Abstract
Schizophrenia is characterized by impaired cognitive control associated with prefrontal cortex dysfunction, but the underlying pathophysioloical mechanisms remain unknown. Higher cognitive processes are associated with cortical oscillations in the gamma range, which are also impaired in chronic schizophrenia. We tested whether cognitive control-related gamma deficits are observed in first-episode patients, and whether they are associated with antipsychotic medication exposure. Fifty-three first-episode schizophrenia patients (21 without antipsychotic medication treatment) and 29 healthy control subjects underwent electroencephalography (EEG) during performance of a preparatory cognitive control task (preparing to overcome prepotency or POP task). The first-episode schizophrenia patient group was impaired (relative to the control group) on task performance and on delay-period gamma power at each of the three subgroups of frontal electrodes. The unmedicated patient subgroup was similarly impaired compared with controls, and was not different on these measures compared with the medicated patient subgroup. In contrast, delay-period theta power was not impaired in the full patient group nor in the unmedicated patient subgroup. Impaired cognitive control-related gamma cortical oscillatory activity is present at the first psychotic episode in schizophrenia, and is independent of medication status. This suggests that altered local circuit function supporting high-frequency oscillatory activity in prefrontal cortex ensembles may serve as the pathophysiological substrate of cognitive control deficits in schizophrenia.
Keywords: schizophrenia, gamma oscillations, cognitive control, first episode, prefrontal cortex
INTRODUCTION
Schizophrenia is a disorder of cognition, with a prominent role for dysfunction in higher-order cognitive processes that are supported by the prefrontal cortex (PFC) (Minzenberg et al, 2009; Weinberger et al, 2001). This dysfunction may have a basis in altered cellular and local network structure and function in the PFC involving gamma amino-butyric acid (GABA) neuron subtypes, as well as glutamatergic and monoaminergic disturbances (Lewis and Moghaddam, 2006; Selemon and Goldman-Rakic, 1999).
These findings suggest links across levels of analysis of cortical dysfunction in schizophrenia. Local PFC network dysfunction may form the basis of altered regional PFC activity. This in turn may lead to impaired recruitment of distributed task-appropriate networks, resulting in cognitive and behavioral dysfunction. However, the physiological signature arising from activity in local circuits within the PFC remains unclear. In response to this problem, investigation has turned to brain oscillations as a manifestation of cortical network function. In particular, oscillations in the gamma range are implicated in a range of higher-order cognitive functions. These oscillations (generally defined as 30–80 Hz activity) are observed throughout the cortex of mammals, arising from local circuit interactions, although they can also be generated or modulated by subcortical–cortical interactions (Whittington et al, 2000). They are also strongly associated with the BOLD response measured by fMRI (Logothetis et al, 2001; Mukamel et al, 2005). Gamma-range activity can be detected at the scalp of humans by electroencephalography (EEG), and although it has been studied primarily in association with perceptual processes, it may be a general feature of top-down modulatory processes, including visual feature binding (Tallon-Baudry and Bertrand, 1999), the formation of expectancies in perception (Engel et al, 2001), attentional selection and memory matching and utilization (Herrmann et al, 2004) and working memory (Lisman and Idiart, 1995). Induced gamma oscillations, which occur with a variable latency and phase from trial to trial, may be a particularly important measure of top-down processing (Engel et al, 2001; Herrmann et al, 2004; Kaiser and Lutzenberger, 2003).
Gamma oscillations have been increasingly studied in schizophrenia patients (see Gonzalez-Burgos and Lewis, 2008; Lee et al, 2003; Uhlhaas et al, 2008 for reviews). Most of these studies have employed low-level perceptual processing tasks, finding impaired evoked gamma power during a visual backward-masking task (Wynn et al, 2005), decreased evoked gamma power (Haig et al, 2000; Symond et al, 2005) and synchrony (Slewa-Younan et al, 2004; Symond et al, 2005) in auditory oddball paradigms, decreased evoked gamma power and synchrony in auditory steady-state potential paradigms using click-train stimuli (Kwon et al, 1999; Light et al, 2006) or sustained tones (Brenner et al, 2003), and decreased gamma phase-locking, observed in visual (Spencer et al, 2008a) and auditory oddball tasks (Roach and Mathalon, 2008), with auditory tones (Teale et al, 2008; Krishnan et al, 2009), and immediately before spontaneous movement (Staykova et al, 2008). Impaired evoked gamma activity has also been found in response to transcranial magnetic stimulation (Ferrarelli et al, 2008), in association with sensory gating deficits in schizophrenia (Hong et al, 2004a), and in the unaffected relatives of schizophrenia patients (Hong et al, 2004b). Gamma activity has also been studied in schizophrenia using tasks with more complex processing demands. These studies have found schizophrenia patients to exhibit relatively lower frequency (Spencer et al, 2004) and decreased synchrony (Spencer et al, 2003) of an oscillation that is phase-locked to motor response in a Gestalt perception task, although others have found preserved gamma power in a different Gestalt perception task (Uhlhaas et al, 2006). Task demands with a stronger PFC-dependence have also revealed gamma deficits in schizophrenia patients, including impaired frontal gamma power (measured by magnetoencephalography) during mental arithmetic performance (Kissler et al, 2000) and an inability to mount increased delay-period gamma power in response to increasing working memory load during the N-Back (Basar-Eroglu et al, 2007). Our research group has reported that schizophrenia patients exhibit impaired frontal induced gamma power during preparatory cognitive control processes (Cho et al, 2006), suggesting a physiological mechanism to link impaired BOLD responses in the DLPFC with impaired cognitive control (Barch et al, 2001; Holmes et al, 2005; MacDonald and Carter, 2003; MacDonald et al, 2005; Perlstein et al, 2003; Snitz et al, 2005; Yoon et al, 2008).
