Abstract
Bovine seminal ribonuclease (BS-RNase) is a dimer in which the subunits are cross-linked by disulfide bonds between Cys31 of one subunit and Cys32 of the other. Dimeric BS-RNase is resistant to ribonuclease inhibitor (RI), a protein endogenous to mammalian cells, and is toxic to a variety of cell types. Monomeric BS-RNase (like its homolog, RNase A) is bound tightly by RI and is not cytotoxic. The three-dimensional structure of the RI·RNase A complex suggests that carboxymethylation of C32S BS-RNase (to give MCM31) or C31S BS-RNase (MCM32) could diminish affinity for RI. We find that MCM31 and MCM32 are not only resistant to RI, but are also aspermatogenic to mice. In contrast to the aspermatogenic activity of dimeric BS-RNase, that of MCM31 and MCM32 is directed only at spermatogenic layers. Intratesticular injection of MCM31 or MCM32 affects neither the diameter of seminiferous tubules nor the weight of testes. Also in contrast to wild-type BS-RNase, MCM31 and MCM32 are not toxic to other cell types. Direct immunofluorescence reveals that MCM31 and MCM32 bind only to spermatogonia and primary spermatocytes. This cell specificity makes MCM31 and MCM32 of potential use in seminoma therapy and contraception.
Keywords: Bovine Seminal Ribonuclease, Carboxymethylation, Cytotoxic, Mice, Ribonuclease, Ribonuclease Inhibitor, Seminoma, Spermatogenesis
INTRODUCTION
Spermatogenesis is a complex developmental process that begins with the differentiation of male primordial germ cells arising from the embryonic ectoderm. During embryogenesis, these cells stop dividing and remain quiescent within the seminiferous tubules. Following birth, the primordial germ cells (which are referred to as gonocytes) differentiate into three principal types of spermatogonia: stem cell spermatogonia, proliferative spermatogonia and differentiating spermatogonia (46). The first two types are able to undergo self-renewal to produce differentiating spermatogonia. These cells form the primary spermatocytes that initiate meiosis and produce secondary spermatocytes and spermatids. Round spermatids undergo spermiogenesis, yielding morphologically and functionally distinct mature spermatozoa. The recent generation of rat spermatozoa in mouse testes indicates that spermatogenesis can be xenogeneic (8).
The seminal plasma and seminal vesicle fluid of bulls contain an unusual ribonuclease (15,17). Bovine seminal ribonuclease (BS-RNase) has aspermatogenic activity in mice and other animals (14,19,30). Fluorescently-labeled enzyme injected subcutaneously into sexually mature male mice appears in the seminiferous tubules of testes within 2 hr, and remains there for 24 hr (34). Cells of the spermatogenic epithelium, including secondary spermatocytes, spermatozoa and Sertoli cells, do not attract BS-RNase (34). Instead, BS-RNase binds to the spermatogonia and primary spermatocytes.
The aspermatogenic activity of BS-RNase appears to result from its ability to catalyze the cleavage of cellular RNA. For example, the aspermatogenic activity of BS-RNase coincides with the degradation of cellular RNA (35). Further, the ribonucleolytic activity of BS-RNase is necessary for its aspermatogenic activity (23).
What is the molecular basis for the toxicity of BS-RNase to mammalian cells? The enzyme is a small protein (2 × 13.6 kDa) of known three-dimensional structure (39). To begin our exploration, we synthesized a gene that codes for BS-RNase, expressed this gene in Escherichia coli, and isolated active dimeric enzyme (22). To illuminate the role of the two intersubunit disulfide bonds of BS-RNase, we prepared C31S and C32S BS-RNase, which have only one intersubunit disulfide bond. Replacing Cys31 or Cys32 with a serine residue does not compromise the enzymatic activity of dimeric BS-RNase, but reduces its cytotoxicity. The loss of cytotoxic activity correlates with the fraction of enzyme that becomes monomeric in the reducing environment of the cytosol. Mammalian cells contain ribonuclease inhibitor (RI) (45), a protein that binds tightly to monomeric but not dimeric BS-RNase (24,41,42). RI appears to act as a sentry, protecting cellular RNA from invading secretory ribonucleases (6,20). We and others therefore proposed that one key to the cytotoxicity of BS-RNase is likely to be its ability to evade cellular RI (7,13,21,25).
