Abstract
Some sources suggest that significant misuse of opioid drugs exists among patients with chronic pain. However, the risk factors and motivation behind their abuse may differ from those of other opioid abusers. This study sought to examine the abuse liability of oxycodone among patients with chronic, non-malignant pain who met the DSM-IV criteria for opioid abuse. Eighteen opioid-dependent patients with chronic pain lived on an in-patient unit of the New York State Psychiatric Institute during the 7-week study. Participants were given oral oxycodone (0, 10, 20, 40, and 60 mg/70 kg) while maintained on various doses of sublingual buprenorphine/naloxone (Bup/Nx; 2/0.5, 8/2, and 16/4 mg/day). Doses of both medications were administered under double-blind conditions. Oxycodone produced an overall positive, but less robust, subjective profile than previously reported in recreational opioid users without pain. Furthermore, unlike our findings in recreational opioid users and more similar to effects in non-drug-abusing individuals, oxycodone failed to serve as a reinforcer. As for the maintenance drug, Bup/Nx produced a dose-related reduction in some of the effects of acutely administered oxycodone. These data suggest that sublingual Bup/Nx has the potential as an analgesic medication and further research should investigate its use in treating patients with chronic pain who abuse opioids.
Keywords: chronic pain, opioid abuse, oxycodone, buprenorphine, suboxone, self-administration
INTRODUCTION
Opioid drugs are some of the most effective tools for chronic pain management (American Academy of Pain Medicine, 1997). The use of opioids for the treatment of chronic, non-cancer pain has escalated in recent years, and it is estimated that opioids are used to manage a significant percentage of cases involving moderate to severe persistent pain (Ballantyne and Mao, 2003; Trescot et al, 2006). Despite their medical utility, many opioid analgesics including morphine, hydrocodone, hydromorphone, fentanyl, and oxycodone have significant abuse liability (Comer et al, 2008; Walsh et al, 2008; Zacny and Lichtor, 2008). Aberrant opioid use behaviors among patients with pain include: obtaining prescriptions from multiple prescribers, forging prescriptions, ‘borrowing' or stealing opioids, aggressively seeking more medication from physicians, and escalating doses without the physician's knowledge (Cowan et al, 2001; Martell et al, 2007; Passik et al, 2006). The exact prevalence of opioid abuse among patients with chronic pain is difficult to determine, although two studies conducted in the United States estimated that over 40% of patients with chronic pain exhibited aberrant drug-related behavior (Katz et al, 2003; Passik et al, 2006).
Owing to the risk of aberrant use behaviors, and the perceived lack of knowledge to manage them, there is often considerable trepidation involved in the initiation of long-term opioid therapy. To address this clinical concern, researchers have attempted to identify factors that may increase the likelihood of patients with chronic pain transitioning from normal to problematic use. Retrospective reviews of medical records and prospective self-report studies have indicated that characteristics such as: a history of poly-substance abuse, legal problems, and psychiatric disorders are all significant predictors of drug abuse among this population (Edlund et al, 2007; Passik et al, 2006). Other variables that have yet to be fully investigated in patients with chronic pain are the subjective and behavioral responses to opioid medications.
Oxycodone is one of the most commonly prescribed and abused opioids (Davis et al, 2003; Katz et al, 2008; Rosenblum et al, 2007). Multiple laboratories have consistently shown that oxycodone produces dose-related increases in positive subjective effects among heroin abusers, prescription opioid abusers, and non-drug abusers (Comer et al, 2008, 2009, 2010b; Walsh et al, 2008; Zacny and Gutierrez, 2009; Zacny and Lichtor, 2008). Oxycodone serves as a robust reinforcer among participants who abuse heroin or prescription opioids (Comer et al, 2008; Comer et al, 2009), yet non-drug-abusing volunteers only self-administer oxycodone when they are exposed to experimentally induced pain (Comer et al, 2010). These data suggest that although subjective response to oxycodone (eg, greater drug liking and/or lesser adverse effects) may predispose individuals to abuse it, this factor alone is not sufficient to motivate abuse.
Although a number patients under long-term opioid therapy for pain develop abusive patterns of use, the impetus behind their abuse may differ significantly from that of other populations of opioid abusers (for a review, see Ballantyne (2006) or Fishbain et al (1992)). Misuse of a drug does not necessarily equate to recreational use, which is typically driven by the positive subjective effects of the drug of choice. Other factors such as self-medication of other psychiatric issues, insufficient pain management, and avoidance of withdrawal also may be responsible for the misuse of opioids. To date, there have been few investigations in the peer-reviewed literature characterizing the subjective and reinforcing effects of opioids in patients with chronic pain. Pain has been shown to modulate the subjective effects of opioids in some studies (Zacny et al, 1996), but few studies have attempted to quantify the subjective effects of opioids among chronic pain sufferers (Lasagna et al, 1955). A more comprehensive assessment of the effects of opioids among patients with chronic pain may provide critical insight into the motivating factors behind opioid abuse within this particular population.
The current investigation sought to assess the contribution of the subjective and reinforcing effects of oxycodone to its abuse liability in patients with chronic pain meeting the DSM-IV criteria for opioid abuse and/or dependence. All patients were maintained on fixed doses of sublingual buprenorphine/naloxone (Bup/Nx) (total daily doses of 2/0.5, 8/2, and 16/4 mg); all of the participants received all of the maintenance doses. Thus, a secondary aim of the study was to evaluate the degree to which Bup/Nx managed chronic pain. Although an injectable formulation of buprenorphine is available in the United States for post-surgical pain and transdermal buprenorphine is prescribed in Europe for pain management, sublingual buprenorphine and Bup/Nx are not approved by the United States Food and Drug Administration for treating pain (Caplan and Southam, 1990). Nevertheless, the off-label use of sublingual buprenorphine (Subutex or Suboxone) to treat pain has been described in the clinical literature (Heit and Gourlay, 2008; Malinoff et al, 2005). These reports indicate that patients with pain who were responding poorly to other opioid analgesics were successfully treated with sublingual buprenorphine. Therefore, in addition to identifying variables that may predict abuse in patients on opioid maintenance therapy, this study may also add to the growing body of literature on the utility of sublingual buprenorphine for managing chronic pain.
METHODS
Participants
Participants who were seeking treatment for their chronic pain were recruited from the New York City metropolitan area through various print media advertisements. Those respondents who met the study criteria, based on the initial telephone interview, were scheduled to come to the New York State Psychiatric Institute for additional screening procedures. Screening consisted of both self-report and clinical interviews administered by a team of research assistants, psychologists, nurses, and physicians. Additional procedures included assessments of: drug use, general health, medical history, and multiple laboratory tests (hematology, blood chemistry panel, liver and thyroid functioning, urinalysis, syphilis serology). Rapid urine drug screens assessed recent use of opioids, benzoylecgonines, benzodiazepines, cannabinoids, and amphetamines.