In evaluating a particular pathophysiological mechanism in schizophrenia, a critical issue to address is whether observed neural dysfunction is present at the outset of the disorder, thus predating significant antipsychotic medication exposure and the numerous deleterious effects of chronic illness. A few studies to date have examined gamma activity in schizophrenia patients during the first episode of illness, finding impaired fronto-central gamma coherence in the resting state of unmedicated patients (Yeragani et al, 2006), and impaired late evoked gamma power in an auditory oddball task in unmedicated (mostly first-episode) patients (Gallinat et al, 2004). Other above-mentioned studies found decreased impaired steady-state gamma power and phase-locking factor in response to 40 Hz click trains (Spencer et al, 2008b), evoked gamma power (Symond et al, 2005) and synchrony (Slewa-Younan et al, 2004; Symond et al, 2005) in auditory oddball paradigms, each in medicated first-episode schizophrenia patients.
No studies to date have examined induced gamma activity during PFC-dependent cognitive task performance in early-course (medicated or unmedicated) schizophrenia patients. In addition, it remains unclear whether gamma dysfunction in early-course schizophrenia is isolated to this frequency band or whether it is coincident with altered theta activity. Accordingly, we evaluated cognitive control-related gamma and theta-range oscillatory activity in schizophrenia patients within the first year of psychotic illness, including a relatively large subsample of unmedicated patients. We hypothesized that first-episode schizophrenia patients exhibit impaired frontal induced gamma activity during cognitive control processing, which is independent of medication status.
PATIENTS AND METHODS
Subjects
Fifty-three schizophrenia patients (SZ group) and 29 healthy age-matched healthy controls (HC group) participated in this study. The schizophrenia patients were recruited through the Early Diagnosis and Preventive Treatment of Psychosis clinic of the Department of Psychiatry at UC Davis School of Medicine (http://www.earlypsychosis.ucdavis.edu). The onset of psychotic illness for all patients was <1 year before study.
Subjects were between 13 and 30 years of age, with the following exclusion criteria: (1) IQ of <70 (by Wechsler Abbreviated Scale of Intelligence), (2) history of neurological illness, including head injury or peripheral sensorimotor disturbances, (3) history of substance-related disorder (by DSM-IV-TR) in the previous 6 months, (4) uncontrolled medical illness, (5) history of electroconvulsive therapy and (6) pregnancy. Healthy controls were recruited from the community through advertisements. In addition to the criteria above, control participants were evaluated with the SCID-non-patient version to exclude those with a history of an Axis I disorder or a first-degree relative with a psychotic disorder. Informed consent was obtained from all the subjects, using a protocol approved by the local Institutional Review Board at the University of California, Davis. Subjects were compensated for their participation.
Of the 53 patients with schizophrenia diagnoses, 32 were on antipsychotic medication (all on atypical antipsychotics and two additionally with typical antipsychotic medication) and 21 were unmedicated. Two of these patients experienced <6 months of antipsychotic medication treatment, and each were unmedicated for at least 6 months before study. The remainder of the unmedicated patient sample was medication-naïve. Table 1 shows the characteristics of the patient and control subjects. The full patient group had significantly lower full-scale IQ (p<0.0005); all other demographic variables were not significantly different between groups (all p>0.10 by t-test or χ2-test). None of these variables were significantly different between the medicated and unmedicated patient subgroups (all p>0.20).
Table 1. Demographic and Clinical Characteristics of Schizophrenia Patients and Healthy Comparison Subjects.
| Measure |
Schizophrenia group (n=53) |
Healthy control group (n=29) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 20.0 | 3.8 | 20.7 | 3.8 |
| Parental education | 14.5 | 2.6 | 14.6 | 3.4 |
| Subject education | 12.4 | 2.2 | 13.6 | 4.4 |
| Full-scale IQ (WAIS) | 101* | 13 | 115 | 11 |
| Clinical measures | ||||
| BPRS | 41.9 | 12.8 | NA | |
| SANS | 30.4 | 18.2 | NA | |
| SAPS | 22.4 | 15.2 | NA | |
| Strauss-Carpenter Outcome Scale | 8.9 | 3.1 | NA | |
| Global Assessment Scale | 45.6 | 9.9 | NA | |
| N | % | N | % | |
| Male | 38 | 72 | 18 | 62 |
| Right-handed | 50 | 94 | 28 | 97 |
* p<0.0005 by t-test.
