Abstract
During constant work rate (CWR) exercise above the lactate threshold (LT), the exponential kinetics of oxygen uptake (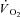 ) are supplemented by a
) are supplemented by a 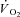 slow component (
slow component (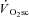 ) which reduces work efficiency. This has been hypothesised to result from ‘fatigue and recruitment’, where muscle fatigue during supra-LT exercise elicits recruitment of additional, but poorly efficient, fibres to maintain power production. To test this hypothesis we characterised changes in the power–velocity relationship during sub- and supra-LT cycle ergometry in concert with
) which reduces work efficiency. This has been hypothesised to result from ‘fatigue and recruitment’, where muscle fatigue during supra-LT exercise elicits recruitment of additional, but poorly efficient, fibres to maintain power production. To test this hypothesis we characterised changes in the power–velocity relationship during sub- and supra-LT cycle ergometry in concert with 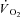 kinetics. Eight healthy participants completed a randomized series of 18 experiments consisting of: (1) a CWR phase of 3 or 8 min followed immediately by; (2) a 5 s maximal isokinetic effort to characterize peak power at 60, 90 and 120 rpm. CWR bouts were: 20 W (Con); 80% LT (Mod); 20%Δ (H); 60%Δ (VH); where Δ is the difference between the work rate at LT and
kinetics. Eight healthy participants completed a randomized series of 18 experiments consisting of: (1) a CWR phase of 3 or 8 min followed immediately by; (2) a 5 s maximal isokinetic effort to characterize peak power at 60, 90 and 120 rpm. CWR bouts were: 20 W (Con); 80% LT (Mod); 20%Δ (H); 60%Δ (VH); where Δ is the difference between the work rate at LT and 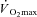 . The
. The 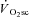 was 238 ± 128 and 686 ± 194 ml min−1 during H and VH, with no discernible
was 238 ± 128 and 686 ± 194 ml min−1 during H and VH, with no discernible 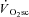 during Mod. Peak power in Con was 1025 ± 400, 1219 ± 167 and 1298 ± 233 W, at 60, 90 and 120 rpm, respectively, and was not different after Mod (P > 0.05). Velocity-specific peak power was significantly reduced (P < 0.05) by 3 min of H (−103 ± 46 W) and VH (−216 ± 60 W), with no further change by 8 min. The
during Mod. Peak power in Con was 1025 ± 400, 1219 ± 167 and 1298 ± 233 W, at 60, 90 and 120 rpm, respectively, and was not different after Mod (P > 0.05). Velocity-specific peak power was significantly reduced (P < 0.05) by 3 min of H (−103 ± 46 W) and VH (−216 ± 60 W), with no further change by 8 min. The 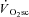 was correlated with the reduction in peak power (R2= 0.49; P < 0.05). These results suggest that muscle fatigue is requisite for the
was correlated with the reduction in peak power (R2= 0.49; P < 0.05). These results suggest that muscle fatigue is requisite for the 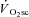 . However, the maintenance of velocity-specific peak power between 3 and 8 min suggests that progressive muscle recruitment is not obligatory. Rather, a reduction in mechanical efficiency in fatigued fibres is implicated.
. However, the maintenance of velocity-specific peak power between 3 and 8 min suggests that progressive muscle recruitment is not obligatory. Rather, a reduction in mechanical efficiency in fatigued fibres is implicated.
Non-technical summary
The mechanisms determining exercise intolerance are poorly understood. A reduction in work efficiency in the form of an additional energy cost and oxygen requirement occurs during high-intensity exercise and contributes to exercise limitation. Muscle fatigue and subsequent recruitment of poorly efficient muscle fibres has been proposed to mediate this decline. These data demonstrate in humans, that muscle fatigue, generated in the initial minutes of exercise, is correlated with the increasing energy demands of high-intensity exercise. Surprisingly, however, while muscle fatigue reached a plateau, oxygen uptake continued to increase throughout 8 min of exercise. This suggests that additional recruitment of inefficient muscle fibres may not be the sole mechanism contributing to the decline in work efficiency during high-intensity exercise.
Introduction
In the steady state of constant work rate exercise, ATP provision is met mainly by aerobic metabolism. Immediately following exercise onset, however, the rate of oxidative phosphorylation (and subsequently pulmonary O2 uptake (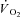 )) responds with finite kinetics. During this ∼2–3 min period (for moderate-intensity work rates below the lactate threshold (LT)) energy provision is supplemented by substrate level phosphorylation, which is both limited in its capacity and has deleterious consequences for the maintenance of muscle function. At work rates above LT, however, the attainment of a steady state is delayed even further, which necessitates additional and progressive anaerobic contributions to the energy transfer. Here,
)) responds with finite kinetics. During this ∼2–3 min period (for moderate-intensity work rates below the lactate threshold (LT)) energy provision is supplemented by substrate level phosphorylation, which is both limited in its capacity and has deleterious consequences for the maintenance of muscle function. At work rates above LT, however, the attainment of a steady state is delayed even further, which necessitates additional and progressive anaerobic contributions to the energy transfer. Here, 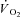 kinetics are slowed by the action of a supplemental, slowly developing phase termed the
kinetics are slowed by the action of a supplemental, slowly developing phase termed the 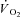 slow component (
slow component (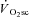 ) (Poole, 1994), which reflects a reduction in efficiency during supra-LT exercise. During exercise between LT and the ‘critical power’ (CP) asymptote (termed heavy intensity) the
) (Poole, 1994), which reflects a reduction in efficiency during supra-LT exercise. During exercise between LT and the ‘critical power’ (CP) asymptote (termed heavy intensity) the 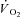 can still attain a steady state; however, this is delayed by as much as 15 min (Poole et al. 1988). During exercise above CP (very heavy intensity), however, a steady state is never achieved (Poole et al. 1988), and the rates of both substrate level and oxidative phosphorylation continue to rise until the work rate is reduced or
can still attain a steady state; however, this is delayed by as much as 15 min (Poole et al. 1988). During exercise above CP (very heavy intensity), however, a steady state is never achieved (Poole et al. 1988), and the rates of both substrate level and oxidative phosphorylation continue to rise until the work rate is reduced or 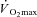 attained (Jones et al. 2008). As such, the
attained (Jones et al. 2008). As such, the 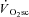 has been closely linked with the development of exercise intolerance (Rossiter et al. 2002a; Whipp & Rossiter, 2005). The mechanism(s) responsible for the
has been closely linked with the development of exercise intolerance (Rossiter et al. 2002a; Whipp & Rossiter, 2005). The mechanism(s) responsible for the 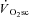 , however, remain uncertain.
, however, remain uncertain.
It is well understood that the majority of the 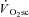 (>85%) originates from the locomotor muscles (Poole et al. 1991; Rossiter et al. 2002b) and is coincident with a progressive rise in blood [lactate]/[pyruvate] and muscle [inorganic phosphate] (Pi) and [H+] (Poole et al. 1988; Jones et al. 2008). In addition, contributions to the
(>85%) originates from the locomotor muscles (Poole et al. 1991; Rossiter et al. 2002b) and is coincident with a progressive rise in blood [lactate]/[pyruvate] and muscle [inorganic phosphate] (Pi) and [H+] (Poole et al. 1988; Jones et al. 2008). In addition, contributions to the 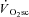 from processes such as increased cardiac and ventilatory work, lactate clearance, β-adrenergic stimulation and increased temperature appear to be minimal in comparison to that from skeletal muscle O2 consumption (Gaesser, 1994; Poole, 1994; Koga et al. 1997). The most prevalent hypothesis, therefore, is that the
from processes such as increased cardiac and ventilatory work, lactate clearance, β-adrenergic stimulation and increased temperature appear to be minimal in comparison to that from skeletal muscle O2 consumption (Gaesser, 1994; Poole, 1994; Koga et al. 1997). The most prevalent hypothesis, therefore, is that the 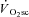 is consequent to a progressive increase in motor unit recruitment during supra-LT exercise (Whipp, 1994). This increased recruitment, it is suggested, is required to maintain force production in the face of muscle fatigue, and results in an increased reliance on poorly efficient type II fibres (Crow & Kushmerick, 1982; Hunter et al. 2001; Han et al. 2003). This ‘fatigue and recruitment’ hypothesis, therefore, suggests that the ATP and O2 cost of constant work rate (CWR) exercise rises during fatigue, which is manifest as the
is consequent to a progressive increase in motor unit recruitment during supra-LT exercise (Whipp, 1994). This increased recruitment, it is suggested, is required to maintain force production in the face of muscle fatigue, and results in an increased reliance on poorly efficient type II fibres (Crow & Kushmerick, 1982; Hunter et al. 2001; Han et al. 2003). This ‘fatigue and recruitment’ hypothesis, therefore, suggests that the ATP and O2 cost of constant work rate (CWR) exercise rises during fatigue, which is manifest as the 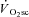 (Rossiter et al. 2002b). However, evidence supporting either the muscle fatigue or the progressive recruitment during supra-LT exercise in humans is equivocal.
(Rossiter et al. 2002b). However, evidence supporting either the muscle fatigue or the progressive recruitment during supra-LT exercise in humans is equivocal.
