Abstract
Anorexia nervosa (AN) is a highly heritable young-onset psychiatric illness the etiology of which remains unknown. Estrogen alpha and beta receptors, encoded by ESR1 and ESR2 genes, are involved in food intake regulation and eating behavior, and may have a potential role in AN. We performed a family-based association study of 17 single-nucleotide polymorphisms (SNPs) encompassing ESR1 and ESR2 genes in a cohort of 321 French AN families. We attempted to replicate this finding in a cohort of 41 restrictive AN (RAN) families and in a population-based study of 693 young women. Using the transmission disequilibrium test, a significant over-transmission was detected between AN and ESR1 rs726281 and rs2295193. These SNPs and another among ESR1 were more specifically associated with the RAN subtype (rs726281, p=0.005, odds ratio (OR)=2.1, 95% confidence interval (95% CI)=1.2–3.6; rs3798577, p=0.021, OR=1.6, 95% CI=1.1–2.3; and rs2295193, p=0.007, OR=1.7, 95% CI=1.2–2.5). A large eight-SNPs haplotype of ESR1 gene was also associated with AN (p<0.0001, OR=3.1, 95% CI=1.8–5.1). Association of ESR1 SNPs and RAN was driven by paternal over-transmissions (p<0.0001, OR=3.7, 95% CI=1.9–7.3). Furthermore, we confirmed the preferential paternal over-transmission of the ESR1 rs726281 on the independent German sample of 41 RAN trios (p=0.025, OR=3, 95% CI=1.1–8.3). Finally, rs3798577 was associated with eating disorders in a population-based sample of 693 women (p<0.01). Our findings are strongly in favor of an association between ESR1 polymorphisms and AN. In particular, ESR1 gene confers a high risk of vulnerability to the restrictive subtype of AN, and suggests that the estrogen pathway has to be further analyzed in AN.
Keywords: anorexia nervosa, restrictive type, binge-eating/purging type, estrogen receptors, transmission disequilibrium test, population-based sample
INTRODUCTION
Anorexia nervosa (AN, MIM 606788) is an eating disorder characterized by weight loss (body mass index (BMI) <17.5 kg/m2) or failure to make expected weight gain during period of growth, intense fear of gaining weight or becoming fat, body image disturbance and amenorrhea (American Psychiatric Association, 1994; Gorwood et al, 2003; Ramoz et al, 2007). The prevalence of AN is between 0.3 and 0.6% (Hudson et al, 2007; Ramoz et al, 2007), with one of the highest mortality rate (approximately 10% per decade) mainly because of cachexia and suicide (Harris and Barraclough, 1994; Hoek, 2006). Furthermore, the heritability of AN is high (70%), thus increasing the potential interests of genetic approaches (Gorwood et al, 2003; Ramoz et al, 2007). Studies have found evidence of a high genetic risk for AN, especially in the RAN subgroup probably because of heterogeneity reduction of the phenotype, with, therefore, an increased power to detect an association (Grice et al, 2002; Gershon et al, 1984).
According to the DSM-IV, AN patients are subdivided in two categories: restrictive type (RAN), characterizing patients with restricted food intake without binge eating or purging episodes, and binge eating/purging type (BPAN), characterizing patients with binge eating/purging episodes during anorexia and bulimia phases (American Psychiatric Association, 1994). RAN and BPAN patients represent two subtypes with specific clinical features, including significant lower BMI in RAN than in BPAN, and an increased impulsivity and a higher rate of self-harm and suicide in BPAN than RAN (Eddy et al, 2002; Foulon et al, 2007; Milos et al, 2004). Hormone concentrations may also help to distinguish these two subtypes, because leptin is decreased in RAN compared with BPAN (Eddy et al, 2002).
Biological pathways of sexual hormones were poorly analyzed in AN, although the involvement of the estrogenic pathway in AN is supported by several cues. First a female predominance is observed in patients with a ratio of 9 women to 1–3 men (Hudson et al, 2007; Ramoz et al, 2007). Second, the onset of AN around puberty is correlated to the presence of estrogenic peaks). Third, recent studies suggested that sex hormones have a role in the risk of eating disorders (Culbert et al, 2008; Procopio and Marriott, 2007) but this observation was not replicated by other authors (Raevuori et al, 2008; Baker et al, 2009). Fourth, the anorexic effect of high estrogen levels was found in animal models (Couse and Korach, 1999; Wade and Gray, 1979). Moreover, estradiol levels were lower in anorexia patients than in controls (Ohwada et al, 2007; Brambilla et al, 2003). Fifth, the ESR1 and ESR2 genes, which code for estrogen alpha and beta receptors and are expressed in non-overlapping brain regions (Osterlund et al, 2000; Osterlund and Hurd, 2001), colocalize with corticotrophin-releasing factor and modulate its expression (Dagnault and Richard, 1997; Bao et al, 2005). They participate in the regulation of the hypothalamic pituitary adrenal (HPA) axis (Licinio et al, 1996). Thus, the disruption of the HPA axis reported in AN might be due to an alteration in the estrogen pathway in patient (Van de Stolpe et al, 2004). Finally, the involvement of the estrogen alpha receptor in the regulation of food intake and eating behavior was confirmed in mouse models (Musatov et al, 2006, 2007). Two case–control studies of AN with ESR1 and ESR2 genes were previously published (Eastwood et al, 2002; Rosenkranz et al, 1998). They reported no association of the ESR1 gene with AN, and an inconsistent association of AN with different variants within ESR2 gene.