Clinical evaluation of patients
Diagnoses of schizophrenia were established through administration of the Structured Clinical Interview for DSM-IV-TR (First et al, 2002) and for patients under 18 years of age, the Kiddie-SADS-Present and Lifetime Version (K-SADS-PL; http://www.wpic.pitt.edu/ksads/ksads-pl.pdf). Diagnoses were established and confirmed by consensus conferences involving research psychiatrists and psychologists. Intra- and inter-rater reliability for these instruments were maintained through monthly reliability rounds. Clinical symptom scores for schizophrenia patients were assessed with the Brief Psychiatric Rating Scale (Overall, 1974) and the Scales for the Assessment of Positive and Negative Symptoms (SANS (Andreasen, 1983a) and SAPS (Andreasen, 1983b)). The medicated vs unmedicated patient subgroups were not different on any clinical (symptom) measures (all p>0.5), which are summarized for the full patient group in Table 1.
Cognitive Paradigm
EEG data were acquired during performance of the preparing to overcome prepotency (POP) task (Snitz et al, 2005). The cognitive task was presented using E-Prime (Psychological Software Tools, Pittsburgh, PA). The trial structure was as follows: cue (a green or red square, 500 ms duration), delay period (1000 ms duration), probe (a centrally presented white arrow pointing left or right, randomized with equal frequency between right and left directions; 500 ms duration) and a randomly variable intertrial interval (1000–2000 ms). Over the cue-probe delay, subjects were required to maintain the appropriate rule (represented by the cue) to guide stimulus–response (S–R) mappings. For the low-control condition (green-cued trials), subjects were required to respond with a button-press in the congruent direction of the subsequent arrow (eg, for a right-pointing arrow, press the right button, and left for left). For the high-control condition (red-cued trials), subjects responded in the incongruent direction (eg, for a right-pointing arrow, press the left button, and vice versa). Subjects were instructed to ‘go as fast as you can without making mistakes.' To increase the control requirements during the high-control trials, we reinforced the prepotency of the low-control S–R mappings by using a prevalence of green cues (55%). Participants received 8 blocks of 82 trials each. All subjects completed practice before EEG. This involved one self-paced block of 20 trials, and at least one automatically paced (as in the EEG experiment) block of 20 trials. All subjects were required to complete one of each block with errors on no more than two successive trials, with blocks repeated as necessary. This criterion was achieved on two blocks total for most subjects, and three blocks total for all subjects.
EEG
Data acquisition
EEG data were acquired in a shielded room using a Neuroscan 128-electrode Quik-Cap and Neuroscan SynAmps2 hardware, with a sampling frequency of 1000 Hz and a 100 Hz low-pass hardware filter. Data were collected using 32-bit encoding software, eliminating the need for high-pass recording filters. Electrode impedances were kept at <5 kΩ. All channels were referenced to Cz.
Offline processing
Malfunctioning electrodes were determined and excluded on the basis of the impedence map and by visual inspection of the recorded waveforms. The remaining data were then imported into EEGLab (Delorme and Makeig, 2004), re-referenced against the average reference, downsampled to 250 Hz and high-pass filtered at 0.5 Hz. Epochs to be extracted from the continuous EEG data were defined from −400 to +1700 ms relative to cue onset. Each epoch was baseline-corrected using the prestimulus interval (−400 to 0 ms) in order to account for stimulus-independent (‘background') fluctuations potentially present across the trial. All trials with incorrect responses were removed. Artifact rejection was then conducted through the use of a probability-based criterion: First, the distribution of voltages averaged across all electrodes for a given trial was compared with the voltage for each individual electrode on that trial. If the individual electrode's voltage within that trial exceeded a voltage equal to 5 SDs from the mean of all electrodes, then the electrode was removed from that trial. A problematic case could arise if the majority of electrodes exhibited excessive noise on a given trial, thereby making it difficult to discriminate an individual electrode's degree of noise from the full electrode set. However, this special case could be detected and resolved upon the use of independent components analysis (ICA), which followed this artifact rejection step (see Supplementary Methods for details of ICA procedure). We also used ICA to address potential microsaccade-related oscillatory activity, finding no evidence that the gamma power measures used for inferential testing were contaminated by microsaccade-related gamma power (see Supplementary Methods for this evaluation).
Time-frequency transformation of the data
Time-frequency transformations of the data were performed using EEGLab (Delorme and Makeig, 2004). The transformation was accomplished by convolving the continuous EEG time series with the complex Morlet wavelet function. These were performed on individual trial segments to identify time-frequency components in the gamma and theta ranges. Forty frequency subbands were calculated separately, with each subband defined by a logarithmically increasing central frequency and a range subject to a Gaussian kernel defined by the constant c, which is the ratio of the central frequency to the standard deviation. For gamma band (27.91–83.74 Hz) decompositions, c=6 and the period from −200 to 0 ms relative to cue onset was defined as a baseline; average gamma power during the baseline period was subtracted from delay-period gamma power determined during the trial. Time-frequency decomposition of the theta band (4.55–8.36 Hz) was performed identically, but with c=4. To obtain a summary measure of induced oscillatory power, the power values (wavelet coefficients in dB) obtained from the frequency decomposition of the trial segments were averaged according to task condition.
