Abstract
The study aimed to investigate the role that four populations of spinocerebellar neurones play in forwarding information on descending commands relayed by feline reticulospinal neurones. Both intracellular and extracellular recording was used from medially located ventral spinocerebellar tract (VSCT) neurones as well as from spinal border (SB) subpopulation of VSCT neurones and from dorsal spinocerebellar tract neurones located in Clarke's column (CC DSCT) and in the dorsal horn (dh DSCT) in the lumbosacral enlargement. Axons of reticulospinal neurones were stimulated within the ipsilateral and contralateral medial longitudinal fascicle (MLF). We found striking differences in synaptic input from reticulospinal neurones to these four populations of spinocerebellar neurones. Both monosynaptic and disynaptic excitatory input was found in VSCT and SB neurones, only disynaptic in CC DSCT neurones and none in dh DSCT neurones. Discharges of VSCT and SB neurones were potently modulated by inhibitory actions of group I and II afferents. Following application of single stimuli to peripheral nerves these neurones ceased to respond for about 5 ms and thereafter discharged at a lower incidence rate. As inhibition of spinocerebellar neurones and of α-motoneurones is evoked by the same premotor interneurones, VSCT neurones may provide the cerebellum with information on the likely outcome of reticulospinal actions on motoneurones depending on the degree to which they are inhibited. They may thereby enable the cerebellum to adjust descending commands relayed by reticulospinal neurones to the requirements of a given situation and thus prevent errors in the centrally initiated movements.
Non-technical summary
Voluntary limb movements are initiated in the brain but the neurones responsible for activating the muscles (motoneurones and interneurones) are located in the spinal cord. The spinal cord also contains neurones that provide the brain, and especially the cerebellum, with continuous information on effects of the descending commands. We show that one population of such neurones provide the cerebellum with information on how likely the brain's commands (mediated by descending reticulospinal neurones) are to be executed as planned, depending on the degree of inhibition of motoneurones. They may therefore play an important role in preventing errors in activation of motoneurones and thereby help the brain to correct its signals to the spinal cord before such errors have been committed.
Introduction
Feedback information on descending commands forwarded by reticulospinal neurones may reach the cerebellum via several parallel pathways. While some reticulospinal neurones were reported to contact cerebellar neurones via their axon collaterals (Waltzer & Martin, 1984) information forwarded by the majority is relayed via reticulo-olivo-cerebellar, spino-reticulo-cerebellar, spino-olivo-cerebellar and spino-cerebellar pathways (see Jansen & Brodal, 1954). We have undertaken the study of the contribution of spinocerebellar neurones to the feedback information on descending commands relayed by reticulospinal neurones considering that information provided by spinocerebellar neurones may be of particular importance for the cerebellar control of spinal activity. It is well established that spinocerebellar neurones forward different kinds of information, (i) on peripheral afferent input, via both extero- and proprioceptors, (ii) on the operation of intrinsic spinal neuronal networks, ensuring appropriate responses to these stimuli, as well as of centrally initiated movements, and (iii) on results of these movements. All these issues have been analysed in a number of studies and reviewed over the years (see e.g. Oscarsson, 1965, 1973; Lundberg, 1971; Lindström, 1973; Arshavsky et al. 1978b; Bosco & Poppele, 2001). However, very little attention has been paid to the contribution of spinocerebellar neurones to monitoring of descending commands. In fact input from descending tract neurones (an ‘efferent copy’, see Arshavsky et al. 1978a,b; Bosco & Poppele, 2001) was analysed in detail in only very few studies: from reticulospinal, vestibulospinal and corticospinal neurones to VSCT neurones (Baldissera & Weight, 1969; Baldissera & Roberts, 1975; Fu et al. 1977) and from corticospinal neurones to CC DSCT neurones (Hongo & Okada, 1967; Hantman & Jessell, 2010). In addition it has not been established how information on this input is forwarded to the cerebellum. In view of the highly differentiated input to various populations of spinocerebellar neurones and their multiple cerebellar target cells (see e.g. Matsushita et al. 1979; Matsushita & Xiong, 2001), complex neuronal networks are likely to be needed to decode signals forwarded by spinocerebellar neurones. The spinal border subpopulation of VSCT neurones may be an exception in this respect because of a more restricted input as well as more specific cerebellar projection areas than of other spinocerebellar neurones. Stimulation of primary afferents often fails to excite SB neurones, being instead followed by IPSPs (Burke et al. 1971), and the only regularly found excitatory input to these neurones appears to be from reticulospinal or vestibulospinal neurones (Baldissera & Roberts, 1975). Furthermore, SB neurones have distinct cerebellar projection areas in the paramedian lobules to which other spinocerebellar neurones do not project (Matsushita & Ikeda, 1980; Matsushita & Yaginuma, 1989; Xu & Grant, 1990).
We therefore hypothesized that SB neurones are links in a highly specialized loop between neurones in the reticular formation and the cerebellum (see the diagram of the postulated neuronal connections in such a loop in the Discussion). We further hypothesized that they are involved in informing the cerebellum about the likely outcome of reticulospinal actions on motoneurones based on the degree to which motoneurones are inhibited by premotor interneurones. As the same premotor interneurones (interposed in reflex pathways from group Ia, Ib and II muscle afferents) act in parallel on motoneurones and on SB neurones (Lundberg, 1971; Lindström, 1973; Lindström & Schomburg, 1974; Jankowska et al. 2010), the modulation of monosynaptic actions of reticulospinal neurones on SB neurones should provide a good prediction of the effects of actions of reticulospinal neurones on motoneurones. On the basis of such information cerebellar output neurones could adjust the degree of activation of reticulospinal neurones, thereby ensuring that it is neither too strong nor too week. Such predictive functions could be closely related to, but distinct from, predictions of the sensory consequences of motor acts and their other effects (Arshavsky et al. 1978b; Tolbert et al. 1980; Bosco & Poppele, 2001; Hantman & Jessell, 2010).
The coupling required for the loop we hypothesize has already been partly demonstrated. For example the coupling between neurones in the SB projection area in the paramedian lobules and the interposital and fastigial cerebellar nuclei as well as between neurones in these nuclei and reticulospinal neurones has been demonstrated morphologically using both anterograde and retrograde transport of various markers (Trott et al. 1998b) and by antidromic activation of individual neurones and monosynaptic actions of these neurones in electrophysiological studies (see e.g. Eccles et al. 1975 and Homma et al. 1995 for nucleus fastigius; McCrea et al. 1977 for nucleus interpositus; Bantli & Bloedel 1975 and Tolbert et al. 1980 for nucleus dentatus).
