Abstract
C-type lectins (CTLs) are proteins that contain one or more carbohydrate-recognition domains (CRDs) that require calcium for sugar binding and share high degree of sequence homology and tertiary structure. CTLs whose CRD contain EPN (Glu-Pro-Asn) tripeptide motifs have potential to bind mannose (Man), N-acetylglucosamine (GlcNAc), glucose (Glc) and l-fucose (Fuc), whereas those with QPD (Glu-Pro-Asp) tripeptide motifs bind galactose (Gal) and N-acetylgalactosamine (GalNAc). We report here for the first time a direct comparison of monosaccharide (and some di- and trisaccharides)-binding characteristics of 11 EPX-containing (X = N, S or D) immune-related CTLs using a competition assay and an enzyme-linked immunosorbent assay, and neoglycoproteins as ligand. The EPX CTLs studied are DC-SIGN, L-SIGN, mSIGNR1, human and mouse mannose receptors, Langerin, BDCA-2, DCIR, dectin-2, MCL and MINCLE. We found that: (1) they all bound Man and Fuc; (2) binding of Glc and GlcNAc varied considerably among these lectins, but was always less than Man and Fuc; (3) in general, Gal and GalNAc were not bound. However, dectin-2, DCIR and MINCLE showed ability to bind Gal/GalNAc; (4) DC-SIGN, L-SIGN, mSIGNR1 and Langerin showed enhanced binding of Manα2Man over Man, whereas all others showed no enhancement; (5) DC-SIGN bound Lex trisaccharide structure, which has terminal Gal and Fuc residues, more avidly than Fuc, whereas L-SIGN, mSIGNR1, DCIR and MINCLE bound Lex less avidly than Fuc. BDCA-2, dectin-2, Langerin, MCL and mannose receptor did not bind Lex at all.
Keywords: C-type lectin, mannose/fucose-binding, immune receptor, binding specificity
Introduction
C-type lectin-like receptors (CTLRs) are transmembrane (TM) proteins that contain one or more C-type lectin-like domains in the extracellular region. A CTLR can be a genuine lectin that binds sugars in a calcium-dependent manner, or it may lack the calcium- and sugar-binding capabilities. Many immune-related cells are endowed with particularly large variety of CTLRs. Natural killer cells express many CTLRs that are mostly not the canonical sugar-binding type (Weis et al. 1998), whereas the surface of antigen-presenting cells, particularly that of dendritic cells (DCs), is known to harbor plethora of CTLRs, many of which are of the sugar-binding type (Robinson et al. 2006).
C-type lectins (CTLs) can be classified into two groups with respect to their sugar-binding specificity. Drickamer (1992) showed that galactose/N-acetylgalactosamine (Gal/GalNAc)-recognizing carbohydrate-recognition domains (CRDs) invariably contain QPD tripeptide motifs in the CRD, whereas CTLs that contain EPN motifs, such as mannose-binding protein (MBP) and macrophage mannose receptor (MMR), can bind Man, Glc, GlcNAc and l-Fuc (Lee et al. 1991a; Engering et al. 1997). X-ray crystallographic structure of the bound carbohydrate showed that CRDs with QPD motifs engage the equatorial/axial configuration of 3-OH/4-OH in H-bonding and calcium chelation (Kolatkar and Weis 1996), whereas those with EPN motifs engage the equatorial/equatorial arrangement of 3-OH/4-OH of the bound sugar residue (Ng et al. 1996). The former configuration thus specifies Gal/GalNAc among common hexoses, whereas the latter configuration opens a door for possible binding of Man, Glc and GlcNAc as well as l-Fuc, on the account of the latter being stereochemically equivalent to d-Man, if the C1 and C5 positions are exchangeable (Lee et al. 1991b).
DC-SIGN and MMR, perhaps the two best-studied of EPN-containing CTLRs on DCs, carry out capture and endocytosis of pathogens for antigen presentation. DC-SIGN binds high-mannose-type structures as well as Lex and related structures (Guo et al. 2004), whereas MMR binds various mannose-containing structures. Both are also known to be usurped by some pathogens to actually help their survival (van Kooyk et al. 2004). For instance, human immunodeficiency virus (HIV) appears to hitch a ride on the peripheral DCs via binding of HIV's high-mannose-type glycans and be transported from mucosal surface to the lymphoid system, where the transfer of HIV to T-cell is consummated. On the other hand, pathogenic mycobacterial species, such as Mycobacterium tuberculosis and M. leprae, cause immunosuppression in the host which is mediated at least partially by the binding of their virulence factor, ManLAM (mannose-capped lipoarabinomannan), to DCs (Nigou et al. 2001; Geijtenbeek et al. 2003). Some helminth pathogens promote conversion of the host immune response from Th1 response to the Th2-type and to regulatory responses via induction of IL-10 through binding of their Fuc-rich glycans to DC-SIGN (Okano et al. 1999, 2001; van Die and Cummings 2010).
It is interesting to note that among all the CTLRs represented on DCs, the group that contains EPX (where X is usually N, but can be S or D and maybe others) greatly outnumbers the QPD-containing type. This may reflect the fact that the EPX group has the capability to bind many natural neutral sugars and thus its members have overlapping but distinct binding specificities. In this study, we investigated the sugar-binding characteristics of 11 EPX-containing CTLs primarily at the level of monosaccharide, using highly sugar-conjugated bovine serum albumin (BSA) neoglycoproteins as ligands. We found that while some EPX-CTLs had a rather stringent binding specificity of binding only Man and Fuc, others bound, in addition to Man and Fuc, GlcNAc and Glc quite well, and some even bound Gal/GalNAc, albeit at much lower levels. We extended the comparison study of these CTLs to some di- and trisaccharide structures.
