Abstract
Glutamate is the major excitatory neurotransmitter in the central nervous system. Although glutamate mediates synaptically confined point-to-point transmission, it has been suggested that under certain conditions glutamate may escape from the synaptic cleft (glutamate spillover), accumulate in the extrasynaptic space, and mediate volume transmission to regulate important brain functions. However, the inability to directly measure glutamate dynamics around active synapses has limited our understanding of glutamatergic volume transmission. The recent development of a family of fluorescent glutamate indicators has enabled the visualization of extrasynaptic glutamate dynamics in brain tissues. In this topical review, we examine glutamate as a volume transmitter based on novel results of glutamate imaging in the brain.

Yohei Okubo received his PhD from the Department of Pharmacology, Graduate School of Medicine, the University of Tokyo in 2005. After a short-time training as a post-doctoral fellow, he became a Research Associate at the University in 2006. He has studied functions and the spatiotemporal dynamics of calcium, inositol 1,4,5-trisphosphate and glutamate in neurons and glial cells using the fluorescence imaging technique. Masamitsu Iino has been the head of the present department since 1995. He has studied the basic principles of spatiotemporal regulation of Ca2+ signalling, and showed the importance of the regenerative property of intracellular Ca2+ release in the shaping of Ca2+ signals. To characterize the spatiotemporal features of signalling molecules upstream and downstream of Ca2+ signals such as IP3, nitric oxide and glutamate, his laboratory imaged these signalling molecules, developing their new indicators. The results shed new light on the spatiotemporal signalling mechanism of these important molecules. Based on these results, his laboratory is now searching for new cell functions that are regulated by Ca2+ signals in the brain.
Glutamate is the major excitatory synaptic transmitter in the mammalian central nervous system. Upon synaptic glutamate release, glutamate concentration within the narrow synaptic cleft between the pre- and postsynaptic membranes rapidly reaches millimolar concentrations, and then decays within a few milliseconds by diffusion and reuptake mechanisms (Clements et al. 1992; Rothstein et al. 1996; Asztely et al. 1997; Rusakov & Kullmann, 1998; Barbour, 2001; Mashimo et al. 2010). These rapid and spatially confined glutamate dynamics ensure precise point-to-point transmission at excitatory synapses. However, spatiotemporally dense synaptic activities may increase glutamate concentration in the extrasynaptic space, which is referred to as ‘glutamate spillover.’ Glutamate spillover is expected to activate extrasynaptic glutamate receptors distributed around the active synapse to mediate volume transmission (Barbour & Hausser, 1997). It has been proposed that glutamatergic volume transmission regulates a variety of important neural and glial functions (Sem’yanov, 2005; Haydon & Carmignoto, 2006).
The spatiotemporal dynamics of neurotransmitter in the extrasynaptic space is the key determinant of volume transmission. However, the details of extrasynaptic spatiotemporal glutamate dynamics have remained elusive; they have been estimated by numerical modelling studies or inferred indirectly using electrophysiological and other measurements. The notion that synaptically released glutamate functions as a volume transmitter had not been confirmed by direct measurements of extrasynaptic glutamate dynamics. Recently, several groups have developed new fluorescent indicators of glutamate (Okumoto et al. 2005; Namiki et al. 2007; Hires et al. 2008). One of these indicators has been found to have a sufficient sensitivity and dynamic range for the measurement of glutamate dynamics in brain tissues during physiologically relevant neural inputs (Namiki et al. 2007; Okubo et al. 2010). In this topical review, we first summarise the physiological functions that involve glutamatergic volume transmission. We then compare the properties of glutamatergic volume transmission estimated by previous studies with those revealed by new glutamate imaging techniques.
