Abstract
The capacity to detect changes in the causal efficacy of actions is mediated by a number of brain areas, including the entorhinal cortex (EC) and the posterior part of the dorsomedial striatum (pDMS). In this study we examined whether interactions between the EC and pDMS are required to detect changes in the instrumental contingency. Rats that received EC–pDMS disconnection lesions, that is, unilateral cell body lesions of the EC and contralateral dopamine depletions of the pDMS, were trained to press two levers, with one delivering food pellets and the other a sucrose solution. Thereafter, we tested whether rats were sensitive (1) to a selective devaluation of the value of one of two outcomes using a specific satiety procedure, and (2) to a selective degradation of one of two contingencies controlling instrumental choice behavior. Our results reveal that rats with EC–pDMS disconnection lesions were sensitive to outcome devaluation. However, unlike rats with sham lesions or unilateral EC and pDMS lesions, rats with EC–pDMS disconnection lesions showed a reduced sensitivity to contingency degradation. These findings suggest that EC and pDMS may be part of a neural system that supports the detection of changes in the causal relationship between an action and its consequences.
Keywords: entorhinal cortex, posterior dorsomedial striatum, instrumental conditioning, dopamine, rat
INTRODUCTION
It is well known that animals can not only encode the causal relationship between actions and their consequences, but also detect changes in the causal efficacy of their actions. For instance, in instrumental conditioning post-training degradation of one of two action–outcome contingencies selectively reduced performance of the action that is no longer causal to the delivery of a particular reward (see Balleine and O'Doherty, 2010 for an excellent recent review).
A flexible control of actions in the pursuit of goals is crucial for adapting goal-directed behavior to changing environments. Recent studies suggest that the capacity to detect changes in the causal efficacy of actions is mediated by a number of brain areas, including the dorsomedial striatum (DMS). For example, using functional magnetic resonance imaging in humans, Tanaka et al (2008) revealed that neural responses in the caudate nucleus, which is the human homolog of the rat DMS, were modulated as a function of the contingency between the rate of button pressing and the amount of money earned. Furthermore, Yin et al (2005) implicated a particular subregion of the DMS in mediating the sensitivity to changes in the instrumental contingency. The researchers showed in rats that cell body lesions of the posterior part of the DMS (pDMS) but not of the anterior part produced an insensitivity to contingency degradation. Similarly, Lex and Hauber (2010) recently revealed that 6-hydroxydopamine (6-OHDA) lesions in pDMS also produced an insensitivity to contingency degradation. In addition, another lesion study in rats indicated that among the major components of the hippocampal formation, the entorhinal cortex (EC) seems to be critical for the detection of changes in the instrumental contingency (Corbit et al, 2002). It has been suggested that deficits in context conditioning rendered animals with entorhinal lesions insensitive to contingency degradation because they have deficits in forming or representing associations between context and reward. Therefore, contextual cues did not compete with other predictors of reward during contingency degradation (Corbit and Balleine, 2000; Corbit et al, 2002). Notably, behavioral studies using maze tasks (Mulder et al, 2004; Ragozzino et al, 2002a; Yin and Knowlton, 2006) provided support to the notion that DMS and hippocampal formation may form a functional circuit that mediates goal-directed behavior based on a representation of the context (see Yin and Knowlton, 2006 for a review). In addition, anatomical studies revealed that DMS, including its posterior part, receives direct projections from the EC (McGeorge and Faull, 1987, 1989). Therefore, we wondered whether in instrumental conditioning the capacity to detect changes in the causal efficacy of actions depends on interactions between the EC and pDMS.
In this study we tested rats that received disconnection lesions of the unilateral EC and contralateral pDMS for their sensitivity to contingency degradation. Specifically, rats subjected to disconnection lesions received unilateral cell body lesions of the EC and contralateral dopamine depletion of the pDMS. A pDMS dopamine depletion was used because dopamine in the pDMS has been implicated in the detection of causal relationships between an action and its consequences (Lex and Hauber, 2010). Furthermore, reward-directed behavior critically depends on a striatal integration of hippocampal inputs mediated by dopamine (Goto and Grace, 2005). If interactions between EC and pDMS are important for monitoring action–outcome contingencies underlying goal-directed behavior, then lesions that disconnect EC and pDMS should render rats insensitive to contingency degradation. In instrumental conditioning, rats also encode the contingency between their actions and the incentive value of the outcomes (eg, Balleine and Dickinson, 1998; Dickinson and Balleine, 1994). Therefore, we also examined lesion effects on the sensitivity to outcome devaluation; that is, whether post-training devaluation of the value of one of two outcomes contingent upon distinct actions selectively reduced performance of the action that leads to the devalued outcome.
MATERIALS AND METHODS
All animal experiments were conducted according to the German Law on Animal Protection and were approved by the appropriate authorities.