During screening, participants were provided detailed information concerning study aims and procedures. Informed consent was obtained before screening as well as before initiation of study procedures. Participants were informed that they would receive sublingual Bup/Nx to manage their pain and that they would be maintained on various doses throughout the study. They were also informed that various doses of oxycodone would be administered acutely during laboratory sessions.
All participants were currently under the care of a physician for their pain conditions. All participants were required to meet the DSM-IV criteria for opioid abuse and prescription opioid physical dependence, but were not necessarily seeking treatment for their opioid abuse/dependence. Potential participants were excluded from the study if they were physiologically dependent on heroin, methadone, alcohol, or other drugs, had a severe Axis I psychiatric diagnosis, or had a primary diagnosis of neuropathic pain, malignant pain, headache, or chronic lower back pain with failed surgeries. Current buprenorphine maintenance and history of failed treatment with buprenorphine maintenance for pain also were exclusionary.
Participants were paid US$25/day, with a US$25/day bonus for completing the study. In addition to the per diem payment, participants had the opportunity to earn money during the experimental sessions (US$20 per sample session plus up to US$20 per self-administration session). All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
Laboratory Sessions
Testing consisted of two types of laboratory sessions, a sample session during which participants were provided with one of the possible doses of drug (oxycodone) and US$20, and a self-administration (choice) session that occurred a few hours later. The sample and choice sessions for each dose of oxycodone occurred on the same day. During the choice session, participants were given the opportunity to work for either the dose of drug that was given during the sample session or money.
Sample session
During the first 45 min (min) of each sample session before drug administration, baseline vital signs were determined, and opioid withdrawal was assessed. During this period, participants completed performance tasks, subjective-effects batteries, assessments of pain, and a cold pressor test (CPT), and had a pupil photograph taken. Participants received drug and money at 0 min. Pupil photographs were taken and pain assessments were administered 5, 15, 30, 60, 120, and 210 min after drug/money administration; the subjective-effects battery began 45 min before and 5, 15, 30, 60, and 120 min after drug/money administration. The second performance battery began 60 min after drug/money administration along with a post-drug CPT at 120 min.
Self-administration (choice) sessions
The baseline assessments during each choice session were identical to a sample session. Participants then completed a self-administration task (see below) to receive portions of the dose of drug or money they sampled (0–100% in increments of 10%). Immediately following the self-administration task, participants completed the subjective-effects battery. At time 0, money and/or the total amount of drug earned during the task was administered. At 4 min after receiving drug and/or money, participants again completed the subjective-effects battery. At 10 min after drug/money administration, participants began the performance battery, followed by the subjective-effects battery.
Self-administration task
During choice sessions, participants were told that they could work for all or part of the sampled dose or the sampled money amount (US$20) by choosing the drug or money option each time a choice was available. The alternative money value (US$20) was chosen based on previous studies conducted in our laboratory (Comer et al, 1997). Drug and money were available at each choice trial. Thus, if the dose for that day was 40 mg, at each opportunity participants could respond for 4 mg (10% of 40 mg) or US$2 (10% of $20). Completion of the ratio requirement for each choice trial was accompanied by a visual stimulus on the computer screen. After a choice was made for one option, by clicking on its visual representation on the computer screen, responding for the other option was not possible until the ratio was completed and another trial was initiated. Responses to complete the ratio requirement consisted of finger presses on a computer mouse. The response requirement for each of the two options increased independently such that the initial ratio requirement for each option was 50 responses; the ratio increased progressively each time the option was selected (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800). To receive all of the drug or money available that day, participants were required to emit 11 550 responses within 40 min. Fewer total responses were required if choices were distributed between the two options. At the end of the self-administration task, the participant received whatever he/she had chosen: money (added to their study payment) and/or drug. During all laboratory sessions, vital signs, computer activities, and behaviors were continuously monitored by the experimenters.
Participants resided on a locked in-patient unit during the 7-week study (up to two 72-h outpatient passes were available). All participants were admitted to the unit and maintained on sublingual Bup/Nx. During the first week after admission, participants were withdrawn from their previous opioid analgesic regimen and stabilized on one of three doses of Bup/Nx (2/0.5, 8/2, or 16/4 mg/day). Participants were treated for emergent withdrawal symptoms with various supplemental medications until withdrawal symptoms dissipated based on self-report and observer ratings. Each Bup/Nx dose was maintained for approximately 2 weeks, 1 week of stabilization followed by 1 week of laboratory testing. Each participant was tested for 2-week periods, with all three Bup/Nx doses in random order; doses were administered under double-blind conditions.
Apparatus and Questionnaires
Subjective measures
Three questionnaires were used to assess subjective drug effects and a fourth questionnaire was used to assess opioid withdrawal symptoms. The first questionnaire was a 26-item visual analog scale (VAS). The first 18 lines were labeled with adjectives describing mood states (eg, ‘I feel.' ‘Mellow') and four additional lines were labeled with questions about the dose just received (eg, ‘I liked the dose,' ‘For this dose, I would pay.'). Participants rated each item on the VAS from ‘Not at all' (0 mm) to ‘Extremely' (100 mm), except for the ‘For this dose, I would pay' question, which ranged between US$0 (0 mm) and US$20 (100 mm). The second questionnaire was a six-item Drug Effects Questionnaire (DEQ). Participants described drug effects by selecting among a series of possible answers ranging from 0 (‘No (good, bad, etc) effects at all') to 4 (‘Very Much'). Ratings of drug liking ranged between −4 (‘Dislike very much') and 4 (‘Like very much'). The third questionnaire was a shortened form of the 550-item Addiction Research Center Inventory, which measures a broad range of subjective drug effects (Haertzen, 1974). No statistically significant results were found with this measure, so the results will not be reported below. Lastly, the Subjective Opioid Withdrawal Scale (SOWS) was used to assess the presence and severity of opioid withdrawal (Handelsman et al, 1987).
Performance measures
The task battery consisted of two tasks: a 3-min digit-symbol substitution task (DSST) and a 10-min divided attention task (DAT). Custom-made software was used for these performance tasks (see Comer et al, 1999 for details). Briefly, the DSST consisted of nine 3-row by 3-column squares (with one black square per row) displayed across the top of the computer screen. A randomly generated number indicated which of the nine patterns should be emulated on a keypad by the participant on a particular trial. Participants were required to emulate as many patterns as possible by entering the pattern associated with randomly generated numbers appearing on the bottom of the screen. The DAT consisted of concurrent pursuit-tracking and vigilance components. Participants tracked a moving stimulus on the video screen using the mouse and also signaled when a small black square appeared at any of the four corners of the video screen. The distance between the cursor and moving stimulus was measured, as was the speed of the moving stimulus (with greater accuracy, the stimulus moved at a faster rate).
