Abstract
The cannabinoid CB1 receptor-mediated modulation of γ-aminobutyric acid (GABA) release from inhibitory interneurons is important for the integrity of hippocampal-dependent spatial memory. Although adenosine A1 receptors have a central role in fine-tuning excitatory transmission in the hippocampus, A1 receptors localized in GABAergic cells do not directly influence GABA release. CB1 and A1 receptors are the main targets for the effects of two of the most heavily consumed psychoactive substances worldwide: Δ9-tetrahydrocannabinol (THC, a CB1 receptor agonist) and caffeine (an adenosine receptor antagonist). We first tested the hypothesis that an A1–CB1 interaction influences GABA and glutamate release in the hippocampus. We found that A1 receptor activation attenuated the CB1-mediated inhibition of GABA and glutamate release and this interaction was manifested at the level of G-protein activation. Using in vivo and in vitro approaches, we then investigated the functional implications of the adenosine–cannabinoid interplay that may arise following chronic caffeine consumption. Chronic administration of caffeine in mice (intraperitoneally, 3 mg/kg/day, for 15 days, >12 h before trials) led to an A1-mediated enhancement of the CB1-dependent acute disruptive effects of THC on a short-term spatial memory task, despite inducing a reduction in cortical and hippocampal CB1 receptor number and an attenuation of CB1 coupling with G protein. A1 receptor levels were increased following chronic caffeine administration. This study shows that A1 receptors exert a negative modulatory effect on CB1-mediated inhibition of GABA and glutamate release, and provides the first evidence of chronic caffeine-induced alterations on the cannabinoid system in the cortex and hippocampus, with functional implications in spatial memory.
Keywords: adenosine, cannabinoid, hippocampus, caffeine, THC, memory
INTRODUCTION
The fine tuning of neuronal activity to suit specific cognitive functions is a major task of endogenous neuromodulators, of which adenosine and the endocannabinoids are two important examples. Both modulators are released by neurons and activate G-protein-coupled receptors (GPCRs) that represent some of the most widely and densely expressed GPCRs in the brain (Dunwiddie and Masino, 2001; Herkenham et al, 1990). In the hippocampus, the predominant adenosine and cannabinoid receptors are the A1 and CB1 receptors, respectively. Several forms of learning, memory, and other cognitive functions require the integrity of the hippocampal circuitry, in which A1 and CB1 receptors were shown to have important roles owing to their presynaptic regulation of neurotransmitter release (eg, Ohno and Watanabe, 1996; Wise et al, 2009). Moreover, in the hippocampus, these receptors are the main targets for the cognitive effects of two of the most heavily consumed psychoactive substances worldwide: caffeine and Δ9-tetrahydrocannabinol (THC) (Barone and Roberts, 1996; Leggett, 2006).
Caffeine is present in various dietary sources, such as coffee, tea, and soft drinks, and at moderate doses is an adenosine receptor antagonist with cognitive-enhancing properties (Fredholm et al, 1999; Ribeiro and Sebastião, 2010). As customary in most coffee consumers, long-term intake of caffeine leads to the development of tolerance to some of its acute effects by mechanisms not yet fully understood, although most studies found an increased number of A1, but not A2A receptors, in several brain areas (Jacobson et al, 1996). Chronic caffeine intake has also been associated with increased behavioral effects of some drugs of abuse, for example, amphetamine and cocaine (Gasior et al, 2000; Justinova et al, 2009). THC is the main psychoactive constituent of the cannabis plant, which is consumed recreationally or used for medicinal purposes; it mainly activates cannabinoid CB1 receptors in the central nervous system to produce motor- and cognitive-disrupting effects (see Pertwee, 2008).
Hippocampal CB1 receptors are primarily found in presynaptic terminals of cholecystokinin (CCK)-expressing γ-aminobutyric acidergic (GABAergic) interneurons from the CA1 and CA3 subfields (Hájos et al, 2000; Katona et al, 1999). CCK-expressing GABAergic interneurons regulate the temporal coordination in the activity of principal cell assemblies, which is critical for the integrity of hippocampal-dependent memory (Freund and Katona, 2007). Accodingly, it was recently shown that presynaptic CB1 receptors at GABAergic, but not glutamatergic, neurons are required for THC-induced amnesia (Puighermanal et al, 2009). In contrast, A1 receptors mostly affect excitatory synaptic transmission (Dunwiddie and Fredholm, 1989; Sebastião et al, 1990), having no direct influence upon GABAergic transmission in mature hippocampal neurons (Jeong et al, 2003; Lambert and Teyler, 1991; Li and Henry, 2000; Yoon and Rothman, 1991) or on GABA release from isolated nerve terminals (Cunha and Ribeiro, 2000). However, A1 receptors are present in hippocampal GABAergic interneurons (Ochiishi et al, 1999), in which they control the actions of vasoactive intestinal peptide (Cunha-Reis et al, 2008).
Both A1 and CB1 receptors regulate synaptic transmission through activation of G-protein αi/o-subunits (Straiker et al, 2002), which inhibit adenylyl cyclase, block voltage-gated calcium channels (VGCCs), and activate inwardly rectifying potassium channels (Dunwiddie and Masino, 2001; Howlett, 2005). In the cerebellum, A1 receptors modulate the motor incoordination effects induced by acute administration of THC or CB1 receptor agonist CP55,940 (Dar, 2000; Dar and Mustafa, 2002; DeSanty and Dar, 2001). Furthermore, prolonged intracerebellar administration of a CB1 or A1 agonist induces cross-tolerance (DeSanty and Dar, 2001), and similar observations were obtained in two subsequent studies (Kouznetsova et al, 2002; Selley et al, 2004). A more recent study observed that CB1-mediated inhibition of excitatory synaptic transmission in the hippocampus is modulated by endogenous adenosine, through A1 receptor activation (Hoffman et al, 2010; but see Serpa et al, 2009). These previous findings raised the hypothesis that a functional interaction between A1 and CB1 receptors in the hippocampus may have cognitive and pathophysiological implications, particularly for the effects of cannabis and caffeine consumption in humans.
