Abstract
Single-nucleotide polymorphisms (SNPs) in the FKBP5, GRIK4, and HTR2A genes have been shown to be associated with response to citalopram treatment in the STAR*D sample, but only associations with FKBP5 have so far been tested in the Munich Antidepressant Response Signature (MARS) project. Response and remission of depressive symptoms after 5 weeks of antidepressant treatment were tested against 82 GRIK4 and 37 HTR2A SNPs. Association analysis was conducted in about 300 depressed patients from the MARS project, 10% of whom had bipolar disorder. The most predictive SNPs from these two genes and rs1360780 in FKBP5 were then genotyped in a total of 387 German depressed in-patients to analyze potential additive and interactive effects of these variants. We could not replicate previous findings of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study in our sample. Although not statistically significant, the effect for the best GRIK4 SNP of STAR*D (rs1954787, p=0.076, pcorrected=0.98) seemed to be in the same direction. On the other hand, the nominally significant association with the top HTR2A SNPs of STAR*D (rs7997012, allelic, p=0.043, pcorrected=0.62) was with the opposite risk allele. The GRIK4 SNP (rs12800734, genotypic, p=0.0019, pcorrected=0.12) and the HTR2A SNP (rs17288723, genotypic, p=0.0011, pcorrected=0.02), which showed the strongest association with remission in our sample, had not been reported previously. Associations across all genetic markers within the GRIK4 (genotypic, p=0.022) or HTR2A (genotypic, p=0.012) locus using the Fisher's product method (FPM) were also significant. In all 374 patients, the best predictive model included a main effect for GRIK4 rs12800734 and two significant interactions between GRIK4 rs12800734 and FKBP5 rs1360780, and GRIK4 rs12800734 and HTR2A rs17288723. This three SNP model explained 13.1% of the variance for remission after 5 weeks (p=0.00051 for the model). Analyzing a sub-sample of 194 patients, plasma ACTH (p=0.002) and cortisol (p=0.021) responses of rs12800734 GG (GRIK4) carriers, who also showed favorable treatment response, were significantly lower in the second combined dexamethasone (dex)/corticotrophin-releasing hormone (CRH) test before discharge compared with the other two genotype groups. Despite large differences in ethnicity and design compared with the STAR*D study, our results from the MARS study further support both independent and interactive involvement of GRIK4, HTR2A and FKBP5 in antidepressant treatment response.
Keywords: GRIK4, HTR2A, FKBP5, interaction, antidepressant treatment response, affective disorder
INTRODUCTION
Major depressive disorder (MDD) has a high prevalence in the general population (16–20%) and an enormous effect not only on the life of affected individuals but also on the society as a whole (Fava and Kendler, 2000). Despite the availability of modern antidepressant drugs that primarily target monoaminergic neurotransmitter systems, only about one-third of patients experience full remission after the first treatment trial. The other two-thirds require several treatment attempts or do not respond at all (Fava and Davidson, 1996; Trivedi et al, 2006). Evidence suggests that such marked individual variation in antidepressant treatment response might to some degree be determined genetically (Angst, 1961; O'Reilly et al, 1994; Franchini et al, 1998). Pharmacogenetic association studies have implicated a series of candidate genes in antidepressant treatment outcome (Malhotra et al, 2004; Binder and Holsboer, 2006; Kato and Serretti, 2008; Horstmann and Binder, 2009). However, until recently, most genetic association studies have been fraught with small samples sizes, insufficient marker density, and inconsistent replications. Starting in 2006, pharmacogenetic association studies have been published from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) sample. For these studies, 1953 individuals within this study with non-psychotic major depressive disorder, who were exclusively treated with the selective serotonin reuptake inhibitor citalopram for up to 14 weeks at level 1, have been analyzed.
In an initial screen in a reduced sample (n=1360), analyzing 768 single-nucleotide polymorphisms (SNPs) in 68 candidate genes, rs7997012 in the HTR2A gene locus encoding the serotonin 2A receptor was found to be associated with treatment outcome at level 1 of STAR*D (McMahon et al, 2006). The researchers used a split-sample design with two-thirds of the sample randomly assigned to the discovery and one-third to the replication sample. The A allele of rs7997012 was associated with a better treatment outcome and this association withstood correction for multiple testing. In a second study from the same group that now used the full sample (n=1816) for association, the HTR2A association result could be reproduced and a new marker, rs1954787, in the GRIK4 gene has been found to be predictive for antidepressant treatment outcome in the discovery as well as replication sample (Paddock et al, 2007). The GRIK4 gene encodes the kainic acid-type glutamate receptor 1 (KA1) subunit, which co-assembles with other glutamate receptor subunits to form cation-selective ion channels, but might also possess metabotropic function (Rodriguez-Moreno and Sihra, 2007). The effect size of the GRIK4 marker alone was modest, but homozygote carriers of the treatment-response-associated marker alleles of both the GRIK4 and HTR2A genes were 23% less likely to experience non-response to citalopram treatment when compared with participants who did not carry any of these marker alleles (Paddock et al, 2007).
The FK-506 binding protein (FKBP5) is a co-chaperone of hsp-90 and regulates glucocorticoid receptor (GR) sensitivity (Schiene-Fischer and Yu, 2001). An impaired negative feedback regulation of the hypothalamus–pituitary–adrenocortical (HPA) system through the GR receptor seems to be the cause for the hyperactivity of the stress hormone system that is consistently found in acutely depressed patients (Reul et al, 1993; Raison and Miller, 2003). Irrespective of their primary pharmacological mode of action, clinical response to antidepressant treatment is often accompanied by a normalization of HPA axis hyperactivity, as measured by the combined dexamethasone (dex) suppression/corticotrophin-releasing hormone (CRH) stimulation (dex/CRH) test (Ising et al, 2005). A decrease in cortisol response in this test has been found to predict clinical improvement, making the dex-CRH test a potential biomarker for antidepressant treatment response (Ising et al, 2007). Genetic associations with the dex/CRH test outcome could thus serve as potential biological support for association with clinical response. In 2004, our group analyzed possible associations between genes regulating the HPA axis and response to antidepressants in the Munich Antidepressant Response Signature (MARS) sample and found a strong association between polymorphisms in FKBP5 and treatment outcome (Binder et al, 2004). In the meantime, these results have been replicated in the STAR*D sample (Lekman et al, 2008) as well as in another German sample (Kirchheiner et al, 2008).
The aims of our study were to:
analyze whether genetic markers in HTR2A and GRIK4 that show association with treatment outcome in a monotherapy antidepressant study would also show associations in a naturalistic European in-patient setting, and
whether polymorphisms in HTR2A, GRIK4, and FKBP5 show additive or interactive effects in treatment response prediction.
Our sample was collected within the MARS project (Hennings et al, 2009). In contrast with the STAR*D cohort, these patients were all in-patients with moderate-to-severe as well as psychotic depressive symptoms. About 10% of our depressed patients suffered from bipolar disorder. Only a fraction of patients were treated with antidepressant monotherapy, whereas the majority received combinations of antidepressants, as well as adjunct mood stabilizers, antipsychotics, and benzodiazepines. In this study, we analyzed the association of the most significant SNPs from the STAR*D results and additional SNPs in the GRIK4 and HTR2A gene region with response and remission after 5 weeks of in-patient treatment. This was accomplished by genotyping a dense set of markers for both loci to account for possible differences in genetic structure because of ethnic differences. This approach also allowed us to evaluate the association across all genetic markers within the GRIK4 or HTR2A locus with treatment response and possible interaction effects among GRIK4, HTR2A, and FKBP5. Furthermore, because successful treatment of depression is associated with a normalization of the endocrine response of the HPA system (Holsboer, 2000; Ising et al, 2005, 2007), we analyzed genotype-dependent differences in plasma ACTH and cortisol responses in the combined dex/CRH test between admission and discharge in a sub-sample of 194 depressed patients.