Thus, our hypothesis is to identify an association between estrogen receptor and AN, especially in RAN subgroup, because a diminution of estradiol level was more important in the restrictive compared with the binging–purging subtype (Ohwada et al, 2007). We more precisely expect to observe an association between AN and ESR1 gene because ovariectomized rat showed an implication of ESR1 but not of ESR2 receptor in food intake, body weight and meal size (Santollo et al, 2007). Finally, we have been also analyzing the role of parental imprinting of this estrogen receptor in AN, because ESR1 belongs to the 6q25 region, which could be subjected to parental imprinting according to database (Morison et al, 2005). Furthermore, the absence of ESR1 transcription could be a result of aberrant methylation of promoter CpG islands, as shown, for example, in breast cancers (Wilson et al, 2008).
In this study, we performed the first family-based association study on a large number of AN families of French origin looking for an association between ESR1 gene and AN, and we took advantage of the narrowing definition of the disorder to show that this association is driven by the restrictive subtype of AN. We attempted to replicate the family-based association in an independent sample of German families (replication of the TDT approach), as well as in an independent population-based sample recruited from the general population.
MATERIALS AND METHODS
Subjects and Phenotype
The study was approved by each national ethics committee. All participants (and if underage, their parents) gave written informed consent.
The first family-based cohort was composed of 321 families of French origin, including 210 complete trios, of whom 102 were described previously (Gorwood et al, 2003). All participants were assessed by clinicians using the face-to-face semi-structured Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al, 1994). Diagnosis of AN were made according to DSM-IV criteria (American Psychiatric Association, 1994), and included RAN with no lifetime binge-eating/purging episode (N=153 families), and BPAN with at least one lifetime bingeing/purging episode, during anorexia or bulimia phases (N=154 families). Information for subtype was incomplete for 14 probands.
Furthermore, the eating disorder inventory-2 (Garner et al, 1983) and the Eating Attitude Test-26 (Garner et al, 1982) were used to measure the psychological and behavioral dimensions and the broad range of symptoms of AN respectively.
A second family-based cohort was composed of 41 RAN trios of German origin, the probands being assessed with the Composite International Diagnostic Interview (CIDI) (Robins et al, 1988). The clinical features of the two family-based cohorts and subgroups are described in Table 1.
Table 1. Demographic and Clinical Data for the French and German Family-Based Cohorts and the French RAN and BPAN Subgroups.
| Population disorder |
French |
German |
||
|---|---|---|---|---|
| AN | RAN | BPAN | RAN | |
| Families number (N) | 321 | 153 | 154 | 41 |
| Female (%) | 97 | 97 | 97 | 95 |
| Age (years) | 22.3±7.4 | 19.4±6.5 | 25.2±7.2a | 16.0±1.9b |
| Age of onset of anorexia (years) | 16.2±4.5 | 15.6±4.4 | 16.8±4.4a | 14.6±1.2 |
| Duration of anorexia (years) | 6.1±6.0 | 3.9±4.8 | 8.3±6.2a | 1.5±1.3b |
| BMI minimum (kg/m2) | 13.6±2.2 | 12.9±1.7 | 14.4±2.2a | 13.5±0.2b |
| Major depressive episode (%) | 68 | 68 | 69 | n.a. |
| Suicide attempt (%) | 28 | 9 | 47a | n.a. |
| Alcohol and drug abuse/dependence (%) | 6 | 2 | 11a | n.a. |
| EATc dieting | 16.4±10.6 | 13.4±10.2 | 19.5±10.3a | n.a. |
| EAT bulimia | 7.6±6.0 | 4.6±4.3 | 10.6±5.8a | n.a. |
| EAT oral control | 7.4±5.3 | 7.7±5.2 | 7.2±5.3 | n.a. |
Abbreviation: n.a., not available.
Significant difference between RAN and BPAN from the French cohort.
Significant difference between French RAN subset and German RAN cohort.
Eating attitude test. Significant difference indicates a p-value inferior at 0.05 in one-way analysis of variance or t-test.
Data are indicated as N, %, or mean±SD.
A third sample was composed of young women (mean age 20.30±0.05 years) with Caucasian origin recruited for a genetic analysis of addictive behaviors, including eating disorders (SAGE study) (Le Strat et al, 2009). Eating disorders were assessed using the SCOFF scale, a brief and reliable questionnaire developed as a screening test of eating disorders in the general population with a sensitivity of 84.6% and a specificity of 89.6% (Hill et al, 2009). Participants were asked the following questions: 1. Do you make yourself sick because you feel uncomfortably full? 2. Do you worry you have lost control over how much you eat? 3. Have you recently lost more than 6 kg in a 3-month period? 4. Do you believe yourself to be fat when others say you are too thin? 5. Would you say that food dominates your life?
Participants were considered as having an eating disorder if they had a SCOFF score equal or above 2 (n=126), and were considered as control if they had a SCOFF score <2 (n=567).