Neural and glial functions mediated by glutamatergic volume transmission
Activation of neuronal extrasynaptic glutamate receptors by glutamate spillover underlies many physiological functions (Fig. 1). Homosynaptic and/or heterosynaptic activation of presynaptic group II metabotropic glutamate receptors (mGluRs) regulates presynaptic release probability at excitatory and inhibitory synapses (Scanziani et al. 1997; Mitchell & Silver, 2000). Activation of postsynaptic group I mGluRs localised at perisynaptic locations, on the other hand, regulates several physiological functions, including synaptic plasticity and synaptic maintenance (Furutani et al. 2006; Luscher & Huber, 2010). Activation of axonal kainate receptors by glutamate spillover mediates biphasic regulation of the excitability of presynaptic fibres (Schmitz et al. 2001). N-methyl-d-aspartate receptors (NMDARs) containing the NR2B subunit localise in extrasynaptic structures, and they may play a role distinct from that of the synaptic NMDARs, e.g. the extrasynaptic NMDARs, but not the synaptic NMDARs, are involved in neuronal survival (Hardingham et al. 2002) and Huntington's disease (Milnerwood et al. 2010). Neuronal extrasynaptic glutamate receptors are activated not only via glutamate spillover but also by glutamate released from glial cells (see below).
Figure 1. Neural and glial functions mediated by glutamatergic volume transmission.

Left, neuronal functions mediated by glutamatergic volume transmission. AMPAR, NMDAR, kainate receptor and mGluR localised to various extrasynaptic locations or within neighbouring synapses are activated by glutamate spillover to mediate physiological and pathophysiological events. Right, astrocytic functions mediated by glutamatergic volume transmission. Astrocytic mGluR is activated by glutamate spillover to induce gliotransmitter release and haemodynamic response.
Glutamate spillover may be involved in excitatory transmission activating ionotropic glutamate receptors, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), NMDARs and kainite receptors. Synaptic crosstalk, i.e. activation of ionotropic glutamate receptors in neighbouring synapses by glutamate spillover, has been found in several types of synapse (Carter & Regehr, 2000; Arnth-Jensen et al. 2002; DiGregorio et al. 2002; DeVries et al. 2006). Excitatory current induced by synaptic crosstalk contributes significantly to the transmission at cerebellar glomeruli (DiGregorio et al. 2002) and retinal cone photoreceptor synapses (DeVries et al. 2006). Furthermore, glutamate spillover can mediate transmission between neurons that have no direct synaptic contact. Such neurotransmission, mediated purely by glutamate spillover, is observed in the olfactory bulb (Isaacson, 1999) and the cerebellum (Szapiro & Barbour, 2007).
Glutamate spillover may mediate not only interactions between neurons but also signal transmission from neurons to glial cells (Fig. 1). Although glial cells do not usually receive direct synaptic contacts from neurons, glutamate released from neurons induces intracellular Ca2+ responses in astrocytes, the major type of glial cells in the brain, through activation of glutamate receptors (Cornell-Bell et al. 1990; Porter & McCarthy, 1996; Wang et al. 2006). The intracellular Ca2+ increase in astrocytes is thought to regulate various astrocytic functions. One of such functions is the release of gliotransmitters, such as ATP (Guthrie et al. 1999), d-serine (Schell et al. 1995) and glutamate (Parpura et al. 1994). Astrocytic-derived ATP and its hydrolysed product, adenosine, suppress adjacent glutamatergic synapses to mediate negative feedback regulation (Koizumi et al. 2003; Zhang et al. 2003; Pascual et al. 2005). d-Serine functions as an endogenous co-agonist of NMDARs (Schell et al. 1995), and d-serine released by astrocytes is required for the NMDAR-dependent classical hippocampal long-term potentiation (Henneberger et al. 2010). Glutamate released by astrocytes preferentially activates extrasynaptic NMDARs in multiple adjacent neurons, which enhances neuronal excitability (Parri et al. 2001; Fellin et al. 2004). Furthermore, group I mGluRs in neurons are activated by astrocytic-derived glutamate to potentiate presynaptic release probability (Fiacco & McCarthy, 2004; Perea & Araque, 2007). Thus, glutamate spillover plays an important role regulating gliotransmitter release from astrocytes, although controversy remains regarding the physiological relevance of astrocytic gliotransmitter release (Agulhon et al. 2010; Hamilton & Attwell, 2010). Another important astrocytic function triggered by glutamate spillover is the regulation of blood flow in the brain (Fig. 1). Glutamate spillover-triggered Ca2+ responses in astrocytes induce dilatation or constriction of adjacent blood vessels to induce haemodynamic responses (Zonta et al. 2003; Mulligan & MacVicar, 2004; Gordon et al. 2008).
