Abstract
We investigated here whether an opposing interplay between the subthreshold currents A-type potassium (IA) and T-type calcium (IT) influences membrane excitability in presympathetic neurones of the hypothalamic paraventricular nucleus (PVN) that innervate the rostral ventrolateral medulla (RVLM). Moreover, we assessed whether a shift in the balance between these two subthreshold currents contributed to increased neuronal activity in hypertension. To this end, we obtained simultaneous electrophysiological recordings, confocal Ca2+ imaging, and single-cell RT-PCR samples from identified PVN-RVLM neurones in sham and renovascular hypertensive rats. Our results indicate that IA and IT, displaying overlapping voltage-dependent and kinetic properties, are present in PVN-RVLM neurones. We found that the relative predominance of each current at hyperpolarized membrane potentials dictates whether PVN-RVLN neurones express a low-threshold spike (LTS) or a transient outward rectification (TOR). Moreover, we report the IA/IT balance to be correlated with the relative expression of Kv4.3 and Cav3.1 subunit mRNA within individual neurones. Pharmacological blockade of IA resulted in an enhanced IT-mediated LTS, as well as LTS-mediated somatodendritic Ca2+ transients. In hypertensive rats, we found a shift in the IT/IA balance, towards an IT predominance, due in part to a diminished Kv4.3 and enhanced Cav3.1 mRNA subunits expression. The imbalanced IT/IA relationship resulted in enhanced LTS, LTS-mediated somatodendritic Ca2+ transients, and increased firing activity in hypertensive rats. Taken together, our results support that a balanced IT/IA interaction influences membrane excitability and Ca2+ dynamics in PVN-RVLM neurones. Moreover, an imbalanced relationship favouring IT results in enhanced neuronal excitability and firing discharge in hypertensive rats, constituting thus a likely mechanism contributing to the characteristic sympathoexcitation observed in this disease.
Non-technical summary
Despite the importance of brain-mediated sympathetic activation in the morbidity and mortality of patients with high blood pressure, the precise cellular mechanisms involved remain largely unknown. We show that an imbalanced interaction between two opposing currents mediated by potassium (IA) and calcium (IT) channels occurs in sympathetic-related hypothalamic neurons in hypertensive rats. We show that this imbalance contributes to enhanced membrane excitability and firing activity in this neuronal population. Knowledge of how these opposing ion channels interact in normal and disease states increases our understanding of underlying brain mechanisms contributing to the high blood pressure condition.
Introduction
Neuronal excitability and firing activity within central neuronal circuits are tightly controlled by a fine-tuned balance between intrinsic membrane properties and synaptic inputs. Based on their voltage-dependent and kinetic properties, subthreshold currents such as the A-type potassium current (IA) and the T-type calcium current (IT) play a pivotal role in the control of membrane excitability within a critical range of membrane potentials near the threshold, influencing thus the frequency and pattern of Na+-dependent action potentials fired by neurones.
IA is a rapidly activating and inactivating transient outward current. Activation of IA transiently decreases membrane excitability, delaying the onset of firing activity, restricting the duration of the action potential waveform, and increasing the interspike interval (Segal et al. 1984; Rogawski, 1985; Rudy, 1988; Fisher et al. 1998). Conversely, IT is a transient inward current that when activated results in an increased neuronal excitability. A low-threshold spike (LTS) and burst firing discharge have been attributed to IT (Llinas & Yarom, 1981; Tasker & Dudek, 1991; Stern, 2001). In addition, IT has also been shown to regulate continuous firing activity (Raman & Bean, 1999), and the repolarization phase of the action potential waveform (McCobb & Beam, 1991; Lambert et al. 1998; Monteil et al. 2000).
Based on their overlapping voltage- and time-dependent properties, an opposing interplay between IA and IT has been shown to be critical in the control of neuronal excitability (Pape et al. 1994; Meis et al. 1996; Cavelier et al. 2003; Li et al. 2005; Molineux et al. 2005). Moreover, an altered balance in the IT/IA interaction was reported to contribute to hyperexcitability in pathological conditions, such as epilepsy (Meis et al. 1996).
The hypothalamic paraventricular nucleus (PVN) is a pivotal autonomic and neuroendocrine integrative centre, which plays a major role in the control of sympathetic outflow to the cardiovascular system (Swanson & Sawchenko, 1983; Coote et al. 1998; Dampney et al. 2005; Guyenet, 2006). These actions are mediated by presympathetic neurones that send direct projections to preganglionic neurones in the intermediolateral column of the spinal cord (Swanson & Kuypers, 1980; Lovick & Coote, 1988; Hosoya et al. 1991; Ranson et al. 1998), as well as to the rostral ventrolateral medulla (RVLM) (Ciriello et al. 1985; Dampney et al. 1987; Coote et al. 1998; Yang & Coote, 1998; Tagawa & Dampney, 1999; Kubo et al. 2000; Hardy, 2001; Allen, 2002). Accumulating evidence supports enhanced PVN neuronal activity as a key contributor to enhanced sympathetic outflow during pathological conditions, including hypertension, heart failure and diabetes (Patel, 2000; Allen, 2002; Zhu et al. 2004; Li & Pan, 2006; Zheng et al. 2006). Despite the impact of sympathoexcitation in the morbidity and mortality of these prevalent disorders (Esler & Kaye, 1998; Nesto, 2004), the precise underlying cellular mechanisms contributing to elevated PVN neuronal activity remain unknown.
Recently, we showed that IA is a key factor regulating neuronal excitability in PVN-RVLM neurones (Sonner & Stern, 2007), and similarly to previous reports in other parvocellular PVN neurones (Luther & Tasker, 2000; Stern, 2001), we demonstrated the presence of IT within this presympathetic neuronal population (Lee et al. 2007; Sonner et al. 2007; Lee et al. 2008). However, whether the interplay between IA and IT influences PVN-RVLM activity, and whether a shift in the balance between the two subthreshold currents contributes to increased excitability in disease conditions is at present unknown. We therefore, investigated in this study (a) whether there is an active interplay between IA and IT in PVN-RVLM neurones, (b) whether this interplay affects membrane properties and intracellular Ca2+ dynamics, and (c) whether a shift in the balance between these two conductances contributes to enhanced PVN neuronal activity during hypertension. To this end, we obtained simultaneous electrophysiological recordings, confocal Ca2+ imaging and single-cell RT-PCR samples from identified PVN-RVLM neurones in control rats, as well as in a well-established renovascular hypertensive animal model, in which a major contributing role for the PVN has been demonstrated (Earle et al. 1992; Earle & Pittman, 1995; Jung et al. 2004).
Methods
Ethical approval
Male Wistar rats (n= 69, 120–140 g) were purchased from Harlan laboratories (Indianapolis, IN, USA), and housed in a 12 h–12 h light–dark cycle with access to food and water ad libitum. All procedures were carried out in agreement with the Medical College of Georgia Animal Care and Use Committee's guidelines, and in compliance with NIH guidelines. Our experiments also comply with the policies and regulations of The Journal of Physiology (Drummond, 2009).
Renovascular surgery
Rats weighing between 150 and 180 g (approximately 5–6 weeks old) were used to induce the renovascular 2K1C Goldblatt hypertension model, a well characterized and widely used model (Martinez-Maldonado, 1991; Bergamaschi et al. 1995). As previously reported (Sonner et al. 2008), rats weighing between 150–180 g were maintained under anesthesia with isoflurane (3%). Following an abdominal incision, the left kidney was exposed, and a 0.2 mm clip was placed over the left renal artery, partially occluding it (Carvalho et al. 2003). The sham procedure was the same, except the artery was not occluded. Post-operative care included proper management of associated pain (buprenorphine, 0.25 mg kg−1, subcutaneous, as needed). All rats were used for experiments during the sixth to seventh week post-surgery. Blood pressure was measured at the beginning of the sixth week post-surgery, using a tail-cuff method. Values obtained were 137.9 ± 2.3 mmHg and 208.4 ± 3.1 mmHg in sham and hypertensive rats, respectively (P < 0.0001).
Retrograde labelling of PVN-RVLM neurones
Preautonomic RVLM-projecting PVN neurones were identified by injecting rhodamine beads unilaterally into the brainstem region containing the RVLM as previously described (Stern, 2001; Li et al. 2003). Rats were anaesthetized (ketamine–xylazine mixture, 90 and 50 mg kg−1, respectively, i.p.) and a stereotaxic apparatus was used to pressure inject 200 nl of rhodamine-labelled microspheres (Lumaflor, Naples, FL, USA) into the RVLM (starting from Bregma: 12 mm caudal along the lamina, 2 mm medial lateral, and 8 mm ventral). In general, RVLM injection sites were contained within the caudal pole of the facial nucleus to ∼1 mm more caudal, and were ventrally located with respect to the nucleus ambiguous. The location of the tracer was verified histologically (Stern, 2001; Li et al. 2003). As previously reported, retrogradely labelled neurones were found in the ventromedial (VM), dorsal cap (DC) and posterior (PaPo) PVN subnuclei (Armstrong et al. 1980; Swanson & Sawchenko, 1983; Stern, 2001; Sonner & Stern, 2007).