PATIENTS AND METHODS
Subjects
In accordance with previous pharmacogenetic studies from our group (Binder et al, 2004; van Rossum et al, 2006; Uhr et al, 2008) 387 depressed in-patients (57.4% women) of the Max Planck Institute of Psychiatry and of the psychiatric hospitals in Augsburg and Ingolstadt, 18 years or older (M=49.5 years, SD=14.3) who participated in the MARS project (http://www.mars-depression.de; Hennings et al, 2009), were recruited for the pharmacogenetic study. Inclusion criteria were a major depressive episode with a minimum total score of 14 in the Hamilton Depression Rating Scale (HAM-D, 21 items version) at study inclusion (M=27.2, SD=6.3). Diagnoses were ascertained by experienced psychiatrists in a semistructured interview according to the criteria of DSM-IV. Patients with neurological or neurodegenerative disorders or severe somatic diseases were excluded. We recorded ethnicity in patients and controls using a self-report questionnaire for perceived nationality, native language, and ethnicity of the subject and of all four grandparents. All patients were Caucasians from German or Western European descent. Patients were included after the details of the study were explained and after written informed consent was given by the participants. The study protocols were approved by the local ethics committee of the Medical Faculty at the Ludwig Maximilians University, Munich.
Fine-mapping for GRIK4 was conducted in 300 and for HTR2A in 305 depressed patients, of which 30 patients suffered from bipolar disorder. Details on demographic and clinical data of our sample of unipolar patients (n=275), in comparison with the STAR*D sample, are presented in Table 1, and data are analyzed including as well as excluding patients with bipolar disorder. Within the unipolar depressed patients, 14.1% suffered from psychotic depression, and the following comorbid mental disorders were diagnosed: anxiety disorders (6.3%), substance abuse disorder (10.4%), eating disorder (2.2%), dysthymia (4.4%), and personality disorders (4.4%). The best SNPs from GRIK4 (rs12800734) and HTR2A (rs17288723) as well as rs1360780 in FKBP5 (Binder et al, 2004; Kirchheiner et al, 2008; Lekman et al, 2008) were genotyped in all 387 patients, and valid genotypes for all three SNPs were available in 374 patients. Demographic and clinical measures as well as comorbid mental disorders from the fine-mapping sample did not differ significantly from the whole sample (n=374; 90.7% unipolar, 9.3% bipolar, and 14.3% with psychotic depression).
Table 1. Demographic and Clinical Data of the MARS Unipolar Patients in Comparison with the STAR*D Patients.
| STAR*D outpatients(n=1953) | MARS in-patients (n=275) | χ2 or t-test (p-value) | |
|---|---|---|---|
| N (%) | |||
| Female | 1205 (63.6) | 153 (57.4) | 0.17 |
| Caucasiana | 1526 (73.4) | 275 (100.0) | <0.001 |
| Recurrent depression | 1378 (70.6) | 174 (63.2) | 0.04 |
| Anxious depressionb | 1038 (53.2) | 179 (65.1) | <0.001 |
| Chronic depressionc | 498 (25.5) | 30 (11.0) | <0.001 |
| Family history of depressiond | 1096 (56.1) | 104 (37.8) | <0.001 |
| History of attempted suicide | 293 (15.0) | 77 (28.0) | <0.001 |
| Age at onset <18 years | 657 (33.6) | 29 (10.5) | <0.001 |
| Psychotic depression | 0 | 39 (14.1) | <0.001 |
| Mean (SD) | |||
| Age (years) | 42.7 (13.4) | 49.5 (14.3) | <0.001 |
| Age at onset (years) | 26.1 (14.9) | 38.3 (15.8) | <0.001 |
| Number of previous episodes | 6.4 (12.5) | 2.2 (3.1) | <0.001 |
| Length of current episode (month) | 24.8 (53.1) | 10.8 (17.7) | <0.001 |
| Length of illness (years) | 16.6 (13.9) | 11.2 (12.2) | <0.001 |
| HAM-D (17 items) score at inclusion | 18.4 (6.2) | 24.6 (6.6) | <0.001 |
In the STAR*D sample, 17.6% of patients were African Americans and 13% of Hispanic origin.
Baseline HAM-D anxiety/somatization factor score ⩾7 (Fava et al, 2004).
Recurrent major depressive episode lasting ⩾24 months.
First-degree relatives with MDE or bipolar disorder.
Procedure and Psychopharmacological Treatment
Ratings of depressive symptoms (HAM-D, 21 items version) were performed within 3 days after admission and then weekly until discharge. Response was defined as a 50% reduction in the baseline HAM-D score, and remission was defined as reaching the HAM-D score of <10 after 5 weeks of in-patient treatment. In our study, the 21-item HAM-D is used so that a cutoff of 10 is similar to the previously proposed cutoff of 7 using the HAMD 17-item scale (Rush et al, 2006). Although major depression often necessitates treatment trials longer than 5 weeks, this time point was chosen to be consistent with previous pharmacogenetic studies from this cohort (Binder et al, 2004; van Rossum et al, 2006), and because this duration of treatment yielded similar remission rates of approximately 30% between the in-patient MARS study and the outpatient STAR*D study (Trivedi et al, 2006). The study was designed as a naturalistic pharmacogenetic study to discover genotypes that are predictive of clinical outcome in relationship to treatment, which was according to the choice of the doctors. All types and combinations of antidepressants were allowed, as well as concomitant treatment (5 weeks time point) with mood stabilizers (18%), antipsychotics (21%), and benzodiazepines (35%). The plasma antidepressant concentrations were monitored to ascertain clinically efficient drug levels.
DNA Preparation, SNP Selection, Genotyping, Quality Control, and CEU Coverage
DNA was extracted from 30 ml of EDTA blood using the Puregene whole-blood DNA extraction kit (Gentra Systems, Minneapolis, MN). SNPs in HTR2A (NM_000621, 13q14.2) and 30-kb downstream of the 5′ and upstream of the 3′ UTR were originally selected from dbSNP (http://www.ncbi.nlm.nih.gov/sites/entrez?db=snp) using tagging SNP information from the HAPMAP project (http://www.hapmap.org). HTR2A SNPs and the intronic FKBP5 SNP, rs1360780, (Binder et al, 2004; Ising et al, 2008) were analyzed on a Sequenom platform using the iPlex technology (Sequenom, San Diego, CA, USA). SNPs in the GRIK4 (NM_014619, 11q23.3) gene region, including markers up to 40-kb 5′ and 3′ of the gene were selected from genotypes generated using the Illumina Sentrix Human-1 100k and 300k BeadChips (Illumina, San Diego, CA, USA). Genotyping was performed according to the standard protocols of the manufacturer. rs12800734 in GRIK4 was additionally analyzed on the Roche 480 Lightcycler by using a hybridization-probe assay (Roche Diagnostics, Basel, Switzerland). All primers are available on request. Only SNPs showing a call rate higher than 98%, a minor allele frequency (MAF) above 10%, and no deviation from Hardy–Weinberg equilibrium (HWE, error level below 10−2 exact test; Wigginton et al, 2005) were included into the analyses. Two SNPs were genotyped by two different methods: rs10892608 on the Illumina platform and using the iPlex technology of Sequenom and rs12800734 on the Illumina platform and the Roche 480 Lightcycler. The genotype results were congruent in 100%. The concordance rate between our Illumina 100K and 300K platforms was 0.998.
To asses the genetic coverage of our SNP panel, we compared them with tag SNPs selected for the CEU population in HapMap phase III data using an R2 cutoff of 0.8 and a MAF higher than 10% implemented in ‘Tagger' (de Bakker et al, 2005). In all, 117 tagging SNPs were selected for the transcribed region of the GRIK4 gene and 39 tagging SNPs for HTR2A. Our SNP panel provided 62% of the GRIK4 (mean R2=0.393) and 78% of the HTR2A (mean R2=0.322) gene information in CEU population for the latest version III of HapMap (February 2009). Our panel yielded higher coverage rates than the genome-wide SNP-array (Illumina 300k; GRIK4 38% and HTR2A 46%) used in Ising et al (2009).
