Abstract
Caffeine, a widely consumed adenosine A1 and A2A receptor antagonist, is valued as a psychostimulant, but it is also anxiogenic. An association between a variant within the ADORA2A gene (rs5751876) and caffeine-induced anxiety has been reported for individuals who habitually consume little caffeine. This study investigated whether this single nucleotide polymorphism (SNP) might also affect habitual caffeine intake, and whether habitual intake might moderate the anxiogenic effect of caffeine. Participants were 162 non-/low (NL) and 217 medium/high (MH) caffeine consumers. In a randomized, double-blind, parallel groups design they rated anxiety, alertness, and headache before and after 100 mg caffeine and again after another 150 mg caffeine given 90 min later, or after placebo on both occasions. Caffeine intake was prohibited for 16 h before the first dose of caffeine/placebo. Results showed greater susceptibility to caffeine-induced anxiety, but not lower habitual caffeine intake (indeed coffee intake was higher), in the rs5751876 TT genotype group, and a reduced anxiety response in MH vs NL participants irrespective of genotype. Apart from the almost completely linked ADORA2A SNP rs3761422, no other of eight ADORA2A and seven ADORA1 SNPs studied were found to be clearly associated with effects of caffeine on anxiety, alertness, or headache. Placebo administration in MH participants decreased alertness and increased headache. Caffeine did not increase alertness in NL participants. With frequent consumption, substantial tolerance develops to the anxiogenic effect of caffeine, even in genetically susceptible individuals, but no net benefit for alertness is gained, as caffeine abstinence reduces alertness and consumption merely returns it to baseline.
Keywords: caffeine, adenosine, polymorphism, anxiety, alertness, headache
INTRODUCTION
Caffeine is prized for its alerting effect that may, at least in part, explain the worldwide popularity of tea, coffee, and other caffeine-containing products. However, although frequent consumers feel alerted by caffeine, especially by their morning tea, coffee, or other caffeine-containing drink, evidence suggests that this is actually merely the reversal of the fatiguing effects of acute caffeine withdrawal (James and Rogers, 2005; Sigmon et al, 2009). That is, little or perhaps no net benefit for alertness is gained. In addition, caffeine has the undesirable effects of, for example, increasing anxiety and raising blood pressure (Alsene et al, 2003; Goldstein et al, 1969; James, 2004). These behavioral and physiological effects of caffeine occur primarily through antagonism by caffeine of the action of endogenous adenosine at adenosine A1 and A2A receptors (Fredholm et al, 1999). Both receptors have a role in miscellaneous biological processes, particularly the cAMP-protein kinase A signaling cascade and the fine-tuning of glutamatergic information flow (Schiffmann et al, 2007; van Calker and Biber, 2005). They are considered to be modulators of glial function, neuronal communication and neuronal activity, and to be involved in sleep and arousal, and cognition, as well as different psychiatric disorders, including anxiety and other mood disorders (Ribeiro et al, 2003; Cunha et al, 2008; Freitag et al, 2010). In mice, genetic knockout of adenosine A1 or A2A receptors has been linked to increased anxiety (Ledent et al, 1997; Johansson et al, 2001), implicating the corresponding genes or, more precisely, polymorphisms within these genes, as promising candidates for increased anxiety reactions. Consistent with this, recent studies have discovered an association between caffeine-induced anxiety and a single nucleotide polymorphism (SNP) in the gene coding for the adenosine A2A receptor (ADORA2A). Specifically, it was found that 150 mg caffeine (equivalent to the amount of caffeine present in, eg, 1½ cups of ground coffee) increased anxiety in individuals carrying the TT genotype of the ADORA2A SNP rs5751876, but not in the CT and CC genotype groups (Alsene et al, 2003; Childs et al, 2008).
The significance of these findings on anxiety for assessing the balance of benefit and harm of everyday caffeine consumption is, however, uncertain, because all the participants in these two genetic studies were infrequent consumers of caffeine—no individual reported consuming more than three cups of coffee or equivalent per week, and many were recorded as consuming no caffeine. It is possible, for example, that the experience of increased anxiety after caffeine may cause vulnerable individuals to avoid caffeine subsequently (cf Evans and Griffiths, 1992; Stern et al, 1989), so that actually there are rather few such individuals among populations of frequent caffeine consumers. Indeed, even among infrequent consumers of caffeine a minority (19–29%) carry the rs5751876 TT genotype (Alsene et al, 2003; Childs et al, 2008). Furthermore, anxiety effects of caffeine might be diminished with frequent consumption due to tolerance.
Accordingly, the present study investigated the effects of caffeine on anxiety in both frequent and infrequent consumers. It also measured habitual caffeine intake and caffeine consumption status (‘frequent' vs ‘infrequent' consumption) in relation to rs5751876 genotype group. The primary hypotheses were that administration of caffeine would increase anxiety to a greater extent in infrequent consumers of caffeine (lack of tolerance) and in individuals carrying the rs5751876 TT genotype, and that this genotype would be associated with caffeine avoidance (especially avoidance of drinks containing higher amounts of caffeine, eg, coffee) or at least relatively low habitual caffeine consumption. Associations between both the anxiogenic and alerting effects of caffeine and other ADORA2A SNPs and ADORA1 SNPs were also examined. These further SNPs were selected according to their potential functional relevance, and to extend the findings of Alsene et al (2003) and Childs et al (2008). A secondary objective of the study was to test the withdrawal reversal hypothesis (James and Rogers, 2005), alluded to above, which predicts that administration of caffeine to frequent caffeine consumers after acute (overnight) caffeine deprivation will increase their alertness, but not to above the level of alertness experienced by infrequent caffeine consumers not given caffeine. In view of the ubiquitous consumption of caffeine worldwide, the potential impact on human well-being of the alerting effects of caffeine and vulnerability to its anxiogenic action is potentially very significant.
Caffeine was administered in two doses, 100 mg late morning, and 150 mg 90 min later. This was done to test the anxiogenic and alerting effects of the amount of caffeine relevant to the consumption of caffeine-containing drinks (eg, a cup of ground coffee). The second dose ensured that systemic caffeine concentration later in the test session modeled that expected for frequent caffeine consumers. Finally, it is noteworthy that the target sample size was 400, which is substantially higher than the number of participants tested in typical studies of the behavioral effects of caffeine.