Clinical pain assessments
Participants' ratings of clinical pain were measured with three instruments: a 15-item Short-form McGill Pain Questionnaire (Clinical Pain MPQ; Melzack, 1987), a 100-mm visual analog pain scale, and a Smiley Scale. The MPQ provided participants with various pain descriptors such as ‘throbbing' and ‘sharp' and asked them to describe the degree to which they felt each type of pain from ‘1=none', ‘2=mild', ‘3=moderate', and ‘4=severe'. The MPQ assesses 15 specific sensory and affective pain descriptors providing a cumulative score that could range between 15 and 60. Along with the MPQ, participants rated their current level of pain using a VAS. This scale asked participants to quantify their clinical pain by responding to the phrase ‘I feel pain' on a 100-mm line; ratings ranged between 0=not at all and 100=extremely. These first two assessment tools have been validated in clinical trials involving chronic pain patients (Dworkin et al, 2005; Kerns et al, 1985, Melzack, 1987). In addition to these measures, the Smiley Scale consists of a series of five faces ranging from a smiling face (1), to an extremely upset, crying face (5). Participants were asked to identify the face that best represented their current level of pain (Pain Associates International Network, 2007).
Cold pressor test
The analgesic effects of oxycodone and Bup/Nx also were evaluated with experimentally induced pain using the CPT, a commonly used and well-established model for producing pain (Comer et al, 2009; Zacny et al, 1996). Crushed ice was added to the cold tank, and warm water was placed in the warm tank. The temperature was maintained at 4°C in the cold tank (additional ice was added, if necessary) and 37°C in the warm tank. Each participant was asked first to immerse the hand in the warm tank for 2 min (to equalize baseline skin temperature across participants). Next, they were asked to immerse the same hand in the cold tank for up to 2 min. Standard instructions were read to each participant before administration of the CPT. During the second water immersion, subjective ratings of pain were measured. Immediately following the CPT, subjective ratings of pain again were measured using the MPQ (CPT-MPQ) and the Pain Intensity/Bothersome Scales (‘Not at all' (0) to ‘Extremely' (10)) during which participants were asked to rate the ‘Intensity' and ‘Bothersomeness' of the acute pain experienced during immersion in cold (4°C) water during the CPT. Objective-dependent measures included: pain threshold (time in seconds to the first report of pain) and pain tolerance (time in seconds to removal of the hand from water).
Physiological effects
A blood pressure cuff was attached to the non-dominant arm, and blood pressure was recorded automatically every 5 min throughout the sessions. A soft sensor attached to a pulse oximeter was placed on a finger of the non-dominant hand to measure arterial oxygen saturation. These data were collected primarily for safety purposes, and since no significant effects of oxycodone or Bup/Nx were found, the data were not reported below. A NeurOptics™ Pupillometer was used to measure changes in pupil diameter under ambient lighting conditions.
Drugs
Bup/Nx tablets (Reckitt Benckiser Pharmaceuticals, Richmond, VA) were administered sublingually at daily doses of 2/0.5, 8/2, and 16/4 mg. The two higher doses are within the recommended dose range for treating opioid abuse (USDH, 2004). The total daily dose was divided (0.5/0.125, 2/0.5, and 4/1 mg) and administered on a QID dosing regimen at 0830, 1230, 1730, and 2130 hours. Bup/Nx tablets in a commercially available dose of 2/0.5 mg were quartered by the research pharmacy in order to provide the correct Bup/Nx dose. At each dosing, participants received two whole tablets and one quartered tablet to maintain the blind. When necessary, whole or quartered placebo tablets were utilized to insure that 2 and ¼ tablets were administered at each dosing time.
Oxycodone (Sigma-Aldrich, St Louis, MO) was administered in oral doses of 0, 10, 20, 40, and 60 mg/70 kg under each Bup/Nx maintenance dose condition (Figure 1). For participants with higher body weights, an absolute maximum amount of 90 mg oxycodone was imposed. All doses of oxycodone were mixed into an orange-flavored drink with 1 ml peppermint oil floated on top in order to maintain a dosing blind. A total volume of 200 ml was administered at each dosing time (1100 and 1500 hours), and participants were required to consume the entire beverage within 5 min. The sample oxycodone dose was administered at 1100 hours and the self-administered oxycodone dose was administered at 1500 hours.
Figure 1.
Time points throughout the day at which buprenorphine/naloxone (Bup/Nx) and oxycodone were administered during the second week of maintenance.
Naloxone HCl (Narcan) for injection, obtainable from DuPont Pharma (Wilimington, PA) was administered in intramuscular doses between 0.2 and 0.8 mg to all participants before admission into the hospital to confirm opioid dependence.
Various concomitant medications were administered, as needed, to participants during the study. To reduce their impact on our study measures, these medications were given during the evening hours.
Statistical Analyses
Repeated-measures ANOVAs were used to assess differences among the dosing conditions (Bup/Nx and oxycodone) over the various time points and to compare peak (or trough) drug effects. Both peak and time-course data were analyzed for all relevant variables, but peak comparisons are primarily reported for the sake of brevity and because the peak data are the most pertinent to the aims of the study. The significance level of α was set at 0.05. All data analyses were performed using SPSS version 15 (SPSS I, 2006) and SuperANOVA (Gagnon et al, 1990).
RESULTS
Participants Demographics
Eighteen opioid-dependent individuals completed the in-patient study. The sample consisted of 11 men and seven women: eight Latinos, seven African-Americans, and three Caucasians (Table 1). The participants' average age was 47±7 years, ranging from 36 to 58 years, and their mean body weight was 92.1 kg (±22.3). The medical conditions cited by participants as the cause of their clinical pain included: accident-related injuries (n=7), osteoarthritis or osteoporosis (n=6), scoliosis or spinal curvature (n=4), nerve damage (n=5), hernia (n=3), spinal stenosis (n=2), sciatica (n=2), disk compression hernia (n=1), neurofibromatosis (n=1), Lyme disease (n=1), bursitis (n=1), and migraine (n=1). Forty-four percent (n=8) of the 18 total participants reported multiple causes of pain. When asked to assess the severity of their clinical pain before the initiation of Bup/Nx maintenance, the participants' average pain rating was 6.98±0.4 on a 1–10 scale, and 24.6±2.9 on the short form of the MPQ. The majority of participants (16 of 18) held a high school diploma or had earned their General Educational Development (GED) credentials.
Table 1. Participant Demographic Characteristics.
| Demographic variable | Statistic |
|---|---|
| Gender, N (%) male | 11 (61%) |
| Age, M (SD) (years) | 47 (7) |
| Education, N (%) completing high school | 16 (89%) |
| Weight, M (SD) (kg) | 92.1 (22.3) |
| Race, N (%) Latino, African-American, Caucasian | 8 (44%), 7 (39%), 3 (17%) |
| Self-reported pain severity (1–10; before Bup/Nx maintenance), M (SD) | 6.98 (0.4) |
| Rx opioid use per day in morphine equivalents, M (SD) (mg) | 161.9 (204.8) |
| Rx opioid use, M (SD) duration in months | 43.6 (48.6) |
| Tobacco use, M (SD) cigarettes per day | 15 (13) |
| Alcohol use, M (SD) drinks per month | 3.3 (3.5) |
All participants were physiologically dependent on opioids upon entry into the study and were not currently seeking treatment for their substance abuse. All participants were daily oral opioid users and had been using opioid medications for a mean duration of 43.6 months. Opioids being used just before study initiation included: Vicodin (n=9), Percocet (n=4), Tramadol (n=2), Endocet (n=2), Dilaudid (n=1), Oxycontin (n=1), and Motrin w/ codeine (n=1). Thirty-three percent (n=6) of the participants indicated that they were currently using multiple opioids. For all participants, their daily opioid use was equivalent to an average of 161.9 mg (±204.8, range: 15–505 mg) of morphine (Anderson et al, 2001; Pereira et al, 2001).