Combined dex/CRH Test
The combined dex/CRH test administered 2–10 days (M=6.4 days, SD=2.1) after admission and before discharge was available for 194 depressed patients. Patients with concomitant treatment with mood stabilizers, such as lithium or carbamazepine, were excluded from the analysis because of the potential of these drugs to influence the outcome of the dex/CRH test (Bschor et al, 2002; Kunzel et al, 2003). The procedure of the combined dex/CRH test has been described in detail elsewhere (Heuser et al, 1994; Kunzel et al, 2003). In brief, patients are pretreated with 1.5 mg dexamethasone p.o. at 2300 h and HPA axis is stimulated with 100 μg human CRH at 1500 h the next day. Blood samples are drawn at 1500, 1530, 1545, 1600, and 1615 h through an intravenous catheter. CRH is injected shortly after the first blood collection. The subjects rest supine and awake throughout the test. Plasma hormone concentrations in the first specimen collected at 1500 h (baseline) reflect the suppressive effect of the dexamethasone upon the HPA axis, whereas the other four samples represent the plasma ACTH and cortisol responses to CRH stimulation under the dexamethasone pretreatment condition. As response indicators to the combined dex/CRH test, total areas under the curve (auc) values, which are defined as the natural logarithms of the trapezoidal integration of the five consecutive plasma ACTH and cortisol concentrations, were used. Plasma ACTH concentrations were analyzed using an immunometric assay (Roche Diagnostics, Mannheim, Germany), and plasma cortisol concentrations were determined using radioimmunoassay (ICN Biomedicals, Carson, CA, USA).
Statistical Analysis
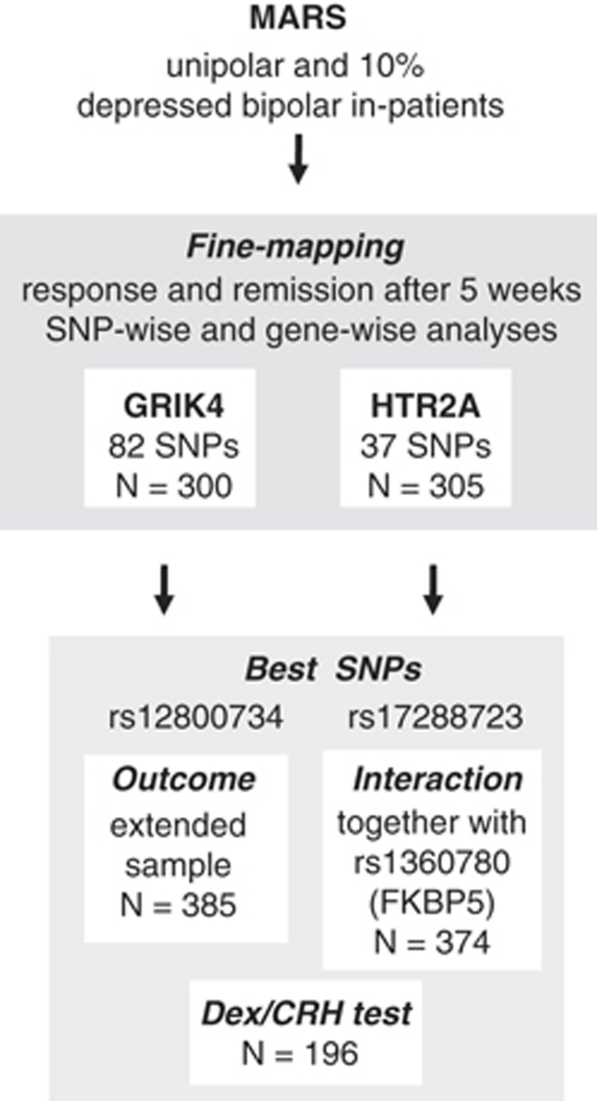
For clarity of our analysis procedure we created a flow chart (Figure 1). Demographic and clinical data, including illness-related variables, were compared between samples and between genotypes using chi-square (χ2) tests for qualitative data and t-tests or analyses of variance (ANOVA) for quantitative data. Genetic effects were evaluated by regression-based models using permutation-based significance estimates using the WG Permer software (http://www.wg-permer.org). Empirical p-values, which are insensitive to potential violations of the regression prerequisites, are reported for 106 permutations of random distribution of phenotypes over genotypes. SNPs were tested for genotypic and allelic models of inheritance. We used the method described by Westfall and Young (WY, Westfall, 1993) to correct for the number of SNPs within each gene. In addition to single SNP by phenotype association analyses, we also performed a priori the multivariate Fisher's product method (FPM) for all variants genotyped within a gene (Fisher, 1932). FPM includes the residuals of the phenotype variables remission or response after 5 weeks and the genotype information from all SNPs of the GRIK4 or HTR2A locus. It noteworthy that there is no need to correct these FPM association results for the number of SNPs tested per gene, because only one statistical operation is being performed. Furthermore, we corrected for the two phenotype variables and Bonferroni-corrected the WY p-values with factor 2. For the most significant SNP within each of the two genes, a repeated-measures ANOVA (age and gender as covariates) with weekly HAMD scores as the within-subject factor was used to describe genotype-related between-subject effects over the first 5 weeks of treatment. We also tested the association of these two SNPs with neuroendocrine measures (ACTH and cortisol) of the dex/CRH test between admission and discharge of individual patients using repeated-measures ANOVA corrected for effects of age and gender after log transformation of the data (to approach normality and homogeneity). To test for interaction between the best SNP in GRIK4, HTR2A and rs1360780 in FKBP5, respectively, we applied first a step forward inclusion and then with the significant coefficients subsequently a hierarchical logistic regression model with remission after 5 weeks as binary outcome. The most strongly associated SNPs within GRIK4 and HTR2A and rs1360780 in FKBP5 as well as their 2 × 2 interaction terms were used as predictors. The rare homozygous carriers were coded as 1 and the heterozygous carriers of all three SNPs were coded as 2. Threshold for inclusion of a coefficient was a p-value of <0.05 in the likelihood ratio test. We used FKBP5 SNP rs1360780 for this analysis, as this SNP yielded the most consistent results in German samples (Binder et al, 2004; Kirchheiner et al, 2008), and was in very high linkage disequilibrium (LD) with rs4713916 in Germans, showing the strongest association in the STAR*D sample (Lekman et al, 2008). To further strengthen our interaction results, we applied a re-sampling to the original sample and conducted a bootstrapping analysis (re-sampling method; Efron, 1979). A total of 1000 bootstrapping samples were created by drawing 374 individuals with replacement from the original data set. For each bootstrapping sample, we reran the association analysis and calculated Nagelkerke's R2 for the logistic regression model including the disadvantageous AG genotype of GRIK4 and two interaction effects between the protective genotypes of GRIK4 and FKBP5 and GRIK4 and HTR2A, respectively. On the basis of the empirical distribution of these Nagelkerke's R2 measurement, we derived an estimator and a confidence interval for the variance explained by the model.
Figure 1.
Flow chart of the analysis procedure. Associations of response and remission (not independent) after 5 weeks with single GRIK4 or HTR2A SNPs (fine mapping) are our primary hypotheses and we considered the two tested genes as independent hypotheses. A Westfall–Young correction was used to correct for all the genetic variants tested within a gene and this p-value was Bonferroni corrected for the number of tested phenotypes (k=2). We also tested (a priori) gene-wise associations between all GRIK4 and all HT2RA SNPs and remission and response to antidepressant treatment. Secondary analyses included our analyses with the best GRIK4 and HTR2A SNPs in the extended uni-/bipolar sample for (1) outcome, (2) dex/CRH test, and (3) interaction analyses of the best SNP within GRIK4 and HTR2A together with the FKBP5 SNP rs1360780.
Power was estimated using the Quanto software version 1.1. We had 80% power to detect association with remission (N=300, 30% remitters) with an effect size of ⩾2.3 for GRIK4 or ⩾2.2 for HTR2A for variants with a minor allele frequency of ⩾0.2 and using an additive genetic model and α-levels adjusted for the number of tested SNPs per gene. Owing to the restricted number of cases, our power calculation shows adequate power only for huge effect sizes. This effect size is clearly smaller than the one reported for FKBP5 (Binder et al, 2004), but larger than the modest effect sizes reported for the best HTR2A and GRIK4 SNPs of STAR*D (McMahon et al, 2006; Paddock et al, 2007). Our odds ratio (OR) estimates agrees well with the one reported on the serotonin transporter (SLC6A4) 5-HTTLP variant (l-allele vs s-allele carriers) in the meta-analysis by Serretti et al (2007). However, results of a genome-wide pharmacogenetic association study supposes that effect sizes of single SNPs to predict antidepressant drug treatment may be lower than expected previously (Ising et al, 2009).
