Abstract
Vago-vagal reflex circuits modulate digestive functions from the oral cavity to the transverse colon. Previous articles in this series have described events at the level of the sensory receptors encoding the peripheral stimuli, the transmission of information in the afferent vagus, and the conversion of this data within the dorsal vagal complex (DVC) to impulses in the preganglionic efferents. The control by vagal efferents of the postganglionic neurons impinging on the glands and smooth muscles of the target organs has also been illustrated. Here we focus on some of the mechanisms by which these apparently static reflex circuits can be made quite plastic as a consequence of the action of modulatory inputs from other central nervous system sources. A large body of evidence has shown that the neuronal elements that constitute these brain stem circuits have nonuniform properties and function differently according to status of their target organs and the level of activity in critical modulatory inputs. We propose that DVC circuits undergo a certain amount of short-term plasticity that allows the brain stem neuronal elements to act in harmony with neural systems that control behavioral and physiological homeostasis.
Keywords: dorsal motor nucleus of the vagus, nucleus of the solitary tract, brain stem, gastrointestinal motility
Vago-Vagal reflexes consist essentially of three components: sensory vagal afferents, second-order integrative neurons of the nucleus of the solitary tract (NTS), and efferent vagal neurons of the dorsal motor nucleus of the vagus (DMV). The sensory limb is comprised of chemo- and mechanosensory elements linked to vagal afferent nerves (reviewed in Refs. 27 and 37). Data received by these sensory elements are funneled mainly via glutamatergic synapses into the brain stem and converge on the NTS (13, 27, 32).
The rat NTS is organized such that sensory information from the gastrointestinal (GI) tract is received and processed in distinct, viscerotopically organized subnuclei. Many of the gastric vagal afferents synapse at the level of the medial subnucleus of the solitary tract and in the subnucleus gelatinosus. Intestinal afferent vagal fibers terminate primarily in the subnucleus commissuralis (2), and esophageal sensory fibers terminate mainly in the subnucleus centralis (cNTS) (2, 14, 26). The NTS, then, sends projections to the efferent vagal neurons in the DMV. The NTS also sends a copy of this immense volume of visceral afferent activity to integrative structures in the pons and the diencephalon.
The viscerotopic organization of the DMV is less rigid than that of the NTS. In fact, the DMV is organized in a mediolateral columnar manner such that neurons in the medial tips of the nucleus project to the stomach and neurons in the lateral tips project to the intestine. Most of the projections from the NTS to the DMV appear to be inhibitory. There are numerous phenotypes of neurons in the NTS that can contribute input to the DMV, although indirect evidence suggests that it consists mainly of a GABAergic input (20, 31, 36) and glutamatergic afferents (5, 13, 31, 36).
Vagally mediated GI motor functions are controlled by two separate populations of DMV preganglionic motoneurons. The cholinergic-muscarinic pathway induces an increase of GI functions such as motility and acid output. Even at “rest,” this subpopulation provides a significant contractile and secretory “tone.” The second, nonadrenergic, noncholinergic (NANC) pathway causes a decrease in gastric motility when activated, mainly via release of nitric oxide onto gastric smooth muscle (1, 3, 11, 33).
There are, therefore, two parallel vagally mediated mechanisms through which changes in the gastric function may be effected: a vagal cholinergic excitatory pathway that, when active, stimulates gastric functions and an inhibitory NANC path that, when active, suppresses gastric functions. These vagal effector mechanisms are under reflex control from the NTS. In most (but not all) cases, gastric control reflexes act to inhibit gastric function. Distension of the esophagus, antrum, and duodenum powerfully inhibit the stomach, as does the presence of acid in the duodenum. Although an oversimplification, it is generally believed that these inhibitory vago-vagal reflexes involve the parallel inhibition of cholinergic (excitatory) vagal efferent pathways and the activation of the NANC (inhibitory vagal) pathway.
From this introduction, one could infer that these brain stem autonomic reflexes that control the stomach are relatively static and perform in much the same way in all conditions. However, new evidence suggests that these reflexes are under modulatory influences that are powerful enough to dramatically alter and possibly even invert their function. This review will outline what is known about the plastic nature of these reflexes and discuss some of the physiological mechanisms thought to be responsible for this plasticity.
CENTRAL NERVOUS SYSTEM MODULATION OF BASIC VAGO-VAGAL GASTRIC CONTROL REFLEXES
Thyrotropin-releasing hormone (TRH) is a neuropeptide released onto vago-vagal reflex circuit elements (NTS/DMV) during periods of cold stress and in anticipation of feeding (cephalic phase). The result of TRH action is a large and sustained increase in gastric motility and acid secretion that 1) augments heat production in response to cold stress and 2) augments gastric functions in anticipation of the arrival of a meal. Activation of TRH receptors on DMV neurons leads to the depolarization of vagal neurons that activate the stomach. These DMV neurons are also under the powerful influence of inhibitory NTS neurons that form the afferent limb of a number of vago-vagal gastric control reflexes (Fig. 1).
