Abstract
In anautogenous mosquitoes, vitellogenesis, the key event in egg maturation, requires a blood meal. Consequently, mosquitoes are vectors of numerous devastating human diseases. After ingestion of blood, 20–hydroxyecdysone activates yolk protein precursor (YPP) genes in the metabolic tissue, the fat body. An important adaptation for anautogenicity is the previtellogenic developmental arrest (the state-of-arrest) preventing the activation of YPP genes in previtellogenic females prior to blood feeding. Here, we show that a retinoid X receptor homolog, Ultraspiracle (AaUSP), which is an obligatory partner in the functional ecdysteroid receptor, exists at the state-of-arrest as a heterodimer with the orphan nuclear receptor AHR38, a homolog of Drosophila DHR38 and nerve growth factor-induced protein B. Yeast two-hybrid and glutathione S-transferase pull-down assays demonstrate that AHR38 can interact strongly with AaUSP. AHR38 also disrupts binding of ecdysteroid receptor to ecdysone response elements. Cell co-transfection of AHR38 with AaEcR and AaUSP inhibits ecdysone-dependent activation of a reporter gene by the ecdysteroid receptor. Co-immunoprecipitation experiments indicate that AaUSP protein associates with AHR38 instead of AaEcR in fat body nuclei at the state-of-arrest.
Keywords: AHR38/20–hydroxyecdysone/mosquito vitellogenesis/nuclear receptor/protein–protein interaction
Introduction
Mosquito-borne diseases are among the most threatening in modern times. Malaria is particularly devastating, taking a heavy toll on the human population in many parts of the world (Collins and Paskewitz, 1995; Bruno et al., 1997; Butler, 1997; Beier, 1998); therefore, there is an urgent need to explore every possible avenue for developing novel control strategies against the disease pathogens and their vectors.
Mosquitoes serve as vectors for many harmful human diseases because they require a blood feeding for their egg development. In these so-called anautogenous mosquitoes, vitellogenesis, the key event in egg maturation, is initiated only after a female mosquito ingests vertebrate blood. A blood meal triggers a hormonal cascade with 20–hydroxyecdysone (20E) as the terminal signal, which in turn activates yolk protein precursor (YPP) genes in the metabolic tissue, the fat body (Hagedorn, 1983, 1985; Raikhel, 1992; Dhadialla and Raikhel, 1994; Deitsch et al., 1995).
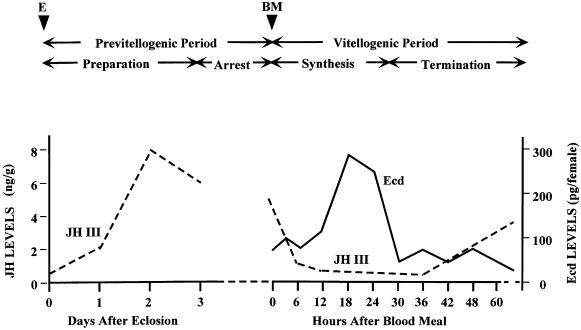
In the anautogenous mosquito Aedes aegypti, which is being used as a model vector, vitellogenesis proceeds through two periods (Figure 1). A preparatory, previtellogenic period, including an increase in ploidy (Dittmann et al., 1989) and ribosome proliferation (Raikhel and Lea, 1990), is required for the fat body to attain both the responsiveness to 20E and competence for massive yolk protein synthesis and secretion. The 3-day-long previtellogenic development of both the ovary and fat body is under the control of juvenile hormone III. Its levels are high during the previtellogenic period and fall immediately following the blood meal (Figure 1). The fat body then produces the yolk protein precursors (YPPs), which are internalized by developing oocytes (Raikhel, 1992). In addition to vitellogenin (Vg), the major YPP, the mosquito fat body synthesizes two other YPPs: vitellogenic carboxypeptidase (VCP) and vitellogenic cathepsin B (VCB) (Cho et al., 1991, 1999). YPP synthesis reaches its maximum levels at 24 h post-blood meal (PBM). Vitellogenesis proceeds until 30–32 h PBM, when it is rapidly terminated (Raikhel, 1992). The hemolymph levels of ecdysteroids in mosquito females correlate with the rate of YPP synthesis in the fat body (Figures 1 and 3A). The 20E levels are only slightly elevated at 4 h PBM; however, they rise sharply at 6–8 h PBM, reaching their maximum level at 18–24 h PBM (Hagedorn et al., 1975).
Fig. 1. Hormonal titers during the first vitellogenic cycle of the anautogenous mosquito A.aegypti. BM, blood meal; E, eclosion; JH III, juvenile hormone III titers (modified from Shapiro et al., 1986); Ecd, ecdysteroid titers (modified from Hagedorn et al., 1975).
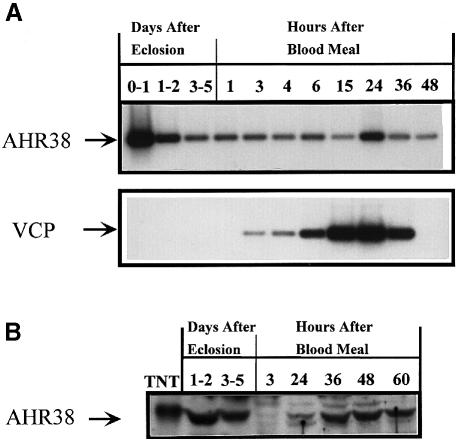
Fig. 3. AHR38 mRNA and protein during the first vitellogenic cycle in the anautogenous mosquito A.aegypti. (A) AHR38 transcript in the fat body of female adults detected by RT–PCR. RNA was isolated from fat bodies of female A.aegypti at different stages. The RT–PCR products were analyzed by agarose electrophoresis and Southern blot hybridization. Primers and RT–PCR conditions are described in Materials and methods. VCP, the transcript of the YPP, vitellogenic carboxypeptidase gene in the fat body. (B) The AHR38 protein in the fat body detected by Western blot hybridization. Nuclear extracts were prepared from the fat bodies of 250 adult females for each time point. An aliquot equivalent to 50 mosquitoes was loaded in each lane and resolved by SDS–PAGE. The AHR38 protein was detected using anti-AHR38 polyclonal antibodies. TNT represents the in vitro translated AHR38 protein that was used as a control in Western blot analysis.
Consistent with the proposed role of 20E in activating mosquito vitellogenesis (Hagedorn and Fallon, 1973; Hagedorn et al., 1973; Fallon et al., 1974), experiments using an in vitro fat body culture have shown that physiological doses of 20E (10–7–10–6 M) activate two YPP genes: vg and vcp (Deitsch et al., 1995). Utilization of the protein synthesis inhibitor cycloheximide has demonstrated that activation of YPP genes in the mosquito fat body requires protein synthesis (Deitsch et al., 1995). Thus, it is likely that a regulatory cascade similar to those in Drosophila development mediates the action of 20E in the mosquito fat body.
The functional ecdysteroid receptor is a heterodimer of the ecdysone receptor (EcR) and a retinoid X receptor (RXR) homolog, Ultraspiracle (USP) (Yao et al., 1992, 1993). In A.aegypti, one isoform of the ecdysone receptor (AaEcR) and two USP isoforms (AaUSP-A and AaUSP-B) have been cloned (Cho et al., 1995; Kapitskaya et al., 1996). The mosquito EcR–USP heterodimer has been shown to bind to various ecdysone response elements (EcREs) to modulate ecdysone-responsive target genes (Wang et al., 1998). More recently, we have demonstrated that a mosquito homolog of the Drosophila early gene E75 is involved in mediating the ecdysone response during vitellogenesis (Pierceall et al., 1999).