Hypothalamic slices
Two to three days after the retrograde injection, rats were deeply anaesthetized with nembutal (50 mg kg−1, i.p.), and perfused through the heart with a cold sucrose solution (containing in mm: 200 sucrose, 2.5 KCl, 3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 1 CaCl2 and 2 pyruvic acid (290–310 mosmol l−1). Rats were then quickly decapitated, the brains were dissected out, and coronal slices cut (300 μm thick) using a vibroslicer (D.S.K. Microslicer, Ted Pella, Redding, CA, USA). An oxygenated ice cold artificial cerebrospinal fluid (ACSF) was used during slicing (containing in mm: 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2 CaCl2 and 2 pyruvic acid; pH 7.4; 290–310 mosmol l−1). Slices were placed in a holding chamber containing ACSF and kept at room temperature until used (see Stern, 2001 for details).
Electrophysiological recordings
Once in the recoding chamber, slices were bathed with solutions (∼3.0 ml min−1) that were continuously bubbled with 95% O2–5% CO2 and maintained at room temperature (∼22–24°C). Patch pipettes (4–7 MΩ) composed of thin walled (1.5 mm outer diameter, 1.17 mm inner diameter) borosilicate glass (GC150T-7.5, Clark, Reading, UK), were pulled on a horizontal electrode puller (P-97, Sutter Instrument Co., Novato, CA, USA). The internal solution contained (mm): 140 potassium gluconate, 0.2 EGTA, 10 Hepes, 10 KCl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP and 20 sodium phosphocreatine; pH 7.2–7.3. Recordings were obtained with a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA, USA) from fluorescently labelled PVN-RVLM neurons, visualized with a combination of fluorescence illumination and infrared differential interference contrast (IR-DIC) videomicroscopy. The voltage output was digitized at 16-bit resolution, 10 kHz (Digidata 1320A, Molecular Devices), and saved on a computer for offline analysis. Data were discarded if the series resistance (12.8 ± 0.2 MΩ, n= 106) was not stable throughout the entire recording. For voltage-clamp recordings, all protocols were run with an output gain of 2, a Bessel filter of 2 kHz, were leak subtracted (P/4), and the series resistance was electronically compensated at least 60% throughout the recordings. Data were corrected for the liquid junction potential (6.5 mV), which was experimentally determined using a 2 m KCl agar bridge. For current-clamp recordings, all protocols were run using an output gain of 10 and a Bessel filter of 10 kHz.
Voltage-clamp recordings of IA and IT
IT was pharmacologically isolated using an ACSF containing blockers of voltage-gated sodium and potassium channels. The ACSF contained in mm: 92 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 5 CaCl2, 2 pyruvic acid, 0.5 μm TTX, 30 TEA and 5 4-AP; pH 7.4; 290–310 mosmol l−1). To study the voltage-dependent activation properties of IT, we used a protocol consisting of a hyperpolarized conditioning pulse (−96.5 mV, 340 ms) followed by a series of depolarizing command pulses (from −76.5 to −26.5 mV, 5 mV increments, 400 ms). At voltages more positive than −20 mV, a slower activating and non-inactivating Ca2+ current component (likely to represent high-threshold voltage-activated Ca2+ currents, HVA) was generally observed. Thus, to prevent contamination of IT with HVA Ca2+ currents, the maximum command pulse was restricted to −20 mV. IA was isolated, as previously described (Sonner et al. 2007), by using pharmacological methods. In order to block calcium channels, sodium channels and delayed rectifier potassium channels (IKDR), an ACSF with nominal Ca2+ (0 mm) was used (containing in mm: 102 NaCl, 2.5 KCl, 3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2 pyruvic acid, 3 EGTA, 200 μm CdCl2, 0.5 μm TTX and 30 mm TEA; pH 7.4; 290–310 mosmol l−1). The protocol used was the same as above.
In both cases, the normalized peak current amplitudes were plotted as a function of the conditioning step potentials, and fitted with a Boltzmann function, to determine their half-activation potential. IA and IT activation thresholds were defined as the membrane potential at which a transient current ≥10 pA was detected.
To study the direct interaction between IA and IT we selected recording conditions that allowed the detection of both currents simultaneously. For these experiments, we used a modified ACSF containing (in mm): 97 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 5 CaCl2, 2 pyruvic acid, 0.5 μm TTX and 30 TEA; pH 7.4; 290–310 mosmol l−1), and used the same electrophysiological protocol mentioned above. The current density of IA and IT was determined by dividing the current amplitude at each command potential by the cell capacitance, obtained by integrating the area under the transient capacitive phase of a 5 mV depolarizing step pulse, in voltage clamp mode. The rate of activation of IT was determined by measuring the 10–90% rise time from the baseline to the peak of the current at a command pulse of −40 mV. The time constant (τ) of inactivation of IT was determined by fitting a monoexponential function to the decay phase of the current activated at a command pulse of –40 mV.
Low-threshold spikes
Low-threshold spikes (LTSs) were evoked in current-clamp mode in the presence of TTX. Current was injected to maintain the membrane potentential (Vm) at ∼–90 mV, and the LTSs evoked by injecting depolarizing pulses (20–100 pA, 220 ms). The LTS threshold was obtained by fitting a monoexponential function to the trace and determining the Vm at which the fitted curve no longer fitted the trace.
Spontaneous repetitive firing activity
Spontaneous repetitive firing activity was recorded from PVN-RVLM neurones in continuous mode. The mean firing frequency (2–3 min period) obtained before and after bath application of 100 μm NiCl2 was calculated and compared using Mini Analysis software (Synaptosoft, Fort Lee, NJ, USA). It is known that 4-AP facilitates presynaptic release of neurotransmitter (Flores-Hernandez et al. 1994), and thus direct effects of 4-AP on intrinsic properties could be masked by this presynaptic effect. Therefore, all current-clamp experiments were performed in the presence of receptor blockers of the main excitatory (glutamate) and inhibitory (GABA) neurotransmitters in this system. The ACSF used for current-clamp experiments contained (in mm): 110 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 5 CaCl2, 2 pyruvic acid, 0.5 μm TTX, 300 μm picrotoxin and 2 mm kynurenic acid; pH 7.4; 290–310 mosmol l−1).
Confocal calcium imaging
As previously described (Sonner et al. 2008), Fluo-5F pentapotassium salt (100 μm; Invitrogen/Molecular Probes, Carlsbad, CA, USA) was incorporated into the internal solution in order to allow for dye-loading into identified PVN-RVLM neurones. Once in the whole-cell mode, the dye was allowed to dialyse into the cell for at least 20 min before the initiation of the recordings, in order to allow complete dialysis of the dye. Calcium imaging was conducted using the Yokogawa real time live cell laser confocal system combined with a highly sensitive EMCCD camera (iXON+885, Andor Technology, South Windsor, CT, USA). Fluorescence images were obtained using diode-pumped solid-state laser (Melles Griot, Carlsbad, CA, USA), and fluorescence emission was collected at >495 nm. Images were acquired at a rate of 40–50 Hz. The fractional fluorescence (F/F0) was determined by dividing the fluorescence intensity (F) within a region of interest (ROI; 6 × 6 pixels ≈ 4.8 × 4.8 μm) by a baseline fluorescence value (F0) determined from 30 images before a LTS was evoked (a period showing no change in intracellular calcium levels) (Sonner et al. 2008). Between three and four traces of calcium transients evoked by LTS were averaged in order to increase the signal to noise ratio. Data were analysed using Andor IQ software (Andor Technology, Belfast, UK).
Single cell real time, reverse transcription-polymerase chain reaction
To quantitatively assess changes in the expression of Kv4.3 and Cav3.1 K+ and Ca2+ channel subunits, respectively, as well as a to establish correlation between the expression of these channel subunits and the properties of IA and IT, we perform single cell real-time PCR from individually recorded PVN-RVLN neurons. For our studies, we have followed quantitative approaches described in details by (Livak & Schmittgen, 2001). A similar approach was recently used in other laboratories to successfully assess mRNA levels within individually identified GnRH neurons (Parhar et al. 2003). Briefly, the cytoplasm of a single neurone was gently pulled into a pipette with negative pressure, taking care not to contain the nucleus. The cytoplasm in the pipette was dissipated into a prepared tube containing (in μl) 18 of nuclease free water, 3 of 7× genomic DNA Wipeout Buffer and stored at −70°C. After finishing all recordings, the tubes were heated to 42°C for 2 min and incubated on ice for at least 1 min. The mixture of (in μl) 6 of 5× Quantiscript RT Buffer, 1 of RT Primer Mix, 1 of Quantiscipt reverse transcriptase was subsequently added and incubated at 42°C for 15 min. The reaction was terminated by heating at 95°C for 3 min and stored at −20°C. All reagents for reverse transcription were purchased from Qiagen (Valencia, CA, USA). Real time PCR amplification was induced by using a fraction of the single cell cDNA as a template. The cDNA of the single neurone was split by 4 μl for three primer sets of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Kv4.3 and Cav3.1. The reaction mixture (20 μl total volume) contained (in μl): 1 of 25 μm each forward and reverse primer, 4 of nuclease free water and 4 of the cDNA template, 10 of 2× SYBR Green Master Mix (Applied Biosystems, Foster City, CA, US). The annealing temperature in the thermal cycler was 60°C and 50 cycles were performed using an ABI Prism 7700 sequence detector (Applied Biosystems). Quantification was conducted by determining the relative changes in gene expression, to a chosen reference gene, using the 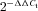 method (Livak & Schmittgen, 2001). Statistical significance was determined by comparing raw ΔCt values. The standard deviations were used to calculate range values (
method (Livak & Schmittgen, 2001). Statistical significance was determined by comparing raw ΔCt values. The standard deviations were used to calculate range values (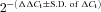 ) for graphs. We compared the efficiency between the target gene (Kv4.3 or Cav3.1) and GAPDH by plotting the ΔCtversus the log of the dilution ratio. The primers were optimized for each reaction, and considered efficient if the absolute value of the slope was less than 1. The slopes of Kv4.3 and Cav3.1 were determined to be 0.07 and 0.005, respectively. All primers used in this study, except GAPDH (purchased from Qiagen, Valencia, CA, USA), were designed by Primer 3 (Whitehead Institute, Cambridge, MA, USA) and synthesized by Bioneer (Alameda, CA, USA). The primer set used in this study is presented in Table 1. The product size was adjusted around 150 bp that is suitable for the real time PCR reaction. Controls used in our studies included harvested neurons that were processed similarly, but without the RT step, as well as buffer without harvested neurons.