Results
In this study, our main goal was to map out the monosaccharide-binding pattern of EPX-type (also referred as Man/Fuc-type) CTLs from myeloid cells, since the EPX-type CTLs have potential to bind Man, l-Fuc, GlcNAc and Glc, so that they may express binding specificities that are overlapping, but distinct for each lectin. Among the 11 EPX-type CTLs we surveyed, nine had the EPN motif, which are DC-SIGN, L-SIGN, MMR, Langerin, BDCA-2, dectin-2 and MINCLE of human kind, and SIGNR1 and MMR of mouse. DCIR contains an EPS tripeptide and MCL contains an EPD tripeptide. In addition, we investigated one QPD-type CTL, hMGL, and MDL-1 that contains neither EPX nor QPD tripeptide motif. Most of the CTLs we studied were in the form of Fc chimera (see Materials and methods), with the exceptions of dectin-2 and MMRs, which were in the His-tagged form. Both Fc-chimera and His-tagged forms of Langerin were used. In addition to the six common monosaccharides, we tested a number of disaccharides and trisaccharides, including Manα2Man and Lex structures, both of which are known good ligands for DC-SIGN.
In our assays, we used glycans in the form of BSA-conjugated neoglycoproteins. Our earlier studies on CTLs showed that the binding avidity of neoglycoproteins increased exponentially with increasing sugar density on BSA (e.g. Man-BSA binding to MBP), reaching maximum plateau avidity around the conjugation level of 30 residues per molecule of BSA (Lee et al. 1991a). For this reason, we used neoglycoproteins of six different hexoses with sugar-conjugation level higher than 30. These were Man51-, Fuc42-, GlcNAc44-, Glc44-, Gal34- and GalNAc35-BSA. In the remainder of this article, we will refer to the neoglycoproteins without mentioning the conjugation level (for instance, GlcNAc-BSA means GlcNAc44-BSA). In addition to the six neoglycoproteins, we routinely screened avidity of Man33- and Fuc30-BSA to find out whether indeed the binding avidity has reached plateau. Using enzyme-linked immunosorbent assay (ELISA), differences in the bound amounts between Fuc42-BSA and Fuc30-BSA and between Man33-BSA and Man51-BSA for all the CTLs were within 10% of each other. Only in one case (MCL), Man33-BSA was bound 22% less than Man51-BSA.
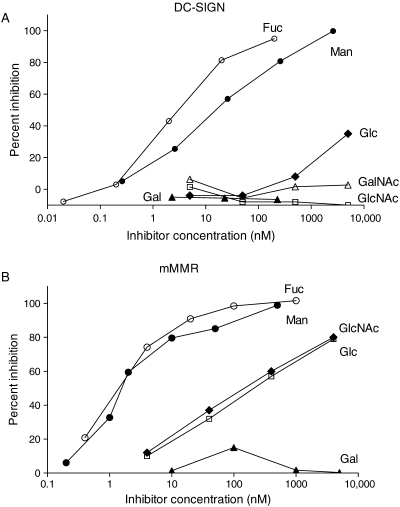
Two types of assays, both using the 96-well format, were employed: Assay I is an inhibition assay in which various neoglycoproteins were used as inhibitors for the binding of a tagged high-affinity ligand (Eu-DTPA-labeled Man51-BSA) to lectin immobilized to the well via antibodies (anti-human Fc or anti-His tag); Assay II is a direct binding of CTL to immobilized neoglycoprotein surface, assayed with the enzyme-linked detection method. Assay I gives quantitative binding avidity data as IC50 values. However, among 11 EPX-type CTLs we tested, only DC-SIGN and human and mouse MMRs gave consistently reproducible data. The results for DC-SIGN and MMRs using Assay I were shown in Figure 1A and B and summarized in Table I. The results show that: (1) binding avidity is higher for MMRs than for DC-SIGN; (2) Fuc-BSA bound 10-fold better than Man-BSA to DC-SIGN, but no preference was shown by either of the MMRs; (3) binding affinities of Glc- and GlcNAc-BSA to MMRs and that of Glc-BSA to DC-SIGN were approximately a 1000-fold worse than Man-BSA, and DC-SIGN was totally devoid of GlcNAc-BSA-binding ability; (4) DC-SIGN, but not MMRs, showed enhanced binding affinity for (Manα2Man)-BSA over Man-BSA (of the PM-type) and higher binding of Lex-BSA over Fuc-BSA of a similar sugar-conjugation level.
Fig. 1.
Examples of inhibition curves generated by Assay I. (A) DC-SIGN-Fc: filled circle, Man-BSA; open circle, Fuc-BSA; open square, GlcNAc-BSA; filled diamond, Glc-BSA; filled triangle, Gal-BSA; open triangle, GalNAc-BSA. (B) His-tagged mMMR: filled circle, Man-BSA; open circle, Fuc-BSA; open square, Glc-BSA; filled diamond, GlcNAc-BSA; filled triangle, Gal-BSA.
Table I.
IC50 values of various neoglycoproteins obtained using the competition assay (Assay I) with Eu-Man51-BSA as reference ligand
| Neoglycoprotein inhibitors | IC50 (nM) |
||
|---|---|---|---|
| DC-SIGN | hMMR | mMMR | |
| Man51-AI-BSA | 43 | 5 | 2 |
| Man33-AI-BSA | 35 | 10 | 0.8 |
| Fuc42-AI-BSA | 3.4 | 9 | 1.4 |
| Fuc30-AI-BSA | 3.3 | 10 | 2.0 |
| GlcNAc44-AI-BSA | NIa | 1000 | 153 |
| Glc44-AI-BSA | 30000 | 2400 | 235 |
| Gal34-AI-BSA | NI | NI | NI |
| GalNAc35-AI-BSA | NI | NI | NI |
| Man38-PM-BSA | 100 | 50 | 8 |
| (Manα2Man)36-PM-BSA | 0.8 | 40 | 10 |
| (Manα2Manα2Man)31-PM-BSA | 1.4 | 120 | 11 |
aNI, not inhibitory.