An important adaptation for anautogenicity is the previtellogenic developmental arrest (the state-of-arrest), which prevents the activation of YPP genes in previtellogenic females prior to blood feeding. In contrast to in vitro incubation of the previtellogenic fat body, in which the YPP genes are activated by physiological doses of 20E, only the injection of 20E at supra-physiological dosages could stimulate some expression of these genes in vivo (Raikhel, 1992; Deitsch et al., 1995). Therefore, the state-of-arrest can be interpreted as the hindrance of a 20E signaling pathway that may be maintained in vivo by undetermined humoral factors; additionally, unidentified humoral factors secreted in response to a blood meal may play a crucial role in the release of vitellogenic tissues from the state-of-arrest. Indeed, we have demonstrated elsewhere that in A.aegypti both AaEcR and AaUSP proteins are abundant in nuclei of the previtellogenic female fat body during the state-of-arrest (Wang et al., 2000). In contrast, the EcR–USP heterodimer capable of binding to the specific EcREs is barely detectable in these nuclei (Miura et al., 1999). In this context, we have considered whether the ecdysteroid receptor could be a primary target of the 20E signaling modification in the mosquito target tissues at the state-of-arrest.
One mechanism through which the formation of ecdysteroid receptor might be regulated is by competitive binding of other factors to either EcR or USP. To investigate this possibility, we cloned a cDNA encoding a repressor of 20E signaling identified as AHR38, the Drosophila counterpart of which has been reported to dimerize with USP and repress ecdysone-dependent activation of a reporter gene by the ecdysteroid receptor (Sutherland et al., 1995). Here, we report that AHR38 (NR4A4 according to the nomenclature proposed by Nuclear Receptors Nomenclature Committee, 1999), the mosquito homolog of DHR38 and vertebrate nerve growth factor-induced protein B (NGFI–B)/Nurr1 orphan receptors, interacts strongly with the AaUSP protein. Our data show that during the state-of-arrest factors in the fat body nuclei block the dimerization of AaEcR and AaUSP. The evidence presented here suggests that AHR38 is one of the key factors inhibiting the ecdysone response in the fat body of the anautogenous mosquito A.aegypti at the state-of-arrest.
Results
Isolation and characterization of the mosquito AHR38 cDNA
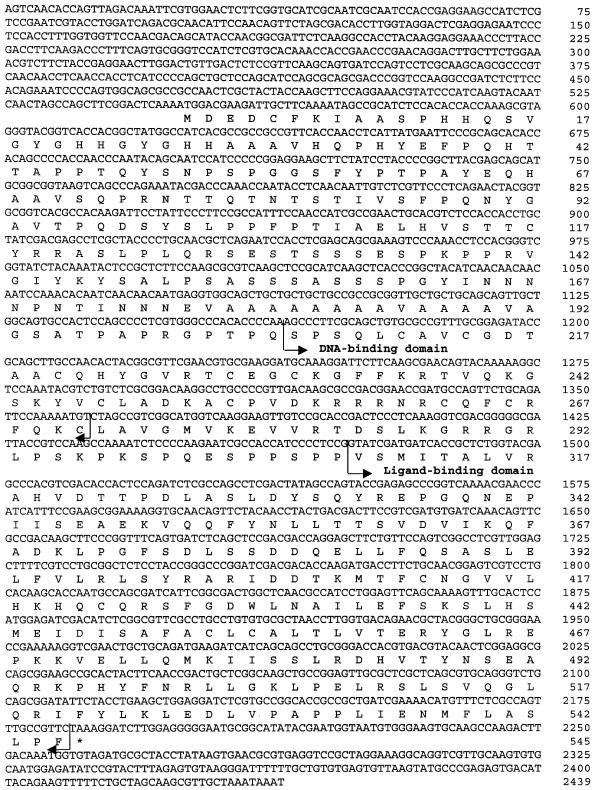
To isolate the cDNA encoding a mosquito homolog of DHR38 and NGFI–B, a pair of degenerate primers was designed based on highly conserved regions in the DNA-binding domain of Drosophila DHR38 and other NGFI–B members (Pd-F 5′-GGITGIAARGGITTYTTYAARMGIACIGTNC-3′, Pd-R 5′-TCIGTICKIACIACYTCYTTIACCATNCC-3′). RT–PCR of the fat body total RNA from the adult female at either 0–3 days post-eclosion (PE) or 24 h PBM yielded a 164 bp fragment whose translated sequence predicted a protein that shared significant homology with the DNA-binding domain of DHR38 (data not shown). A putative clone encoding the mosquito AHR38 was obtained by a combination of cDNA library screening and 5′-rapid amplification of cDNA ends (5′-RACE) PCR. These sequence data have been submitted to the DDBJ/EMBL/GenBank database under accession No. AF165528. The AHR38 mRNA extended over 2400 bp and contained a 5′ untranslated region (UTR) of at least 550 bp, a continuous open reading frame (ORF) of 1638 bp and a 3′ UTR of at least 252 bp (Figure 2). The complete ORF encoded a protein of 545 amino acids, which exhibited a structural domain organization similar to that of DHR38. The C (DNA-binding), D (hinge) and E/F (dimerization/ligand-binding) domains were highly conserved; AHR38 shared 97, 100 and 79.7% identity with the respective domains of DHR38, and 89.4, 100 and 50.8% identity with rat NGFI–B (data not shown).
Fig. 2. Nucleotide and deduced amino acid sequences of AHR38 from the mosquito A.aegypti. Both nucleotides and deduced amino acids are numbered. Borders of the DNA-binding and ligand-binding domains are marked with bent arrows.
Developmental kinetics of AHR38 mRNA transcription and protein synthesis during the first vitellogenic cycle
RT–PCR was used to evaluate the levels of AHR38 mRNA in the fat body of female mosquitoes throughout the first vitellogenic cycle. The maximum levels of AHR38 mRNA were present in the fat body of newly emerged females (Figure 3A). The mRNA levels declined during previtellogenic development, declined further following the blood feeding and showed some elevation at 24 h PBM (Figure 3A).
We then investigated the presence of the AHR38 protein in the fat bodies of female mosquitoes at different stages of the vitellogenic cycle. Polyclonal antibodies were prepared against bacterially expressed AHR38 protein. Fat body nuclei were isolated and subjected to Western blot analysis using anti-AHR38 antibodies. These analyses revealed that the levels of AHR38 protein were high in the fat body of previtellogenic females (Figure 3B). However, the levels diminished drastically after blood feeding, and increased slightly at ∼24 h PBM. They were restored back to the previtellogenic levels during the post-vitellogenic stage, 36–60 h PBM (Figure 3B).