Statistical Analyses
Mean power was thus derived for each individual electrode for each experimental condition, ie, green-cue and red-cue trials, in the task phase represented by the cue-probe delay period. For inferential testing of both gamma and theta oscillatory power, we proceeded as follows. Because we were interested in delay period, preparatory control-related oscillatory activity, without specific hypotheses about the timing of gamma or theta activity during this delay, or about subbands within these oscillatory bands, we averaged power throughout the delay period (ie, from cue offset to probe onset) and throughout the gamma range, to establish a single summary measure for gamma power at each electrode, as the red cue minus green cue difference. Theta power was summarized in the same manner, by averaging throughout both the full range of this frequency band and throughout the full delay period. We then grouped the electrodes into nine subgroups, following scalp topography with the goal of maintaining approximately equal numbers of electrodes across the various subgroups: frontal, central and posterior, each into left, middle and right (each subgroup with 11–15 electrodes). We averaged power across all electrodes within each subgroup, so that each subject had a grand mean gamma power and grand mean theta power, for each electrode subgroup. Our inferential testing was conducted on the total of six frontal and central electrode subgroups bilaterally, which span the topography of the scalp overlying the frontoparietal cortical regions that subserve cognitive control processes, as they are engaged in the POP task. Initial inferential testing proceeded by parallel analyses of variance (ANOVA) for gamma and theta bands, with delay-period control-related (red cue minus green cue) power as the dependent variable, subject as the random factor, electrode subgroup (nine total) as within-subject factor and diagnostic group (SZ, HC) as the between-subject factor. The threshold for statistical significance was set at p<0.05. Significant effects involving the diagnostic group factor were followed up by t-tests on individual frontal electrode subgroups, as appropriate. Cognitive performance was tested by comparing the accuracy and RT costs (ie, decrements in accuracy and speed to red-cued trials relative to green-cued trials) by t-test. Each of the above-mentioned analyses was also conducted to compare the unmedicated SZ subgroup to both the HC group and to the medicated SZ subgroup.
RESULTS
Full Sample: All Schizophrenia Patients VS Healthy Control Subjects
Task performance
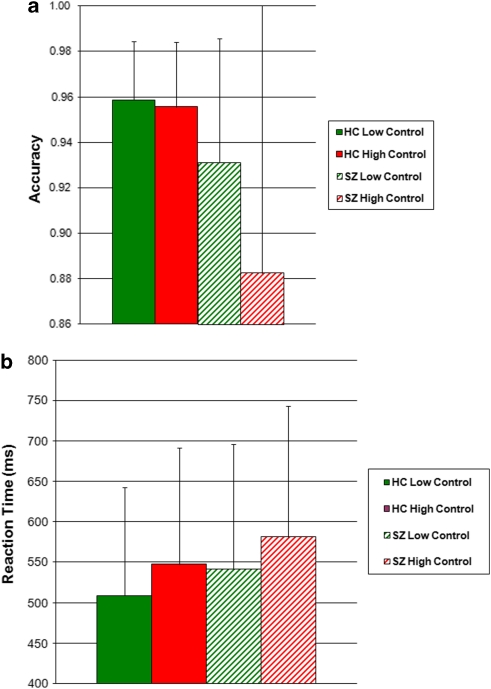
The SZ group showed impaired cognitive control task performance, manifest as increased accuracy cost in the high-control (red cued) condition: SZ group, 4.9±10.8%, vs HC group, 0.3±1.9% (t=2.25, df=80, p=0.027 in two-tailed t-test). The SZ group was not significantly impaired on RT cost compared with the HC group: SZ group, 39±28 ms, vs HC group, 39±22 ms (t=0.09, df=80, p=0.93) (See Figure 1 for group means).
Figure 1.
Cognitive control task performance. Mean group task performance for SZ and HC groups. (a) Accuracy, (b) reaction time.
Control-related gamma power
The SZ group had significantly fewer malfunctioning electrodes that were excluded from analysis: HC, 21.1±9.3; SZ, 13.5±7.6 (t=3.98, df=80, p<0.0005). The number of valid trials retained for analysis was as follows: HC group, green cue, 202±66; red cue, 161±51; SZ group, green cue, 188±53; red cue, 143±45. In ANOVA of the number of valid trials retained, there was no significant main effect of diagnostic group (F=2.06, df=1,74; p=0.16) nor a significant diagnostic group-by-task condition interaction (F=0.41, df=1,74; p=0.52); by t-test, no significant between-group differences were found for individual task conditions (all p-values >0.11). These results indicate that comparable numbers of trials were used for analysis.