In addition to daily opioid use, 67% (n=12) of our sample were daily tobacco smokers, averaging 15 cigarettes per day. Alcohol was used less regularly, with 50% (n=9) of the participants reporting occasional alcohol use, averaging three drinks each month. Marijuana use was quite rare, with only one participant endorsing regular use. None of the participants reported regular use of cocaine. However, urine drug testing (performed repeatedly during screening) revealed that four participants tested positive for cocaine and eight participants admitted to cocaine use within the past 30 days. Similarly, none of the participants reported regular use of heroin, yet two participants reported heroin use within the last 30 days. Unfortunately, our urine drug screen was not able to distinguish between recent use of certain prescription opioids and heroin.
Physiological, Analgesic, and Subjective Effects of Buprenorphine/Naloxone
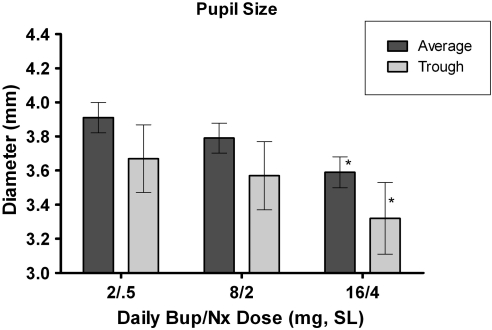
Repeated-measures ANOVA revealed that the largest Bup/Nx maintenance dose (16/4 mg) significantly decreased pupil diameter (miosis) when compared with the 2/0.5 and 8/2 mg doses (which did not significantly differ from one another). These comparisons were made under the oxycodone placebo condition (0 mg/kg) and were statistically significant when comparing trough values and averages across the various time points (p's<0.01; Figure 2).
Figure 2.
Mean and trough (±standard error of mean) pupil diameter as a function of buprenorphine/naloxone maintenance dose (following placebo administration of oxycodone). *A significant difference from the 2/0.5 mg sublingual maintenance dose condition.
Experimental pain
Analyses also revealed dose-dependent increases in the analgesic effects of Bup/Nx in response to experimentally induced pain (CPT). When compared with the 2/0.5 mg dose, the 8/2 mg maintenance dose significantly increased the participants' latency to withdraw their hand from cold water (F(1, 17)=4.93, p<0.05). Interestingly, the responses to the CPT after the 16/4 mg dose did not significantly differ from the 2/0.5 mg dose. A similar effect was observed on ‘latency to feel pain'. That is, relative to the 2/0.5 mg dose, the 8/2 mg dose significantly increased the time in which participants reported the perception of pain following cold-water immersion (F(1, 17)=5.77, p<0.05), but the 16/4 mg dose did not (Table 2). The CPT-MPQ summary scores, assessed immediately following the CPT, did not significantly vary as a function of Bup/Nx maintenance dose.
Table 2. Effects of Bup/Nx Dose on Various-Dependent Measures.
| Dependent measure | Bup/Nx (2/0.5) | Bup/Nx (8/2) | Bup/Nx (16/4) | Significance (main effect) |
|---|---|---|---|---|
| CPT | ||||
| Latency to withdraw (s) | 43.72 (12.47)a | 59.72 (15.73)b | 55.44 (14.39) | p<0.05 |
| Latency to feel pain (s) | 32.00 (12.78)a | 46.39 (14.89)b | 40.83 (13.10) | p<0.05 |
| CPT-MPQ (15–60) | ||||
| MPQ Sum | 43.22 (3.49) | 43.94 (3.56) | 46.00 (3.33) | p=0.16 |
| Pain intensity/bothersomeness (1–10) | ||||
| Bothersome | 9.66 (0.24) | 9.39 (0.37) | 9.83 (0.17) | p=0.10 |
| Intensity | 9.67 (0.24) | 9.27 (0.43) | 9.83 (0.16) | p=0.06 |
| VAS (0–100) | ||||
| Alert | 57.72 (7.08) | 54.39 (6.91) | 54.61 (6.73) | p=0.22 |
| Anxious | 28.78 (7.84) | 25.88 (6.22) | 25.66 (7.01) | p=0.22 |
| Bad Effects | 14.61 (6.84) | 11.78 (4.03) | 7.50 (2.56) | p=0.43 |
| Confused | 13.44 (5.59) | 14.56 (5.59) | 11.22 (4.34) | p=0.67 |
| Depressed | 16.83 (6.24) | 16.89 (5.32) | 16.83 (6.08) | p=0.55 |
| Difficulty concentrating | 20.11 (6.61)a | 30.50 (8.74)b,c | 17.56 (5.39)a | p<0.01 |
| Dizzy | 13.83 (5.27) | 16.56 (5.92) | 10.83 (4.02) | p=0.67 |
| Drug effect | 18.94 (7.04) | 30.89 (7.89) | 19.50 (5.84) | p=0.22 |
| Floating | 11.33 (4.49) | 14.27 (5.69) | 8.50 (3.92) | p=0.92 |
| High | 19.73 (2.74) | 19.68 (2.60) | 17.54 (2.42) | p=0.69 |
| I feel pain | 34.22 (3.75) | 33.58 (3.33) | 32.17 (3.01) | p=0.88 |
| Irritable | 34.78 (8.17) | 29.00 (6.82) | 25.06 (7.56) | p=0.40 |
| Lightheaded | 17.50 (6.37) | 15.00 (5.52) | 10.67 (4.44) | p=0.81 |
| Mellow | 22.22 (7.97)a,c | 34.22 (7.87)b | 37.17 (8.72)a | p<0.05 |
| Muscle pain | 29.33 (7.14) | 26.72 (7.46) | 24.89 (6.13) | p=0.06 |
| Nauseous | 8.05 (4.03) | 13.61 (5.55) | 8.16 (3.07) | p=0.92 |
| Sedated | 17.33 (5.82)a | 22.89 (6.48)b,c | 17.56 (6.44)a | p<0.05 |
| Stimulated | 24.11 (2.85) | 22.67 (2.82) | 20.12 (2.69) | p=0.34 |
| Uncomfortable | 26.56 (6.74)a | 43.83 (8.62)b,c | 22.17 (6.54)a | p<0.01 |
| Clinical pain MPQ (15–60) | ||||
| MPQ sum | 25.56 (2.18) | 24.67 (2.77) | 23.89 (2.19) | p=0.39 |
| SOWS (0–64) | ||||
| SOWS sumd | 5.22 (1.39)a | 7.22 (2.28)b,c | 5.11 (1.61)a | p<0.05 |
| DAT | ||||
| False alarms | 6.44 (2.07) | 8.56 (3.25) | 10.56 (3.16 | p=0.36 |
| Hits | 20.78 (0.65) | 20.67 (0.62) | 20.56 (0.84) | p=0.32 |
| Misses | 1.28 (0.47) | 1.06 (0.49) | 1.61 (0.62) | p=0.67 |
| Max. speed | 4.44 (0.47) | 4.72 (0.47) | 4.22 (0.40) | p=0.49 |
| DSST | ||||
| Total attempted | 59.72 (3.34) | 56.83 (3.33) | 56.00 (2.79) | p=0.28 |
| Total correct | 57.78 (3.19) | 53.61 (3.38) | 51.94 (2.96) | p=0.26 |
Values represent means (±SEM) from peak comparisons.