Subjects and Apparatus
Subjects were 40 naive male Lister-Hooded rats (Harlan-Winkelmann, Borchen, Germany) weighing between 200 and 230 g upon arrival. The rats were housed in groups of four in transparent plastic cages (55 × 39 × 27 cm, Ferplast, Nürnberg, Germany) in a temperature- and humidity-controlled room (20±2 °C, 50–60%) on a 12 : 12-h light–dark cycle (lights on at 0700 h). Throughout the experiment the rats had ad libitum access to water. Standard laboratory maintenance chow (Altromin, Lage, Germany) was given ad libitum for 2 days after arrival, after which food was restricted to 15 g per animal per day to maintain them at ∼85% of their free-feeding weight.
Training and testing took place in identical operant chambers (24 × 21 × 30 cm; Med Associates, St Albans, VT) housed within sound-attenuating cubicles. Each operant chamber was equipped with a pellet dispenser that delivered 45 mg Noyes Pellets (formula A/I; Sandown Scientific, Hampton, Middlesex, UK) into a dual pellet/liquid cup receptacle that was positioned in the middle of the right wall, and a syringe pump that delivered 0.1 ml of a 20% sucrose solution into the same receptacle. Each chamber also contained two retractable levers located on either side of the dual pellet/liquid cup receptacle. A 24V/3W houselight mounted on the top center of the opposite wall illuminated the chambers and an electric fan integrated into the cubicle provided a constant background noise (∼70 dB).
Surgery
Before surgery, animals were assigned to four treatment groups (sham lesion, disconnection lesion, unilateral pDMS lesion, and unilateral EC lesion). Animals of the sham lesion group (n=11) received unilateral vehicle infusions into the pDMS of one hemisphere and unilateral vehicle infusions into the EC of the contralateral hemisphere. Animals of the disconnection lesion group (n=9) received unilateral 6-OHDA infusions into the pDMS of one hemisphere and unilateral N-methyl--aspartic acid (NMDA) infusions into the EC of the contralateral hemisphere. Animals of the unilateral pDMS group (n=11) were subjected to a unilateral 6-hydroxydopamine (6-OHDA) infusion into the pDMS of one hemisphere and a unilateral vehicle infusion into the EC of the contralateral hemisphere; animals of the unilateral EC group (n=9) received unilateral NMDA infusions into the EC of one hemisphere and vehicle infusions into the pDMS of the contralateral hemisphere. The sides of the lesions were balanced in each group, such that there were approximately equal numbers of rats with seven drug/vehicle infusions in the left or right hemispheres.
After pre-treatment with atropine (0.2 mg/kg i.p.; WDT, Garbsen, Germany), the animals were anesthetized with sodium pentobarbital (60 mg/kg i.p.; Medial GmbH, Hallbergmoos, Germany) and xylazine (4 mg/kg i.m.; Bayer AG, Leverkusen, Germany) before being placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) with the toothbar −3.3 mm below the interaural line. The skull surface was exposed and small holes were drilled above the respective sides. Infusions were made using a 1 μl Hamilton syringe at the following coordinates as determined from the atlas of Paxinos and Watson (2007): pDMS=AP −0.4; ML ±2.6; DV −5.0; EC=AP −7.5; ML ±4.6; DV −7.0 and at AP −8.2; ML ±4.6; DV −5.5. 6-OHDA hydrobromide. 6 μg 6-OHDA hydrobromide were dissolved in 0.4 μl saline containing 0.01% ascorbic acid (Sigma Aldrich, Steinheim, Germany) and then infused into the pDMS. 8 μg NMDA were dissolved in 0.4 μl saline (Sigma Aldrich, Steinheim, Germany) and then infused into the EC. Intra-pDMS vehicle infusions consisted of 0.4 μl saline containing 0.01% ascorbic acid and intra-EC vehicle infusions consisted of 0.4 μl saline.
Procedure
The behavioral procedure was similar to a protocol used previously by Corbit and Balleine (2003).
Magazine and lever-press training
On the first day all animals received two magazine training sessions for 20 min, in which both outcomes (pellets and 20% sucrose solution) were delivered on independent random-time schedules (RT-60). Thereafter, lever-press training was started; for half of the animals of each group pressing the left lever earned one pellet and pressing the right lever earned 0.1 ml 20% sucrose solution. The other half received the opposite action–outcome pairings. All animals received two daily 20-min lever-press training sessions, in which only one lever and one outcome was available. After the first training session, there was at least a 2-h break before the second training session with the other lever-outcome pairing began. The order of the pellet and sucrose sessions was alternated each day. Lever-press training was conducted for 8 consecutive days with progressively leaner random ratio (RR–) schedules of reinforcement, except for the first 2 days in which a continuous reinforcement schedule (CRF) was used (days 1 and 2=CRF, P (O∣A)=1.0; days 3–5=RR-5, P (O∣A)=0.2; and days 6–8=RR-10, P (O∣A)=0.1).
