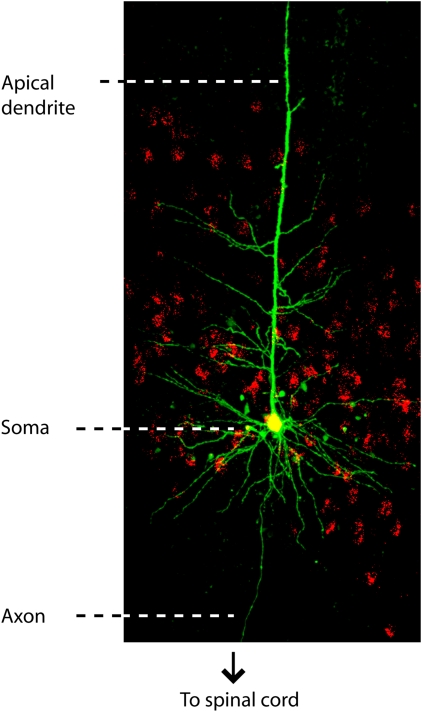
Voluntary control of movement relies on activity in a subclass of layer 5B pyramidal neurons projecting to the spinal cord—corticospinal neurons (also called Betz cells or upper motor neurons) (Figure 1). In motor cortex, corticospinal neurons are one of the many classes of pyramidal neurons distributed over multiple layers and sublayers with diverse local and long-range projections. Studies in primates, the motor systems of which so closely resemble our own, have provided a wealth of information about the spiking activities of motor cortex neurons during action planning and execution. However, in vivo recording methods usually preclude identification of the precise laminar locations of neurons and their synaptic connectivity, and in vitro analysis is impractical in primates. Consequently, much remains to be learned about how local circuits are organized according to projection target and sublayer (Brown and Hestrin, 2009; Anderson et al, 2010), and how this organization influences spiking activity in different subclasses of projection neurons in motor cortex.
Figure 1.
Corticospinal neurons in layer 5B of mouse motor cortex. Neurons were retrogradely labeled in vivo by injecting red fluorescent microspheres into the cervical spinal cord. Brain slices containing motor cortex were prepared, and a single corticospinal neuron was targeted for whole-cell patch recording with biocytin in the pipette solution. The slice was fixed and processed with streptavidin-conjugated green fluorescent dye for visualizing dendritic morphology. Red and green fluorescence images were acquired by 2-photon microscopy, and merged for display. Image provided by L Trapp and B Suter.
Recently, several groups have established the feasibility of motor behavior experiments with rodents, using not only electrophysiology, but with calcium imaging, also large-scale optical recordings (Dombeck et al, 2009). It is remarkable that this has now been combined with sophisticated within-session learning paradigms (Komiyama et al, 2010). There are, of course, limitations. One of the limitations is depth: only neurons in the upper layers of the cortex are easily imaged. However, this is hardly uninteresting, as microcircuit mapping studies have shown that layer 2/3 is the main source of synaptic output within motor cortex circuits, and is the major source of input to corticospinal neurons (Anderson et al, 2010).
Another intriguing issue is whether circuits in the remaining frontal agranular cortex—presumably involved in more ‘cognitive' aspects of motor control—are organized in the form of primary motor cortex. If “thinking is the evolutionary internalization of movement” (Llinás, 2002), then one might expect so. In this view the cortical extent of the motor system extends beyond primary motor areas. Behavior, after all, is essentially synonymous with the activity of the motor system. Indeed, a compelling case can be made for the viewpoint that ‘layer 5 is motor everywhere' (Diamond, 1979); in other words, layer 5B is ‘motor cortex'. Should we regard layer 2/3 as ‘premotor cortex'?
We expect that as research on motor/frontal cortex goes forward, a general framework for understanding behavioral ‘control' mechanisms at the level of cortical circuits will emerge, which will link multiple levels of neural organization and have useful roles both in assimilating the rapidly accruing new information and in inspiring testable hypotheses. New opportunities for the neuropsychopharmacology of movement disorders are also likely to arise. For example, many cognitive disorders have a motor component, and vice versa; apraxias are classic examples. Also, the expression patterns of ion channel and intracellular signaling molecules is highly diverse in layer 5B (Allen Brain Atlas). This diversity presents a fertile substrate for exploring pharmacological strategies to selectively target pathological mechanisms in specific subclasses of cortical projection neurons involved in different aspects of voluntary movement.
Acknowledgments
Supported by grants from the NIH (NS061963, NS066675) and Whitehall Foundation.
The authors declare no conflict of interest.
References
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT.1979The subdivision of neocortex: a proposal to revise the traditional view of sensory, motor and association areasIn: Sprague JM, Epstein AN, (eds).Progress in Psychobiology and Physiological Psychology Academic Press: New York; 1–43. [Google Scholar]
- Dombeck DA, Graziano MS, Tank DW. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J Neurosci. 2009;29:13751–13760. doi: 10.1523/JNEUROSCI.2985-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Llinás R. i of the vortex. MIT Press: Cambridge, Massachusetts; 2002. [Google Scholar]