Significant difference when compared against the 8/2 maintenance condition.
Significant difference when compared against the 2/0.05 maintenance condition.
Significant difference when compared against the 16/4 maintenance condition.
Sum total of the participants' ratings of all 16 opioid withdrawal symptoms.
Clinical pain
In contrast to several of the experimental pain measures, a lack of a dose-dependent effect of Bup/Nx was observed for MPQ, VAS, and Smiley-Face ratings of participants' clinical pain. All comparisons among the three Bup/Nx maintenance conditions were performed following placebo administration of oxycodone.
The effects of Bup/Nx maintenance dose were evident on a number of VAS measures (Table 2). There was a significant dose-dependent increase in VAS ratings of ‘Mellow' (2 vs 8 mg: p<0.05; 8 vs 16 mg: p=0.46; 2 vs 16 mg: p<0.01). In additionally, an inverted-U shaped function was observed on VAS ratings of ‘Sedated' (2 vs 8 mg: p<0.05; 8 vs 16 mg: p<0.05; 2 vs 16 mg: p=0.96), ‘Difficulty Concentrating' (2 vs 8 mg: p<0.05; 8 vs 16 mg: p<0.05; 2 vs 16 mg: p=0.63), ‘Uncomfortable' (2 vs 8 mg: p<0.01; 8 vs 16 mg: p<0.01; 2 vs 16 mg: p=0.42), and the SOWS sum score (2 vs 8 mg: p<0.05; 8 vs 16 mg: p<0.05; 2 vs 16 mg: p=0.90).
Physiological, Analgesic, and Subjective Effects of Oxycodone
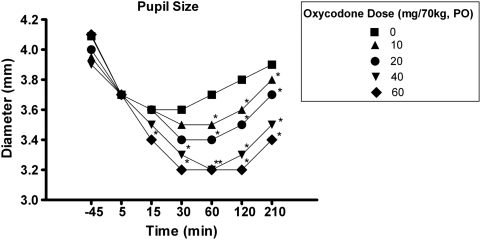
Repeated-measures ANOVAs comparing the effects of oxycodone on miosis found a significant dose-dependent decrease in pupil diameter (oxycodone main effect, F(4, 17)=10.36, p<0.001; oxy × time interaction, F(4, 7)=5.17, p<0.001). As shown in Figure 3, all active doses of oxycodone significantly decreased pupil size in comparison to placebo (0 mg).
Figure 3.
Mean pupil diameter as a function of oxycodone dose and time. *A significant difference from placebo (0 mg/70 kg) at that particular time point. Error bars were removed for clarity.
Experimental pain
Dose-dependent changes also were observed in the analgesic effects of oxycodone as measured by the CPT (oxycodone main effect: F(4, 17)=3.88, p<0.01). The 20 mg (p<0.05), 40 mg (p<0.01), and 60 mg (p<0.01) doses all significantly increased the amount of time participants kept their hand immersed in cold water (Table 3). However, CPT-MPQ ratings of cold pressor-induced pain did not significantly vary as a function of oxycodone dose.
Table 3. Effects of Oxycodone Dose on Various-Dependent Measures.
| Dependent measure | Placebo (0) | Oxy (10) | Oxy (20) | Oxy (40) | Oxy (60) | Significance (main effect) |
|---|---|---|---|---|---|---|
| CPT | ||||||
| Latency to withdraw | 52.96 (8.13) | 53.88 (8.08) | 62.32 (8.58)* | 63.70 (8.56)* | 63.78 (8.09)* | p<0.01 |
| Latency to feel pain | 39.74 (7.67) | 41.96 (7.86) | 41.44 (7.46) | 44.07 (7.59) | 43.59 (7.21) | p=0.46 |
| CPT MPQ (15–60) | ||||||
| MPQ sum | 44.38 (1.96) | 44.68 (1.98) | 44.26 (1.89) | 43.72 (1.95) | 44.26 (1.87) | p=0.67 |
| Pain intensity/bothersomeness (1–10) | ||||||
| Bothersome | 9.63 (0.16) | 9.59 (0.15) | 9.63 (0.15) | 9.50 (0.21) | 9.59 (0.16) | p=0.66 |
| Intensity | 9.53 (0.17) | 9.57 (0.16) | 9.65 (0.15) | 9.62 (0.15) | 9.59 (0.16) | p=0.93 |
| Clinical pain MPQ (15–60) | ||||||
| MPQ sum | 24.70 (1.37) | 23.29 (1.31) | 24.20 (1.410 | 24.48 (1.51) | 23.81 (1.48) | p=0.53 |
| SOWS (0–64) | ||||||
| SOWS suma | 5.85 (1.00) | 5.87 (0.89) | 6.22 (0.97) | 5.04 (0.89) | 5.85 (0.81) | p=0.15 |
| VAS (0–100) | ||||||
| Alert | 55.57 (3.92) | 58.50 (4.21) | 53.76 (4.36) | 58.22 (4.00) | 55.96 (4.42) | p=0.47 |
| Anxious | 27.11 (4.01) | 31.06 (4.44) | 31.41 (4.28) | 29.67 (4.18) | 27.41 (4.01) | p=0.62 |
| Bad effects | 11.29 (2.75) | 12.27 (2.57) | 13.19 (2.79) | 30.06 (18.43) | 15.33 (3.24) | p=0.50 |
| Confused | 13.07 (2.95) | 14.77 (3.06) | 16.68 (3.34) | 14.68 (2.98) | 15.76 (3.23) | p=0.53 |
| Depressed | 16.85 (3.33) | 13.50 (3.05) | 15.96 (3.35) | 16.52 (3.17) | 15.87 (3.38) | p=0.78 |
| Dizzy | 13.74 (2.92 | 16.51 (3.14)* | 21.03 (3.82)* | 18.52 (3.37)* | 20.59 (3.54)* | p<0.05 |
| Drug effect | 23.11 (4.01) | 25.78 (3.56) | 27.98 (4.34) | 31.79 (3.99)* | 33.89 (4.06)* | p<0.05 |
| Energetic | 34.53 (3.82) | 40.61 (4.41) | 36.11 (4.33) | 39.48 (4.33) | 41.22 (4.28) | p=0.09 |