SNPs and Genotyping
Genomic DNA from probands and parents was extracted from peripheral blood leukocytes. Thirteen single-nucleotide polymorphisms (SNPs) encompassing ESR1 gene and four SNPs within ESR2 gene were screened using TaqMan SNP genotyping assays (Applied Biosystems, Les Ullis, France). The selection of SNPs is based on tagged SNPs that depict the haplotype blocks across the genes, according to available databases (Perlegen and Hapmap) and positive associations with diseases.
Details of SNPs are indicated in Table 2 and Supplementary Table 2 and their position within genes are shown on Figure 1 and Supplementary Figure 1 for ESR1 and ESR2 genes, respectively. A total of 362 AN patients and 613 parents were genotyped. Furthermore, 30 samples were genotyped in duplicate to assess the accuracy of the allelic call. SNP rs3798577 was genotyped using SNPlex technology (Applied Biosystems) for 693 women from the population-based sample (Tobler et al, 2005).
Table 2. Transmission Disequilibrium Test of ESR1 SNPs with AN in the French Family-Based Cohort.
| SNP# | Markers | Position | MAF |
TDT |
|||
|---|---|---|---|---|---|---|---|
| OT | T:U | p | OR (CI95) | ||||
| 1 | rs488133 | 152167137 | T (0.39) | C | 102:102 | 1 | 1 (0.8–1.3) |
| 2 | rs11155819 | 152241052 | C (0.31) | C | 105:100 | 0.727 | 1.1 (0.8–1.4) |
| 3 | rs12199722 | 152276593 | G (0.32) | G | 99:88 | 0.421 | 1.1 (0.8–1.5) |
| 4 | rs1884051 | 152324972 | G (0.3) | A | 81:71 | 0.417 | 1.1 (0.8–1.6) |
| 5 | rs726281 | 152344271 | G (0.26) | A | 85:58 | 0.024a | 1.5 (1.0–2.0) |
| 6 | rs3020407 | 152348954 | G (0.29) | A | 95:76 | 0.146 | 1.3 (0.9–1.7) |
| 7 | rs17081994 | 152358440 | C (0.09) | T | 36:23 | 0.091 | 1.6 (0.9–2.6) |
| 8 | rs2982712 | 152399872 | C (0.39) | T | 106:95 | 0.438 | 1.1 (0.8–1.5) |
| 9 | rs3020371 | 152425513 | T (0.31) | C | 93:87 | 0.655 | 1.1 (0.8–1.4) |
| 10 | rs2228480 | 152461788 | A (0.17) | G | 55:50 | 0.626 | 1.1 (0.8–1.6) |
| 11 | rs3798577 | 152462823 | C (0.46) | T | 116:91 | 0.082 | 1.3 (1.0–1.7) |
| 12 | rs2295193 | 152494787 | G (0.47) | G | 117:85 | 0.024 | 1.4 (1.0–1.8) |
| 13 | rs2252837 | 152510513 | T (0.35) | C | 106:87 | 0.171 | 1.2 (0.9–1.6) |
Abbreviations: MAF, minor allele and its frequency in the parentheses; OR (CI95), odds ratio and the 95% confidence interval; OT, over-transmitted allele; T:U, transmitted vs untransmitted allele.
Significant p-values are indicated in bold.
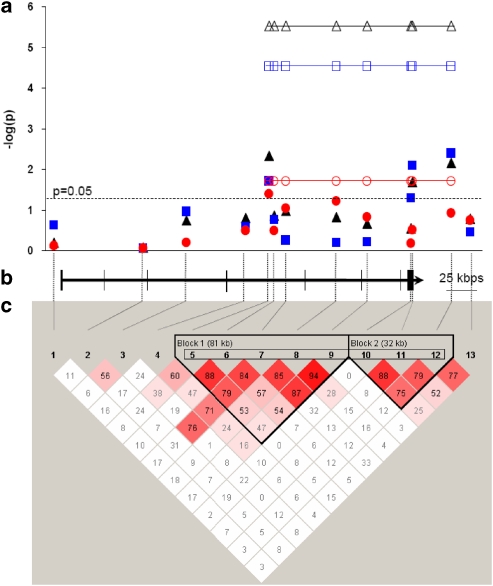
Figure 1.
ESR1 SNPs, mapping, and association with restrictive AN. (a) Association between restrictive AN and SNPs (filled symbol) or haplotypes (open symbol) for combined parental (triangle), maternal (circle), and paternal (square) transmissions. The strongest association is observed for the eight-SNPs haplotype (p=6.10−6). (b) Genomic organization of ESR1 gene (bars correspond to exons and arrowhead indicates the orientation of transcription) and position of the 13 encompassing SNPs (interrupted bars) at scale. (c) Pairwise linkage disequilibrium of SNPs. Haplotype blocks 1 and 2 are indicated. The haplotype of eight-SNPs is boxed.
Statistical Analysis
Comparisons of clinical features between cohorts and subsets were performed using Student's t-test and χ2 test. Hardy–Weinberg equilibrium tests, linkage disequilibrium D' values and minor allele frequencies were computed using Haploview 4.1 software (Barrett et al, 2005). Transmission disequilibrium tests (TDTs) for SNPs and haplotypes were carried out with FBAT and PLINK programs (Horvath et al, 2001; Purcell et al, 2007). Parental origin of the association was calculated using ASPEX 2.5 package (Hinds and Risch, 1996). Gene–gene interaction was computed using the gene-based test of the PLINK program. The power of sample size for association tests was calculated using the Genetic Power Calculator program (http://pngu.mgh.harvard.edu/∼purcell/gpc/) (Purcell et al, 2003). To take account the linkage disequilibrium between each SNP, we computed correction for multiple testing for SNPs using Single-Nucleotide Polymorphism Spectral Decomposition (SNPSpD) (http://gump.qimr.edu.au/general/daleN/SNPSpD/) (Nyholt, 2004).