Fig. 1.
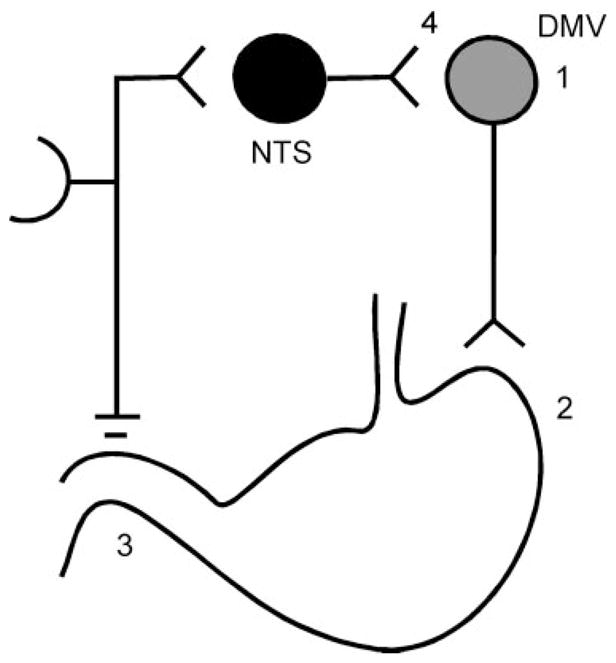
Differential effects of thyrotropin-releasing hormone (TRH) in the nucleus of the solitary tract (NTS). Plasticity in the NTS responses to TRH allows TRH to “command” large changes to gastric functions unopposed by vago-vagal reflexes. DMV, dorsal motor nucleus of the vagus. 1, TRH activates DMV cholinergic neurons; 2, activated cholinergic pathway increases contractility and acid secretion; 3, increased contractility increases wall tension, chyme contact with antral mechanoreceptors and duodenal chemo- and mechanosensors. No modulation of NTS by TRH: TRH “command” to the DMV to increase gastric motility and secretion is blocked by reflex action (4 = activated vagal afferents activate GABAergic NTS neurons that inhibit DMV neurons, thus blocking the effects of TRH). Modulation of NTS by TRH: TRH command to the DMV to produce large and sustained increases in gastric motility and secretion (4 = the consequence of vagal afferent activation by TRH action on the stomach is blocked by TRH action at the NTS. The TRH-mediated inhibition of the reflex allows TRH to be far more effective).
These inhibitory reflexes are activated by the very events caused by TRH action on the DMV: increases in gastric wall stress, chyme contact with the antral wall, and chyme and acid accumulation in the duodenal bulb secondary to the TRH-induced increase in motility and secretion. Clearly then, normal vago-vagal reflex mechanisms would blunt, if not completely antagonize, the effect of TRH on DMV neurons to augment digestive functions.
However, TRH has an additional effect on NTS neurons that completely changes the way vago-vagal reflexes function. TRH “gates out” the potential inhibitory effects of reflex activation by altering the state of NTS intracellular transduction mechanisms (Fig. 1). Here, TRH receptor activation increases cAMP/PKA to levels that allow normally ineffectual, tonic, inhibitory serotonergic inputs to the NTS to have a physiological effect (Fig. 2). The result is that TRH now causes the continuous activation of vagal cholinergic inputs to the stomach while filtering out the reflex inhibition that would normally develop as a consequence of this TRH action on vagal efferents (37). Evidence now suggests that TRH, and other factors that modulate cAMP/PKA levels in vago-vagal reflex circuits, can also have dramatic effects on how these circuits respond to opiates, neuropeptide Y (NPY), and even natural afferent input.
Fig. 2.