This study initially focused upon the possibility that an A1–CB1 interaction influences GABA and glutamate release. We found that A1 receptor activation attenuated the CB1-mediated inhibition of GABA and glutamate release, and that this interaction is manifested at the level of G-protein activation. We then evaluated the functional consequences of chronic caffeine administration on the memory deficits induced by acute THC administration. Caffeine (intraperitoneally, 3 mg/kg/day, for 15 days, >12 h before trials) increased A1 receptor levels, and did not by itself cause measurable effects on spatial memory, but led to an A1-mediated exacerbation of the CB1-dependent acute effects of THC in a spatial memory task.
MATERIALS AND METHODS
Animals
Adult male 6–8 weeks old Wistar rats (Harlan Interfauna Iberica, Barcelona, Spain) and 12–16 weeks old C57Bl/6J mice (Harlan-Olac, Bicester, UK) were used. Animals were housed in a temperature- and humidity-regulated room with a 12 h dark/light cycle, and free access to food and water. Experiments were performed during the light phase. All experimentation followed the UK Animals (Scientific Procedures) Act, 1986, Portuguese and European Union law concerning animal care. C57Bl/6J mice were used in all experiments involving chronic caffeine administration for logistic advantages and because mice have been extensively used in behavioral studies where the systemic effects of cannabinoids on motor and cognitive function have been assessed (Lichtman et al, 2002).
Drugs
4-Amino-[2,3-3H]butyric acid ([3H]GABA), -[G-3H]glutamic acid ([3H]glutamate), 1,3-[3H]-dipropyl-8-cyclopentylxanthine ([3H]-DPCPX), and [3H]SR141716A were obtained from GE Healthcare Life Sciences (Buckinghamshire, UK). Guanosine 5′-[γ-35S]-thio)triphosphate ([35S]-GTPγS) was from Perkin-Elmer NEN Radiochemicals (Boston, MA, USA). Adenosine deaminase (ADA, EC 3.5.4.4) was from Roche Diagnostics (Indianapolis, IN, USA). THC (>98% purity) was from THC Pharm (Frankfurt, Germany) or Tocris Bioscience (Bristol, UK). CdCl2, caffeine (anhydrous base), GABA, aminooxyacetic acid (AOAA), guanosine diphosphate (GDP), and guanosine 5′-O-[γ-thio]triphosphate (GTPγS) were from Sigma (St Louis, MO, USA). 1-(4,4-Diphenyl-3-butenyl)-3-piperidinecarboxylic acid hydrochloride (SKF89976A), N6-cyclopentyladenosine (CPA), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 8-[4-[(2-aminoethyl) amino]carbonylmethyloxyphenyl] xanthine (XAC), (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone mesylate (WIN55,212-2), (3S)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone monomethanesulfonate (WIN55,212-3), and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) were from Tocris Bioscience. (RS)-4-amino-3-(4-chlorophenyl)butanoic acid (baclofen) was from Ascent Scientific (Bristol, UK). All other reagents were from Sigma. For in vitro experiments, non-water-soluble drugs were dissolved in dimethyl sulfoxide (DMSO), aliquoted, and stored at −20 °C. The amount of DMSO in solutions was normalized for all conditions in any given experiment, and always <0.02%.
Synaptosomal Preparation
For each experiment, hippocampal synaptosomes were prepared from two rats, or cortico-hippocampal synaptosomes from one mouse. Animals were decapitated under halothane anesthesia and synaptosomes were prepared as described previously (Assaife-Lopes et al, 2010), with modifications. Briefly, tissue was dissected in a continuously oxygenated (95% O2, 5% CO2) ice-cold artificial cerebrospinal fluid (aCSF) of the following composition (mM): NaCl 125, KCl 3, NaH2PO4 1, NaHCO3 25, CaCl2 1.5, MgSO4 1.2, glucose 10, pH 7.4. Samples were homogenized in ice-cold 0.32 M sucrose solution containing 1 mM EDTA and 10 mM HEPES, pH 7.4. The homogenate was then centrifuged at 3000 g for 10 min, and the supernatant obtained was centrifuged at 14 000 g for 12 min to obtain a stratified pellet, containing synaptosomes (McMahon et al, 1992; Phelan and Gordon-Weeks, 1997).