The LD structure and haplotype block definitions (Gabriel et al, 2002) were evaluated with HAPLOVIEW 4.1 using the ∣D′∣ method with a threshold of 0.80 (http://www.broad.mit.edu/mpg/haploview) (Barrett et al, 2005). When not otherwise stated, means (M) and SD are reported. Analyses were performed with SPSS (16.0, SPSS Chicago, IL), Sigma Plot (11.0, Systat Software, Chicago, IL, USA), or WG Permer (http://www.wg-permer.org).
RESULTS
GRIK4 and Treatment Response
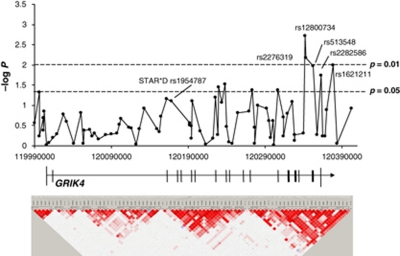
We analyzed 82 informative SNPs in a 412-kb spanning region on chromosome 11 that contains the GRIK4 encoding locus with an average inter-marker distance of 5 kb. Although we observed nominally significant associations for both remission and response in the unipolar (n=270) and the extended uni/bipolar sample (n=300; see Table 2), none of the GRIK4 association results withstood WY correction for the number of SNPs tested. Genotypic, permutation-based −log p results for remission after 5 weeks of all 82 GRIK4 SNPs in the extended sample plotted against their physical location (hg18) as well as the corresponding LD map based on D′ are shown in Figure 2. SNPs showing the strongest association with remission in our study are located at the distal end of the GRIK4 gene region. In contrast, rs1954787, the best SNP of STAR*D resides in intron 1, about 173 kb proximal to our best SNP, rs12800734 (D′=0.42; R2=0.001).
Table 2. Association of Markers in GRIK4 with Treatment Response and Remission.
| GRIK4 dbSNP ID |
Unipolar (n=270) |
Uni-/Bipolar (n=300a) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Responseb |
Remissionc |
Response |
Remission |
SNP information |
||||||||
|
pemp |
pemp |
|||||||||||
| Genotypic | Allelic | Genotypic | Allelic | Genotypic | Allelic | Genotypic | Allelic | Alleles | Protective genotype | Function | MAF | |
| rs12800734 | 0.015 | 0.047 | 0.0043 | 0.087 | 0.0035 | 0.077 | 0.0019 | NS | A/G | GG | Intron 16 | 0.45 |
| rs2276319 | 0.025 | 0.045 | 0.014 | 0.084 | 0.0073 | 0.076 | 0.0067 | NS | C/T | CC | Intron 16 | 0.45 |
| rs1621211 | 0.0076 | NS | 0.017 | NS | 0.0091 | NS | 0.011 | NS | A/G | AA | 5′ end | 0.26 |
| rs513548 | 0.062 | NS | 0.016 | NS | 0.066 | NS | 0.011 | NS | T/G | GG | Intron 17 | 0.27 |
| rs2282586 | NS | NS | 0.014 | 0.097 | NS | NS | 0.018 | NS | A/G | GG | 5′ end | 0.37 |
| rs2156633 | 0.0021 | 0.048 | NS | NS | 0.0010 | 0.081 | NS | NS | A/G | GG | Intron 11 | 0.22 |
| rs1944522 | 0.013 | 0.088 | NS | NS | 0.0044 | NS | NS | NS | A/G | AA | 5′ end | 0.46 |
| rs1954787d | NS | NS | NS | NS | NS | NS | 0.079 | NS | C/T | CC | Intron 1 | 0.42 |
| FPM (82 SNPs) | 0.088 | NS | 0.026 | NS | 0.028 | NS | 0.029 | NS | ||||
Abbreviations: pemp, empirical p-value; NS, not significant; MAF, minor allele frequency; FPM, Fisher's product method (Fisher, 1932), gene-wise association results.
270 unipolar and 30 depressed bipolar patients.
A 50% reduction in the baseline HAM-D score after 5 weeks.
HAM-D score of <10 (21 items version) after 5 weeks.
Best GRIK4 SNP of the STAR*D study (Paddock et al, 2007).
Figure 2.
Association of SNPs in the GRIK4 gene region with remission after 5 weeks in the genetic model and pairwise LD structure, measured as D′ using the method of Gabriel as implemented in Haploview (n=300). The −log p-values of the associations were plotted against the physical location of the GRIK4 SNPs according to version hg18 of the University of California Santa Cruz genome draft.
We observed the highest nominal significances with remission in the genotypic model for rs12800734 (p=0.0019, pcorrected=0.12) and rs2276319 (p=0.0067, pcorrected=0.33). rs12800734 and rs2276319 reside about 1 kb apart in intron 17 of GRIK4. Both SNPs are closely linked and strongly correlated (D′=0.93; R2=0.99). Two additional SNPs, rs2156633 (intron 11) and rs1944522 (5′ UTR), showed associations with response only. The SNP predictive for treatment outcome in STAR*D, rs1954787, showed a nominally significant association with remission in the same direction (C allele associated with better outcome; genotypic, p=0.076, pcorrected=0.98).
Applying the FPM over all 82 SNPs of the GRIK4 locus, the genotypic model reached significance with remission in both samples, the unipolar (p=0.026) and the uni-/bipolar sample (p=0.029). For association with response status, the results were only significant in the larger sample (Table 2).
Analysis of rs12800734 in the extended unipolar/bipolar patient sample (n=387)
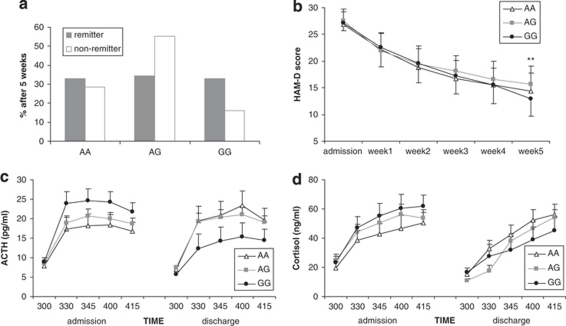
For rs12800734, only 16.2% (n=47) of the non-remitters vs 25.8% (n=25) of the remitters carried the protective GG genotype, whereas 55.2% (n=160) of the non-remitters and 40.2% (n=39) of the remitters carried the disadvantageous AG genotype with impaired treatment response (n=300). Thus, rs12800734 GG homozygotes are over-represented, whereas AG heterozygotes are under-represented among remitters (odds ratio (OR) 2.18, 95% confidence interval (CI) 1.20−3.97, p=0.009; OR GG vs AG/AA: 1.79, CI 1.03−3.12, p=0.036; Figure 3a). A repeated-measures ANOVA for change in HAM-D score over the first 5 weeks showed a significant time by genotype effect for this polymorphism in the extended unipolar/bipolar patient sample (n=387; Greenhouse–Geisser (GG): F6.12= 2.25, p=0.036; Figure 3b).
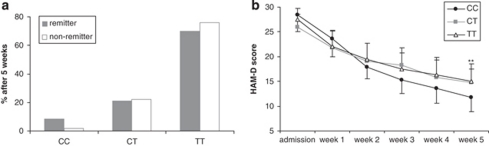
Figure 3.
Association of GRIK4 rs12800734 genotypes with antidepressant treatment outcome and neuroendocrine measures in depressed individuals. (a) Remission after 5 weeks (n=387, p=0.036). (b) HAM-D scores over the first 5 weeks (n=387; repeated-measures ANOVA: p=0.036); ** p<0.01. (c) ACTH (auc; repeated-measures ANOVA: p=0.002) and (d) cortisol (p=0.021) response in the dex/CRH test at admission (left) and discharge (right) in 193 depressed patients.