Much can be learned about BS-RNase from consideration of its monomeric homologs, bovine pancreatic ribonuclease A (RNase A) and onconase (4). RNase A is bound tightly by RI and is not cytotoxic. Onconase resists RI and is cytotoxic (1,52). In 1995, the three-dimensional structure of a crystalline RI·RNase A complex was determined by X-ray diffraction analysis (26). Confirming the results of chemical modification studies (5), this structure shows that extensive contacts between RI and RNase A occur in the region near Lys31 and Ser32 of RNase A (Fig. 1). In particular, the favorable Coulombic interaction between the cationic side chain of Lys31 of RNase A and the anionic side chain of Asp31 of RI is likely to contribute to the stability of the RI·RNase A complex. We reasoned that placing an anionic side chain at position 31 (or position 32) could compromise the ability of RI to bind to monomeric BS-RNase, and that the resulting protein could be cytotoxic. Here, we report the results of this study.
FIG. 1.
Interactions between ribonuclease inhibitor and ribonucleases. (A) Interactions between residues 6, 7 and 31 of porcine RI, and residues 31 and 32 of bovine RNase A in the RI·RNase A complex (26). (B) Residues 31 and 32 of MCAM, MCM31 and MCM32. In human angiogenin, which like RNase A binds tightly to RI (29), the residues analogous to 31 and 32 are both arginine.
MATERIALS AND METHODS
Ribonuclease Preparation
Wild-type BS-RNase was isolated from bull seminal vesicle fluid as described (14). Wild-type, C31S and C32S BS-RNase were produced in E. coli strain BL21(DE3) by using expression vector pLSR1 and purified as described (22,25).
The nitrogen of a lysine side chain is separated from the main chain by four atoms. In contrast, the oxygens of an aspartate or glutamate side chain are separated by only two or three atoms, respectively. Thus, mutation of a lysine residue to an aspartate or glutamate residue not only exchanges a positive for a negative charge, but also changes the location of that charge. To place carboxylate oxygens 4 atoms from the main chain, we resorted to cysteine elaboration, which we had used previously to probe the role of the active-site lysine residue in RNase A (40).
The sulfhydryl groups of BS-RNase monomers can be alkylated to prevent the dimerization of this protein. Previously, alkylation was accomplished by reacting monomers of wild-type BS-RNase with iodoacetamide (11,25). The resulting carbamoylmethylated monomers were referred to as “M”. Here, we use the more precise “MCAM” to refer to monomers of wild-type BS-RNase with carbamoylmethyl groups [–CH2C(O)NH2] on Cys31 and Cys32, as indicated in Fig. 1 and Table 1. We use “MCM” to refer to monomers with a carboxymethyl group (–CH2CO2–) on Cys31 or Cys32. The forms of BS-RNase listed in Table 1 were prepared from wild-type, C31S or C32S BS-RNase by procedures analogous to those described previously (11,40). The additional negative charge on MCM31 and MCM32 enabled us to use cation exchange chromatography (40) to separate these enzymes completely from any unmodified monomers.
Table 1.
Forms of monomeric bovine seminal ribonuclease
| Name | BS-RNase | Residue 31 | Residue 32 |
|---|---|---|---|
| MCAM (or M*) | Wild-type | S-carbamoylmethylcysteine | S-carbamoylmethylcysteine |
| MCM31 | C32S | S-carboxymethylcysteine | Serine |
| MCM32 | C31S | Serine | S-carboxymethylcysteine |
Inhibition by RI
The ability of RI to inhibit the ribonucleolytic activity of various ribonucleases was assessed by a qualitative assay analogous to that of Youle and coworkers (52). Briefly, a ribonuclease (1 pmol) was added to 20 mM Hepes buffer, pH 7.0, containing RI (0 or 60 units, where 1 unit is the amount of RI required to inhibit the activity of 5 ng = 0.4 pmol of RNase A by 50%) (Promega, Madison, WI), calf liver rRNA (3.0 μg; Sigma Chemical, St. Louis, MO), NaCl (125 mM), DTT (10 mM) and EDTA (1 mM). The reaction mixture was incubated at 25°C for 20 min. Then, an aliquot (20 μL) of the reaction mixture was quenched by the addition of a solution (4 μL) of sucrose (40% w/v), bromophenol blue (0.25% w/v) and diethylpyrocarbonate (0.2% v/v). The quenched reaction mixture was subjected to electrophoresis in an agarose (1.2% w/v) gel.