RESULTS
Association of ESR1 Gene with AN and RAN Subset
We first genotyped 17 SNPs, encompassing ESR1 and ESR2 genes, in a French cohort of 321 AN families. All SNPs analyzed in this study were in Hardy–Weinberg equilibrium in the patients and parents. No discrepancy was observed in the genotyping of 30 duplicated samples. Two families were excluded because of Mendelian inheritance errors in the paternal transmissions.
Using TDTs, we found a significant over-transmission in AN with ESR1 SNPs rs726281 (p=0.024, odds ratio (OR)=1.5, 95% confidence interval (95% CI)=1–2) and rs2295193 (p=0.024, OR=1.4, 95% CI=1–1.8), (Table 2). We showed that RAN and BPAN subjects differed significantly for several clinical variables, particularly regarding the age of onset of anorexia and the minimum lifetime BMI (Table 1). Therefore, we performed TDT analyses according to the RAN and BPAN subsets. Three ESR1 SNPs were significantly over-transmitted in the RAN subset, rs726281 (p=0.005, OR=2.1, 95% CI=1.2–3.6), rs3798577 (p=0.021, OR=1.6, 95% CI=1.1–2.3), and rs2295193 (p=0.007, OR=1.7, 95% CI=1.2–2.5) (Table 3). In contrast, no SNP was associated with the BPAN subgroup (Supplementary Table 1).
Table 3. Transmission Disequilibrium Test of ESR1 SNPs with Restrictive AN Subset of French Family-Based Cohort.
| SNP# | Markers |
TDT |
Maternal TDT |
Paternal TDT |
|||||
|---|---|---|---|---|---|---|---|---|---|
| OT | T:U | p | OR (CI95) | p | OR (CI95) | p | OR (CI95) | ||
| 1 | rs488133 | C | 56:51 | 0.629 | 1.1 (0.8–1.6) | 0.758 | 0.9 (0.5–1.7) | 0.237 | 1.5 (0.8–2.9) |
| 2 | rs11155819 | C | 51:49 | 0.841 | 1 (0.7–1.5) | 0.866 | 1.1 (0.5–2.1) | 0.873 | 1.1 (0.6–2.0) |
| 3 | rs12199722 | G | 53:40 | 0.178 | 1.3 (0.9–2.0) | 0.631 | 1.2 (0.6–2.2) | 0.114 | 1.7 (0.9–3.2) |
| 4 | rs1884051 | A | 42:30 | 0.157 | 2.0 (1–3.8) | 0.330 | 1.4 (0.7–2.6) | 0.257 | 1.5 (0.7–3.3) |
| 5 | rs726281 | A | 48:24 | 0.005a | 2.1 (1.2–3.6) | 0.041 | 2.2 (1.0–4.9) | 0.024 | 2.3 (1.1–4.8) |
| 6 | rs3020407 | A | 52:38 | 0.140 | 1.4 (0.9–2.1) | 0.317 | 1.4 (0.7–2.7) | 0.170 | 1.6 (0.8–3.2) |
| 7 | rs17081994 | T | 20:11 | 0.106 | 1.8 (0.9–3.8) | 0.090 | 2.4 (0.8–6.8) | 0.564 | 1.4 (0.4–4.4) |
| 8 | rs2982712 | T | 61:46 | 0.147 | 1.3 (0.9–1.9) | 0.064 | 1.8 (1.0–3.4) | 0.647 | 1.2 (0.6–2.1) |
| 9 | rs3020371 | C | 53:41 | 0.216 | 1.3 (0.9–1.9) | 0.150 | 1.6 (0.8–3.0) | 0.622 | 1.2 (0.6–2.2) |
| 10 | rs2228480 | G | 31:23 | 0.276 | 1.3 (0.8–2.3) | 0.670 | 0.8 (0.4–1.9) | 0.050 | 2.3 (1.0–5.2) |
| 11 | rs3798577 | T | 66:42 | 0.021 | 1.6 (1.1–2.3) | 0.307 | 1.4 (0.8–2.4) | 0.008 | 2.4 (1.2–4.7) |
| 12 | rs2295193 | G | 68:40 | 0.007 | 1.7 (1.2–2.5) | 0.123 | 1.6 (0.9–3.0) | 0.004 | 2.6 (1.3–5.3) |
| 13 | rs2252837 | C | 57:43 | 0.162 | 1.3 (0.9–2.0) | 0.182 | 1.6 (0.8–3.1) | 0.355 | 1.3 (0.7–2.5) |
Abbreviations: OR (CI95), odds ratio and the 95% confidence interval; OT, over-transmitted allele; T:U, transmitted vs untransmitted allele.
Significant p-values are indicated in bold.
No preferential transmission was found for the ESR2 SNPs in any of the AN subgroups (Supplementary Table 2). Furthermore, only one haplotype block encompasses the 112 kb of the ESR2 gene that renders unlikely the association between ESR2 gene with AN, or with the RAN or BPAN subsets (Supplementary Figure 1).