| Floating | 11.37 (2.71) | 12.01 (2.70) | 13.04 (3.11) | 15.80 (3.04)* | 17.67 (3.39)* | p<0.01 |
| Good effects | 21.96 (3.97) | 27.83 (4.34)* | 24.00 (4.37) | 29.24 (4.17)* | 29.00 (4.50)* | p<0.05 |
| High | 13.39 (2.69) | 19.17 (3.45)* | 18.39 (3.42)* | 21.78 (3.62)* | 21.61 (3.41)* | p<0.05 |
| I feel pain | 32.90 (3.74) | 30.02 (3.57) | 32.78 (3.58) | 32.79 (4.14) | 38.11 (4.17) | p=0.09 |
| Irritable | 29.61 (4.31) | 28.02 (4.44) | 28.35 (4.05) | 35.04 (4.89) | 31.32 (4.31) | p=0.24 |
| Lightheaded | 16.02 (3.17) | 17.61 (3.09) | 18.82 (3.59) | 20.11 (3.19) | 22.07 (3.11) | p=0.15 |
| Mellow | 32.53 (4.68) | 39.19 (4.59) | 35.28 (4.74) | 37.53 (4.39) | 40.35 (5.02) | p<0.05 |
| Muscle pain | 26.98 (3.94) | 28.13 (3.93) | 28.61 (4.12) | 28.94 (3.98) | 29.21 (4.31) | p=0.89 |
| Nauseous | 9.94 (2.48) | 14.94 (3.42) | 17.70 (3.79) | 14.82 (3.02) | 19.78 (3.53) | p=0.14 |
| Restless | 31.98 (4.29) | 31.39 (4.19) | 37.89 (4.24) | 35.65 (4.30) | 33.74 (4.36) | p=0.25 |
| Sedated | 19.25 (3.56) | 25.56 (3.91)* | 23.29 (4.04) | 26.94 (3.81)* | 29.19 (4.33)* | p<0.05 |
| Stimulated | 20.15 (3.34) | 20.22 (3.22) | 19.33 (3.43) | 24.70 (3.97)* | 27.07 (3.94)* | p<0.05 |
| Uncomfortable | 30.85 (4.36) | 33.85 (4.14) | 38.14 (4.66) | 36.68 (4.63) | 35.94 (4.20) | p=0.23 |
| DEQ | ||||||
| Good effect | 1.01 (0.23) | 1.16 (0.17) | 1.16 (0.18) | 1.52 (0.23)* | 1.32 (0.19) | p<0.05 |
| Drug liking | 1.04 (0.23) | 1.04 (0.17) | 0.94 (0.17) | 1.28 (0.23) | 1.11 (0.17) | p=0.36 |
| Strong drug effect | 1.24 (0.21) | 1.19 (0.15) | 1.22 (0.16) | 1.63 (0.22)* | 1.48 (0.17) | p<0.05 |
| Would take the dose again | 1.35 (0.24) | 1.32 (0.18) | 1.26 (0.19) | 1.43 (0.23) | 1.28 (0.19) | p=0.73 |
| DAT | ||||||
| False alarms | 8.51 (1.65) | 9.02 (1.95) | 9.13 (1.87) | 10.74 (2.47) | 8.91 (1.86) | p= 0.70 |
| Hits | 20.66 (0.40) | 20.77 (0.37) | 20.65 (0.42) | 20.70 (0.41) | 21.13 (0.44) | p=0.07 |
| Misses | 1.32 (0.30) | 1.00 (0.20) | 0.907 (0.21) | 1.17 (0.25) | 1.32 (0.27) | p=0.59 |
| Max speed | 4.46 (0.25) | 4.42 (0.27) | 4.37 (0.26) | 3.87 (0.24)* | 3.89 (0.26)* | p<0.01 |
| Tracking distance | 38 661 (4219) | 36 414 (3924) | 36 235 (4190) | 42 808 (4561) | 45 889 (5343)* | p<0.05 |
| DSST | ||||||
| Total attempted | 57.52 (1.81) | 55.93 (1.87) | 55.63 (1.91) | 55.63 (1.88) | 53.82 (1.76) | p=0.09 |
| Total correct | 54.44 (1.78) | 51.93 (2.04) | 51.41 (2.06) | 51.11 (2.08) | 51.04 (1.89) | p=0.20 |
Values represent means (±SEM) from peak comparisons.
*Significant difference when compared against the placebo condition.
Sum total of the participants' ratings of all 16 opioid withdrawal symptoms.
Clinical pain
Clinical MPQ, VAS, and Smiley-Face assessments of clinical pain did not vary as a function of oxycodone dose. Peak values on a number of subjective measures did show significant oxycodone main effects. Participants' ratings of ‘Dizzy' (F(4, 17)=2.53, p<0.05), ‘Drug Effect' (F(4, 17)=3.36, p<0.05), and ‘Floating' (F(4, 17)=4.17, p<0.01) all showed significant oxycodone dose-related increases when compared against placebo.
Furthermore, repeated-measures ANOVA comparisons revealed statistically significant main effects of oxycodone dose for peak VAS ratings of: ‘Good Effect' (F(4, 17)=2.83, p<0.05), ‘High' (F(4, 17)=3.23, p<0.05), ‘Mellow' (F(4, 17)=2.80, p<0.05), ‘Sedated' (F(4, 17)=3.44, p<0.01), and ‘Stimulated' (F(4, 17)=2.90, p<0.05). In addition to these measures, peak DEQ ratings of ‘Good Drug Effect' (F(4, 17)=2.55, p<0.05) and ‘Strong Drug Effect' (F(4, 17)=2.80, p<0.05) both significantly increased as a function of oxycodone dose.
In addition to altering subjective responses, larger doses of oxycodone also produced detrimental effects on performance of the DAT (Table 3). Repeated-measures ANOVA revealed a significant main effect of oxycodone dose upon tracking distance (F(4, 17)=2.94, p<0.05) and performance speed (F(4, 17)=5.18, p<0.01). Post hoc analysis found that when compared with the placebo condition, the 60 mg dose significantly increased tracking distance (p<0.05). In addition, the two highest doses of oxycodone both decreased performance speed (p values <0.01).