A progressive increase in regional muscle activation, as estimated by magnetic resonance imaging (Saunders et al. 2000; Endo et al. 2007), and an increased type II fibre recruitment following glycogen depletion in type I fibres (Krustrup et al. 2004a), have each been shown to relate to the magnitude of the 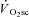 . This is consistent with the ‘recruitment’ component of the hypothesis. However, observations using surface electromyographic (EMG) activity or mean/median power frequency (MPF) are more variable, with some findings being consistent with a progressive recruitment (Shinohara & Moritani, 1992; Borrani et al. 2001; Perrey et al. 2001; Burnley et al. 2002; Sabapathy et al. 2005; Bernasconi et al. 2006; Osborne & Schneider, 2006; Vercruyssen et al. 2009; Hirai et al. 2010) and others showing no change in recruitment during the
. This is consistent with the ‘recruitment’ component of the hypothesis. However, observations using surface electromyographic (EMG) activity or mean/median power frequency (MPF) are more variable, with some findings being consistent with a progressive recruitment (Shinohara & Moritani, 1992; Borrani et al. 2001; Perrey et al. 2001; Burnley et al. 2002; Sabapathy et al. 2005; Bernasconi et al. 2006; Osborne & Schneider, 2006; Vercruyssen et al. 2009; Hirai et al. 2010) and others showing no change in recruitment during the 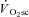 (Lucia et al. 2000; Scheuermann et al. 2001; Pringle & Jones, 2002; Avogadro et al. 2003, 2004; Tordi et al. 2003; Cleuziou et al. 2004; Garland et al. 2006; Migita & Hirakoba, 2006). Moreover, when all motor units are recruited using electrical stimulation, an increase in the O2 cost of force production has been observed in canine (Zoladz et al. 2008) and frog (Nagesser et al. 1993; Hepple et al. 2010) muscle, suggesting that recruitment is not a necessary event for the
(Lucia et al. 2000; Scheuermann et al. 2001; Pringle & Jones, 2002; Avogadro et al. 2003, 2004; Tordi et al. 2003; Cleuziou et al. 2004; Garland et al. 2006; Migita & Hirakoba, 2006). Moreover, when all motor units are recruited using electrical stimulation, an increase in the O2 cost of force production has been observed in canine (Zoladz et al. 2008) and frog (Nagesser et al. 1993; Hepple et al. 2010) muscle, suggesting that recruitment is not a necessary event for the 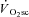 . Rather, these findings from animal preparations suggest that the
. Rather, these findings from animal preparations suggest that the 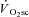 is due to an increased ATP and/or O2 cost of force production in fatigued muscle.
is due to an increased ATP and/or O2 cost of force production in fatigued muscle.
In humans, muscle fatigue has been reported at exercise intensities and over durations for which a 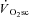 would be expected (Sargeant & Dolan, 1987), a finding consistent with the ‘fatigue’ part of the hypothesis. Furthermore, peak power production during an all-out 10 s, Wingate-style test was reduced after supra-LT, but not moderate intensity, exercise (Yano et al. 2001). Additionally, when all-out exercise is sustained for up to 3 min,
would be expected (Sargeant & Dolan, 1987), a finding consistent with the ‘fatigue’ part of the hypothesis. Furthermore, peak power production during an all-out 10 s, Wingate-style test was reduced after supra-LT, but not moderate intensity, exercise (Yano et al. 2001). Additionally, when all-out exercise is sustained for up to 3 min, 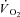 continues to rise (at least until
continues to rise (at least until 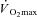 is reached) despite a fall in power output, consistent with a high O2 cost of power production in fatigue (Burnley et al. 2006). While these data are consistent with the ‘fatigue and recruitment’ hypothesis of the
is reached) despite a fall in power output, consistent with a high O2 cost of power production in fatigue (Burnley et al. 2006). While these data are consistent with the ‘fatigue and recruitment’ hypothesis of the 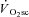 , estimation of muscle fatigue by these latter methods is complicated by the fact that peak power is dependent on the contraction velocity at which it is measured (Beelen & Sargeant, 1991).
, estimation of muscle fatigue by these latter methods is complicated by the fact that peak power is dependent on the contraction velocity at which it is measured (Beelen & Sargeant, 1991).
Thus, the aim of this study was to address whether muscle fatigue precedes the 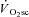 during dynamic exercise in humans. This was done by characterising the changes in velocity-specific peak power during moderate, heavy and very heavy-intensity cycling in concert with the kinetic features of
during dynamic exercise in humans. This was done by characterising the changes in velocity-specific peak power during moderate, heavy and very heavy-intensity cycling in concert with the kinetic features of 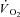 . To achieve this we used instantaneous switching between constant work rate and isokinetic (constant velocity) cycling with pedal force measurement, at discrete time points during exercise with and without a
. To achieve this we used instantaneous switching between constant work rate and isokinetic (constant velocity) cycling with pedal force measurement, at discrete time points during exercise with and without a 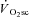 . We hypothesized that the magnitude and time-course of muscle fatigue during exercise above LT would be related to the kinetics of the
. We hypothesized that the magnitude and time-course of muscle fatigue during exercise above LT would be related to the kinetics of the 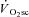 .
.
Methods
Ethical approval
Both the San Diego State University Institutional Review Board and The Biological Sciences Faculty Research Ethics Committee, University of Leeds, approved this study, and all procedures complied with the latest revision of the Declaration of Helsinki. Written informed consent was obtained from all volunteers prior to their participation in the study.
Participants
Eight healthy university students (2 females, 6 males) volunteered to participate in this study. Physical characteristics are presented in Table 1. All participants were undertaking a regular exercise regimen ranging from recreational fitness to amateur competitive sport. Volunteers were screened for cardiovascular disease with a health history and physical activity readiness questionnaire.
Table 1.
Physical characteristics for the eight participants
| Participant | Sex | Age | Height (cm) | Weight (kg) | LT (l min−1) |
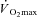 (l min−1) (l min−1) |
|---|---|---|---|---|---|---|
| 1 | F | 24 | 175 | 61 | 1.63 | 2.83 |
| 2 | M | 25 | 171 | 71 | 1.60 | 3.03 |
| 3 | F | 24 | 178 | 64 | 1.55 | 2.71* |
| 4 | M | 27 | 183 | 71 | 3.20 | 4.87 |
| 5 | M | 25 | 167 | 54 | 1.38 | 3.10 |
| 6 | M | 28 | 172 | 68 | 1.90 | 3.54 |
| 7 | M | 24 | 178 | 70 | 1.68 | 3.60* |
| 8 | M | 23 | 171 | 70 | 1.50 | 3.31 |
| Mean (s.d.) | 25 (2) | 174 (6) | 66 (6) | 1.80 (0.58) | 3.37 (0.68) |
Exercise protocols
All exercise tests were undertaken using a computer-controlled electromagnetically braked cycle ergometer (Excalibur Sport PFM, Lode BV, Groningen, the Netherlands). Initially, participants completed a ramp incremental (RI) exercise test to the limit of tolerance. For this, participants sat at rest on the ergometer for ∼1 min, followed by a low work rate exercise (20 W) for ∼4 min. The work rate was then increased as a smooth function of time at 20 W min−1 until reaching the limit of tolerance. Participants were allowed to self-select a comfortable pedal cadence, but thereafter encouraged to maintain it within approximately ±10 rpm (typically 80–100 rpm). The RI was terminated upon the participant being unable to maintain a pedal cadence of 50 rpm, despite strong verbal encouragement. Upon reaching the limit of tolerance, participants were given a 5 min period of active recovery at 20 W after which a step increase in work rate was made to 105% of the peak work rate achieved during RI (SE105) (Rossiter et al. 2006). Again, participants were given strong verbal encouragement until the limit of tolerance, where the work rate was reduced to 20 W for at least 5 min of active recovery. The results of this RI and SE105 test were used to estimate LT and determine 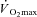 , and to calculate work rates for the subsequent tests.
, and to calculate work rates for the subsequent tests.
A series of exercise tests was then completed in a randomized order (Fig. 1). Each test consisted of two phases: (1) a constant work rate (CWR) phase, followed immediately by; (2) a maximal isokinetic effort, maintained for five crank revolutions, to measure velocity-specific peak torque and power.
Figure 1. A schematic showing the exercise protocols for measurement of peak isokinetic torque (and power) over time during exercise at 80% LT (Mod), 20%Δ (H) and 60%Δ (VH).
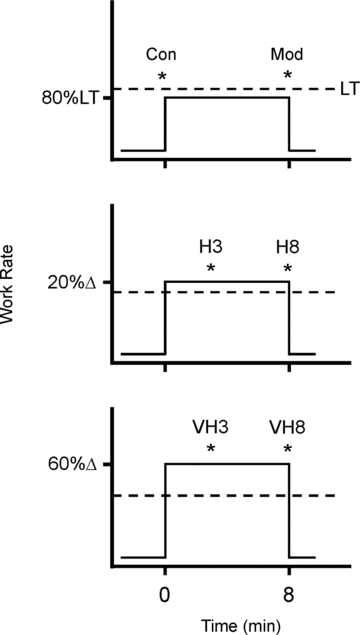
Maximal efforts for 5 crank revolutions were performed at time points represented by *. Velocity-specific peak power was measured at each of three target pedalling cadences (60, 90 and 120 rpm) with each test completed on a different day and in a randomized order. This required a total of 18 laboratory visits for each participant. 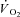 was measured breath-by-breath throughout each test.
was measured breath-by-breath throughout each test.
The participants completed CWR bouts at each of: (1) 4 min at 20 W (Con); (2) 8 min at 80% LT (Mod); (3) 20%Δ (H), where Δ is the difference between the work rates at LT and peak RI; and (4) 60%Δ (VH). H and VH conditions were completed for each of 3 and 8 min (i.e. H3, H8, VH3 and VH8). These time points were chosen to represent the earliest time at which the 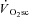 could be readily identified and to allow a sufficient duration for its evolution. The Con trial acted as a baseline characterization, or ‘time 0’ (Fig. 1). The work rates were chosen to span the thresholds of the moderate (Mod), heavy (H) and very heavy (VH) intensity domains and were verified post hoc from the dynamic features of
could be readily identified and to allow a sufficient duration for its evolution. The Con trial acted as a baseline characterization, or ‘time 0’ (Fig. 1). The work rates were chosen to span the thresholds of the moderate (Mod), heavy (H) and very heavy (VH) intensity domains and were verified post hoc from the dynamic features of 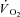 .
.
Immediately upon completion of the CWR phase of the test, the ergometer was instantaneously switched into an isokinetic mode that limited the peak pedalling frequency to either 60, 90 or 120 rpm. The participants were given an auditory cue to begin a maximal effort in the seated position and strong verbal encouragement was given throughout the five crank revolutions. On completion of the maximal isokinetic effort the ergometer was returned to the CWR, cadence-independent mode at 20 W for 5 min of active recovery.