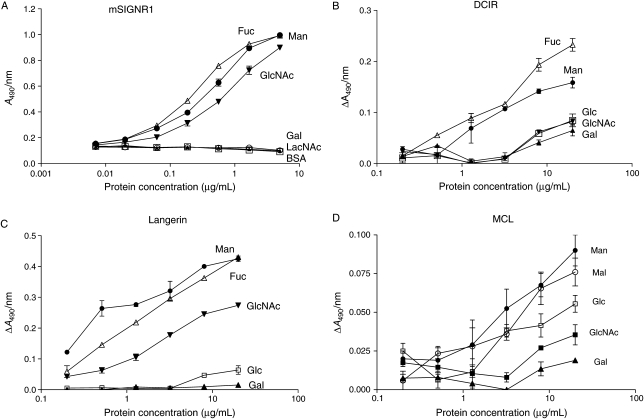
For the remaining CTLs, it was necessary to use ELISA (Assay II) to generate relative affinity of various neoglycoproteins for each lectin. Using the sequential Assay II, all members of the DC-SIGN family, i.e. DC-SIGN, L-SIGN and mSIGNR1, produced strong signal and reproducible data. For the remainder of type II TM CTLs, precomplexing protocol for enhancing binding avidity was necessary to achieve measurable binding signal. These include all the CTLs that have short stalk (43 to 52 amino acid residues), as well as Langerin and MGL, both of which have a segment of coiled-coil structure in the stalk region, albeit shorter and maybe structurally different from that of DC-SIGN-type tetrameric structure. An example of the binding curves generated by the sequential ELISA for mSIGNR1 and three examples of precomplex-forming ELISA binding curves are shown in Figure 2A–D. Note that the error bars for the precomplex-forming ELISA are larger than those of the sequential ELISA, due to higher blank values and weaker signal. This is particularly true for BDCA-2 and MCL, which produced very weak binding signal (see Figure 2D for MCL).
Fig. 2.
Examples of binding curves using Assay II. (A) Binding curves for mSIGNR1-Fc generated by sequential ELISA. Immobilized proteins: open circle, BSA (blank); filled circle, Man-BSA; filled triangle, Gal-BSA; filled inverted triangle, GlcNAc-BSA; open triangle, Fuc-BSA; open square, LacNAc-BSA. The immobilization concentration of proteins was expressed in logarithmic scale on the x-axis. The highest concentration was 5 μg/mL, from which 3-fold serial dilutions were made. Examples of using the precomplex-forming ELISA. (B) DCIR-Fc; (C) Langerin-(His)6; (D) MCL-Fc. Immobilized proteins: filed circle, Man-BSA; open triangle, Fuc-BSA; filled triangle, Gal-BSA; filled inverted triangle or filled square, GlcNAc-BSA; open square, Glc-BSA; open circle, Mal-BSA. The highest immobilization concentration was 20 μg/mL, from which 2.5-fold serial dilutions were made.
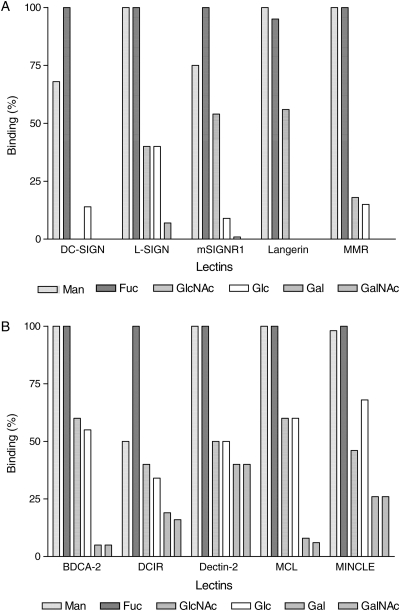
In order to compare binding avidity of six monosaccharide-containing neoglycoproteins, the highest net bound amount (i.e. ΔA490 nm) of the six neoglycoproteins for each lectin obtained by Assay II was set as 100%, and the percent bound amounts of remaining neoglycoproteins were calculated. Always either Fuc42-BSA or Man51-BSA had the highest binding signal. Figure 3A shows side-by-side comparison of all the EPX-type CTLs other than the short-stalk variety and Figure 3B shows comparison of all the short-stalk Man/Fuc-type CTLs. An Fc-chimera of MDL-1, a CTL-like receptor that lacks calcium- and sugar-binding amino acid residues, bound none of the neoglycoproteins to a significant degree (not shown). We tested one QPD-type CTL, i.e. human MGL-Fc. Surprisingly, it produced rather poor binding signal, and GalNAc-BSA was the only neoglycoprotein that bound to MGL-Fc (not shown). It is quite clear from such fingerprint-like presentation of the binding pattern that each CTL possesses rather unique binding characteristics.
Fig. 3.
Comparison of binding patterns of 10 CTLRs for 6 hexoses. Bar graph for each CTLR was generated as described in the text, setting the highest bound neoglycoprotein as 100% on the y-axis. (A) DC-SIGN family members, Langerin and hMMR; (B) short-stalk, Type II TM CTLs.
From Figure 3, one can conclude that: (1) Fuc-BSA and Man-BSA are the two best ligands for all the EPX-type CTLs. DCIR, DC-SIGN and mSIGNR1 bound Fuc-BSA better than Man-BSA, and among them DCIR exhibited the largest differential in the bound amount. (2) Glc-BSA was always bound less strongly than its 2-epimeric counterpart, Man-BSA. (3) GlcNAc-BSA and Glc-BSA were usually bound to a similar extent, with two exceptions: mSIGNR1 and Langerin hardly bound Glc-BSA, but bound GlcNAc-BSA with good avidity. (4) Usually Gal- and GalNAc-BSA were not bound, as expected for the EPX-type lectins. However, dectin-2, DCIR and MINCLE showed ability to bind Gal- and GalNAc-BSA, in addition to the usual Man-, Fuc-, GlcNAc- and Glc-BSA. (5) DC-SIGN had the most restrictive binding pattern, essentially binding only Man- and Fuc-BSA.