AHR38 interacts directly with AaUSP
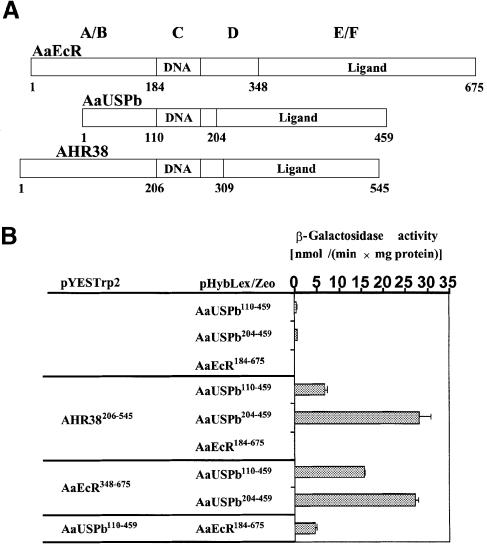
To assess the possibility that AHR38 interacts physically with AaUSP, we used the yeast two-hybrid assay. LexA and B42 fusion constructs were prepared for AaEcR, AaUSPb and AHR38 (Figure 4A); these constructs were tested in the yeast assays. LexA fusions with AaUSPb or with AaEcR alone were transcriptionally inert (Figure 4B). However, co-expression of the LexA fusion to AaUSP (LexA–AaUSP110–459) with either B42 fusion to AaEcR (B42–AaEcR348–675) or to AHR38 (B42–AHR38206–545) led to significant activation of the LacZ reporter gene, indicating that AaUSP is able to interact directly with both AaEcR and AHR38. Interestingly, utilization of the AaUSP fragment containing only the ligand/dimerization (E/F) domain (LexA–AaUSP204–459) resulted in even higher activation of the reporter via either B42 fusion to AaEcR (B42–AaEcR348–675) or to AHR38 (B42–AHR38206–545) in similar tests. Testing of the B42 fusion to AaUSPb (B42–AaUSP110–459) showed positive interaction with AaEcR (LexA–AaEcR184–675). In contrast, no activation of the reporter was observed when LexA–AaEcR184–675 was tested with B42–AHR38206–545 (Figure 4B).
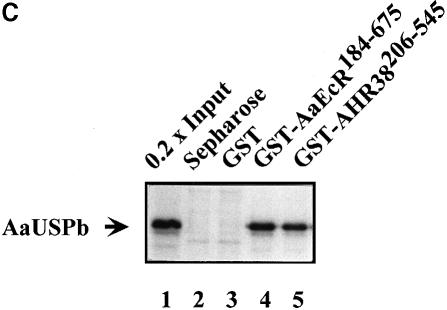
Fig. 4. Interaction of AHR38 with AaUSP. (A) Schematic representation of domain structure of AaEcR, AaUSPb and AHR38 used in generating fusion protein constructs for the yeast two-hybrid assays. (B) Yeast two-hybrid assays reveal interactions between AHR38 and AaUSP. Yeast cells were co-transformed with the indicated pHybLex/Zeo and pYESTrp2 constructs. β-galactosidase activity was measured and normalized for protein concentration. The data represent means ± SD of three experiments. (C) AHR38 binds directly to AaUSP. [35S]methionine-labeled AaUSPb was incubated with glutathione–Sepharose beads (lane 2), beads bound with GST (lane 3), GST–AaEcR (lane 4) or GST–AHR38 (lane 5). The beads were washed in phosphate-buffered saline and collected by centrifugation. Bound proteins were eluted in SDS sample buffer, resolved by SDS–PAGE and visualized by autoradiography.
This AHR38–AaUSP protein interaction was further confirmed by in vitro glutathione S-transferase (GST) pull–down assays. AaEcR and AHR38 were fused to GST (GST–AaEcR184–675 and GST–AHR38206–545) and expressed in Escherichia coli. These proteins were purified on glutathione–Sepharose beads and used as affinity matrixes to test possible interactions with in vitro translated 35S-labeled AaUSP. Whereas no significant binding was observed with GST alone, AaUSPb was efficiently retained by GST–AaEcR184–675 or GST–AHR38206–545 (Figure 4C). Similar experiments carried out with 35S-labeled AaUSPa showed identical results (data not shown).
Analysis of the AHR38–AaUSP heterodimer interaction with EcRE using electrophoretic mobility shift assays (EMSA)
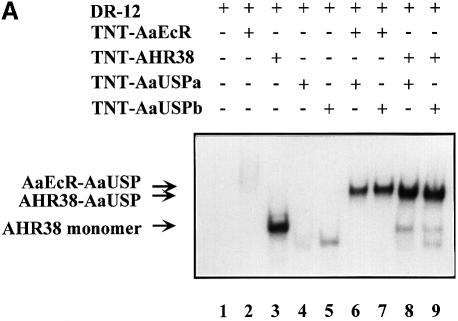
The interaction between AHR38 and AaUSP in their cooperative DNA binding was examined by EMSA using 32P-labeled oligonucleotides containing consensus EcRE sequences. For these tests, we used in vitro translated, as well as bacterially expressed and purified GST fusion proteins of AaEcR, AHR38 and AaUSP. In controls, the AaEcR–AaUSP heterodimer could bind to both the ng EcRE (EcRE of Drosophila ng-1 and ng-2 intermolt genes, direct repeat of AGGTCA half-sites with a 12 bp spacer; DR-12) and hsp27 EcRE (EcRE of Drosophila heat shock protein-27, inverted repeat of imperfect consensus half-sites with a 1 bp spacer; IR-1) (Figure 5A, lanes 6 and 7 and Figure 5C, lane 2) as observed previously (Wang et al., 1998). AHR38 alone bound to DR-12 (Figure 5A, lane 3), but not to IR-1 (data not shown). Similarly, the AHR38–AaUSP heterodimer was bound to DR-12 (Figure 5A, lanes 8 and 9), but not to IR-1 (Figure 5C, lane 3). The specificity of these binding complexes was confirmed by competition with an excess of unlabeled DR-12 and a putative EcRE of AaVg gene (DR-1 with imperfect half-sites), as well as by super-shift analyses with either anti-AHR38 or anti-AaUSP antibodies (Figure 5B). In similar experiments, we also observed the specific binding of AHR38–AaUSP heterodimer to DR-5 (direct repeat of AGGTCA half-sites with a 5 bp spacer) (data not shown).
