GABA receptors to extinction of memory traces in normal conditions and in a depression-like state. Neurosci Behav Physiol. 2008;38:775–779. doi: 10.1007/s11055-008-9045-y. [DOI] [PubMed] [Google Scholar]
- Durand VM, Barlow DH.2006Essentials of Abnormal Psychology4th edn.,Thomson-Wadsworth: Belmont [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Esmaeili A, Lynch JW, Sah P. GABAA receptors containing gamma1 subunits contribute to inhibitory transmission in the central amygdala. J Neurophysiol. 2009;101:341–349. doi: 10.1152/jn.90991.2008. [DOI] [PubMed] [Google Scholar]
- Evans MS, Viola-McCabe KE. Midazolam inhibits long term potentiation through modulation of GABAA receptors. Neuropharmacology. 1996;35:347–357. doi: 10.1016/0028-3908(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Farkash L, Cranney J.2010Functional Mechanisms Underlying the Midazolam-Induced Deficits in Contextual Fear RespondingUnpublished manuscript,University of New South Wales: Sydney, Australia [Google Scholar]
- File SE, Pellow S. Low and high doses of benzodiazepine receptor inverse agonists respectively improve and impair performance in passive avoidance but do not affect habituation. Behav Brain Res. 1988;30:31–36. doi: 10.1016/0166-4328(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Foa EB, Keane MT, Friedman MJ. Guidelines for the treatment of PTSD. J Traumatic Stress. 2000;13:539–588. doi: 10.1023/A:1007802031411. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding H, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. 5 subtypes of type-A gamma-aminobutyric-acid receptors identified in neurons by double and triple immunofluoresence staining with subunit specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Takagi H, Iwasaki T. Effects of chlordiazepoxide on passive avoidance learning in Wistar-Imamichi and F344/Du strain rats. Japn J Psychopharmacol. 1987;7:307–312. [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Effects of post-training hippocampal injections of midazolam on fear conditioning. Learn Mem. 2005;12:573–578. doi: 10.1101/lm.51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS. Effect of chlordiazepoxide administered early in extinction on subsequent extinction of a conditioned emotional response in rats: Implications for human clinical use. Psychol Reports. 1977;40:783–786. doi: 10.2466/pr0.1977.40.3.783. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Hefner MA, Robichaud RC, Dubinksy B. Effects of delta9-tetrahydrocannabinol (THC) and chlordiazepoxide (CDP) on state-dependent learning: EVidence for assymetrical dissociation. Psychopharmacologia. 1973;30:173–184. doi: 10.1007/BF00421432. [DOI] [PubMed] [Google Scholar]
- Gorman JE, Dyak JD, Reid LD. Methods of deconditioning persisting avoidance: Diazepam as an adjunct to response prevention. Bull Psychon Soc. 1979;14:46–48. [Google Scholar]
- Graham B.2006The Effect of Gamma-Aminobutyric Acid Antagonist on the Acquisition and Consolidation of Extinction of Pavlovian FearUnpublished honours thesis,University of New South Wales: Sydney, Australia [Google Scholar]
- Graham RM. Adrenergic receptors: structure and function. Cleve Clinic J Med. 1990;57:481–491. doi: 10.3949/ccjm.57.5.481. [DOI] [PubMed] [Google Scholar]
- Hackmann A, Clark DM, McManus F. Recurrent images and early memories in social phobia. Behav Res Ther. 2000;38:601–610. doi: 10.1016/s0005-7967(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Hackmann A, Holmes EA. Reflecting on imagery: a clinical perspective and overview of the special issue of Memory on mental imagery and memory in psychopathology. Memory. 2004;12:389–402. doi: 10.1080/09658210444000133. [DOI] [PubMed] [Google Scholar]
- Haefely WE. Pharmacology of the benzodiazepine receptor. Eur Arch Psychiatry Neurol Sci. 1989;238:294–301. doi: 10.1007/BF00449811. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998a;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Benzodiazepine-induced amnesia in rats: reinstatement of conditioned performance by noxious stimulation on test. Behav Neurosci. 1998b;112:183–192. doi: 10.1037//0735-7044.112.1.183. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. The benzodiazepine midazolam does not impair Pavlovian fear conditioning but regulates when and where fear is expressed. J Exp Psychol: Animal Behavior Processes. 1999;25:236–246. doi: 10.1037//0097-7403.25.2.236. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Contextual control over the expression of fear in rats conditioned under a benzodiazepine. Psychopharmacology. 2001;156:92–97. doi: 10.1007/s002130100757. [DOI] [PubMed] [Google Scholar]