Assay for Immunosuppressive Activity
The effect of the various ribonucleases on the viability of human lymphocytes, which had been stimulated by mixed lymphocyte culture (MLC), was assessed as described (23,25,49).
Assay for Antitumor Activity
The effect of the various ribonucleases on the viability of human leukemic cell lines K-562 and HL-60 (derived from human erythroid leukemia and human myeloid leukemia, respectively) was assessed as described (23,25).
Assay for Embryotoxic Activity
The effect of the various ribonucleases on the viability of 6-day bovine embryos was evaluated as described (23). The hatching ability of the embryos (that is, their ability to leave from the zona pellucida) was assessed 90 hr after exposure to a ribonuclease.
Assay for Aspermatogenic Activity
The effect of ribonucleases on spermatogenesis in Institute for Cancer Research (Fox Chase Cancer Center) mice was evaluated as described (32). Briefly, the left testes of male mice were injected with a solution containing a ribonuclease (50 μg). Ten days later, the mice were sacrificed. The mice and the isolated testes were weighed. The testes were stained with haematoxilin and eosin, and subjected to histological examination. Aspermatogenic activity was assessed by the diameter of seminiferous tubules and the width of spermatogenic layers, and by the index weight (which is 104 × testes weight/body weight). The degree of testicular damage was evaluated on a scale of 0 (normal histological appearance) to 4 (disappearance of all cells of the spermatogenic layers except spermatogonia).
In Vitro Direct Immunofluorescence
Direct immunofluorescence was used to identify the cells of testicular tissue that bind to MCM31 and MCM32. IgG from the antiserum of a rabbit twice immunized with native BS-RNase without adjuvant was conjugated with fluorescein isothiocyanate (FITC) by the Institute of Sera and Vaccines (Prague, Czech Republic). Six male mice were injected intratesticularly with a solution containing MCM31 or MCM32 (80 μg). Two mice were killed and their testes examined 2, 4 and 24 hr after ribonuclease injection.
Statistical Analyses
Results in figures are reported as the mean ± SEM. Data were analyzed for statistical significance with Fisher's t-test.
RESULTS
Inhibition by RI
RI is known to form a complex with RNase A having Kd = 10–15 M (47). RI is also known to bind more tightly to carbamoylmethylated monomers of BS-RNase than to the dimeric enzyme (5). Here, we assessed the ability of RI to bind to various monomeric forms of BS-RNase. As shown in Fig. 2, RI does not inhibit significantly the ribonucleolytic activity of wild-type dimeric BS-RNase from either seminal plasma (lane 2) or E. coli (lane 3). In contrast, RI does inhibit the activity of unmodified monomers of C31S BS-RNase (lane 4) and C32S BS-RNase (lane 5), and of RNase A (data not shown). This inhibition of BS-RNase is eliminated by carboxymethylation. RI does not inhibit significantly the ribonucleolytic activity of MCM32 (lane 6) or MCM31 (lane 7). Thus, the binding of RI to monomers of BS-RNase is weakened by the presence of an S-carboxymethylcysteine residue at either position 31 or 32.
FIG. 2.
Inhibition of ribonucleolytic activity by RI. Inhibition was assessed by observing the ability of a ribonuclease to degrade rRNA in the absence (A) or presence (B) of RI. Lane 1, no ribonuclease; lane 2, BS-RNase from seminal plasma; lane 3, dimeric BS-RNase from E. coli; lane 4, monomeric C32S BS-RNase; lane 5, monomeric C31S BS-RNase; lane 6, MCM31; and lane 7, MCM32.
Immunosuppressive Activity
Previously, we reported that dimeric BS-RNase from either seminal plasma or E. coli displayed strong immunosuppressive activity, and that MCAM displayed weak activity (23,25,48). Now, we assessed the immunosuppressive activities of MCM31 and MCM32 and the dimeric enzymes. As shown in Fig. 3, both MCM31 and MCM32 display immunosuppressive activities that are weak, like that of MCAM.
FIG. 3.

Effect of various forms of BS-RNase on the proliferation in culture of MLC-stimulated human lymphocytes. Proliferation was evaluated by the incorporation of [6-3H]thymidine into cellular DNA. Values are the mean from three cultures and are reported as a percent of the control, which was the mean value from cultures containing no exogenous ribonuclease. Data were recorded 6 days after addition of ribonuclease to the culture.