Interaction of Bup/Nx and Oxycodone
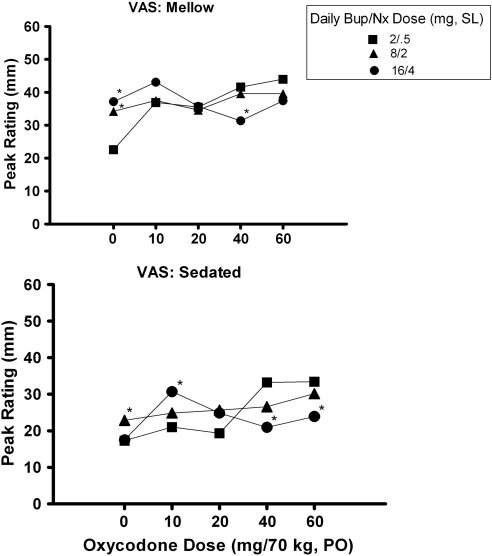
On some measures, ANOVA revealed a significant interaction between the maintenance dose of Bup/Nx and the acutely administered dose of oxycodone. Figure 4 depicts the peak oxycodone ratings for VAS measures of ‘Mellow' (Bup × Oxy interaction: F(2, 4)=2.68, p<0.01) and ‘Sedated' (Bup × Oxy interaction: F(2, 4)=2.24, p<0.05), as a function of the Bup/Nx maintenance dose. When maintained on 2/0.5 mg of Bup/Nx, a significant oxycodone dose-related increase was observed on both measures, but when maintained on the 16/4 mg dose of Bup/Nx, the influence of larger oxycodone doses was eliminated (Mellow: 40 mg (p<0.05); Sedated: 40 mg (p<0.01) and 60 mg (p<0.01)).
Figure 4.
Mean visual analog scale ratings of ‘Mellow' and ‘Sedated' as a function of the acutely administered oxycodone dose and the buprenorphine/naloxone (Bup/Nx) maintenance dose. *A significant difference from the 2/.5 mg maintenance dose for that particular dose of oxycodone. Error bars were removed for clarity.
Self-Administration
None of the self-administration measures significantly differed as a function of oxycodone dose or Bup/Nx maintenance condition. Progressive ratio breakpoint values for drug were low (<300 responses) and did not vary among the different oxycodone doses that were tested, including placebo.
DISCUSSION
Our investigation suggests that Bup/Nx has notable analgesic properties. In comparison to the smallest dose, the 8/2 mg daily maintenance condition induced significant analgesia in response to experimentally induced pain. Consistent with some preclinical reports, this study found an inverted U-shaped dose–response function with respect to several effects of Bup/Nx (Cowan et al, 1977; Dum and Herz, 1981). In addition to having the strongest effects on acute pain measures (CPT), when assessing its aversive effects, the 8/2 mg dose produced the highest reports of: ‘Sedation', ‘Uncomfortable', and reports of withdrawal severity. Clinical pain as assessed by the MPQ, VAS, and Smiley-Face scales did not significantly vary as a function of Bup/Nx dose. Interestingly, there appears to be a discrepancy between the analgesic effects of Bup/Nx as assessed by self-report and objective measures. However, our assessments of the analgesic effects of oxycodone may provide insight into our results with Bup/Nx. When the analgesic effects of oxycodone were assessed using the CPT, it appears as though objective behavioral measures (eg, latency to withdraw) are more sensitive than verbal reports (eg, latency to feel pain). These data argue that our assessments of Bup/Nx effects upon clinical pain may have benefited from the use of objective measures as they may be more sensitive in detecting analgesic response. Unfortunately, assessments of clinical pain are typically self-report. As such, our study may not have had sufficient power to detect the effects of Bup/Nx for the management of clinical pain.
Although the doses of Bup/Nx currently tested produced some aversive effects (see VAS assessments of ‘Difficulty Concentrating' and ‘Uncomfortable' in Table 2), no discernable positive subjective effects were observed. It is difficult to determine conclusively that Bup/Nx had no positive subjective effects without a placebo maintenance control condition. However, a 0 mg maintenance phase was judged to be unethical by our Institutional Review Board because of the possible emergence of opioid withdrawal and of clinical pain. Nevertheless, no significant dose–response effects were reported on any measures of positive subjective effects typically associated with abuse liability, such as ratings of drug liking, good drug effects, and high. Combined, our findings suggest that, under these experimental conditions (sublingual administration to chronic pain sufferers), Bup/Nx has a relatively low abuse liability.
Bup/Nx failed to significantly impair psychomotor functioning, although some studies have found that buprenorphine can disrupt performance on the DSST in participants with a limited opioid use history (Zacny et al, 1997). In contrast, oxycodone did cause significant impairments on psychomotor task performance, as in other investigations (Zacny and Gutierrez, 2009). When compared with Bup/Nx, oxycodone had similar effects on experimentally induced pain and clinical pain assessments, yet with minimal aversive effects and a number of positive subjective effects. This observation is consistent with recent reports of an overall positive subjective profile of oxycodone among opioid abusers and non-abusers (Comer et al, 2008; Walsh et al, 2008; Zacny and Lichtor, 2008). Yet, in comparison to other investigations noting the positive subjective effects of similar oxycodone doses, those reported in this study were relatively small. In their investigation with non-opioid abusers, Zacny and Gutierrez (2003, 2009) reported that compared with placebo, a 10 mg dose of oxycodone produced an approximately 80% increase in peak VAS ratings of ‘High' and 20 mg oxycodone had an even stronger effect, increasing ratings up to 90%. In this study, these same oxycodone doses only produced a 27% (10 mg) and 24% (20 mg) increase in subjective ratings of the same measure. In addition, the fact that oxycodone did not alter subjective ratings on measures, such as ‘drug liking' and ‘would take the dose again', further argues that the ‘abuse' of prescription opioids among this population may not be for recreational purposes. Yet, only moderate effects of oxycodone were found on those subjective measures that reached statistical significance. In order to more definitely answer this question, future studies are needed to compare directly the abuse liability of oxycodone in abusers with and without clinical pain, using the same experimental parameters.
Despite the statistically significant increases in its positive subjective effects, oxycodone failed to serve as a reinforcer in this study. These results are in marked contrast with previous reports observed with heroin-dependent participants and prescription opioid abusers who self-administered similar oxycodone doses at significantly higher levels (Comer et al, 2008, 2009; Comer et al, 2010). The Bup/Nx dosing regimen used in this study was most likely responsible for the marked differences in outcomes between prescription opioid-abusing pain patients and heroin abusers. In an effort to address the unique needs (eg, pain management) of the current population, this study employed a QID Bup/Nx dosing regimen, whereas in our previous studies, sublingual buprenorphine was administered once daily in the evening, 15 h before administration of oxycodone. It is likely that this more frequent pattern of dosing contributed to the robust interfering actions of Bup/Nx in this study compared with our previous studies in heroin abusers. Therefore, it is possible that oxycodone's lack of reinforcing value and blunted positive subjective profile may not be owing to population differences, but owing to varying parametric conditions. Future studies in our laboratory will address this important point.