This series of exercise tests required a total of 18 laboratory visits to characterise peak power at each of the three pedalling frequencies (60, 90 and 120 rpm) for the six different CWR trials (Con, Mod, H3, H8, VH3 and VH8). Each test was completed on a separate day with at least 24 h free of strenuous physical activity and at least 12 h abstinence from alcohol or caffeine.
Measurements
The cycle ergometer was instrumented for pedal force measurement. Crank torque was measured independently from the left and right crank arms by strain gauge transducers (peak force 2000 N, <0.5 N resolution and measurement uncertainty of <3%). Instantaneous angular velocity of the crank (rad s−1) was measured every 2 deg using three independent sensors sampling in series (measurement uncertainty of <1%). The isokinetic velocity was limited by the electromagnetic breaking system of the flywheel and computer controlled to the value programmed by the experimenter. Maintenance of the target pedalling frequency was confirmed post hoc from the data output.
The peak torque in each isokinetic phase was used to characterize the velocity-specific muscle torque in the left and right crank arms. Velocity-specific peak power at each constant pedalling frequency was then calculated from the product of peak torque and instantaneous angular velocity.
Respired gases were measured breath-by-breath throughout the exercise protocols via a metabolic measurement system (Oxycon Mobile, VIASYS Healthcare GmbH, Höchberg, Germany). Participants breathed through a low-resistance (<0.1 kPa l−1 s−1 at 15 l s−1), low dead space (155 ml) mouthpiece, while gas concentrations were measured with a fast-response electrochemical oxygen measuring cell and a thermal conductivity carbon dioxide analyser (both <80 ms 90% response time after digital filtering). The gas analysers were calibrated using atmospheric air and precision-verified calibration gases to span the range of respired gas concentrations. Flow rates and volumes were measured with an infrared turbine flow sensor that was calibrated before each test across a range of physiological flow rates (0.1–2.0 l s−1) using an electronic pump. Arterial saturation (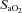 ) was measured at the earlobe using pulse oximetry (Nonin 8000Q 1m, Nonin Medical Inc., Plymouth, MN, USA) and heart rate was measured from the R–R interval (Polar Electro Oy, Finland).
) was measured at the earlobe using pulse oximetry (Nonin 8000Q 1m, Nonin Medical Inc., Plymouth, MN, USA) and heart rate was measured from the R–R interval (Polar Electro Oy, Finland).
Data analyses
The LT was estimated non-invasively using multiple gas exchange criteria through consideration of the profiles of 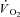 , carbon dioxide output (
, carbon dioxide output (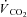 ), end-tidal partial pressures for CO2 and O2, as well as ventilatory equivalents (
), end-tidal partial pressures for CO2 and O2, as well as ventilatory equivalents (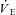 /
/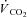 and
and 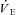 /
/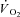 ) and the respiratory exchange ratio (RER) (Beaver et al. 1986). Peak pulmonary gas exchange values were determined from the highest 12 breath mean, and
) and the respiratory exchange ratio (RER) (Beaver et al. 1986). Peak pulmonary gas exchange values were determined from the highest 12 breath mean, and 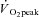 during RI and SE105 were compared within each participant to establish
during RI and SE105 were compared within each participant to establish 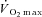 .
.
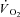 kinetics were modelled using non-linear least-squares regression (OriginPro 7.5, OriginLab Corp., Northampton, MA, USA). Breath-by-breath
kinetics were modelled using non-linear least-squares regression (OriginPro 7.5, OriginLab Corp., Northampton, MA, USA). Breath-by-breath 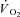 was filtered for errant breaths (i.e. values resulting after sighs, swallows, coughs etc., defined as residing outside of 99% prediction limits) and interpolated to 1 s intervals. Responses from like transitions were ensemble averaged to improve the signal-to-noise and averaged into 5 s bins for non-linear regression fitting according to the following function:
was filtered for errant breaths (i.e. values resulting after sighs, swallows, coughs etc., defined as residing outside of 99% prediction limits) and interpolated to 1 s intervals. Responses from like transitions were ensemble averaged to improve the signal-to-noise and averaged into 5 s bins for non-linear regression fitting according to the following function:
where 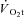 ,
, 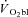 and Δ
and Δ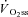 are the time variant form, baseline and fundamental amplitude of
are the time variant form, baseline and fundamental amplitude of 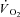 , respectively. The model includes a time delay (d) and a time constant (τ). The fitting window was determined from an iterative process (Rossiter et al. 2001) to ensure the exclusion of the cardiopulmonary phase (generally the first 15–25 s) and phase III (steady-state or slow component, depending on the intensity domain). The magnitude of the
, respectively. The model includes a time delay (d) and a time constant (τ). The fitting window was determined from an iterative process (Rossiter et al. 2001) to ensure the exclusion of the cardiopulmonary phase (generally the first 15–25 s) and phase III (steady-state or slow component, depending on the intensity domain). The magnitude of the 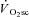 was expressed as the difference in
was expressed as the difference in 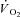 between the amplitude of the fundamental (
between the amplitude of the fundamental (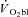 +Δ
+Δ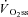 ) and the end-exercise values.
) and the end-exercise values.
Statistical analyses
The difference between the 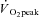 values from RI and SE105 were examined using unpaired t tests, to establish the attainment of
values from RI and SE105 were examined using unpaired t tests, to establish the attainment of 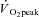 or
or 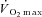 . The relationship between the velocity-specific peak power, used to measure fatigue, and
. The relationship between the velocity-specific peak power, used to measure fatigue, and 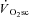 were assessed with a Pearson correlation coefficient. The torque–velocity and power–velocity relationship for Mod, H and VH exercise were compared with a two-factor (time × pedalling frequency) repeated measures ANOVA. Bonferroni corrected t tests (α=α.κ−1; where κ is the number of post hoc comparisons) were used in the case of a significant ANOVA to identify the locus of the differences. Statistical significance was set at P < 0.05. All analyses were completed using the Statistical Package for the Social Sciences (SPSS v.15.0, SPSS Inc., Chicago, IL, USA).
were assessed with a Pearson correlation coefficient. The torque–velocity and power–velocity relationship for Mod, H and VH exercise were compared with a two-factor (time × pedalling frequency) repeated measures ANOVA. Bonferroni corrected t tests (α=α.κ−1; where κ is the number of post hoc comparisons) were used in the case of a significant ANOVA to identify the locus of the differences. Statistical significance was set at P < 0.05. All analyses were completed using the Statistical Package for the Social Sciences (SPSS v.15.0, SPSS Inc., Chicago, IL, USA).
Results
Participants attained a peak work rate during RI of 293 ± 58 W, thus the SE105 work rate was 307 ± 66 W. 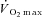 (3.37 ± 0.68 l min−1) was confirmed in 6 of the 8 participants completing the RI SE105 protocol (the other two achieving
(3.37 ± 0.68 l min−1) was confirmed in 6 of the 8 participants completing the RI SE105 protocol (the other two achieving 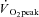 ). Estimated LT averaged 1.80 ± 0.58 l min−1, or 52 ± 7% of
). Estimated LT averaged 1.80 ± 0.58 l min−1, or 52 ± 7% of 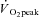 (Table 1). Work rates determined for Mod (80% LT), H (20%Δ) and VH (60%Δ) exercise were 93 ± 33, 148 ± 46 and 222 ± 51 W, respectively.
(Table 1). Work rates determined for Mod (80% LT), H (20%Δ) and VH (60%Δ) exercise were 93 ± 33, 148 ± 46 and 222 ± 51 W, respectively.
Oxygen uptake kinetics
Representative examples of the 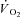 responses during Mod, H and VH exercise for one participant are superimposed in Fig. 2, and the parameter estimates describing
responses during Mod, H and VH exercise for one participant are superimposed in Fig. 2, and the parameter estimates describing 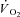 kinetics for all participants are presented in Table 2. The fundamental τ was similar across the intensity domains (Mod, H and VH) (P > 0.05). A
kinetics for all participants are presented in Table 2. The fundamental τ was similar across the intensity domains (Mod, H and VH) (P > 0.05). A 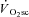 was not discernible in Mod (38 ± 88 ml min−1, which was not different from zero; P > 0.05), but became apparent during exercise above LT with the
was not discernible in Mod (38 ± 88 ml min−1, which was not different from zero; P > 0.05), but became apparent during exercise above LT with the 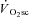 in H (238 ± 128 ml min−1) being significantly less than that in VH (686 ± 194 ml min−1) (P < 0.05).
in H (238 ± 128 ml min−1) being significantly less than that in VH (686 ± 194 ml min−1) (P < 0.05).
Figure 2. Superimposed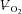 responses during Mod (•), H (□) and VH (▪) for a representative individual.
responses during Mod (•), H (□) and VH (▪) for a representative individual.
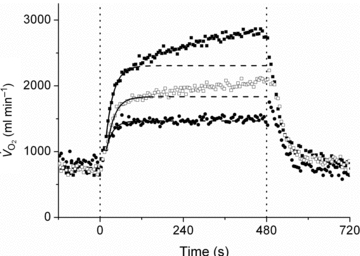
Fundamental (phase II) kinetics are characterised with non-linear least-squares regression modelling (continuous line), with the fit extrapolated (dashed line) to the end of the CWR bout.
Table 2.
Kinetic parameters 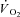 during moderate (Mod), heavy (H) and very heavy (VH) intensity exercise
during moderate (Mod), heavy (H) and very heavy (VH) intensity exercise
| Mod | H | VH | |
|---|---|---|---|
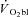 (ml min−1) (ml min−1) |
744 (112) | 729 (94) | 765 (97) |
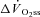 (ml min−1) (ml min−1) |
774 (356) | 1349 (533)1 | 1876 (433)1,2 |
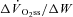 (ml min−1 W−1) (ml min−1 W−1) |
10.6 (0.9) | 10.5 (0.8) | 9.3 (0.8)1,2 |
| τ (s) | 21.4 (7.8) | 22.6 (3.8) | 26.4 (7.2) |
| CI95τ (s) | 3.6 (2.7) | 1.5 (0.7) | 1.1 (0.5)2 |
| d (s) | 16.0 (4.0) | 11.4 (4.7) | 7.5 (3.3)1 |
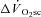 (ml min−1) (ml min−1) |
38 (88) | 238 (128)1 | 686 (194)1,2 |
Significantly (P < 0.05) different from Mod.