MATERIALS AND METHODS
Participants and Randomization
The participants were 218 women and 198 men. Many (44%) were recruited from a list of respondents to a postal survey of caffeine consumption habits and health carried out during the previous year (Heatherley et al, 2006b). These respondents were provisionally divided into infrequent and (more) frequent caffeine consumers (caffeine intake of <40 mg per day and ⩾40 mg per day) and younger and older participants (<30 years and ⩾30 years), and within these groups they were selected for contact at random. The aim of this division was to achieve similar numbers of infrequent and frequent caffeine consumers of similar ages in the final sample, despite the fact that only 10% of this population consumed <40 mg caffeine daily, and older participants tended to have higher habitual caffeine intake. The remaining participants were recruited using local advertisements and by word of mouth, targeting infrequent and frequent caffeine consumers, as required. Suitability for the study was assessed in a telephonic or face-to-face interview. Key inclusion criteria were: age between 18 and 65 years, good general physical and mental health, availability and willingness to attend an experiment lasting 7 h, which might include consumption of caffeine, willingness to give a blood sample (for genotyping), being a nonsmoker or a light smoker (⩽5 cigarettes or equivalent a day), normal blood pressure, not pregnant, not planning to become pregnant, and not breastfeeding.
Randomization to receive caffeine or placebo on the test day was stratified according to self-reported habitual caffeine intake of <40 mg per day and ⩾40 mg per day and age (<30 years and ⩾30 years) recorded during the recruitment interview. Final assignment to caffeine group (see Data Analysis) was done on the basis of information recorded in a caffeine intake questionnaire completed during the week preceding testing, and analyzed after the participant had been tested. This assessed frequency of consumption of teas, coffees, colas, other caffeine-containing drinks (eg, Red Bull) and products (eg, Pro Plus and Anadin Extra), and chocolate. Mean daily caffeine intake was calculated from these data using dietary and manufacturers' information on caffeine content (Heatherley et al, 2006a); for example, instant coffee 54 mg, ground coffee 105 mg, tea (bags, loose leaf, instant, and green) 40 mg.
The research was presented to participants as a study on ‘genetic variation, caffeine consumption habits, and caffeine effects.' It was reviewed and approved by the University of Bristol's Department of Experimental Psychology Human Research Ethics Committee.
Design and Procedure
This was a double-blind, parallel groups, repeated-measures study. After overnight caffeine abstinence, participants received one of two treatments: either 100 mg of caffeine followed 90 min later by a further 150 mg of caffeine, or placebo on both the occasions. This two-stage dosing regimen was used to model, as far as practicable, real-life consumption of caffeine and to allow assessment of effects at lower and higher plasma caffeine concentrations. Various measurements were conducted before treatment, again after the first dose of caffeine or placebo, and finally twice after the second dose of caffeine or placebo.
The procedures are summarized in Table 1. The behavioral data described here are self-rated anxiety, alertness, and headache assessed using the Mood, Alertness and Physical Sensations Scales (MAPSS, see below). This was presented as part of a battery of ‘computer tasks' programmed using E-Prime 1.0 (Psychology Software Tools, Science Plus Group, Groningen, the Netherlands), which also included tests of psychomotor performance, memory, attention, and vigilance. Results for these latter tests and for other measures (hand steadiness, heart rate and blood pressure, taste sensitivity, etc) will be reported elsewhere.
Table 1. Test Day Schedule.
| Time (hours) | Activity |
|---|---|
| 0930a | Briefing and consent Blood pressure, height, weight Venous blood sample Practice computer tasksb and hand-steadiness |
| 1030 | Baseline (pre-treatment) test session Computer tasksb, hand-steadiness, blood pressure, saliva sample (1110 hours) |
| 1115 | 100 mg caffeine/placebo |
| Rest break | |
| 1200 | Post-treatment test session 1 Computer tasksb, blood pressure, hand steadiness |
| 1245 | Saliva sample 150 mg caffeine (if caffeine at 1115 hours)/placebo (if placebo at 1115 hours) |
| Lunch | |
| 1315 | Attention and impulsivity tasks |
| 1345 | Post-treatment test session 2 Computer tasksb, saliva sample (1415 hours), hand-steadiness, blood pressure, sweet and bitter taste sensitivity |
| Rest break | |
| 1500 | Post-treatment test session 3 Computer tasksb, attentional bias for caffeine-related stimuli |
| Rest break | |
| 1600 | Debriefing, participants paid £50 each |
| 1615 | Saliva sample, participants leave |
Caffeine intake was prohibited from 1900 hours the previous evening.
This battery of computer tasks included MAPSS, which measured anxiety, alertness and headache. MAPSS was completed after a tapping task, which lasted 30 s.
On any single day, between two and six participants were tested. They were previously told not to consume alcohol or caffeine-containing products from 1900 hours on the evening before testing, to replace any caffeine-containing drinks with water, and to eat their normal breakfast. If they were a smoker they were asked not to smoke on the test day until after they left the laboratory. They were informed that compliance with the instruction to avoid caffeine would be assessed by measurement of the concentration of caffeine present in their saliva at the start of testing (saliva sample taken at 1110 hours). The initial briefing session was held in a communal room in the laboratory, and this same room was used for rest periods, lunch, and debriefing. Blood collection and the computer tasks were carried out in rooms close by. For the computer tasks, each participant was accommodated in a separate, private booth within the larger of these rooms. Lunch consisted of a sandwich, a small cake, and fruit (total energy content 580–740 kcal). Participants had access to bottled water throughout their stay in the laboratory, and the amount they consumed was recorded.
Drug Administration
Caffeine BP (caffeine anhydrous powder; Courtin and Warner, Lewes, East Sussex, UK) and placebo (cornflour) were administered in white, size 1 cellulose capsules (Capsuline, Pompano Beach, Florida, FL, USA). These caffeine and placebo capsules were identical in appearance, and were swallowed with 50 ml of room temperature water. Each dose was contained in a single capsule.
Mood, Alertness and Physical Sensations Scales
MAPSS was used to measure anxiety, alertness, and headache. It was adapted from similar instruments used in previous studies on the effects of caffeine (Rogers et al, 2005, 2008). It comprised 24 items (single or groups of descriptors, eg I feel mentally alert/attentive/able to concentrate/observant; I feel tense/anxious/nervous/on edge; My head aches/I feel headachy), which were rated on a nine-point unipolar scale using the horizontal number pad on the computer keyboard, where 1 represented ‘not at all' and 9 represented ‘extremely' (adjusted to a 0–8 scale for the presentation of results here). Participants were instructed: ‘There are no right or wrong answers. Do not spend too much time on any one statement but give the rating which seems to best describe your present feeling.' The order of presentation of the items was determined randomly for each participant on each occasion. See Supplementary Materials and Methods for full details of MAPSS.