[3H]Neurotransmitter Release Assays
For [3H]GABA release assays, the pellet was resuspended and synaptosomes were incubated for 20 min, at 37 °C, with [3H]GABA (1.5 μCi/ml, 1.85 nM), and 0.625 μM of unlabelled GABA to decrease specific activity of [3H]GABA to 2.3 μCi/nmol. Incubation and superfusion solutions consisted of oxygenated aCSF containing the GABA transaminase inhibitor AOAA. For [3H]glutamate experiments, the synaptosomal pellet was resuspended in aCSF, which did not contain AOAA, and synaptosomes were incubated for 5 min, at 37°C with 10 μCi/ml [3H]glutamate. Synaptosomes were then layered over GF/C filters (Milipore, MA, USA) on an eight-chamber superfusion (0.8 ml/min) apparatus (Raiteri et al, 1974). This constant and rapid flow rate washes out endogenously released substances, thus ensuring drug effect specificity (see Raiteri and Raiteri, 2000). After a 30-min washout period, samples were continuously collected for 36 min, in 2-min fractions. Synaptosomes were stimulated during 2 min with 15 mM K+ (isomolar substitution of Na+ with K+) at the 6th (S1) and 24th (S2) minutes of collection time. CB1 agonists were added to the superfusion medium from the 18th minute onward, to measure effects on S2. The CB1 antagonist, AM251, or A1 and GABAB receptor ligands were added from the 15th minute of the washout period onward, that is, present during S1 and S2 in order to assess their ability to modify the effect of WIN55,212-2 (applied before S2). Each condition was tested in duplicate, as commonly accepted in this paradigm (eg, Cunha and Ribeiro, 2000). Under similar conditions, the percentage of GABA and glutamate in the K+-evoked outflow is >90% of the total tritium in the sample (Cunha et al, 1997; Lopes et al, 2002). Fractional [3H]neurotransmitter release was expressed as the percentage of total radioactivity present in the synaptosomes at each time point (fractional release). The amount of tritium released after each pulse of K+ (S1 or S2) was calculated by integration of the peak area. Effects were calculated by normalizing the S2/S1 values of corresponding controls from the same batch of synaptosomes to 0% effect. For example, the effect of WIN55,212-2 (added before S2), in the presence of CPA (during S1 and S2), was calculated using the S2/S1 of CPA alone (during S1 and S2) as a control, which was obtained from the same experiment and batch of synaptosomes.
Binding Assays
Rat hippocampal or mouse cortico-hippocampal membranes were prepared as described previously (eg, Cunha et al, 1999), with modifications. Tissue was homogenized in ice-cold 0.32 M sucrose solution containing 2 mM EGTA, 1 mM DTT, and 50 mM Tris, pH 7.6. The homogenate was centrifuged at 1000 g for 10 min, and the supernatant obtained was centrifuged at 14 000 g for 12 min. The pellets were resuspended in assay buffer and incubated with 4 U/ml ADA for 30 min at 37 °C, followed by centrifugation at 14 000 g for 12 min and resuspension in assay buffer. Assay buffer composition, in mM, for radioligand binding assays was: Tris 50, MgCl2 2, pH 7.4 and for [35S]GTPγS binding assays it was: Tris 50, MgCl2 5, NaCl 100, EGTA 0.2, pH 7.4. Protein content was determined by the Bradford method (Bradford, 1976). For [3H]DPCPX binding assays, membranes (40 μg protein) were incubated for 1 h at room temperature in a final incubation volume of 300 μl containing 4 U/ml ADA, and using 2 μM of XAC to measure nonspecific binding. For [3H]SR141716A binding, membranes (50 μg protein) were incubated for 1 h at 30 °C in a final volume of 300 μl containing 1 mg/ml BSA and using 1 μM of AM251 to measure nonspecific binding. For [35S]GTPγS binding assays, membranes (10 μg of protein) were incubated with 0.1 nM [35S]GTPγS and 0.1 nM–10 μM of CB1 agonist, in the absence or presence of 100 nM CPA or 100 μM baclofen, in assay buffer containing 30 μM GDP, in a total volume of 500 μl, for 30 min at 37 °C. At this GDP concentration, WIN55,212-2 has been shown to induce high-affinity [35S]GTPγS binding (Breivogel et al, 1998). Specific binding was calculated by subtracting nonspecific binding obtained by incubation with 10 μM GTPγS. The effect of co-application of CPA or baclofen with CB1 agonists was calculated by subtracting the increase in [35S]GTPγS induced by CPA or baclofen alone. The reactions were stopped by vacuum filtration through GF/C filters, followed by washing with ice-cold buffer.
In Vivo Drug Administration
Mice were randomly assigned to various groups and habituated to the handling during 5 days before testing began. For chronic treatment with caffeine, animals received caffeine (3 mg/kg/day), or vehicle (saline: 0.9% NaCl), >12 h before trials, for at least 15 days before experimental days, and throughout the course of behavioral testing, in order to avoid withdrawal effects. Total caffeine exposure was for 22–24 days, and euthanization occurred 24 h after last injection. The half-life of caffeine for doses lower than 10 mg/kg ranges from 0.7 to 1.2 h in the rat and mouse (Fredholm et al, 1999); therefore, the estimated concentrations of caffeine present in plasma or brain during behavioral testing were negligible. For acute administration, animals received a single dose of vehicle (8% Tween-80 in saline), THC (5 mg/kg), AM251 (3 mg/kg), DPCPX (1 mg/kg), or WIN55,212-2 (1 mg/kg). THC was prepared in Tween-80 as described previously (Pertwee et al, 1992); AM251, DPCPX, and WIN55,212-2 were suspended in the vehicle and carefully sonicated. All drugs were given by intraperitoneal injection in a volume of 2 ml/kg weight. The concentration of Tween-80 used was previously shown not to affect motor activity in mice (Castro et al, 1995).
Water Maze Experiments (Trials to Criterion Task)
We performed two separate sets of water maze experiments, in which mice were randomly assigned to four experimental groups of seven to eight subjects (total of 57 animals). The protocol is a version of the Morris water maze test that is sensitive to hippocampal-dependent short-term spatial learning (Chen et al, 2000; Daumas et al, 2007). To form a stable representation of the environment, mice were first trained to quickly find a hidden platform at a fixed platform location for 5 consecutive days. Subjects then performed several tasks, each consisting of a new platform position. Each animal was given a maximum of eight trials per day, to perform the task until reaching a performance criterion of ⩽7 s average latency on three consecutive trials. A 15 min intertrial interval was applied, during which animals were allowed to dry under a ceramic heat lamp. Once the criterion was reached, trials stopped and a new task began on the following testing day. Animals first performed 4–5 training tasks in order to learn to optimize their search strategies, and then the effects of acute drug administration were tested in separate tasks, as described in the Results section.