) for graphs. We compared the efficiency between the target gene (Kv4.3 or Cav3.1) and GAPDH by plotting the ΔCtversus the log of the dilution ratio. The primers were optimized for each reaction, and considered efficient if the absolute value of the slope was less than 1. The slopes of Kv4.3 and Cav3.1 were determined to be 0.07 and 0.005, respectively. All primers used in this study, except GAPDH (purchased from Qiagen, Valencia, CA, USA), were designed by Primer 3 (Whitehead Institute, Cambridge, MA, USA) and synthesized by Bioneer (Alameda, CA, USA). The primer set used in this study is presented in Table 1. The product size was adjusted around 150 bp that is suitable for the real time PCR reaction. Controls used in our studies included harvested neurons that were processed similarly, but without the RT step, as well as buffer without harvested neurons.
Table 1.
Primers used in the single cell real time PCR reactions
Immunohistochemistry
In a subpopulation of recordings, PVN-RVLM neurones were intracellularly filled with biocytin (0.2%) and processed for immunohistochemical detection of oxytocin and vasopressin immunoreactivities. Slices were briefly fixed overnight in a 4% paraformaldehyde–0.2% picric acid solution, dissolved in 0.3 m phosphate buffered saline (PBS; pH ∼7.3) and then thoroughly rinsed with 0.01 m PBS. Slices were then dehydrated using increasing concentrations of ethanol (60–100%; 10% increments; 10 min each step except 100% for 20 min), and then incubated in xylene for 10 min followed by the reverse ethanol procedure (100–60%). Slices were then rinsed in 0.01 m PBS with 0.5% Triton X-100 (TX) for 10 min, and incubated for 45 min in 10% normal horse serum with 0.01 m PBS, 0.5% TX and 0.04% NaN3. Slices were then thoroughly rinsed with 0.01 m PBS, 0.5% TX and 0.04% NaN3, followed by a 48 h incubation with a cocktail containing a combination of primary antibodies for oxytocin and vasopressin (both raised in guinea pig, used at 1:50,000 dilution; Bachem, Torrance, CA, USA) in 0.01 m PBS, 0.5% TX and 0.04% NaN3. Slices were then rinsed in 0.01 m PBS, 0.5% TX and 0.04% NaN3 for 30 min and then incubated overnight with the secondary antibodies: CY5-streptavidin (1:10,000) and FITC-guinea pig (1:400; both from Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 0.01 m PBS, 0.5% TX and 0.04% NaN3. Slices were then thoroughly rinsed in 0.01 m PBS for 20 min, mounted, and visualized using fluorescence microscopy (20×; Olympus America Inc., Melville, NY, USA).
Chemicals
All chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA), with the exceptions of pyruvic acid (MP Biomedicals, Aurora, OH, USA) and TTX (Alomone Labs, Jerusalem, Israel).
Statistical analysis
All values are expressed as means ±s.e.m. In most cases, Student's unpaired or paired t test was used, as indicated. Two-way ANOVA with Bonferroni post hoc test was used as needed. Pearson's correlation test was used to determine if correlations existed between two parameters. Differences were considered significant at P < 0.05. All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Whole-cell patch clamp recordings were obtained from retrogradely labelled PVN-RVLM neurones in sham (n= 80) and hypertensive (n= 90) rats. The mean input resistance was 1003 ± 84.0 MΩ and 1034 ± 79.2 MΩ for sham and hypertensive PVN-RVLM neurones, respectively (P= 0.8). Neuronal cell capacitance was slightly, though significantly reduced in PVN-RVLM neurones during hypertension (sham: 20.6 ± 1.0 pF; hypertensive: 17.6 ± 0.9 pF, P < 0.05), as previously reported (Sonner et al. 2008).
Interplay between IA and IT within individual PVN-RVLM neurones
In response to depolarizing steps from a hyperpolarized Vm, and under conditions where Ca2+ or K+ currents were pharmacologically isolated, both a transient outward K+ current (IA) and a transient inward Ca2+ current (IT) were observed, respectively, in PVN-RVLM neurones (Fig. 1A). Both currents were observed over the voltage range tested. Plots of normalized IA and IT current amplitude vs. command potentials were generated and fitted with a Boltzmann function (Fig. 1B). Note that the current–voltage plot for IT was negatively shifted with respect to IA. IT activated at a significantly more hyperpolarized Vm than IA (mean activation threshold: IT=−54.6 ± 1.6 mV; IA=−46.3 ± 1.2 mV, P= 0.001, Fig. 1C), and the half-activation Vm of IT was significantly more hyperpolarized than that of IA (IT, −40.8 ± 4.0 mV; IA, −33.1 ± 4.0 mV, (P < 0.01). The steepness of the activation curve was not significantly different between IT an IA (slope factor, k, for IT= 6.0 ± 1.2 mV vs. IA= 7.5 ± 0.4; P= 0.1).
Figure 1. IA and IT are expressed in PVN-RVLM neurons.
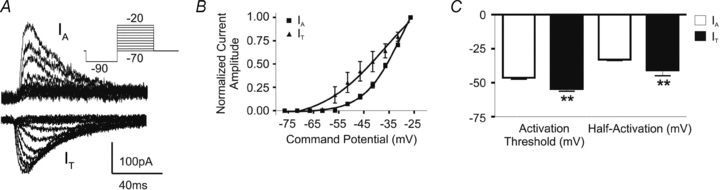
A, representative examples of isolated IA (upper trace) and IT (lower trace) in PVN-RVLM neurones. B, mean plots of IA (squares) and IT (triangles) normalized current amplitudes vs. the command potential. Boltzmann functions were fitted to the I–V plots. C, summary of the activation thresholds and half-activation potentials of IA and IT in sham PVN-RVLM neurons. Note the more hyperpolarized activation threshold and half-activation potential for IT when compared to IA. **P < 0.01. All traces and data shown from sham rats.
IT rise time (10–90%) and τinactivation ranged from 3.2 to 25.4 ms (mean rise time = 14.6 ± 3.1 ms) and from 8.4 to 50.6 ms (mean τinactivation= 31.9 ± 5.5 ms), respectively, at a command potential of −40 mV (n= 8). These values were similar to those we recently reported for IA (Sonner & Stern, 2007).
As we previously showed (Stern, 2001; Sonner & Stern, 2007), IA and IT were sensitive to block by 4-AP (2–5 mm) and NiCl2 (100 μm), respectively (not shown). No differences in current density between IA and IT were observed over the voltage range tested (P= 0.12, 2-way ANOVA, not shown).
Based on the large overlap in their voltage-dependent and kinetic properties, we investigated whether these currents with opposing polarities interacted at similar membrane potentials. To this end, IA and IT were simultaneously recorded in the same PVN-RVLM neurones. Both transient currents were observed in each individually tested neurone (n= 16) (Fig. 2). In most cases (13/16), IT became apparent at a significantly more hyperpolarized Vm than IA (IT=−51.8 ± 1.9 mV and IA=−36.2 ± 2.3 mV; P < 0.0005; see also Fig. 2A and B). As the Vm became more depolarized (−45 through −20 mV), IA grew in amplitude at a much larger rate than IT. Thus, at relatively hyperpolarized Vm, a relative dominance of IT over IA was observed (∼−50 mV =IT: 48.5 ± 10.1 pA, IA: 11.0 ± 4.8 pA; P < 0.05); the opposite was true at depolarized Vm (∼−25 mV =IT: 92.5 ± 32.3 pA, IA: 213.7 ± 32.5 pA; P < 0.05) (Fig. 2B and C). The competitive interaction between IT and IA was further confirmed by studies showing that pharmacological blockade of IA (5 mm 4-AP) unmasked a significantly larger IT (2-way ANOVA, F= 23.8, and 38.6, for 4-AP treatment and voltage, respectively, n= 16, P < 0.001 in both cases, Fig. 2B).
Figure 2. IA and IT overlap at similar membrane potentials.