Imaging extrasynaptic glutamate dynamics in the brain
There have been efforts to directly measure glutamate concentration in the brain. Microdialysis and amperometric measurements have been carried out to measure glutamate concentration changes within the brain tissue (Baker et al. 2002; Dash et al. 2009). However, these methods have limitations with regard to spatiotemporal resolution. To gain greater spatiotemporal resolution, fluorescent indicators of glutamate have been developed to directly visualise glutamate dynamics (Okumoto et al. 2005; Namiki et al. 2007; Hires et al. 2008). FLIPE (Okumoto et al. 2005) and SuperGluSnFR (Hires et al. 2008) are genetically encoded, fluorescence resonance energy transfer (FRET)-based indicators, in which cyan fluorescent protein (CFP) and a variant of yellow fluorescent protein (YFP) are fused to the N- and C-termini of a bacterial glutamate binding protein ybeJ, respectively. The FRET intensity between CFP and the variant YFP changes with the concentration of glutamate, with an apparent dissociation constant (Kd) of 630 nm for FLIPE and 2.5 μm for SuperGluSnFR. These indicators have a maximum FRET ratio change (ΔRmax) of 11% for FLIPE and 44% for SuperGluSnFR, and have been expressed on the plasma membrane to image glutamate concentration changes on the extracellular surface of neurons in culture (Okumoto et al. 2005; Hires et al. 2008). However, these indicators have not been successfully used to image glutamate dynamics in brain tissues with intact structures. FLIPE has been modified so that CFP is inserted within the ybeJ sequence to yield FLII81PE-1μ, which has a Kd of 1 μm and ΔRmax of 48% (Deuschle et al. 2005). Purified FLII81PE-1μ protein was introduced into the extracellular space of cerebral slice preparations by diffusion, and was used to image glutamate dynamics before the indicator was washed out. Although a nerve stimulation-induced FRET intensity change of FLII81PE-1μ was imaged under a wide-field fluorescence microscope with sampling frequencies of 50 Hz or lower (Dulla et al. 2008), a cell-level spatial resolution was not attained.
Another type of fluorescent glutamate indicator has been generated (Namiki et al. 2007) and successfully used to image fine extrasynaptic glutamate dynamics in brain tissue preparations (Okubo et al. 2010). The new hybrid-type fluorescent glutamate indicator consists of the glutamate-binding domain of AMPAR subunit GluR2 and a fluorescent small molecule (Oregon Green 488) conjugated near the glutamate-binding pocket (Fig. 2A). This indicator, the glutamate (E) optical sensor (EOS), shows high affinity (Kd of 148 nm) and selectivity for glutamate, and achieves a large dynamic range (maximum fluorescence intensity change, ΔFmax of 21% measured on the cell surface) (Namiki et al. 2007; Okubo et al. 2010). EOS can be evenly and densely labelled on the cell surface through a tight biotin–streptavidin interaction, and can be used for high-resolution imaging of glutamate dynamics around neurons in culture (Namiki et al. 2007). Variants of EOS have been generated with a different Kd and a greater dynamic range: K716A-EOS has a Kd of 174 nm and ΔFmax of 29%, and L401C-EOS has a Kd of 1.57 μm and ΔFmax of 48%. The sister indicators (collectively called EOS), in combination with two-photon microscopy, opened the door to visualizing extrasynaptic glutamate dynamics in preparations with intact brain structures (Namiki et al. 2007; Okubo et al. 2010). EOS labelling in the extracellular space of acute cerebellar slice preparations through biotin–streptavidin interaction resulted in roughly even and bright staining with silhouettes of the cellular components (Fig. 2C). When the parallel fibres (PFs) are stimulated with a physiologically relevant pulse train (5 pulses at 100 Hz; Chadderton et al. 2004), the EOS fluorescence intensity under a two-photon microscope increases within a circular area on a sagittal plane (Fig. 2D). The observed EOS signal predominantly reports extrasynaptic glutamate concentration changes, because a negligible amount of EOS is immobilised within the synaptic cleft (Namiki et al. 2007; Okubo et al. 2010). Thus, the EOS imaging method allows direct measurement of extrasynaptic glutamate dynamics adjacent to excitatory synapses.
Figure 2. Imaging of extrasynaptic glutamate dynamics by EOS.