Outcome devaluation test
On the day after the last instrumental training session, all animals were given a 1-h ad libitum access to one of the two outcomes in the feeding cages (1 animal per cage). Half of the animals of each group received pellets in a glass bowl and the other half received the sucrose solution in a drinking bottle. Immediately after pre-feeding, the rats were placed into the operant chambers and a 10-min choice extinction test was conducted, in which both levers were inserted but no outcomes were given. On the next day the rats received two retraining session (RR-10; one session on each lever) after which they were given a second choice extinction test. This second devaluation test was identical to the first one except that those animals that had received free access to the pellets on the first test were given free access to the sucrose solution and vice versa.
Contingency degradation training
Rats received 3 days of retraining under the RR-10 schedule (two sessions/day, one session on each lever), after which they were trained for another 3 days on an RR-20 schedule of reinforcement (P (O∣A)=0.05; two sessions/day, one session on each lever). Thereafter, the contingency training started. To assess whether the rats were sensitive to a degradation of the instrumental contingency, a protocol was applied in which the same outcome earned by pressing one of the two levers was given additionally in a non-contingent manner with the same probability in each second without a response; that is, the probability of outcome delivery by pressing a lever was P (O∣A)=0.05 and the probability of outcome delivery by not pressing a lever was P (O∣no A)=0.05. Thus, the experienced probability of outcome delivery was the same regardless of whether the animal performed that action or not, a protocol that degrades this action–outcome association (see Corbit and Balleine, 2003). The animals received two training sessions each day, one with each action–outcome pairing. The outcome given non-contingently was the same in both sessions; therefore, one action–outcome association was degraded whereas the other action–outcome association was not degraded. For half of the animals of each group the lever-pellet contingency was degraded (ie, pellets were the outcome given non-contingently), whereas for the other half the lever-sucrose contingency was degraded. The rats received two 30-min training sessions each day (one on each lever) and had a break of at least 2 h between the two sessions. The order of the two sessions was alternated each day and training continued for 6 days.
Contingency degradation test
On the day after the last contingency training session, a 10-min choice extinction test was given. Both levers were inserted but no rewards were given.
Statistical Analysis
Lever training was given for 8 days and contingency degradation training for 6 days. Respective data were subjected to repeated-measures ANOVA with two within-subject factors (days of testing and degradation, ie, degraded vs non-degraded) and one between-subject factor (treatment groups: sham lesion, disconnection lesion, unilateral EC lesion, and unilateral pDMS lesion). Outcome devaluation and contingency degradation effects were tested in extinction in a single test day, respectively. The data were subjected to a priori comparisons to assess the sensitivity to outcome devaluation and contingency degradation of each treatment group. Presses for the devalued vs valued lever and the degraded vs not-degraded lever in each treatment group were analyzed separately using planned contrasts. All statistical computations were carried out with STATISTICA (version 7.1, StatSoft, Inc., Tulsa, OK).
Histology
After the behavioral testing, animals were killed by an overdose of isoflurane (cp-pharma, Burgdorf, Germany) and perfused transcardially as described previously (Lex and Hauber, 2010).
Tyrosine hydroxylase immunohistochemistry in the pDMS
Coronal brain sections were cut (40 μm; HM550, Microm GmbH, Walldorf, Germany) in the region of the pDMS. Tyrosine-hydroxylase (TH) staining was performed as described previously (Calaminus and Hauber, 2009; Lex and Hauber, 2010). To determine the size and placement of the lesions, the TH immunoreactivity was analyzed under a microscope with reference to the atlas of Paxinos and Watson (2007).
Nissl staining in the EC
Coronal brain sections were cut (40 μm; Microm HM550, Microm GmbH) in the region of the EC, mounted on coated slides, and stained with cresyl violet. Size and placement of lesions was analyzed under a microscope with reference to the atlas of Paxinos and Watson (2007).