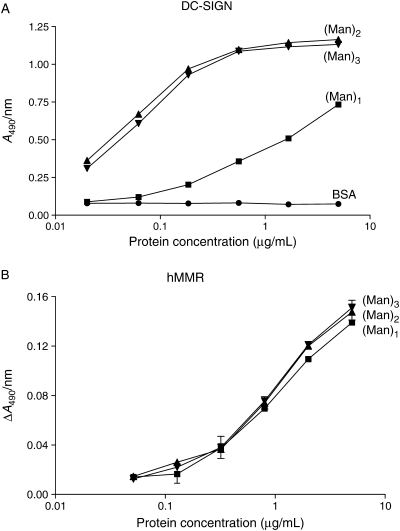
DC-SIGN is perhaps the best-studied CTL out of all the myeloid cell-associated CTLs in structural, biochemical and immunological aspects. It has dual high-affinity binding structures of high-mannose-type and Lex and related structures (Guo et al. 2004). We compared the binding to all the CTLs of Man-BSA, (Manα2Man)-BSA and (Manα2Manα2Man)-BSA attached to BSA by the same linkage of PM-type (see Materials and methods). Manα2Man is the outermost disaccharide structure of Man9 oligosaccharide, which is a high-affinity ligand for DC-SIGN. DC-SIGN exhibited a very large enhancement effect by the presence of the second mannose as shown in Figure 4A, whereas MMR showed no affinity enhancement at all by the second mannose (Figure 4B). Table II summarizes the binding enhancement by the presence of the second and third mannose residues. The presence of the second mannose enhanced the binding of DC-SIGN, L-SIGN, mSIGNR1 and Langerin, but there was no further enhancement by the third mannose residue. All other CTLs showed no affinity enhancement by the presence of the second mannose residue. Among the four that exhibited the enhancement effect, DC-SIGN had the highest enhancement, which is also evident in the Assay I results (Table I), showing a 100-fold increase in the binding affinity by the presence of the second mannose residue.
Fig. 4.
Comparison of binding affinity of BSA derivatives containing Man (filled square), Manα2Man (filled triangle), and Manα2Manα2Man (filled inverted triangle) structures. (A) DC-SIGN-Fc by sequential ELISA; filled circle, BSA (blank). The highest immobilization concentration was 5 μg/mL, from which 3-fold serial dilutions were made. (B) hMMR-(His tag) by precomplex-forming ELISA. The highest immobilization concentration was 5 μg/mL, from which 2.5-fold serial dilutions were made.
Table II.
Affinity enhancement effect of the second and third mannose residues
| CTL | Affinity enhancement (fold)a |
|
|---|---|---|
| Disaccharide | Trisaccharide | |
| DC-SIGN | 7.2 | 6.8 |
| L-SIGN | 4.4 | 4.6 |
| mSIGNR1 | 2.8 | 3.5 |
| Langerin | 2.5 | 2.7 |
| hMMR | 1.0 | 1.0 |
| BDCA-2 | 0.31 | 0.38 |
| DCIR | 0.54 | 0.77 |
| Dectin-2 | 0.84 | 0.77 |
| MCLb | ≪1 | NTc |
| MINCLE | 0.86 | NT |
aRatio of bound amount of BSA derivative containing Manα2Man or Manα2Manα2Man to the bound amount of Man-BSA, all linked to BSA via PM-linkage. The bound amounts were determined as ΔA490 nm using Assay II.
bWhile Man38-PM-BSA showed definite binding, the binding of (Manα2Man)36-PM-BSA was nil.
cNot tested.
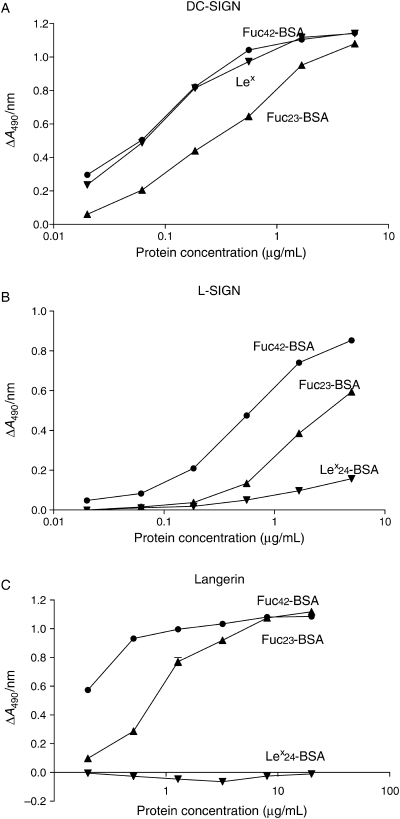
To investigate possible affinity enhancement by the Lex structure over Fuc, we tested Lex24-BSA versus Fuc23-BSA. Three examples of binding curves are shown in Figure 5. Figure 5A shows the result for DC-SIGN, in which a significant affinity enhancement was seen for Lex24-BSA over Fuc23-BSA. Figure 5B shows the case (L-SIGN) of Lex24-BSA binding less effectively than Fuc23-BSA. Figure 5C shows the pattern seen for many CTLs, in which Lex24-BSA did not bind at all, despite decent binding exhibited by Fuc23-BSA. The CTLs devoid of binding capacity for Lex-BSA are BDCA-2, dectin-2, Langerin, MCL and MMR. For those CTLs that bound Lex-BSA, the ratios of the bound amount of Lex24-BSA over Fuc23-BSA were 1.57, 0.25, 0.74, 0.21 and 0.74 for DC-SIGN, L-SIGN, mSIGNR1, DCIR and MINCLE, respectively. Thus, DC-SIGN was the only CTL that showed higher binding of Lex24-BSA over Fuc23-BSA.
Fig. 5.
Comparison of Lex24-BSA (filled inverted triangle) versus Fuc23-BSA (filled triangle). Fuc42-BSA (filled circle) is also included for reference. (A) DC-SIGN-Fc; (B) L-SIGN-Fc; (C) Langerin-Fc. For (A) and (B), sequential ELISA was used with the highest protein concentration at 5 μg/mL, from which 3-fold serial dilutions were made. For (C), precomplex-forming ELISA was used with the highest immobilization concentration at 20 μg/mL, and 2.5-fold serial dilutions thereafter.