A, a schematic of the EOS structure. EOS is composed of the recombinant S1S2 domain of GluR2 (in magenta) and a conjugated fluorescent dye (in green). B, dose–response relationship between glutamate concentration and fractional change in fluorescence intensity (ΔF/F0) of EOS sister indicators. Estimated Kd values of K716A-EOS and L401C-EOS were 174 nm and 1.57 μm, respectively. C, EOS immobilised in the molecular layer of cerebellar slices. The magnified image (lower panel) shows a roughly even distribution of EOS in the extracellular space with silhouettes of cellular components. D, imaging of EOS signals in response to PF stimulation (5 pulses at 100 Hz). ΔF/F0 is colour coded as indicated. Modified from Okubo et al. (2010), copyright (2010) National Academy of Sciences, USA.
Spatiotemporal summation of glutamate spillover
Modelling studies predicted that glutamate released from a single exocytotic vesicle is immediately diluted by passive diffusion and active reuptake via glutamate transporters, which confines the activation of glutamate receptors within the synaptic cleft (Barbour, 2001). On the other hand, model-based analyses also showed that glutamate released within a narrow time window from a single synapse in succession and/or from neighbouring synapses may be summed in the extrasynaptic space (Barbour & Hausser, 1997). Thus, spatially and temporally packed synaptic activity would be expected to induce extrasynaptic glutamate dynamics that may be sufficient for volume transmission. Indeed, electrophysiological measurements suggested that both the density of stimulated nerve fibres and the number of repetitive stimulation pulses were critical for the activation of extrasynaptic glutamate receptors (Carter & Regehr, 2000; Arnth-Jensen et al. 2002; Marcaggi & Attwell, 2005). However, the extrasynaptic glutamate dynamics had not been directly measured.
EOS-based glutamate imaging of the PF–Purkinje cell synapse in the cerebellar cortex has been used to address this issue. While a single PF stimulation pulse generated only a faint EOS signal, two to five pulses at 100 Hz induced a significant EOS response (Fig. 3A, upper panel). Thus, the extrasynaptic glutamate dynamics around PF–Purkinje cell synapses had been finally caught in action, and the results indicated that the extrasynaptic glutamate dynamics indeed undergo temporal summation. Another important advancement made with EOS-based measurement was the ability to directly estimate the extrasynaptic glutamate concentration. Due to a relatively low rate constant of glutamate–EOS dissociation, EOS is a leaky integrator of the glutamate concentration. Therefore, the glutamate concentration during synaptic inputs can be estimated by the numerical deconvolution of EOS signals. The time course of the estimated glutamate transients reaches micromolar concentrations for tens of milliseconds in response to two to five pulses of stimulation (Fig. 3A, lower panel). Such extrasynaptic glutamate dynamics are sufficient to activate high-affinity glutamate receptors, including NMDAR (EC50≈ 2 μm; Clements & Westbrook, 1991) and mGluR (EC50≈ 10 μm; Hayashi et al. 1993). This is consistent with previous findings that at least two pulses of repetitive PF input are required to activate perisynaptic mGluRs in Purkinje cells (Finch & Augustine, 1998; Takechi et al. 1998; Okubo et al. 2004; Marcaggi & Attwell, 2005). The EOS measurements were subsequently performed in hippocampal and neocortical slice preparations, with essentially the same results (Okubo et al. 2010).
Figure 3. Direct evaluation of extrasynaptic glutamate dynamics by EOS imaging.