No significant epistatic association was found for combinations of ESR1 and ESR2 SNPs and the AN cohort, neither for the RAN nor for the BPAN subgroups (data not shown).
Haplotype Association in the RAN Subset
Linkage disequilibrium analysis of pairwise SNPs revealed several haplotype blocks across the 296 kb of ESR1 gene (Figure 1c). Over-transmissions in the RAN subset were found for common haplotypes based on five SNPs (block 1: rs726281*A-rs3020407*A-rs17080994*T-rs2982712*T-rs3020371*C, 0.503 frequency, p=0.06), three SNPs (block 2: rs2228480*G-rs3798577*T-rs2295193*G, 0.410 frequency, p=0.002), and the eight SNPs of the two blocks merged (rs726281*A-rs3020407*A-rs17080994*T-rs2982712*T-rs3020371*C-rs2228480*G-rs3798577*T-rs2295193*G, 0.230 frequency, p=6.10−6). This eight-SNPs haplotype presents an OR of 3.1 (95% CI=1.8–5.1) for RAN.
Parental Origin of Association
In addition, we analyzed the parental origin of the association with RAN subset. Considering the combined TDT of the 13 ESR1 SNPs, we detected a highly significant excess of paternal transmission (p=9.10−7) and a significant excess of maternal transmission (p=0.0004). More precisely in RAN, a paternal over-transmission of four SNPs (rs726281 p=0.024, rs2228480 p=0.05, rs3798577 p=0.008 and rs2295193 p=0.004) and a maternal over-transmission of rs726281 only (p=0.041) were observed (Table 3). Interestingly, the rs726281 over-transmitted by both parents is located in haplotype block 1, while the three SNPs solely transmitted in excess by the father constitute block 2 (Figure 1c). Regarding the eight-SNPs haplotype in RAN, in comparison with the maternal over-transmission (p=0.02, OR=2.1, CI95=1.1–3.8), we found a higher statistical significance for the paternal over-transmission (p=3.10−5, OR=3.7, 95% CI=1.9–7.3) (Figure 1a).
ESR1 Association with an Independent RAN German Cohort
As a second step, we attempted to replicate the association by genotyping ESR1 SNPs in an independent German cohort of 41 RAN trios (Table 4). Although no allele was transmitted in excess in the probands of this replication cohort, we found a preferential paternal over-transmission of the same SNP, which was initially associated in our sample, namely the rs726281 (p=0.025), and a trend of significance for rs3798577 (p=0.059), conferring a higher risk for RAN in this sample (OR=3.0, 95% CI=1.1–8.3, and OR=2.6, 95% CI=0.9–7.3, respectively).
Table 4. Transmission Disequilibrium Test of ESR1 SNPs with Restrictive AN of German Family-Based Cohort.
| SNP# | Markers | MAF |
Parental TDT |
Maternal TDT |
Paternal TDT |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OT | T:U | p | OR (CI95) | T:U | p | OR (CI95) | T:U | p | OR (CI95) | |||
| 4 | rs1884051 | G (0.30) | G | 20:15 | 0.398 | 1.3 (0.7–2.6) | 09:09 | 1 | 1 (0.4–2.5) | 11:06 | 0.225 | 1.8 (0.7–5.0) |
| 5 | rs726281 | G (0.27) | G | 23:14 | 0.139 | 1.6 (0.8–3.2) | 08:09 | 0.808 | 0.9 (0.3–2.3) | 15:05 | 0.025a | 3 (1.1–8.3) |
| 6 | rs3020407 | G (0.33) | A | 17:16 | 0.862 | 1.1 (0.5–2.1) | 11:06 | 0.225 | 1.8 (0.7–5.0) | 06:10 | 0.317 | 0.6 (0.2–1.7) |
| 7 | rs17081994 | C (0.09) | C | 08:05 | 0.405 | 1.6 (0.5–4.9) | 01:02 | 0.564 | 0.5 (0–5.5) | 06:02 | 0.157 | 3 (0.6–14.9) |
| 9 | rs3020371 | T (0.32) | T | 22:16 | 0.330 | 1.4 (0.7–2.6) | 09:08 | 0.808 | 1.1 (0.4–2.9) | 12:07 | 0.251 | 1.7 (0.7–4.4) |
| 10 | rs2228480 | A (0.19) | G | 15:13 | 0.705 | 1.2 (0.5–2.4) | 08:06 | 0.593 | 1.3 (0.5–3.8) | 08:07 | 0.796 | 1.1 (0.4–3.2) |
| 11 | rs3798577 | C (0.40) | T | 23:13 | 0.096b | 1.8 (0.9–3.5) | 10:08 | 0.637 | 1.3 (0.5–3.2) | 13:05 | 0.059 | 2.6 (0.9–7.3) |
| 12 | rs2295193 | A (0.45) | – | 21:21 | 1 | 1 (0.5–1.8) | 11:11 | 1 | 1 (0.4–2.3) | 10:10 | 1 | 1 (0.4–2.4) |
Abbreviations: MAF, minor allele and its frequency in the parentheses; OR (CI95), odds ratio and the 95% confidence interval; OT, over-transmitted allele; T:U, transmitted vs untransmitted allele.