Antitumor Activity
Dimeric BS-RNase from either seminal plasma or E. coli inhibits the growth of mouse and human tumor cells of a lymphoid type in culture (23,25,38,50). Here, we chose K562 (which are sensitive to wild-type BS-RNase) and HL-60 (which are less sensitive) to test the anti-proliferative activity of MCM31 and MCM32. Neither of these enzymes is toxic to the leukemic cells (data not shown).
Embryotoxic Activity
BS-RNase from either E. coli or seminal plasma is toxic to 6-day bovine embryos, as shown in Fig. 4. In contrast, incubation with MCM31 or MCM32 is not toxic. MCM31 and MCM32 do, however, decrease the ability of bovine embryos to hatch. After 90 hr, the fraction of embryos hatching from each incubation was PBS: 12 of 15; MCM31: 2 of 15; MCM32: 1 of 15; wild-type (E. coli): 0 of 15; wild-type (seminal plasma): 0 of 7.
FIG. 4.

Effect of various forms of BS-RNase on the survival of 6-day bovine embryos in culture. Each assay began with 15 embryos except for that of BS-RNase (E. coli), which began with 7 embryos.
Aspermatogenic Activity
Intratesticular injection of BS-RNase from either E. coli or seminal plasma blocks spermatogenesis in male mice. As shown in Fig. 5, the diameter of seminiferous tubules, width of spermatogenic layers and weight of testes are all diminished significantly. In contrast to the broad antitesticular effects of wild-type BS-RNase, MCM31 and MCM32 decrease significantly only the width of spermatogenic layers (Fig. 5). This effect is also evident in Fig. 6. The degree of testicular damage caused by each ribonuclease was judged to be PBS: 0, MCAM: 0; MCM31: 2–3; MCM32: 1–3; wild-type (E. coli): 1–3; wild-type (seminal plasma): 1–3.
FIG. 5.
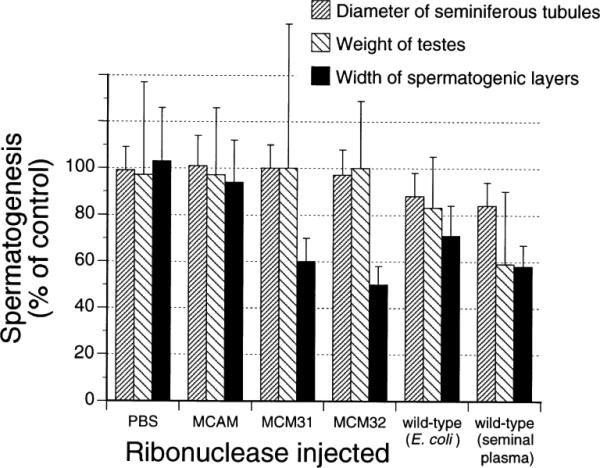
Effect of various forms of BS-RNase on mouse spermatogenesis. Values are an average from 13 (MCM31), 12 (MCM32), 6 (BS-RNase, seminal plasma; PBS) or 4 (BS-RNase, E. coli) injected testes and are reported as a percent of the control, which is from the non-injected testes of the same mice. Data were recorded 10 days after injection. Data for MCAM are from (25).
FIG. 6.
Effect of MCM31 and MCM32 on mice testes. Images were obtained 10 days after intratesticular injection of PBS (A). Spermatogenic epithelium, including all spermatogenic cells and spermatozoa, are normal. (B) MCM31 (50 μg); (C) MCM32 (50 μg). Spermatogonia are intact but primary spermatocytes are degenerated. Bar, 20 μm.
Binding of MCM31 and MCM32 to Spermatogenic Cells
MCM31 and MCM32 injected into testes localize in spermatogonia and primary spermatocytes. Fig. 7 shows the fluorescence emitted from a section of testes probed with FITC-conjugated IgG against native BS-RNase. Fluorescence was observed 2 hr (Fig. 7B) and 4 hr (data not shown) after injection, but not 24 hr after injection (data not shown). Cells of the spermatogenic epithelium, including spermatozoa and Sertoli cells, did not fluoresce.
FIG. 7.