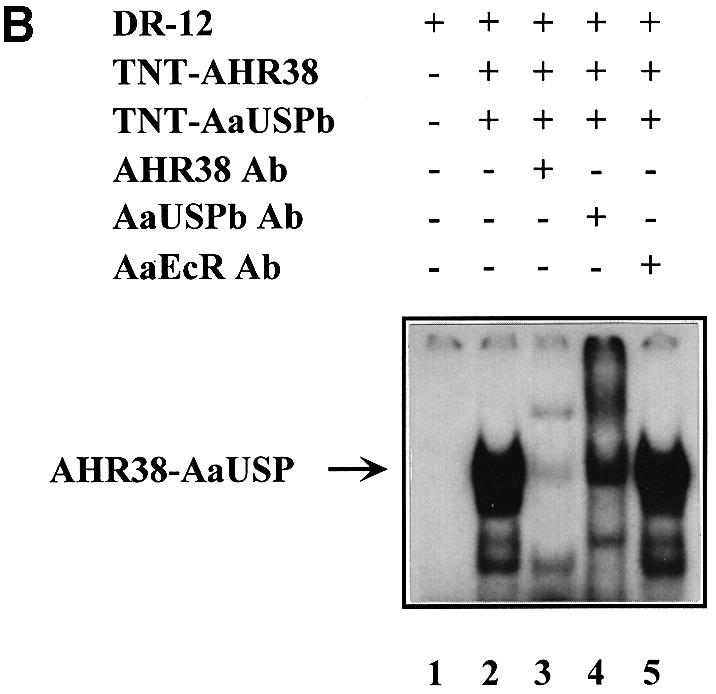
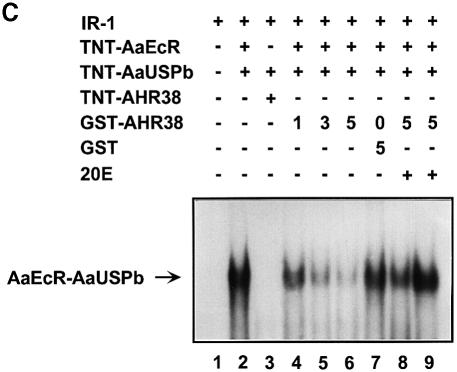
Fig. 5. Effect of AHR38 protein on binding to EcREs. EMSAs were performed using the radiolabeled ng-EcRE (DR-12) (A and B) or hsp27-EcRE (IR-1) (C). Nuclear receptor proteins were synthesized in vitro by the coupled transcription–translation reactions. (A) AHR38 was able to bind to DR-12 as either a monomer (lane 3) or an AHR38–AaUSP heterodimer (lanes 8 and 9). (B) The binding of AHR38–AaUSP to DR-12 was confirmed by super-shifting with anti-AHR38 (lane 3) and anti-AaUSP (lane 4), but not with anti-AaEcR (lane 5). (C) AHR38 repressed the binding of AaEcR–AaUSP to IR-1. Ten nanograms of AaEcR, AaUSP or AHR38 (in vitro translated) were used in each reaction. GST and GST–AHR38 fusion proteins were expressed in E.coli and partially purified. Increasing amounts of GST–AHR38 protein (1–5 μl, 10 ng/μl, lanes 4–6; 5 μl, lanes 8 and 9), GST protein (5 μl, 10 ng/μl, lane 7) and 20E (2 × 10–7 M, lane 8; 2 × 10–6 M, lane 9) were added to the reactions as indicated.
As shown in Figure 5C, the in vitro binding of AaEcR–AaUSPb to IR-1 was inhibited by GST–AHR38206–545 in a dose-dependent manner (lanes 4–6), but not by GST alone (lane 7). Addition of 20E to the reaction alleviated this inhibition (Figure 5C, compare lane 6 with lanes 8 and 9). Similar experiments were also carried out with AaUSPa; the results were identical to those seen with AaUSPb (data not shown).
AHR38 attenuates the AaEcR–AaUSP-mediated transactivation
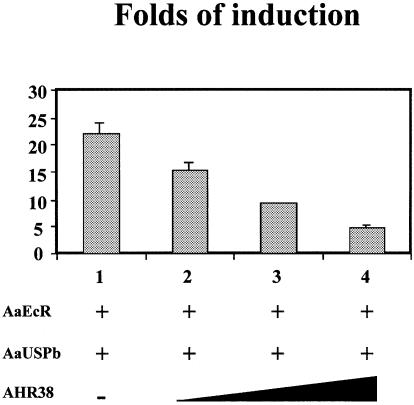
For transient transfection experiments, we employed an hsp27-EcRE-Luc reporter construct that contains the IR–1 EcRE sequence from the hsp27 gene. The reporter itself was not activated by the ecdysteroid ponasterone A in CV-1 cells (data not shown). Co-transfection of AaEcR and AaUSPb led to an ∼22-fold activation of the reporter gene expression by 1 × 10–6 M ponasterone A (Figure 6). The addition of increasing amounts of AHR38 resulted in a decrease in the ecdysteroid-dependent transactivation of the reporter gene by AaEcR–AaUSPb in a dose-dependent manner, suggesting that AHR38 was able to inhibit the AaEcR–AaUSP-mediated transactivation in mammalian cells (Figure 6). Co-transfection of AHR38 alone resulted in a weak constitutive stimulation of the luciferase activity (data not shown).
Fig. 6. AHR38 represses AaEcR–AaUSP-mediated transactivation. A combination of 0.3 μg of cytomegalovirus β-galactosidase, 0.6 μg of reporter plasmid ΔMTV-1×hsp27-EcRE-Luc, and 0.3 μg each of AaEcR and AaUSPb expression vectors was used to transfect CV-1 cells (column 1), with 0.3 μg (column 2), 0.9 μg (column 3) or 1.5 μg (column 4) of AHR38 expression vectors. After transfection, cells were incubated in the presence of the ethanol vehicle or 1 × 10–6 M ponasterone A for 36 h and harvested for luciferase assay. Luciferase activity was normalized by β-galactosidase activity. Data represent means ± SD of triplicate samples.
AaEcR and AaUSP proteins are sequestered by some factors during the previtellogenic state of developmental arrest
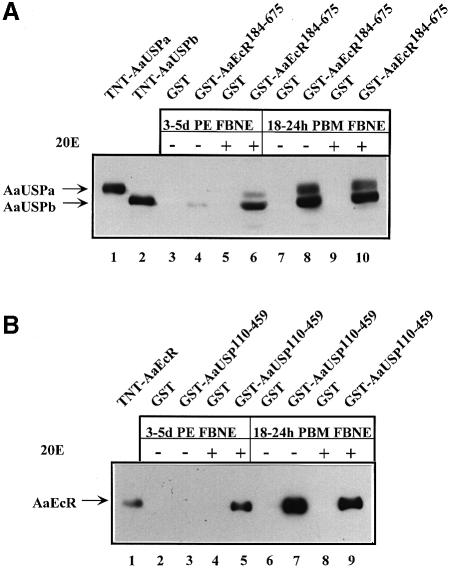
RT–PCR and Western blot analyses have shown that AaEcR and AaUSP are present at both the mRNA and protein levels in the fat body of female mosquitoes during previtellogenic developmental arrest, 3–5 days PE (Wang et al., 2000). Therefore, we investigated whether the interaction between AaEcR and AaUSP was inhibited by some factors active in the fat body at this stage. We utilized GST pull-down assays, which were performed by incubating GST fusion proteins of AaEcR or AaUSP with fat body nuclear extracts (FBNE). GST–AaEcR184–675 was able to retain AaUSP protein, predominantly AaUSPb, from FBNE of 18–24 h PBM mosquitoes. However, the interaction between GST–AaEcR and AaUSP from FBNE of 3–5 days PE female mosquitoes was not detected unless 5 × 10–6 M 20E was added to the incubation buffer. Under these conditions, interaction with AaUSPb was observed (Figure 7A). Similarly, GST–AaUSP110–459 was able to bind to AaEcR from FBNE of female mosquitoes 18–24 h PBM. However, no interactions were detected from FBNE of previtellogenic females 3–5 days PE. Addition of 5 × 10–6 M 20E to the incubation buffer enabled GST–AaUSP110–459 to interact with AaEcR from FBNE of 3–5 days PE mosquitoes (Figure 7B). These results suggested that both AaEcR and AaUSP proteins were sequestered by some other factors during the state-of-arrest, and that the presence of 20E helped the release of AaEcR and AaUSP.
Fig. 7. GST pull-down analysis with nuclear proteins extracted from the fat body of A.aegypti at the pre- and vitellogenic periods. Nuclear extracts were prepared from the fat bodies of 250 adult females for each time point. An aliquot equivalent to 50 mosquitoes was incubated with glutathione–Sepharose beads bound with equal amounts of either GST or an indicated GST fusion protein. 20E (5 × 10–6 M) was added to the indicated reactions. Beads were washed, specifically bound material was resolved by SDS–PAGE, and detected on Western blots with anti-AaUSP antibodies (A) and anti-AaEcR antibodies (B). In vitro translated proteins (TNT-AaUSPa, -AaUSPb and -AaEcR) were used as controls in Western blot analysis.