SNP Selection and Genotyping
Single nucleotide polymorphism selection was based on previous studies (Alsene et al, 2003; Childs et al, 2008; Deckert et al, 1998), and their regulatory potential (UCSC, http://genome.ucsc.edu), linkage disequilibrium (LD) and tagging capabilities (Hapmap, http://www.hapmap.org), and minor allele frequencies. Eight SNPs were selected to cover the 25 kb ADORA2A variant resulting in mRNA X68486 (rs5751862, rs5760405, New3, rs11704959, rs2298383, rs3761422, rs2267076, and rs5751876), and nine SNPs were selected to cover the 76 kb ADORA1 variant resulting in mRNA L22214 (rs9660662, rs1874142, rs10920568, rs12135643, rs3766566, rs3766560, rs3753472, rs16851030, and rs12744240). Participants were genotyped by custom TaqMan SNP genotyping assays (Applied Biosystems, Darmstadt, Germany) for all SNPs except rs2298383 and rs10920568 (see below), with PCR setup (5 μl reactions) as recommended by the manufacturer and performed on a Genesis Workstation RSP150 (Tecan, Crailsheim, Germany). The ABI Prism 7900 Sequence Detection System with SDS software version 2.1 (Applied Biosystems) was used for PCR amplification and allelic discrimination. To minimize the risk of genotyping errors, about 10% of randomized participants (n=45) were additionally genotyped for all SNPs by RFLP assays using independent primer-sets different to those used for the TaqMan assays, which resulted in concordance rates of 100%. Genotyping assay conditions and primer-probe sequences from all TaqMan and RFLP assays are available on request. ADORA2A SNP rs2298383 and ADORA1 SNP rs10920568, which failed TaqMan assay design, were genotyped by RFLP assays as previously described (Deckert et al, 1998; Freitag et al, 2010). Overall, genotyping resulted in averaged call rates of 100% (range 99.5–100.0), and all genotypes from TaqMan and RFLP analysis were assigned blind regarding group assignment and the measured phenotypic characteristics of the participants.
Collection of Saliva and Analysis of Methylxanthine Concentrations
Saliva collections were carried out at the times shown in Table 1. Caffeine and its metabolites, paraxanthine, theophylline and theobromine, were analyzed using an HPLC method adapted from Hartley et al (1985). The limit of detection for all analytes was 0.02 μg/ml. For full details see Supplementary Materials and Methods.
Data Analysis
Initial assessment of influences of habitual level of caffeine consumption (consumer status) was conducted by dividing the <40 mg caffeine per day participants into two groups based on a median split of consumption level to produce ‘nonconsumer' (N) and ‘low' (L) consumer groups, and doing the same for ⩾40 mg per day participants to produce ‘medium' (M) and ‘high' (H) consumer groups. Subsequent analyses involving consumer status were conducted on a <40 mg per day (non-/low, NL) vs ⩾40 mg per day (medium/high, MH) split.
ANOVA, run using SPSS 12.0.1 for Windows, was used to analyze the data on anxiety, alertness, and headache. Age as a covariate and gender as a factor were included in all these analyses. In addition, for certain analyses of the effects of caffeine vs placebo on anxiety, baseline (pretreatment) anxiety score was also included as a covariate. This is similar to the approach of calculating change from baseline scores (Childs et al, 2008), and was done to control for preexisting individual differences in anxiety in these analyses. Baseline differences in anxiety as a function of consumer status and genotype group were small (see Results).
For genotype data, Hardy–Weinberg equilibrium and LD analyses were performed using Haploview 4.0 (Barrett et al, 2005). Because Childs et al (2008) found that 150 mg but not a higher dose of caffeine differentially increased anxiety in ADORA2A rs5751876 genotype groups, initial analysis of anxiety effects in relation to this SNP was carried out separately for post-treatment session 1 data (collected after administration of 100 mg of caffeine) alone. In analyses which included dose/session as a (within subjects) factor mean values were calculated for the data for post-treatment sessions 2 and 3 to give equal weight to the data collected after administration of the first (session 1) and second doses (sessions 2 and 3) of caffeine. Dose/session was included in the analyses involving caffeine and consumer status, and caffeine, consumer status and genotype. In the latter analyses, data from ADORA2A rs5751876 CC and CT genotype groups (which were similar in their behavior) were combined to simplify the presentation of the results.
The Bonferroni procedure was used to adjust for multiple testing at eight ADORA2A loci (α=0.00625) and seven ADORA1 loci (α=0.00714). Two of the nine ADORA1 SNPs (rs9660662 and rs10920568) were excluded from the analyses due to deviation from Hardy–Weinberg expectations (see Results). Bonferroni t-test (Howell, 1997) was used following ANOVA for making multiple paired comparisons (caffeine vs placebo within consumer and/or genotype group).
RESULTS
Ethnic origin of the participants was predominantly (95%) white European. A total of 416 individuals were randomized to receive caffeine or placebo. Genetic data were not available for four participants due to problems encountered in extracting DNA. Fifteen NL participants were excluded because their baseline saliva sample contained >0.2 μg/ml caffeine and/or paraxanthine. This concentration of caffeine and its major metabolite, paraxanthine, suggested that caffeine consumption the previous day was substantially higher than the <40 mg criterion. Five MH participants were excluded because their baseline caffeine sample contained >2 μg/ml caffeine. This high salivary concentration of caffeine suggested that they had failed to comply with the instruction to be overnight caffeine abstinent. The same criterion was used in a previous study (Rogers et al, 2005). Of the remaining participants, eight withdrew after having received at least the first caffeine or placebo capsule, and there were missing data for five participants due to equipment malfunction. Of the eight participants who withdrew, five were from the MH group, all of whom received placebo (reasons given for withdrawing were headache and feeling sick (n=4), no reason (n=1)), and three were from the NL group, of whom two received caffeine and one received placebo (no reasons given for withdrawing). There was no difference in the ADORA2A rs5751876 genotype distribution for the 33 participants excluded, who withdrew or had missing data compared with the remaining 379 participants for whom genetic data were available (CC=13, CT=14, TT=6 vs CC=146, CT=168, TT=65; χ2=0.05, df=2, P>0.1). All analyses reported below were carried out on these 379 participants or on subsets of these participants.
Participant Characteristics
Participant characteristics, summarized by consumer status group, are shown in Table 2. Participants were aged between 18 and 62 years, 47% were male and 16.5% were smokers. Age varied among the groups, with L and M participants being somewhat younger. Smokers tended to be overrepresented in the higher caffeine consumption groups. The lowest recorded mean daily caffeine intake was 0 mg (43 participants), and the highest was 778 mg, with 55 participants consuming more than 300 mg per day. Lowest mean daily caffeine intake in group H was 203 mg.
Table 2. Participant Characteristics by Level of Habitual Caffeine Consumption.
| Participant characteristic |
Caffeine consumer status group |
Statistic | |||
|---|---|---|---|---|---|
| Nonconsumers (N) | Low (L) | Medium (M) | High (H) | ||
| n | 81 | 81 | 109 | 108 | |
| Age (years) | 34.1±13.3 | 29.9±11.2 | 32.5±12.4 | 35.4±13.1 | F(3, 375)=3.23, P=0.023 |
| Gender (male/female) | 35/46 | 39/42 | 49/60 | 57/51 | χ2=2.10, df=3, P>0.1 |
| Weight (kg) | 70.6±12.3 | 71.2±16.3 | 74.4±15.1 | 73.9±15.3 | F(3, 375)=1.47, P>0.1 |
| Nonsmokers/smokers | 73/7 | 67/13 | 87/22 | 86/20 | χ2=4.99, df=3, P>0.1 |
| Habitual caffeine intake (mg per day) | 1.3±1.7 | 19 ±10 | 128±46 | 346±129 | |
Data are means±SD, and n for gender and smoking (data for smoking were missing for 4 participants).