At the spinal level, previous investigations of the excitatory input from the reticular formation to spinocerebellar neurones were limited to one subpopulation of VSCT neurones, and to actions evoked via reticulospinal fibres descending within the ipsilateral ventral quadrant of the spinal cord, with only preliminary observations on SB neurones (Baldissera & Weight, 1969; Baldissera & Roberts, 1975). Considering that preliminary observations on discharges of CC DSCT neurones led to the conclusion that they are under presynaptic rather than postsynaptic control (Kubota et al. 1978) and that descending input to dh DSCT neurones has not yet been investigated, the main aims of this study were threefold. The first aim was to compare effects of stimulation of axons of reticulospinal neurones on various populations of spinocerebellar neurones within the lumbosacral enlargement. In particular, we aimed to compare these effects in two populations of DSCT neurones, CC DSCT and dh DSCT neurones, and in two populations of VSCT neurones, located in laminae VII–VIII and in the lateral part of lamina IX (referred to as VSCT and SB neurones, respectively). Previous studies revealed several functional differences between neurones of these populations and we therefore expected them to be differently involved in interrelations between reticulospinal and cerebellar neurones. The second aim was to compare effects of stimulation of axons of reticulospinal neurones descending within the ipsilateral and contralateral MLF which is of particular interest as effects relayed ipsilaterally or contralaterally are likely to be secondary to activation of different interneuronal populations. The third aim was to investigate to what extent modifications of the original descending reticulospinal commands before they reach spinal motoneurones may be reflected in the information forwarded to the cerebellum.
Methods
Ethical approval
All experiments were approved by the Ethics Committee for Animal Research at the University of Göteborg (Göteborgs Djurförsöksetiska Nämnd) and comply with the ethical policies and regulations of The Journal of Physiology (Drummond, 2009). The animals were bred and housed under veterinary supervision at the Experimental Biomedicine Unit at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on 10 deeply anaesthetised adult cats weighing 2.6–3.8 kg. Anaesthesia was induced with sodium pentobarbital (Apoteksbolaget, Sweden; 40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg kg−1; administered i.v., every 1–2 h up to 30 mg kg−1 and every 2–3 h thereafter). Additional doses of α-chloralose were given whenever increases in the continuously monitored blood pressure or heart rate occurred during surgery or any of the experimental procedures. Atropin (0.05–0.2 mg kg−1i.m.) was administered during the preliminary surgical procedures to reduce tracheal secretion. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden) and the animals were artificially ventilated. Neuromuscular relaxation was induced only after several hours of surgery and when the animal had reached a deep and stable level of anaesthesia by an i.v. injection (0.3 mg kg−1) and thereafter maintained by adding pancuronium bromide to the buffer infusion (see below) at doses corresponding to about 0.2 mg kg−1 h−1i.v. The effectiveness of synaptic transmission was increased by intravenous application of 4-amino-pyridine (4-AP, Sigma, St Louis, MO, USA) in doses of 0.1–0.2 mg kg−1, i.v. Mean blood pressure was maintained at 100–140 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 38°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of sodium pentobarbital (i.v.) and cardiac arrest verified by ECG.
The spinal cord was exposed by a laminectomy from the third to the sixth lumbar (L3–L6) segments and at the level of the low thoracic (Th10–Th12) segments. The dura mater was either left intact, except for small holes (about 1 mm2) over the dorsal columns through which both the stimulating and recording electrodes were inserted, or cut open along the whole length of the lumbosacral enlargement (in 2 experiments).
The caudal part of the cerebellum was exposed to allow insertion of electrodes used to stimulate axons of spinocerebellar neurones in order to activate them antidromically and to stimulate reticulospinal neurones running in the MLF. The cerebellar stimulation sites were located ipsilaterally to DSCT and contralaterally to VSCT and SB neurones, just rostral to or within the ipsilateral or contralateral nucleus interpositus (at Horsley–Clarke coordinates about P 7, L 3.0–3.5, H 0 to −1). The axons within the MLF were stimulated either ipsilaterally or contralaterally to the location of the neurones recorded from (see Results). The stimuli were applied at Horsley–Clarke coordinates P 7–9, L 0.5–0.8, H about −5. All electrode positions were adjusted while recording descending volleys from the surface of the spinal cord at the Th11–12 level. The electrodes were left at locations from which distinct descending volleys were evoked at thresholds of 10–20 μA and were near maximal at 50–100 μA (for cerebellar stimulation sites) or 100–150 μA (for MLF stimulation sites). The stimulation sites were marked by electrolytic lesions made at the end of each experiment and reconstructed histologically. The location of these sites is indicated in Fig. 1.
Figure 1. Examples of monosynaptic actions evoked from the MLF in SB and VSCT neurons.
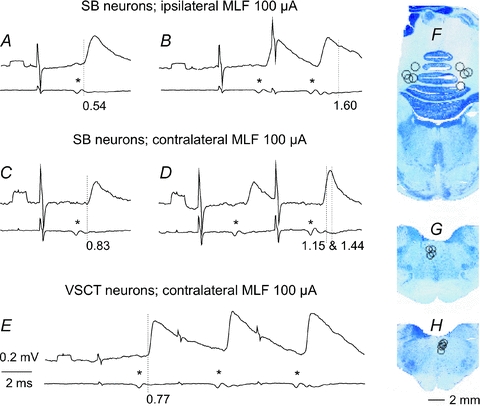
In each pair of records upper traces are representative averaged intracellular records (n= 10–40) from 2 SB neurones (A and B, C and D) and from 1 VSCT neurone (E), while lower traces are records of descending volleys from the surface of the spinal cord. A and B, after contralateral hemisection. C–E, after ipsilateral hemisection. Asterisks indicate the first component of the MLF volleys. Dotted lines in A, C and E mark the onset of monosynaptic EPSPs evoked by these volleys while dotted lines in B and D mark the onset of disynaptic PSPs following them. Note that the second monosynaptic EPSP in B is followed by disynaptic EPSPs (as indicated by a hump in its declining phase) and that the second monosynaptic EPSP in D is followed by both disynaptic EPSP during its rising phase and disynaptic IPSP starting just after its peak. Latencies of these PSPs as from the volleys (segmental latencies) are given below the records. Rectangular pulses at the beginning of the records are 0.2 mV calibration pulses. Time calibration (2 ms) is for all records. In this and the following figures the negativity is downwards in intracellular records and upwards in cord dorsum records. F, G and H, reconstruction of stimulation sites in the cerebellum and in the ipsilateral and contralateral MLF, respectively. The circles indicate locations of electrolytic lesions made to mark stimulation sites in the cerebellum in 8 experiments and in the MLF in 9 experiments. They are indicated on the transverse sections from one of these experiments with respect to the midline and the location of the nucleus interpositus in F and the midline and the dorsal border of the medulla in G and H.
In order to restrict the effects of the stimulated MLF fibres to those descending either ipsilaterally or contralaterally (in spite of the unavoidable spread of current to fibres on the other side of the midline), fibres descending on the non-investigated side were eliminated by hemisection of the spinal cord at a Th11 or L2 level (see Results).
Nerves of the left hindlimb were dissected free, transected and mounted on stimulating electrodes. They included: quadriceps (Q) and sartorius (Sart) branches of the femoral nerve mounted in subcutaneous cuff electrodes; the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), sural (Sur), gastrocnemius–soleus (GS), plantaris (PL) and flexor digitorum and hallucis longus (FDL).