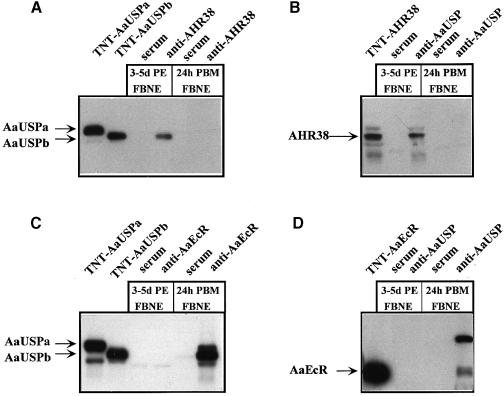
Co-immunoprecipitation experiments implicated AHR38 as blocking the ecdysone signaling during the previtellogenic arrest via dimerization with AaUSP
To investigate whether AHR38 interacts directly with AaUSP during the previtellogenic period in the A.aegypti female, immunoprecipitation studies were performed using FBNE from mosquitoes 3–5 days PE. FBNE from vitellogenic mosquitoes 24 h PBM were used as a control. First, the putative complex was immunoprecipitated using the anti-AHR38 antibody and analyzed by Western blotting with anti-AaUSP antibodies (Figure 8A); in the second test, we used the anti-AaUSP antibody to precipitate the putative complex and anti-AHR38 antibody for the Western blot (Figure 8B). Both of these tests showed that the AHR38–AaUSP interactions occurred in the fat body nuclei of previtellogenic female mosquitoes during the state of arrest. In contrast, no AHR38–AaUSP complex was found in the FBNE of vitellogenic female mosquitoes 18–24 h PBM (Figure 8A and B). To detect the presence of the AaEcR–AaUSP heterodimer in FBNE, we used the anti-AaEcR and anti-AaUSP antibodies (Figure 8C and D). In contrast to the AHR38–AaUSP complex, the AaEcR–AaUSP complex only immunoprecipitated from FBNE of vitellogenic female mosquitoes, but not from FBNE of previtellogenic females (Figure 8C and D). The results were the same irrespective of which antibodies were used for immunoprecipitations. These analyses suggested that in the fat body of a previtellogenic female mosquito during the state-of-arrest, AaUSP predominantly existed in a complex with AHR38 and not with AaEcR. On the contrary, in the fat body of vitellogenic female mosquitoes AaUSP was able to form a complex with AaEcR. Interestingly, Western blot analyses indicated that in both stages AaUSPb was the predominant isoform in these complexes (Figure 8A and C), while for AaEcR the analyses showed the existence of two immunopositive polypeptides in the vitellogenic fat bodies (Figure 8D). The smaller polypeptide corresponds to AaEcR; however, the identity of the larger immunopositive polypeptide remains to be elucidated.
Fig. 8. Co-immunoprecipitation analyses of interactions between AHR38 and AaUSP, AaEcR and AaUSP in the fat body of A.aegypti at the pre- and vitellogenic periods. Nuclear extracts were prepared from the fat bodies of 250 adult females for each time point. An aliquot equivalent to 100 mosquitoes was pre-cleared with protein A–agarose and then incubated with rabbit polyclonal anti-AHR38 (A), mouse monoclonal anti-DmUSP (B and D), rabbit polyclonal anti-AaEcR antibodies (C) or the respective pre-immune sera (serum). The resulting immune complexes were then precipitated by the addition of protein A–agarose beads. After extensive washing, immune complexes were dissociated by boiling the beads in 1× SDS sample buffer. Protein samples were separated by SDS–PAGE, followed by immunoblot analyses with mouse anti-DmUSP (A and C), rabbit anti-AHR38 (B) or rabbit anti-AaEcR (D) antibodies. In vitro translated proteins (TNT-AaUSPa, -AaUSPb, -AHR38 and -AaEcR) were used as controls in Western blot analysis.
Discussion
In this paper, we report the cloning of AHR38, the mosquito homolog of the Drosophila nuclear receptor DHR38. DHR38 and AHR38 are both insect members of the NGFI–B/Nurr1/Nor1 subgroup of nuclear receptors (Sutherland et al., 1995). The mosquito homolog exhibits structural organization typical for a nuclear receptor with the C (DNA-binding), D (hinge) and E/F (dimerization/ligand-binding) domains highly conserved with those of DHR38 and NGFI–B receptors. The evolutionary conservation of this nuclear receptor subgroup has been demonstrated by the finding of NGFI–B homologs not only in insects but also in Caenorhabditis elegans (Kostrouch et al., 1995).
The NGFI–B, Nurr1 and Nor1 orphan nuclear receptors have been implicated as transcriptional regulators, which exert their effects via binding as monomers to a DNA recognition sequence called the NGFI–B-response element, AAAGGTCA (Wilson et al., 1993; Glass, 1994; Zetterstrom et al., 1996). A recent report, which has determined an important role of DHR38 in the formation of the adult insect cuticle, has also suggested that it may act as a monomer (Kozlova et al., 1998).
The unique feature of the NGFI–B/Nurr1/Nor1 orphan nuclear receptors is that in addition to acting as monomers they can heterodimerize with RXR and bind to recognition response elements arranged as direct repeats of AGGTCA spaced by five nucleotides (Forman et al., 1995; Perlmann and Jansson, 1995; Zetterstrom et al., 1996). RXR is a promiscuous nuclear receptor, which serves as a heterodimeric partner for the retinoic acid receptor (RAR), the thyroid hormone receptor (TR), the vitamin D receptor (VDR) and the fatty acid/peroxisome proliferator-activated receptor (PPAR) (Ribeiro et al., 1993; Glass, 1994; Tsai and O'Malley, 1994; Kastner et al., 1995; Mangelsdorf and Evans, 1995; Mangelsdorf et al., 1995). Likewise, DHR38 is an alternative partner of USP, the insect homolog of RXR, which is an obligatory partner of EcR (Sutherland et al., 1995). DHR38 can compete in vitro with EcR for dimerization with USP and consequently disrupts EcR–USP binding to an EcRE. Transfection experiments in Schneider cells show that DHR38 can repress ecdysteroid-dependent transcriptional activation (Sutherland et al., 1995). Interestingly, both DHR38–USP and EcR–USP heterodimers can bind to EcRE of Drosophila ng-1 and ng-2 intermolt genes, containing directly repeated half-sites spaced by 12 bp (Crispi et al., 1998). This suggests that DHR38 may interfere with the ecdysone-signaling pathway not only by disrupting the formation of the EcR–USP heterodimer, but also by competing for binding to selected EcREs. Similarly, we demonstrated here that AHR38 interacted strongly with AaUSP, and that AHR38–AaUSP was able to recognize EcREs such as the DR-12 of the ng genes and DR-5. DNA-binding and transient transfection assays showed that AHR38 disrupted the binding of the AaEcR–AaUSP heterodimer to a specific EcRE and inhibited the transactivation of a reporter gene by the AaEcR–AaUSP heterodimer.
Sutherland et al. (1995) have demonstrated that DHR38 inhibits ecdysone action through its heterodimerization with USP; however, its biological role in Drosophila remains obscure. We propose here that in the anautogenous mosquito AHR38 plays an important function by blocking ecdysone responsiveness in the target tissues at the state-of-arrest through heterodimerization with AaUSP as well as by possibly competing for binding EcREs.