Localization of MCM31 in mouse testis. Images were obtained by direct immunofluorescence 2 hr after injection of MCM31 (80 μg). (A) Visualization with FITC-conjugated normal IgG. (B) Visualization with FITC-conjugated IgG against BS-RNase. Bar, 20 μm.
DISCUSSION
Bovine seminal ribonuclease (BS-RNase) belongs to a group of ribonucleases with special biological activities (9,10). This enzyme, like other ribonucleases, catalyzes the transphosphorylation of RNA and the hydrolysis of the resulting 2′,3′-cyclic phosphodiester intermediate (31,51). In addition, BS-RNase is cytotoxic, which gives rise to aspermatogenic, immunosuppressive, antiembryo and antitumor activities (33).
The cytotoxic activity of bovine ribonucleases has long been associated with their dimeric form (2,3,12,13). Still, the cytotoxicity of onconase (1,52,53) and angiogenin (36,37), two monomeric ribonucleases, suggests that a dimeric form is not essential for a ribonuclease to be cytotoxic. The aspermatogenic activity of MCM31 and MCM32, which are monomers, supports further the possibility that a dimeric structure is not a requirement for ribonuclease cytotoxicity. Instead, the key to ribonuclease cytotoxicity appears to be the evasion of cellular RI (24). All data are consistent with the hypothesis that ribonucleases, whether monomeric or dimeric, are cytotoxic if they can gain access to cellular RNA without being bound by RI. Further, our finding that a noncytotoxic monomer can be endowed with cytotoxic activity presages the creation of new ribonucleases with useful cytotoxic activities.
The difference in the aspermatogenic activity of wild-type BS-RNase and that of MCM31 and MCM32 is substantial. The dimeric enzyme decreases the diameter of seminiferous tubules, the width of spermatogenic layers and the weight of testes (Fig. 5). In contrast, the monomers damage only the spermatogenic layers (Fig. 5 and 6). The binding of the cytotoxic monomers to spermatagonia and primary spermatocytes (Fig. 7) is essentially identical to that of wild-type BS-RNase (34). This result suggests that differential binding to a cell-surface receptor is not responsible for the difference in cytotoxicity.
What is the molecular basis for this difference in cytotoxicity? Carboxymethylated monomers of wild-type BS-RNase are susceptible to inactivation by RI, but less so than is RNase A (42). We find that adding a carboxymethyl group to either Cys31 (in C32S BS-RNase) or Cys32 (in C31S BS-RNase) increases the resistance of monomers to RI (Fig. 2), consistent with a simple Coulombic depiction of binding (Fig. 1). Different tissues have been found to contain different concentrations of RI (29). A possible explanation for the toxicity of MCM31 and MCM32 for spermatogenic layers is that these cells do not contain enough RI to inactivate invading MCM31 and MCM32. This explanation leads to the prediction that the susceptibility of a cell to a cytotoxic ribonuclease correlates with its RI level. The level of RI in spermatogenic layers is now unknown. Nevertheless, this prediction is borne out with lymphocytes, which are vulnerable to BS-RNase (Fig. 3) and which contain a low concentration of RI (27,28). Further, angiogenin is toxic to lymphocytes (36,37). Angiogenin appears to differ from BS-RNase and onconase in being bound tightly by RI (29) and in entering cells by receptor-mediated endocytosis (18,44). An influx of angiogenin may be able to overwhelm the low RI level in lymphocytes. Similarly, a carboxymethylated monomer of wild-type BS-RNase is cytotoxic in the presence of retinoic acid or monensin (53), which disrupt the Golgi apparatus and could thereby enhance access to cellular RNA.
By mutagenesis and chemical modification, we have been able to refine the broad cytotoxicity of BS-RNase. In MCM31 and MCM32, this toxicity is directed specifically to the cells of spermatogenic layers. Because of this narrow specificity, MCM31 and MCM32 may be useful in the contraceptive blocking of spermatogenesis. In addition, these ribonucleases may serve in the treatment of seminomas (which constitute approximately 40% of testicular cancer) and other types of germ cell neoplasia (16,43). Finally, our results suggest that mutations that modulate the interaction of ribonucleases with RI may lead to new molecules with useful cytotoxicity profiles.