- Hart G, Harris JA, Westbrook RF. Systemic or intra-amygdala injection of a benzodiazepine (midazolam) impairs extinction but spares re-extinction of conditioned fear responses. Learn Mem. 2009;16:53–61. doi: 10.1101/lm.1154409. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson BG, Jarbe T. Effects of diazepam on conditioned avoidance learning in rats and its transfer to normal state conditions. Psychopharmacologia. 1971;20:186–190. doi: 10.1007/BF00404372. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav Neural Biol. 1994;61:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Ishitobi S, Ayuse T, Yoshida H, Oi K, Toda K, Miyamoto T. Effects of midazolam on acquisition and extinction of conditioned taste aversion memory in rats. Neurosci Lett. 2009;450:270–274. doi: 10.1016/j.neulet.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Da Cunha C, Huang CH, Walz R. Post-training down-regulation of memory consolidation by a GABAA mechanism in the amygdala modulated by endogenous benzodiazepines. Behav Neural Biol. 1990a;54:105–109. doi: 10.1016/0163-1047(90)91282-g. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Pereira ME, Medina JH. Benzodiazepine receptor ligand influences on acquisition: Suggestion of an endogenous modulatory mechanism mediated by benzodiazepine receptors. Behav Neural Biol. 1990b;54:27–41. doi: 10.1016/0163-1047(90)91221-v. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann Cryan JF. GABA-B(1) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26:8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RA, Martinez JL, Vasquez BJ, McGaugh JL. Benzodiazepines alter acquisition and retention of an inhibitory avoidance response in mice. Psychopharmacology. 1979;64:125–126. doi: 10.1007/BF00427358. [DOI] [PubMed] [Google Scholar]
- Jin XC, Lu YF, Yang XF, Ma L, Li BM. Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci. 2007;25:3702–3712. doi: 10.1111/j.1460-9568.2007.05621.x. [DOI] [PubMed] [Google Scholar]
- Johnston GAR. GABAA receptor pharmacology. Pharmacology and Ther. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Kamano DK. Using drugs to modify the effect of response prevention on avoidance extinction. Behav Res Ther. 1972;10:367–370. doi: 10.1016/0005-7967(72)90059-9. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1039. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim JH, McNally G, Richardson R. Recovery of fear memories in rats: role of GABA in infantile amnesia. Behav Neurosc. 2006;120:40–48. doi: 10.1037/0735-7044.120.1.40. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. Expression of renewal is dependent on the extinction-test interval rather than the acquisition-extinction interval. Behav Neurosci. 2009;123:641–649. doi: 10.1037/a0015237. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear in rats. Soc Neurosci Abstracts. 2008;34:487.415. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Conditioned Reflexes and Neuronal Organization. Cambridge University Press: London; 1948. [Google Scholar]
- Lan NC, Gee KW. Neuroactive steroid actions at the GABAA receptor. Hormones Behav. 1994;28:537–544. doi: 10.1006/hbeh.1994.1052. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-Cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, et al. Synaptic protein Degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15:876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Pereira GS, Cammarota M, Izquierdo I. Different time course for the memory facilitating effect of bicuculline in hippocampus, entorhinal cortex, and posterior parietal cortex of rats. Neurobiol Learn Mem. 2004;82:52–56. doi: 10.1016/j.nlm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Cranney J, Zhang S.2010Mechanisms Underlying Midazolam Disruption of Reconsolidation of Contextually Conditioned FearUnpublished manuscript,University of New South Wales: Sydney, Australia [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marsciano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Hermann H, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci. 1990;104:264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- McNamara RK, dePape GE, Skelton RW. Differential effects of benzodiazepine receptor agonists on hippocampal long-term potentiation and spatial learning in the Morris water maze. Brain Res. 1995;626:63–70. doi: 10.1016/0006-8993(93)90563-3. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;116:389–395. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milekic HM, Pollonini G, Alberini CM. Temporal requirement of C/EBPß in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn Mem. 2007;14:504–511. doi: 10.1101/lm.598307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Atemus M, Debiec J, LeDoux JE.2004Propranolol impairs reconsolidation of conditioned fear in humans Soc Neurosci Abstracts208.2.