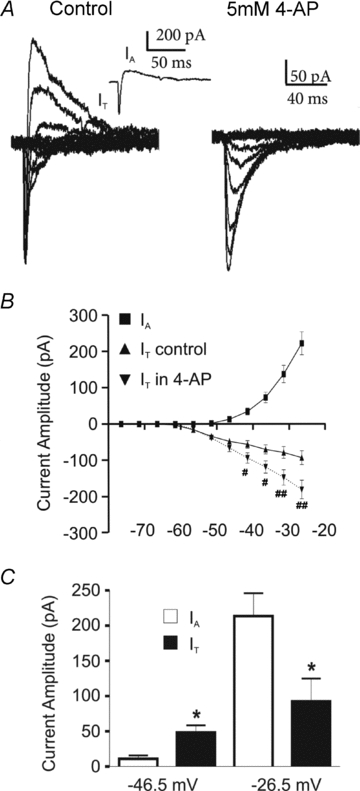
A, representative example of a PVN-RVLM neurone in which both IA and IT were simultaneously recorded at the same command potentials. Note that when the IA inhibitor 4-AP was applied, IA was blocked and IT became larger in amplitude. Inset, currents evoked at –41.5 mV are shown. B, plot of the mean IA and IT amplitude versus command potential, before and during 4-AP (n= 16; IA control: squares; IT control: triangles; IT in 4-AP: inverted triangles). #P < 0.05 and ##P < 0.001, IT control vs. IT 4-AP. C, summary of current amplitudes of IA and IT at a relatively hyperpolarized (–46.5 mV) and a depolarized (–26.5 mV) command potentials. Note that at the hyperpolarized potential the IT amplitude was larger than that of IA, whereas the opposite was observed at the more depolarized potential.
In recent studies, we showed that Kv4.3 and Cav3.1 are the K+ and Ca2+ subunits more predominantly expressed in PVN-RVLM neurones (Lee et al. 2007; Sonner et al. 2007; Sonner & Stern, 2007), likely to mediate IA and IT, respectively. To determine whether the relative expression of these subunits is an important factor influencing the IT/IA balance, we simultaneously recorded IA and IT in a subset of neurones (n= 15, note that for this analysis, data from sham and hypertensive rats were pooled) in which the cytoplasmic content was subsequently aspirated, and the Kv4.3 and Cav3.1 mRNA levels were quantitatively assessed using single-cell real-time RT-PCR (Methods). mRNA levels were quantified using a ΔCt approach (Methods). Thus, the less Cav3.1 relative to Kv4.3 mRNA expressed within a cell, the larger the ΔCt Cav3.1/Kv4.3 ratio will be. The ratio of the amplitude of the evoked IT and IA at a Vm of ∼−20 mV was plotted as a function of the Cav3.1/Kv4.3 subunit expression ratio for that particular neurone (Fig. 3A,B). As expected, a negative correlation between the IT/IA and the ΔCt Cav3.1/Kv4.3 ratios was observed (r2= 0.61; P < 0.001, Fig. 3B).
Figure 3. The relative expression of Kv4.3 and Cav3.1 mRNAs correlates with the relative balance between IA and IT.
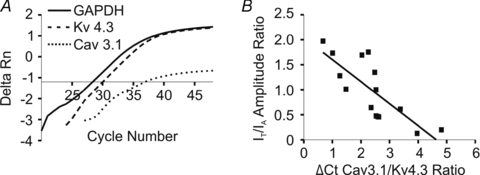
A, representative example of real-time PCR amplifications from a single PVN-RVLM neuron from a sham rat, in which the A-type channel subunit Kv4.3 (dashed line) and the T-type channel subunit Cav3.1 (dotted line) mRNAs were tested against the reference gene, GAPDH (continuous line). The cycle numbers that the respective lines crossed the x-axis are their threshold values (Ct). Note that Cav3.1 mRNA crossed the x-axis at a later cycle number than did Kv4.3, indicating less Cav3.1 mRNA expression compared to Kv4.3. B, summary of the relative expression of Cav3.1/Kv4.3 single-cell mRNA compared to the IT/IA amplitude ratio measured at –20 mV. Note that the ΔCt value is used in the plot. Thus, the less Cav3.1 relative to Kv4.3 mRNA expressed, the larger the ΔCt Cav3.1/Kv4.3 ratio will be. A significant correlation between current amplitude and mRNA expression ratios was observed (r2= 0.61, P < 0.001). For this analysis, neurons from both sham and hypertensive rats were used.
The IT/IA balance influences the expression and magnitude of low threshold spikes (LTS)
It is well established that IT mediates low threshold spikes (LTS), which promotes bursting firing patterns (Llinas & Yarom, 1981; Stern, 2001). IA, on the other hand, is known to mediate a transient outward rectification (TOR), delaying spike firing onset (Bourque, 1988; Fisher et al. 1998; Luther & Tasker, 2000). Given our results supporting the presence of both opposing transient currents in PVN-RVLM neurones, we aimed to determine whether and how their balance influenced neuronal membrane voltage behaviour. Therefore, we simultaneously recorded IA and IT in the voltage-clamp mode, and then determined whether a LTS or a TOR was observed in the current-clamp mode (Methods). In all cases where IT was more evident than IA at hyperpolarized command potentials, n= 40/49), a LTS was evoked upon membrane depolarization. Conversely, in the few cases where IA was more evident than at IT (n= 9/49), a TOR was observed (Fig. 4A). The differential incidence between LTS/IT and TOR/IA was significant (P < 0.0001, Fisher's exact test).
Figure 4. The IT/IA balance influences the expression and magnitude of LTSs.
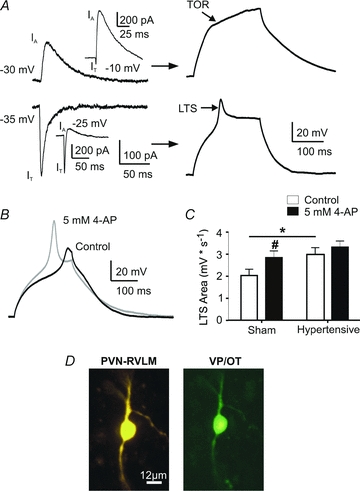
A, left, summary data showing that all neurons in which IT activated at more hyperpolarized potentials than IA showed a LTS, whereas those in which IA activated at more hyperpolarized potentials than IT showed a TOR in response to membrane depolarization. Right, representative traces for each of these conditions are shown (upper panels: more hyperpolarized IA/TOR; lower panels: more hyperpolarized IT/LTS). The insets in both cases show the presence of both currents at more depolarized command potentials. B, representative example of LTSs before (black) and after (grey) application of the IA inhibitor 4-AP (5 mm). Note the increased peak and area of the LTS in 4-AP. C, summary data of the mea LTS area in PVN-RVLM neurons from sham and hypertensive rats, before and after application of 4-AP. Note that the LTS area was significantly increased in the sham group upon 4-AP application. Also, note the larger basal area in the hypertensive group compared to sham, and the lack of 4-AP effects on the former. D, representative photomicrograph (20×) of a PVN-RVLM neuron from a hypertensive rat showing a positive vasopressin/oxytocin (VP/OT) immunoreactivity. #P < 0.05 (sham control vs. sham 4-AP); *P < 0.05 (sham control vs. hypertensive control).
We then investigated whether pharmacologically altering the IT/IA balance towards an inward current predominance affected the magnitude of the LTS. Thus, LTS properties were analysed before and after application of 5 mm 4-AP (n= 22). While the LTS threshold was not affected by IA blockade (Δ= 0.62 ± 0.65 mV, P= 0.9), both the LTS peak amplitude and area were significantly enhanced during IA blockade (200.1 ± 18.1%, P < 0.0005 and 144.9 ± 16.2%, P= 0.01, respectively) (Fig. 4B and C).
The IT/IA balance in PVN-RVLM neurones is dependent on their neurochemical phenotype
Preautonomic PVN neurones, including those innervating the RVLM, are neurochemically heterogeneous, and a proportion of them express the neurohormone vasopressin (VP) or the neurohormone oxytocin (OT) (Swanson & Sawchenko, 1983; Yang et al. 2001; Mack et al. 2002; however, see Stocker et al. 2006). Since magnocellular neurosecretory OT and VP neurones of the PVN and SON express a robust IA and prominent TOR (Fisher et al. 1998; Luther & Tasker, 2000), we hypothesized that PVN-RVLM neurones showing a relative dominance of IA over IT and a TOR displayed a VP and/or OT phenotype. Therefore, in a subset of recordings (n= 12), we simultaneously recorded IA and IT in voltage-clamp, and determined whether a TOR or a LTS was observed in current-clamp. Subsequently, the neurochemical phenotype of the recorded neurones was immunohistochemically determined, using a combination of antibodies against oxytocin and vasopressin peptides (Methods, Fig. 4D). We found all those neurones in which IA activated at a more hyperpolarized potential than IT and displayed TOR were VP/OT immunoreactive (n= 5/5) (P= 0.01, Fisher's exact test). Conversely, most neurones (n= 6/7) in which IT was evident at a more hyperpolarized potential than IA and displayed LTS failed to display VP/OT immunoreactivity.