HTR2A and Treatment Response
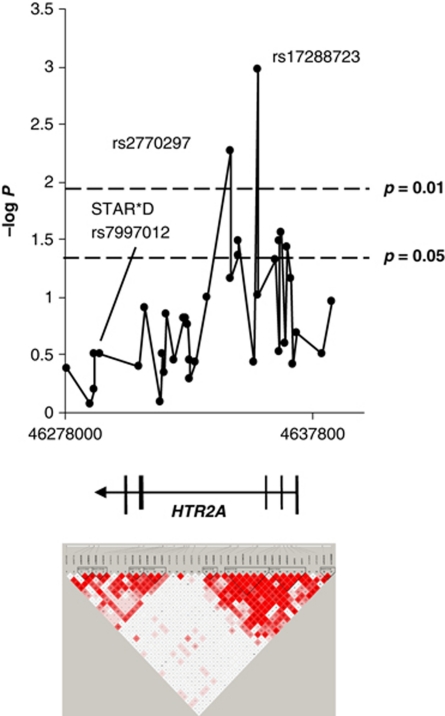
We tested 37 informative SNPs spanning a 106-kb long region encompassing the HTR2A gene on chromosome 13 with an average inter-marker distance of 2.8 kb for genotypic and allelic associations with remission and response after 5 weeks of treatment, as shown in Table 3. For two SNPs in intron 2 of HTR2A, rs17288723 and rs2770297, we observed nominally significant genotypic associations with remission but not response after 5 weeks in the unipolar (n=275) and the uni-/bipolar sample (n=305). In addition, three close-by SNPs in intron 1 were associated with remission after 5 weeks with higher significances in the allelic model. However, only the association of rs17288723 (genotypic, p=0.0011) remained significant after WY correction for the number of SNPs tested within this locus (pcorrected=0.02) and after additional conservative correction for the two phenotypes response and remission (pcorrected=0.04). rs7997012, the SNP identified and replicated as predictor of treatment response in the STAR*D sample, was nominally associated in our cohort; however, with the opposite allele predicting beneficial outcome (n=305; G allele protective; allelic, p=0.043, pcorrected=0.62). The genotype distribution of rs7997012 was 27 GG (40.9%), 33 AG (50.0%), and 6 AA (9.1%) for remitters, and 73 GG (30.5%), 125 AG (52.3%), and 41 AA (17.2%) for non-remitters after 5 weeks.
Table 3. Association of Markers in HTR2A with Treatment Response and Remission.
| HTR2A dbSNP ID |
Unipolar (n=270) |
Uni-/Bipolar (n=305a) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Responseb |
Remissionc |
Response |
Remission |
SNP information |
||||||||
|
pemp |
pemp |
|||||||||||
| Genotypic | Allelic | Genotypic | Allelic | Genotypic | Allelic | Genotypic | Allelic | Alleles | Prot. allele/ genotype | Function | MAF | |
| rs17288723 | NS | NS | 0.0040 | 0.046 | NS | NS | 0.0011/0.02d | 0.017 | C/T | CC | Intron 2 | 0.14 |
| rs2770297 | NS | NS | 0.028 | 0.099 | NS | NS | 0.0055 | 0.087 | C/T | CC | Intron 2 | 0.27 |
| rs2070037 | NS | NS | NS | 0.074 | NS | 0.074 | 0.027 | 0.011 | C/T | C | Intron 1 | 0.23 |
| rs2770302 | NS | NS | NS | 0.099 | NS | NS | 0.033 | 0.015 | T/A | T | Intron 1 | 0.24 |
| rs2296973 | NS | NS | 0.050 | 0.056 | NS | NS | 0.033 | 0.018 | T/G | T | Intron 1 | 0.29 |
| rs7997012e | NS | NS | NS | NS | NS | NS | NS | 0.043 | A/G | G | Intron 2 | 0.45 |
| FPM (37 SNPs) | NS | NS | NS | NS | NS | NS | 0.012 | 0.024 | ||||
Abbreviations: pemp, empirical p-value; NS, not significant; prot., protective; MAF, minor allele frequency; FPM, Fisher's product method (Fisher, 1932), gene-wise association results.
275 unipolar and 30 depressed bipolar patients.
A 50% reduction in the baseline HAM-D score after 5 weeks.
HAM-D score of <10 (21 items version) after 5 weeks.
After Westfall–Young correction for the number of SNPs tested.
Best HTR2A SNP of the STAR*D study (McMahon et al, 2006).
Testing the overall association of variation in the HTR2A locus with remission after 5 weeks by combining the results of all 37 SNPs according to FPM and obtained significant results for both models: genotypic (p=0.012) and allelic (p=0.024; Table 3).
Genotypic, permutation-based −log p results for remission after 5 weeks of all 37 HTR2A SNPs plotted against their physical location (hg18) as well as the corresponding LD map based on D′ are shown in Figure 4. Our LD structure analysis revealed two major LD blocks of approximately 48.6 kb and 41.3 kb. A 18.7-kb long gene region with low LD separates these two large blocks. rs7997012 resides between two smaller blocks, about 45.7 kb distal from rs17288723 in the larger distal (left) block. rs7997012 SNP is neither in LD nor correlated with any of our tested SNPs, including rs6313, rs1928040, and rs17288723 (D′=0.029; R2=0.0). All SNPs, which are significantly associated with remission from depression in our study, are in LD with moderate R2 (0.42−0.59).
Figure 4.
Association of SNPs in the HTR2A gene region with remission after 5 weeks in the genetic model and pairwise LD structure, measured as D′ using the method of Gabriel as implemented in Haploview (n=305). The −log p-values of the associations were plotted against the physical location of the HTR2A SNPs according to version hg18 of the University of California Santa Cruz genome draft.
Analysis of rs17288723 in the extended unipolar/bipolar patient sample (n=385)
Of the patients analyzed, 8.5% of the remitters and only 1.7% of the non-remitters were of the rare CC homozygous genotype. The genotype distribution for remitters was 8 CC (8.5%), 20 CT (21.37%), and 66 TT (70.2%), and was 5 CC (1.7%), 65 CT (22.3%), and 221 TT (75.9%) for non-remitters (χ2, p=0.007). In a post hoc analysis, homozygous carriers of the rare C-allele vs CT/TT genotypes explained best the association with remission after 5 weeks (χ2, p=0.002; carrier T model). The association of rs17288723 with remission reflects an over-representation of CC homozygotes among remitters with an OR of 5.36 (95% CI 1.69−16.93; Figure 5a). There was a significant rs17288723 CC vs CT/TT genotype effect change in HAM-D score over the first 5 weeks of treatment in a repeated-measures ANOVA (n=381; GG: F6.20=2.25, p=0.035; Figure 5b).
Figure 5.
Association of HTR2A rs17288723 genotypes with antidepressant treatment outcome. (a) Remission after 5 weeks (n=385, p=0.007). (b) HAM-D scores over the first 5 weeks (n=381; repeated-measures ANOVA: p=0.035); **p<0.01.
Association with Demographic, Disease-Related Variables, and Drug Plasma Levels
We further analyzed whether GRIK4 rs12800734 or HTR2A rs17288723 genotypes were significantly associated with various subject, disease-related variables, and drug plasma levels. We observed no genotype-dependent differences in sex, age, diagnostic subgroup (unipolar, bipolar, and psychotic), illness duration, number of previous depressive episodes, number of former hospitalizations, duration of current episode, chronic depression (current episode lasting ⩾2 years), anxious depression (baseline HAM-D anxiety/somatization factor score ⩾7, Fava et al, 2004), HAM-D score at inclusion, plasma drug levels (corrected for age, gender, drug dosage, and body weight) of mirtazapine (n=89), trimipramine (n=62), venlafaxine (n=57), citalopram (n=49), and paroxetine (n=23) at week 5, and comedication with mood stabilizers, antipsychotics, or benzodiazepines at week 5. However, we found a nominally significant effect on age of onset for GRIK4 rs12800734, with AG heterozygotes suffering from their first depressive episode earlier in life (p=0.044).
Association with Endocrine Variables
We next analyzed genotype-dependent differences in plasma ACTH and cortisol responses in the combined dex/CRH test (n=194) from admission to discharge for rs12800734 (GRIK4) and rs17288723 (HTR2A). We found interaction between GRIK4 rs12800734 genotype and change in endocrine response to the dex/CRH test between admission and discharge. The plasma ACTH (auc ACTH; GG: F2=9.95, p=0.002) and cortisol (auc cortisol; GG: F2=5.40, p=0.021) responses were significantly lower in the second dex/CRH test before discharge in homozygous GG carriers compared with the other two genotype groups (Figure 3c and d). No genotype-dependent differences were observed in basal plasma cortisol levels or plasma cortisol levels measured on the morning after treatment with dexamethasone. There were also no differences between genotype groups in the severity of depressive symptoms or plasma endocrine responses at the time of the first dex/CRH test. No significant interaction or main effects were observed for rs17288723 (HTR2A).