RESULTS
Histology
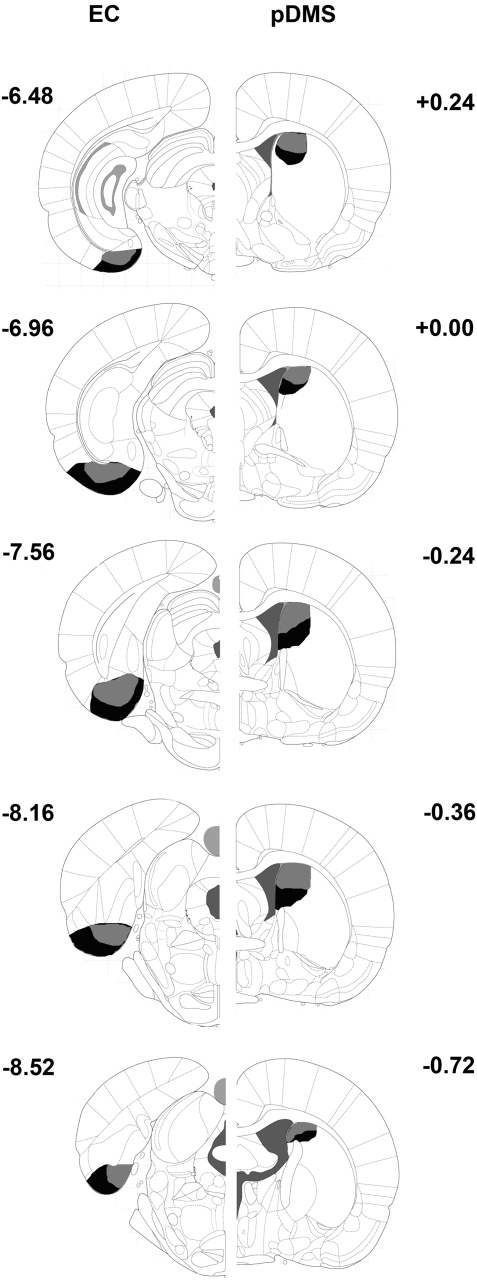
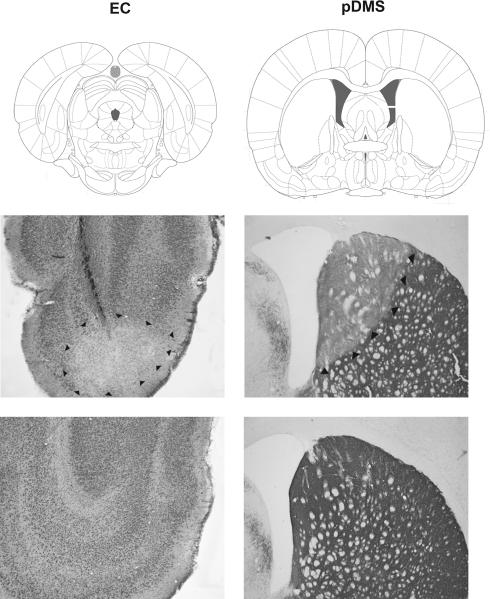
Figures 1 and 2 provide photomicrographs of brain sections with lesions and schematic representations of the minimum and maximum extent of NMDA-induced cell body lesions of the EC and 6-OHDA-induced DA depletions of the pDMS across all lesion groups in one hemisphere, respectively.
Figure 1.
Schematic representations of the extent of neuronal damage in the EC (left hemisphere) and the areas devoid of TH immunoreactivity in the pDMS (right hemisphere) across all lesion groups. Animals received disconnection lesions using NMDA infusion into the ipsilateral EC, 6-OHDA infusion into the contralateral pDMS, unilateral NMDA infusions into the EC, or unilateral 6-OHDA into the pDMS. Gray areas represent the smallest extent and black areas the largest extent of lesions. Numbers indicate distance from bregma in mm.
Figure 2.
Representative photomicrographs of an NMDA-induced cell body lesion of the EC and a 6-OHDA-induced loss of tyrosine-immunoreactive fibers in the pDMS. The panels on the left side show an EC lesion (middle) and an EC sham lesion (bottom), and the panels on the right side show a pDMS DA depletion (middle) and a pDMS sham lesion (bottom). The boxed regions in the top panel are shown at high magnification in the middle and bottom panels. Plates are adaptations from the atlas of Paxinos and Watson (2007); scales are relative to bregma.
In animals with EC–pDMS disconnection lesions, we observed a marked reduction of neuronal density in the unilateral EC accompanied by gliosis and an atrophy of neurons in the target area. Damage was mostly restricted to the caudomedial, medial, and ventral intermediate EC with occasional minimal damage to the parasubiculum. Unilateral damage of the EC seen here was comparable to damage observed in single hemispheres of bilaterally EC-lesioned animals in studies using similar infusion parameters (Corbit et al, 2002; Coutureau et al, 2000; Majchrzak et al, 2006; Traissard et al, 2007). In the contralateral side, TH-positive fibers in the pDMS were markedly reduced. There was no evidence of damage outside the pDMS, that is, to areas more anterior than +0.2 mm from bregma and to areas more lateral than 3.2 mm from the midline. The volume and concentration of 6-OHDA in this experiment (6 μg/0.4 μl) was the same as in previous studies from our lab in which we found a marked reduction of TH immunoreactivity in the pDMS (Calaminus and Hauber, 2009; Lex and Hauber, 2010). A number of earlier studies reported that intrastriatal infusion of solutions with lower concentrations of 6-OHDA (<7 μg/μl), as used in this study (15 μg/μl), profoundly reduced tissue concentrations of DA (>85%) (Baunez and Robbins, 1999; Blandini et al, 2007; Brown and Robbins, 1991; Faure et al, 2005; Moukhles et al, 1994). Similarly, intrastriatal infusions of solutions containing 3.5 μg/μl 6-OHDA caused a significant DA denervation (≈90%), as assessed by DA transporter autoradiography (Winkler et al, 2002). Together, these findings provide support to our observation that rats subjected to a 6-OHDA infusion had an almost complete unilateral pDMS DA depletion. One caveat to note, however, is that we did not quantify the exact extent of the DA depletion, for example, by measuring striatal tissue levels of DA (Hauber et al, 1994). Thus, we cannot exclude that the extent of DA depletion induced in our present study differed from those quantified in previous studies using lower concentrations of 6-OHDA (Baunez and Robbins, 1999; Blandini et al, 2007; Brown and Robbins, 1991; Winkler et al, 2002).