Significantly (P < 0.05) different from H.
Velocity and torque measurements
The mean isokinetic pedalling frequency was close to the target cadence (60, 90 and 120 rpm) during Con: 59.6 ± 2.2, 90.4 ± 1.1 and 120.5 ± 2.3 rpm. There was no difference between the measured pedalling frequencies (within each target) across the six conditions (P > 0.05; Table 3). Peak torque was similar between the left and right crank in all conditions (P > 0.05); thus, the measurements from both crank arms were averaged for all further analyses. Peak torque measured during all trials is presented in Table 3. In Con, as expected, peak torque was reduced at each increment in pedalling frequency (F= 52.9; P < 0.05; η2= 88). The peak torque and angular velocity measurements were then used to calculate peak power.
Table 3.
Pedalling frequency and peak crank torque measured during a maximal isokinetic effort performed instantaneously following constant work rate exercise of varied intensity and duration
| Intensity | Con | Mod | H | VH | ||
|---|---|---|---|---|---|---|
| Time (min) | 0 | 8 | 3 | 8 | 3 | 8 |
| Cadence (rpm) | ||||||
| 60 | 59.6 (2.2) | 58.3 (1.8) | 58.4 (2.4) | 60.0 (2.0) | 58.6 (2.9) | 59.1 (3.0) |
| 90 | 90.4 (1.1) | 90.8 (1.4) | 90.1 (0.6) | 90.8 (1.4) | 91.2 (1.1) | 91.6 (1.0) |
| 120 | 120.5 (2.3) | 120.5 (1.9) | 120.5 (1.3) | 122.4 (1.7) | 121.2 (2.6) | 120.0 (4.3) |
| Torque (Nm) | ||||||
| 60 | 144.0 (24.7) | 138.5 (20.2) | 133.8 (20.3) | 134.0 (15.4) | 132.1 (18.1) | 126.9 (16.1) |
| 90 | 128.9 (18.2) | 125.5 (20.0) | 119.7 (25.3) | 116.2 (19.9) | 102.4 (18.8) | 105.4 (28.1) |
| 120 | 103.0 (18.9) | 103.7 (20.2) | 94.2 (19.5) | 101.0 (22.6) | 78.2 (18.7) | 77.6 (19.5) |
| Mean (s.d.) | 125.3 (26.3) | 122.6 (24.2) | 116.0 (26.8)1 | 117.1 (23.2)1 | 104.2 (28.7)1,2 | 103.3 (29.3)1,3 |
Significantly (P < 0.05) different from Con.
Significantly (P < 0.05) different from H3.
Significantly (P < 0.05) different from H8.
Peak power after constant work rate exercise
Velocity-specific peak power increased as a function of pedalling frequency in Con (1025 ± 400, 1219 ± 167 and 1298 ± 233 W for 60, 90 and 120 rpm, respectively) (F= 5.08; P < 0.05; η2= 0.42). Following Mod, the main effect of pedalling frequency remained (P < 0.05); however, no effect of time (i.e. between 0 (Con) and 8 (Mod) minutes; F= 2.89; P > 0.05; η2= 0.29) or interaction was detected (F= 0.79; P > 0.05; η2= 0.10). Therefore, velocity-specific peak power in Mod was similar to Con (mean peak power 1155 ± 240 W vs. 1181 ± 246 W, respectively; Fig. 3).
Figure 3. Velocity-specific peak power developed during a maximal isokinetic effort, plotted as a function of pedalling frequency.
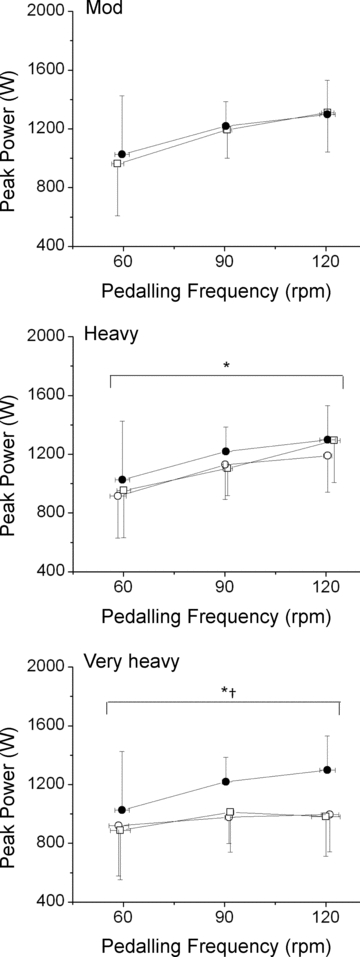
Panels: Mod:•, Con; □, Mod. Heavy:•, Con; ○, H3; □, H8. Very Heavy: •, Con; ○, VH3; □, VH8. *Significant (P < 0.05) main effect of time; power during H3/VH3 and H8/VH8 significantly (P < 0.05) lower than Con. †Significant (P < 0.05) interaction (time × pedalling frequency) was present. Error bars are s.d.
Measurements made in H (Fig. 3) revealed a main effect of pedalling frequency (F= 10.6; P < 0.05; η2= 0.60) and time (F= 30.3; P < 0.05; η2= 0.81), but no interaction was detected (F= 1.45; P > 0.05; η2= 0.17). Post hoc analyses revealed a significant (P < 0.05) reduction in velocity-specific peak power in both H3 (1078 ± 243 W vs. 1181 ± 246 W) and H8 (1117 ± 243 W vs. 1181 ± 246 W) compared with Con. However, H3 and H8 did not differ (P > 0.05; 95% confidence interval (CI95) of the difference; CIDiff–39, 12 W), indicating that velocity-specific peak power was maintained between 3 and 8 min in heavy-intensity exercise. In one case (at 120 rpm) there was a small increase in peak power between H3 and H8; however, this small adjustment was apparently too limited for a significant interaction between pedalling frequency and time to be detected (Fig. 3).
The reduction in velocity-specific peak power over time in VH revealed a significant interaction (F= 3.60; P < 0.05; η2= 0.34), indicating a flattening of the power–velocity relationship. Post hoc analyses revealed a significant (P < 0.05) reduction in peak power at all pedalling frequencies in VH3 compared with Con, with the greatest reduction in peak power occurring at 120 rpm (−302 ± 117 W) compared with 60 rpm (−105 ± 64 W) or 90 rpm (−241 ± 85 W) (Fig. 3). However, VH3 and VH8 did not differ (P > 0.05; CIDiff–51, 60 W), indicating that velocity-specific peak power was maintained between 3 and 8 min in very heavy-intensity exercise (Fig. 3).
Additionally, comparison of the H and VH conditions revealed an interaction between velocity-specific peak power and time at both 3 (F= 8.4; P < 0.05; η2= 0.55) and 8 (F= 10.4; P < 0.05; η2= 0.60) minutes. The difference in velocity-specific peak power between H3 and VH3 was greatest at 120 rpm (−194 ± 76 W), compared with 60 rpm (−5 ± 129 W) and 90 rpm (−151 ± 81 W). These differences were similar in magnitude between H8 and VH8 (Fig. 3).
Skeletal muscle fatigue and the slow component
Across intensities, the overall reduction in peak power (i.e. the magnitude of muscle fatigue) was correlated (P < 0.05) to the 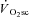 (R2= 0.49; Fig. 4). Additionally, the relationship between skeletal muscle fatigue and the
(R2= 0.49; Fig. 4). Additionally, the relationship between skeletal muscle fatigue and the 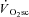 was strongest at the higher contraction velocities (Fig. 5), consequent to the interaction (time × pedalling frequency) in the VH condition.
was strongest at the higher contraction velocities (Fig. 5), consequent to the interaction (time × pedalling frequency) in the VH condition.
Figure 4. Relationship (P < 0.05) between the 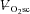 and muscle fatigue (reduction in peak power across all contraction velocities) after constant work rate exercise.
and muscle fatigue (reduction in peak power across all contraction velocities) after constant work rate exercise.
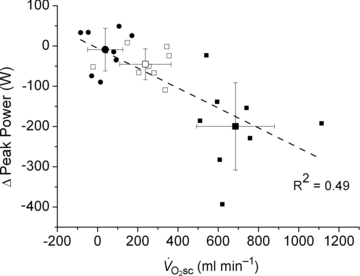
•, Mod; □, H8; ▪, VH8. Error bars are s.d.
Figure 5. Reduction in velocity-specific peak power (indicative of muscle fatigue) from Con to end exercise, plotted as a function of the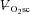 during CWR exercise.
during CWR exercise.
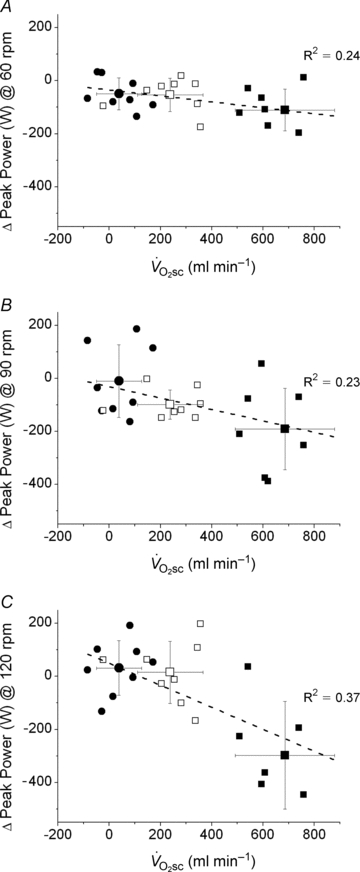
A, 60 rpm; B, 90 rpm; C, 120 rpm. •, Mod; □, H8; ▪, VH8. Error bars are s.d.