A black infrared-translucent Perspex tank (1 m in diameter) of water (temperature, 22±1 °C) was placed over an infrared lightbox (Tracksys, Nottingham, UK) in a room with various visible external cues. A transparent platform was ∼0.5 cm below the water surface and its position varied between several possible locations, on two concentric circles, according to the original protocol. An infrared-sensitive automated tracking system (Noldus Ethovision 7.0, Noldus Information Technology, Wageningen, the Netherlands) monitored all performances.
Statistical Analysis
Statistical significance was tested using paired Student's t-test, one- or two-way analysis of variance (ANOVA), followed by Dunnett's or Tukey's post hoc tests, as indicated. The two-way ANOVA and post hoc tests were performed using the Predictive Analytics Software 18.0 (SPSS, an IBM Company, Chicago, IL). GraphPad Prism 5.0 (GraphPad Prism Software, San Diego, CA, USA) was used for all other statistical tests and nonlinear regression curve fitting. Differences in parameters between binding curves were tested using extra sum-of-squares F test.
RESULTS
A1 Receptor Activation Attenuates the CB1 Receptor-Dependent Inhibition of [3H]GABA and [3H]Glutamate Release from Rat Hippocampal Nerve Terminals
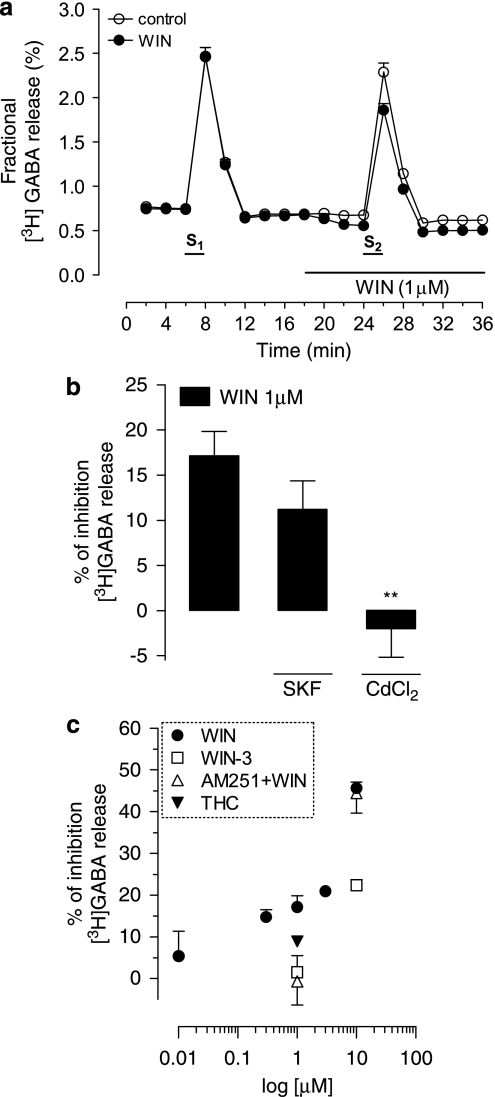
In control conditions, pooling data from all experiments performed, the average basal release of [3H]GABA from rat hippocampal synaptosomes was 0.76±0.02% (n=31, average of first 6 min of collection; Figure 1a) of the total tritium retained by synaptosomes at the same time points. Depolarization of the hippocampal synaptosomes with K+ (15 mM) for 2 min induced a threefold increase in the [3H]GABA release with an average peak of 2.5±0.1% during S1, and 2.3±0.1% (n=31; Figure 1a) during S2, giving an average S2/S1 of 0.94±0.01. Depolarization by K+ mainly induced a calcium-dependent release of [3H]GABA, as blockade of VGCCs by Cd2+ (CdCl2, 200 μM) inhibited its release by 70±3.0% (n=8, p<0.001, data not shown). The GABA transporters account for the remaining percentage of [3H]GABA released upon K+ depolarization, as blockade of GABA transporters with SKF89976a (20 μM) inhibited its release by 34±1.6% (n=8, p<0.001, data not shown).
Figure 1.
Inhibition of K+-evoked, Ca2+-dependent release of [3H]GABA from rat hippocampal synaptosomes by WIN55,212-2 (WIN). (a) Fractional release of [3H]GABA evoked by two 15 mM K+ stimuli of 2-min duration, as indicated (S1 and S2); in the test assay, WIN (1 μM) was applied before S2, as indicated by the horizontal bar. Data represent mean±standard error of mean (SEM) from 31 experiments performed in duplicate. (b) Percentage inhibition of [3H]GABA release induced by WIN (1 μM) in the absence or in the presence of the calcium channel blocker, CdCl2 (Cd2+, 200 μM), or the GABA transporter inhibitor, SKF89976A (SKF, 20 μM), as indicated below each bar. Note that Cd2+ fully blocked the effect of WIN (n=5, **p<0.01, paired Student's t-test vs effect of WIN alone within the same batch of synaptosomes), whereas SKF did not alter the WIN-induced inhibition (n=5, p>0.05). Data represent mean±SEM from five experiments, performed in duplicate. (c) Concentration-dependent inhibition of K+-evoked release of [3H]GABA induced by WIN (0.01–10 μM) in the absence or in the presence of the CB1 receptor antagonist, AM251 (1 μM); the effect of partial CB1 receptor agonist, Δ9-tetrahydrocannabinol (THC) (1 μM), as well as of a WIN enantiomer that is inactive at the CB1 receptor, WIN55,212-3 (WIN-3; 1–10 μM), is also shown. WIN significantly inhibited [3H]GABA release at all concentrations (p<0.01), except for the lowest concentration tested (0.01 μM, p>0.05); THC (1 μM) also significantly inhibited [3H]GABA release (p<0.01). Note that WIN-3 was devoid of the effect at 1 μM (p>0.05), but not at 10 μM (p<0.01), and that AM251 antagonized the effect of 1 μM, but not of 10 μM WIN, indicating that WIN is CB1 receptor selective at 1 μM, but not at 10 μM. Each point represents the mean±SEM of 4–10 independent experiments performed in duplicate, except (n=2) for 0.01 and 3 μM WIN, and 1 μM WIN-3. The S2/S1 values from corresponding controls were taken as 0% within each experiment. P-values were obtained by a one-way analysis of variance (ANOVA) test with Dunnett post hoc, compared with control (0%). SKF, Cd2+, and AM251 were applied 15 min before the start of sample collection and were continuously perfused throughout the experiment, being therefore present during S1 and S2 (S1+S2); WIN, WIN-3, and THC were added before S2 (see Materials methods for further details).