In animals with control lesions, that is, unilateral pDMS and unilateral EC lesions, neuronal density in the EC and TH immunoreactivity in the pDMS was reduced to a similar extent as observed in animals with disconnection lesions. In contrast, in animals subjected to sham lesions we observed only minimal damage along the injection cannulae tracks with no damage to the respective target area. We did not include a further control group with combined unilateral lesions of the pDMS and EC in the same hemisphere, as numerous studies using disconnection designs showed that ipsilateral lesions of two interconnected structures in the same hemisphere do not impair behavior relative to the effect of crossed disconnection lesions (Chudasama et al, 2003; Dunnett et al, 2005; Hauber and Sommer, 2009; Olton et al, 1982; Warburton et al, 2000).
Lever-Press Training
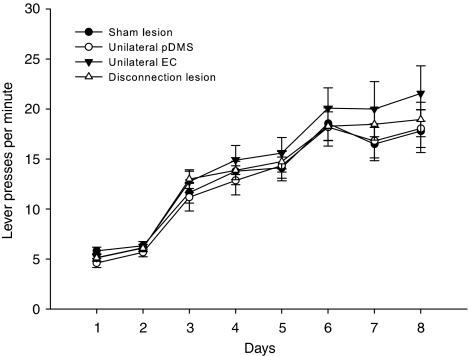
All animals acquired the instrumental task during training (Figure 3). First, we analyzed within each group whether the side of the lesions had an effect on performance. Separate repeated-measures ANOVA from data of each group (sham lesion, disconnection lesion, unilateral EC, and unilateral pDMS lesion) showed no significant effect of lesion side on lever pressing during training (sham lesion; lesion side F(1, 9)=0.55, n.s.; day F(7, 63)=32.73, p<0.001; unilateral pDMS lesion: lesion side F(1, 9)=0.02, n.s.; day F(7, 63)=49.15, p<0.001; unilateral EC lesion: lesion side F(1, 7)=0.15, n.s.; day F(7, 49)=33.28, p<0.001; disconnection lesion: lesion side F(1, 7)=0.03, n.s; day F(7, 49)=51.97, p<0.001; no lesion side × day interactions were observed) as well as in other tests (data not shown). Therefore, the possible effects of the lesion side were not considered further in subsequent statistical analyses. A repeated-measures ANOVA of lever pressing during training revealed a significant effect of day (F(7, 252)=171.58, p<0.001), but no effect of group (F<1, n.s.) and no group × day interaction (F<1, n.s.).
Figure 3.
Lever training. Mean lever presses±SEM per minute averaged for both actions from animals of all four groups. Ratio schedules increased across training days as follows: days 1 and 2=CRF, days 3–5=RR-5, and days 6–8=RR-10.
Outcome Devaluation
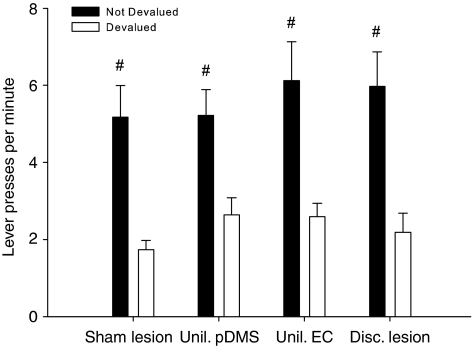
A clear outcome devaluation effect was observed in all treatment groups. Planned contrast analysis revealed that lever press rates in each treatment group were significantly different for the valued vs devalued reward (Figure 4).
Figure 4.
Outcome devaluation. Mean lever presses per minute±SEM on the devalued and not-devalued lever during the 10-min choice extinction test, after one of the outcomes was devalued using a specific satiety procedure. Both levers were inserted but no outcomes were given. Planned contrast analysis revealed that lever-press rates in each treatment group were significantly different for the valued vs devalued reward (#p<0.01).
Contingency Degradation
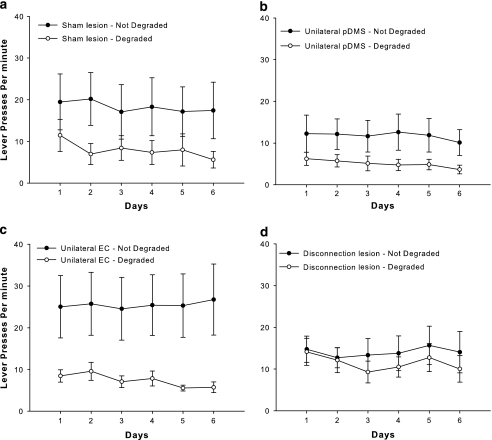
A selective degradation of the instrumental contingency over 6 days of degradation training had distinctive effects in treatment groups as shown in Figure 5. A repeated-measures ANOVA of the contingency training data revealed a significant effect of degradation (F(1, 34)=13.53, p<0.001), no effect of group (F<1, n.s.), and no group × degradation interaction (F<2, n.s.). Although it is possible that the failure to find a reliable interaction reflects no difference between groups, an analysis of simple effects can be warranted even in the face of a nonsignificant interaction (Howell, 2007). Accordingly, an explorative simple effects analysis revealed that the sham lesion group (F(1, 10)=5.93, p<0.05), the unilateral pDMS lesion group (F(1, 9)=5.54, p<0.05), and the unilateral EC lesion group (F(1, 8)=6.06, p<0.05) showed a degradation effect, but this was not the case for the disconnection lesion group (F<2, n.s.). Hence, animals with a disconnection of EC and the pDMS seemed to be less sensitive to the contingency degradation training procedure.