Data on the developmental kinetics of AHR38 at both the RNA and protein levels support the presumed role of this mosquito nuclear receptor during the state-of-arrest. Maximal levels of AHR38 mRNA were present in the fat body of newly emerged females, the mRNA levels declined during previtellogenic development, and levels of its protein were high during the previtellogenic developmental stage, including the state-of-arrest. The AHR38 protein levels, which decreased after blood feeding, were restored to the previtellogenic levels during post-vitellogenic stage 36–60 h PBM. This suggests that AHR38 may play a similar role in preventing the ecdysone response via heterodimerization with AaUSP in post-vitellogenic mosquitoes until another blood meal activates a second vitellogenic cycle.
GST pull-down assays utilizing GST fusion proteins of AaEcR or AaUSP and FBNE showed that GST–AaEcR was able to bind AaUSP from FBNE of vitellogenic mosquitoes. However, the interaction between GST–AaEcR and AaUSP, from FBNE of previtellogenic female mosquitoes during the state-of-arrest, was not detectable. The same was also true for the interaction of GST–AaUSP and AaEcR from FBNE. These results suggest that the AaEcR and AaUSP proteins may be sequestered by other partners in the fat body of previtellogenic female mosquitoes during the state-of-arrest and therefore are not capable of forming the AaEcR–AaUSP heterodimers. Co-immunoprecipitation experiments clearly showed that AaUSP protein interacted with AHR38 instead of AaEcR in nuclei of the fat body at that stage.
Interestingly, the interaction between GST–AaEcR and AaUSP from FBNE of 3–5 days PE female mosquitoes was detectable in the presence of 5 × 10–6 M 20E. Likewise, GST–AaUSP was able to bind to AaEcR from this FBNE after the addition of 20E. This observation suggests that 20E is capable of shifting the preference of AaUSP to heterodimerization with AaEcR. However, in vivo in the fat body of previtellogenic female mosquitoes at the state-of-arrest, an undetermined factor probably maintains AaUSP binding to AHR38. It has been shown that RXR, which is a silent heterodimeric partner with RAR and TR with respect to its own ligand-binding activity, is active in a complex with NGFI–B or Nurr1 (Kurokawa et al., 1994; Forman et al., 1995; Perlmann and Jansson, 1995). At present, it is not known whether heterodimerization of AaUSP with AHR38 could impart similar allosteric changes on the USP LBD, modifying the USP into an active ligand-binding receptor. Elucidation of the nature of this possible USP-specific ligand represents a challenging question for future studies.
Materials and methods
Animals
Aedes aegypti mosquitoes were reared as described in Hays and Raikhel (1990). Vitellogenesis was initiated by allowing females 3–5 days PE to feed on an anesthetized white rat. Fat bodies were dissected from females at previtellogenic and vitellogenic stages, quickly frozen in liquid nitrogen and stored at –80°C.
RNA isolation and RT–PCR/Southern blot analysis
Fat bodies were dissected from female mosquitoes at different time points ranging from 0 to 5 days PE at the previtellogenic stage or from the vitellogenic stage ranging from 1 to 60 h PBM. Total RNA was prepared from the fat body throughout the first vitellogenic cycle, using the guanidine isothiocyanate method as described previously (Miura et al., 1998) with the modification that all isopropanol precipitation steps were performed without low-temperature incubation to avoid co-precipitation of glycogen and salts. Total RNA was also extracted with Trizol reagent (Gibco-BRL). RNA yields were determined spectrophotometrically. A260/A280 and A260/A230 of RNA preparations were always above 1.7 and 2.0, respectively, and quite consistent irrespective of developmental stages of the mosquitoes. The temporal profile of AHR38 transcripts in the mosquito fat body was examined using RT–PCR followed by Southern blotting with an AHR38-specific radioactive probe. Five microgram aliquots of each RNA preparation were reverse-transcribed by the Superscript II reverse transcriptase (Gibco-BRL) and random hexamer (Promega) in a reaction volume of 20 μl. The reverse transcription product was diluted to 40 μl with TE buffer (10 mM Tris–HCl, 1 mM EDTA pH 8.0) and stored at –20°C as a cDNA pool until use. For the developmental profile analysis, 0.025 fat body equivalents of cDNA pools were used as PCR templates. A 285 bp AHR38-specific fragment was amplified with the following primer pair: forward, 5′-AGCTCACCCGGCTACATCAAC-3′; and reverse, 5′-GCAGGCCTTGTCCGCGAGAC-3′. A 463 bp VCP-specific fragment (Cho et al., 1991) was amplified with the following primer pair: forward, 5′-AGCGCCCATTCTTGTTTGG-3′; and reverse, 5′-CAGCTCATACAGGTATTCTCC-3′.
Thermal cycling conditions were as follows: the reaction was incubated at 94°C for 3 min followed by 17 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 40 s. As a reference of the developmental changes of the fat body, the same cDNA was subjected to the 10–15 cycles of PCR with a primer pair specific to the mosquito VCP. After PCR amplification, 10 μl out of the total 25 μl reaction were fractionated on a 2% agarose gel, and transferred onto Hybond N+ membrane (Amersham) under alkaline conditions. The RT–PCR products were visualized by Southern hybridization with AHR38- or VCP-specific probes.
5′-RACE
A Gibco-BRL 5′-RACE kit was used according to the manufacturer's protocol. The primers used were: gene-specific primer1 (GSP1) (5′TGGAATCTGCAGAACTG-3′); gene-specific primer2 (GSP2) (5′-CGTTCGAACGCCGTAGTGTTG-3′); and abridged anchor primer (AP) (5′-GGCCACGCGTCGACTAGTACGGIIGGGIIGGGIIG-3′).
Antibodies
cDNA fragments encoding AaEcR, AaUSP (Cho et al., 1995; Kapitskaya et al., 1996) and AHR38 were individually subcloned in pGEX-4T-1 (Pharmacia) to create GST fusions. The fusion proteins were induced by isopropyl-β-d-thiogalactopyranoside (IPTG) in E.coli strain BL21 and purified by GST Purification Modules from Pharmacia. Proteins were further purified by SDS–PAGE followed by electroelution, and sent to Cocalico Biologicals Inc., where New Zealand white rabbits and Leghorn chickens were immunized. The monoclonal antibody against DmUSP (anti-DmUSP), described in Christianson et al. (1992), was a gift from Dr F.C.Kafatos (European Molecular Biological Laboratories, Heidelberg, Germany). For Western blot assays, the following dilutions of antibodies were used: anti-AaEcR polyclonal antibodies, 1:100; anti-AaUSP polyclonal antibodies, 1:100; anti-DmUSP monoclonal antibodies, 1:200; anti-AHR38 polyclonal antibodies, 1:100; anti-rabbit IgG antibodies conjugated with horseradish peroxidase (HRP), 1:5000 (Cappel); anti-mouse IgG antibodies conjugated with HRP, 1:5000 (Cappel); anti-chicken IgG antibodies conjugated with HRP, 1:160 000 (Sigma).