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli at intensities expressed in multiples of threshold (T) for the activation of the most excitable fibres. Intracerebellar axonal branches of spinocerebellar neurones were stimulated by using 0.2 ms long constant current pulses at intensities ≤100 μA. Axons of these neurons ascending within the ipsilateral or contralateral funiculus were stimulated extradurally at the Th12–13 level, with a pair of silver ball electrodes in contact with their surface, using constant current pulses of 100–200 μA. The stimulators used (designed by E. Eide, D. Magnusson and N. Pihlgren, University of Gothenburg) allowed the use of square constant voltage or constant current pulses via inbuilt isolation units, setting the constant voltage stimuli in multiples of threshold for activation of nerve fibres. Both intracellular and extracellular records from VSCT neurones were obtained using glass micropipettes, with tips broken to external diameters of 1.2 to 1.5 μm and an impedance of about 4–6 MΩ and 2–3 MΩ, respectively.
Analysis
Both original data and averages of 10–40 single records (with the time resolution of 30 μs per address) were stored on-line using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg). Latencies of postsynaptic potentials (PSPs) evoked by stimulation of the MLF and peripheral nerves were measured from the first positive peak of nerve volleys recorded from the cord dorsum close to the recording electrode penetration site while those evoked by intraspinal stimuli were measured from stimulus artefacts. Peristimulus time histograms and cumulative sums were constructed as described by Jankowska et al. (1997). Differences between data sets were assessed for statistical significance by using Student's t test (for unpaired or paired samples assuming equal variances and the two tail distribution).
Samples
Intracellular records were obtained from 124 SB and 61 VSCT neurones in the L4 and L5 segments and from 53 CC DSCT and 36 dh DSCT neurones in the L3 and L4 segments. An additional 43 SB neurones were recorded from extracellularly. The neurones were identified on the basis of antidromic activation from the cerebellum, as well as a number of additional criteria specific for each population of neurones. These were: (i) antidromic activation from the ipsilateral lateral funiculus at Th12–13 for CC and dh DSCT neurones and from the contralateral lateral funiculus for SB and VSCT neurones, (ii) location within the most lateral and most medial parts of the dorsal horn (separated by 500–600 μm) for dh DSCT and CC neurones, at depths at which the largest focal field potentials were evoked from Q group II muscle afferents or group I muscle afferents of any nerves, respectively; location just lateral to the location of motor nuclei in L4 for SB neurones and dorso-medial to motor nuclei in the regions just ventral to intermediate zone field potentials from group I afferents for VSCT neurones; (iii) input from group Ia afferents to CC DSCT and SB neurones, from group Ib or both Ia and Ib to VSCT neurones and from group II and cutaneous afferents to dh DSCT neurones. For further details see Results. When the neurones were recorded from extracellularly prior to penetration, a collision test was performed to ensure that the shortest latency spike potentials were indeed evoked antidromically. The spikes were classified as evoked antidromically when they collided with spike potentials evoked by stimulation of a peripheral nerve at a critical time interval (about twice conduction time from the Th segment plus refractory period of the axons; see e.g. Fuller & Schlag, 1976; Lipski, 1981; Asif & Edgley, 1992; Krutki et al. 2003). In intracellular records a constant latency, usually coinciding with the descending volleys induced by the cerebellar and Th stimuli, lack of preceding EPSPs and the appearance of these spikes at an all-or-none fashion were used as criteria for antidromic activation.
Results
The results of this study show that only two populations of the investigated spinocerebellar neurones may serve to couple reticulospinal and cerebellar neurones. As summarized in Table 1, we found that reticulospinal neurones with axons descending within the ipsilateral MLF make direct contacts with SB as well as with VSCT neurones, in accordance with previous findings by Baldissera & Weight (1969) and Baldissera & Roberts (1975). In addition we found monosynaptic EPSPs from the contralateral MLF in a small proportion (12%) of SB neurones and in a larger proportion (38%) of VSCT neurones. The monosynaptic coupling is indicated by latencies of EPSPs not exceeding 1 ms and by the lack of temporal facilitation of EPSPs. As illustrated in Fig. 1A–E such EPSPs were evoked by single stimuli and the amplitudes of EPSPs evoked by the second, third or later stimuli were similar, unless followed by disynaptically evoked ones (as in Fig. 1D). In contrast, we failed to find monosynaptic EPSPs in CC DSCT or dh DSCT neurones.
Table 1.
Proportions and mean latencies of PSPs evoked in various populations of spinocerebellar tract neurones by stimuli applied in the MLF
| SB | VSCT | CC DSCT | dh DSCT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | % | Latency (ms) mean ±s.e.m. | Incidence | % | Latency (ms) mean ±s.e.m. | Incidence | % | Latency (ms) mean ±s.e.m. | Incidence | % | Latency (ms) | ||
| 26/93″ | 28% | 0.66 ± 0.03 | |||||||||||
| EPSPs | Mono ipsi | 10/23 | 43% | 0.69 ± 0.06 | 5/17 | 29% | 0.77 ± 0.14 | 0/21 | 0% | 0/13 | 0% | ||
| Mono contra | 6/51 | 12% | 0.94 ± 0.05* | 17/44 | 38% | 0.79 ± 0.03* | 0/14 | 0% | 0/23 | 0% | |||
| 25/93″ | 27% | 1.46 ± 0.04 | |||||||||||
| EPSPs | Di-, trisyn ipsi | 8/23 | 35% | 1.43 ± 0.06 | 11/17 | 65% | 1.58 ± 0.12 | 0/21 | 0% | 0/13 | 0% | ||
| Di-, trisyn contra | 15/51 | 29% | 1.98 ± 0.19** | 23/44 | 52% | 1.46 ± 0.07 ns | 7/14 | 50% | 1.78 ± 0.06 | 1/23 | 4% | 2.43 | |
| 14/93″ | 15% | 1.42 ± 0.11 | |||||||||||
| IPSPs | Di-, trisyn ipsi | 18/23 | 78% | 1.46 ± 0.10 | 12/17 | 71% | 1.34 ± 0.13 | 8/21 | 38% | 1.87 ± 0.13 | 0/13 | 0% | |
| Di-, trisyn contra | 26/51 | 51% | 1.56 ± 0.04 ns | 29/44 | 66% | 1.59 ± 0.05 ns | 3/14 | 21% | 3.37 ± 0.11*** | 1/23 | 4% | 2.82 | |
Vertical columns, proportions and mean latencies (with s.e.m.) of PSPs evoked in 74 SB neurones, 61 VSCT neurones, 35 CC DSCT neurones and 36 dh DSCT neurones investigated in this study in preparations in which the spinal cord was transected either ipsilaterally or contralaterally. The data are for monosynaptic EPSPs and for oligosynaptic EPSPs and IPSPs, including only those with segmental latencies not exceeding 3.5 ms. Data from additional SB, VSCT and DSCT neurones analysed in preparations in which no hemisections were performed, or were found to be incomplete, are not included in the table but are referred to in the text. Additional data of Baldissera & Roberts (1975) for VSCT neurones are given in italics and indicated by ″. Statistical significance of differences between PSPs evoked from the ipsilateral and contralateral MLF is indicated to the right of the contra MLF data (Student's t test for two samples assuming equal variance)
P 0.01–0.05;
P 0.001–0.01;
P < 0.001; ns, not statistically significant.