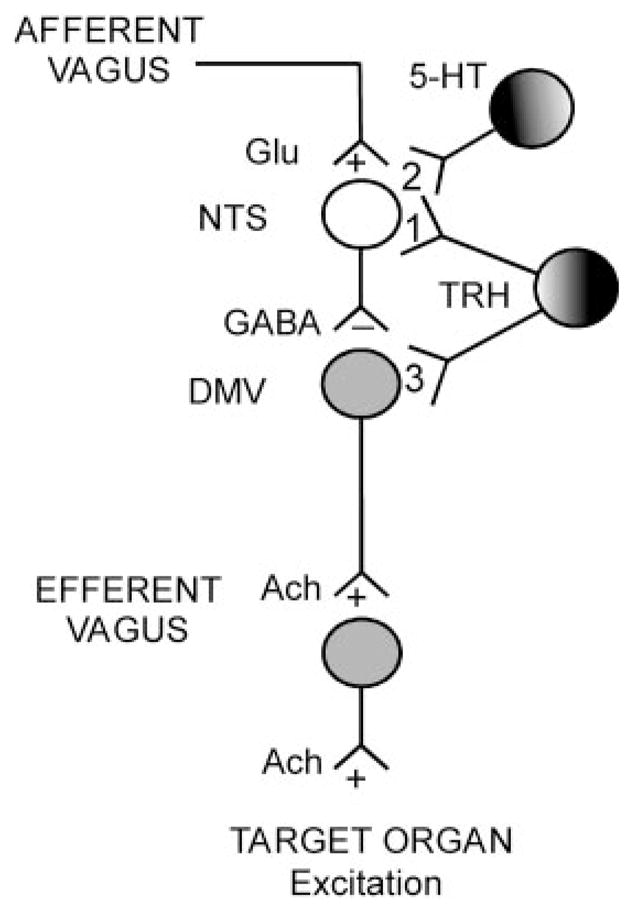
Schematic representation of an example of dorsal vagal complex (DVC) plasticity. TRH modulation of gastric vago-vagal reflexes depends on its increase of the cAMP/PKA pathway. Elevation of the cAMP intracellular levels uncovers the function of previously silent inhibitory synapses. The activation of the cAMP/PKA pathway is probably a common mechanism responsible for the plasticity of vago-vagal reflexes in a variety of different circumstances. Ach, acetylcholine; Glu, glutamate; 1, TRH increases cAMP/PK activity; 2, Local 5-HT circuit with partial tonic activity. Fully active when NTS transduction mechanisms are modulated. An increase in cAMP levels permits the inhibitory 5-HT1A receptors to operate by opening a potassium conductance resulting in inhibition of NTS neurons; 3, TRH activates DMV neurons via closure of potassium conductances.
THE RECEPTIVE RELAXATION REFLEX FROM THE BRAIN STEM PERSPECTIVE
There are a wide variety of vago-vagal reflexes activated by mechano- and chemotransmission from the GI tract; it is not the purpose of this article to review them all, and we refer the reader to the several manuscripts that have dealt with them in recent years. Rather we will discuss, in more detail, one of these reflexes that has drawn the attention of both of our laboratories in the recent past.
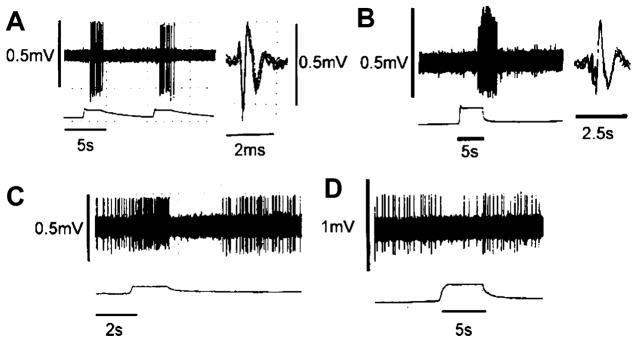
It has long been known that the esophageal distension produced by swallowing elicits a powerful proximal gastric relaxation. This reflex was termed the “receptive relaxation reflex” and is an important mechanism that permits increases in gastric volume and reduction of intragastric pressure to ensure that swallowed food is transported efficiently to the stomach. Several laboratories have shown that this potent gastroinhibition results from stimulation of low-threshold mechanoreceptive afferents of the vagus nerve and that the reflex requires intact vagal connections between the brain stem, esophagus, and stomach (22, 29). The cNTS sends a dense projection of axons to the compact formation of the nucleus ambiguus, the site containing vagal motor neurons projecting to the esophagus and lower esophageal sphincter (reviewed in Ref. 14). The relationship between the cNTS and the vagal motor neurons that regulate gastric functions has, however, been described only recently (26). Our anatomic and electrophysiological data provided evidence of an esophageal-cNTS-DMV-gastric pathway whose activation by mechanodistension of the esophagus induces a pronounced gastric relaxation. Gastro-inhibitory control by the esophagus involves neural pathways from esophageal distension-sensitive neurons in the cNTS with connections to virtually all levels of the DMV. Esophageal distension can elicit two different patterns of electrophysiological responses in both the cNTS and the DMV (Fig. 3). In cNTS, both responses are excitatory (consistent with a glutamatergic vagal afferent fiber input) but can be distinguished by their different patterns of firing. The majority of cNTS neurons are silent when unstimulated but are strongly activated by esophageal distension. Another group of cNTS neurons has resting activity that increases following esophageal distension; upon relief, however, these cells are completely inhibited for few seconds before recovering to prestimulus basal activity.
Fig. 3.
Electrophysiological responses in NTS and DMV following esophageal distension. A: representative trace from a subnucleus centralis (cNTS) neuron responding to repeated esophageal distension (0.4 ml). The majority of cNTS neurons did not have resting basal activity but, when stimulated by esophageal distension, responded with a robust increase in firing rate that returned to baseline upon cessation of the stimulus. B: another group of cNTS neurons had resting firing activity that increased following esophageal distension. Upon relief, these cells were completely inhibited for few seconds before recovering to prestimulus basal activity. C: representative trace from a DMV neuron responding to esophageal distension (0.4 ml). Typically, neurons located in the most caudal and rostral portions of the DMV responded to the stimulus with a delayed increase in firing rate. D: conversely, neurons located in the intermediate portion of the DMV responded to esophageal distension with a decrease in the spontaneous firing rate. Spike amplitudes are distorted by the slow sampling rate. Reprinted from Ref. 26 with permission.