Systemic Caffeine Concentrations
Mean±SD baseline salivary caffeine concentrations for N, L, M, and H participants were 0.014±0.031, 0.024±0.040, 0.18±0.26, and 0.39±0.45 μg/ml, respectively. For participants who received caffeine, mean±SD salivary caffeine concentrations 90 min after the first dose of caffeine and 90 min after the second dose of caffeine were 1.64±0.68 and 2.86±1.21 μg/ml, respectively.
Consumer Status by Caffeine Effects
The analyses of the effects of caffeine as a function of level of habitual caffeine consumption (four consumer status groups) revealed no effects (P>0.05) involving dose/session for either anxiety, alertness, or headache, so for the results shown in Figure 1 mean values were calculated across post-treatment sessions using the formula (session 1+((session 2 +session 3)/2))/2. For all three variables there was a caffeine by consumer status interaction effect (anxiety, F(3, 362)=7.03, P=0.0001; alertness, F(3, 362)=6.06, P=0.0005; headache, F(3, 362)=9.52, P<0.0001). In addition, there was an effect of treatment for anxiety (F(1, 362)=12.37, P=0.0005) and alertness (F(1, 362)=17.94, P<0.0001), and there was a consumer status effect for alertness (F(3, 362)=6.30, P=0.0004). Because smoking tended to be associated with caffeine intake (Table 2), and smoking was not permitted on the test day, analyses were conducted to examine differences in anxiety, alertness, and headache between smokers and nonsmokers. Smokers did not report more headache or anxiety than nonsmokers, but they were less alert (data not shown). Therefore, the analysis of effects of consumer status and caffeine on alertness was repeated with smoking additionally included as a covariate. Controlling for smoking in this way had little effect on the outcome (caffeine by consumer status interaction F(3, 357)=6.31, P=0.0004).
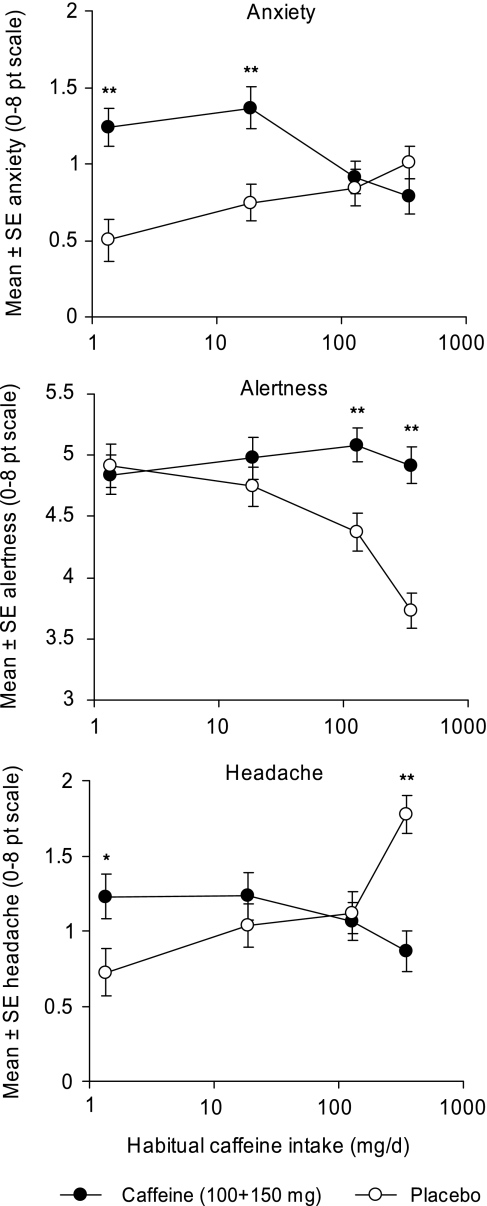
Figure 1.
Effects of caffeine on anxiety, alertness, and headache as a function of level of habitual caffeine consumption (corresponding to the non- (N), low (L), medium (M) and high (H) consumer groups described in Table 2). The data are for session 1 (after 100 mg caffeine) pooled with sessions 2 and 3 (after a second dose, 150 mg, of caffeine). Note that smoking was included as a covariate in the analysis of the data for alertness (see text for rationale). **P<0.01 and *P<0.05 for caffeine vs placebo within consumer group (Bonferroni t).
Figure 1 shows that caffeine increased anxiety in N and L participants. It did not affect anxiety in either M or H participants. In contrast, alertness declined with increasing level of habitual caffeine consumption in participants who received placebo, but not in those who received caffeine. Caffeine did not increase alertness in any group above that of the nonconsumers (N) who received placebo. Headache was increased in H participants who received placebo, and increased by caffeine in L participants.
At baseline there were consumer status effects for anxiety (F(3, 370)=2.66, P=0.048), alertness (F(3, 365)=5.74, P=0.001, smoking also included as a covariate), and headache (F(3, 370)=5.05, P=0.002). Higher habitual caffeine intake was associated with greater anxiety, lower alertness, and more headache at baseline. The placebo group in Figure 1 shows continuation and worsening of these effects.
Genotype Distributions and Genotype by Caffeine Effects
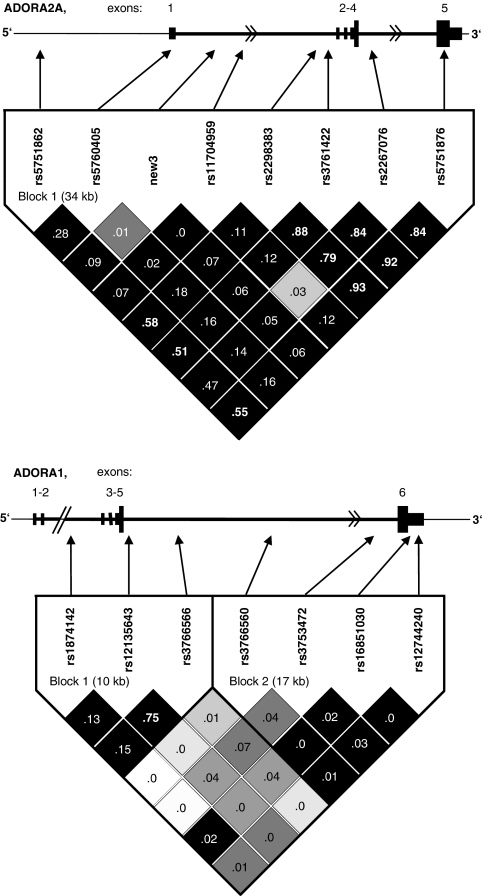
Genotype frequencies conformed to Hardy–Weinberg expectations for all eight ADORA2A SNPs (smallest P=0.075; Table 3) and for seven ADORA1 SNPs (smallest P=0.093; Supplementary Table S1). Genotype frequencies did not conform to Hardy–Weinberg expectations for ADORA1 rs9660662 and rs10920568 (P=0.029 and 0.023, respectively), therefore these SNPs were excluded from subsequent analyses. LD analysis revealed one block of high LD in ADORA2A spanning the eight SNPs, and two blocks of high LD in ADORA1, spanning three and four SNPs, respectively (Figure 2).