A relationship between the maintenance drug (Bup/Nx) and the acutely administered drug (oxycodone) was observed in this study. On certain measures, the 8/2 and 16/4 mg doses of Bup/Nx appeared to reduce the effects of oxycodone. Oxycodone dose-dependent increases in participants' ratings of ‘Mellow' and ‘Sedated' observed under the 2/.5 mg Bup/Nx maintenance dose disappeared during the 8/2 and 16/4 mg maintenance dose. It is likely that the reduced subjective effects of oxycodone observed here were functions of Bup/Nx maintenance schedule and may extend to other full mu-opioid agonists. For example, other studies have shown that 8/2 and 32/8 mg Bup/Nx and also 8 and 16 mg buprenorphine alone are capable of antagonizing the effects of heroin (Comer et al, 2005; Mello et al, 1982, 1983; Mello and Mendelson, 1980).
Future Directions and Clinical Implications
The ability of Bup/Nx to reduce the subjective effects of oxycodone, combined with its minimal positive subjective effects, argues in favor of the utility of sublingual buprenorphine as an opioid abuse treatment and a pain management tool. Of clinical relevance to many chronic pain patients, Bup/Nx is associated with the absence of psychomotor task impairment. Comparisons of subjective pain ratings (MPQ) pre- and post-Bup/Nx maintenance revealed no significant differences between the analgesic effectiveness of the participants' previous analgesic regimen and that achieved on the three Bup/Nx doses. This result suggests that a QID dosing regimen of sublingual Bup/Nx may be as efficacious for pain management as traditional opioid analgesics, although future studies should be conducted to more definitively establish this finding. Furthermore, a maximum dose of 16/4 mg was used in the present study. Although Bup/Nx produced an inverted U-shaped dose–response pattern for analgesic and some subjective responses in this study, a more linear dose–response relationship for many of buprenorphine's effects have been reported in other studies (Duke et al, 2010). As buprenorphine doses of 32 mg and higher have been shown to be safe and effective for treating opioid dependence (Johnson et al, 1992, 2000), it is possible that higher doses also may prove to be more effective than lower doses for the treatment of pain. The ability of Bup/Nx to reduce the effects of oxycodone raises concerns with the use of additional opioids to treat breakthrough pain. Nevertheless, this clinical issue may be circumvented by the use of more potent opioids or additional break-through doses of buprenorphine. Future studies should address these important issues.
Acknowledgments
The medical assistance of Robert Vorel MD, Ben Bryan MD, Elias Dakwar MD, David Mysels MD, Janet Murray RN, and Claudia Tindall RN, along with the technical assistance of Elisa Payne BA, Debra Wolkenfeld BA, Phillip Saccone BA, and Joseph Lazar BA, is gratefully acknowledged. Funding for this investigation was provided by National Institute on Drug Abuse Grant DA020448 to Dr Maria Sullivan.
The authors declare that over the past 3 years SDC, JDJ, SKV, JMM, and MAS have all received compensation (in the form of partial salary support) from separate investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals and Schering-Plough Corporation, manufacturers of the Bup/Nx used in this investigation. Other companies that provided partial salary support to the manuscript's authors over the past 3 years for other investigations include: Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and Avigen. However, none of these companies manufactured any drug, product, or device used in this work. In addition, SDC has served as a consultant to the following companies: Abbott, Alpharma, Analgesic Research, BioDelivery Sciences, Cephalon, Inflexxion, King, Neuromed, Purdue, and Shire.
References
- American Academy of Pain Medicine (APM) and American Pain Society (APS) The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- Anderson R, Saiers JH, Abram S, Schlicht C. Accuracy in equianalgesic dosing: conversion dilemmas. J Pain Symptom Manage. 2001;21:397–406. doi: 10.1016/s0885-3924(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC. Opioids for chronic nonterminal pain. South Med J. 2006;99:1245–1255. doi: 10.1097/01.smj.0000223946.19256.17. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Caplan RA, Southam M. Transdermal drug delivery and its application to pain control. Adv Pain Res. 1990;14:233–240. [Google Scholar]
- Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manage. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intranasal and intravenous heroin self-administration by morphine-maintained humans. Psychopharmacology. 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacolgy. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, MacFarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DT, Allan LG, Libretto SE, Griffiths P. Opioid drugs: a comparative survey of therapeutic and ‘Street' use. Pain Med. 2001;2:193–203. doi: 10.1046/j.1526-4637.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- Davis MP, Varga J, Dickerson D, Walsh D, LeGrand SB, Lagman R. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer. 2003;11:84–92. doi: 10.1007/s00520-002-0385-9. [DOI] [PubMed] [Google Scholar]
- Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacology (Berl) 2010;211:303–312. doi: 10.1007/s00213-010-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence and addiction in chronic pain patients. Clin J Pain. 1992;8:77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, et al. Superanova accessible general linear modeling. Yale J Biol Med. 1990;63:191–192. [Google Scholar]
- Haertzen CA. An Overview of Addiction Research Center Inventory Scales (ARCI): An Appendix and Manual of Scales. National Inst. on Drug Abuse (DHEW/PHS): Rockville, MD; 1974. [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Heit HA, Gourlay DL. Buprenorphine: new tricks with an old molecule for pain management. Clin J Pain. 2008;24:93–97. doi: 10.1097/AJP.0b013e31815ca2b4. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. New Eng J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. J Am Med Assoc. 1992;267:2750–2755. [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Katz NP, Sherburne S, Beach M, Rose RJ, Vielguth J, Bradley J, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097–1102. doi: 10.1213/01.ANE.0000080159.83342.B5. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, Rudy TE. The West Haven–Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23:345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Lasagna L, Von Felsinger JM, Beecher HK. Drug-induced mood changes in man. I. Observations on healthy subjects, chronically-ill patients, and postaddicts. JAMA. 1955;157:1006–1020. doi: 10.1001/jama.1955.02950290026009. [DOI] [PubMed] [Google Scholar]
- Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- Martell BA, O'Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, et al. Systematic review. Opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther. 1983;225:378–386. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther. 1982;223:30–39. [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Pain Associates International Network 2007. Retrieved from: http://www.pain-initiative.com/pain_services/ pain_management_tools (January 2007).
- Passik SD, Kirsh KL, Donaghy KB, Portenoy RK. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain. 2006;22:173–181. doi: 10.1097/01.ajp.0000161525.48245.aa. [DOI] [PubMed] [Google Scholar]
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- SPSS I . SPSS 15.0.0 for Windows. Pearson-Prentice Hall: Chicago, IL; 2006. [Google Scholar]
- Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Phys. 2006;9:1–39. [PubMed] [Google Scholar]
- US DEPARTMENT OF HEALTH AND HUMAN SERVICES: Center for Substance Abuse Treatment 2004Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid AddictionTreatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939Substance Abuse and Mental Health Services Administration: Rockville, MD; 2004 [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 2003;170:242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend. 2009;101:107–114. doi: 10.1016/j.drugalcdep.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor S. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berl) 2008;196:105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, et al. The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alcohol Depend. 1996;42:133–142. doi: 10.1016/0376-8716(96)01274-4. [DOI] [PubMed] [Google Scholar]