Figure 5.
Contingency degradation training. Mean lever presses per minute±SEM during contingency degradation training. (a) Animals with sham lesions, (b) animals with unilateral pDMS lesions, (c) animals with unilateral EC lesions, and (d) animals with EC–pDMS disconnection lesions. One action–outcome contingency was degraded by unpaired delivery of an outcome that was the same as that what was earned by pressing the degraded lever (RR-20).
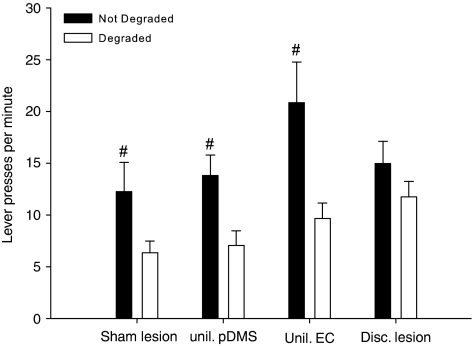
The critical contingency degradation test performed in extinction confirmed this observation and indicated that the sensitivity to contingency degradation was impaired in animals with a disconnection of the EC and the pDMS. Planned contrast analysis of the data from the degradation test in extinction revealed that, except for the disconnection lesion group, animals performed significantly less responses on the lever for which the contingency had been degraded (Figure 6).
Figure 6.
Contingency degradation test. Mean lever presses±SEM per minute during the 10-min choice extinction test from animals of all treatment groups. Both levers were inserted but no outcomes were given. Planned contrast analysis revealed that, except for the disconnection lesion group, animals performed significantly less responses on the lever for what the contingency had been degraded (#p<0.01).
DISCUSSION
Our results reveal that rats subjected to a disconnection of the EC and the pDMS were sensitive to outcome devaluation, but showed a reduced sensitivity to the degradation of the instrumental contingency. Previous studies already showed that bilateral cell body lesions of the EC (Corbit et al, 2002) and the pDMS (Yin et al, 2005) as well as bilateral DA depletions of the pDMS (Lex and Hauber, 2010) impaired the sensitivity to contingency degradation. Therefore, our present data suggest that interactions between EC and pDMS support the detection of changes in the causal relationship between an action and its consequences.
Animals with EC–pDMS disconnection lesions responded selectively in the outcome devaluation test, indicating that their ability to discriminate between the two actions and the two outcomes was not compromised. Furthermore, lesioned rats were sensitive to changes in the outcome value and could integrate such a change in value into an association between a specific action and the related outcome. Hence, encoding the current value of an outcome as well as encoding and retrieval of action–outcome associations that guide selective responding does not depend on an interaction between the EC and the pDMS. Given the fact that the prelimbic region of the prefrontal cortex is critical for instrumental learning (Balleine and Dickinson, 1998; Balleine and O'Doherty, 2010; Corbit and Balleine, 2003; Killcross and Coutureau, 2003), interactions between prefrontal cortex and pDMS may have a crucial role in encoding actions and outcomes.
In contrast, animals with a disconnection lesion of the EC and the pDMS pressed the levers as if reward delivery was still dependent on their actions, even if one action was no longer causal to the delivery of a specific reward. This impairment was pronounced if animals with disconnection lesions were tested in extinction, but moderate if tested during contingency degradation training. Conversely, animals with sham lesions were already sensitive to the effects of the contingency degradation on the first day of training, which is in accordance with earlier observations (Corbit et al, 2001, 2002; Corbit and Balleine, 2003). It is important to note that in the contingency degradation procedure the critical test is the extinction test, as it examines previously learned associations without contamination by new learning (Dickinson and Balleine, 1994; Niv et al, 2006). Thus, lesions that impair the sensitivity to contingency degradation tested in extinction can have no (Corbit and Balleine, 2003) or moderate effects (eg, Corbit and Balleine, 2000; Corbit et al, 2002; Lex and Hauber, 2010) on contingency degradation training. The impaired sensitivity might not reflect an impairment in encoding action–outcome associations per se as the same animals showed an outcome devaluation effect that relies on an intact action–outcome association. It has been argued that in normal rats reduced instrumental performance associated with contingency degradation results from a comparison of the validity that an action is predictive of reward and the validity that the background or context is predictive of reward (eg, Colwill and Rescorla, 1986; Corbit and Balleine, 2000). Previous studies showed that bilateral EC lesions impaired context conditioning (eg, Majchrzak et al, 2006; Maren and Fanselow, 1997) and produced an insensitivity to a degraded instrumental contingency, but left intact the sensitivity to outcome devaluation (Corbit et al, 2002). Therefore, Corbit et al (2002) suggested that animals with bilateral EC lesions are insensitive to the contingency degradation procedure because they have deficits in forming or representing associations between context and reward. As a result, contextual cues did not compete with other predictors of reward during contingency degradation. Importantly, anatomical studies in rats using retrograde labeling revealed that the pDMS receives significant direct projections from the EC (McGeorge and Faull, 1987, 1989). Thus, the sensitivity to contingency degradation as tested here could rely on the contextual information flow from the EC to the pDMS. Accordingly, it is conceivable that the reduced sensitivity to contingency degradation in animals with EC–pDMS disconnections was due to an impaired processing of reward-related contextual information.