To induce a CB1 receptor-dependent effect on [3H]GABA release, we used the potent cannabinoid receptor agonist WIN55,212-2, which has been previously shown to inhibit evoked [3H]GABA release from hippocampal synaptosomes through a CB1-specific mechanism, having a maximum CB1-selective effect at 1 μM (Köfalvi et al, 2007). Application of 1 μM WIN55,212-2 6 min before S2 caused a decrease of basal [3H]GABA outflow and inhibited evoked GABA release (Figure 1a) with an average S2/S1 of 0.78±0.01 (n=31) that represents an inhibition of 16.7±1.4% (n=31), when compared with control S2/S1 within each experiment. Blockade of VGCCs by Cd2+ (CdCl2, 200 μM) completely abolished the effect of WIN55,212-2 at its maximum CB1-specific concentration (n=5, p<0.01; Figure 1b). Conversely, blockade of GABA transporters with SKF89976a (20 μM) did not alter the effect of WIN55,212-2 (n=5; Figure 1b), which suggests that the effect of 1 μM WIN55,212-2 upon [3H]GABA release is exerted through the inhibition of Ca2+-dependent exocytotic release. The effect of WIN55,212-2 (0.01–10 μM) on K+-evoked [3H]GABA release was concentration dependent (Figure 1c). As WIN55,212-2 is known to directly block N-type VGCCs at concentrations above 1 μM (Németh et al, 2008; Shen and Thayer, 1998), we tested the specificity of its effect in our preparation using the CB1 antagonist AM251, as well as WIN55,212-3, an enantiomer of WIN55,212-2 that does not activate the CB1 receptor, but maintains the Ca2+ channel-blocking properties (Shen and Thayer, 1998). AM251 (1 μM) fully blocked the effect of 1 μM, but not of 10 μM of WIN55,212-2 (Figure 1c). Higher concentrations of AM251 were not used to avoid loss of selectivity (see Köfalvi, 2007, 2008). The enantiomer had no significant effect applied at 1 μM, but it inhibited evoked [3H]GABA release by 22.4±1.4% at 10 μM (n=5, p<0.05; Figure 1c), which indicates that the effect of 1 μM WIN55,212-2 upon [3H]GABA release is CB1 receptor dependent. It is worth noting that the effect of 10 μM WIN55,212-2 was larger than the effect of 10 μM WIN55,212-3, which indicates that the effect of 10 μM WIN55,212-2 still encompasses a CB1 receptor-dependent component. The partial CB1 agonist THC (1 μM) inhibited K+-evoked [3H]GABA release by 8.9±0.9% (n=8, p<0.05; Figure 1c).
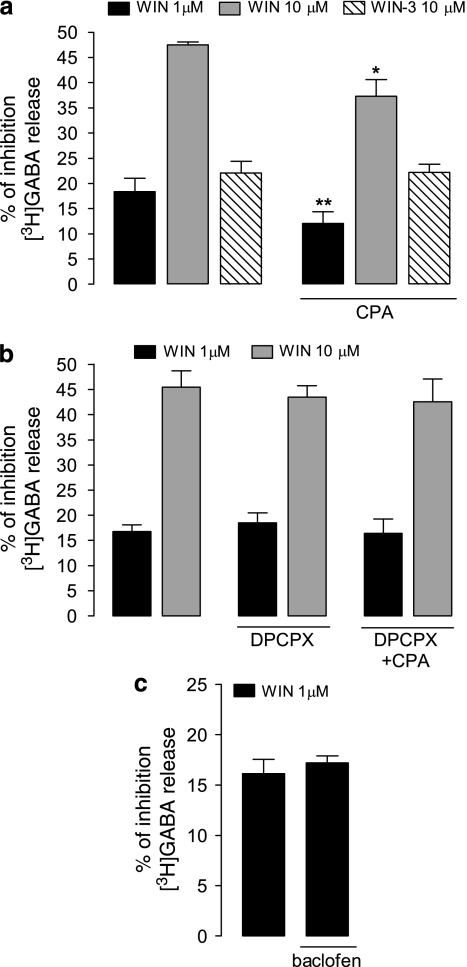
Consistent with previous observations (Cunha and Ribeiro, 2000), adenosine A1 receptor activation with the selective agonist CPA (100 nM) before S2 did not affect K+-evoked [3H]GABA release (2.7±3% of control S2/S1, n=3, p>0.05, data not shown). To evaluate the influence of A1 receptors on the CB1-mediated inhibition of GABA release, we tested the effect of WIN55,212-2 (applied before S2) in the presence of CPA (100 nM, applied throughout the experiment, S1+S2). Under these conditions, the effect of WIN55,212-2 at 1 μM (n=10) and at 10 μM (n=7) was significantly attenuated (p<0.01 and <0.05, respectively; Figure 2a). The average effect of 1 μM WIN55,212-2 was 18±3% (n=10) and CPA attenuated this effect to 12±2% (p<0.01, n=10), which represents a 33±13% decrease in the average effect of WIN55,212-2 alone. To test if the attenuation of the effect of 10 μM WIN55,212-2 caused by CPA occurred through a Ca2+ channel-dependent mechanism and not involving CB1 receptors, we performed the experiments using WIN55,212-3. Application of WIN55, 212-3 (10 μM, S2) by itself inhibited [3H]GABA release by 22±2%, whereas in the presence of CPA (100 nM), its effect was unaltered (22±2%, n=3; Figure 2a). This indicates that the CPA-induced attenuation of the effect of WIN55, 212-2 was exerted at the CB1 receptor-dependent component, but not upon the Ca2+channel-dependent mechanisms, affected by high micromolar concentrations of WIN55,212-2. The blockade of A1 receptors with the antagonist DPCPX (50 nM) did not, on its own, alter the effects of 1 and 10 μM WIN55,212-2, but it fully prevented the CPA-induced attenuation (p>0.05, n=7, paired Student's t-test; Figure 2b).