The IT/IA balance influences LTS-mediated increases in somatic and dendritic intracellular Ca2+ levels
In addition to an increase in membrane conductance and induction of LTS, activation of T-type Ca2+ channels also results in changes in intracellular Ca2+ levels ([Ca2+]i), and activation of downstream Ca2+-dependent signals (Goldberg et al. 2004; Egger et al. 2005; Pinato & Midtgaard, 2005). Thus, in order to determine whether the IT-mediated LTS in PVN-RVLM neurones evoked changes in [Ca2+]i, and whether this was influenced by the IT/IA balance, we performed simultaneous patch-clamp recordings and confocal Ca2+ imaging. Our results indicate that LTS in PVN-RVLM neurones consistently evoked transient increases in somatic [Ca2+]i (n= 13, Fig. 5). The peak of the Ca2+ transient was delayed with respect to the LTS peak (time to peak LTS: 184.3 ± 24.3 ms; time to peak [Ca2+]i: 305.0 ± 49.4 ms, P= 0.01), and in all cases, the evoked change in [Ca2+]i persisted beyond the duration of the LTS (Fig. 5B). In general, a correlation between the LTS area and the peak of the Ca2+ transient was observed (r2= 0.7, P < 0.005, Fig. 5C). The LTS-evoked change in [Ca2+]i was blocked in nominal 0 Ca2+ ACSF, and subthreshold depolarizations failed to evoke a change in [Ca2+]i (not shown).
Figure 5. IA modulates LTS-mediated changes in intracellular Ca2+.
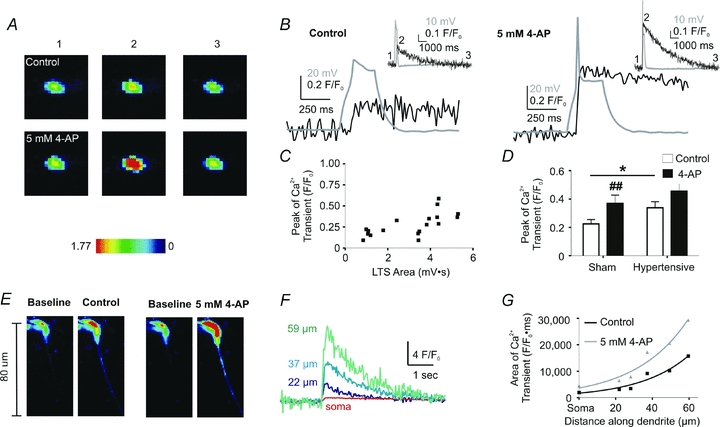
A, representative confocal images of LTS-mediated changes in somatic [Ca2+]i (pseudocolour) in a PVN-RVLM neuron from a sham rat, before and after 4-AP application. Images of the basal [Ca2+]i (1), maximum peaks (2) and subsequent return to basal levels (3) are displayed (scale units are F/F0). B, representative examples of an evoked LTS (grey) and the resulting change in [Ca2+]i (black), before (left) and after 4-AP application (right). Inset, same traces shown at a more expanded time scale. Note that the numbers correspond to the images shown in A. C, plot showing the relationship of the LTS area and the peak of the LTS-evoked Ca2+ transient. A significant positive correlation was observed (r2= 0.7, P < 0.005). Neurons from sham and hypertensive control rats were plotted together. D, summary data of the mean peak of the Ca2+ transient from PVN-RVLM neurons in sham and hypertensive PVN-RVLM rats, before and after 4-AP application. ##P < 0.01 (sham control vs. sham 4-AP); *P < 0.05 (sham control vs. hypertensive control). E, representative confocal images (pseudocolour) of dendritic, LTS-mediated changes in [Ca2+]i before and after 4-AP application. F, representative traces of Ca2+ transients measured at various dendritic distances from the soma of the neuron in A. Note that the magnitude of the Ca2+ transient increased as a function of the distance from the soma. G, summary plot of the area of the Ca2+ transient along the dendritic process, before and after addition of 4-AP. Distances measured along the dendrites were binned in tens of micrometres.
When the IT/IA balance was pharmacologically shifted towards a predominance of inward currents (5 mm 4-AP), the LTS-evoked change in [Ca2+]i was significantly enhanced (P < 0.01, Fig. 5D). On the other hand, the kinetics of the changes in [Ca2+]i (i.e. rise and decay time courses) were not affected by 4-AP (not shown).
In a few instances, relatively long dendritic processes efficiently loaded with Fluo-5 (n= 3) were contained within the same focal plane as the recorded soma. In those cases, we found the somatically evoked LTS to efficiently increase [Ca2+]i along the dendritic process. As shown in the representative example in Fig. 5E, the magnitude of the Ca2+ transient increased as a function of the distance from the soma (P < 0.0001, 2-way ANOVA), and was significantly enhanced in the presence of 4-AP (P < 0.001, 2-way ANOVA) (Fig. 5F and G). In both cases, changes in [Ca2+]i along the dendritic process were best fitted by a monoexponential function (control: r2= 0.94; 4-AP: r2= 0.95).
A shift in the IT/IA balance contributes to enhanced excitability and firing activity in hypertensive rats
Our studies show that a pharmacological shift in the subthreshold balance of IT/IA towards a predominance of IT resulted in enhanced membrane excitability and somatodendritic Ca2+ transients. Based on these findings, we explored whether similar changes in the IT/IA balance could occur intrinsically in a pathophysiological condition (i.e. renovascular hypertension), and whether such imbalance contributed to aberrant neuronal excitability during this condition. We found IT current amplitude and density to be enhanced in PVN-RVLM neurones from hypertensive rats (P < 0.0005 in both cases, 2-way ANOVA, Fig. 6A). No differences in IT voltage-dependent activation properties or kinetic properties were observed between sham and hypertensive rats (not shown). Along with our recent studies showing a diminished IA availability in these neurones during hypertension (Sonner et al. 2008), these results altogether support a shift in the IT/IA balance towards a predominance of IT in hypertensive rats.
Figure 6. Altered IT current magnitude, Cav3.1 and Kv4.3 subunits mRNA expression in PVN-RVLM neurons during hypertension.
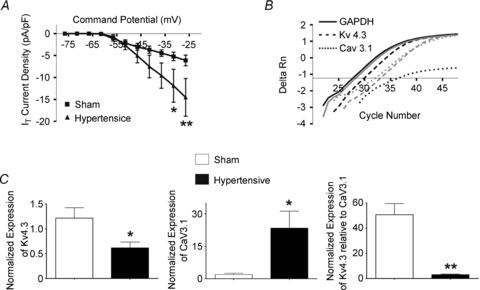
A, mean plots of IT current densities in PVN-RVLM neurons from sham (squares) and hypertensive (triangles) rats. B, representative examples of single-cell, real-time PCR amplifications for Kv4.3 (dashed line), Cav3.1 (dotted line) and GAPDH (whole line) obtained from a PVN-RVLM neuron in sham rat (black) and a PVN-RVLM neuron in a hypertensive rat (grey). C, summary data of the normalized expression of single-cell Kv4.3 mRNA (left) and single-cell Cav3.1 mRNA (middle). The normalized expression of Kv4.3 relative to Cav3.1 within individual cells is displayed in the right panel. Note that the expression of Kv4.3 mRNA was reduced while Cav3.1 mRNA expression was enhanced in neurons from hypertensive rats. Also, note the significant reduction in the relative expression of Kv4.3 to Cav3.1 within individual neurons during hypertension. *P < 0.05, **P < 0.0001.
In agreement with diminished IA and enhanced IT magnitudes, results from single-cell RT-PCR studies in identified PVN-RVLM neurones from control and hypertensive rats (n= 20 and 31 neurones in each group, respectively) indicated a robust reduction in the relative expression levels of Kv4.3 mRNA, and an increase relative expression of Cav3.1 mRNA in hypertensive when compared to sham rats (P < 0.05 in both cases) (Fig. 6B and C). In fact, a nearly 15-fold decrease in the relative expression of Kv4.3 to Cav3.1 mRNA within individual PVN-RVLM neurones was observed in hypertensive, when compared to sham rats (P < 0.0001).
The magnitude of the evoked LTS was significantly larger in PVN-RVLM neurones from hypertensive (n= 24) when compared to control rats (P < 0.05, Fig. 4C). Moreover, blockade of IA in hypertensive rats, in contrast to what was observed in controls, failed to further increase the LTS magnitude (3.3 ± 8.1%, P= 0.4) (Fig. 4C). Likewise, the magnitude of the LTS-evoked change in [Ca2+]i was significantly larger in PVN-RVLM neurones from hypertensive (n= 11) when compared to control rats (P < 0.05, Fig. 5D), and blockade of IA in hypertensive rats failed to further increase the magnitude of the evoked Ca2+ transient (P > 0.1, Fig. 5D). Taken together, these results support an imbalanced IT/IA relationship during hypertension, resulting in an enhanced LTS and associated changes in [Ca2+]i.
Since IT has been shown to regulate spontaneous activity in a variety of CNS neurones (Huguenard, 1996; Raman & Bean, 1999), we addressed whether the subthreshold IT/IA imbalance resulted in and/or contributed to increased PVN-RVLM firing activity in hypertensive rats. All recorded neurons in control and hypertensive rats were spontaneously active. However, PVN-RVLM basal spontaneous firing frequency was significantly higher in hypertensive when compared to control rats (n= 8 and 12, respectively, P < 0.05) (Fig. 7). Bath application of NiCl2 (100 μm), which at this concentration more efficiently blocks IT over high threshold voltage-activated Ca2+ currents (Fox et al. 1987; Narahashi et al. 1987; Hagiwara et al. 1988; Gu & Spitzer, 1993), failed to affect firing discharge in control rats (P= 0.4). On the other hand, the firing rate in neurones from hypertensive rats was significantly reduced in the presence of NiCl2 (P < 0.005), to a value not significantly different from that observed in control rats (P > 0.4). These results support an enhanced IT contribution to spontaneous firing activity in PVN-RVLM neurones from hypertensive rats.