Discussion
These data demonstrate for the first time in humans that muscle fatigue, determined from a velocity-specific reduction in peak power, occurs in heavy- and very heavy-intensity exercise above the LT where a 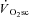 is present. In contrast, during moderate-intensity exercise where a
is present. In contrast, during moderate-intensity exercise where a 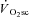 was not evident, the velocity-specific peak power was maintained at the control level (at least between the pedalling frequencies of 60 and 120 rpm).
was not evident, the velocity-specific peak power was maintained at the control level (at least between the pedalling frequencies of 60 and 120 rpm).
As hypothesised, the velocity-specific reduction in peak power was correlated to the 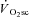 . To our surprise, however, the time course of fatigue was unrelated to the
. To our surprise, however, the time course of fatigue was unrelated to the 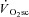 progression, because velocity-specific peak power was unchanged (or even recovered in the rare case; H8 at 120 rpm) between 3 and 8 min of supra-LT exercise where the
progression, because velocity-specific peak power was unchanged (or even recovered in the rare case; H8 at 120 rpm) between 3 and 8 min of supra-LT exercise where the 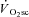 was most evident. Thus, only 3 min of constant work rate exercise at 20%Δ was required to induce fatigue, but the attenuation in velocity-specific peak power developed prior to, and not in concert with, the
was most evident. Thus, only 3 min of constant work rate exercise at 20%Δ was required to induce fatigue, but the attenuation in velocity-specific peak power developed prior to, and not in concert with, the 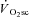 . Additionally, both fatigue and the
. Additionally, both fatigue and the 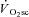 were greater during exercise at 60%Δ (VH) compared with 20%Δ (H) but, again, fatigue was not exacerbated between 3 and 8 min of exercise. Thus, whereas fatigue was generated early in the exercise transient, the apparent consequences of this fatigue continued throughout the exercise. Overall, these data suggest that the increase in the ATP and/or O2 cost of exercise during supra-LT exercise may be more closely related to the metabolic consequences of muscle fatigue rather than to additional recruitment of poorly efficient muscle fibres.
were greater during exercise at 60%Δ (VH) compared with 20%Δ (H) but, again, fatigue was not exacerbated between 3 and 8 min of exercise. Thus, whereas fatigue was generated early in the exercise transient, the apparent consequences of this fatigue continued throughout the exercise. Overall, these data suggest that the increase in the ATP and/or O2 cost of exercise during supra-LT exercise may be more closely related to the metabolic consequences of muscle fatigue rather than to additional recruitment of poorly efficient muscle fibres.
Skeletal muscle fatigue and the 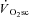
Various mechanisms have been proposed to contribute to the 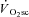 , such as increased ventilatory and cardiac work, lactate clearance, stimulation from circulating catecholamines and increased temperature. However, the total contribution of these processes to the
, such as increased ventilatory and cardiac work, lactate clearance, stimulation from circulating catecholamines and increased temperature. However, the total contribution of these processes to the 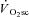 appears to be small (Gaesser, 1994; Poole, 1994; Koga et al. 1997), because locomotor muscle O2 consumption accounts for more than 85% of the pulmonary
appears to be small (Gaesser, 1994; Poole, 1994; Koga et al. 1997), because locomotor muscle O2 consumption accounts for more than 85% of the pulmonary 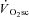 (Poole, 1994) – a feature that is mirrored in the kinetics of intramuscular PCr breakdown (Rossiter et al. 2002b). As the
(Poole, 1994) – a feature that is mirrored in the kinetics of intramuscular PCr breakdown (Rossiter et al. 2002b). As the 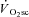 is only observed during exercise above LT where a sustained alteration in muscle and blood acid–base status is manifest, it has been suggested that skeletal muscle fatigue is the event required to initiate the rise in the O2 cost of exercise (Shinohara & Moritani, 1992; Gaesser & Poole, 1996; Yano et al. 2001). However, evidence to support this notion is sparse.
is only observed during exercise above LT where a sustained alteration in muscle and blood acid–base status is manifest, it has been suggested that skeletal muscle fatigue is the event required to initiate the rise in the O2 cost of exercise (Shinohara & Moritani, 1992; Gaesser & Poole, 1996; Yano et al. 2001). However, evidence to support this notion is sparse.
The heavy- and very heavy-intensity work rates used here (∼150 and ∼220 W, respectively) were considerably below the peak power (∼1150 W) and therefore may not be expected to result in substantial muscle fatigue, at least not during the first 3 min of exercise. However, the presence of skeletal muscle fatigue has been reported during cycling exercise at work rates consistent with those used here, i.e. work rates that would be expected to result in a 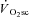 (Sargeant & Dolan, 1987). In addition, it has been shown that a reduction in peak power during a 10 s all-out sprint (an abbreviated ‘Wingate’ style test) was manifest following exercise above, but not below, LT (Yano et al. 2001). However, because peak torque (and power) during cycling is dependent on the muscular contraction velocity, the latter observation is complicated by the fact that the pedalling cadences used to estimate peak power differed between conditions.
(Sargeant & Dolan, 1987). In addition, it has been shown that a reduction in peak power during a 10 s all-out sprint (an abbreviated ‘Wingate’ style test) was manifest following exercise above, but not below, LT (Yano et al. 2001). However, because peak torque (and power) during cycling is dependent on the muscular contraction velocity, the latter observation is complicated by the fact that the pedalling cadences used to estimate peak power differed between conditions.
Thus, isokinetic cycle ergometry has been used in conjunction with torque measurements at the crank to characterise maximal power production across a range of contraction velocities. This approach demonstrated that reductions in peak power after fatigue in cycling are dependent on the pedalling frequency (Beelen & Sargeant, 1991) and, therefore, any estimation of muscle fatigue requires that contraction velocity be controlled. These findings are corroborated in the present data, where peak power was dependent on pedalling frequency during the isokinetic phase, with muscle fatigue being absent in Mod and progressively greater in the H and VH conditions. Additionally, the magnitude of muscle fatigue was strongly correlated with the magnitude of the 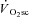 (Fig. 4), which is consistent with the hypothesis that muscle fatigue is a requisite event for the induction of progressive inefficiency that the
(Fig. 4), which is consistent with the hypothesis that muscle fatigue is a requisite event for the induction of progressive inefficiency that the 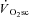 represents.
represents.
Also in line with previous findings, a greater magnitude of fatigue was present at 120 rpm in VH compared with 60 or 90 rpm. This has been suggested to reflect greater fatigue in type II muscle fibres that, compared with type I fibres, have a proportionally greater contribution to absolute power production at high shortening velocities and a greater decline in absolute power during fatigue (Beelen & Sargeant, 1991; Sargeant, 1999). Therefore, because the 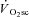 was also larger in the VH condition, this is consistent with the notion that fatigue of type II muscle fibres is associated with a larger
was also larger in the VH condition, this is consistent with the notion that fatigue of type II muscle fibres is associated with a larger 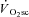 . Such proportionality, however, would only be expected if the effect of fatigue on efficiency were similar in both fibre types (see ‘Fatigue and skeletal muscle contractile efficiency’ for further discussion of this point). While it is not possible to know the contribution of the different fibre types to the overall fatigue profile using this technique, the present data are consistent with the notion that the magnitude of muscle fatigue is related to the magnitude of the
. Such proportionality, however, would only be expected if the effect of fatigue on efficiency were similar in both fibre types (see ‘Fatigue and skeletal muscle contractile efficiency’ for further discussion of this point). While it is not possible to know the contribution of the different fibre types to the overall fatigue profile using this technique, the present data are consistent with the notion that the magnitude of muscle fatigue is related to the magnitude of the 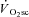 . However, the ‘fatigue and recruitment’ hypothesis suggests that the
. However, the ‘fatigue and recruitment’ hypothesis suggests that the 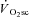 results from a fatigue-induced recruitment of additional motor units (presumably containing poorly efficient type II muscle fibres), which would be necessitated by a progression of muscle fatigue after 3 min of exercise – a feature that was, to our surprise, absent in both H and VH conditions.
results from a fatigue-induced recruitment of additional motor units (presumably containing poorly efficient type II muscle fibres), which would be necessitated by a progression of muscle fatigue after 3 min of exercise – a feature that was, to our surprise, absent in both H and VH conditions.
Motor unit recruitment during supra-LT exercise
While numerous reports in the literature provide evidence in support of progressive motor unit recruitment during constant work rate supra-LT exercise (Shinohara & Moritani, 1992; Saunders et al. 2000; Borrani et al. 2001; Perrey et al. 2001; Burnley et al. 2002; Krustrup et al. 2004a,b; Sabapathy et al. 2005; Bernasconi et al. 2006; Osborne & Schneider, 2006; Endo et al. 2007; Vercruyssen et al. 2009; Hirai et al. 2010), there is also a substantial volume of work that fails to corroborate this hypothesis (Lucia et al. 2000; Scheuermann et al. 2001; Pringle & Jones, 2002; Avogadro et al. 2003, 2004; Tordi et al. 2003; Cleuziou et al. 2004; Garland et al. 2006; Migita & Hirakoba, 2006; Zoladz et al. 2008; Hepple et al. 2010). The lack of a change in MPF or amplitude in the surface EMG signal during supra-LT exercise may have resulted from a low sensitivity of the technique relative to that expected from the changes in the motor unit recruitment pattern. It has been posited that fatiguing type IIa/x fibres may be replaced by proportionally more type I fibres (Pringle et al. 2003), which are less efficient at high shortening velocity and generate less force (He et al. 2000). The net result could be unchanging EMG amplitude and MPF due to the characteristics of type I fibres (Garland et al. 2006). Alternatively, the accumulation of extracellular K+ resulting from muscular contraction may blunt the propagation of action potentials across the cell membrane and reduce MPF (Gamet et al. 1993). Therefore, the use of EMG to characterise activity of different fibre types has been questioned (Farina, 2008; von Tscharner & Nigg, 2008), highlighting the difficulties in confident interpretation of alterations in motor unit activity from changes in the EMG signal. As an alternative, therefore, tools such as magnetic resonance imaging and muscle biopsy have been used to provide a characterisation of recruitment and metabolic changes in contracting skeletal muscle in concert with the 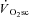 .