Disynaptic EPSPs and IPSPs were evoked in SB and VSCT neurones as well as in CC DSCT neurones, but not, or only exceptionally, in dh DSCT neurones. Examples of disynaptic EPSPs following monosynaptic EPSPs are shown in Fig. 1B and D where they are indicated by dotted lines at the onset of a hump in the declining phase of one of these and of the second component of the other one, with onsets at 1.60 and 1.15 ms. Disynaptic IPSPs following monosynaptic EPSPs were indicated by a faster declining phase of the EPSPs evoked by the 2nd or 3rd stimuli than after the first stimuli (Fig. 1D, and Fig. 2D and F). The most distinct were, however, EPSPs and IPSPs that were not preceded by monosynaptic EPSPs, with examples in Fig. 2A, C and E. They were as a rule evoked only by the 2nd or 3rd stimulus in the train and thus showed potent temporal facilitation.
Figure 2. Examples of disynaptic actions evoked from the ipsilateral and contralateral MLF in SB and VSCT neurons.
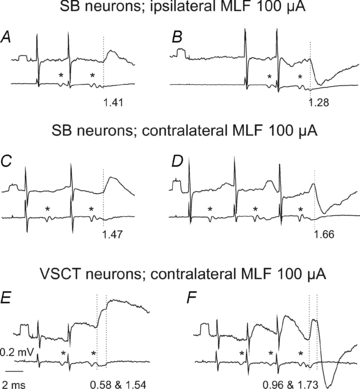
In each panel upper traces are averaged intracellular records (n= 10–40) from 4 SB neurones (A–D) and from 2 VSCT neurones (E and F) while lower traces are records of descending volleys from the surface of the spinal cord. A and B, after contralateral hemisection. C–F, after ipsilateral hemisection. As in Fig. 1, asterisks indicate first component of the MLF volleys. Dotted lines mark onset of disynaptic PSPs in A–D and both monosynaptic and disynaptic PSPs in E and F. Latencies of PSPs from the volleys (segmental latencies) are below the records. Other indications are as in Fig. 1.
The four populations of spinocerebellar neurones differed not only in the presence or absence of disynaptic PSPs from the MLF but also in the origin of these PSPs. In VSCT and SB neurones, disynaptic EPSPs and IPSPs were evoked not only from the ipsilateral but also from the contralateral MLF, with examples in Fig. 2B, D and F. However, in CC DSCT neurones disynaptic EPSPs were evoked exclusively from the contralateral MLF and disynaptic IPSPs from the ipsilateral MLF (Fig. 3) while in dh DSCT neurones disynaptic IPSPs were only found from the contralateral MLF (see Table 1 and the summarizing diagram in Fig. 7).
Figure 3. Examples of disynaptic EPSPs evoked from the contralateral MLF and disynaptic IPSPs from the ipsilateral MLF in CC DSCT neurones.
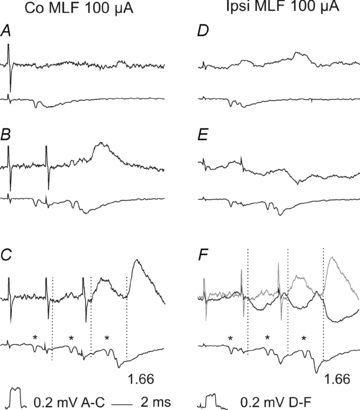
Upper traces in A–C and in D–F are from two CC DSCT neurones located in the left and right Clarke's column, respectively, recorded in the same experiment after the left hemisection of the spinal cord. Lower traces are from the surface of the spinal cord. Averages of 40 records. Dotted lines indicate onset of PSPs evoked by stimuli applied in the right MLF, with latencies given from the first components of the descending volleys (*). Grey trace in F, records from C. Note that EPSPs and IPSPs were evoked at similar latencies (1.66 ms after the 3rd stimulus) compatible with disynaptic coupling via single excitatory or inhibitory interneurones, and that peak amplitudes of PSPs evoked after subsequent stimuli increased.
Figure 7. Diagrams summarizing the most direct (mono-, di- and trisynaptic) coupling between reticulospinal neurones and spinocerebellar neurones investigated in this study.
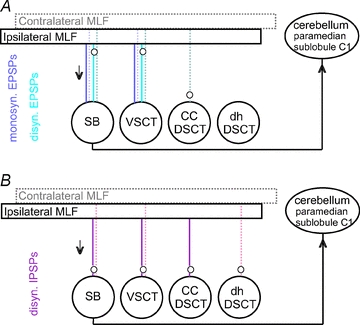
A and B, coupling in excitatory and inhibitory pathways respectively. Large circles represent populations of the indicated spinocerebellar neurones; spinal border neurones are indicated separately even though they constitute a subpopulation of VSCT neurones. Rectangles represent the ipsilateral and contralateral MLF where axons of reticulospinal neurones were stimulated. Direct lines between these rectangles and the spinocerebellar neurones indicate monosynaptic coupling. The dotted lines indicate indirect pathways with relay neurones represented by small circles. For further explanations see text.
The segmental latencies of EPSPs evoked in SB and VSCT neurones plotted in Fig. 4A and B fall into two ranges of about 0.5–1.0 ms (diamonds) and 1.1–2.0 ms (triangles), compatible with monosynaptic and disynaptic coupling from MLF in other spinal neurones (e.g. Krutki et al. 2003), although some EPSPs appeared at longer latencies. The segmental latencies of all EPSPs evoked in CC DSCT neurones fell within the range of longer latencies, in keeping with their appearance only following the second MLF stimuli (Fig. 3B and C) and temporal facilitation characterizing disynaptically evoked PSPs Latencies of all except the two earliest IPSPs plotted in Fig. 5B, D and F likewise fell within the range of disynaptically evoked PSPs. The exception were latencies of the two IPSPs recorded by Baldissera & Roberts (1975) which were classified by them as evoked monosynaptically. However no statistically significant differences were found between the mean latencies of disynaptic EPSP and IPSPs except for longer latencies of EPSPs evoked in SB neurones (see Table 1).
Figure 4. Comparison of latencies of earliest components of EPSP and IPSP evoked from the MLF in three populations of spinocerebellar neurones.