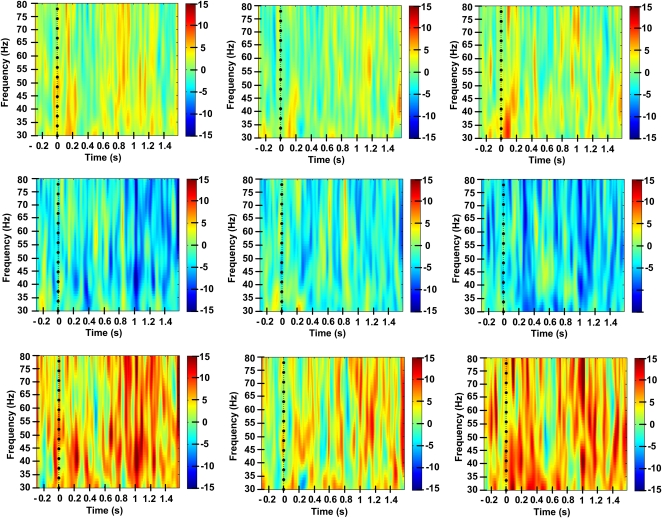
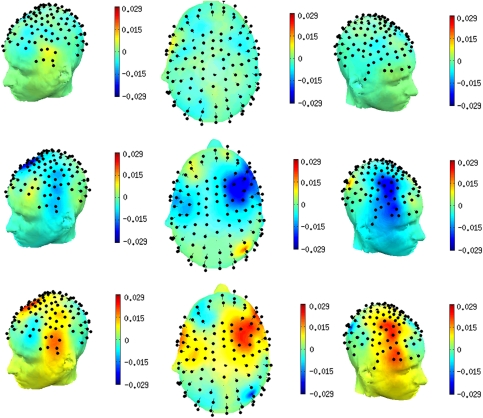
In ANOVA of delay-period Gamma power across the set of six electrode subgroups on the scalp, there were significant main effects of diagnostic group (F=12.94, df=1,80, p=0.001) and electrode subgroup (F=3.34, df=5, 76, p=0.009) and the diagnostic group-by-electrode subgroup interaction (F=2.76, df=5,76, p=0.024). In within-diagnostic group analyses of frontal electrode subgroups, the HC group exhibited control-related (ie, red cue minus green cue) gamma power that was significantly >0 (ie, greater than baseline gamma power, by two-tailed, one-sample t-test) in the left frontal electrode subgroup (t=2.43, df=28, p=0.022) and mid-frontal electrode subgroup (t=2.62, df=28, p=0.014); it was not significant in the right frontal subgroup (t=1.58, df=28, p=0.12). These effects are observed in the time/frequency spectrograms (Figure 2, top panel) as strong and relatively transient increases in gamma power, manifest both during the red cue-on period, as well as throughout the red cue-probe delay period, and generally throughout the 30–80 Hz frequency range. In contrast, the SZ group exhibited consistent control-related gamma power that was negative (relative to baseline) in each of the frontal electrode subgroups, including significantly in the left (t=−2.90, df=52, p=0.005) and middle frontal subgroups (t=−2.07, df=52, p=0.043) and at trend level in the right frontal subgroup (t=−1.72, df=52, p=0.091). The time-frequency spectrograms for the patient group (Figure 2, middle panel) show relatively few, weak gamma power increases in the cue-probe delay period, on a background of largely null or negative changes in power throughout the gamma range, as a function of high-control (ie, red cued) task demands. In between-group comparisons of gamma power in individual frontal electrode subgroups, the SZ group exhibited significant deficits (relative to the HC group) in control-related gamma power in each frontal subgroup: left frontal, t=−3.94, df=80, p<0.0005; middle frontal, t=−3.64, df=80, p<.0005; right frontal, t=−2.43, df=80, p=0.017 (two-tailed, independent samples t-tests). These patient-group deficits are observed in the time-frequency spectrograms (Figure 2, bottom panel) as relatively greater power in the HC group, manifest throughout the delay period and throughout the gamma range. When pooled across the full delay period and 30–80 Hz range, the group differences in gamma power rendered at each electrode exhibited a scalp topography (Figure 3) characterized by two major, approximately symmetrical loci, one in the left ventrolateral frontal region and a contralateral locus in the right ventrolateral frontal region, extending in a more dorsomedial direction than the left hemisphere locus. The SZ group also showed significantly impaired delay-period gamma in the red cue minus green cue difference in each of the central electrode subgroups: left central, t=−3.13, df=80, p=0.002; middle central, t=−2.75, df=80, p=0.007; right central, t=−2.83, df=80, p=0.006.
Figure 2.
Spectrograms of induced gamma power across delay period of cognitive control task performance. Color-coded plots of power (within 30–80 Hz range) vs time during task performance, for high-control vs low-control conditions. Each panel, for left, middle and right frontal electrode subgroups, respectively. Top panel: healthy control group; middle panel: schizophrenia group; bottom panel: healthy control group minus schizophrenia group. Time zero represents the cue onset, t=500 ms cue offset and t=1500 ms probe onset.
Figure 3.