In the DMV, esophageal distension affects two distinct populations of neurons. One population of DMV cells responds to esophageal distension with an increased firing rate, whereas the other subpopulation is inhibited. These and other observations suggest that NTS-DMV synaptic connections use both an excitatory (possibly glutamate) and inhibitory (possibly GABA) neurotransmitter. Thus following esophageal distension: 1) there is a marked increase in activity in cNTS, 2) there is both an increase and a decrease in DMV neuronal activity, and 3) the final result is gastric relaxation. One would thus be tempted to assume that the NTS-DMV inhibitory GABAergic synapses are present only on DMV neurons that comprise the excitatory cholinergic pathway. That is, activation of this pathway would induce gastric relaxation via withdrawal of cholinergic tone to the gastric smooth muscle. The NTS-DMV excitatory glutamatergic synapses would thus be present only on DMV neurons that are part of the NANC pathway. That is, activation of this pathway would induce gastric relaxation via increased release of nitric oxide onto the gastric smooth muscle. Indeed, such a clear-cut separation of pathways and functions seems to be supported by several studies reporting differences in morphological, immunocytochemical, pharmacological, and electrophysiological properties of NTS and DMV neurons (5, 6, 12, 15–17, 24, 35, 38, 41). The parallel operation of these pathways seems to explain much of what we know about the esophageal afferent control of the stomach. However, this reflex is subject to an additional level of modulation, not unlike the action of TRH, that is powerful enough to effectively invert the normal direction of this reflex. This reflex inversion can be observed in the fed vs. fasted state.
FASTED VS. FED: NPY, OPIOIDS, AND THE “STATE OF ACTIVATION”
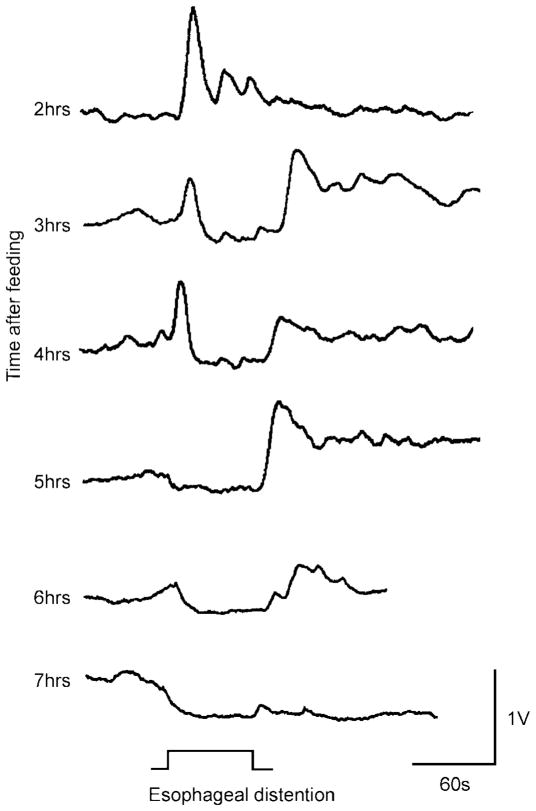
The experiments on the neurophysiology of the receptive relaxation reflex described above were conducted in fasted rats (26). Subsequent preliminary studies have shown that, if animals were allowed ad libitum access to food before anesthesia (i.e., the stomach still contained food), esophageal distension now evoked an increase in gastric contractility (Fig. 4). Over the course of the experiment this effect gradually reversed until, hours later (i.e., in the fasted state), the “classical” gastric relaxation effect followed esophageal distension. These observations are in agreement with previous data (15, 38) that shows gastric emptying to be faster during and just after feeding than compared with the fasted state. These results are interesting given that distension of the esophagus induced by swallowing a meal should cause gastric relaxation. Gastric relaxation reduces intragastric pressure, which reduces gastric transit. Therefore, if gastric transit is rapid as a consequence of feeding, then the normal (fasted) esophageal gastric reflex must be interrupted, or even inverted, by some aspect of the act of feeding to account for rapid gastric emptying during feeding.
Fig. 4.
Plasticity of the receptive relaxation reflex. Representative traces depicting the gastric response to esophageal distension in a fed rat are shown. Note that, 2 h after a meal, a 60-s-long esophageal distension induces an increase in gastric tone but no stomach relaxation. Conversely, the esophageal-induced gastric relaxation increases and the gastric tone decreases with time after meal ingestion. At 7 h after a meal, esophageal distension induces gastric relaxation only.