- Miller RR, Springer AD. Amnesia, consolidation and retrieval. Psychol Rev. 1973;80:69–79. doi: 10.1037/h0033897. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Lalumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2009;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K. A single standard for memory; the case for reconsolidation. Debates Neurosci. 2007;1:2–16. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Iwasaki T, Ishima T, Kimura K. Interaction between benzodiazepine and GABAA receptors in state-dependent learning. Life Sci. 1993;52:1935–1945. doi: 10.1016/0024-3205(93)90634-f. [DOI] [PubMed] [Google Scholar]
- Nomura H, Matsuki N. Ethanol enhances reactivated fear memories. Neuropsychopharmacology. 2008;33:2912–2921. doi: 10.1038/npp.2008.13. [DOI] [PubMed] [Google Scholar]
- Oishi H, Iwahara S, Yang KM, Yoci A. Effects of chlordiazepoxide on passive avoidance responses in rats. Psychopharmacologia. 1972;23:373–386. doi: 10.1007/BF00406740. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long term extinction learning. Proc Natl Acad Sci USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain L, Launoy A, Fouquet N, Oberling P. Mechanisms of action of midazolam on expression of contextual fear in rats. Br J Anaesthesia. 2002;89:614–621. doi: 10.1093/bja/aef228. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JB, Ciofalo B, Iorio LC. Benzodiazepine blockade of passive avoidance task in mice: A state-dependent phenomenon. Psychopharmacology. 1979;61:25–28. doi: 10.1007/BF00426805. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Dover Publications: Mineola, New York; 1960. [Google Scholar]
- Pearce JM. Animal Learning and Cognition: An Introduction. Psychology Press: East Sussex; 1997. [Google Scholar]
- Pereira ME, Rosat R, Huang CH, Godoy MGC, Izquierdo I. Inhibition by diazepam of the effect of additional training and of extinction on the retention of shuttle avoidance behavior in rats. Behav Neurosci. 1989;103:202–205. doi: 10.1037//0735-7044.103.1.202. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block ‘reconsolidation' but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Alcala RA, Diaz del Guante MA, Garin-Aguilar ME, Diaz-Trujillo A, Quirarte GL, McGaugh JL. Amygdala or hippocampus inactivation after retrieval induces temporary memory deficit. Neurobiol Learn Mem. 2006;86:144–149. doi: 10.1016/j.nlm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S. Neo-conditioning and the classical theory of fear acquisition. Clin Psychol Rev. 1991;11:155–173. [Google Scholar]
- Rescorla RA. Deepened extinction from compound stimulus presentation. J Exp Psychol. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR.1972A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcementIn: Black AH, Prokasy WF (eds).Classical Conditioning II: Current Research and Theory Appleton Century Crofts: New York; 64–99. [Google Scholar]
- Roediger HL, III, Dudai Y, Fitzpatrick SM. Science of Memory: Concepts. Oxford University Press: New York; 2007. [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Neurobiology. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bonini JS, Coitinho AS, Vianna MRM, Cammarota M, Medina JH, et al. Retrograde amnesia induced by drugs acting on different molecular systems. Behav Neurosci. 2004;118:563–568. doi: 10.1037/0735-7044.118.3.563. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid-A receptor sybtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABAA receptor sybtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Cur Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Joly D, Zivkovic B. Behavioral effects of nonbenzodiazepine anxiolytic drugs: a comparison of CGS 9896 and zopiclone with chlordiazepoxide. J Pharmacol Exp Thers. 1985;232:831–837. [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena DO, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: towards a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Savic MM, Obradovic DI, Ugresic ND, Cook JM, Yin W, Bokonjic DR. Bidirectional effects of benzodiazepine binding site ligands in the passive avoidance task: differential antagonism by flumazenil and beta-CCt. Behav Brain Res. 2005;158:293–300. doi: 10.1016/j.bbr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Schachtman TR, Threlkeld R, Meyer K. Retention of conditioned inhibition produced by extinction. Learning and Motivation. 2000;31:283–300. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Schiller D, Johansen J. Prelimbic prefrontal neurons drive fear expression: a clue for extinction-reconsolidation interactions. J Neurosci. 2009;29:13432–13434. doi: 10.1523/JNEUROSCI.4299-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Gilman AG. Norepinephrine stimulated increase of cyclic AMP levels in developing mouse brain cell cultures. Science. 1971;174:292. doi: 10.1126/science.174.4006.292. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hatton M. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;13:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acid-A receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sierra-Mercado D, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Spear NE. Retrieval of memory in animals. Psychol Rev. 1973;80:63–94. [Google Scholar]
- Sternfeld F, Carling RW, Jelley RA, Ladduwahetty T, Merchant KJ, Moore KW, et al. Selective, orally active gamma-aminobutyric acidA alpha5 receptor inverse agonists as cognition enhancers. J Med Chem. 2004;47:2176–2179. doi: 10.1021/jm031076j. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Zörner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes, Brain and Behavior. 2003;2:3–10. doi: 10.1034/j.1601-183x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub J, Taylor P, Smith M, Kelley K, Becker B, Reid L. Methods of deconditioning persisting avoidance: drugs as adjuncts to response prevention. Physiol Psychol. 1977;5:67–72. [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Taverna FA, Cameron BR, Hampson DL, Wang LY, MacDonald JF. Sensitivity of AMPA receptors to pentobarbital. Eur J Pharmacol. 1994;267:R3–R5. doi: 10.1016/0922-4106(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nature. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Venault P, Chapouthier G, Prado de Carvahlo L, Simiand J, Morre M, Dodd RH, et al. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature. 1986;321:864–866. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]
- von Hertzen LSJ, Giese KP. Memory reconsolidation engages only a subset of immediate early-genes induced during a consolidation. J Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, et al. GABAA receptors containing the alpha-5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Cranney J, Richardson R. Dopamine antagonists in the orbital prefrontal cortex reduce prepulse inhibition of the acoustic startle reflex in the rat. Pharmacol Biochem Behav. 1999;63:55–61. doi: 10.1016/s0091-3057(98)00234-2. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cranney J. The role of GABA and pre-existing anxiety in the reconsolidation of conditioned fear. Behav Neurosci. 2008;122:1295–1305. doi: 10.1037/a0013273. [DOI] [PubMed] [Google Scholar]