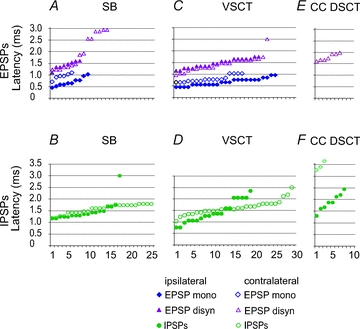
A and B, C and D and E and F, latencies of PSPs (ordinate) evoked in samples of SB, VSCT and CC DSCT neurones (abscissa). In each plot filled symbols are for PSPs evoked from the ipsilateral MLF after contralateral hemisection of the spinal cord (i.e. via ipsilaterally descending MLF fibres) and open symbols are for those evoked after ipsilateral hemisection (i.e. via contralaterally descending MLF fibres). Data for ipsilateral actions on VSCT neurones are estimated from the histogram of latencies of PSPs in Fig. 4 in Baldissera & Roberts (1975). All PSPs were evoked by stimuli ≤100 μA. When both monosynaptic and di- or trisynaptic PSPs were evoked, latencies of both were measured separately. The latencies are ranked from the shortest to longest. Note that in CC DSCT neurones no EPSPs were evoked from the ipsilateral MLF and that only di- or trisynaptic EPSPs followed stimuli applied in the contralateral MLF in contrast to SB and VSCT neurones in which both mono- and disynaptic EPSPs were evoked from the ipsilateral as well as the contralateral MLF. Note also that a considerable proportion of EPSPs and IPSPs were evoked at similar latencies via ipsilaterally or contralaterally descending MLF fibres.
Figure 5. Examples of monosynaptically evoked discharges from the MLF and their suppression by group II muscle afferents.
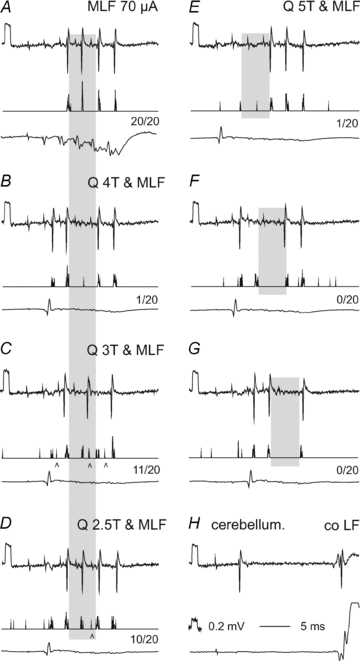
In panels A–G top records are single sweep original extracellular records from a SB neurone, middle traces are peristimulus time histograms of responses evoked from the MLF and bottom traces records from the cord dorsum (at a higher amplification in A). A, control responses evoked by MLF stimuli alone, with spikes evoked 3.5, 3.5, 3.7 and 3.7 ms after the 2nd, 3rd, 4th and 5th stimuli. B–D, responses evoked by the same MLF stimuli but associated with stimulation of the quadriceps (Q) nerve at intensities 4, 3 and 2.5T. Note that 4T stimuli prevented the appearance of spikes after the third MLF stimulus, while 3T and 2.5T stimuli had a weaker effect or delayed them during the 5 ms period indicated by shading; this indicates inhibition by predominantly group II muscle afferents. The relative decreases during this period are indicated below each histogram. E–G, effects of 5T Q stimuli coinciding with the 1st, 2nd and 3rd MLF stimuli. Note that the periods of inhibition (shaded) were then delayed. H, records identifying the investigated neurone as an SB neurone by its antidromic activation from the cerebellum and the contralateral lateral funiculus in addition to its location in the most lateral part of the ventral horn (depth 1.8 mm from the surface of the lateral funiculus). Arrowheads in C and D indicate discharges most likely evoked disynaptically following the monosynaptic ones.
In contrast to CC DSCT neurones, stimulation of the ipsilateral or contralateral MLF consistently failed to evoke either monosynaptic or disynaptic EPSPs in 23 dh DSCT neurones investigated in two experiments prior to (n= 13) and after (n= 10) an ipsilateral hemisection. In both experiments the intensity of the MLF stimuli was increased up to 200 μA, which due to the electrode placement in the proximity of the midline must have resulted in a spread of current that would activate both contralaterally and ipsilaterally descending fibres. The absence of short latency PSPs evoked from the MLF could not be attributed to technical problems because the histologically verified position of the stimulating MLF electrode was similar to that in the other experiments and the descending volleys displayed as distinct early and late components at the lumbar level as in all other preparations. Furthermore, PSPs at longer latencies (2.43 and 2.82 ms from the descending volleys) found in two neurones were evoked by 150 μA stimuli and spread of current to other structures could not be excluded in their case.
Effectiveness of activation of SB neurones by MLF stimulation
The anaesthetised preparation did not provide optimal conditions for estimating the outcome of synaptic actions of reticulospinal neurones on the investigated neurones. Nevertheless, as monosynaptic and/or disynaptic EPSPs were found in a high proportion of SB neurones, we investigated the possibility that short trains of MLF stimuli would evoke action potentials in these neurones even under deep anaesthesia. To this end effects of trains of four to five MLF stimuli up to 100 μA were examined in 43 extracellularly recorded SB neurones. In the great majority of these neurones (34/43, 79%) spike potentials were evoked, with examples in Fig. 5. The most effective stimuli induced action potentials within relatively narrow ranges of latencies (3.3–3.7 or 4.0–4.5 ms from the stimulus, 0.8–1.2 or 1.5–2.0 ms from the descending volleys); in Fig. 5C and D the longer latency responses are indicated by arrowheads. The shortest of these latencies exceeded those of monosynaptic EPSPs evoked from the MLF by only 0.3–0.4 ms, this difference being fully compatible with the delay with which action potentials are generated during the rising phase of the EPSPs (see Fig. 6A). The two ranges of latencies of extracellular spike potentials may thus be taken to indicate monosynaptic as well as disynaptic coupling between the stimulated fibres and the SB neurones. Nevertheless the neurones rarely responded faithfully to each stimulus; the discharges most often appeared only following one to three stimuli in a train of five stimuli and in addition were not always evoked after the same stimuli in the 20 routinely repeated trains. The neurone illustrated in Fig. 5A represents those that responded most regularly, as illustrated with both single trace original records of responses to the 2nd, 3rd, 4th and 5th stimuli and peri-stimulus time histograms compiled from 20 successive trains of five stimuli. However, as the records in Fig. 5B show, it responded also to the first stimulus when MLF stimulation was combined with stimulation of a peripheral muscle nerve, indicating that this particular neurone was not only inhibited but also excited by group I muscle afferents (Burke et al. 1971). On average, the monosynaptic and disynaptic responses were evoked by 37% and 12% of the MLF stimuli, respectively, but these proportions depended to a great extent on experimental conditions and could be either increased or decreased.
Figure 6. Examples of inhibition of intracellularly recorded discharges by group II muscle afferents.