Figure 7. IT contributes to enhanced PVN-RVLM firing activity in hypertensive rats.
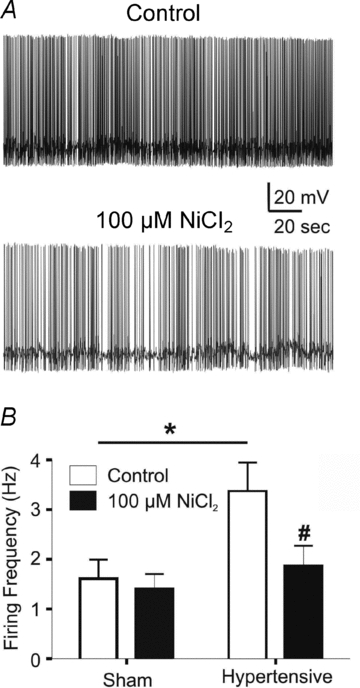
A, representative traces of spontaneous firing activity in a PVN-RVLM neuron from a hypertensive rat, before and after addition of 100 μm NiCl2. B, summary data of mean spontaneous firing activity in PVN-RVLM neurons from sham and hypertensive rats before and after NiCl2 application. #P < 0.05 (hypertensive control vs. hypertensive NiCl2); *P < 0.05 (sham control vs. hypertensive control).
Discussion
The main findings that emerge from our studies can be summarized as follows: (a) the subthreshold currents IA and IT are both present in PVN-RVLM neurones, and display overlapping voltage-dependent and kinetic properties; (b) The relative predominance of each current at hyperpolarized membrane potentials dictates the membrane behaviour upon depolarization (i.e. expression of LTS or TOR); (c) The IT/IA balance within individual neurones correlated with the relative expression of Cav3.1 and Kv4.3 mRNA within the same neurone; (c) pharmacological blockade of IA unmasked a larger IT, resulting in an enhanced IT-mediated LTS as well as LTS-mediated somatodendritic Ca2+ transients; (d) in hypertensive rats, there is a shift in the IT/IA balance towards an IT predominance – this shift is in part mediated by a diminished and enhanced Kv4.3 and Cav3.1 mRNA subunit expression, respectively, and the imbalanced IT/IA relationship results in an enhanced LTS, LTS-mediated somatodendritic Ca2+ transients, and overall increased PVN-RVLM firing activity in hypertensive rats.
Taken together, our results support that a balanced relationship between IA and IT influences membrane excitability and Ca2+ dynamics in PVN-RVLM neurones. Moreover, an imbalanced interaction between these two opposing currents, favouring IT, results in enhanced neuronal excitability and firing discharge in hypertensive rats, constituting thus an important underlying mechanism contributing to the characteristic sympathoexcitation observed in this disease.
Interplay between IA and IT within individual PVN-RVLM neurones
IA and IT are critical inhibitory and excitatory conductances regulating the degree and pattern of spiking activity in the CNS (Segal et al. 1984; Rogawski, 1985; Rudy, 1988; McCobb & Beam, 1991; Raman & Bean, 1999; Stern, 2001). In the PVN, both have been shown to play important roles in influencing neuroendocrine and preautonomic neurones. Elegant work by Tasker and colleagues showed that while both IA and IT are present in magnocellular and parvocellular neuronal types, differences in their relative expression and voltage-dependent properties determine the distinctive membrane properties in these functionally distinct neuronal populations. Thus, magnocellular neurones predominantly express an IA-mediated transient outward rectification (TOR) (Bourque & Renaud, 1991; Stern & Armstrong, 1995; Luther & Tasker, 2000), whereas parvocellular neurones express an IT-dependent LTS (Luther & Tasker, 2000). In line with these studies, we reported PVN-RVLM neurones to also express both types of subthreshold conductances (Stern, 2001; Sonner & Stern, 2006). Thus, whereas IT mediates LTS and promotes bursting firing activity, we found IA to regulate action potential waveform and interspike interval in PVN-RVLM neurones. However, whether and how these two opposing conductances interact with each other, and what the impact of such interplay is on membrane excitability in presympathetic PVN neurones, has not been established. To this end, we simultaneously measured IA and IT in identified PVN-RVLM neurones, and evaluated how their interactions influenced basic membrane properties, intracellular Ca2+ dynamics and firing activity, under both physiological and pathological conditions.
Our results support an active interplay between IA and IT in presympathetic PVN neurones. Firstly, both currents displayed overlapping voltage-dependent and kinetic properties. Secondly, pharmacological block of IA unmasked and/or increased IT magnitude, resulting in turn in larger and prolonged IT-mediated LTS, as well as larger LTS-mediated somatodendritic Ca2+ transients. In a recent study, 4-AP at the concentration used in this study, was reported to directly potentiated high threshold voltage-dependent Ca2+ currents in acutely dissociated spinal cord neurons (Wu et al. 2009). On the other hand, low-threshold T-type currents were unaffected. Thus, it is unlikely that the enhanced IT and the associated LTS reported in our study resulted from a direct action of 4-AP on the T-type channels. Taken together, these results suggest that IA and IT can precisely influence each other's availability over a voltage-range near spike threshold, in a way that, for example, diminished or absent IA will result in the additional recruitment of IT, and consequently a stronger depolarizing drive and increased probability for action potential firing. These results are in agreement with studies in other CNS areas (Pape et al. 1994; Meis et al. 1996; Cavelier et al. 2003; Molineux et al. 2005).
In the majority of PVN-RVLM neurones (81%), and in response to depolarizing steps, IT became evident at a more hyperpolarized membrane potential than IA. This pattern corresponded with the predominant and/or exclusive expression of a LTS. Conversely, in the rest of the neurones, IA became evident before IT, which corresponded with the expression of a TOR, rather than a LTS. Thus, the relative predominance of IT over IA at hyperpolarized membrane potentials seems to strongly influence whether a LTS or a TOR is expressed in PVN-RVLM neurones. Numerous factors could influence the balance or predominance of one conductance over the other. Firstly, it has been suggested that differences in activation threshold can shift the balance between IA and IT (Pape et al. 1994). Our results show that in the majority of cells, IT activates at a more hyperpolarized potential than IA, which may account for the larger proportion of cells expressing LTS that we observed. Secondly, we found a positive correlation between the relative expression of Cav3.1/Kv4.3 mRNA subunits and IT/IA amplitude within single cells. Thus, it could be argued that differences in the relative expression and densities of channels underlying IT and IA may influence the ability of PVN-RVLM neurones to express an excitatory (LTS) or inhibitory (TOR) membrane behaviour. Finally, another factor that could influence the IT/IA balance is the relative distribution of the underlying channels along the somatodendritic plasma membrane. While numerous studies support a heterogeneous distribution of Ca2+ and K+ channels along dendritic trees in various neuronal types (Magee & Johnston, 2005; Nusser, 2009), no information is currently available on topographical distribution of active conductances in PVN-RVLM neurones.
Importantly, we found the IT/IA balance and its impact on membrane properties to be in part dependent on, or correspondent to, the neurochemical phenotype of PVN-RVLM neurones. Preautonomic PVN neurones are known to be neurochemically heterogeneous, with a proportion of them expressing the neuropeptides OT or VP (Swanson & Sawchenko, 1983; Yang et al. 2001; Mack et al. 2002). Surprisingly, our studies indicate that the majority of PVN-RVLM neurones in which IA activated at a more hyperpolarized Vm than IT, and expressed TOR, were immunoreactive for VP/OT. Given the well-established presence of IA and TOR in magnocellular neurosecretory neurones (Armstrong & Stern, 1998a,b; Fisher et al. 1998; Luther & Tasker, 2000), it seems that OT and VP neurones in the SON and PVN, regardless of their functional roles (i.e. presympathetic or neurosecretory), share a similar IT/IA balance and membrane properties.
Functional implications of IT/IA interplay in PVN-RVLM neurones
In addition to dictating whether a LTS or a TOR is expressed upon membrane depolarization, subtle changes in the IT/IA balance may affect the magnitude of these membrane properties. Thus, as previously demonstrated in thalamic neurones of the lateral geniculate nucleus (Pape et al. 1994), we found pharmacological blockade of IA to significantly amplify and/or prolong the IT-dependent LTS magnitude.