Significant p-values are indicated in bold.
Trend of significance for p-values are indicated in italic.
ESR1 Association with Eating Disorders in an Independent Population-Based Sample
In a population-based sample of 693 young French Caucasian women (mean age 20.30±0.05 years), tagged-SNP rs3798577 was found associated with the presence of eating disorders (additive model: p=0.008) (Table 5).
Table 5. Association of ESR1 rs3798577 Tag-SNP with AN in a Population-Based Sample of 693 French Caucasian Women.
|
Genotype |
Total | |||||||
|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | ||||||
| N | % | N | % | N | % | N | % | |
| SCOFF<2 | 128 | 22.6 | 284 | 50.1 | 155 | 27.3 | 567 | 100 |
| SCOFF⩾2 | 19 | 15.1 | 59 | 46.8 | 48 | 38.1 | 126 | 100 |
p-value (additive model)=0.008.
DISCUSSION
In this study, we found an association between ESR1 gene and AN, mainly observed in the restrictive subtype of AN. We showed no association of ESR2 with AN, nor with RAN or BPAN subgroups. Furthermore, we did not identified ESR1–ESR2 gene–gene interaction with either AN or restrictive subtype.
Only two works of AN with ESR1 and ESR2 genes were previously published (Rosenkranz et al, 1998; Eastwood et al, 2002). The initial study has screened for mutation in ESR2 gene (Rosenkranz et al, 1998) and found different distribution of the variant G1082A (rs1256049) in AN compared with BN or to obese–underweight subjects. In the second study, no association was reported between AN and three markers, or haplotypes, of ESR1 gene, but a significant association was reported with the rs1256049 SNP of ESR2 (Eastwood et al, 2002). Furthermore, an association was also reported with rs4986938 and rs928554 (but not with rs1256049) in bulimia nervosa and eating disorders not otherwise specified (Nilsson et al, 2004). In this study, no association was found between ESR2 SNPs, which are in linkage disequilibrium (D'>0.9) with rs1256049 according to databases, and AN, RAN, or BPAN subsets. It is noteworthy that our screening of 321 AN families using the TDT was more powerful (1−β=89%) than the two previous case–control studies (1−β=18 and 63%, respectively) and distinguished RAN and BAN.
We reported a strong association of specific SNPs and haplotypes of the ESR1 gene and AN, and RAN subgroup, with a preferential paternal over-transmission. Although we genotyped a large cohort of families and performed TDTs that reduce the sex and ethnic stratification bias, the probability of a false-positive finding in this study cannot be excluded because of the number of tests performed. After a Bonferroni correction taking into account the 13 SNPs within ESR1 gene for the RAN subgroup, we found only trends of association for two SNPs, rs726281 pcorrected=0.065 and rs2295193 pcorrected=0.091, but still observed a strong statistical significance for the eight-SNPs haplotype (pcorrected=0.00008). It must be kept in mind that the Bonferroni correction is relevant for independent tests. Given that linkage disequilibrium between the 13 SNPs are mainly above zero in our sample, this correction is probably over-conservative. To assess a more appropriate p-value corrected for multiple testing for single SNP association, we carried out the SNPSpD method (Nyholt, 2004). Significance threshold required is a p<0.0051 (rs726281) and effective number of independent SNP is 10 instead of 13. Thus, we found a significant association for rs726281 pcorrected=0.049 and a trend for rs2295193 pcorrected=0.07.
The three SNPs of ESR1 associated with RAN in our sample (rs726281, rs3798577, and rs2295193) are located in introns and 3′ UTR region. To date, they have no known functional consequences. However, the associated haplotype block of eight SNPs covers the C terminal E/F region of ESR1 protein, which corresponds to the ligand fixation domain of nuclear receptor. So, it is tempting to speculate that the association of these SNPs with RAN could suggest changes of ligand fixation in RAN patients.
We also attempted to replicate our results in an independent German cohort of 41 RAN trios, but failed to confirm significant associations between ESR1 SNPs and RAN. Surprisingly, participants in the German RAN cohort showed a significant higher mean of minimal lifetime BMI compared with the French RAN cohort, while they were significantly younger and had shorter disease duration. As up to half of the RAN patients cross-over to binge eating/purging subtype in a 7 years follow-up study (Eddy et al, 2008), this difference may explain the absence of replication in the German sample, because in the latter, young RAN patients could still switch to the BPAN phenotype. Although this difference between the two cohorts decreases any chances of replication, we were able to observe the excess of paternal transmission of the main SNP associated with AN in the first sample. Owing to a small sample size, the RAN German cohort provided a reduce power (power=33%) compared with the RAN French cohort (power=59%) that can explain the low significance of association.
Finally, we tested a tagged-SNP of the ESR1 gene in a large population-based sample, and found an association with eating disorders, as defined by a SCOFF score equal or above 2. However, it should be noted that the SCOFF scale is a screening tool for eating disorders rather that a diagnostic instrument for AN, and therefore no direct comparison should be made between the two first clinical samples and this population-based sample. At most, this epidemiological sample gives indirect evidence for replication, which is usually regarded as needed for genetic association studies in complex disorders.