Figure 2.
A1 receptor activation significantly attenuates the CB1-mediated inhibition of K+-evoked [3H]GABA release from rat hippocampal synaptosomes. (a) Effects of WIN55,212-2 (WIN, 1 and 10 μM) and of its CB1 receptor-inactive enantiomer, WIN55,212-3 (WIN, 3, 10 μM), in the absence or in the presence of the selective A1 receptor agonist, CPA (100 nM), as indicated below each column. Note that CPA significantly attenuated the effect of 1 and 10 μM WIN (**p<0.01, *p<0.05, respectively, compared with the effect of WIN in the absence of CPA, in the same experiments), whereas the effect of WIN55,212-3 (WIN-3) (10 μM) was unaffected by CPA (p>0.05). (b) WIN (1 and 10 μM) was tested in the absence or in the presence of selective A1 receptor antagonist, DPCPX (50 nM), alone and in combination with CPA (100 nM). (c) WIN (1 μM) was tested in the absence or in the presence of the selective GABAB receptor agonist, baclofen (10 μM), as indicated below the column. Note that in the presence of DPCPX, CPA did not attenuate the inhibitory effect of WIN (p>0.05), and that baclofen did not modify the effect of WIN (1 μM) (p>0.05). Bars represent the mean±standard error of mean (SEM) of 3–10 individual experiments performed in duplicate. The S2/S1 values from controls were taken as 0% within each experiment. P-values were obtained by paired Student's t-test, compared with corresponding controls within the same batch of synaptosomes. WIN and WIN-3 were added before S2, whereas the other drugs were applied 15 min (CPA or baclofen) or 30 min (DPCPX) before the start of sample collection, being therefore present during S1 and S2 (see Materials and methods for further details).
The signaling pathways of CB1, A1, and GABAB are known to converge when co-expressed in cerebellar neurons (Selley et al, 2004). Furthermore, both CB1 and GABAB receptors are present in inhibitory interneurons (Katona et al, 1999; Sloviter et al, 1999), couple to the same Gαi/o-subunits (Straiker et al, 2002), and exhibit reciprocal inhibition (Cinar et al, 2008) in hippocampal neurons. We therefore evaluated whether the A1 receptor-dependent attenuation of the effect of WIN55,212-2 was mimicked by the activation of GABAB receptors. As shown in Figure 2c, the effect of WIN55,212-2 (1 μM) was unchanged (p>0.05, n=5, paired Student's t-test) by the presence of the GABAB receptor agonist baclofen (10 μM). Altogether, these results indicate that the cannabinoid CB1 receptors in GABAergic nerve terminals are under the modulatory influence of adenosine A1 receptors, but not GABAB receptors.
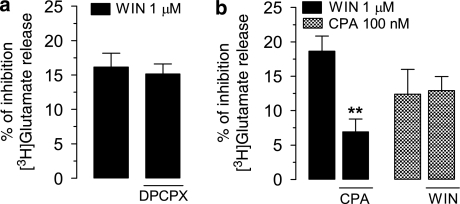
Despite the predominant influence of CB1 receptors in hippocampal circuitry being exerted through the inhibition of GABA release, CB1 receptors at glutamatergic presynaptic nerve terminals (Katona et al, 2006; Kawamura et al, 2006) also inhibit the K+-evoked release of glutamate (eg, Cannizzaro et al, 2006; D'Amico et al, 2004; Köfalvi et al, 2007). Importantly, the regulation of excitatory synaptic transmission is the most relevant role of the hippocampal A1 receptors (Dunwiddie and Fredholm, 1989; Sebastião et al, 1990). To investigate whether A1 receptors also regulate the CB1-dependent effects upon glutamate release, the influence of A1 receptor activation on the CB1-mediated inhibition of K+-evoked [3H]glutamate release was also tested. The absence of a tonic activation of A1 receptors by endogenous adenosine was first assessed by using A1 receptor blocker DPCPX. As Figure 3a shows, WIN55,212-2 (1 μM) inhibited the release of [3H]glutamate by 16±2% and blockade of A1 receptors with DPCPX (50 nM) did not modify the effect of WIN55,212-2 (15±1%, p>0.05, n=4). This indicates that endogenous adenosine was effectively washed out by the continuous vertical flow of superfusion medium (see Materials and methods).
Figure 3.