GST pull-down assay
GST fusion proteins immobilized on glutathione–Sepharose 4B beads (Pharmacia) were resuspended in the binding buffer [20 mM HEPES pH 7.9, 100 mM NaCl, 1 mM EDTA, 4 mM MgCl2, 1 mM dithiothreitol (DTT), 0.02% NP-40, 10% glycerol supplemented before use with 1 mg/ml bovine serum albumin, 0.5 mM phenylmethylsulfonyl fluoride, and 5 mg/ml each of antipain, leupeptin and pepstatin, 9 mg/ml aprotinin and 2 mM benzamidine], and incubated for 2–12 h at 4°C with FBNE (see below) or proteins labeled with [35S]methionine by a coupled in vitro transcription–translation protocol (TNT; Promega). After extensive washing, the bound proteins were recovered by boiling the beads in 1× SDS sample buffer and separated by SDS–PAGE. The proteins were examined by autoradiography or immunoblot analysis. 20E (5 × 10–6 M) was included in the incubation mixture, while only the ethanol vehicle was added to the control incubation.
Electrophoretic mobility shift assay
Assays were carried out as described by Wang et al. (1998). Oligonucleotides used in EMSA were: DR-5, 5′-AGCGGATCCAGGTCACCGAAAGGTCAGGATCCGCG-3′; hsp27-EcRE, 5′-AGCTTCAAGGGTTCA- TGCACTTGTCCATCG-3′; ng-EcRE, 5′-GCGAAAGGTCAAGAGGCCAAATGAAGGTCAGGAA-3′; and vg-EcRE, 5′-AGCGGGAGGCCAATGGTCGAGTGAATCT-3′.
Transient transfections
The KpnI–NotI fragment of the AHR38 cDNA was cloned into the corresponding sites of the expression vector pcDNA 3.1/Zeo(+) (Invitrogen). The green African monkey kidney CV-1 cell line (American Tissue Culture Collection, Bethesda, MD) was transfected as described elsewhere (Wang et al., 1998).
Yeast two-hybrid assay
cDNA fragments of AHR38, AaEcR and AaUSPb were inserted into either pHybLex/Zeo or pYESTrp vector (Invitrogen) to form fusion proteins with the DNA-binding domain of LexA or the activation domain of B42, respectively. The fusion constructs were used to co-transform the L40 strain of Saccharomyces cerevisiae [MATa his3D200 trp1-901 leu2-3112 ade2 LYS2::(4lexAop-HIS3) URA3::(8lexAop-lacZ) GAL4], which has integrated LexA operator-driven HIS3 and lacZ reporter genes. The strength of protein–protein interactions in the yeast nuclei was estimated by either the filter assay with X-gal (Breeden and Nasmyth, 1985) or the liquid β-galactosidase assay with the substrate o-nitrophenyl-β-d-galactoside according to Mori et al. (1992).
Co-immunoprecipitation experiments
FBNE were prepared as described (Miura et al., 1999). The soluble nuclear proteins were diluted to reduce the NaCl concentration to 150 mM and pre-cleared with Sepharose beads (Pharmacia) for 1–2 h at 4°C. The supernatant was then incubated with antiserum or pre-immune serum for 12 h at 4°C followed by incubation with protein A–agarose beads (Boehringer Mannheim) for 3–4 h at 4°C. The binding buffer was composed of 25 mM HEPES pH 7.9, 150 mM NaCl, 10% glycerol, 0.1 mM DTT, 0.2 mM EDTA, 0.1% NP-40 and 1 mM (2-aminoethyl)benzenesulfonyl fluoride (AEBSF). The precipitate was washed three times with binding buffer and once with a solution of 0.1 M Tris (pH 6.7) containing protease inhibitors. The bound proteins were recovered by boiling the beads in 1× SDS sample buffer and separated by SDS–PAGE followed by immunoblotting. Ponceau-S staining of Western blots confirmed the presence of equal amounts of precipitating antibody in each sample. The antigen–antibody complex was detected by chemiluminescence using the SuperSignal Substrate system (Pierce).
Acknowledgments
Acknowledgements
The authors thank Dr M.Fluck for permission to use her laboratory for cell transfection assays and Dr F.C.Kafatos for a generous gift of anti-USP monoclonal antibodies. We are also grateful to Drs S.-F.Wang and J.Sun, and Mr N.Dittmer and Mr C.Ontiveros for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health (RO1 AI-36959).
References
- Beier J.C. (1998) Malaria parasite development in mosquitoes. Annu. Rev. Entomol., 43, 519–543. [DOI] [PubMed] [Google Scholar]
- Breeden L. and Nasmyth, K. (1985) Regulation of the yeast HO gene. Cold Spring Harb. Symp. Quant. Biol., 50, 643–650. [DOI] [PubMed] [Google Scholar]
- Bruno J.-M. et al. (1997). The spirit of Dakar: a call for action on malaria. Nature, 386, 541. [DOI] [PubMed] [Google Scholar]
- Butler D. (1997) Time to put malaria control on the global agenda. Nature, 386, 535–536. [DOI] [PubMed] [Google Scholar]
- Cho W.-L., Deitsch, K.W. and Raikhel, A.S. (1991) An extraovarian protein accumulated in mosquito oocytes is a carboxypeptidase activated in embryos. Proc. Natl Acad. Sci. USA, 88, 10821–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.-L., Kapitskaya, M. and Raikhel, A.S. (1995) Mosquito ecdysteroid receptor: analysis of the cDNA and expression during vitellogenesis. Insect Biochem. Mol. Biol., 25, 19–27. [DOI] [PubMed] [Google Scholar]
- Cho W.-L., Tsao, S.M., Hays, A.R., Walter, R., Chen, J.S., Snigirevskaya, E.S. and Raikhel, A.S. (1999) Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. J. Biol. Chem., 274, 13311–13321. [DOI] [PubMed] [Google Scholar]
- Christianson A.M., King, D.L., Hatzivassiliou, E., Casas, J.E., Hallenbeck, P.L., Nikodem, V.M., Mitsialis, S.A. and Kafatos, F.C. (1992) DNA binding and heteromerization of the Drosophila transcription factor chorion factor 1/ultraspiracle. Proc. Natl Acad. Sci. USA, 89, 11503–11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.H. and Paskewitz, S.M. (1995) Malaria: current and future prospects for control. Annu. Rev. Entomol., 40, 195–219. [DOI] [PubMed] [Google Scholar]
- Crispi S., Giordano, E., D'Avino, P.P. and Furia, M. (1998) Cross-talking among Drosophila nuclear receptors at the promiscuous response element of the ng-1 and ng-2 intermolt genes. J. Mol. Biol., 275, 561–574. [DOI] [PubMed] [Google Scholar]
- Deitsch K.W., Chen, J.S. and Raikhel, A.S. (1995) Indirect control of yolk protein genes by 20–hydroxyecdysone in the fat body of the mosquito, Aedes aegypti.Insect Biochem. Mol. Biol., 25, 449–454. [DOI] [PubMed] [Google Scholar]
- Dhadialla T.S. and Raikhel,A.S. (1994) Endocrinology of mosquito vitellogenesis. In Davey,K.G., Peter,R.E. and Tobe,S.S. (eds), Perspectives in Comparative Endocrinology. National Research Council of Canada, Ottawa, Canada, pp. 275–281. [Google Scholar]
- Dittmann F., Kogan, P.H. and Hagedorn, H.H. (1989) Ploidy levels and DNA synthesis in the fat body cells of the adult mosquito, Aedes aegypti.Arch. Insect Biochem. Physiol., 12, 133–143. [Google Scholar]
- Fallon A.M., Hagedorn, H.H., Wyatt, G.R. and Laufer, H. (1974) Activation of vitellogenin synthesis in the mosquito Aedes aegypti by ecdysone. J. Insect Physiol., 20, 1815–1823. [DOI] [PubMed] [Google Scholar]