Several endogenous neurotransmitters are known to affect the vago-vagal brain stem circuitry and are implicated in altering vagal reflex function in association with feeding. Among these neurotransmitters, NPY and opioids are of particular interest due to their dramatic, but apparently fickle, effects on gastric motility.
Under fasted conditions but just before feeding; i.e., when gut functions are primed to increase the efficiency of the subsequent gastric and intestinal phases, a vagally mediated increase in gastric motility and secretion is observed following central NPY administration (10, 40). Other studies, however, have noted a decrease in GI function following NPY administration to the dorsal vagal complex (DVC) (10, 18). Further investigation revealed that the effects on gastric function of brain stem NPY administration may depend on the activity (or state of activation) of the GI tract at the time of application.
Under fasting (resting) conditions, application of NPY to the DVC causes an increase in gastric motility. If, however, gastric motility is stimulated by central TRH, NPY has no additional stimulatory effects; rather, NPY reduces gastric motility (10). A mechanistic correlate to these observations has been provided in recent electrophysiological studies conducted by our group (7, 34). NPY has no direct effects on DMV neurons or on NTS-induced inhibitory postsynaptic currents (IPSCs). However, if the NTS is stimulated, NPY now inhibits the IPSCs normally evoked in DMV neurons.
The functional result, i.e., the increase in gastric motility observed in vivo under basal (i.e., nonstimulated) conditions, could be explained if one assumes that electrical stimulation of the NTS evokes glutamate-mediated EPSCs on DMV neurons comprising the inhibitory NANC pathway only. In contrast, if the NTS is exposed to TRH (or the animal is in the fed state) a different response is elicited. Under these conditions, perfusion with NPY decreases the amplitude of the evoked EPSCs (as in basal conditions) and inhibits the previously unaffected IPSCs. At this point, then, the only scheme that can explain the reported functional result, i.e., the decreased gastric motility observed in vivo under stimulated conditions, is the one that assumes a contribution of GABA-mediated IPSCs on DMV neurons comprising the NANC pathway only.
A similar relationship (i.e., receptor uncovering or receptor plasticity) can be used to explain the effects of opioids in the DVC. In fasted animals, microinjection of opioids in the DVC induces a marginal gastric relaxation but, following activation of the circuits by TRH, the gastric relaxation following opioid microinjections was significantly larger. In fed animals, microinjection of opioids (which did not cause any gastric effects in fasted animals) induces a large gastric relaxation that was further enhanced by pretreatment with TRH (Rogers et al., unpublished data). Our patch-clamp experiments in identified gastric-projecting DMV neurons have shown that perfusion with opioids does not affect the amplitude of evoked IPSCs; indeed, GABAergic terminals did not express μ-opioid receptor-like immunoreactivity (MOR-IR) (5). Once the preparation is pretreated with TRH or with drugs that increase cAMP levels, however, GABAergic IPSCs are inhibited and MOR-IR is present on the NTS terminals apposing the identified DMV neurons (Browning KN, Kalyuzhny AE, and Travagli RA, unpublished results). Thus both NPY and opioids decrease the amplitude of evoked IPSCs under stimulated, compared with basal, conditions.
Thus the electrophysiological and functional (in vivo) data can be accounted for by assuming that opioid receptors are present on glutamatergic and GABAergic NTS terminals that form synapses on DMV neurons of both the cholinergic and the NANC pathways (Ref. 5 and Browning KN, Rogers EC, Kalyuzhny AE, and Travagli RA, unpublished results). The availability and function of the single synapse would be determined by the state of activation of the circuitry. Specifically under fasted, basal conditions, opioid receptors would be expressed in and be available for interaction with their ligand in a small percentage of DMV neurons (specifically on glutamatergic synapses impinging on DMV neurons of only the cholinergic pathway). Activation of the circuitry (e.g., by feeding-related neurotransmitters like TRH) causes the expression or increases the functionality of opioid receptors in GABAergic terminals apposing the same DMV neurons. Thus in fed animals, opioid receptors would be expressed on glutamatergic synapses and on DMV neurons forming the cholinergic pathway. Activation of the circuitry in fed animals would express opioid receptors on GABA terminals impinging on DMV neurons that comprise the NANC pathway.
The organization of vagal circuits in the DVC schematically depicted in Fig. 5 can be used to explain the gastric responses to esophageal distension observed after NPY or opiates. As can be seen in this (oversimplified) scheme, four separate sets of GABAergic and glutamatergic NTS neurons project to the DMV neurons, forming both the cholinergic excitatory and the NANC inhibitory motor pathways. Even though the same neurotransmitters are used, activation of NTS neurons would have opposite effects on GI functions, i.e., excitation or inhibition. The short-term plasticity that determines receptor availability at the level of the DMV-NTS synaptic connection allows the brain stem circuits to adapt their responses to the state of activation of the animal. This hypothesis is quite appealing because it suggests the existence of a rather economic, though efficient, method of controlling basic reflex functions.