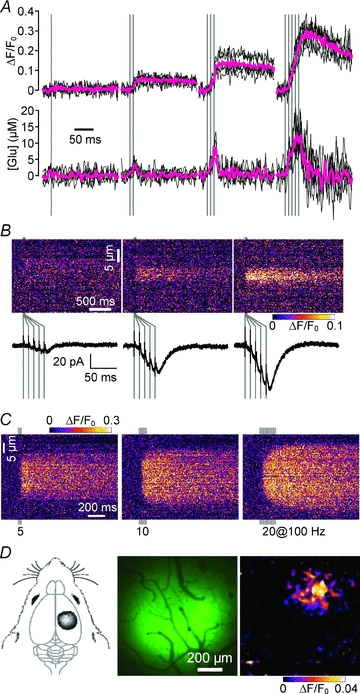
A, temporal summation in extrasynaptic glutamate dynamics upon repetitive PF stimulation in the cerebellar cortex and estimation of corresponding glutamate concentration. Upper traces show the time course of the EOS signal and the lower traces show the corresponding time course of glutamate concentration estimated by the deconvolution method (Okubo et al. 2010). Magenta traces indicate averaged data. B, spatial summation in extrasynaptic glutamate dynamics. The simultaneous measurement of EOS signals and EPSCs upon PF stimulation (5 pulses at 100 Hz) shows a correlation between extrasynaptic glutamate dynamics and PF input density. C, EOS signal upon 5, 10 and 20 pulses of PF stimulation at 100 Hz. Increasing the number of stimulation pulses increased the spatial width of the EOS signal. Peripheral signals emerged after 10 and 20 pulses of stimulation that showed significant latency. D, extrasynaptic glutamate dynamics upon sensory input from the hind paw. EOS was immobilised in the rat sensory cortex corresponding to the hind paw (left and middle panels). Peak EOS signal induced by 200 ms tactile stimulation to the hind paw is shown (right panel). Modified from Okubo et al. (2010), copyright (2010) National Academy of Sciences, USA.
Spatial summation of extrasynaptic glutamate dynamics has also been analysed in the vicinity of small bundles of activated PFs. EOS imaging was carried out simultaneously with PF-induced excitatory postsynaptic current (EPSC) measurements from Purkinje cells as an indicator of the number of active synapses. The EOS signal increased with EPSC, showing the dependence of extrasynaptic glutamate dynamics on the density of stimulated PFs (Fig. 3B). The summation of glutamate released from adjacent PF synapses is consistent with the dependence of mGluR activation on the PF input density (Marcaggi & Attwell, 2005).
Limiting concentration of glutamate spillover
EOS imaging methods provide direct evidence that synaptic activity generates an increase in the local glutamate concentration that exceeds micromolar levels for tens of milliseconds in the extrasynaptic space. Although such extrasynaptic glutamate dynamics are sufficient to activate high-affinity glutamate receptors including NMDAR and mGluR as discussed above, they are insufficient to activate AMPAR (EC50≈ 500 μm; Jonas & Sakmann, 1992). Thus, the activation of extrasynaptic AMPAR must take place only in very close proximity to synaptic clefts or ectopic glutamate release sites, where glutamate concentrations may approach millimolar concentrations (DiGregorio et al. 2002; Matsui & Jahr, 2003).
Spatial spreading distance of glutamate spillover
Prior to the introduction of glutamate imaging techniques, the spatial spreading of glutamate in the extrasynaptic space, i.e. the working distance of glutamatergic volume transmission, had not been directly measured. EOS-based glutamate imaging can provide a direct measurement of spreading distance of glutamate spillover. In response to a physiologically relevant stimulation (5 pulses of PF stimulation at 100 Hz; Fig. 3C, left panel), there was an immediate increase in the EOS signal, the width of which is almost constant during the imaging period. This observation indicated that glutamate concentration increases only within a region that contains active synapses, and that the glutamate signal is effectively contained within that region (core region). However, when prolonged stimulation with 10–20 pulses was applied, additional EOS signals with latency appear on both sides of the core region (Fig. 3C, middle and right panels). This suggested that under such conditions glutamate cannot be sequestered within the core region and overflows laterally for a distance of a few micrometres. However, even in such an extreme condition, the distance of glutamate spreading is no greater than a few micrometres. Thus, glutamatergic volume transmission between components separated by over several micrometres is not expected to occur in physiologically relevant conditions. These data set the upper limit of the effective range of glutamate spillover.
Glutamate spillover imaging in vivo
To further evaluate extrasynaptic glutamate dynamics under physiological conditions, in vivo glutamate imaging has been carried out in the brain (Fig. 3D). In these studies, EOS was immobilised in the rat sensory cortex through the biotin–streptavidin interaction as in slice preparations. In response to a tactile stimulation of the hind paw, significant EOS signals were observed at the appropriate sensory area through a cranial window under a wide-field fluorescence microscope (Fig. 3D, right panel). This indicated that glutamatergic volume transmission takes place under physiological conditions. Together with the finding that sensory input from whiskers induces mGluR-mediated astrocytic Ca2+ responses at the corresponding cortical sensory area in vivo (Wang et al. 2006), these results showed that physiological inputs are sufficient to induce volume transmission via high-affinity glutamate receptors in the brain in vivo.