We also tested BSA derivatives containing Gal-terminated disaccharides, lactose (Lac) and N-acetyllactosamine (LacNAc), as well as glucose disaccharides, maltose (Mal), cellobiose (Cel) and isomaltose (iMal). For all CTLs, binding of Lac- and LacNAc-BSA, if there was any, was less than that of Gal-BSA. Similarly, BSAs containing glucose disaccharide were usually bound less effectively than Glc-BSA, with one exception: MCL bound Mal38-BSA better than Glc44-BSA. The percentage of the bound amount of neoglycoproteins to MCL, with Man-/Fuc-BSA set at 100%, were 60% for Glc- and GlcNAc-BSA and 90% for Mal-BSA (Figure 2D). Binding of Cel-BSA was much less than that of Glc-BSA, and iMal-BSA did not show any binding.
Among the nine type II TM CTLs we surveyed, the binding affinity differed tremendously. When the binding strength was expressed as the ratio of specific binding of Man51-BSA or Fuc42-BSA over nonspecific binding (bound amount to the immobilized BSA), DC-SIGN had the highest specific to nonspecific ratio (≫10), meaning that the background was much less than 10% of the total bound amount. Other DC-SIGN-related CTLs and Langerin also had good specific to nonspecific binding ratio of >6.5. In contrast, for all the short-stalk CTLs of the EPX type, the binding ratio did not reach 2, meaning that the background was higher than 50% of the total binding. Among them, DCIR had the highest binding ratio of 1.9 for Fuc42-BSA, followed by MINCLE and dectin-2 in the range 1.5–1.6. BDCA-2 and MCL had the lowest specific binding, the ratio being ca. 1.35. For MDL-1, a sole non-EPN/QPD CTL we tested, the ratios for all six hexose neoglycoproteins were lower than 1.12, which we considered to be insignificant and nonbinding.
Discussion
As described in the Introduction, the sugar-binding CTLs can be divided into those that contain QPD tripeptide motifs in the CRD that bind Gal/GalNAc and those that contain EPN tripeptide which have a potential to bind Man, GlcNAc, Glc and Fuc. Although EPN is the most common tripeptide sequence for Man/Fuc/GlcNAc/Glc specificity, N can be replaced with S (Ser) or D (Asp), and maybe some other amino acids. A recent survey of CTLs of myeloid cells (Robinson et al. 2006) showed that the EPX-type CTLs outnumber the QPD-type CTLs (nine EPX-type, two QPD-type and three without either motif). Perhaps the versatility of the EPX-type CTL with respect to sugar-binding ability is reflected in the large number it represents among all the CTLs.
While the presence of QPD or EPN may be the primary requirement for specifying the binding preferences, other elements in CRD are needed to produce a strict preference for Gal over Man and vice versa. Conversion of EPN in the CRD of MBP to QPD produced a mutant MBP that bound Gal better than Man, but the higher binding affinity and better selectivity for Gal required additional amino acid replacement and a short peptide insertion (Iobst and Drickamer 1994). Similarly, one can expect amino acids in the CRD other than the EPX tripeptide sequence to influence the binding preferences for the six common hexoses. Indeed, in this paper, we demonstrated that the binding pattern of EPX CTLs for six hexose-containing neoglycoproteins varied considerably, each CTL expressing unique binding characteristics (Figure 3). Interestingly, however, without exception, Man-BSA was bound better than its 2-epimer, Glc-BSA (and GlcNAc-BSA).
Among the 11 EPX CTLs we examined, DC-SIGN showed the most restrictive specificity of binding essentially Man/Fuc only. Glc-BSA was bound with a 1000-fold lower affinity than Man-BSA in Assay I, but due to a very strong signal, DC-SIGN generates in Assay II, Glc-BSA actually appears to be binding quite well (Figure 3). In contrast, GlcNAc-BSA was not bound by DC-SIGN either by Assay I or Assay II. Apparently, the presence of N-acetyl group is obstructing its binding. Dectin-2 represents the opposite case of exhibiting promiscuity in the binding pattern: it bound all sugar-BSAs including Gal- and GalNAc-BSA (Figure 3). To a lesser extent, DCIR and MINCLE also showed capacity to bind Gal- and GalNAc-BSA. DC-SIGN and MMR are the CTLs that exhibited the highest affinity for Man- and Fuc-BSA, with the IC50 in the range of nM up to 50 nM. The high affinity of DC-SIGN is undoubtedly due to clustering of four CRDs by stable tetramer formation through its long stalk region (Feinberg et al. 2005). The fact that both L-SIGN and mSIGNR1 produced less reproducible binding data by Assay I suggests that their multimer formation may not be as strong as that of DC-SIGN. In contrast to DC-SIGN, the high affinity of MMR, a type I TM CTL, is generated by the presence of multiple CRDs that are capable of binding Man-type ligand (Taylor et al. 1992). Perhaps, because of the strong binding affinity, DC-SIGN and MMR may be the first receptors among many CTLs to interact with pathogens. Both receptors are known to capture, endocytose and degrade antigens, but they, DC-SIGN, in particular, are known also to be utilized by pathogens to their benefit, such as increased infectivity and better survival by alteration of the host immune response (Chieppa et al. 2003; van Kooyk et al. 2004).