Scalp topography of control-related gamma power deficits in schizophrenia group. Color-coded power in gamma-range (30–80 Hz) averaged throughout the delay period of cognitive control task performance. Top panel: healthy control group; middle panel: schizophrenia group; bottom panel: healthy control group minus schizophrenia group. Left lateral, superior, and right lateral views, from left to right, respectively.
Control-independent gamma power
We evaluated gamma power that was independent of control demands during task performance, by conducting inferential testing on delay-period green-cue gamma power in a manner completely parallel to that for the red cue minus green cue difference. In ANOVA of the six frontal and central electrode subgroups, the main effect of diagnostic group was significant (F=6.52, df=1,80, p=0.013); there was no significant effect of electrode subgroup (F=1.62, df 5,76, p=0.16) nor the interaction of diagnostic group-by-electrode subgroup (F=1.45, df=5,76, p=0.22). In t-tests on frontal electrode subgroups, the SZ group was significantly impaired in the left frontal subgroup (t=−2.21, df=80, p=0.029), with a trend-level impairment at the middle frontal subgroup (t=−1.86, df=80, p=0.066) and no difference at the right frontal subgroup (t=−0.84, df=80, p=0.42).
In light of these results, the effect of diagnostic group on red cue minus green cue gamma power was re-evaluated by ANCOVA. Each of the frontal electrode subgroups maintained a significant effect of diagnostic group after covarying for green cue gamma in the corresponding frontal subgroup: left frontal (F=9.82, df=1,79, p=0.002), middle frontal (F=9.33, df=1,79, p=0.003), right frontal (F=5.26, df=1,79, p=0.025).
Control-related theta power
In ANOVA of delay-period theta power, there were no significant effects of diagnostic group (F=0.004, p=0.95), electrode subgroup (F=0.43, p=0.83), nor diagnostic group-by-electrode subgroup interaction (F=1.50, p=0.20). Exploratory t-tests on individual electrode subgroups similarly revealed no significant differences at any frontal electrode subgroup (all p-values >0.55).
Unmedicated Schizophrenia Patients vs Healthy Control Subjects, and vs Medicated Schizophrenia Patients
Task performance
Unmedicated patients vs healthy controls: The unmedicated SZ subgroup showed a trend toward impaired cognitive control task performance compared with the HC group, manifest as increased accuracy cost in the high-control (red cued) condition: unmedicated SZ subgroup, 3.7±9.7%, vs HC group, 0.3±1.9% (t=1.84, df=48, p=0.074 two-tailed). The unmedicated SZ subgroup was not significantly impaired on RT cost compared with the HC group: unmedicated SZ subgroup, 43±22 ms, vs HC group, 39±22 ms (t=0.73, df=48, p=0.47 two-tailed).
Unmedicated patients vs medicated patients. The unmedicated SZ group showed cognitive control task performance that was not significantly different from the medicated SZ subgroup. For accuracy cost in the high-control (red cued) condition: unmedicated SZ subgroup, 2.7±8.4%, vs medicated SZ subgroup, 6.3±12.1% (t=−1.21, df=51, p=0.23 two-tailed). For RT cost in the high-control (red cued) condition: unmedicated SZ group, 43±22 ms, vs medicated SZ subgroup, 37±31 ms (t=0.85, p=0.40 two-tailed).
Control-related gamma power
Unmedicated patients vs healthy controls. The unmedicated patients had numbers of trials retained for analysis that were similar to HC (red-cue trials, p=0.39; green-cue trials, p=0.65), with fewer malfunctioning electrodes than HC (p=0.034). In ANOVA of delay-period gamma power across six electrode subgroups on the scalp, there was a significant effect of diagnostic group (F=6.93, df=1,48; p=0.015). The main effect of electrode subgroup was not significant (F=1.54, df=5,44; p=0.20), nor was the diagnostic group-by-electrode subgroup interaction (F=1.70, df=5,44; p=0.16). At each individual frontal electrode subgroup, the unmedicated schizophrenia patient subgroup exhibited significantly impaired delay-period gamma power (by two-tailed, independent samples t-test) compared with the HC group: left frontal (t=2.71, df=48, p=0.009), middle frontal (t = 2.85, df=48, p=0.006) and a trend at right frontal (t=1.71, df=48, p=0.094).
Unmedicated patients vs medicated patients. The unmedicated patients had numbers of trials retained for analysis that were similar to the medicated patients (p>0.45), and comparable numbers of malfunctioning electrodes (p=0.23). In ANOVA of delay-period gamma power, there was a significant main effect of electrode subgroup (F=4.36, df=5,47; p=0.002) but no significant effects of patient subgroup (F=0.001, df=1,51; p=0.97) nor patient subgroup-by-electrode subgroup interaction (F=0.39, df=5,47; p=0.86). Exploratory t-tests on individual frontal electrode subgroups similarly revealed no significant differences between unmedicated patients and medicated patients at any frontal electrode subgroup (all p-values >0.55).