Multi-SNP Prediction Model
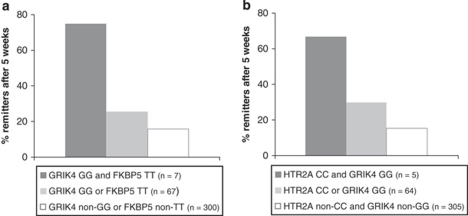
To test for possible additive or interaction effects between genotypes of GRIK4 (rs12800734), HTR2A (rs17288723), and FKBP5 (rs1360780), we applied a logistic regression model with remission after 5 weeks as the binary outcome and the three SNPs and their 2 × 2 interaction terms as predictors corrected for age and gender (Table 4). In 374 patients tested, the best step forward inclusion model included a main effect for the disadvantageous AG genotype of GRIK4 (p=0.004) and two significant interaction effects between the protective genotypes of each SNP: (1) GG of GRIK4 and TT of FKBP5 (p=0.022), and (2) CC of HTR2A and GG of GRIK4 (p=0.039). This three SNP model explained 13.1% of the variance (Nagelkerke's R2) for remission at week 5 (p=0.00051 for the model). Remission rates after 5 weeks for both SNP–SNP interaction effects are depicted in Figure 6a and b. Among patients homozygous for both HTR2A CC and GRIK4 GG (n=5), 66.7% were remitted at week 5 in contrast with 25.7% of patients carrying either HTR2A CC or GRIK4 GG (n=64) and only 15.9% for HTR2A non-CC and GRIK4 non-GG carriers (n=305). We additionally evaluated the gain in model fit of including the two identified interaction terms. A hierarchical regression analysis with the GRIK4 SNP in the first and the two significant SNP × SNP interaction terms in the second block showed that adding the two interaction terms significantly improves the model (p=0.00015). A bootstrapping analysis with 1000 samples that were randomly re-sampled out of the original data set of 374 depressed patients by applying the same model (het./hom. main effect for GRIK4 and two interaction effects: GRIK4 and FKBP5 and GRIK4 and HTR2A) revealed an average Nagelkerke's R2 of 0.138 (95% CI 0.066–0.225). Testing single SNPs for main effects only, the protective TT genotype of FKBP5 explained 4.3%, the adverse AG and the beneficial GG genotypes of GRIK4 explained 2.5%, and the favorable GG of HTR2A explained 1.5% of the variance (Nagelkerke's R2) for remission at week 5.
Table 4. Logistic Regression Model (Step Forward Inclusion) with Remission after 5 Weeks as Binary Outcome.
| Significant model parameters | Exp(B) OR | Standard regression coefficient | p-value |
Omnibus test—model |
Model gain per step |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 (df) | p-value | χ2 (df) | p-value | AIC | Nagelkerke's R2 | |||||
| Step 1 | rs12800734 GG × rs1360780 TT | 20.88 | 2.79 | 0.005 | 12.36 (4) | 0.015 | 12.36 (4) | 0.015 | 412.68 | 0.049 |
| Step 2 | rs12800734 GG × rs1360780 TT | 27.58 | 2.91 | 0.003 | ||||||
| rs17288723 CC × rs12800734 GG | 12.92 | 2.19 | 0.028 | 24.23 (8) | 0.002 | 11.87 (4) | 0.018 | 408.81 | 0.094 | |
| Step 3 | rs12800734 GG × rs1360780 TT | 17.61 | 2.30 | 0.022 | ||||||
| rs17288723 CC × rs12800734 GG | 11.41 | 2.07 | 0.039 | |||||||
| rs12800734 AG | 0.029 | −2.87 | 0.004 | 34.48 (10) | 0.00015 | 10.246 (2) | 0.006 | 402.56 | 0.131 | |
The significant new coefficients of each step are in bold.
Figure 6.
Gene × gene interaction effects according to a logistic regression model (step forward inclusion; n=374). Remission rates after 5 weeks of antidepressant treatment for SNP × SNP interactions between (a) GRIK4 and FKBP5 (p=0.022) and (b) GRIK4 and HTR2A genotypes (p=0.039).
DISCUSSION
This study supports the association of GRIK4 and HTR2A with response to antidepressant treatment and builds on the original identification of these genes as predictors of treatment outcome to citalopram in the STAR*D sample (McMahon et al, 2006; Paddock et al, 2007). Because of substantial differences in study design, these results have to be interpreted with caution, but they may broaden the effect of these genes to a more general psychiatric in-patient population who is treated according to the choice of the doctors.
In contrast with the ethnically diverse, outpatient cohort in STAR*D, our MARS patients are all Caucasians and in-patients with moderate-to-severe depressive symptoms, including a subset with psychotic depression. Furthermore, exit time points, outcome measures, and analytical approaches differed substantially between studies. Moreover, in the majority of our analyses we have included approximately 10% of currently depressed bipolar patients. Only a fraction of our patients had been treated with antidepressant monotherapy, but the majority received combinations of antidepressants, as well as mood stabilizers, antipsychotic drugs, and benzodiazepines. All STAR*D patients were treated only with the selective serotonin reuptake inhibitor citalopram at level 1. The STAR*D and the unipolar MARS samples further varied significantly in almost all examined disease-related parameters. In particular, chronic depression was over twice as common in the STAR*D cohort (see Table 1).
Despite these differences, we observed nominally significant associations for the two top SNPs of the so far published STAR*D pharmacogenetics studies. The GRIK4 SNP (rs195487) was nominally significantly associated with treatment remission in our sample in the same direction as the one previously reported. On the other hand, the HTR2A SNP (rs7997012) was significantly associated with remission after 5 weeks, but the G, not the A allele as in STAR*D seemed to be protective. However, these single SNP associations did not withstand correction for multiple testing, so that the observed associations most likely reflect false-positive findings. In conclusion, neither rs195478 nor rs7997012 of HTR2A replicated in our MARS study. More convincingly, association testing across all variants genotyped within the two genes showed significant associations with response and remission for both loci, supporting the involvement of genetic variation within GRIK4 and HTR2A in general, but not specific variants within these genes, in treatment response. Despite the good genetic coverage of our SNP panel (62% for GRIK4 and 78% for HTR2A), the lack of complete genotype data for all relevant variants makes it possible that further SNPs not genotyped in this study are relevant for antidepressant treatment response.
Although the association of rs12800734 in intron 16 of GRIK4 did not withstand correction for the number of variants tested in this gene, data from the combined dex/CRH test, an established biological marker for antidepressant treatment response, support it's effect on treatment response. Comparing ACTH and cortisol measures in the combined dex/CRH test between admission and discharge in 194 depressed patients, patients carrying the remission-associated GG genotype of rs12800734 showed a significant downregulation of the HPA axis hyperactivity. This pattern has been repeatedly shown to be associated with beneficial treatment outcome (Nickel et al, 2003; Ising et al, 2007; Binder et al, 2009). The additional heterozygote disadvantage of rs12800734 AG carriers is somewhat unusual and not easily to explain, but there are other reports, for example, for P2RX7 that show that the co-expression of both alleles may lead to specific effects not observed in homozygotes (Lucae et al, 2006; McQuillin et al, 2009). Although the two best SNPs in GRIK4 and HTR2A predicted treatment response, we could exclude that this was because of associations with variables that could themselves be predictive for treatment outcome, such as, for example, drug plasma levels, diagnostic subgroup, duration of the current episode, or anxious depression (Serretti et al, 2008).