Myeloid cells, however, seem to express a much larger number of the short-stalk type CTLs, all of which exhibited much lower affinities than DC-SIGN-related CTLs and MMRs in our survey. In fact, most of the EPX-containing short-stalk CTLs we studied (BDCA-2, DCIR, MCL and MINCLE) are listed as having unknown or inconclusive glycan-binding property by the consortium for functional glycomics glycan array assay methods. Although signals were weak, we did show in this paper that all of the above CTLs exhibited highest binding for Man- and Fuc-BSA. The weak binding affinities of short-stalk CTLs suggest that the multimer formation by these CTLs, if exists at all, must be rather weak and could not be sustained during the assay conditions, which involve multiple washing steps. For some short-stalk CTLs, the interactions with pathogens and resulting immunological effects have been demonstrated. For instance, dectin-2 and MINCLE on the cell surface bind Candida albicans, resulting in the induction of inflammatory signal (Sato et al. 2006; Wells et al. 2008). Perhaps the short-stalk CTLs require different mechanisms of clustering for the affinity enhancement, such as sequestration of CTL molecules into certain cell-surface micro-domains, or a large number of pathogen epitopes available for simultaneous engagement by multiple CTL molecules. Most of the type II TM CTLs we examined were in the form of human Fc peptide attached to the extracellular domain of the CTL. In our precomplex-forming format of ELISA, theoretically four CRDs will be in a cluster mediated by the dimerization of Fc peptide and the interaction of Fc with anti-Fc antibody. On the other hand, a His-tagged CTL would be expected to be in a dimeric form. We tested both Fc chimera and the His-tagged form of Langerin. Indeed, ΔA490 nm signal generated by Langerin-Fc was at least 10-fold stronger than that from the His-tagged form. The only other His-tagged short-stalk CTL we tested was dectin-2. Although the signal produced by the His-tagged dectin-2 was sufficient in strength, perhaps its Fc chimeric form would have produced much stronger signal.
DC-SIGN and L-SIGN (DC-SIGNR) are homologous proteins with 77% amino acid identity. DC-SIGN is mainly expressed on DCs, whereas L-SIGN is expressed primarily on endothelial cells of liver and lymph nodes (Pohlmann et al. 2001). Mouse has eight members in the DC-SIGN family, among which SIGNR1 has the longest stalk, although it is still much shorter than that of DC-SIGN and L-SIGN (Powlesland et al. 2006). Interestingly, while DC-SIGN and mSIGNR1 share the ability to bind Fuc-BSA better than Man-BSA, L-SIGN bound the two BSA derivatives with equal avidity. Among all the EPX lectins, the three DC-SIGN-related lectins (and Langerin) are the only ones that exhibited enhanced binding of Manα2Man disaccharide over Man (Table II), suggesting that the second mannose residue makes favorable contact with the binding site of these lectins. Among the four lectins, DC-SIGN exhibited the highest affinity enhancement, which amounted to more than 100-fold (decrease in IC50) in Assay I. With respect to the binding of Lex structure, the three lectins of DC-SIGN family all showed ability to bind Lex-BSA. DCIR and MINCLE, two non-DC-SIGN family lectins, also bound Lex-BSA. However, DC-SIGN was the only one among the five that bound Lex24-BSA better than Fuc23-BSA (Figure 5A and B). Surprisingly, five CTLs, MMR, Langerin, BDCA-2, dectin-2 and MCL, were totally devoid of Lex-BSA binding ability, despite their ability to bind Fuc23-BSA (Figure 5C). It may be that the proximity of Gal to the Fuc residue in the Lex structure hinders binding to these lectins, most likely due to steric interference.
The X-ray structures of DC-SIGN with number of different mannose-containing oligosaccharides as well as lacto-N-fucopentaose (with terminal Lex structure) are available (Guo et al. 2004; Feinberg et al. 2007). X-ray structure of the bound Man6 oligosaccharide (the structure that include outer tri-mannosyl core group) showed that Manα2Man sequence was bound with both sugar residues interacting at the binding site (Feinberg et al. 2007), which explains the strong binding enhancement of Manα2Man over Man. Man9 oligosaccharide, which has the highest affinity among the Man-containing oligosaccharides (Guo et al. 2004), has three terminal Manα2Man sequences. In the case of lacto-N-fucopentaose (a pentasaccharide-containing Lex terminal), the X-ray structure showed Fuc at the main binding site and the Gal residue making contact with protein (Guo et al. 2004). Interestingly, however, Fuc was bound in an alternate configuration, in which 3-OH(eq)/4-OH(ax) pair was bound to the essential calcium ion, instead of the canonical 2-OH(eq)/3-OH(eq) configuration. Since a simple fucoside is bound with 2-OH(eq)/3-OH(eq) configuration, the alternate 3-OH(eq)/4 OH(ax) combination would be expected to generate less affinity than the 2-OH/3-OH configuration. Despite this, we found that Lex24-BSA is bound with higher affinity than Fuc23-BSA to DC-SIGN, suggesting a strong contribution generated by the contact the protein makes with the Gal residue.
Although far outnumbered by the type II TM CTLs, there exist a few known type I TM CTLs of EPX type. Interestingly, they (MMR, DEC205, ENDO180, etc.) are all homologous proteins that contain multiple CRD domains. MMRs, the only one studied here, exhibited very strong binding of both Man- and Fuc-BSA in Assay I. Both human and mouse MMRs had IC50 in nM range for these neoglycoproteins, an order of magnitude higher affinity than that of DC-SIGN. However, MMR generated very weak but very reproducible binding signal in Assay II (Figure 5B). In this assay, a CTL molecule is interacting with glycans on BSA directly attached to the well surface. Perhaps the way a molecule of MMRs lies on the glycan surface for the high-affinity, multi-domain interaction interferes physically with other MMR molecules to be bound optimally thus drastically decreasing the signal generation.