Control-related theta power
Unmedicated patients vs healthy controls. In ANOVA of delay-period theta power, there were no significant effects of diagnostic group, electrode subgroup nor diagnostic group-by-electrode subgroup interaction (all F-values <0.93; all p-values >0.47). Exploratory t-tests on individual frontal electrode subgroups similarly revealed no significant differences between unmedicated patients and the control group at any frontal electrode subgroup (all p-values >0.50).
Unmedicated patients vs medicated patients. In ANOVA of delay-period theta power, there were no significant effects of patient subgroup, electrode subgroup, nor patient subgroup-by-electrode subgroup interaction (all F-values <1.28; all p-values >0.29). Exploratory t-tests on individual frontal electrode subgroups similarly revealed no significant differences between unmedicated patients and medicated patients at any frontal electrode subgroup (all p-values >0.55).
DISCUSSION
In this study, we investigated gamma-range cortical oscillatory power during preparatory cognitive control processing in first-episode schizophrenia patients. As hypothesized, the patient group was impaired in mounting a gamma oscillatory response to high-control demands and in task performance. These impairments were also observed in a patient subgroup that was similar in clinical severity to the treated patient subgroup, but free of concurrent antipsychotic medication treatment. Furthermore, these gamma power deficits were not coincident with changes in theta oscillatory power. These findings extend the literature to date, which has primarily emphasized deficits in various measures of evoked gamma oscillations in response to relatively simple information-processing demands, and primarily in chronic patients with a long history of antipsychotic medication exposure.
Gamma oscillatory deficits have been observed in schizophrenia under a wide range of experimental conditions, including in the resting state (Yeragani et al, 2006), elicited by TMS (Ferrarelli et al, 2008), with relatively simple perceptual task demands (Spencer et al, 2003; Spencer et al, 2004; Spencer et al, 2008a), in relation to motor responses (Ford et al, 2008), and with more complex cognitive demands (Cho et al, 2006), including this study. Taken together, these findings suggest that cortical oscillatory dysfunction may be a general feature of this illness. Other measures of gamma phenomenology that are impaired in schizophrenia include measures of synchrony and phase-locking to environmental stimuli, which suggest that the temporal dynamics of stimulus-related oscillatory activity may be altered. The present measure of gamma power indicates that schizophrenia is characterized by an impaired PFC capacity for establishing dynamic neuronal assemblies, to represent information in the absence of a stimulus in order to guide task-relevant behavior. We found evidence that delay-period gamma power is also impaired to some degree even in a (green cued) task condition that does not have significant control demands. Nevertheless, these gamma deficits did not account for control-related gamma, which was manifest even after controlling for low-control gamma in this patient sample.
Cognitive control is a PFC-dependent process, which is consistently impaired in schizophrenia, appearing at the outset of overt psychotic illness (Barch et al, 2001), and which may be specific to schizophrenia over other psychotic disorders (MacDonald et al, 2005). It is associated with other classically defined executive functions (Cohen et al, 1999; Minzenberg et al, 2009), which strongly predict functional outcome in this illness (Green, 1996). Because gamma oscillations are related to BOLD signal change measured by fMRI (Logothetis et al, 2001; Mukamel et al, 2005), the presently observed gamma deficits may form the basis of these other well-established neuroimaging measures of PFC dysfunction in schizophrenia. There is now an emerging empirical literature in schizophrenia demonstrating disturbances in GABAergic cortical interneurons (Gonzalez-Burgos and Lewis, 2008), particularly fast-spiking, parvalbumin-positive interneurons, such as basket cells and chandelier cells, and associated decrements in cortical GABA levels (Yoon et al, 2010). These interneurons are critical cellular elements that subserve a signal gating role to initiate and maintain high-frequency cortical oscillatory activity (Freund, 2003). Therefore, gamma oscillatory deficits may represent the link between altered molecular/cellular processes and the deficits in complex cognition that appears ubiquitous in schizophrenia.
Interestingly, we also found that theta power is intact during preparatory cognitive control processes in this sample, in both medicated and unmedicated first-episode patients. The anterior cingulate cortex (ACC) can exhibit theta rhythms (Onton et al, 2005), exhibits altered BOLD signal change in association with disturbed performance monitoring in schizophrenia (Carter et al, 2001; Kerns et al, 2005; Snitz et al, 2005), and there is preliminary evidence for impaired theta power in association with error monitoring during N-Back performance in schizophrenia (Schmiedt et al, 2005). Nevertheless, the cue-probe delay period of the present task is more directly dependent on the lateral PFC (where the rule, or context, is represented), than the ACC. The present evidence suggests that gamma deficits during lateral PFC-dependent cognitive processes are not merely a function of altered theta power. However, it remains unknown whether schizophrenia is characterized by a disturbed modulation of gamma oscillatory activity by theta phase, which could in principle arise from altered theta timing. Because theta oscillations are most powerfully driven (in the hippocampus at least) by activity at the dendrites of primary cells (Buzsaki, 2002), the present results are again most consistent with a perisomatic locus of pathology in local PFC circuitry, which is where basket cells and chandelier cells exert their influence on high-frequency cortical oscillations (Freund, 2003).