A1 receptors modulate the CB1-mediated inhibition of [3H]glutamate. (a) Blockade of A1 receptors by DPCPX (50 nM) did not modify (p>0.05, n=4) the effect of WIN55,212-2 (WIN, 1 μM). (b) The inhibition induced by WIN (1 μM) was significantly (**p<0.01, n=5) attenuated by CPA (100 nM), but the effect of CPA alone (applied before S2) was not modified (p>0.05, n=4) when applied in the presence of WIN (1 μM). Bars represent the mean±standard error of mean of 4–5 individual experiments performed in duplicate. The S2/S1 values from controls were taken as 0% within each experiment. P-values were obtained by paired Student's t-test, compared with corresponding controls within the same batch of synaptosomes.
As shown in Figure 3b, the inhibitory effect of 1 μM WIN55,212-2 upon glutamate release (19±2%) was significantly attenuated to 7±2% (n=5, p<0.01; Figure 3b) in the presence of CPA. We then evaluated whether the A1 receptor-mediated inhibition of glutamate release is also under the modulatory control of CB1 receptors, by comparing the effect of CPA (before S2) in the absence and in the presence of 1 μM WIN55,212-2. CPA (100 nM) inhibited [3H]glutamate release by 12±4%, and this effect was not modified in the presence of 1 μM WIN55,212-2 (13±2%, n=4, p>0.05; Figure 3b). These findings further indicate that A1 receptors negatively modulate the CB1-mediated effects in the hippocampus and support recent evidence that CB1-mediated inhibition of excitatory synaptic transmission in the hippocampus is modulated by A1 receptor activation (Hoffman et al, 2010).
A1 Receptor Activation Attenuates CB1 Receptor-Induced Stimulation of G Proteins in Rat Hippocampal Membranes
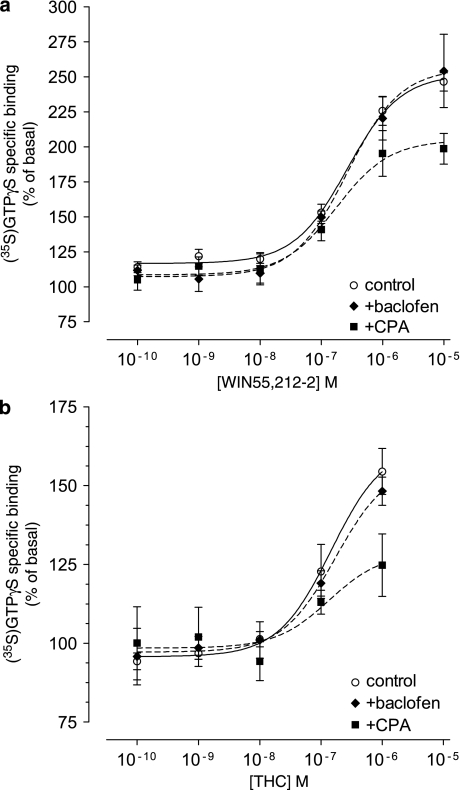
To test whether the adenosine–cannabinoid interaction occurs at the level of G-protein activation, we measured agonist-stimulated [35S]GTPγS binding in hippocampal membranes with the full agonist of CB1 receptors, WIN55, 212-2, or with the partial agonist THC, in the absence and in the presence of A1 receptor agonist CPA. The basal [35S]GTPγS binding in the absence of WIN55,212-2 (Figure 4a) or THC (Figure 4b) is represented as 100% in the ordinates, which corresponds to (fmol/mg protein): Figure 4a—149±16 in control (○, n=7), 311±50 in the presence of CPA (▪, n=4), and 331±74 in the presence of baclofen (♦, n=4); and Figure 4b—98±4 in control (○, n=4), 255±25 in the presence of CPA (▪, n=4), and 161±15 in the presence of baclofen (♦, n=4). When applied alone, WIN55,212-2 (0.1 nM–10 μM) concentration dependently stimulated [35S]GTPγS binding, with an EC50 ≈255 nM and Emax=251±7% (n=7; Figure 4a and Table 1). CPA (100 nM) by itself induced a 150±12% net increase from basal [35S]GTPγS binding (n=4). Co-application of WIN55,212-2 (0.1 nM–10 μM) with 100 nM CPA (Figure 4a) significantly decreased the Emax of WIN55,212-2 to 204±10% (p<0.001, n=4; Table 1), but not the EC50 (≈177 nM; Table 1). This indicates a functional interaction between colocalized CB1 and A1 receptors in hippocampal membranes, which impacts on the ability of CB1 receptors to activate Gαi/o proteins. Similarly, the co-application of THC (0.1 nM–1 μM) with 100 nM CPA (Figure 4b) significantly decreased the Emax of THC from 163±8% (when applied alone) to 129±13% (n=4, p<0.05; Table 1), but not the EC50.
Figure 4.
Influence of A1 or GABAB receptor activation on CB1-induced stimulation of G proteins, as assayed by WIN55,212-2 or Δ9-tetrahydrocannabinol (THC)-induced [35S]GTPγS binding. Rat hippocampal membranes (10 μg protein) were incubated for 30 min at 37 °C with 30 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of (a) WIN55,212-2 (0.1 nM–10 μM) or (b) THC (0.1 nM–1 μM), alone (○) or in combination with 100 nM CPA (▪), or 100 μM baclofen (♦). Emax and log EC50 values are shown in Table 1. Data represent mean percentage of basal stimulation±SEM of n=7 (○) and n=4 (▪, ♦), performed in duplicate. Non-visible error bars are within symbols.