Fig. 5.
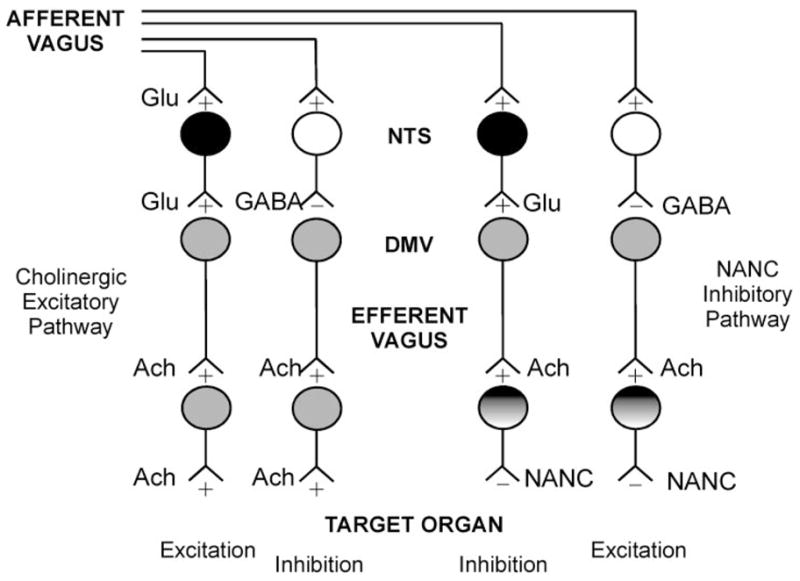
Schematic and oversimplified representation of brain stem vago-vagal circuits. Sensory information from the gastrointestinal (GI) tract is carried to the brain stem by afferent vagal fibers. The main neurotransmitter from the afferent vagus to the sub-nuclei of the NTS is Glu. NTS neurons use, in the main, Glu or GABA as neurotransmitters to convey modulated sensory information to the DMV. The mainly cholinergic DMV neurons transmit parasympathetic motor innervation to the GI tract via 2 pathways: a cholinergic excitatory pathway and a noncholinergic, nonadredergic (NANC) inhibitory pathway. Increased activity in NTS neurons can cause either excitation or inhibition of the GI functions. Excitation (i.e., increased motility and/or secretion) can be achieved via activation of the cholinergic excitatory pathway or via inhibition of the NANC pathway. Conversely, inhibition of GI functions can be obtained by withdrawal of cholinergic excitatory tone or by activation of the inhibitory NANC pathway. The location and the unmasking capabilities of receptors for neurotransmitters along this brain stem pathway would thus allow the same neurotransmitter to have both excitatory and inhibitory effects.
PERSPECTIVE: MODULATION OF NTS TRANSDUCTION MECHANISMS EQUALS VAGO-VAGAL REFLEX PLASTICITY
The scheme represented in Fig. 5 is an oversimplification of the organization of brain stem vagal circuits. Furthermore, we have only scant detail as to the number of other ways central nervous system (CNS) input to DVC circuitry can alter circuit function and responsiveness. Indeed, many neuromodulators are known to be present in, and released by, the NTS neurons onto DMV cells; these are probably released differentially under a vast array of conditions. Additionally, the NTS is outside the blood-brain diffusion barrier and therefore is accessible to a multitude of circulating hormones, cytokines, and chemokines, which can themselves dramatically alter vago-vagal reflex responsiveness (37). Therefore, the number of mechanisms by which vago-vagal reflex selectivity and sensitivity can be modulated is theoretically limitless.
Studies of vago-vagal reflex circuitry and neural circuitry elsewhere in the brain, however, show that one particular mechanism may be common to a variety of circuit elements and agonists. Modulation of synaptic transmission by variations in the cAMP levels has been observed in multiple neural networks, including the hippocampus, nodose ganglia, cerebellar granule cells, enteric plexus, and sympathetic ganglia. In cerebellar granule cells, it has been shown that activation of the cAMP/PKA pathway causes an increase in presynaptic vesicular turnover so that previously “silent” synapses show spontaneous transmitter release (9). Similarly, in other CNS areas, increases in the cAMP levels induce a pronounced augmentation of the exocytotic activity in synaptic terminals with low baseline vesicular activity (9, 28). These factors suggest that cAMP levels may increase the response of synapses to numerous neuromodulatory substances. Such variations in the activation levels of the adenylate cyclase/PKA pathway occur during opioid withdrawal and underlie the different electrophysiological responses to opioid agonists (reviewed in Ref. 39). Thus changes in the intracellular levels of cAMP are likely to cause altered sensitivity of neural circuits to a variety of inputs.