Materials and methods
Neoglycoproteins
All of the neoglycoproteins used were based on BSA, and most had the AI-type linkage, which is a thiosugar linked to the BSA amino groups through a short amidino linkage, [sugar-SCH2C( = NH)NH-] (Stowell and Lee 1982). In order to assure high affinity, generally we used BSA derivatives that contain sugar residues more than 30 residues per molecule of BSA. The monosaccharide-containing AI-type BSA derivatives used are: Man51- and Man33-BSA, Fuc42- and Fuc30-BSA, GlcNAc44-, Glc44-, Gal34 and GalNAc35-BSA. Reductive amination of α-1,2-linked manno-biose, -triose and -tetraose (generous gifts from Dr. C. Ballou, University of California, Berkeley, CA) to BSA produced BSA derivatives that contained mannose, α1,2-linked di- and trisaccharide of mannose, respectively, all linked by the same linking group of sugar-O-[(CHOH)3CH2OH]CHCH2- to the amino groups of BSA. This type of neoglycoproteins is designated as PM type. Reductive amination was carried out using ∼400-fold excess of the α1,2-linked manno-oligosaccharides over BSA on molar basis in 0.2 M sodium borate buffer, pH 8.5, at 37°C for 4 days, using pyridine borane as a reducing agent. Pyridine borane was added initially to make ∼50 mM, and was replenished once on the third day with the same amount. Protein solutions were dialyzed successively in the cold against phosphate-buffered saline, 0.15 M NaCl and finally against two changes of water, and then freeze-dried. Sugar incorporation was determined using phenol–sulfuric acid method (McKelvy and Lee 1969). The three PM-type BSA derivatives produced contained 38, 36 and 33 residues of mannose, α1,2-linked mannobiose and mannotriose, respectively. Similarly, a BSA derivative containing α1,6-linked glucose disaccharide was prepared by reductive amination using 350-fold excess of isomaltotriose as described for mannotriose. The conjugation level of iMal(isomaltose)-BSA was 42. The AI-type BSA derivatives containing disaccharides are as follows: Lac34-, LacNAc33-, Mal(maltose)38- and Cel(cellobiose)36-BSA. A BSA derivative containing 24 residues of Lex-terminated structure was purchased from V-Labs (Covington, LA). Assay I (see below) requires a tagged, high-affinity ligand as the reference compound. For this purpose, Man51-AI-BSA was reacted with diethylenetriamine pentaacetic acid dianhydride in 0.25 M sodium borate buffer, pH 8.6, and then chelated with europium ion (Kawasaki and Lee 1997).
CTLRs and other protein reagents
All CTLR proteins were recombinant proteins containing only the exocellular domain, to which either human Fc chain or His tag was attached. DC-SIGN, L-SIGN (DC-SIGNR) and mouse SIGNR1 as Fc chimera, and human and mouse mannose receptors (MMRs), dectin-2 (CLEC7A) and Langerin in His-tagged form are from R&D (Minneapolis, MN). Langerin, BDCA-2 (CLEC4C), DCIR (CLEC4A), MCL (CLEC4D), MINCLE (CLEC4E), MDL-1 (CLEC5A) and MGL (CLEC10A), all of human kind, as Fc chimera were produced in-house (Hsu et al. 2009). Briefly, the DNA fragments encoding extracellular domains of CTLRs were fused with the gene that encodes human IgG1 Fc to generate N-terminally Fc-tagged fusion proteins. The CTLR/Fc chimeras were overexpressed via the FreeStyleTM293 Expression System (Invitrogen, Carlsbad, CA) and purified by Protein A column (GE Healthcare, Piscataway, NJ). Goat anti-hIgG IgG and goat anti-(His tag) IgG were from US Biological (Swampscott, MA), horseradish peroxidase (HRP) conjugate of goat anti-hFc-IgG was from Pierce (Rockford, IL) and HRP conjugate of mouse anti-(His tag)-IgG was from AbD Serotec (Oxford, UK).
Ligand-binding assays
Two types of binding assay methods using 96-well microplates were employed. For both assays, incubation buffers were 15 mM HEPES [N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)] buffer, pH 7.2, containing 0.15 M NaCl and 0.1% BSA with or without 20 mM CaCl2. Wash buffers were same as above, except that 0.1% BSA was replaced with 0.05% Tween 20. Blocking of well surface was done using SuperBlock® (Pierce) according to the manufacturer's protocol.
Assay I (competition assay using Eu fluorescence for detection): Wells of the microplate were coated with anti-Fc-IgG or anti-(His tag) IgG at 3 μg/mL (100 μL) in 0.1 M HEPES buffer, pH 8.5, containing 0.15 M NaCl, for overnight in the cold. BSA at the same concentration was used as blank. After blocking and washing, the wells were incubated with 100 μL of CTL-Fc or CTL-(His tag) at 1 μg/mL for 1 h at room temperature with shaking. After washing, wells were incubated for 1 h at room temperature with Eu-labeled Man51-AI-BSA at 50 nM, containing inhibitors at various concentrations. Bound fluorescence (Eu-Man51-AI-BSA) was determined using time-resolved fluorescence counting (EnVision; Perkin-Elmer, Turku, Finland) after treating the wells with 0.2 mL of Eu-enhancing solution (Hemmila et al. 1984). After subtracting blank fluorescence from all the experimental fluorescence counts, the net fluorescence counts were converted to percent inhibition by setting the fluorescence counts without inhibitor as 0%. A plot of percent inhibition on the y-axis and the inhibitor concentration on the x-axis in logarithmic scale produced a sigmoidal curve for each inhibitor, and the IC50 value (the concentration of inhibitor that causes 50% inhibition) was obtained from each curve.
Assay II is based on ELISA. The immobilized neoglycoprotein surface was incubated sequentially with CTL-Fc or His-tagged CTL and then with an HRP conjugate of either anti-Fc IgG or anti-(His-tag) IgG. For immobilization of neoglycoproteins, wells of a 96-well plate were incubated for overnight in the cold with 0.1 mL of 3-fold sequentially diluted neoglycoprotein solutions (starting from 5 μg/mL) in 50 mM sodium carbonate buffer, pH 9.5. For blanks, BSA solutions of the same dilutions were used. After proper blocking and washing, the wells were incubated first with 0.1 mL of CTL-Fc (or His-tagged CTL) at 0.5 μg/mL for 2 h at room temperature and then with the appropriate IgG-HRP conjugate (20,000-fold dilution, 0.1 mL) for 1 h. Stable peroxide substrate buffer (Pierce) and o-phenylene diamine were used to develop the colored enzymatic reaction product according to the manufacturer's protocol. The absorbance at 490 nm was determined at a proper time of color development (between 5 and 30 min) using a microplate reader (Benchmark; Bio-Rad, Hercules, CA). The speed of color development differed considerably between various CTLs. When the sequential addition of CTLs and IgG-HRP gives very low binding signal, the precomplexing protocol is used. A CTL-Fc (or His tag) is mixed with the corresponding IgG-HRP at approximate molar ratio of 2:1. This solution is then placed on neoglycoprotein surface for incubation for 2 h at room temperature. For this assay, 2.5-fold sequential dilutions of neoglycoprotein starting from 20 μg/mL were used. The concentration of CTL in the incubation mixture was 0.5 μg/mL. The enzyme reaction was carried out as in the sequential assay.