Our results also suggest that in addition to affecting membrane potential and firing patterns (i.e. tonic and bursting) (Stern, 2001; Lee et al. 2008), the LTS also influences somatic and dendritic Ca2+ dynamics in PVN-RVLM. This is indicated by our results showing that the LTS, even in the absence of functional Na+ channels, is sufficient to induce an increase in [Ca2+]i, which can propagate along neuronal dendrites. A similar phenomenon was recently reported in olfactory bulb granule cells and in cortical interneurones (Goldberg et al. 2004; Egger et al. 2005). We found the magnitude of the dendritic LTS-evoked Ca2+ transient to increase as a function of the distance from the soma. A possible interpretation is that IT channels underlying the LTS are expressed at increasingly higher densities along the dendritic trees, as observed in other CNS bursting neurones (Destexhe et al. 1998; Sherman, 2001; Goldberg et al. 2004). Alternatively, our results could be confounded by a progressive decrease in dendritic volume with distance from the soma, which is expected to result in an enhanced Ca2+ transient (Komendantov et al. 2007).
Importantly, we found pharmacological blockade of IA to result in larger LTS-mediated Ca2+ transients, in both somata and dendrites of PVN-RVLM. These results suggest that IA plays an important role in antagonizing LTS-dependent Ca2+ signalling in these neurones. A similar LTS-mediated Ca2+ signalling has been shown to be important for boosting distal synaptic inputs (Goldberg et al. 2004) and for lateral inhibition in the olfactory bulb (Egger et al. 2005). While the functional significance of the LTS-mediated Ca2+ signalling in PVN-RVLM neurones is at present unclear, our studies suggest that through this mechanism, these neurones have at least the potential for a subthreshold form of associative plasticity, in which the efficacy of incoming dendritic synaptic inputs may be influenced by recent neuronal activity.
Taken together, our results support that in PVN-RVLM neurones, IA and IT act in a concerted manner to regulate subthreshold membrane excitability, action potential firing degree/pattern, as well as somatic and dendritic intracellular Ca2+ dynamics. Moreover, they suggest that a diminished IA availability, as a result of a pharmacological blockade, or alternatively, during a pathological condition, could shift the IT/IA balance towards a predominance of IT, resulting in an overall enhanced excitatory drive
Altered IT/IA balance contributes to increased neuronal activity in hypertensive rats
Sympathoexcitation of a central origin is characteristic of hypertension (Judy et al. 1976; Guyenet, 2006), and mounting evidence supports an important role for the PVN in enhanced sympathetic outflow in this disease (Earle et al. 1992; Earle & Pittman, 1995; Jung et al. 2004). Moreover, increased neuronal excitability, associated with elevated blood pressure and sympathetic activity, has recently been demonstrated in PVN-RVLM neurones (Allen, 2002; Li & Pan, 2006). While alterations in various PVN neurotransmitter systems have been reported during hypertension (Petty & Reid, 1977; Martin & Haywood, 1998; Haywood et al. 2001; Jung et al. 2004), the contribution of altered intrinsic membrane properties to PVN neuronal hyperactivity, during hypertension, remains less investigated. In a recent study, we demonstrated an overall reduction in the magnitude of IA in PVN-RVLM neurones in renovascular hypertensive rats, which contributed to their enhanced excitability in this condition (Sonner et al. 2008). In addition to the previously demonstrated enhanced voltage-dependent inactivation and diminished single channel conductance, our present studies support a diminished expression of Kv4.3 mRNA, the most abundant subunit expressed in PVN-RVLM neurones (Sonner & Stern, 2007), as another contributing factor to blunted IA function in PVN-RVLM neurones during hypertension.
Importantly, we found in the present study the current amplitude and density of IT to be enhanced during hypertension. Moreover, expression of Cav3.1 mRNA, the subunit underlying IT and LTS in PVN neurones (Lee et al. 2008), was elevated in PVN-RVLM neurones in hypertensive rats. Thus, a diminished IA along with an increased IT magnitude indicates a shift in the IT/IA balance towards an inward current predominance during hypertension. This is supported by our results showing an augmented magnitude of the IT-mediated LTS, as well as the LTS-mediated increase in intracellular Ca2+ levels in PVN-RVLM neurones in hypertensive rats. The blunted effect of 4-AP on the LTS and LTS-mediated changes in intracellular Ca2+ levels in hypertensive rats supports a contribution of the diminished IA to the IT predominance in this condition. Finally, a shift in the IT/IA balance in hypertensive rats is supported by our single-cell RT-PCR studies, showing that the relative expression of Kv4.3 to Cav3.1 mRNA in a given cell was significantly reduced during hypertension, shifting from a Kv4.3 predominance towards an equal relative expression of both subunits.
In addition to mediating LTSs, IT has been shown to regulate spontaneous firing activity in some CNS neurones (Huguenard, 1996; Raman & Bean, 1999). Thus, we aimed to determine whether the shift in the IT/IA balance towards an IT predominance contributed to an enhanced ongoing firing discharge of PVN-RVLM neurones during hypertension (Allen, 2002; Li & Pan, 2006; Sonner et al. 2008). Our studies indicate that in hypertensive rats, the basal spontaneous firing activity in PVN-RVLM neurones was significantly increased when compared to sham rats. Moreover, a low concentration of NiCl2 (100 μm), which more selectively blocks IT over high-threshold voltage-dependent Ca2+ channels (Fox et al. 1987; Gu & Spitzer, 1993), significantly reduced PVN-RVLM firing activity in hypertensive rats, to levels observed in sham rats. Conversely, NiCl2 had no effect on PVN-RVLM firing discharge in sham rats. Taken together, these results support that under normal conditions, IT does not contribute to ongoing PVN-RVLM firing activity. On the other hand, the IT/IA imbalance in hypertensive rats resulted in a larger IT predominance, contributing in turn to the elevated spontaneous firing activity observed in this condition. A similar IT/IA imbalance was shown to contribute to altered neuronal activity in epileptic conditions (Meis et al. 1996). While low micromolar NiCl2 concentations more selectively blocks IT over high-threshold voltage-dependent Ca2+ channels (Fox et al. 1987; Narahashi et al. 1987; Hagiwara et al. 1988; Gu & Spitzer, 1993), the R-type high threshold voltage-gated Ca2+ current could have also be affected by NiCl2 (Lee et al. 1999). Thus, while R-type currents have not yet been reported in PVN neurons, we cannot completely rule out their involvement in the NiCl2 effects reported here.
It is important to take into consideration that, given the absence of peripheral afferent inputs in the slice preparation, the reported firing activities in these studies reflect only the actions of intrinsic properties and local synaptic inputs preserved in this in vitro preparation. Nonetheless, the reported changes in intrinsic neuronal properties reported here are expected to also affect neuronal responsiveness to activation of such afferent inputs.
The underlying mechanisms leading to the IT/IA imbalance reported here are at present unknown. Angiotensin II (AngII), however, stands as a likely candidate. Within the PVN, AngII has been shown to increase sympathetic activity (Chen & Toney, 2001; Li et al. 2006), and abundant evidence supports enhanced PVN AngII activity during hypertension (Gutkind et al. 1988; Jung et al. 2004). Importantly, AngII has been shown to inhibit IA (Nagatomo et al. 1995; Li & Ferguson, 1996; Wang et al. 1997) and to enhance the IT-dependent LTS activity (Spanswick & Renaud, 2005). Moreover, AngII has been shown to diminish IA current amplitude and diminish Kv4.3 mRNA in the RVLM of rats with congestive heart failure (Gao et al. 2006). Thus, future studies evaluating the contribution of AngII to altered IT/IA balance in PVN-RVLM neurones during hypertension are warranted.
In summary, our results support a dynamic interplay between the subthreshold currents IA and IT in the control of PVN-RVLM membrane properties, firing activity and intracellular Ca2+ dynamics. Moreover, our studies support an imbalanced IT/IA interplay, favouring IT, to contribute to increased neuronal excitability and firing discharge in PVN-RVLM neurones in renovascular hypertensive rats.
Acknowledgments
This work was supported by NIH R01 HL68725 (J.E.S.) and the National Research Foundation Grant 2010-0015531 (S.Y.L.).
Glossary
Abbreviations
- 4-AP
4 aminopyridine
- IA
A-type potassium current
- IT
T-type calcium current
- LTS
low-threshold spike
- OT
oxytocin
- PVN
paraventricular nucleus
- RVLM
rostral ventrolateral medulla
- TEA
tetraethylammonium
- TOR
transient outward rectification
- VP
vasopressin
Author contributions
P.M.S.: collection, analysis and interpretation of electrophysiological, immunohistochemical and confocal imaging data; drafting of the article. S.L.: collection and analysis of PCR data. S.Y.L.: conception and design of PCR studies, interpretation of data, critical revision of the manuscript for intellectual content. P.D.R.: conception and design of PCR studies, interpretation of data, critical revision of the manuscript for intellectual content. J.E.S.: conception and design of electrophysiological and PCR studies; interpretation of data; drafting of the article. All authors approved the final version of the manuscript. This work was done at the Medical College of Georgia.