The relevance of the HTR2A and GRIK4 genes in antidepressant response is supported by preclinical, imaging, and genetic studies. Although not covering the locus systematically, several previous studies have provided suggestive evidence for an involvement of other HTR2A variants in antidepressant outcome (Minov et al, 2001; Cusin et al, 2002; Peters et al, 2004; Choi et al, 2005; Hong et al, 2006; Kato et al, 2006; McMahon et al, 2006; Paddock et al, 2007; Perlis et al, 2009). Post-mortem (McKeith et al, 1987; Lopez-Figueroa et al, 2004) and positron emission tomography (PET) (Yates et al, 1990; Yatham et al, 2000; Mintun et al, 2004) studies have reported both increased and decreased HTR2A receptor number and binding in different brain regions of depressed individuals. It has also been shown that cortical HT2RA signaling modulates anxiety-like behaviors in mice (Weisstaub et al, 2006). HTR2A is expressed in a series of brain regions relevant for depressive behavior, including the hippocampus and the paraventricular nucleus of the hypothalamus (Barnes and Sharp, 1999; Hanley and Van de Kar, 2003). Moreover, HTR2A is a well-documented activator of the HPA axis (Van de Kar et al, 2001; Hanley and Van de Kar, 2003), and is in turn regulated by stress hormones (Torda et al, 1990; Kuroda et al, 1992; McKittrick et al, 1995; Fernandes et al, 1997). A large number of drugs mediate their actions, at least partially by interactions directly or indirectly (increasing 5-HT in the synaptic cleft) with HTR2A. Downregulation of HTR2A is a common characteristic after chronic exposure in a variety of pharmacologically distinct antidepressant drugs (Peroutka and Snyder, 1980; Blackshear and Sanders-Bush, 1982). Antidepressant drugs therefore potentially affect the function of neuronal prefrontal-subcortical circuits, as well as the HPA system through downregulation of HTR2A.
A series of studies have also implicated the glutamatergic system in the pathogenesis, pathophysiology, and treatment of mood disorders (Sanacora et al, 2008). Two gene families code for several kainate receptors subunits: GRIK1-3 (GLUR5-7) for the low-affinity and GRIK4/GRIK5 (KA1/KA2) for the high-affinity subunits. These subunits act as tetrameric assemblies pre- and post-synaptically. Kainate receptors are involved in the regulation of neurotransmitter release, fast excitatory neurotransmission, in the control of neuronal excitability, and in synaptic integration and plasticity (Pinheiro and Mulle, 2006). Glutamatergic abnormalities have been reported in plasma (Altamura et al, 1993; Hashimoto et al, 2007), cerebrospinal fluid (Levine et al, 2000; Frye et al, 2007), brain tissue (Francis et al, 1989; Hashimoto et al, 2007), and in brain imaging studies (Auer et al, 2000; de Graaf et al, 2003) of individuals affected with mood disorders. Finally, ketamine, an NMDA receptor antagonist, as well as riluzole, a drug currently approved for the treatment of amyotrophic lateral sclerosis (ALS) that decreases glutamate release, show antidepressant and anxiolytic effects (Zarate et al, 2004; Mathew et al, 2005).
In the past few years, much effort has been directed toward the search for genetic predictors of antidepressant drug efficacy. The effect of genetic variation has been studied at the pharmacokinetic (eg, CYP2D6; Kirchheiner and Seeringer, 2007; Peters et al, 2008; Tomalik-Scharte et al, 2008; Uhr et al, 2008), the pharmacodynamic (Binder and Holsboer, 2006; Kato and Serretti 2008; Horstmann and Binder, 2009), and the bioavailability level (eg, ABCB1; Uhr et al, 2008). Up till now all studies were driven by hypothesis, analyzing candidate genes in the main systems currently implicated in the etiology of major depression and mechanism of action of antidepressant drugs (Holsboer, 2008). The first whole genome association study has recently been reported by our group. Although no single genetic variant was consistently replicated, the combination of 46 risk alleles, together with the presence of anxious depression, predicted treatment outcome in MARS and STAR*D (Ising et al, 2009). Although preliminary, our data from a multi-marker model also support the use of SNP combinations in the pharmacogenetics of antidepressant drugs and underline the importance of gene × gene interaction. Our three-marker model, which included main effects as well as two gene × gene interaction, predicted over 13% of the variance in our sample, whereas models that solely included main effects for FKBP5, GRIK4, or HTR2A only explained 4.3%, 2.5%, and 1.5% of the variance, respectively. In comparison, the variance explained by 5-HTTLPR (SLC6A4), a polymorphism showing significant association with response to SSRIs in a recent meta-analysis, has been estimated to be approximately 2–3% (Serretti et al, 2008). The interaction of genes from the monoamine, glutamatergic, and HPA axis system indicate that several systems are likely disturbed in major depression and their function restored with clinically effective antidepressant treatment. Drugs having a combined effect, either directly or indirectly, on all systems might yield more rapid antidepressant response.
Although our results seem to support data from the STAR*D study, caution is needed in the interpretation of the results, as the power of the study is limited, especially for gene × gene interaction analyses. Although we found several SNPs in HTR2A and GRIK4 to be associated with treatment response with nominally significant p-values, only one HTR2A SNP withstood correction for multiple testing. However, both genes showed significant associations in gene-wise tests. Further limitations of this study are summarized below. (1) Our results may be confounded by population stratification. This is, however, unlikely as previous studies using genomic control SNPs (Binder et al, 2004) as well as a GWAS study (Ising et al, 2009) did not find any indication of population admixture in the MARS sample (λ=1.023). (2) Because our study did not include a placebo treatment arm, we cannot distinguish whether our associations are related with the specific effects of antidepressant treatment or are a reflection of the natural course of the disease in individual patients. (3) The inclusion of patients suffering from bipolar depression may be regarded as confounder. However, as reported, we did not detect differences between patients with unipolar or bipolar depression with respect to the genotype frequencies of our best HTR2A and GRIK4 SNPs. In addition, we could show that in the MARS study the diagnosis of unipolar vs bipolar depression had no effects on the short-term treatment outcome in the MARS study (Hennings et al, 2009), and hence we feel that the combined analyses are permissible. (4) A further limitation is the heterogeneity of antidepressant treatment types used in the MARS sample. However, given the fact that the primary mode of action of all antidepressants is likely related with an enhancement of monoaminergic neurotransmission and that despite differences in the profile of receptor occupancy, antidepressants show comparable efficacy across drug classes (Mathew et al, 2008), a combined analyses across antidepressant treatments seem justifiable. Side effects and consecutive change of medication are likely to influence the speed of response and have to be further considered as potential confounding variables. (5) A further limitation is the choice of a 5-week time point, which is much shorter than the maximal possible observation time in level 1 of the STAR*D study. The choice of this shorter treatment time was based on well-documented differences in time until response/remission between antidepressant treatment of in-patients and outpatients (Mauskopf et al, 2009) and yielded similar remission rates between the MARS and the STAR*D study. Approximately 30% of patients remit to antidepressant therapy within 5 weeks in our MARS study (Hennings et al, 2009), close to the remission rate of 28% (HAM-D) and 33% (QIDS-SR) observed after the first treatment trial of up to 14 weeks in the STAR*D study (Trivedi et al, 2006).
Although our study is limited by power and sample size and despite substantial differences in study design as compared with the STAR*D trial, our findings support a central role of HTR2A, GRIK4, and FKBP5 in the pharmacogenetics of antidepressant drugs and these effects do not seem to be restricted to citalopram treatment. However, further replications in independent samples, in particular replication of the interaction results, are warranted to corroborate our findings.
Acknowledgments
We thank all former raters of the MARS studies for collecting the data, and thank Gertrud Ernst-Jansen, Gisela Gajewsky, Melanie Hartung, Johannes Huber, and Elisabeth Kappelmann for excellent technical assistance in performing the dex/CRH tests and for help in documenting medical history. We are grateful to Thomas Bettecken, Manfred Uhr, Stephan Ripke, Benno Pütz, and Hildegard Pfister for their valuable help in performing the studies, and to Sabine Damast, Maik Koedel, Susann Sauer, and Alina Tontsch for excellent technical assistance. We are grateful to the research teams at the BKH Augsburg (Professor Dr Max Schmauß) and the Zentrum für psychische Gesundheit at the Klinikum Ingolstadt (Professor Dr Thomas Pollmächer) for contributing cases to the Munich Antidepressant Response Signature (MARS) project.
Footnotes
DISCLOSURE
This study is supported by a grant of the Exzellenz-Stiftung of the Max Planck Society. This work has also been funded by the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN), FKZ 01GS0481. Sonja Horstmann, Susanne Lucae, Andreas Menke, Johannes Hennings, Marcus Ising, and Darina Roeske have nothing to disclose. Drs Binder, Holsboer, and Müller-Myhsok are co-inventors of the following pending patent applications: (1) FKBP5: a novel target for antidepressant therapy (International publication number: WO 2005/05450), and (2) Polymorphisms in ABCB1 associated with a lack of clinical response to medicaments (International application number: PCT/EP2005/005194). Elisabeth B Binder receives grant support from NIMH and Doris Duke Charitable foundation, and Florian Holsboer receives grant support from the NGFN and Bristol Myers Squibb. He is also a founder and shareholder of Affectis and a shareholder of Corcept and Neurocrine.