Our recent paper (8) provided a mechanistic explanation for the dramatic synergistic effects of brain stem TRH and serotonin (5-HT) on gastric function (19). Electrophysiological studies revealed that TRH permits 5-HT1A receptors to function on NTS GABAergic terminals (8). This unmasking activity is triggered by the elevation of the activity of the cAMP/PKA pathway induced by TRH. The increased activation of this second-messenger pathway “primes” the GABAergic synapses and allows their modulation by 5-HT, with the consequent increase in parasympathetic excitatory output to the stomach (8, 37). The widespread use by different neurotransmitters of the cAMP/PKA pathway would, then, argue in favor of its use in a more general manner (i.e., not only restricted to TRH and 5-HT) in the control of vago-vagal circuits.
Both opioids and NPY have receptors that are coupled to the pertussis toxin-sensitive G proteins, Gi/Go. Receptor activation can potentially affect several distinct pathways. The most commonly reported action has been the inhibition of adenylate cyclase with a consequent reduction in cAMP levels and decrease in neurotransmitter release (reviewed in Refs. 21 and 39).
If variations in the levels of cAMP in the synaptic terminals of brain stem circuits affect the sensitivity of the synapses to modulation by neurotransmitters, and if variations of the cAMP levels are determined by the different state of activation induced by the change from fasted to fed status (and vice versa), then the effects of NPY and opioids, for example, can vary according to the cAMP levels within the synapse itself. This would imply, then, that the contrasting effects observed upon DVC administration of NPY or opioids (and, for our purposes, by esophageal distension) might be the result of variations in the cAMP levels at GABAergic NTS-DMV synapses.
Specifically, the lack of effect of these neuromodulators on inhibitory GABAergic transmission under basal conditions may be due to low intracellular levels of cAMP. Under these conditions, activation of Gi/Go-coupled receptors (such as μ-opioid or NPY receptors) evokes no discernible effects. Following an increase in cAMP levels, however, the activation of these Gi/Go-coupled receptors can now cause a perceptible decrease in cAMP levels, with a consequent decrease in GABA-ergic synaptic transmission. Depending on whether the affected GABAergic NTS-DMV synapse controls cholinergic excitatory pathways or NANC inhibitory pathways, a decrease in synaptic transmission would result in an increased or decreased vagal motor output, respectively.
The physiological correlate of these experimental conditions would be modulation of the vagal complex by neurotransmitters (e.g., TRH) that are positively coupled to adenylate cyclase and generate cAMP. Alterations in sensory inputs from the GI tract, such as following activation of vago-vagal reflexes or changes in the feeding status, for example, would be expected to modify the ability of tonic GABAergic inputs controlling the vagal brain stem circuits to be modulated. It is possible that the constant perception of ongoing GI tract activity exerts a tonic inhibition of cAMP/PKA pathways in the DVC. Activation of vago-vagal reflexes or changes in the state of activation (from fasted to fed or vice versa) would change the levels of cAMP and influence the ability of circulating hormones or locally released neurotransmitters to modulate inhibitory synaptic transmission between the NTS and DMV. Changes in an intracellular second-messenger system would thus provide an efficient means by which the receptors can be made available for interaction with their receptor ligands. One possible mechanism is stimulus-dependent insertion of proteins and receptors in the plasma membrane, which has been shown to occur in different systems, including the CNS (4, 23, 25, 30). For example, Shuster and colleagues (30) showed that κ-opioid receptors are localized on internal membranes on the nerve terminals of vasopressin cell bodies in basal conditions, but the receptors are quickly (minutes) and transiently (1 h) translocated to plasma membranes once a stimulus (specifically, salt loading) is applied. We propose that activation of the cAMP/PKA pathway stimulates the transport of peptide receptors present in GABAergic terminals of the NTS to the plasma membrane. The cAMP/PKA-mediated receptor trafficking allows the detection of the receptors by locally released neurotransmitters, such as NPY or opioids, thereby explaining the variations in the response to esophageal distension in fed vs. fasted rats and the electrophysiological observations reported above.
Thus we hypothesize that the afferent information associated with the “basal” state of the GI tract ultimately exerts a tonic inhibition of cAMP/PKA pathways in the DVC. Any changes in the status of this basal condition (e.g., food in the gut, circulating chemicals released as a consequence of feeding, elevated circulating cytokine levels, medications such as opiates) would affect the activity of the cAMP/PKA pathways. Changes in cAMP levels could, in turn, alter the efficacy of neurotransmitters to modulate inhibitory synaptic transmission between the NTS and DMV. The end result would be that the tonically active, inhibitory, GABAergic synapse is now “available” for a more efficient modulation by a variety of neurotransmitters. Such a system of modulating background cAMP levels might help to explain the diverse gastric and electrophysiological responses that can be elicited by esophageal distension or central application of NPY or opioids dependent on the state of activation of the animal.
Acknowledgments
We thank the National Institute of Diabetes and Digestive and Kidney Diseases (DK-55530, DK-56373, and DK-52142) and the National Science Foundation (IBN-9816662) for their support.