Table 3. Genotype Distributions of ADORA2A Polymorphisms and their Association with Caffeine-Induced Anxiety.
| Polymorphism | Genotype | Anxiety at session 1, genotype by treatment effecta | ||
|---|---|---|---|---|
| rs5751862 | 105 (GG) | 184 (AG) | 90 (AA) | F(2, 365)=1.10, P>0.1 |
| rs5760405 | 234 (CC) | 133 (CT) | 12 (TT) | F(2, 365)<1 |
| rs new3 | 310 (GG) | 68 (AG) | 1 (AA)b | F(1, 368)=1.12, P>0.1 |
| rs11704959 | 328 (CC) | 46 (AC) | 5 (AA)b | F(1, 364)=3.24, P=0.073 |
| rs2298383 | 140 (TT) | 167 (CT) | 72 (CC) | F(2, 365)=4.08, P=0.018 |
| rs3761422 | 150 (CC) | 170 (CT) | 59 (TT) | F(2, 365)=5.58, P=0.004 |
| rs2267076 | 156 (CC) | 176 (CT) | 47 (TT) | F(2, 365)=1.04, P>0.1 |
| rs5751876 | 146 (CC) | 168 (CT) | 65 (TT) | F(2, 365)=6.57, P=0.002 |
After 100 mg caffeine. Pretreatment (baseline) anxiety included as covariate. α=0.00625 after correction for multiple tests.
These rare genotypes were excluded from the analyses of the effects of genotype and treatment.
Figure 2.
Genomic organization and linkage disequilibrium (LD) structure of the ADORA2A gene (NM_000675), and the ADORA1 (NM_000674) gene. SNP positions relative to the 5′ promoter region, exons (numbered consecutively; ADORA2A coding exons are 4 and 5, and ADORA1 coding exons are 5 and 6) and introns are shown by arrows. Shades of gray represent extent of LD (black denotes D′=1) and numbers in boxes give R2 values (>0.5 denotes high LD, >0.9 denotes nearly complete LD in bold).
None of the ADORA2A or ADORA1 SNPs was significantly associated with baseline anxiety, headache, or alertness (largest F ratios were for anxiety and ADORA2A rs3761422, F(2, 372)=2.84, P=0.060; and for anxiety and ADORA2A rs5751876, F(2, 372)=2.63, P=0.073; uncorrected for multiple tests).
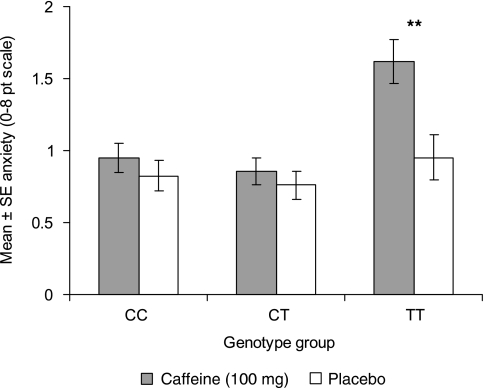
For the ADORA2A rs5751876 SNP, which has previously been found to be associated with caffeine-induced anxiety (see Introduction), there was an effect of caffeine (F(1, 366)=8.97, P=0.003), an effect of genotype (F(2, 366)=7.12, P=0.0009), and a nonsignificant caffeine by genotype interaction (F(2, 366)=2.72, P=0.067) for anxiety in session 1 (after 100 mg caffeine). Figure 3 shows that caffeine increased anxiety in the TT genotype group but not in the CC or CT genotype groups. When baseline anxiety was included as a covariate in the analysis, the caffeine by genotype interaction was significant (Table 3). Similar results were found for rs3761422 (Table 3), with the TT genotype group showing the largest increase in anxiety after caffeine (mean±SE for caffeine=1.65±0.15 and for placebo=0.95±0.17, P<0.01). The LD analysis showed rs5751876 and rs3761422 to be in strong LD (D′=1, R2=0.92; Figure 2, top panel).
Figure 3.
Effect of 100 mg caffeine (session 1 data, see text) on anxiety as a function of ADORA2A rs5751876 genotype group. **P<0.01 for caffeine vs placebo within TT genotype group (Bonferroni t).
The effects involving genotype described above remained significant when the analysis was confined to participants who were of European descent (n=361).
None of the ADORA1 SNPs was associated with caffeine-induced anxiety (P>0.1; data not shown), and none of the ADORA1 or ADORA2A SNPs was associated with the effects of caffeine on alertness or headache (P>0.05; data not shown).
Consumer Status by ADORA2A Genotype by Caffeine Effects: Anxiety
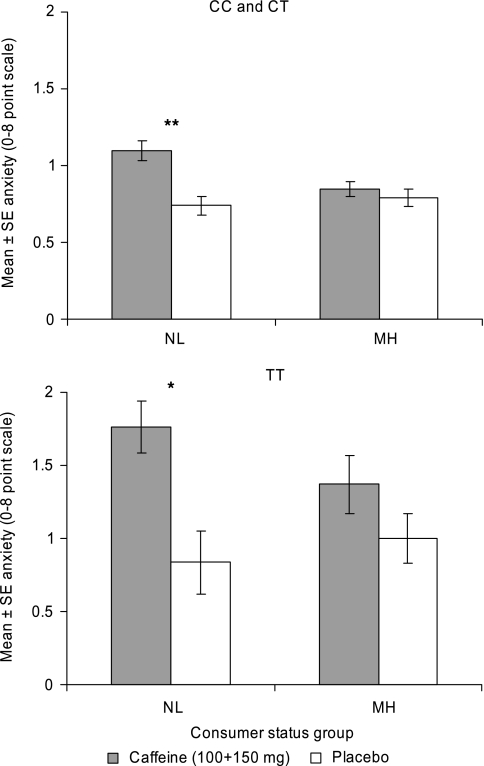
Given that both consumer status and ADORA2A genotype were found to modify the anxiogenic response to caffeine, these factors were included together in a further analysis of the effects of caffeine on anxiety. This consumer status (NL vs MH) by ADORA2A rs5751876 genotype (CC and CT vs TT) by caffeine by dose/session analysis (baseline anxiety included as a covariate) revealed an effect of caffeine (F(1, 361)=28.36, P<0.0001) and a caffeine by consumer status interaction (F(1, 361)=7.00, P=0.009). The effect of genotype (F(1, 361)=6.44, P=0.012) and the caffeine by genotype interaction (F(1, 361)=7.16, P=0.008) were marginally insignificant after correction for multiple testing (α=0.00625). The consumer status by genotype by caffeine interaction was not significant (F<1). Because of the unequal variances in the combined CC and CT genotype group vs the TT genotype group, planned comparisons investigating the above effects were conducted following ANOVA performed separately for these two groups. These analyses showed that caffeine increased anxiety in NL participants in both TT and combined CC and CT genotype groups (Figure 4). Although caffeine also somewhat increased anxiety in MH participants possessing the TT genotype, this effect was not significant (P>0.1) in this relatively small sample (Figure 4, lower panel).