Acknowledgments
We thank Drs. T. Slavík and L. W. Schultz, and M. Hokesová and G. Lindnerová for technical assistance; and Dr. B. Kobe for providing the atomic coordinates of the RI·RNase A complex. This work was supported by grant 523/96/1738 from the Grant Agency of the Czech Republic (to J.M.) and by the National Institutes of Health. J.-S.K was a Steenbock predoctoral fellow. P.A.L. is a molecular biosciences predoctoral trainee (NIH T32 GM07215).
REFERENCES
- 1.Ardelt W, Mikulski SM, Shogen K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. J. Biol. Chem. 1991;266:245–251. [PubMed] [Google Scholar]
- 2.Bartholeyns J, Baudhuin P. Inhibition of tumor cell proliferation by dimerized ribonuclease. Proc. Natl. Acad. Sci. USA. 1976;73:573–576. doi: 10.1073/pnas.73.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholeyns J, Zenebergh A. In vitro and in vivo antitumor effect of dimerized ribonuclease A. Eur. J. Cancer. 1979;15:85–91. doi: 10.1016/0014-2964(79)90209-3. [DOI] [PubMed] [Google Scholar]
- 4.Beintema JJ, Schüller C, Irie M, Carsana A. Molecular evolution of the ribonuclease superfamily. Prog. Biophys. Molec. Biol. 1988;51:165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn P, Gavilanes JG. Identification of lysine residues in the binding domain of ribonuclease A for the RNase inhibitor from human placenta. J. Biol. Chem. 1982;255:10959–10965. [PubMed] [Google Scholar]
- 6.Blackburn P, Moore S. Pancreatic ribonuclease. The Enzymes. 1982;XV:317–433. [Google Scholar]
- 7.Cafaro B, De Lorenzo C, Piccoli R, Bracale A, Mastronicola MR, Di Donato A, D'Alessio G. The antitumor action of seminal ribonuclease and its quaternary conformations. FEBS Lett. 1995;359:31–34. doi: 10.1016/0014-5793(94)01450-f. [DOI] [PubMed] [Google Scholar]
- 8.Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–421. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alessio G. New and cryptic biological messages from RNases. Trends Cell Biol. 1993;3:106–109. doi: 10.1016/0962-8924(93)90166-x. [DOI] [PubMed] [Google Scholar]
- 10.D'Alessio G, Di Donato A, Parente A, Piccoli R. Seminal RNase: a unique member of the ribonuclease superfamily. Trends Biochem. Sci. 1991;16:106–108. doi: 10.1016/0968-0004(91)90042-t. [DOI] [PubMed] [Google Scholar]
- 11.D'Alessio G, Malorni MC, Parente A. Dissociation of bovine seminal ribonuclease into catalytically active monomers by selective reduction and alkylation of the intersubunit disulfide bridges. Biochemistry. 1975;14:1116–1122. doi: 10.1021/bi00677a004. [DOI] [PubMed] [Google Scholar]
- 12.Di Donato A, Cafaro V, D'Alessio G. Ribonuclease A can be transformed into a dimeric ribonuclease with antitumor activity. J. Biol. Chem. 1994;269:17394–17396. [PubMed] [Google Scholar]
- 13.Di Donato A, Cafaro V, Romeo I, D'Alessio G. Hints on the evolutionary design of a dimeric RNase with special bioactions. Protein Sci. 1995;4:1470–1477. doi: 10.1002/pro.5560040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dostál J, Matoušek J. Isolation and some chemical properties of aspermatogenic substance form bull seminal vesicle fluid. J. Reprod. Fertil. 1973;33:263–274. doi: 10.1530/jrf.0.0330263. [DOI] [PubMed] [Google Scholar]
- 15.Dostál J, Matoušek J. Purification of aspermatogenic substance (AS) from the bull seminal vesicle fluid. J. Reprod. Fert. 1972;31:273–275. doi: 10.1530/jrf.0.0310273. [DOI] [PubMed] [Google Scholar]
- 16.Elbe JN. Spermatocytic seminoma. Hum. Pathol. 1994;25:1035–1042. doi: 10.1016/0046-8177(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 17.Floridi A, D'Alessio G. Compartamento chromatografico della ribonucleasi seminale. Bull. Soc. Ital. Biol. Sper. 1967;43:32–36. [PubMed] [Google Scholar]