.
The proton transverse relaxation time (T2) of the magnetic resonance image increases with the accumulation of osmotically active ions (and therefore water), and correlates with local muscle activity in humans (Adams et al. 1992). Using this technique, proportional increases in muscle activation (and presumably recruitment) and the 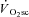 have been demonstrated during supra-LT exercise in humans (Saunders et al. 2000; Endo et al. 2007). However, changes in T2 are influenced by both the metabolic changes in active muscle fibres and recruitment (Meyer & Prior, 2000), and therefore, the coincidence of lengthened T2 and
have been demonstrated during supra-LT exercise in humans (Saunders et al. 2000; Endo et al. 2007). However, changes in T2 are influenced by both the metabolic changes in active muscle fibres and recruitment (Meyer & Prior, 2000), and therefore, the coincidence of lengthened T2 and 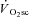 may not solely reflect de novo muscle fibre recruitment.
may not solely reflect de novo muscle fibre recruitment.
Using invasive techniques such as muscle biopsy, it has been demonstrated that reductions in [PCr] were present in type II fibres only during exercise above, not below, LT, suggesting that their recruitment is limited to the higher-intensity domains (Krustrup et al. 2004b). This method shows that the number of fibres expressing a low [PCr] increases during the 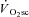 , consistent with increased fibre recruitment. However, because it is only possible to assess a small sample of tissue using this technique, and that the location varies with each serial biopsy, it is a matter of surmise whether the increased incidence of single fibres with reduced [PCr] between 3 and 6 min of exercise actually results from increased motor unit recruitment.
, consistent with increased fibre recruitment. However, because it is only possible to assess a small sample of tissue using this technique, and that the location varies with each serial biopsy, it is a matter of surmise whether the increased incidence of single fibres with reduced [PCr] between 3 and 6 min of exercise actually results from increased motor unit recruitment.
Therefore, while these data are consistent with progressive motor unit recruitment during the 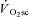 , evidence supporting a mechanistic link has remained elusive. It is of interest, therefore, that the dynamics of muscle fatigue in the present study suggest that progressive recruitment may not be obligatory to initiate a
, evidence supporting a mechanistic link has remained elusive. It is of interest, therefore, that the dynamics of muscle fatigue in the present study suggest that progressive recruitment may not be obligatory to initiate a 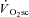 , at least after the first 3 min of exercise.
, at least after the first 3 min of exercise.
The fatigue dynamics during H and VH exercise in the present study (a rapid onset followed by a maintenance of peak torque) are very similar to previous reports in the literature (Sargeant & Dolan, 1987), and follow a time course that is consistent with changes in fatigue-related intramuscular phosphates. For example, intramuscular [Pi] and [ADP] accumulate most rapidly during the first 3 min of constant work rate exercise, with only small changes thereafter (Hultman et al. 1967; Rossiter et al. 2002a). Furthermore, in skinned skeletal muscle fibres, low levels of Pi accumulation (<20 mm) are associated with large reductions in force production (>40%), whereas further increases in [Pi] alone have little additional effect on force (Cooke & Pate, 1985). Also, the recovery of peak power has been estimated to have a half-time of 32 s, mirroring the resynthesis kinetics of PCr (Sargeant & Dolan, 1987). Whether fatigue occurs via disruption at the crossbridge itself, or through Pi entry to the sarcoplasmic reticulum and Ca2+ precipitate formation (Allen et al. 2008) remains unclear, but the dynamics of fatigue in this study appear to reflect well the kinetics of known fatigue-related metabolites.
Thus, while we originally reasoned that the dynamics of muscle fatigue would mirror those of the 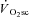 , this was not the case, suggesting that power output from active fibres was sufficient to meet the demands of the exercise during this phase, and that additional fibre recruitment would therefore be unnecessary. Using the present data it is not possible to rule out that a cyclic, or selective, motor unit recruitment occurred during the
, this was not the case, suggesting that power output from active fibres was sufficient to meet the demands of the exercise during this phase, and that additional fibre recruitment would therefore be unnecessary. Using the present data it is not possible to rule out that a cyclic, or selective, motor unit recruitment occurred during the 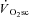 (at the cost of contractile efficiency) in order to protect against a reduction in force output. Such a migration of motor unit activity without concomitant changes in the velocity-specific peak power production seems unlikely and is inconsistent with the size principle (Henneman, 1957; De Luca & Hostage, 2010). Selective recruitment has been observed where a rapid increase in power production is required, but it seems unlikely during the CWR exercise of the present study (Hodson-Tole & Wakeling, 2009). This suggests that cyclical recruitment of type II muscle fibres may not be a major contributor to the
(at the cost of contractile efficiency) in order to protect against a reduction in force output. Such a migration of motor unit activity without concomitant changes in the velocity-specific peak power production seems unlikely and is inconsistent with the size principle (Henneman, 1957; De Luca & Hostage, 2010). Selective recruitment has been observed where a rapid increase in power production is required, but it seems unlikely during the CWR exercise of the present study (Hodson-Tole & Wakeling, 2009). This suggests that cyclical recruitment of type II muscle fibres may not be a major contributor to the 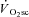 in this study. Rather, the present data are more consistent with a degradation in muscle efficiency during the
in this study. Rather, the present data are more consistent with a degradation in muscle efficiency during the 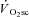 that occurs without recruitment changes, not unlike in situ observations available in the literature (Nagesser et al. 1993; Barclay, 1996; Zoladz et al. 2008; Hepple et al. 2010). This suggests that the
that occurs without recruitment changes, not unlike in situ observations available in the literature (Nagesser et al. 1993; Barclay, 1996; Zoladz et al. 2008; Hepple et al. 2010). This suggests that the 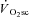 may be consequent to the metabolic disturbances from fatigue in muscle fibres recruited from exercise onset.
may be consequent to the metabolic disturbances from fatigue in muscle fibres recruited from exercise onset.
Fatigue and skeletal muscle contractile efficiency
An increased rate of O2 consumption has been shown in isolated Xenopus laevis fibres following fatigue without additional fibre recruitment (Nagesser et al. 1993). In these experiments, force output fell substantially but the rate of O2 consumption persisted at its maximum (Nagesser et al. 1993), akin to the increase in O2 cost of power output seen during the 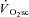 in humans. This slow component-like response has also been shown in an electrically stimulated pump-perfused canine hindlimb model, where the time–tension integral was reduced but
in humans. This slow component-like response has also been shown in an electrically stimulated pump-perfused canine hindlimb model, where the time–tension integral was reduced but 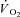 was maintained (Zoladz et al. 2008). Together these data show that a progressive reduction in efficiency can occur without alterations in motor unit recruitment (as all motor units are recruited during electrical stimulation) (Zoladz et al. 2008). Moreover, a greater change in intracellular
was maintained (Zoladz et al. 2008). Together these data show that a progressive reduction in efficiency can occur without alterations in motor unit recruitment (as all motor units are recruited during electrical stimulation) (Zoladz et al. 2008). Moreover, a greater change in intracellular 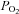 has been observed in slowly fatiguing single Xenopus type III muscle fibres compared with their fast-fatiguing counterparts (Hepple et al. 2010). The implication from these data is that the O2 cost of force production may increase during fatigue in human type I fibres, whereas it remains constant during fatigue in type II fibres. The present data, showing maintenance of velocity-specific peak power production during the
has been observed in slowly fatiguing single Xenopus type III muscle fibres compared with their fast-fatiguing counterparts (Hepple et al. 2010). The implication from these data is that the O2 cost of force production may increase during fatigue in human type I fibres, whereas it remains constant during fatigue in type II fibres. The present data, showing maintenance of velocity-specific peak power production during the 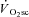 , are consistent with this mechanism.
, are consistent with this mechanism.
It is important to note that estimates of contractile velocity during cycling at 90 rpm (such as in the present study) relate to ∼50% of whole muscle Vmax (Sargeant, 1999). Using estimates from mouse fibre bundles, it has been suggested that type I fibres exhibit a reduction in mechanical efficiency during fatigue at contraction velocities greater than ∼10%Vmax (Barclay, 1996). Mechanical efficiency of type II fibres in a fatigued state, on the other hand, is relatively well protected up to ∼60%Vmax (Barclay, 1996). Therefore, at contraction velocities appropriate for comparison to cycling exercise in humans (i.e. accounting for the differences in Vmax between mouse and human fibres), a moderate degree of fatigue (reduction in peak force) in type I fibres may have a greater influence on efficiency than comparable fatigue in type II. In other words, at contraction velocities corresponding to 90 rpm, human type I fibres are likely to reside on the descending limb of their efficiency–velocity relationship (Sargeant, 1999), which is highly sensitive to fatigue because they may exhibit appreciably more force-generating states that resist filament sliding (Barclay, 1996). Type II fibres, however, may be on the ascending limb of their efficiency–velocity relationship, which is relatively well protected during fatigue.
The present data are consistent with the notion that fatigue is necessary to initiate the 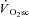 and, while speculative, in situ observations from muscle preparations (Nagesser et al. 1993; Barclay, 1996; Zoladz et al. 2008; Hepple et al. 2010) suggest this increase in ATP and/or O2 cost of power production may derive, in large part, from a reduced mechanical efficiency in type I fibres during fatigue. Therefore, the large fall in power production at 120 rpm during VH in the present study presumably reflects type II fibre fatigue that is not expected to have a large impact on efficiency during exercise at 90 rpm. Rather fatigue in type I fibres, exposed by isokinetic cycling at 60 rpm, may have a greater impact on efficiency and the
and, while speculative, in situ observations from muscle preparations (Nagesser et al. 1993; Barclay, 1996; Zoladz et al. 2008; Hepple et al. 2010) suggest this increase in ATP and/or O2 cost of power production may derive, in large part, from a reduced mechanical efficiency in type I fibres during fatigue. Therefore, the large fall in power production at 120 rpm during VH in the present study presumably reflects type II fibre fatigue that is not expected to have a large impact on efficiency during exercise at 90 rpm. Rather fatigue in type I fibres, exposed by isokinetic cycling at 60 rpm, may have a greater impact on efficiency and the 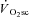 during supra-LT CWR exercise.
during supra-LT CWR exercise.