Figure 4.
Effect of caffeine on anxiety as a function of habitual level of caffeine consumption and ADORA2A rs5751876 genotype group (with baseline anxiety included as a covariate). The data are for session 1 (after 100 mg caffeine) pooled with sessions 2 and 3 (after a second dose, 150 mg, of caffeine). NL, non- and low consumers; MH, medium and high consumers. Top panel: CC and CT genotype groups combined. Bottom panel: TT genotype group. **P<0.01 and *P<0.05 for caffeine vs placebo within consumer and genotype group (Bonferroni t).
Effects involving the above variables (caffeine, consumer status, and genotype) and dose/session were not significant (P>0.1) after controlling for multiple testing (Figure 4).
Results were similar for the ADORA2A rs3761422 SNP (data not shown).
ADORA2A Genotype and Habitual Caffeine Consumption
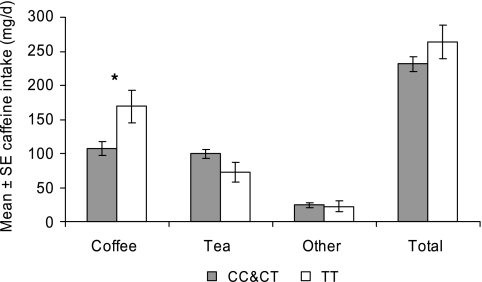
ADORA2A rs5751876 genotype distribution did not differ between the NL (CC=62, CT=70, TT=30) and MH (CC=84, CT=98, TT=35) groups (χ2=0.39, df=2, P=0.82; for CC and CT combined vs TT, χ2=0.37, df=1, P=0.54). Genotype distribution in the H group was CC=43, CT=46, TT=19. In the participants who habitually consumed at least moderate amounts of caffeine (ie, the MH group), caffeine intake from coffee was higher in the TT genotype group compared with the combined CC and CT group (F(1, 212)=5.91, P=0.016; adjusting for multiple comparisons α=0.017), but tea intake was somewhat, though not significantly, lower in the TT group (F(1, 212)=2.89, P=0.09) (Figure 5). As a result, total caffeine intake did not differ between genotype groups (P>0.1; Figure 5). Again, results were similar for rs3761422, including higher habitual coffee consumption in the TT genotype group (mean±SE for CC and CT combined=106±10 mg per day and for TT=181±24 mg per day; F(1,212)=8.03, P=0.005; adjusting for multiple comparisons α=0.017).
Figure 5.
Habitual caffeine consumption as a function of ADORA2A rs5751876 genotype group. ‘Other' sources of caffeine were cola, energy drinks, and chocolate. *P<0.05 (adjusted for multiple tests).
DISCUSSION
The present results confirm that the ADORA2A rs5751876 SNP is associated with variation in the anxiogenic response to caffeine (Alsene et al, 2003; Childs et al, 2008). Furthermore, they show that this differential response of the TT vs CC and CT genotypes is apparent at a fairly modest dose of caffeine (100 mg—approximately the amount of caffeine in a cup of ground coffee). The effect was observed after 150 mg, but not after 50 and 450 mg, by Alsene et al (2003) and Childs et al (2008). Similar results were found for the nearly completely linked SNP rs3761422, and less certainly so for rs2298383 (not significant after adjustment for multiple testing; Table 3). In the present population rs2298383 was completely linked with rs4822492 (D′=1, R2=0.995) and the latter was therefore dropped from the analysis. These results are in partial agreement with Childs et al (2008), who found rs5751876, rs2298383, and rs4822492, but not rs3761422 to predict caffeine-induced anxiety. SNPs rs2298383 and rs3761422 are potentially functional variants reflected by rs5751876, which is coding but does not cause an amino-acid exchange and therefore is unlikely to represent the causal variant. Both rs2298383 and rs3761422 are located in potential promoter regions upstream of several newly identified variants of ADORA2A exon 1 (Yu et al, 2004) near the corresponding transcription start sites. SNPs located within or immediately around these sites have been highlighted as having high potential to alter gene function (Veyrieras et al, 2008).
In contrast to ADORA2A SNPs, no association of caffeine-induced anxiety was found with ADORA1 SNPs. This is consistent with and extends the findings of Alsene et al (2003) who investigated 716T>G (rs10920568), a synonymous SNP located within the first coding exon. This study included further SNPs of high potential to regulate gene function, however none of them moderated caffeine-induced anxiety.
Another key finding of this study is that a clear anxiogenic effect of caffeine, larger in individuals with the ADORA2A rs5751876 TT genotype, was observed only for people who habitually consumed little or no caffeine (the N and L groups). Higher caffeine consumption appears to lead to substantial tolerance to this effect. Previously, Evans and Griffiths (1992) found that caffeine consumers chronically withdrawn from caffeine responded to a caffeine challenge with increased anxiety, whereas caffeine consumers maintained on 900 mg of caffeine daily did not. What is striking about the present results is that substantial tolerance to caffeine-induced anxiety appears to occur at much lower daily caffeine intakes than this. Indeed, caffeine (100+150 mg) failed to increase anxiety even in the medium (M) consumer group (Figure 1, top panel), whose habitual caffeine consumption averaged just 128 mg per day of caffeine, which is equivalent to, for example, a little more than one cup of ground coffee or three cups of tea a day. It is worth noting that 90% of the population from which many of the present participants were recruited (Heatherley et al, 2006a) had habitual caffeine intakes within the range represented by the MH group (40–778 mg per day), and only 10% had caffeine intakes within the range represented by the NL group (<40 mg per day).