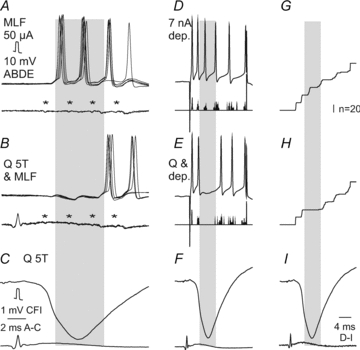
A and B, superimposed single records of responses of a SB neurone evoked from the MLF (upper traces) and from the cord dorsum (with the first components of the descending volleys indicated by asterisks). Note delays of about 0.3–0.8 ms between the onset of the EPSPs and of action potentials. C, averaged record of IPSPs evoked by stimulation of the quadriceps nerve (Q) at the same intensity as in B. D and E, single records from the same SB neurone but of discharges evoked by intracellularly applied depolarizing current pulses (upper traces) and histograms of 20 sequences of such spikes (lower traces) and at a lower time base. G and H, cumulative sums of spikes included in histograms D and E, for control responses and for effects of conditioning stimulation of the quadriceps nerve which evoked IPSPs illustrated in F and I (the same IPSP as in C but at a slower time base, matching that in D, E, G and H). Periods of maximal inhibition are indicated by shading. The total number of spikes in 20 sequences of stimuli was reduced from 60 to 35 (58%) in the series B and from 48 to 23 (48%) in the series E and H.
When the proportion of activated neurones was increased, the neurones were made to respond to stimuli earlier in the train, at a higher incidence rate and at shorter latencies. This occurred with increases in MLF stimulus intensity, or in the length of the train, and when MLF stimulation was combined with stimulation of other sources of input to either SB or reticulospinal neurones, e.g. the cuneiforme nucleus (the mesencephalic locomotor region, MLR) or of the pyramidal tract (E. Jankowska, E. Nilsson and I. Hammar, in preparation). However, even under optimal experimental conditions VSCT neurones responded with only one or two spikes to individual MLF stimuli (see linking of the responses to individual stimuli in Figs 5 and 6) and only exceptionally with a brief train of spikes.
Interactions between synaptic actions of reticulospinal neurones and peripheral afferents on SB neurones
The analysis of interactions between reticulospinal neurones and peripheral afferents focused on SB neurones located in the L4 segment where most neurones are inhibited by group I and/or II afferents (Burke et al. 1971). The main effects of stimulation of peripheral nerves on activation of these neurones from the MLF were therefore expected to be depressive, as was indeed found.
Three different approaches were used to analyse the modulation of responses of VSCT neurones by peripheral input. Firstly, we examined effects of stimuli suprathreshold for group II afferents (Fig. 5A–D) on extracellularly recorded discharges of VSCT neurones evoked by a train of MLF stimuli. The peripheral nerve stimulation was timed so that MLF evoked spikes would fall within the rising phase of disynaptic IPSPs evoked by group I and/or II afferents. However, the optimal timing could not be predicted as such IPSPs could be evoked by either group I afferents (at shorter latency, with steeper rising phase and shorter duration) or group II afferents (at longer latency, with less steep rising phase and longer duration). A few different intervals between the MLF and peripheral stimuli were therefore routinely tried. When the effects of peripheral afferent stimulation were maximal, the number of responses evoked by at least one of the MLF stimuli in the train was reduced to naught in 12 of 16 SB neurones tested and in the remaining neurones the numbers of responses were reduced to 10–30%. However, the maximal reduction only occurred when the peripheral nerves were stimulated at intensities of 4–5T, near-maximal for group II afferents. At threshold for group II afferents (at 2–3T for the quadriceps nerve, tested in 4 neurones) the reduction was much less marked (to 15–80%), as illustrated in Fig. 5C and D. The maximal inhibition of MLF evoked responses following single stimuli applied to group II afferents occurred within periods of about 5 ms (indicated by shading in Fig. 5). Facilitation was seen only rarely and was then limited to a very narrow range of intervals, with an example in Fig. 5B and F with the appearance of responses to the first MLF stimulus when MLF volleys coincided with those from Q. Such facilitation was seen even when Q was stimulated at low stimulus intensities, as indicated by peri-stimulus time histograms in Fig. 5C and D.
The second approach involved a similar comparison of responses that were or were not associated with stimulation of group I or group II afferents but were analysed in intracellularly recorded neurones. These tests (in two neurones) allowed us to relate the duration of the inhibition to the actually recorded IPSPs following nerve stimulation, with records from one of these neurones shown in Fig. 6A and B. The inhibition was maximal during 4.5–5 ms (during almost the whole rising phase of the IPSPs and the first two-thirds of their declining phase) but the reduction of the total number of spikes evoked from the MLF occurred during periods lasting at least 10 ms.
The third approach likewise involved intracellularly recorded neurones (n= 4) but in these neurones the action potentials were induced by intracellularly applied depolarizing current pulses. As shown in Fig. 6E and H, the generation of these action potentials was prevented during IPSPs evoked by stimulation of peripheral nerves as effectively as of those induced by MLF stimuli. In future studies modulatory actions of peripheral afferents on VSCT neurones and how these actions are decoded by cerebellar neurones might thus be analysed interchangeably by using either synaptic activation of these neurones, or intracellularly applied current pulses.
Discussion
Major differences were found between effects of stimulation of axons of reticulospinal neurones on four populations of spinocerebellar neurones in the lumbosacral enlargement. As summarized in Table 1 and in Fig. 7, monosynaptic and disynaptic EPSPs evoked from the MLF were found in SB and VSCT neurones, only disynaptic EPSPs in CC DSCT neurones and only longer latency EPSPs in dh DSCT neurones. Feedback information concerning the intended actions of reticulospinal neurones, at least those descending within the MLF, must thus be more essential for the cerebellar target neurones of SB and VSCT neurones than for those of either CC DSCT neurones or dh DSCT neurones. It is also likely that disynaptic EPSPs evoked in SB and VSCT neurones are to a great extent relayed by reticulospinal neurones activated via axon collaterals of fibres stimulated in the MLF (see evidence for this possibility in Edgley et al. 2004). Also disynaptic EPSPs might thus reflect direct input from reticulospinal neurones to SB and VSCT neurones. Taking this possibility into account, the relay neurones along the lines from the MLF to SB and VSCT neurones in Fig. 7A (indicated by small circles) have been placed close to the MLF. Disynaptic and any longer latency EPSPs evoked in CC and dhDSCT neurones would on the other hand most likely be mediated by spinal neurones, as they lack direct connections from RS neurones.
A consistent feature of EPSPs evoked in both SB and VSCT neurones was that they were evoked by reticulospinal tract fibres descending both ipsilaterally (after contralateral hemisection of the spinal cord) and contralaterally (after ipsilateral hemisection). In a previous study, monosynaptic EPSPs evoked in motoneurones by contralaterally descending reticulospinal fibres (Jankowska et al. 2003) were attributed to crossed intraspinal axon collaterals of these fibres (Matsuyama et al. 1999). Monosynaptic EPSPs from the contralateral MLF evoked in SB neurones could be similarly explained, especially as the small numbers of both motoneurones (9%; Jankowska et al. 2003) and SB neurones (12%; present study) in which they were found could be related to the small number of reticulospinal fibres with crossed collaterals (14% in the sample of Matsuyama et al. 1999). The slightly longer latencies of EPSPs evoked from the contralateral than from the ipsilateral MLF (cf. Table 1) are in keeping with the most likely longer axon collaterals and/or slower conduction velocity of crossed collaterals. The finding of a considerably larger proportion of VSCT neurones (38%) in which EPSPs were evoked from the contralateral MLF at latencies ≤1.0 ms was therefore unexpected and we cannot at present account for it. However, the probability of synaptic contacts made by crossing collaterals might be higher for VSCT neurones located medially and within the main terminal projection areas of crossed collaterals in laminae VII and VIII (Matsuyama et al. 1999) than for SB neurones and motoneurones, both located more laterally. The larger fraction of neurones with crossed monosynaptic actions found in this than in previous studies indicates that they might be a regular rather than an exceptional feature. Consequently it weakens the probability that disynaptic EPSPs and IPSPs evoked from the contralateral MLF are mediated predominantly by commissural interneurones while those evoked from the ipsilateral MLF are mediated by ipsilaterally located interneurones, except when disynaptic EPSPs are evoked from the contralateral but not the ipsilateral MLF (in CC DSCT neurones).