Additional considerations
After exercise onset, the time at which the 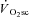 is initiated is unknown. It has been recently highlighted (Stirling & Zakynthinaki, 2009; Whipp, 2009) that the observation of the
is initiated is unknown. It has been recently highlighted (Stirling & Zakynthinaki, 2009; Whipp, 2009) that the observation of the 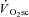 at 2–3 min after exercise onset may reflect its emergence from the large-magnitude fundamental phase rather than its time of onset. That is, the slow kinetics inherent in the
at 2–3 min after exercise onset may reflect its emergence from the large-magnitude fundamental phase rather than its time of onset. That is, the slow kinetics inherent in the 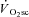 may mean that it is only observable (with current techniques) after the first few minutes of exercise, but that it may actually begin closer to, or at, exercise onset (Stirling & Zakynthinaki, 2009). This is an important consideration in our study, as the temporal association (or dissociation) of skeletal muscle fatigue and the
may mean that it is only observable (with current techniques) after the first few minutes of exercise, but that it may actually begin closer to, or at, exercise onset (Stirling & Zakynthinaki, 2009). This is an important consideration in our study, as the temporal association (or dissociation) of skeletal muscle fatigue and the 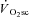 is dependent on this time delay. The absence of a distorting influence in the exponential fundamental phase has been used as evidence to suggest that the onset of the
is dependent on this time delay. The absence of a distorting influence in the exponential fundamental phase has been used as evidence to suggest that the onset of the 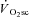 is actually delayed from exercise onset (Whipp, 2009). An absence of the
is actually delayed from exercise onset (Whipp, 2009). An absence of the 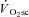 in the recovery kinetics of fatiguing exercise lasting less than ∼60 s (Turner et al. 2006) suggests that the
in the recovery kinetics of fatiguing exercise lasting less than ∼60 s (Turner et al. 2006) suggests that the 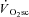 requires at least a finite duration to become manifest. This latter finding is in accordance with the present data suggesting that muscle fatigue, which takes time to develop, is required to initiate the
requires at least a finite duration to become manifest. This latter finding is in accordance with the present data suggesting that muscle fatigue, which takes time to develop, is required to initiate the 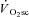 .
.
Additionally, we were unable to determine whether changes in muscle temperature affected the power production during cycling. It has been reported in the literature that passive warming or cooling of the legs can modulate power output (Sargeant, 1987). This effect may be quite large, as an average of 2.7°C increase (although this was heterogeneous depending on the muscle depth) in muscle temperature with passive warming results in an 11% increase in power output (Sargeant, 1987). Therefore, our data could reflect an underestimation of fatigue and a normalisation of muscle power production with increasing time and intensity, especially during the VH8 condition where heat production was at its greatest. However, this effect has also been observed during moderate-intensity exercise (Sargeant & Dolan, 1987), yet velocity-specific peak power was unaffected in the Mod condition in our experiments. Therefore, although the 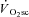 is unaffected by passive heating (Koga et al. 1997), temperature would probably influence our measurement of muscle fatigue. The extent to which muscle temperature influenced the relationship between muscle fatigue and
is unaffected by passive heating (Koga et al. 1997), temperature would probably influence our measurement of muscle fatigue. The extent to which muscle temperature influenced the relationship between muscle fatigue and 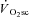 is unknown.
is unknown.
Conclusions
In this investigation we have shown that skeletal muscle fatigue is requisite for the development of the 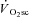 and is absent during moderate-intensity exercise at 80% LT. Surprisingly, fatigue occurred early in the exercise transient, and velocity-specific peak power was maintained after 3 min exercise, even at very heavy-intensity work rates, despite the majority of the
and is absent during moderate-intensity exercise at 80% LT. Surprisingly, fatigue occurred early in the exercise transient, and velocity-specific peak power was maintained after 3 min exercise, even at very heavy-intensity work rates, despite the majority of the 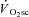 evolving during this time. Consequently, skeletal muscle fatigue was temporally dissociated from the
evolving during this time. Consequently, skeletal muscle fatigue was temporally dissociated from the 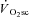 .
.
The ‘fatigue and recruitment’ hypothesis for the 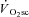 suggests that muscle fatigue occurring during supra-LT exercise demands an increased fibre recruitment in order for power output to be maintained. This recruitment is presumed to be of poorly efficient type II fibres which results in an increased ATP and O2 cost of exercise, manifest as the
suggests that muscle fatigue occurring during supra-LT exercise demands an increased fibre recruitment in order for power output to be maintained. This recruitment is presumed to be of poorly efficient type II fibres which results in an increased ATP and O2 cost of exercise, manifest as the 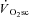 . While our initial observation of muscle fatigue during exercise above LT is, therefore, consistent with the ‘fatigue’ part of this hypothesis, our observation of maintained velocity-specific peak power after 3 min of exercise is inconsistent with the necessity for progressive fibre recruitment. Rather, these data suggest that an increased ATP and/or O2 cost of power production in fatigued fibres is responsible for the
. While our initial observation of muscle fatigue during exercise above LT is, therefore, consistent with the ‘fatigue’ part of this hypothesis, our observation of maintained velocity-specific peak power after 3 min of exercise is inconsistent with the necessity for progressive fibre recruitment. Rather, these data suggest that an increased ATP and/or O2 cost of power production in fatigued fibres is responsible for the 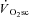 during dynamic exercise in humans.
during dynamic exercise in humans.
Acknowledgments
We wish to thank Tim Burnett and Laura Palombo for their assistance with data collection, and Scott Bowen, Scott Murgatroyd and Rob Wüst for their helpful discussion and comments.
Glossary
Abbreviations
- CIDiff
95% confidence interval of the difference
- Con
control
- CP
critical power threshold
- CWR
constant work rate
- d
time delay
- EMG
electromyography
- LT
lactate threshold
- Mod
moderate intensity
- MPF
mean/median power frequency
- H
heavy intensity
- H3
heavy intensity of 3 min duration
- H8
heavy intensity of 8 min duration
- κ
number of post hoc comparisons
- PCr
phosphocreatine
- Pi
inorganic phosphate
- RER
respiratory exchange ratio
- RI
ramp incremental exercise test
- SE
step exercise test
- τ
time constant
- T2
1H transverse relaxation time
- VH
very heavy intensity
- VH3
very heavy intensity of 3 min duration
- VH8
very heavy intensity of 8 min duration
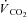
rate of carbon dioxide output
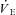
minute ventilation
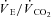
ventilatory equivalent for carbon dioxide
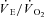
ventilatory equivalent for oxygen
- Vmax
maximal shortening velocity
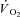
rate of oxygen uptake
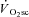
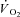 slow component
slow component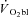
baseline
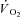
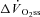
fundamental amplitude of
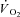
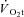
time variant form of
Author contributions
H.B.R., D.T.C. and F.W.K. conceived of, and designed the experiments. D.T.C., A.C.W. and M.F.A. completed the data collection in the Fred W. Kasch Exercise Physiology Laboratory at San Diego State University. D.T.C., H.B.R., A.C.W. and M.F.A. analysed the data for this experiment. D.T.C., H.B.R. and F.W.K. drafted and critically revised the manuscript. All authors approved the final version of the manuscript.
References
- Adams GR, Duvoisin MR, Dudley GA. Magnetic resonance imaging and electromyography as indexes of muscle function. J Appl Physiol. 1992;73:1578–1583. doi: 10.1152/jappl.1992.73.4.1578. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Avogadro P, Dolenec A, Belli A. Changes in mechanical work during severe exhausting running. Eur J Appl Physiol. 2003;90:165–170. doi: 10.1007/s00421-003-0846-y. [DOI] [PubMed] [Google Scholar]
- Avogadro P, Kyrolainen H, Belli A. Influence of mechanical and metabolic strain on the oxygen consumption slow component during forward pulled running. Eur J Appl Physiol. 2004;93:203–209. doi: 10.1007/s00421-004-1200-8. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol. 1996;497:781–794. doi: 10.1113/jphysiol.1996.sp021809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Beelen A, Sargeant AJ. Effect of fatigue on maximal power output at different contraction velocities in humans. J Appl Physiol. 1991;71:2332–2337. doi: 10.1152/jappl.1991.71.6.2332. [DOI] [PubMed] [Google Scholar]
- Bernasconi S, Tordi N, Perrey S, Parratte B, Monnier G. Is the VO2 slow component in heavy arm-cranking exercise associated with recruitment of type II muscle fibers as assessed by an increase in surface EMG? Appl Physiol Nutr Metab. 2006;31:414–422. doi: 10.1139/h06-021. [DOI] [PubMed] [Google Scholar]
- Borrani F, Candau R, Millet GY, Perrey S, Fuchslocher J, Rouillon JD. Is the VO2 slow component dependent on progressive recruitment of fast-twitch fibers in trained runners? J Appl Physiol. 2001;90:2212–2220. doi: 10.1152/jappl.2001.90.6.2212. [DOI] [PubMed] [Google Scholar]
- Burnley M, Doust JH, Ball D, Jones AM. Effects of prior heavy exercise on VO2 kinetics during heavy exercise are related to changes in muscle activity. J Appl Physiol. 2002;93:167–174. doi: 10.1152/japplphysiol.01217.2001. [DOI] [PubMed] [Google Scholar]
- Burnley M, Doust JH, Vanhatalo A. A 3-min all-out test to determine peak oxygen uptake and the maximal steady state. Med Sci Sports Exerc. 2006;38:1995–2003. doi: 10.1249/01.mss.0000232024.06114.a6. [DOI] [PubMed] [Google Scholar]
-
Cleuziou C, Perrey S, Borrani F, Lecoq AM, Courteix D, Germain P, Obert P.