Although frequent caffeine consumers experience minimal increased anxiety after caffeine consumption, they are at risk of at least two clear adverse effects of acute caffeine abstinence, namely low alertness and increased headache. These are the most commonly found symptoms of caffeine withdrawal reviewed by Juliano and Grifftihs (2004). In the present study both symptoms were evident after less than 24 h caffeine abstinence in the group with the highest level of caffeine consumption (H), whose average caffeine intake was 346 mg per day (equivalent to about three cups of ground coffee per day). The caffeine-withdrawn M group showed only decreased alertness. It is noteworthy that all the five MH participants who dropped out during the test day had been given placebo, and four of these were high consumers who complained of headache or related symptoms. If caffeine was consumed, the adverse effects of lowered alertness and headache were avoided, but even after 100+150 mg of caffeine their alertness was not raised above the level of alertness showed by nonconsumers of caffeine (group N) who received placebo (Figure 1, middle panel). This result is similar to that from an early study comparing responses to caffeine of coffee drinkers and abstainers (Goldstein et al, 1969), and is consistent with the claim, supported by a variety of subsequent findings, that regular caffeine consumption provides little or no net benefit for alertness or performance on tests of vigilance (James and Rogers, 2005; Sigmon et al, 2009). Another interpretation could be that frequent caffeine consumers are ‘constitutionally' less alert or more fatigued, and they use caffeine to remedy this state of affairs. This, though, does not readily explain why caffeine failed to increase alertness in individuals consuming little or no caffeine (ie, group N and L participants in Figure 1, middle panel).
Vasodilation leading to increased cerebral blood flow appears to be the cause of headache that occurs on withdrawal of caffeine in frequent caffeine consumers (Couturier et al, 1997). It may be that vasoconstriction and reduced cerebral blood flow was responsible for the increased headache observed in this study in the group N participants given caffeine (Figure 1, bottom panel).
Critical to the above conclusions concerning the alerting (and headache) effects of caffeine and caffeine withdrawal is the assignment of caffeine consumer status. In several previous studies, participants who were classified variously as ‘infrequent caffeine users', ‘light, nondependent caffeine users', and ‘nonconsumers', based on their self-reported intake of caffeine-containing drinks and foodstuffs, have been found to respond to caffeine vs placebo with increased alertness and improved cognitive performance (Alsene et al, 2003; Childs and de Wit, 2006; Haskell et al, 2005; Rogers et al, 2003). Although the studies differ in various ways, the discrepancy between these results and those of this study could be explained if these groups included at least some participants who nonetheless habitually consumed significant amounts of caffeine. Evidence in support of this comes from pretreatment salivary caffeine concentrations measured in two of the studies. The mean concentrations were 0.11 μg/ml (Childs and de Wit, 2006) and 0.36 μg/ml (Haskell et al, 2005). The corresponding results for the N and L groups in this study, after exclusion of 15 individuals with values >0.2 μg/ml (see above), were 0.014 and 0.024 μg/ml, indicating much lower dietary caffeine intakes in these participants. Indeed, the value reported by Haskell et al (2005) is twice of that of the present M group (0.18 μg/ml). It would seem, therefore, that data on caffeine intake can prove an unreliable guide to consumer status. Similarly, using salivary caffeine concentration to verify compliance with intake restrictions in frequent consumers is important for accurate determination of the effects of acute caffeine withdrawal. These effects will be underestimated if even a small minority of participants do not abstain as instructed.
As discussed above, individuals carrying the TT genotype of the ADORA2A SNP rs5751876 were more susceptible to caffeine-induced anxiety than individuals carrying the CC and CT genotypes. However, they were not, as hypothesized, less likely to be frequent caffeine consumers. Indeed, among frequent caffeine consumers (MH group), TT individuals' caffeine intake from coffee was greater than that of CC and CT individuals, and they had a slightly greater (not statistically significant) total caffeine intake (Figure 5). In other words, the anxiogenic effect of caffeine does not appear to deter individuals from becoming or being caffeine consumers. There are several possible reasons for this. First, although reliable, the increase in anxiety after a dose of caffeine equivalent to one cup of ground coffee is not large. In this study, after 100 mg of caffeine (vs placebo) anxiety measured on an eight-point scale (ranging from ‘not at all' to ‘extremely') increased by an average of 0.67 points in the TT genotype group and 0.11 points in the combined CC and CT genotype group (Figure 3). The statistically significant effects of caffeine (150 mg) on anxiety in TT individuals observed by Alsene et al (2003) and Childs et al (2008) were similarly modest in size. Second, lower doses of caffeine, which are more likely to correspond to early experiences of caffeine-containing drinks (eg, cola or weak milky coffee or, in the UK, tea), may have very little or no effect on anxiety even in individuals with the TT genotype (Childs et al, 2008). Third, the anxiety effect of caffeine might be outweighed by withdrawal reversal or an (unidentified) positive effect of caffeine. Fourth, anxiety-related feelings experienced after caffeine might be appraised positively (eg, as a ‘buzz' or ‘excitement'). This would be consistent with Thayer's (1989) conceptualization of mood and arousal that sees a modest level of ‘tense arousal', resulting from an external threat or challenge, or drug, as pleasant. Indeed, notwithstanding the second point above, this might explain why among the present sample of frequent caffeine consumers, individuals with the TT genotype consumed more caffeine from coffee than CC and CT individuals. If 100 mg is around the ‘threshold' dose for anxiety induction, then coffee is the only widely consumed caffeine-containing drink that would produce such an effect—albeit a very modest effect in frequent caffeine consumers. Caffeine avoidance, predicted by caffeine-induced anxiety, is readily observed only at higher acute doses (300 mg) (Evans and Griffiths, 1992; Stern et al, 1989).
A caveat to the above arguments is that ‘impression management' may have caused participants to be reluctant to report their feelings of anxiety. However, because caffeine was administered double blind, it is unlikely that this can account even for the differences between infrequent and frequent caffeine consumers' anxiety response to caffeine, rather it would have reduced the size of this effect (and the size of the effect of genotype).
Although the various considerations above help explain why the ADORA2A rs5751876 TT genotype was not associated with lower dietary caffeine intake, this is different from the result reported by Cornelis et al (2007). They found that individuals with the rs5751876 TT genotype were less likely to be heavier (>200 mg per day) caffeine consumers than CC and CT individuals. There are several potentially crucial differences between this and the current study. The Cornelis et al (2007) study population was a large sample (n=2735) of Hispanic Americans who were survivors of a first acute myocardial infarction between 1994 and 2004 and their case controls. The frequency of the rs5751876 TT genotype was much higher in this population (30.6%) than in the present sample (17.2%), and the proportion of current smokers was higher (36% vs 16.5% with those smoking more than five cigarettes or equivalent per day being excluded). The latter might be especially important, as Cornelis et al (2007) found the association between rs5751876 genotype and caffeine intake to be stronger in smokers. Conversely, in this study the small number of caffeine-consuming (MH group) smokers with the TT genotype, like the whole sample of caffeine-consuming TT individuals, consumed more caffeine from coffee than their CC and CT counterparts (287 vs 123 mg per day, P<0.05, after adjusting for multiple comparisons).