Since Assay II does not give quantitative affinity values, a set of highly sugar-modified BSA derivatives was used to compare the relative binding affinity of six different hexoses to all the CTLs. Conjugation levels were 51, 42, 44, 44, 34 and 35 residues of Man, Fuc, GlcNAc, Glc, Gal and GalNAc, respectively, per molecule of BSA. For each CTL, the absorbance at 490 nm or the net absorbance (ΔA490 nm, experimental A490 nm minus A490 nm produced on the BSA surface) was plotted against neoglycoprotein concentration on the x-axis. The relative binding of six different monosaccharides (as BSA derivatives) was calculated from non-plateau region of the binding curves. In this region, ΔA490 nm is assumed to be proportional to the bound amount of lectin. The monosaccharide-binding profile was then presented as a bar graph with the highest bound amount of the six monosaccharide neoglycoproteins set at 100%.
Funding
This work was supported in part by NIH grants, AI052468 and AI073610, and Taiwan Merit Scholarship from National Science Council, Taiwan, TMS-094-1-B-003 (to T.S.H.).
Abbreviations
BSA, bovine serum albumin; Cel, cellobiose; CRD, carbohydrate-recognition domain; CTL, C-type lectin; CTLR, C-type lectin-like receptor; DC, dendritic cell; DC-SIGN, DC-specific, ICAM-grabbing non-integrin; DC-SIGNR (L-SIGN), DC-SIGN-related; ELISA, enzyme-linked immunosorbent assay; Gal, galactose; GalNAc, N-acetylgalactosamine; HIV, human immunodeficiency virus; HRP, horseradish peroxidase; iMal, isomaltose; Lex, Lewis x; Mal, maltose; ManLAM, mannose-capped lipoarabinomannan; MBP, mannose-binding protein; MMR, macrophage mannose receptor; Th, T helper; TM, transmembrane.
References
- Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. doi:10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. doi:10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- Feinberg H, Castelli R, Drickamer K, Seeberger PH, Weis WI. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J Biol Chem. 2007;282:4202–4209. doi: 10.1074/jbc.M609689200. doi:10.1074/jbc.M609689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H, Guo Y, Mitchell DA, Drickamer K, Weis WI. Extended neck regions stabilize tetramers of the receptors DC-SIGN and DC-SIGNR. J Biol Chem. 2005;280:1327–1335. doi: 10.1074/jbc.M409925200. doi:10.1074/jbc.M409925200. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. doi:10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. doi:10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- Hemmila I, Dakubu S, Mukkala VM, Siitari H, Lovgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984;137:335–343. doi: 10.1016/0003-2697(84)90095-2. doi:10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Hsu T-L, Cheng SC, Yang WB, Chin SW, Chen BH, Huang MT, Hsieh S-L, Wong C-H. Profiling carbohydrate-receptor interaction with recombinant innate immunity receptor-Fc fusion proteins. J Biol Chem. 2009;284:34479–34489. doi: 10.1074/jbc.M109.065961. doi:10.1074/jbc.M109.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iobst ST, Drickamer K. Binding of sugar ligands to Ca2+-dependent animal lectins. J Biol Chem. 1994;269:15512–15519. [PubMed] [Google Scholar]
- Kawasaki N, Lee YC. Europieum labeling of natural and synthetic glycopeptides. Anal Biochem. 1997;250:260–262. doi: 10.1006/abio.1997.2233. doi:10.1006/abio.1997.2233. [DOI] [PubMed] [Google Scholar]
- Kolatkar AR, Weis WI. Structural basis of galactose recognition by C-type animal lectins. J Biol Chem. 1996;271:6679–6685. doi:10.1074/jbc.271.12.6679. [PubMed] [Google Scholar]
- Lee RT, Ichikawa Y, Fay M, Drickamer K, Shao MC, Lee YC. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A): Homology of binding site architecture with mammalian and chicken hepatic lectins. J Biol Chem. 1991a;266:4810–4815. [PubMed] [Google Scholar]
- Lee YC, Lee RT, Rice K, Ichikawa Y, Wong T-C. Topography of binding sites of animal lectins: Ligands' view. Pure Appl Chem. 1991b;63:499–506. doi:10.1351/pac199163040499. [Google Scholar]
- McKelvy JF, Lee YC. Microheterogeneity of the carbohydrate group of Aspergillus oryzae α-amylase. Arch Biochem Biophys. 1969;132:99–110. doi: 10.1016/0003-9861(69)90341-5. doi:10.1016/0003-9861(69)90341-5. [DOI] [PubMed] [Google Scholar]
- Ng KK-S, Drickamer K, Weis WI. Structural analysis of monosaccharide recognition by rat liver mannose-binding protein. J Biol Chem. 1996;271:663–674. doi: 10.1074/jbc.271.2.663. doi:10.1074/jbc.271.2.663. [DOI] [PubMed] [Google Scholar]
- Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: Evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA., Jr Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–6717. [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Harn DAJ. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. doi:10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. doi:10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. doi:10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- Sato K, Yang X-L, Yudate T, Chung J-S, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Jr, Arizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. doi:10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- Stowell CP, Lee YC. Preparation of neoglycoproteins using 2-imino-2-methoxyethyl 1-thioglycosides. Methods Enzymol. 1982;83:278–288. doi: 10.1016/0076-6879(82)83021-8. doi:10.1016/0076-6879(82)83021-8. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Bezouska K, Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biol Chem. 1992;267:1719–1726. [PubMed] [Google Scholar]
- van Die I, Cummings RD. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology. 2010;20:2–12. doi: 10.1093/glycob/cwp140. doi:10.1093/glycob/cwp140. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TBH. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16:488–493. doi: 10.1016/j.coi.2004.05.010. doi:10.1016/j.coi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. doi:10.1111/j.1600-065X.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. The macrophage-inducible C-type lectin, MINCLE, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]