There are several limitations of this study. First, the instruments assessing the eating disorders are different in the three cohorts. French and German trios families were assessed with semi-structured interview, respectively, DIGS and CIDI, while the self-evaluation SCOFF scale was used to screen epidemiological eating disorders in a large woman population sample. Second, the sample size of the German trios is small with a shorter disease evolution period, although a cohort with a larger sample size and a disease duration equivalent to the French cohort is recommended to replicate our results. Third, the three SNPs of ESR1 (rs726281, rs3798577, and rs2295193), associated with RAN, have no known functional consequences up to now and are located in non-coding regions. These SNPs required a functional analysis regarding their potential effects of associated alleles in ESR1 receptor proprieties.
Although there is a lack of report on the genetic aspects of hormonal status as a risk factor for AN, recent investigations suggest that some hormonal environment might be associated with eating disorders (Procopio and Marriott, 2007; Young, 1991). Observations between same-sex and opposite-sex twins allowed to find a high AN prevalence in adult life in female compares with male, suggesting an influence of intrauterine exposure level to sex hormone (Procopio and Marriott, 2007). Interestingly, significant deficiency of serum levels for estradiol, the ligand of estrogen receptor, has been described in AN patients compared with controls, and a lower concentration in restrictive subtype rather than the binge eating/purging subtype has been reported (Ohwada et al, 2007). It is tempting to speculate that the association between ESR1 gene and RAN may lead to specific biological effects, such as a modified expression and/or an altered function of the estrogen receptor alpha. Furthermore, the paternal transmission of the association that we observed also supports a genetic imprinting, which might be influenced by sex hormones. In addition, it is important to consider DNA methylation in psychiatric disorder because it could help to the understanding of mechanism of the pathology and could constitute potential therapeutic targets. Acute administration of a selective ER alpha agonist leads to ovariectomized rats that showed a decrease in daily food intake and body weight (Santollo et al, 2007). Furthermore, in female mice, agonists of ER receptors produce different effects on social learning of food preferences (Clipperton et al, 2008). Then, ER alpha antagonist treatment failed to reduce food intake decrease in chemically ovariectomized rats (Santollo and Eckel, 2009). Finally, in female rat, the level of ESR1 protein expression and estrogen sensitivity are modulated by oxytocin (Perry et al, 2009). Thus, it is difficult up to now to suggest a pharmacological treatment for AN. Further functional studies are needed to decipher the biological effect of the association identified in our study, which may influence the number or affinity of ESR1 receptor in RAN patients.
Our study suggests evidences that common variants in ESR1 gene are associated with eating disorders, and more specifically RAN, highlighting the possible involvement of estrogenic hormonal pathway in AN that might open novel avenues of neuroendocrinopharmacological approaches in this neuropsychiatric disorder.
Acknowledgments
Overall, we acknowledge the kind assistance of the families, students, and patients who participated in the studies. This work received grants from EC Framework V ‘Factors in Healthy Eating' (a consortium coordinated by Janet Treasure and David Collier), and from INRA/INSERM (4M406D). AV is supported by grants from ‘Région Ile-de-France'. YLS is funded by the ‘Société Française de Tabacologie'. The SAGE study was supported by grants from the Institut de Recherche sur les Boissons (IREB), and from the ‘Mission Interministérielle de Lutte contre la Drogue et la Toxicomanie' (MILDT). JH, AN, and SS were supported by the German Ministry of Education and Research (BMBF, EDNET 01GV0905).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- American Psychiatric Association 1994Diagnostic and Statistical Manual of Mental Disorders4th edn., Text Revision (DSMIV-TR).American Psychiatric Press: Washington, DC [Google Scholar]
- Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, Kendler KS. Genetic risk factors for disordered eating in adolescent males and females. J Abnorm Psychol. 2009;118:576–586. doi: 10.1037/a0016314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Monteleone P, Bortolotti F, Dalle Grave R, Todisco P, Faravo A, et al. Persistent amenorrhoea in weight-recovered anorexics: psychological and biological aspects. Psychiatry Res. 2003;118:249–257. doi: 10.1016/s0165-1781(03)00074-x. [DOI] [PubMed] [Google Scholar]
- Clipperton AE, Spinato JM, Chernets C, Pfaff DW, Choleris E. Differential effects of estrogen receptor alpha and beta specific agonists on social learning of food preferences in female mice. Neuropsychopharmacology. 2008;33:2362–2375. doi: 10.1038/sj.npp.1301625. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnault A, Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. Am J Physiol. 1997;272:R311–R317. doi: 10.1152/ajpregu.1997.272.1.R311. [DOI] [PubMed] [Google Scholar]
- Eastwood H, Brown KM, Markovic D, Pieri LF. Variation in the ESR1 and ESR2 genes and genetic susceptibility to anorexia nervosa. Mol Psychiatry. 2002;7:86–89. doi: 10.1038/sj.mp.4000929. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Keel PK, Dorer DJ, Delinsky SS, Franko DL, Herzog DB. Longitudinal comparison of anorexia nervosa subtypes. Int J Eat Disord. 2002;31:191–201. doi: 10.1002/eat.10016. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon C, Guelfi JD, Kipman A, Adès J, Romo L, Houdeyer K, et al. Switching to the bingeing/purging subtype of anorexia nervosa is frequently associated with suicidal attempts. Eur Psychiatry. 2007;22:513–519. doi: 10.1016/j.eurpsy.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int J Eat Disord. 1983;2:15–34. [Google Scholar]
- Gershon ES, Schreiber JL, Hamovit JR, Dibble ED, Kaye W, Nurnberger JI, Jr, et al. Clinical findings in patients with anorexia nervosa and affective illness in their relatives. Am J Psychiatry. 1984;141:1419–1422. doi: 10.1176/ajp.141.11.1419. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Kipman A, Foulon C. The human genetics of anorexia nervosa. Eur J Pharmacol. 2003;480:163–170. doi: 10.1016/j.ejphar.2003.08.103. [DOI] [PubMed] [Google Scholar]
- Grice DE, Halmi KA, Fichter MM, Strober M, Woodside DB, Treasure JT, et al. Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. Am J Hum Genet. 2002;70:787–792. doi: 10.1086/339250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EC, Barraclough BM. Suicide as an outcome for medical disorders. Medicine (Baltimore) 1994;73:281–296. doi: 10.1097/00005792-199411000-00001. [DOI] [PubMed] [Google Scholar]
- Hill LS, Reid F, Morgan JF, Lacey JH.2009SCOFF, the development of an eating disorder screening questionnaire Int J Eat Disordin press. [DOI] [PubMed]
- Hinds DA, Risch N.1996The ASPEX package: affected sib-pair exclusion mapping . http://aspex.sourceforge.net/ .
- Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry. 2006;19:389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype—phenotype associations. Eur J Hum Genet. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Horwood J, Falissard B, Hassler C, Romo L, et al. First positive reactions to cannabis constitute a priority risk factor for cannabis dependence. Addiction. 2009;104:1710–1717. doi: 10.1111/j.1360-0443.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML, Gold PW. The hypothalamic-pituitary-adrenal axis in anorexia nervosa. Psychiatry Res. 1996;62:75–83. doi: 10.1016/0165-1781(96)02991-5. [DOI] [PubMed] [Google Scholar]
- Milos G, Spindler A, Hepp U, Schnyder U. Suicide attempts and suicidal ideation: links with psychiatric comorbidity in eating disorder subjects. Gen Hosp Psychiatry. 2004;26:129–135. doi: 10.1016/j.genhosppsych.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrog. Proc Natl Acad Sci USA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Naessen S, Dahlman I, Linden-Hirschberg A, Gustafsson JA, Dahlman-Wright K. Association of estrogen receptor beta gene polymorphisms with bulimic disease in women. Mol Psychiatry. 2004;9:28–34. doi: 10.1038/sj.mp.4001402. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. 1994Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative Arch Gen Psychiatry 51849–859.discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada R, Hotta M, Sato K, Shibasaki T, Takano K. The relationship between serum levels of estradiol and osteoprotegerin in patients with anorexia nervosa. Endocr J. 2007;54:953–959. doi: 10.1507/endocrj.k07-034. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Perry AN, Paramadilok A, Cushing BS. Neonatal oxytocin alters subsequent estrogen receptor alpha protein expression and estrogen sensitivity in the female rat. Behav Brain Res. 2009;205:154–161. doi: 10.1016/j.bbr.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Procopio M, Marriott P. Intrauterine hormonal environment and risk of developing anorexia nervosa. Arch Gen Psychiatry. 2007;64:1402–1407. doi: 10.1001/archpsyc.64.12.1402. [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raevuori A, Kaprio J, Hoek HW, Sihvola E, Rissanen K, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: lack of association with twin type in a nationwide study of Finnish twins. Am J Psychiatry. 2008;165:1604–1610. doi: 10.1176/appi.ajp.2008.08030362. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Versini A, Gorwood P. Eating disorders: an overview of treatment responses and the potential impact of vulnerability genes and endophenotypes. Expert Opin Pharmacother. 2007;8:2029–2044. doi: 10.1517/14656566.8.13.2029. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The composite international diagnostic interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Hinney A, Ziegler A, Hermann H, Fichter M, Mayer H, et al. Systematic mutation screening of the estrogen receptor beta gene in probands of different weight extremes: identification of several genetic variants. J Clin Endocrinol Metab. 1998;83:4524–4527. doi: 10.1210/jcem.83.12.5471. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Effect of a putative ERalpha antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009;97:193–198. doi: 10.1016/j.physbeh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler AR, Short S, Andersen MR, Paner TM, Briggs JC, Lambert SM, et al. The SNPlex genotyping system: a flexible and scalable platform for SNP genotyping. J Biomol Tech. 2005;16:398–406. [PMC free article] [PubMed] [Google Scholar]
- Van de Stolpe A, Slycke AJ, Reinders MO, Zomer AW, Goodenough S, Behl C, et al. Estrogen receptor (ER)-mediated transcriptional regulation of the human corticotropin-releasing hormone-binding protein promoter: differential effects of ERalpha and ERbeta. Mol Endocrinol. 2004;18:2908–2923. doi: 10.1210/me.2003-0446. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Prewitt AK. Dynamic regulation of estrogen receptor-alpha gene expression in the brain: a role for promoter methylation. Front Neuroendocrinol. 2008;29:375–385. doi: 10.1016/j.yfrne.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK. Estrogen and the etiology of anorexia nervosa. Neurosci Biobehav Rev. 1991;15:327–331. doi: 10.1016/s0149-7634(05)80025-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.