Another possible source of bias in this study is that anticipated anxiety or a related effect in susceptible individuals (ie, individuals with the rs5751876 TT genotype compared with individuals with the rs5751876 CC or CT genotype) might have deterred them from taking part, thus leading to an underrepresentation of these susceptible individuals in the sample. Again, though, there is evidence against this, as in both the infrequent (NL) and frequent (MH) caffeine consumer groups the TT genotype frequency did not differ from Hardy–Weinberg predictions (observed=30 and 35, expected=26 and 33, for NL and MH groups, respectively). Furthermore, very few (<2%) known infrequent caffeine consumers who were contacted but not successfully recruited to the study indicated concern about possible adverse effects of caffeine as a reason for not wanting to take part. The difference between this study and that of Cornelis et al (2007) in relation to association between caffeine intake and ADORA2A rs5751876 genotype, therefore, remains unexplained.
In conclusion, the present results are consistent with the proposal that frequent caffeine consumption is maintained by avoidance of the negative effects of withdrawal (negative reinforcement), and they also show that caffeine consumption is little affected by the tendency of caffeine to increase anxiety, at least in part, because substantial tolerance develops to this effect even at modest levels of habitual intake and even in susceptible individuals.
Acknowledgments
This research was funded by a grant (BBS/B/01855) from the Biotechnology and Biological Sciences Research Council, UK.
The authors declare that over the past 3 years PJR has received consulting fees from Unilever; and grants for research from Barry Callebaut, Cadbury, DSM, GSK, and Unilever. DJN has received consulting fees from Pfizer, GSK, MSD, BMS, Esteve, Novartis, Asahi, Organon, Cypress, Lilly, Janssen, Takeda, Phamacia, Therasci, Passion for Life, Hythiam, Servier, Roche, sanofi-aventis, Actelion, Lundbeck, and Wyeth; speaker's fees from Reckitt-Benkiser and Cephalon; grants for research or clinical trial payments from MSD, GSK, Novartis, Servier, Janssen, Yamanouchi, Lundbeck, Pfizer, Wyeth, Organon, AZ, Cephalon, P1vital, MoD, and NHS; and he has worked for the UK Government's Committee on Safety of Medicines, the Advisory Council on the Misuse of Drugs, and the British National Formulary. JD has received speaker's fees for educational seminars from AstraZeneca, BMS, GSK, Janssen-Cilag, Pfizer, and Wyeth. The other authors declare no conflicts of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Subjective, behavioural, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology. 2006;185:514–523. doi: 10.1007/s00213-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–2800. doi: 10.1038/npp.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–244. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- Couturier EGM, Laman DM, van Duijn MAJ, van Duijn H. Influence of caffeine and caffeine withdrawal on headache and cerebral blood flow velocities. Cephalalgia. 1997;17:188–190. doi: 10.1046/j.1468-2982.1997.1703188.x. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ferré S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Nothen MM, Franke P, Delmo C, Fritze J, Knapp M, et al. Systematic mutation screening and association study of the A1 and A2A adenosine receptor genes in panic disorder suggest a contribution of the A2A gene to the development of disease. Mol Psychiatry. 1998;3:81–85. doi: 10.1038/sj.mp.4000345. [DOI] [PubMed] [Google Scholar]
- Evans SM, Griffiths RR. Caffeine tolerance and choice in humans. Psychopharmacology. 1992;108:51–59. doi: 10.1007/BF02245285. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartan EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, Meyer J, et al. Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:67–74. doi: 10.1007/s00787-009-0043-6. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Kaizer S, Whitby O. Psychotropic effects of caffeine in man. IV. Quantitative and qualitative differences associated with habituation to coffee. Clin Pharmacol Ther. 1969;10:489–497. doi: 10.1002/cpt1969104489. [DOI] [PubMed] [Google Scholar]
- Hartley R, Smith IJ, Cookman JR. Improved high-performance liquid-chromatographic method for the simultaneous determination of caffeine and its n-demethylated metabolites in plasma using solid-phase extraction. J Chromatogr. 1985;342:105–117. doi: 10.1016/s0378-4347(00)84493-x. [DOI] [PubMed] [Google Scholar]
- Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology. 2005;179:813–825. doi: 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- Heatherley SV, Mullings EL, Tidbury MA, Rogers PJ. Caffeine consumption among a sample of UK adults. Appetite. 2006a;47:266. [Google Scholar]
- Heatherley SV, Mullings EL, Tidbury MA, Rogers PJ. The Dietary Caffeine and Health Study: administration of a large postal survey in Bristol. Appetite. 2006b;47:266. [Google Scholar]
- Howell DC. Statistical Methods for Psychology. Duxbury Press: Belmont, CA; 1997. [Google Scholar]
- James JE. Critical review of dietary caffeine and blood pressure: a relationship that should be taken more seriously. Psychosom Med. 2004;66:63–71. doi: 10.1097/10.psy.0000107884.78247.f9. [DOI] [PubMed] [Google Scholar]
- James JE, Rogers PJ. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacology. 2005;182:1–8. doi: 10.1007/s00213-005-0084-6. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Grifftihs RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity and associated features. Psychopharmacology. 2004;126:1–29. doi: 10.1007/s00213-004-2000-x. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2003;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Heatherley SV, Hayward RC, Seer HC, Hill J, Kane M. Effects of caffeine and caffeine withdrawal on mood and cognitive performance degraded by sleep restriction. Psychopharmacology. 2005;179:742–752. doi: 10.1007/s00213-004-2097-y. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Martin J, Smith C, Heatherley SV, Smit HJ. Absence of reinforcing, mood and psychomotor performance effects of caffeine in habitual non-consumers of caffeine. Psychopharmacology. 2003;167:54–62. doi: 10.1007/s00213-002-1360-3. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Smith JE, Heatherley SV, Pleydell-Pearce CW. Time for tea: mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology. 2008;195:569–577. doi: 10.1007/s00213-007-0938-1. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Herning RI, Better W, Cadet JL, Griffiths RR. Caffeine withdrawal, acute effects, tolerance, and absence of net beneficial effects of chronic administration: cerebral blood flow velocity, quantitative EEG, and subjective effects. Psychopharmacology. 2009;204:573–585. doi: 10.1007/s00213-009-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern KN, Chait LD, Johanson CE. Reinforcing and subjective effects of caffeine in normal human subjects. Psychopharmacology. 1989;98:81–88. doi: 10.1007/BF00442010. [DOI] [PubMed] [Google Scholar]
- Thayer RE. The Biopsychology of Mood and Arousal. Oxford University Press: New York; 1989. [Google Scholar]
- van Calker D, Biber K. The role of glial adenosine receptors in neural resilience and the neurobiology of mood disorders. Neurochem Res. 2005;30:1205–1217. doi: 10.1007/s11064-005-8792-1. [DOI] [PubMed] [Google Scholar]
- Veyrieras J-B, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, Stephens M, et al. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;5:e1000214. doi: 10.1371/journal.pgen.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Frith MC, Suzuki Y, Peterfreund RA, Gearan T, Sugano S, et al. Characterization of genomic organization of the adenosine A2A receptor gene by molecular and bioinformatics analyses. Brain Res. 2004;1000:156–173. doi: 10.1016/j.brainres.2003.11.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.