References
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- Angst J. A clinical analysis of the effects of tofranil in depression. Longitudinal and follow-up studies. Treatment of blood-relations. Psychopharmacologia. 1961;2:381–407. doi: 10.1007/BF00407438. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Binder EB, Holsboer F. Pharmacogenomics and antidepressant drugs. Ann Med. 2006;38:82–94. doi: 10.1080/07853890600551045. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kunzel HE, Nickel T, Kern N, Pfennig A, Majer M, et al. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34:99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Blackshear MA, Sanders-Bush E. Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J Pharmacol Exp Ther. 1982;221:303–308. [PubMed] [Google Scholar]
- Bschor T, Adli M, Baethge C, Eichmann U, Ising M, Uhr M, et al. Lithium augmentation increases the ACTH and cortisol response in the combined DEX/CRH test in unipolar major depression. Neuropsychopharmacology. 2002;27:470–478. doi: 10.1016/S0893-133X(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Choi MJ, Kang RH, Ham BJ, Jeong HY, Lee MS. Serotonin receptor 2A gene polymorphism (-1438A/G) and short-term treatment response to citalopram. Neuropsychobiology. 2005;52:155–162. doi: 10.1159/000087847. [DOI] [PubMed] [Google Scholar]
- Cusin C, Serretti A, Zanardi R, Lattuada E, Rossini D, Lilli R, et al. Influence of monoamine oxidase A and serotonin receptor 2A polymorphisms in SSRI antidepressant activity. Int J Neuropsychopharmacol. 2002;5:27–35. doi: 10.1017/S1461145701002711. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- Efron BL. Bootstrap methods: another look at the jackknife. Ann Statist. 1979;7:1–26. [Google Scholar]
- Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–1308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Oliver and Boyed: London; 1932. [Google Scholar]
- Franchini L, Serretti A, Gasperini M, Smeraldi E. Familial concordance of fluvoxamine response as a tool for differentiating mood disorder pedigrees. J Psychiatr Res. 1998;32:255–259. doi: 10.1016/S0022-3956(98)00004-1. [DOI] [PubMed] [Google Scholar]
- Francis PT, Poynton A, Lowe SL, Najlerahim A, Bridges PK, Bartlett JR, et al. Brain amino acid concentrations and Ca2+-dependent release in intractable depression assessed antemortem. Brain Res. 1989;494:315–324. doi: 10.1016/0006-8993(89)90600-8. [DOI] [PubMed] [Google Scholar]
- Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic—pituitary-adrenal axis in health and disease. Vitam Horm. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hennings JM, Owashi T, Binder EB, Horstmann S, Menke A, Kloiber S, et al. Clinical characteristics and treatment outcome in a representative sample of depressed inpatients—findings from the Munich Antidepressant Response Signature (MARS) project. J Psychiatr Res. 2009;43:215–229. doi: 10.1016/j.jpsychires.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F. How can we realize the promise of personalized antidepressant medicines. Nat Rev Neurosci. 2008;9:638–646. doi: 10.1038/nrn2453. [DOI] [PubMed] [Google Scholar]
- Hong CJ, Chen TJ, Yu YW, Tsai SJ. Response to fluoxetine and serotonin 1A receptor (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J. 2006;6:27–33. doi: 10.1038/sj.tpj.6500340. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol Ther. 2009;124:57–73. doi: 10.1016/j.pharmthera.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression—a potential biomarker. Biol Psychiatry. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, et al. A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry. 2009;66:966–975. doi: 10.1001/archgenpsychiatry.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, et al. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology. 2006;53:186–195. doi: 10.1159/000094727. [DOI] [PubMed] [Google Scholar]
- Kato M, Serretti A.2008Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder Mol Psychiatrye-pub ahead pf print. [DOI] [PubMed]
- Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, et al. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. doi: 10.2217/14622416.9.7.841. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Seeringer A. Clinical implications of pharmacogenetics of cytochrome P450 drug metabolizing enzymes. Biochim Biophys Acta. 2007;1770:489–494. doi: 10.1016/j.bbagen.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Binder EB, Nickel T, Ising M, Fuchs B, Majer M, et al. Pharmacological and nonpharmacological factors influencing hypothalamic-pituitary-adrenocortical axis reactivity in acutely depressed psychiatric in-patients, measured by the Dex-CRH test. Neuropsychopharmacology. 2003;28:2169–2178. doi: 10.1038/sj.npp.1300280. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Mikuni M, Ogawa T, Takahashi K. Effect of ACTH, adrenalectomy and the combination treatment on the density of 5-HT2 receptor binding sites in neocortex of rat forebrain and 5-HT2 receptor-mediated wet-dog shake behaviors. Psychopharmacology (Berl) 1992;108:27–32. doi: 10.1007/BF02245281. [DOI] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barde N, Harvey M, Gagné B, Labbé M, et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–796. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162:2379–2381. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26:83–97. doi: 10.1002/da.20505. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Marshall EF, Ferrier IN, Armstrong MM, Kennedy WN, Perry RH, et al. 5-HT receptor binding in post-mortem brain from patients with affective disorder. J Affect Disord. 1987;13:67–74. doi: 10.1016/0165-0327(87)90075-9. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, Lawrence J, et al. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginiene in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psych. 2009;14:614–620. doi: 10.1038/mp.2008.6. [DOI] [PubMed] [Google Scholar]
- Minov C, Baghai TC, Schule C, Zwanzger P, Schwarz MJ, Zill P, et al. Serotonin-2A-receptor and -transporter polymorphisms: lack of association in patients with major depression. Neurosci Lett. 2001;303:119–122. doi: 10.1016/s0304-3940(01)01704-9. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry. 2004;55:217–224. doi: 10.1016/j.biopsych.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Nickel T, Sonntag A, Schill J, Zobel AW, Ackl N, Brunnauer A, et al. Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol. 2003;23:155–168. doi: 10.1097/00004714-200304000-00008. [DOI] [PubMed] [Google Scholar]
- O'Reilly RL, Bogue L, Singh SM. Pharmacogenetic response to antidepressants in a multicase family with affective disorder. Biol Psychiatry. 1994;36:467–471. doi: 10.1016/0006-3223(94)90642-4. [DOI] [PubMed] [Google Scholar]
- Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, et al. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Fijal B, Adams DH, Sutton VK, Trivedi MH, Houston JP. Variation in catechol-O-methyltransferase is associated with duloxetine response in a clinical trial for major depressive disorder. Biol Psychiatry. 2009;65:785–791. doi: 10.1016/j.biopsych.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science. 1980;210:88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, Kraft JB, Jenkins GD, Reinalda MS, McGrath PJ, et al. Pharmacokinetic genes do not influence response or tolerance to citalopram in the STAR*D sample. PLoS ONE. 2008;3:e1872. doi: 10.1371/journal.pone.0001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Soder M, Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology. 1993;133:312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Sihra TS. Metabotropic actions of kainate receptors in the CNS. J Neurochem. 2007;103:2121–2135. doi: 10.1111/j.1471-4159.2007.04924.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene-Fischer C, Yu C. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001;495:1–6. doi: 10.1016/s0014-5793(01)02326-2. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psych. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, Kennedy JL. Pharmacogenetic studies in depression: a proposal for methodologic guidelines. Pharmacogenomics J. 2008;8:90–100. doi: 10.1038/sj.tpj.6500477. [DOI] [PubMed] [Google Scholar]
- Tomalik-Scharte D, Lazar A, Fuhr U, Kirchheiner J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J. 2008;8:4–15. doi: 10.1038/sj.tpj.6500462. [DOI] [PubMed] [Google Scholar]
- Torda T, Murgas K, Cechova E, Kiss A, Saavedra JM. Adrenergic regulation of [3H]ketanserin binding sites during immobilization stress in the rat frontal cortex. Brain Res. 1990;527:198–203. doi: 10.1016/0006-8993(90)91138-7. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–209. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Young SS. Resampling-Based Multiple Testing. John Wiley & Sons: New York; 1993. [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN. 5HT2 receptor changes in major depression. Biol Psychiatry. 1990;27:489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, et al. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]