Author's present address
P. M. Sonner: Department of Neuroscience, Cell Biology and Physiology, Wright State University, Dayton, OH, USA.
References
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Stern JE. Electrophysiological distinctions between oxytocin and vasopressin neurons in the supraoptic nucleus. Adv Exp Med Biol. 1998a;449:67–77. doi: 10.1007/978-1-4615-4871-3_7. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Stern JE. Phenotypic and state-dependent expression of the electrical and morphological properties of oxytocin and vasopressin neurones. Prog Brain Res. 1998b;119:101–113. doi: 10.1016/s0079-6123(08)61564-2. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Res. 1991;540:349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- Carvalho TH, Bergamaschi CT, Lopes OU, Campos RR. Role of endogenous angiotensin II on glutamatergic actions in the rostral ventrolateral medulla in Goldblatt hypertensive rats. Hypertension. 2003;42:707–712. doi: 10.1161/01.HYP.0000086524.35251.2D. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Desplantez T, Beekenkamp H, Bossu JL. K+ channel activation and low-threshold Ca2+ spike of rat cerebellar Purkinje cells in vitro. Neuroreport. 2003;14:167–171. doi: 10.1097/00001756-200302100-00001. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Caverson MM, Calaresu FR. Lateral hypothalamic and peripheral cardiovascular afferent inputs to ventrolateral medullary neurons. Brain Res. 1985;347:173–176. doi: 10.1016/0006-8993(85)90908-4. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Phyiol Regul Integr Comp Physiol. 2001;281:R1844–1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Czachurski J, Dembowsky K, Goodchild AK, Seller H. Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst. 1987;20:73–86. doi: 10.1016/0165-1838(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle ML, Boorman R, Takahashi Y, Pittman QJ. Lesions of the paraventricular nucleus alter the development and intensity of chronic renal hypertension. Can J Physiol Pharmacol. 1992;70:Avii–Aviii. [Google Scholar]
- Earle ML, Pittman QJ. Involvement of the PVN and BST in 1K1C hypertension in the rat. Brain Res. 1995;669:41–47. doi: 10.1016/0006-8993(94)01222-4. [DOI] [PubMed] [Google Scholar]
- Egger V, Svoboda K, Mainen ZF. Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J Neurosci. 2005;25:3521–3530. doi: 10.1523/JNEUROSCI.4746-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Kaye D. Increased sympathetic nervous system activity and its therapeutic reduction in arterial hypertension, portal hypertension and heart failure. J Auton Nerv Syst. 1998;72:210–219. doi: 10.1016/s0165-1838(98)00107-6. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Voisin DL, Bourque CW. Density of transient K+ current influences excitability in acutely isolated vasopressin and oxytocin neurones of rat hypothalamus. J Physiol. 1998;511:423–432. doi: 10.1111/j.1469-7793.1998.423bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Galarraga E, Pineda JC, Bargas J. Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol. 1994;72:2246–2256. doi: 10.1152/jn.1994.72.5.2246. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Wang W, Mann E, Finch M, Li Y, Liu D, Schultz HD, Zucker IH. Sympathoexcitation in chronic heart failure: AngII induced inhibition of voltage-gated K+ channel, an in vivo and in vitro study. FASEB J. 2006;20:A1202–A1203. [Google Scholar]
- Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J Neurosci. 1993;13:4936–4948. doi: 10.1523/JNEUROSCI.13-11-04936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. γ-Aminobutyric acid (GABA)-A function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension. 2001;37:614–618. doi: 10.1161/01.hyp.37.2.614. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Sugiura Y, Okado N, Loewy AD, Kohno K. Descending input from the hypothalamic paraventricular nucleus to sympathetic preganglionic neurons in the rat. Exp Brain Res. 1991;85:10–20. doi: 10.1007/BF00229982. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976;38:21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- Jung JY, Lee JU, Kim WJ. Enhanced activity of central adrenergic neurons in two-kidney, one clip hypertension in Sprague-Dawley rats. Neurosci Lett. 2004;369:14–18. doi: 10.1016/j.neulet.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Komendantov AO, Trayanova NA, Tasker JG. Somato-dendritic mechanisms underlying the electrophysiological properties of hypothalamic magnocellular neuroendocrine cells: a multicompartmental model study. J Comput Neurosci. 2007;23:143–168. doi: 10.1007/s10827-007-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Hagiwara Y, Sekiya D, Chiba S, Fukumori R. Cholinergic inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Brain Res Bull. 2000;53:275–282. doi: 10.1016/s0361-9230(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lambert RC, McKenna F, Maulet Y, Talley EM, Bayliss DA, Cribbs LL, Lee JH, Perez-Reyes E, Feltz A. Low-voltage-activated Ca2+ currents are generated by members of the CavT subunit family (α1G/H) in rat primary sensory neurons. J Neurosci. 1998;18:8605–8613. doi: 10.1523/JNEUROSCI.18-21-08605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Han TH, Ryu PD, Lee SY. 2007 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. Molecular identification of ion channels for electrophysiological properties of rat hypothalamic neurons. Programme No. 784.716. [Google Scholar]
- Lee S, Han TH, Sonner PM, Stern JE, Ryu PD, Lee SY. Molecular characterization of T-type Ca2+ channels responsible for low threshold spikes in hypothalamic paraventricular nucleus neurons. Neuroscience. 2008;155:1195–1203. doi: 10.1016/j.neuroscience.2008.06.055. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1110–1119. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- Li M, Hansen JB, Huang L, Keyser BM, Taylor JT. Towards selective antagonists of T-type calcium channels: design, characterization and potential applications of NNC 55-0396. Cardiovasc Drug Rev. 2005;23:173–196. doi: 10.1111/j.1527-3466.2005.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience. 2003;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Inegr Comp Physiol. 2006;290:R1035–1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: modulation of IA by angiotensin II. Neuroscience. 1996;71:133–145. doi: 10.1016/0306-4522(95)00434-3. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res. 1988;454:123–130. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2000;523:193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr Opin Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1523–1529. doi: 10.1152/ajpregu.1998.275.5.R1523. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707–719. doi: 10.1161/01.hyp.17.5.707. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Beam KG. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991;7:119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- Meis S, Biella G, Pape HC. Interaction between low voltage-activated currents in reticular thalamic neurons in a rat model of absence epilepsy. Eur J Neurosci. 1996;8:2090–2097. doi: 10.1111/j.1460-9568.1996.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Molineux ML, Fernandez FR, Mehaffey WH, Turner RW. A-type and T-type currents interact to produce a novel spike latency-voltage relationship in cerebellar stellate cells. J Neurosci. 2005;25:10863–10873. doi: 10.1523/JNEUROSCI.3436-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human α1G subunit that forms T-type calcium channels. J Biol Chem. 2000;275:6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- Nagatomo T, Inenaga K, Yamashita H. Transient outward current in adult rat supraoptic neurones with slice patch-clamp technique: inhibition by angiotensin II. J Physiol. 1995;485:87–96. doi: 10.1113/jphysiol.1995.sp020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Tsunoo A, Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto RW. Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med. 2004;116(Suppl 5A):11S–22S. doi: 10.1016/j.amjmed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. [Google Scholar]
- Pape HC, Budde T, Mager R, Kisvarday ZF. Prevention of Ca2+-mediated action potentials in GABAergic local circuit neurones of rat thalamus by a transient K+ current. J Physiol. 1994;478:403–422. doi: 10.1113/jphysiol.1994.sp020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Hamada T, Sakuma Y. Single-cell real-time quantitative polymerase chain reaction of immunofluorescently identified neurons of gonadotropin-releasing hormone subtypes in cichlid fish. Endocrinology. 2003;144:3297–3300. doi: 10.1210/en.2003-0386. [DOI] [PubMed] [Google Scholar]
- Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- Petty MA, Reid JL. Changes in noradrenaline concentration in brain stem and hypothalamic nuclei during the development of renovascular hypertension. Brain Res. 1977;136:376–380. doi: 10.1016/0006-8993(77)90814-9. [DOI] [PubMed] [Google Scholar]
- Pinato G, Midtgaard J. Dendritic sodium spikelets and low-threshold calcium spikes in turtle olfactory bulb granule cells. J Neurophysiol. 2005;93:1285–1294. doi: 10.1152/jn.00807.2004. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Segal M, Rogawski MA, Barker JL. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984;4:604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sonner P, Stern JE. 2006 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006. Functional interactions between subthreshold A-type potassium (IA) and T-type calcium currents (IT) in RVLM-projecting PVN neurons in normotensive and hypertensive rats. Programme No. 454.11. [Google Scholar]
- Sonner PM, Filosa JA, Stern JE. 2007 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. Activity-dependent changes in intracellular Ca2+ dynamics in hypothalamic presympathetic PVN neurons. Programme No. 85.88. [Google Scholar]
- Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol. 2008;586:1605–1622. doi: 10.1113/jphysiol.2007.147413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol. 2007;582:1219–1238. doi: 10.1113/jphysiol.2007.134379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Renaud LP. Angiotensin II induces calcium-dependent rhythmic activity in a subpopulation of rat hypothalamic median preoptic nucleus neurons. J Neurophysiol. 2005;93:1970–1976. doi: 10.1152/jn.00769.2004. [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Dampney RA. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sumners C, Posner P, Gelband CH. A-type K+ current in neurons cultured from neonatal rat hypothalamus and brain stem: modulation by angiotensin II. J Neurophysiol. 1997;78:1021–1029. doi: 10.1152/jn.1997.78.2.1021. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Li DP, Chen SR, Pan HL. Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel β subunit. J Biol Chem. 2009;284:36453–36461. doi: 10.1074/jbc.M109.075523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res. 2001;908:99–103. doi: 10.1016/s0006-8993(01)02593-8. [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Mayhan WG, Bidasee KR, Patel KP. Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R992–R1002. doi: 10.1152/ajpregu.00363.2005. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol. 2004;97:1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]