Conclusion and perspectives
In conclusion, the research summarised here indicates that the extrasynaptic glutamate concentration reaches sufficient levels to activate high-affinity glutamate receptors in response to spatiotemporally summable synaptic activities. Thus, glutamate is a physiological volume transmitter and may regulate important neuronal, glial and vascular functions. The EOS-based glutamate imaging method may be used to obtain further information regarding glutamatergic volume transmission. One of the most promising future applications of EOS is the direct evaluation of glutamate release by astrocytes as a gliotransmitter. As described above, controversy exists regarding Ca2+-triggered glutamate release under the physiologically relevant conditions. Since the extracellular glutamate concentration upon agonist activation of astrocytes may reach 1–6 μm (Lee et al. 2007), glutamate release from astrocytes may be detected by EOS. Furthermore, in vivo glutamate imaging may be used to locate active synapses during physiological responses of the brain, providing a new modality for functional brain imaging.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CFP
cyan fluorescent protein
- EOS
glutamate (E) optical sensor
- EPSC
excitatory postsynaptic current
- FRET
fluorescence resonance energy transfer
- mGluR
metabotropic glutamate receptor
- NMDAR
N-methyl-d-aspartate receptors
- PF
parallel fibre
- YFP
yellow fluorescent protein
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B. An evaluation of synapse independence. J Neurosci. 2001;21:7969–7984. doi: 10.1523/JNEUROSCI.21-20-07969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci. 2000;20:4423–4434. doi: 10.1523/JNEUROSCI.20-12-04423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005;14:2304–2314. doi: 10.1110/ps.051508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- DiGregorio DA, Nusser Z, Silver RA. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron. 2002;35:521–533. doi: 10.1016/s0896-6273(02)00787-0. [DOI] [PubMed] [Google Scholar]
- Dulla C, Tani H, Okumoto S, Frommer WB, Reimer RJ, Huguenard JR. Imaging of glutamate in brain slices using FRET sensors. J Neurosci Methods. 2008;168:306–319. doi: 10.1016/j.jneumeth.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Furutani K, Okubo Y, Kakizawa S, Iino M. Postsynaptic inositol 1,4,5-trisphosphate signaling maintains presynaptic function of parallel fiber-Purkinje cell synapses via BDNF. Proc Natl Acad Sci U S A. 2006;103:8528–8533. doi: 10.1073/pnas.0600497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters ? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hires SA, Zhu Y, Tsien RY. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc Natl Acad Sci U S A. 2008;105:4411–4416. doi: 10.1073/pnas.0712008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol. 2007;581:1057–1081. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo M, Okubo Y, Yamazawa T, Yamasaki M, Watanabe M, Murayama T, Iino M. Inositol 1,4,5-trisphosphate signaling maintains the activity of glutamate uptake in Bergmann glia. Eur J Neurosci. 2010;32:1668–1677. doi: 10.1111/j.1460-9568.2010.07452.x. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, Ko RW, Vasuta OC, Graham RK, Hayden MR, Murphy TH, Raymond LA. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Namiki S, Sakamoto H, Iinuma S, Iino M, Hirose K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur J Neurosci. 2007;25:2249–2259. doi: 10.1111/j.1460-9568.2007.05511.x. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Kakizawa S, Hirose K, Iino M. Cross talk between metabotropic and ionotropic glutamate receptor-mediated signaling in parallel fiber-induced inositol 1,4,5-trisphosphate production in cerebellar Purkinje cells. J Neurosci. 2004;24:9513–9520. doi: 10.1523/JNEUROSCI.1829-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M. Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci U S A. 2010;107:6526–6531. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci U S A. 2005;102:8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Sem’yanov AV. Diffusional extrasynaptic neurotransmission via glutamate and GABA. Neurosci Behav Physiol. 2005;35:253–266. doi: 10.1007/s11055-005-0003-7. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