- 18.Harper JW, Vallee BL. A covalent angiogenin/ribonuclease hybrid with a fourth disulfide bond generated by regional mutagenesis. Biochemistry. 1989;28:1875–1884. doi: 10.1021/bi00430a067. [DOI] [PubMed] [Google Scholar]
- 19.Hlinák A, Matoušek J, Madlafoušek J. The effect of bull seminal ribonuclease on reproductive organs and sexual behaviour in male rats. Physiol. Bohem. 1981;30:539–542. [PubMed] [Google Scholar]
- 20.Hofsteenge J. “Holy” proteins I: ribonuclease inhibitor. Current Opin. Struct. Biol. 1994;4:807–809. doi: 10.1016/0959-440x(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 21.Kim J-S. Ph.D. Thesis. University of Wisconsin–Madison; 1994. [Google Scholar]
- 22.Kim J-S, Raines RT. Bovine seminal ribonuclease produced from a synthetic gene. J. Biol. Chem. 1993;268:17392–17396. [PubMed] [Google Scholar]
- 23.Kim J-S, Souček J, Matoušek J, Raines RT. Catalytic activity of bovine seminal ribonuclease is essential for its immunosuppressive and other biological activities. Biochem. J. 1995;308:547–550. doi: 10.1042/bj3080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J-S, Souček J, Matoušek J, Raines RT. Mechanism of ribonuclease cytotoxicity. J. Biol. Chem. 1995;270:31097–31102. doi: 10.1074/jbc.270.52.31097. [DOI] [PubMed] [Google Scholar]
- 25.Kim J-S, Souček J, Matoušek J, Raines RT. Structural basis for the biological activities of bovine seminal ribonuclease. J. Biol. Chem. 1995;270:10525–10530. doi: 10.1074/jbc.270.18.10525. [DOI] [PubMed] [Google Scholar]
- 26.Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186. doi: 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- 27.Kraft N, Shortman K. A suggested control function for the animal tissue ribonuclease – ribonuclease inhibitor system, based on studies of isolated cells and phytohaemagglutinin-transformed lymphocytes. Biochim. Biophys. Acta. 1970;217:164–175. doi: 10.1016/0005-2787(70)90133-4. [DOI] [PubMed] [Google Scholar]
- 28.Kyner D, Christman JK, Acs G. The effect of 12-O-tetradecanoyl-phorbol 13-acetate on the ribonuclease activity of circulating human lymphocytes. Eur. J. Biochem. 1979;99:395–399. doi: 10.1111/j.1432-1033.1979.tb13268.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee FS, Vallee BL. Structure and action of mammalian ribonuclease (angiogenin) inhibitor. Progr. Nucl. Acid Res. Mol. Biol. 1993;44:1–30. doi: 10.1016/s0079-6603(08)60215-9. [DOI] [PubMed] [Google Scholar]
- 30.Leone E, Greco L, Rastogi RK, Ieala L. Antispermatogenic properties of bull seminal ribonuclease. J. Reprod. Fert. 1973;34:197–200. doi: 10.1530/jrf.0.0340197. [DOI] [PubMed] [Google Scholar]
- 31.Markham R, Smith JD. The structure of ribonucleic acids. 1. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem. J. 1952;52:552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matoušek J. Aspermatogenic effect of the bull seminal ribonuclease (BS RNase) in the presence of anti BS RNase antibodies in mice. Animal Genet. 1994;25(Suppl. 1):45–50. doi: 10.1111/j.1365-2052.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 33.Matoušek J, D'Alessio G. Bull seminal ribonuclease (BS RNase), its immunosuppressive and other biological effects—a review. Anim. Sci. Papers Rep. 1991;7:5–14. [Google Scholar]
- 34.Matoušek J, Grozdanovic J. Specific effect of bull seminal ribonuclease (AS RNase) on cell systems in mice. Comp. Biochem. Physiol. 1973;46A:241–248. doi: 10.1016/0300-9629(73)90415-5. [DOI] [PubMed] [Google Scholar]
- 35.Matoušek J, Pavlok A, Dostál J, Grozdanovic J. Some biological properties of bull seminal vesicle aspermatogenic substance and its effect on mice. J. Reprod. Fert. 1973;34:9–22. doi: 10.1530/jrf.0.0340009. [DOI] [PubMed] [Google Scholar]