References
- 1.Abrahamsson H. Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. Arch Int Pharmacodyn Ther. 1986;280:50–61. [PubMed] [Google Scholar]
- 2.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 3.Boeckxstaens GE, Pelckmans PA, Bogers J, Bult H, De Man JG, Oosterbosch L, Herman AG, Van Maercke YM. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991;256:441–447. [PubMed] [Google Scholar]
- 4.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA. 1998;95:5573–5578. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning KN, Travagli RA. NPY in the rat dorsal motor nucleus of the vagus (DMV) inhibit excitatory but not inhibitory synaptic transmission. Dig Dis Week; Atlanta, GA. 19–23 May 2001. [Google Scholar]
- 8.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavis P, Mollard P, Bockaert J, Manzoni O. Visualization of cyclic AMP-regulated presynaptic activity at cerebellar granule cells. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 11.Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- 12.Guo JJ, Browning KN, Travagli RA. Tyrosine hydroxylase-immunoreactive dorsal motor nucleus of the vagus neurons project to discrete areas of the rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;280:G361–G367. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- 13.Hornby PJ. Receptors and transmission in the brain-gut axis. II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055–G1060. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- 14.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 15.Kawai Y, Senba E. Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J Comp Neurol. 1996;373:309–321. doi: 10.1002/(SICI)1096-9861(19960923)373:3<309::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MW, Travagli RA. Effects of Substance P (SP) on identified neurons of the rat dorsal motor nucleus of the vagus (DMV) Am J Physiol Gastrointest Liver Physiol. 2001;281:G164–G172. doi: 10.1152/ajpgi.2001.281.1.G164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maley BE. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses. 1996;21:367–376. doi: 10.1093/chemse/21.3.367. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered NPY inhibits gastric emptying and intestinal transit in the rat. Dig Dis Sci. 1993;38:845–850. doi: 10.1007/BF01295910. [DOI] [PubMed] [Google Scholar]
- 19.McCann MJ, Hermann GE, Rogers RC. Dorsal medullary serotonin and gastric motility: enhancement of effects by thyrotropin-releasing hormone. J Auton Nerv Syst. 1988;25:35–40. doi: 10.1016/0165-1838(88)90005-7. [DOI] [PubMed] [Google Scholar]
- 20.McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall TC. XVI. International union of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 22.Miolan JP, Roman C. The role of oesophageal and intestinal receptors in the control of gastric motility. J Auton Nerv Syst. 1984;10:235–241. doi: 10.1016/0165-1838(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 23.Passafaro M, Rosa P, Sala C, Clementi F, Sher E. N-type Ca2+ channels are present in secretory granules and are transiently translocated to the plasma membrane during regulated exocytosis. J Biol Chem. 1996;271:30096–30104. doi: 10.1074/jbc.271.47.30096. [DOI] [PubMed] [Google Scholar]
- 24.Paton JF, Li YW, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the sub-diaphragmatic vagus nerve. Neuroscience. 2000;95:141–153. doi: 10.1016/s0306-4522(99)00416-9. [DOI] [PubMed] [Google Scholar]
- 25.Quick MW, Corey JL, Davidson N, Lester HA. Second messengers, trafficking-related proteins, and amino acid residues that contribute to the functional regulation of the rat brain GABA transporter GAT1. J Neurosci. 1997;17:2967–2979. doi: 10.1523/JNEUROSCI.17-09-02967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers RC, McTigue DM, Hermann GE. Vagovagal reflex control of digestion: afferent modulation by neural and “endoneurocrine” factors. Am J Physiol Gastrointest Liver Physiol. 1995;268:G1–G10. doi: 10.1152/ajpgi.1995.268.1.G1. [DOI] [PubMed] [Google Scholar]
- 28.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 30.Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travagli RA, Browning KN. The state of activation of NTS neurons determines the effects of pancreatic polypeptides on inhibitory synaptic transmission within the rat dorsal vagal complex (Abstract) Soc Neurosci Abstr. 2001;25:380.1. [Google Scholar]
- 35.Travagli RA, Gillis RA. Hyperpolarization-activated currents IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol. 1994;71:1308–1317. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- 36.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 37.Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001;281:G595–G601. doi: 10.1152/ajpgi.2001.281.3.G595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent A, Tell F. Postnatal development of rat nucleus tractus solitarius neurons: morphological and electrophysiological evidence. Neuroscience. 1999;93:293–305. doi: 10.1016/s0306-4522(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 39.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 40.Yoneda M, Yokohama S, Tamori K, Sato Y, Nakamura K, Makino I. Neuropeptide Y in the dorsal vagal complex stimulates bicarbonate-dependent bile secretion in rats. Gastroenterology. 1997;112:1673–1680. doi: 10.1016/s0016-5085(97)70050-7. [DOI] [PubMed] [Google Scholar]
- 41.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience. 1999;90:685–694. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]