Our previous study showed that a bilateral DA depletion of the pDMS impaired the sensitivity to contingency degradation, but left the sensitivity to outcome devaluation intact. Therefore, we suggested that DA in the pDMS contributes to instrumental conditioning by supporting the detection of the causal relationship between an action and its consequences (Lex and Hauber, 2010). Consistent with this notion, the present disconnection study that involved pDMS DA depletions produced a similar pattern of impairments. Furthermore, our present results point to the possibility that DA signaling in the pDMS is important in enabling the integration of contextual information within the pDMS. In a similar vein, electrophysiological studies showed that reward-directed behavior depends on the interaction between hippocampal inputs and neurons in the ventral striatum that is modulated by DA (Goto and Grace, 2005). Specifically, it has been suggested that a phasic DA release evoked when an animal encounters unexpected reward facilitates the hippocampal drive of nucleus accumbens neurons through action on D1 receptors. In contrast, a suppression of DA release could decrease tonic DA stimulation of D2 receptors and, in turn, input integration in the nucleus accumbens is shifted favoring prefrontal over hippocampal signals (Goto and Grace, 2008). Correspondingly, contingency degradation as tested here represents a situation involving an unexpected transition of the predictive value of the context. Such a change of the instrumental contingency could entail a prediction error signaled by a phasic DA release (Schultz, 1997, 1998) that facilitates the integration of entorhinal information by activation of D1 receptors. As phasic DA signaling was impaired in animals with EC–pDMS disconnection lesions, striatal processing of entorhinal signals might have been compromised such that the context did not become a predictor for the reward during contingency degradation. However, it is also evident that a large variety of behavioral processes simply rely on an appropriate level of tonic DA receptor stimulation (see Schultz, 2007 for a review). Given this enabling function of tonic DA transmission, a striatal integration of entorhinal signals underlying the sensitivity to contingency degradation could merely depend on tonic DA release. Thus, from our results it is not clear whether tonic or phasic DA release or an interaction between these DA states could be involved in the striatal regulation of EC input.
Notably, studies that examined spatial navigation in mazes provided support to the notion that DMS and hippocampal formation may form a functional circuit (Mulder et al, 2004; Ragozzino et al, 2002b). For instance, unlike sham controls, animals with pDMS cell body lesions tested in a cross maze task used a response, instead of a place strategy, possibly because of an impaired representation of contextual cues (Yin and Knowlton, 2004). Remarkably, bilateral DA depletions of the pDMS produced a similar strategy shift in the same task (B Lex and W Hauber, unpublished results). Therefore, the pDMS might represent a key component of a cortico-striatal circuit that mediates flexible goal-directed behavior based on contextual representation (Balleine and O'Doherty, 2010; Yin and Knowlton, 2006). Consistent with this notion, our present findings suggest that, possibly by processing contextual signals, direct pathways between EC and pDMS provide flexibility to goal-directed behavior if the context becomes a reliable predictor of reward. However, goal-directed behavior tested in operant tasks as used here and spatial navigation tasks might involve different forms of learning (see Yin et al, 2008 for a recent review); therefore, respective inferences have to be made with caution.
Notably, studies using functional magnetic resonance imaging in humans showed an involvement of the caudate nucleus in contingency learning. For instance, Tricomi et al (2004) showed that caudate activity is modulated by the action–outcome contingency, as a strong striatal activation occurred when subjects perceived a contingency between a button press response and an outcome. Similarly, Tanaka et al (2008) revealed that neural activity in the caudate nucleus was modulated as a function of the contingency between the rate of button pressing and the amount of money earned. In addition, Delgado et al (2005) showed that during contingency learning the activity in the caudate nucleus was particularly robust during the early phases of learning but decreased as learning progressed. However, it is yet unknown whether a compromised striatal DA transmission, for example, in patients with Parkinson's disease or in patients treated with dopamine antagonists, impairs the sensitivity to a contingency degradation.