Several other central neurotransmitter system have been implicated in schizophrenia, including glutamate, serotonin, catecholamines, acetylcholine and others. These systems also have a role in the initiation and/or modulation of high-frequency cortical oscillations (Whittington et al, 2000), and therefore the present findings do not clearly favor one model of pathophysiology over competing models. Recent preliminary evidence does indicate that GABA-A receptor-specific pharmacological agents may remediate these control-related gamma deficits in stable chronic schizophrenia patients (Lewis et al, 2008), suggesting that the GABA system is one excellent candidate for targeting the neural basis of cognitive dysfunction in schizophrenia. Cortical GABAergic interneurons also mediate some major catecholamine modulatory effects as well (Bacci et al, 2005), suggesting that procatecholamine agents may also exert part of their efficacy for PFC function through cortical interneurons.
Nonetheless, the issues of pharmacological and anatomical specificity in gamma oscillations, and how these might point toward the pathophysiology of schizophrenia, are important questions that remain unresolved. It seems likely that a ubiquitous basis for high-frequency cortical oscillations is found in the local interaction of GABAergic interneurons and glutamatergic principal cells (Whittington et al, 2000). These interactions are strongly influenced by ascending thalamocortical projections, with additional modulatory influences conferred by ascending monoaminergic and cholinergic systems. The anatomic sites of oscillatory activity, and thus the profile of associated modulatory systems' influence, may result primarily from the distinct identity of the cortical ensemble that is engaged by a particular cognitive process. In the present case, this would be the dorsal frontoparietal network, and the various other cortical/subcortical areas that participate in cognitive control. These are targets for each of the ascending subcortical systems indicated above, leaving open the possibility of factors arising in one or more of many different (and interacting) systems contributing to gamma dysfunction in schizophrenia.
It also remains unknown whether these gamma deficits predate the overt manifestation of psychotic illness, or evolve over the course of illness in schizophrenia, and whether clinical remission is accompanied by normalization of this dysfunction. From another perspective, it is unknown to what degree this neural disturbance may represent a good candidate endophenotype for the illness (eg, whether it is found in non-affected biological relatives or independent of clinical status), as a useful phenotype to link to genetic alterations. A related issue is the nosological specificity of gamma dysfunction, which has yet to be fully addressed by comparing gamma phenomena in schizophrenia with that in other major mental disorders. Furthermore, the precise neurochemical systems implicated in this dysfunction in schizophrenia remain to be tested (as alluded to above), and changes in cortical oscillatory activity may ultimately be an expression of multiple interacting neurotransmitter systems, which may serve as concurrent targets of heterogeneous pharmacological agents (Roth et al, 2004). Addressing these unresolved research questions will help to better understand the implications of gamma oscillatory dysfunction in schizophrenia.
Acknowledgments
This work was supported by MH059883 to CSC. This publication was also made possible by Grant number UL1 RR024146 to MJM from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/ overview-translational.asp.
DISCLAIMER
The contents of the study are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andreasen NC. Scale for assessment of negative symptoms. Technical Report. University of Iowa: Iowa City 1983a.
- Andreasen NC. Scale for assessment of positive symptoms. Technical Report. University of Iowa: Iowa City 1983b.
- Bacci A, Huguenard JR, Prince DA. Modulation of neocortical interneurons: extrinsic influences and exercises in self-control. Trends Neurosci. 2005;28:602–610. doi: 10.1016/j.tins.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatr. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Rev. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW.2002Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (SCID-I/P)(New York State Psychiatric Institute: New York; ). [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding. Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, MacDonald A, III, Carter CS, Barch DM, Andrew Stenger V, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophrenia Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004a;15:155–159. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004b;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Induced gamma-band activity and human brain function. Neuroscientist. 2003;9:475–484. doi: 10.1177/1073858403259137. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kissler J, Muller MM, Fehr T, Rockstroh B, Elbert T. MEG gamma band activity in schizophrenia patients and healthy subjects in a mental arithmetic task and at rest. Clin Neurophysiol. 2000;111:2079–2087. doi: 10.1016/s1388-2457(00)00425-9. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. NeuroImage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7±2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnormal Psychol. 2003;112:689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Overall JE.1974. In: Pichot, P (ed). Psychological Measurements in Psychopharmacology: Modern Problems in Pharmacopsychiatry vol. 7. Karger: Basel; 267–301. [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Gordon E, Harris AW, Haig AR, Brown KJ, Flor-Henry P, et al. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2004;161:1595–1602. doi: 10.1176/appi.ajp.161.9.1595. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008a;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008b;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staykova MA, Linares D, Fordham SA, Paridaen JT, Willenborg DO. The innate immune response to adjuvants dictates the adaptive immune response to autoantigens. J Neuropathol Exp Neurol. 2008;67:543–554. doi: 10.1097/NEN.0b013e31817713cc. [DOI] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. Gamma synchrony in first-episode schizophrenia: a disorder of temporal connectivity. Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. NeuroImage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Cashmere D, Miewald J, Tancer M, Keshavan MS. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: A preliminary report. Psychiatry Res. 2006;141:53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.