- 36.Matoušek J, Souček J, Rìha J, Zankel TR, Benner SA. Immunosuppressive activity of angiogenin in comparison with bovine seminal ribonuclease and pancreatic ribonuclease. Comp. Biochem. Physiol. 1995;112B:235–241. doi: 10.1016/0305-0491(95)00075-5. [DOI] [PubMed] [Google Scholar]
- 37.Matoušek J, Souček J, Stratil A, Vallee BL. Immunosuppressive activity of angiogenin. Anim. Genet. Suppl. 1992;23:46. [Google Scholar]
- 38.Matoušek J, Stanek R. Action of bull seminal vesicle ribonuclease on mouse leukaemic cells. Folia Biol. (Prague) 1977;23:56–65. [PubMed] [Google Scholar]
- 39.Mazzarella L, Mattia CA, Capasso S, Di Lorenzo G. Composite active sites in bovine seminal ribonuclease. Gaz. Chim. Ital. 1987;117:91–97. [Google Scholar]
- 40.Messmore JM, Fuchs DN, Raines RT. Ribonuclease A: revealing structure–function relationships with semisynthesis. J. Am. Chem. Soc. 1995;117:8057–8060. doi: 10.1021/ja00136a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murthy BS, De Lorenzo C, Piccoli R, D'Alessio G, Sirdeshmukh R. Effects of protein RNase inhibitor and substrate on the quaternary structures of bovine smeinal RNase. Biochemistry. 1996;35:3880–3885. doi: 10.1021/bi952429m. [DOI] [PubMed] [Google Scholar]
- 42.Murthy BS, Sirdeshmukh R. Sensitivity of monomeric and dimeric forms of bovine seminal ribonuclease to human placental ribonuclease inhibitor. Biochem. J. 1992;281:343–348. doi: 10.1042/bj2810343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabes HM. Proliferation of human testicular tumours. Int. J. Androl. 1987;10:127–137. doi: 10.1111/j.1365-2605.1987.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 44.Raines RT, Toscano MP, Nierengarten DM, Ha JH, Auerbach R. Replacing a surface loop endows ribonuclease A with angiogenic activity. J. Biol. Chem. 1995;270:17180–17184. doi: 10.1074/jbc.270.29.17180. [DOI] [PubMed] [Google Scholar]
- 45.Roth JS. Some observations on the assay and properties of ribonucleases in normal and tumor tissues. Methods Cancer Res. 1967;3:153–242. [Google Scholar]
- 46.Russell LD, Ettlin RA, Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL: 1990. [Google Scholar]
- 47.Shapiro R, Vallee BL. Interaction of human placental ribonuclease with placental ribonuclese inhibitor. Biochemistry. 1991;30:2246–2255. doi: 10.1021/bi00222a030. [DOI] [PubMed] [Google Scholar]
- 48.Souček J, Chudomel V, Potmesilova I, Novak JT. Effect of ribonucleases on cell-mediated lympholysis reaction and on cfv-c colonies in bone marrow culture. Nat. Immun. Cell Growth Regul. 1986;5:250–258. [PubMed] [Google Scholar]
- 49.Souček J, Marinov I, Benes J, Hilgert I, Matoušek J, Raines RT. Immunosuppressive activity of bovine seminal ribonuclease and its mode of action. Immunobiology. 1996;195:271–285. doi: 10.1016/S0171-2985(96)80045-3. [DOI] [PubMed] [Google Scholar]
- 50.Souček J, Matousek J, Chudomel V, Lindnerová G. Inhibitory effect of bovine seminal ribonuclease on activated lymphocytes and lymphoblastoid cell lines in vitro. Folia Biol. (Prague) 1981;27:344–335. [PubMed] [Google Scholar]
- 51.Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic intermediate. Biochemistry. 1994;33:7408–7414. doi: 10.1021/bi00189a047. [DOI] [PubMed] [Google Scholar]
- 52.Wu Y, Mikulski SM, Ardelt W, Rybak SM, Youle RJ. A cytotoxic ribonuclease. J. Biol. Chem. 1993;268:10686–10693. [PubMed] [Google Scholar]
- 53.Wu Y, Saxena SK, Ardelt W, Gadina M, Mikulski SM, De Lorenzo V, D'Alessio G, Youle RJ. A study of the intracellular routing of cytotoxic ribonucleases. J. Biol. Chem. 1995;270:17476–17481. doi: 10.1074/jbc.270.29.17476. [DOI] [PubMed] [Google Scholar]