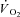 and EMG activity kinetics during moderate and severe constant work rate exercise in trained cyclists. Can J Appl Physiol. 2004;29:758–772. doi: 10.1139/h04-049. [DOI] [PubMed] [Google Scholar]
and EMG activity kinetics during moderate and severe constant work rate exercise in trained cyclists. Can J Appl Physiol. 2004;29:758–772. doi: 10.1139/h04-049. [DOI] [PubMed] [Google Scholar] - Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol. 2010;104:1034–1046. doi: 10.1152/jn.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo MY, Kobayakawa M, Kinugasa R, Kuno S, Akima H, Rossiter HB, Miura A, Fukuba Y. Thigh muscle activation distribution and pulmonary VO2 kinetics during moderate, heavy, and very heavy intensity cycling exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R812–R820. doi: 10.1152/ajpregu.00028.2007. [DOI] [PubMed] [Google Scholar]
- Farina D. Counterpoint: spectral properties of the surface EMG do not provide information about motor unit recruitment and muscle fiber type. J Appl Physiol. 2008;105:1673–1674. doi: 10.1152/japplphysiol.90598.2008a. [DOI] [PubMed] [Google Scholar]
- Gaesser GA. Influence of endurance training and catecholamines on exercise VO2 response. Med Sci Sports Exerc. 1994;26:1341–1346. [PubMed] [Google Scholar]
- Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev. 1996;24:35–71. [PubMed] [Google Scholar]
- Gamet D, Duchene J, Garapon-Bar C, Goubel F. Surface electromyogram power spectrum in human quadriceps muscle during incremental exercise. J Appl Physiol. 1993;74:2704–2710. doi: 10.1152/jappl.1993.74.6.2704. [DOI] [PubMed] [Google Scholar]
- Garland SW, Wang W, Ward SA. Indices of electromyographic activity and the “slow” component of oxygen uptake kinetics during high-intensity knee-extension exercise in humans. Eur J Appl Physiol. 2006;97:413–423. doi: 10.1007/s00421-006-0185-x. [DOI] [PubMed] [Google Scholar]
- Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–2196. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Howlett RA, Kindig CA, Stary CM, Hogan MC. The O2 cost of the tension-time integral in isolated single myocytes during fatigue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R983–R988. doi: 10.1152/ajpregu.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai DM, Roseguini BT, Diefenthaeler F, Carpes FP, Vaz MA, Ferlin EL, Ribeiro JP, Nakamura FY. Effects of altering pedal frequency on the slow component of pulmonary VO2 kinetics and EMG activity. Int J Sports Med. 2010;31:529–536. doi: 10.1055/s-0030-1251989. [DOI] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. Motor unit recruitment for dynamic tasks: current understanding and future directions. J Comp Physiol B. 2009;179:57–66. doi: 10.1007/s00360-008-0289-1. [DOI] [PubMed] [Google Scholar]
- Hultman E, Bergstrom J, Anderson NM. Breakdown and resynthesis of phosphorylcreatine and adenosine triphosphate in connection with muscular work in man. Scand J Clin Lab Invest. 1967;19:56–66. doi: 10.3109/00365516709093481. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24:654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol. 2008;294:R585–R593. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Kondo N, Barstow TJ. Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol. 1997;83:1333–1338. doi: 10.1152/jappl.1997.83.4.1333. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. Slow-twitch fiber glycogen depletion elevates moderate-exercise fast-twitch fiber activity and O2 uptake. Med Sci Sports Exerc. 2004a;36:973–982. doi: 10.1249/01.mss.0000128246.20242.8b. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004b;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Lucia A, Hoyos J, Chicharro JL. The slow component of VO2 in professional cyclists. Br J Sports Med. 2000;34:367–374. doi: 10.1136/bjsm.34.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Prior BM. Functional magnetic resonance imaging of muscle. Exerc Sport Sci Rev. 2000;28:89–92. [PubMed] [Google Scholar]
- Migita T, Hirakoba K. Effect of different pedal rates on oxygen uptake slow component during constant-load cycling exercise. J Sports Med Phys Fitness. 2006;46:189–196. [PubMed] [Google Scholar]
- Nagesser AS, Van Der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. J Muscle Res Cell Motil. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Osborne MA, Schneider DA. Muscle glycogen reduction in man: relationship between surface EMG activity and oxygen uptake kinetics during heavy exercise. Exp Physiol. 2006;91:179–189. doi: 10.1113/expphysiol.2005.031450. [DOI] [PubMed] [Google Scholar]
- Perrey S, Betik A, Candau R, Rouillon JD, Hughson RL. Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J Appl Physiol. 2001;91:2135–2142. doi: 10.1152/jappl.2001.91.5.2135. [DOI] [PubMed] [Google Scholar]
- Poole DC. Role of exercising muscle in slow component of VO2. Med Sci Sports Exerc. 1994;26:1335–1340. [PubMed] [Google Scholar]
- Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31:1265–1279. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- Pringle JS, Doust JH, Carter H, Tolfrey K, Jones AM. Effect of pedal rate on primary and slow-component oxygen uptake responses during heavy-cycle exercise. J Appl Physiol. 2003;94:1501–1507. doi: 10.1152/japplphysiol.00456.2002. [DOI] [PubMed] [Google Scholar]
- Pringle JS, Jones AM. Maximal lactate steady state, critical power and EMG during cycling. Eur J Appl Physiol. 2002;88:214–226. doi: 10.1007/s00421-002-0703-4. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Kowalchuk JM, Whipp BJ. A test to establish maximum O2 uptake despite no plateau in the O2 uptake response to ramp incremental exercise. J Appl Physiol. 2006;100:764–770. doi: 10.1152/japplphysiol.00932.2005. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ. Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol. 2002a;93:2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002b;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy S, Schneider DA, Morris NR. The VO2 slow component: relationship between plasma ammonia and EMG activity. Med Sci Sports Exerc. 2005;37:1502–1509. doi: 10.1249/01.mss.0000182497.43221.e1. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ. Effect of muscle temperature on leg extension force and short-term power output in humans. Eur J Appl Physiol Occup Physiol. 1987;56:693–698. doi: 10.1007/BF00424812. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ. Neuromuscular determinants of human perfornace. In: Whipp BJ, Sargeant AJ, editors. Studies in Physiology: Four. Oxford: Information Press Ltd; 1999. pp. 13–28. [Google Scholar]
- Sargeant AJ, Dolan P. Effect of prior exercise on maximal short-term power output in humans. J Appl Physiol. 1987;63:1475–1480. doi: 10.1152/jappl.1987.63.4.1475. [DOI] [PubMed] [Google Scholar]
- Saunders MJ, Evans EM, Arngrimsson SA, Allison JD, Warren GL, Cureton KJ. Muscle activation and the slow component rise in oxygen uptake during cycling. Med Sci Sports Exerc. 2000;32:2040–2045. doi: 10.1097/00005768-200012000-00012. [DOI] [PubMed] [Google Scholar]
- Scheuermann BW, Hoelting BD, Noble ML, Barstow TJ. The slow component of O2 uptake is not accompanied by changes in muscle EMG during repeated bouts of heavy exercise in humans. J Physiol. 2001;531:245–256. doi: 10.1111/j.1469-7793.2001.0245j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Moritani T. Increase in neuromuscular activity and oxygen uptake during heavy exercise. Ann Physiol Anthropol. 1992;11:257–262. [PubMed] [Google Scholar]
- Stirling JR, Zakynthinaki M. Counterpoint: the kinetics of oxygen uptake during muscular exercise do not manifest time-delayed phases. J Appl Physiol. 2009;107:1665–1667. doi: 10.1152/japplphysiol.00158.2009a. discussion 1667–1668. [DOI] [PubMed] [Google Scholar]
- Tordi N, Perrey S, Harvey A, Hughson RL. Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol. 2003;94:533–541. doi: 10.1152/japplphysiol.00532.2002. [DOI] [PubMed] [Google Scholar]
- Turner AP, Cathcart AJ, Parker ME, Butterworth C, Wilson J, Ward SA. Oxygen uptake and muscle desaturation kinetics during intermittent cycling. Med Sci Sports Exerc. 2006;38:492–503. doi: 10.1249/01.mss.0000188450.82733.f0. [DOI] [PubMed] [Google Scholar]
- Vercruyssen F, Missenard O, Brisswalter J. Relationship between oxygen uptake slow component and surface EMG during heavy exercise in humans: influence of pedal rate. J Electromyogr Kinesiol. 2009;19:676–684. doi: 10.1016/j.jelekin.2008.02.005. [DOI] [PubMed] [Google Scholar]
- von Tscharner V, Nigg BM. Point: spectral properties of the surface EMG can characterize/do not provide information about motor unit recruitment strategies and muscle fiber type. J Appl Physiol. 2008;105:1671–1673. doi: 10.1152/japplphysiol.90598.2008. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26:1319–1326. [PubMed] [Google Scholar]
- Whipp BJ. Point: the kinetics of oxygen uptake during muscular exercise do manifest time-delayed phases. J Appl Physiol. 2009;107:1663–1665. doi: 10.1152/japplphysiol.00158.2009. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Rossiter HB. The kinetics of oxygen uptake: physiological inferences from the parameters. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise, and Medicine. London: Routledge; 2005. pp. 64–94. [Google Scholar]
- Yano T, Yunoki T, Ogata H. Relationship between the slow component of oxygen uptake and the potential reduction in maximal power output during constant-load exercise. J Sports Med Phys Fitness. 2001;41:165–169. [PubMed] [Google Scholar]
- Zoladz JA, Gladden LB, Hogan MC, Nieckarz Z, Grassi B. Progressive recruitment of muscle fibers is not necessary for the slow component of VO2 kinetics. J Appl Physiol. 2008;105:575–580. doi: 10.1152/japplphysiol.01129.2007. [DOI] [PubMed] [Google Scholar]


