Extinction of maladaptive conditioned responses or behaviors is a process of new and active learning, and requires the organism to learn new stimulus–response and action–outcome relationships, and to form new associations between previously hypersalient stimuli (ie, trauma-related cues and contexts) and appropriate cognitive and/or behavioral responses. Much of our knowledge about the neural substrates that underlie extinction processes comes from studies of fear conditioning (Myers and Davis, 2007), but an increasing number of studies have begun to examine the mechanisms underlying extinction of drug-seeking behavior (Cleva and Gass, 2010).
Adult neurogenesis is an ongoing process that occurs in most mammalian species, including humans. This phenomenon occurs primarily in two brain regions: the subgranular layer of the dentate gyrus region of the hippocampus, which gives rise to neurons that migrate and integrate into the granule cell layer (GCL), and the subventricular layer of the lateral ventricles, which supplies newborn neurons to the olfactory system through the rostral migratory stream. Factors that contribute to the birth, differentiation, maturation, migration, and survival of adult-born neurons, as well as the specific function of surviving neurons, are poorly understood. However, adult-born neurons may be involved in certain learning and memory processes.
As extinction is a form of learning, it would follow that adult hippocampal neurogenesis (AHN) might have a role in extinction processes. An initial study showed that ablation of AHN in mice by γ-irradiation or anti-mitotic agent administration failed to affect the extinction of contextual fear memory (Ko et al, 2009). However, it was subsequently showed that conditional ablation of AHN in a nestin-thymidine kinase transgenic mouse line indeed impaired extinction of a contextual fear memory (Deng et al, 2009). In agreement with these latter findings, disruption of AHN by irradiation impaired the extinction of cocaine-seeking behavior in rats following intravenous cocaine self-administration (Noonan et al, 2010).
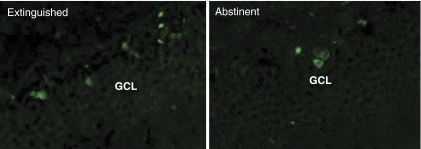
Performance of various learning and memory tasks can increase AHN, further supporting a role for these neurons in experience-dependent neural plasticity. To this end, extinction training following intravenous heroin self-administration increases cell proliferation in the hippocampus (Figure 1; see also Wischerath et al, 2009), and in agreement with the observations of Noonan et al (2010), conditional ablation of AHN in mice impairs extinction learning following heroin self-administration (unpublished observations). Together, these data suggest that in most instances, AHN is involved in extinction learning, and future studies are needed to determine the precise environmental conditions as well as the chemical and molecular regulators of AHN. As a result, AHN could potentially be targeted as an adjunct to extinction-based therapies, such as cue exposure therapy, that are used in the treatment of anxiety and substance use disorders.
Figure 1.
Rats self-administered heroin 3 h daily for 12 days and were then administered 2-bromodeoxyuridine (BrdU) immediately after the first five extinction training sessions or the on first 5 days of forced abstinence. Extinction-trained animals (left) showed approximately twice as many BrdU-labeled cells in the subgranular layer as those that underwent forced abstinence (right). GCL, granule cell layer.
Acknowledgments
This work was supported by NIDA Grant DA024355.
The authors declare no conflicts of interest.
References
- Cleva RM, Gass JT. Neuroanatomical substrates underlying the extinction of drug-seeking behavior. Open Addict J. 2010;3:63–75. [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischerath KC, Cleva RM, Gass JT, Olive MF.2009Positive allosteric modulation of mGluR5 receptors decreases extinction responding following intravenous heroin and cocaine self-administration: correlation with hippocampal neurogenesis Program No 348.11, Neuroscience Meeting Planner Chicago, IL: Society for Neuroscience, 2009. Online.