To conclude, a number of brain areas, such as the prelimbic region of the medial prefrontal cortex (Balleine and Dickinson, 1998; Killcross and Coutureau, 2003), the basolateral amygdala (Balleine et al, 2003), the pDMS (Yin et al, 2005), the mediodorsal thalamus (Corbit et al, 2003), and the EC (Corbit et al, 2002), have been implicated to mediate the sensitivity to contingency degradation. In addition, DA in the pDMS (Lex and Hauber, 2010), but not in the prelimbic subregion of the prefrontal cortex (but see Naneix et al, 2009), mediates the sensitivity to contingency degradation. However, interactions between these brain areas underlying sensitivity to contingency are largely unknown so far. The present results suggest that interactions between EC and pDMS are involved in the detection of changes in the causal relationship between an action and its consequences. Furthermore, our findings suggest that DA in the pDMS is critical to adapt goal-directed behavior to changing environments, possibly by modulating the contextual information flow from the EC to the pDMS.
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (HA2340/8-2).
Wolfgang Hauber has received an honorarium for consulting from Merz Pharmaceuticals GmbH (Frankfurt, Germany) within the past 3 years. The other authors declare no conflict of interest.
References
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Simple and choice reaction time performance following unilateral striatal dopamine depletion in the rat. Impaired motor readiness but preserved response preparation. Brain. 1991;114 (Part 1B:513–525. doi: 10.1093/brain/114.1.513. [DOI] [PubMed] [Google Scholar]
- Calaminus C, Hauber W. Modulation of behavior by expected reward magnitude depends on dopamine in the dorsomedial striatum. Neurotox Res. 2009;15:97–110. doi: 10.1007/s12640-009-9009-1. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–5485. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill R, Rescorla R.1986Associative structures in instrumental learningIn: Bower G (ed).The Psychology of Learning and Motivation Academic Press: New York; Vol 20,55–104. [Google Scholar]
- Corbit LH, Balleine BW. The role of the hippocampus in instrumental conditioning. J Neurosci. 2000;20:4233–4239. doi: 10.1523/JNEUROSCI.20-11-04233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur J Neurosci. 2003;18:1286–1294. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Ostlund SB, Balleine BW. Sensitivity to instrumental contingency degradation is mediated by the entorhinal cortex and its efferents via the dorsal hippocampus. J Neurosci. 2002;22:10976–10984. doi: 10.1523/JNEUROSCI.22-24-10976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Galani R, Jarrard LE, Cassel JC. Selective lesions of the entorhinal cortex, the hippocampus, or the fimbria-fornix in rats: a comparison of effects on spontaneous and amphetamine-induced locomotion. Exp Brain Res. 2000;131:381–392. doi: 10.1007/s002219900301. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine BW. Motivational control of goal directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Dunnett SB, Meldrum A, Muir JL. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience. 2005;135:1055–1065. doi: 10.1016/j.neuroscience.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex impairs motor initiation but not motor execution. Exp Brain Res. 1994;99:524–528. doi: 10.1007/BF00228988. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19:2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- Howell D.2007Statistical Methods for Psychology6th edn.Thomson Wadsworth: Belmont [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex. 2010;20:873–883. doi: 10.1093/cercor/bhp151. [DOI] [PubMed] [Google Scholar]
- Majchrzak M, Ferry B, Marchand AR, Herbeaux K, Seillier A, Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus. 2006;16:114–124. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization and collateralization of corticostriate neurones in the motor and sensory cortex of the rat brain. Brain Res. 1987;423:318–324. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Moukhles H, Amalric M, Nieoullon A, Daszuta A. Behavioural recovery of rats grafted with dopamine cells after partial striatal dopaminergic depletion in a conditioned reaction-time task. Neuroscience. 1994;63:73–84. doi: 10.1016/0306-4522(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Tabuchi E, Wiener SI. Neurons in hippocampal afferent zones of rat striatum parse routes into multi-pace segments during maze navigation. Eur J Neurosci. 2004;19:1923–1932. doi: 10.1111/j.1460-9568.2004.03301.x. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. A role for medial prefrontal dopaminergic innervation in instrumental conditioning. J Neurosci. 2009;29:6599–6606. doi: 10.1523/JNEUROSCI.1234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10:375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Wolf WA. A disconnection analysis of hippocampal function. Brain Res. 1982;233:241–253. doi: 10.1016/0006-8993(82)91200-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2007The Rat Brain in Stereotaxic Coordinates6th edn.Elsevier Academic Press: San Diego [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002a;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002b;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Balleine BW, O'Doherty JP. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci. 2008;28:6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traissard N, Herbeaux K, Cosquer B, Jeltsch H, Ferry B, Galani R, et al. Combined damage to entorhinal cortex and cholinergic basal forebrain neurons, two early neurodegenerative features accompanying Alzheimer's disease: effects on locomotor activity and memory functions in rats. Neuropsychopharmacology. 2007;32:851–871. doi: 10.1038/sj.npp.1301116. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci. 2000;12:1714–1726. doi: 10.1046/j.1460-9568.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]