IPSPs following MLF stimulation have also been found to be distributed unevenly. As summarized in Table 1 and Fig. 7B disynaptic IPSPs were evoked in SB and VSCT neurones from both the ipsilateral and contralateral MLF, but only from the ipsilateral MLF in CC DSCT and only from the contralateral MLF in dh DSCT. As no direct inhibitory actions of MLF stimuli were found in any spinocerebellar neurones of our sample (see however Baldissera & Roberts, 1975), these IPSPs should be mediated by spinal interneurones. The relays in the inhibitory pathways from the MLF have therefore been placed in Fig. 7B close to the spinocerebellar neurones. On the basis of evidence presented by Jankowska et al. (2010), we may further postulate that the IPSPs evoked from the MLF in SB, VSCT and CC DSCT neurones are mediated by the same premotor interneurones that are interposed in inhibitory reflex pathways from group I and II afferents, while in dh DSCT neurones they are mediated by another population of interneurones.
The findings that monosynaptic excitation evoked by MLF stimulation is potent enough to discharge SB neurones and that these discharges are highly efficiently modulated by inhibitory actions of peripheral afferents are fully in support of our original hypothesis that SB neurones may play an important role in relaying information on the likely outcome of actions of reticulospinal neurones on spinal neurones. This hypothesis applies in the first hand to actions of reticulospinal neurones with axons running in the MLF, as effects of those descending outside the MLF (see e.g. Mitani et al. 1988) have not yet been investigated. The potent inhibition of discharges of SB neurones is also of interest as providing a functional meaning for the predominantly inhibitory input from group Ib tendon organs and group Ia and II muscle spindle afferents to many SB neurones. IPSPs from group I and II afferents are evoked in SB and VSCT neurones as collateral actions of interneurones inhibiting motoneurones (Lundberg, 1971; Lundberg & Weight, 1971; Lindström & Schomburg, 1973, 1974; Lindström, 1973; Lindström & Takata, 1977; Hongo et al. 1983; Jankowska & Puczynska, 2008). The modified discharges of the spinocerebellar neurones could therefore be decoded by the cerebellum as an indication that the intended actions of reticulospinal neurones on motoneurones will be counteracted by inhibitory spinal interneurones. The feedback information obtained in this way could then be used to enhance the activation of reticulospinal neurones and thereby ensure that spinal interneurones and motoneurones are neither too weakly nor too strongly activated by reticulospinal neurones and that the intended movements are executed as planned. If so, the modulation of discharges of SB neurones by spinal interneurones in pathways from primary afferents could serve to prevent errors in descending commands, and thereby enable these commands to be adjusted before errors are committed, while corrections of the already committed errors utilize the spino-olivary and climbing fibres route to the cerebellum.
These possibilities are also compatible with the known morphological relations between SB neurones and neurones in both the reticular formation and the cerebellum. The terminal projection areas of SB neurones in the paramedian lobule are very specific and there is apparently a minimal degree of overlap between terminal projection areas of SB and other spino-cerebellar neurones in this lobule, although a considerable overlap occurs within the main projection areas of VSCT neurones in lobules II, III and IV of the anterior lobe. Matsushita and colleagues found discrete areas of termination of SB neurones (in sublobules C1 and 2 of the paramedian lobule) to be separated from those of CC DSCT neurones (in sublobule B) and of most other spinocerebellar neurones (Matsushita & Ikeda, 1980; Matsushita & Yaginuma, 1989). The paramedian sublobules C1 and 2 may thus be specialized in monitoring information provided by SB neurones on how spinal interneurones might shape the descending commands of reticulospinal neurones while neurones in other cerebellar areas process other kinds of information from these as well as a variety of other neurones. Connections between the paramedian sublobules C1 and 2 and cerebellar nuclei have been investigated by Trott et al. (1998a,b); who have found them in distinct parts of the nucleus interpositus and there have also been indications of a projection to the nucleus fastigius (Jansen & Brodal, 1954), which in turn provide monosynaptic input to reticulospinal or other reticular neurones (Eccles et al. 1975; Kitai et al. 1976). Nevertheless, information forwarded by SB neurones to neurones in the paramedian sublobules C1 and 2 would be integrated with information from other sources, in particular via climbing fibres, and further integration should occur at each level. Before knowing how information forwarded by spinocerebellar neurones is decoded by cerebellar neurones, it is thus difficult to predict when and how it is used to adjust motor performance.
By proposing that SB neurones forward information on the likely output of descending commands of reticulospinal neurones on spinal neurones and thus serve to prevent errors in the central activation of spinal neurones, we do not consider this as their only or even main function. However, such a function may add to the functions of VSCT neurones discussed previously, such as comparing input and output from spinal interneurones (Lundberg, 1971; Lindström, 1973; Baldissera & Roberts, 1975), on central spinal mechanisms of rhythmic movements (Arshavsky et al. 1984; for more recent references see Armstrong et al. 1997; Van der Linden et al. 2007), or of functions of spinocerebellar neurones involved with feedback information on proprioception and limb biomechanics (Bosco & Poppele, 2001). Monitoring and adjusting the descending commands as outlined above may, however, constitute an important function of spinocerebellar neurones that should not be overshadowed by their other functions. Whether different functions of SB and other spinocerebellar neurones are subserved by distinct subpopulations, or by the same neurones via different cerebellar target neurones will, however, have to be investigated separately.
Acknowledgments
The study was supported by grants from NINDS/NIH (R01 NS040863) and the Swedish Research Council (15393-01) and 522-2005-7255 for IH.
Glossary
Abbreviations
- ABSM
anterior biceps and semimembranosus
- CC DSCT
Clarke's column dorsal spinocerebellar tract
- dh DSCT
dorsal horn dorsal spinocerebellar tract
- DSCT
dorsal spinocerebellar tract
- FDL
flexor digitorum and hallucis longus
- GS
gastrocnemius–soleus
- MLF
medial longitudinal fascicle
- MLR
mesencephalic locomotor region
- PL
plantaris
- PBST
posterior biceps and semitendinosus
- PSP
postsynaptic potential
- Q
quadriceps
- Sart
sartorius
- SB
spinal border
- Sur
sural
- VSCT
ventral spinocerebellar tract
Author contributions
H.I. and J.E: conception and design of the experiments; collection, analysis and interpretation of data; drafting the article. K.P., D-C.H. and N.E.: collection, analysis and interpretation of data. All authors approved the final version to be published.