- Forman B.M., Umesono, K., Chen, J. and Evans, R.M. (1995) Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell, 81, 541–550. [DOI] [PubMed] [Google Scholar]
- Glass C.K. (1994) Differential recognition of target genes by nuclear receptor monomers, dimers and heterodimers. Endocr. Rev., 15, 391–407. [DOI] [PubMed] [Google Scholar]
- Hagedorn H.H. (1983) The role of ecdysteroids in the adult insect. In Downer,R.G.H. and Laufer,H. (eds), Endocrinology of Insects. Alan R.Liss, New York, NY, pp. 271–304. [Google Scholar]
- Hagedorn H.H. (1985) The role of ecdysteroids in reproduction. In Kerkut,G.A. and Gilbert,L.I. (eds), Comprehensive Insect Physiology, Biochemistry and Pharmacology. Pergamon Press, New York, NY, Vol. 8, pp. 205–261. [Google Scholar]
- Hagedorn H.H. and Fallon, A.M. (1973) Ovarian control of vitellogenin synthesis by the fat body in Aedes aegypti.Nature, 244, 103–105. [DOI] [PubMed] [Google Scholar]
- Hagedorn H.H., Fallon, A.M. and Laufer, H. (1973) Vitellogenin synthesis by the fat body of Aedes aegypti.Dev. Biol., 31, 285–294. [DOI] [PubMed] [Google Scholar]
- Hagedorn H.H., O'Connor, J.D., Fuchs, M.S., Sage, B., Schlaeger, D.A. and Bohm, M.K. (1975) The ovary as a source of α-ecdysone in an adult mosquito. Proc. Natl Acad. Sci. USA, 72, 3255–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays A.R. and Raikhel, A.S. (1990) A novel protein produced by the vitellogenic fat body and accumulated in mosquito oocytes. Roux's Arch. Dev. Biol., 199, 114–121. [DOI] [PubMed] [Google Scholar]
- Kapitskaya M., Wang, S.-F., Cress, D.E., Dhadialla, T.S. and Raikhel, A.S. (1996) The mosquito ultraspiracle homologue, a partner of ecdysteroid receptor heterodimer: cloning and characterization of isoforms expressed during vitellogenesis. Mol. Cell. Endocrinol., 121, 119–132. [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark, M. and Chambon, P. (1995) Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell, 83, 859–869. [DOI] [PubMed] [Google Scholar]
- Kostrouch Z., Kostrouchova, M. and Rall, J.E. (1995) Steroid/thyroid hormone receptor genes in Caenorhabditis elegans.Proc. Natl Acad. Sci. USA, 92, 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova T., Pokholkova, G.V., Tzertzinis, G., Sutherland, J.D., Zhimulev, I.F. and Kafatos, F.C. (1998) Drosophila hormone receptor 38 functions in metamorphosis: a role in adult cuticle formation. Genetics, 149, 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., DiRenzo, J., Boehm, M., Sugarman, J., Gloss, B., Rosenfeld, M.G., Heyman, R.A. and Glass, C.K. (1994) Regulation of retinoid signaling by receptor polarity and allosteric control of ligand binding. Nature, 371, 528–531. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. and Evans, R.M. (1995) The RXR heterodimers and orphan receptors. Cell, 83, 841–850. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Shinoda, T., Yura, M., Nomura, S., Kamiya, K., Yuda, M. and Chizei, Y. (1998) Two hexameric cyanoprotein subunits from an insect, Riptortus clavatus. Sequence, phylogeny and developmental and juvenile hormone regulation. Eur. J. Biochem., 258, 929–940. [DOI] [PubMed] [Google Scholar]
- Miura K., Wang, S.F. and Raikhel, A.S. (1999) Two distinct subpopulations of ecdysone receptor complex in the female mosquito during vitellogenesis. Mol. Cell. Endocrinol., 156, 111–120. [DOI] [PubMed] [Google Scholar]
- Mori K., Sant, A., Kohno, K., Normington, K., Gething, M.J. and Sambrook, J.F. (1992) A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J., 11, 2583–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- Perlmann T. and Jansson, L. (1995) A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI–B and NURR1. Genes Dev., 9, 769–782. [DOI] [PubMed] [Google Scholar]
- Pierceall W.E., Li, C., Biran, A., Miura, K., Raikhel, A.S. and Segraves, W.A. (1999) E75 expression in mosquito ovary and fat body suggests reiterative use of ecdysone-regulated hierarchies in development and reproduction. Mol. Cell. Endocrinol., 150, 73–89. [DOI] [PubMed] [Google Scholar]
- Raikhel A.S. (1992) Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol., 37, 217–251. [DOI] [PubMed] [Google Scholar]
- Raikhel A.S. and Lea, A.O. (1990) Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. Gen. Comp. Endocrinol., 77, 423–434. [DOI] [PubMed] [Google Scholar]
- Ribeiro R.C., Apriletti, J.W., West, B.L., Wagner, R.L., Fletterick, R.J., Schaufele, F. and Baxter, J.D. (1993) The molecular biology of thyroid hormone action. Ann. NY Acad. Sci., 758, 366–389. [DOI] [PubMed] [Google Scholar]
- Shapiro A.B., Wheelock, G.D., Hagedorn, H.H., Baker, F.C., Tsai, L.W. and Schooley, D.A. (1986) Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti.J. Insect Physiol., 32, 867–877. [Google Scholar]
- Sutherland J.D., Kozlova, T., Tzertzinis, G. and Kafatos, F.C. (1995) Drosophila hormone receptor 38: a second partner for Drosophila USP suggests an unexpected role for nuclear receptors of the nerve growth factor-induced protein B type. Proc. Natl Acad. Sci. USA, 92, 7966–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.J. and O'Malley, B.W. (1994) Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem., 63, 451–483. [DOI] [PubMed] [Google Scholar]
- Wang S.-F., Miura, K., Miksicek, R.J., Segraves, W.A. and Raikhel, A.S. (1998) DNA binding and transactivation characteristics of the mosquito ecdysone receptor–Ultraspiracle complex. J. Biol. Chem., 273, 27531–27540. [DOI] [PubMed] [Google Scholar]
- Wang S.-F., Li,C., Zhu,J., Miura,K., Miksicek,R.J. and Raikhel,A.S. (2000) Differential expression and regulation by 20-hydroxyecdysone of mosquito ultraspiracle isoforms. Dev. Biol., in press. [DOI] [PubMed] [Google Scholar]
- Wilson T.E., Fahrner, T.J. and Milbrandt, J. (1993) The orphan receptors NGFI–B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor–DNA interaction. Mol. Cell. Biol., 13, 5794–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.P., Segraves, W.A., Oro, A.E., McKeown, M. and Evans, R.M. (1992) Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell, 71, 63–72. [DOI] [PubMed] [Google Scholar]
- Yao T.P., Forman, B.M., Jiang, Z., Cherbas, L., Chen, J.D., McKeown, M., Cherbas, P. and Evans, R.M. (1993) Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature, 366, 476–479. [DOI] [PubMed] [Google Scholar]
- Zetterstrom R.H., Williams, R., Perlmann, T. and Olson, L. (1996) Cellular expression of the immediate early transcription factors Nurr1 and NGFI–B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Mol. Brain Res., 41, 111–120. [DOI] [PubMed] [Google Scholar]