References
- Armstrong DM, Apps R, Marple-Horvat DE. Aspects of cerebellar function in relation to locomotor movements. Prog Brain Res. 1997;114:401–421. doi: 10.1016/s0079-6123(08)63377-4. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by descending tracts during scratching in the cat. I. Activity of vestibulospinal neurons. Brain Res. 1978a;159:99–110. doi: 10.1016/0006-8993(78)90112-9. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res. 1978b;151:493–506. doi: 10.1016/0006-8993(78)91082-x. [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA, Popova LB. Origin of signals conveyed by the ventral spino-cerebellar tract and spino-reticulo-cerebellar pathway. Exp Brain Res. 1984;54:426–431. doi: 10.1007/BF00235467. [DOI] [PubMed] [Google Scholar]
- Asif M, Edgley SA. Projections of group II-activated midlumbar spinocerebellar tract neurones to the region of nucleus Z in the cat. J Physiol. 1992;448:565–578. doi: 10.1113/jphysiol.1992.sp019058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Roberts WJ. Effects on the ventral spinocerebellar tract neurones from Deiters’ nucleus and the medial longitudinal fascicle in the cat. Acta Physiol Scand. 1975;93:228–249. doi: 10.1111/j.1748-1716.1975.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Weight F. Descending monosynaptic connexions to spinal border cells. Acta Physiol Scand. 1969;76:28A–29A. [PubMed] [Google Scholar]
- Bantli H, Bloedel JR. Monosynaptic activation of a direct reticulo-spinal pathway by the dentate nucleus. Pflugers Arch. 1975;357:237–242. doi: 10.1007/BF00585978. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- Burke R, Lundberg A, Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res. 1971;12:283–294. doi: 10.1007/BF00237921. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Nicoll RA, Schwarz WF, Taborikova H, Willey TJ. Reticulospinal neurons with and without monosynaptic inputs from cerebellar nuclei. J Neurophysiol. 1975;38:513–530. doi: 10.1152/jn.1975.38.3.513. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TC, Jankowska E, Tanaka R. Effects of volleys in cortico-spinal tract fibres on ventral spino-cerebellar tract cells in the cat. Acta Physiol Scand. 1977;100:1–13. doi: 10.1111/j.1748-1716.1977.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Fuller JH, Schlag JD. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;112:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci. 2010;13:1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1995;23:89–102. [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Okada Y. Cortically evoked pre- and postsynaptic inhibition of impulse transmission to the dorsal spinocerebellar tract. Exp Brain Res. 1967;3:163–177. doi: 10.1007/BF00233260. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat. J Neurophysiol. 2010;104:1872–1883. doi: 10.1152/jn.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Puczynska A. Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat. J Neurosci. 2008;28:3615–3622. doi: 10.1523/JNEUROSCI.0466-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Brodal A, editors. Aspects of Cerebellar Anatomy. Oslo: Johan Grundt Tanum Forlag; 1954. [Google Scholar]
- Kitai ST, Kocsis JD, Kiyohara T. Electrophysiological properties of nucleus reticularis tegmenti pontis cells: antidromic and synaptic activation. Exp Brain Res. 1976;24:295–309. doi: 10.1007/BF00235017. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Amano K, Kitamura K, Poppele RE. [Differences of behavior of DSCT neurons in decerebrate and barbiturate anesthetized cats (author's transl)] No To Shinkei. 1978;30:387–391. [PubMed] [Google Scholar]
- Lindström S. Recurrent control from motor axon collaterals of Ia inhibitory pathways in the spinal cord of the cat. Acta Physiol Scand. 1973;89(Suppl. 392):1–43. [PubMed] [Google Scholar]
- Lindström S, Schomburg ED. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973;88:505–515. doi: 10.1111/j.1748-1716.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Lindström S, Schomburg ED. Group I inhibition in Ib excited ventral spinocerebellar tract neurones. Acta Physiol Scand. 1974;90:166–185. doi: 10.1111/j.1748-1716.1974.tb05575.x. [DOI] [PubMed] [Google Scholar]
- Lindström S, Takata M. Lack of recurrent depression from motor axon collaterals of Ia IPSPs in dorsal spinocerebeller tract neurones. Brain Res. 1977;129:158–161. doi: 10.1016/0006-8993(77)90979-9. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12:317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12:295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoya Y, Ikeda M. Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;184:81–106. doi: 10.1002/cne.901840106. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinocerebellar projections to the vermis of the posterior lobe and the paramedian lobule in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1980;192:143–162. doi: 10.1002/cne.901920110. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Xiong G. Uncrossed and crossed projections from the upper cervical spinal cord to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol. 2001;432:101–118. doi: 10.1002/cne.1091. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yaginuma H. Spinocerebellar projections from spinal border cells in the cat as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1989;288:19–38. doi: 10.1002/cne.902880103. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- McCrea RA, Bishop GA, Kitai ST. Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior. II. Mossy fiber system. Brain Res. 1977;122:215–228. doi: 10.1016/0006-8993(77)90290-6. [DOI] [PubMed] [Google Scholar]
- Mitani AK, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol. 1988;268:546–566. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of the opino- and cuneocerebellar tracts. Physiol Rev. 1965;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of spinocerebellar paths. In: Iggo I, editor. Handbook of Sensory Physiology. Berlin: Springer Verlag; 1973. pp. 339–380. [Google Scholar]
- Tolbert DL, Bantli H, Hames EG, Ebner TJ, McMullen TA, Bloedel JR. A demonstration of the dentato-reticulospinal projection in the cat. Neuroscience. 1980;5:1479–1488. doi: 10.1016/0306-4522(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum. 1. The C1 zone. Exp Brain Res. 1998a;118:298–315. doi: 10.1007/s002210050285. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum. 2. The C2 zone. Exp Brain Res. 1998b;118:316–330. doi: 10.1007/s002210050286. [DOI] [PubMed] [Google Scholar]
- Van Der Linden MH, Marigold DS, Gabreels FJ, Duysens J. Muscle reflexes and synergies triggered by an unexpected support surface height during walking. J Neurophysiol. 2007;97:3639–3650. doi: 10.1152/jn.01272.2006. [DOI] [PubMed] [Google Scholar]
- Waltzer R, Martin GF. Collateralization of reticulospinal axons from the nucleus reticularis gigantocellularis to the cerebellum and diencephalon. A double-labelling study in the rat. Brain Res. 1984;293:153–158. doi: 10.1016/0006-8993(84)91462-8. [DOI] [PubMed] [Google Scholar]
- Xu Q, Grant G. The projection of spinocerebellar neurons from the sacrococcygeal region of the spinal cord in the cat. An experimental study using anterograde transport of WGA-HRP and degeneration. Arch Ital Biol. 1990;128:209–228. [PubMed] [Google Scholar]