Table 1. Emax and Log EC50 Values of Agonist-Stimulated [35S]GTPγS Binding in Rat Hippocampal Membranes.
| EC50 | Emax | |
|---|---|---|
| −log, M | % of stimulation | |
| WIN | −6.589±0.11 | 251±7 |
| WIN+CPA | −6.752±0.23 | 204±10a |
| WIN+baclofen | −6.554±0.20 | 257±12 |
| THC | −6.837±0.20 | 163±8 |
| THC+CPA | −6.870±0.72 | 129±13a |
| THC+baclofen | −6.765±0.26 | 157±9 |
Note that the A1 receptor agonist CPA (100 nM) significantly decreased the Emax of WIN55,212-2 (WIN) and THC-stimulated [35S]GTPγS binding, but not the EC50. The GABAB receptor agonist baclofen (100 μM) had no significant influence. Data represent mean values±SEM (n=4) obtained from nonlinear regression analyses of the data shown in Figure 3.
p<0.05, compared with appropriate control, calculated using the extra sum-of-squares F test.
To examine if other Gαi/o-coupled receptors are also capable of interfering with the G-protein coupling of CB1 receptors, we tested whether combined activation of CB1 and GABAB receptors in the hippocampus would also affect the efficacy of WIN55,212-2 in [35S]GTPγS binding. To activate GABAB receptors, we used 100 μM baclofen, which by itself induced a 118±11% net increase from basal [35S]GTPγS binding (n=4), which was not significantly different from the effect of 100 nM CPA. As shown in Figure 4a and b and Table 1, 100 μM baclofen did not affect the WIN55,212-2-induced (Emax=257±12%, n=4) or THC-induced (Emax=157±9%, n=4) stimulation of [35S]GTPγS binding. The reduced efficacy in stimulation of [35S]GTPγS binding by CB1 with A1, but not GABAB, suggests that the adenosine A1 receptors play a specific role in modulating CB1 signaling in hippocampal presynaptic terminals.
Chronic Caffeine Administration Increases Acute THC-Induced Spatial Memory Deficits in Mice
The evidence that A1 receptor activation attenuates CB1 receptor signaling raised the hypothesis that this A1–CB1 interplay has a functional impact upon hippocampal-dependent memory. Chronic caffeine consumption is known to induce an increase in adenosine A1, but not A2A receptors (reviewed by Jacobson et al, 1996). Acute systemic THC administration induces CB1-dependent deficits in working memory (Wise et al, 2009). Therefore, we used a hippocampal-dependent, short-term spatial memory testing protocol (Chen et al, 2000; see Materials and methods) to study the effects of chronic caffeine administration on the memory deficits induced by an acute systemic THC injection in mice. Two separate sets of experiments were performed, in which caffeine (3 mg/kg/day), or vehicle, was administered >12 h before trials, and for at least 15 days before the first test with THC.
During the training phase, all subjects learned to perform efficiently at all test parameters, in both sets of experiments (see Figures 5a–c and 6a–c). The number of trials, the total latency, and total pathlength needed to reach the criterion decreased progressively from task to task reaching a plateau in the last training task. A repeated measures two-way ANOVA on these parameters revealed a significant overall learning effect. For ‘pathlength' (representative parameter of memory performance), significance values were: F(4, 100)=10.2, p<0.0001 (Figure 5c); and F(3, 72)=13.5, p<0.0001 (Figure 6c). The average swim speed (control parameter for motor activity) was constant throughout the training tasks (Figure 5d: F(4, 100)=0.4, p=0.8; Figure 6d: F(3, 72)=1, p=0.4), with no significant differences between groups (Figure 5d: F(3, 25)=1.8, p=0.2; Figure 6d: F(3, 24)=0.2, p=0.9). In both sets of experiments, there were no differences between groups and no ‘group × task' interaction in any parameter during the training period (p>0.05 for all parameters). Thus, chronic caffeine administration by itself did not affect memory performance or motor activity.
Figure 5.
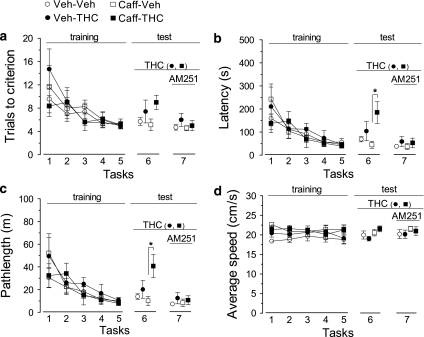
Influence of chronic caffeine administration upon the Δ9-tetrahydrocannabinol (THC)-induced short-term spatial memory deficits in mice. Caffeine was given daily (3 mg/kg, >12 h before trials), for 15 days before testing the effect of THC (see Methods). (a–d) Mice familiarized with the escape strategies during the first 5 tasks (training) and all groups showed improved performance in (a) the number of trials to reach criterion, (b) the escape latency, (c) swim pathlength, whereas (d) average swim speed remained constant. THC (5 mg/kg), or vehicle, was then tested in the absence (task 6) and in the presence (task 7) of AM251 (3 mg/kg). Subjects rested for 1 day off drug after task 6, to allow full metabolization of THC. For clarity of comparison between groups, symbols were nudged at tasks 6 and 7. Note that chronic caffeine exacerbated the spatial memory deficits induced by acute THC, and this effect of THC was fully prevented by previous administration of AM251. All data represent mean±standard error of mean (SEM) of n=7–8. *p<0.05, two-way analysis of variance, followed by Tukey's post hoc test (see text for more details).
Figure 6.
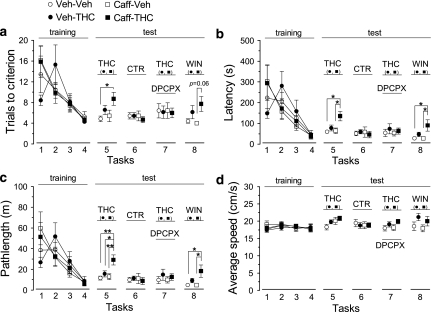
Influence of chronic caffeine administration, and involvement of the adenosine A1 receptors, upon the acute effects of Δ9-tetrahydrocannabinol (THC) and WIN55,212-2 on mice tested in a short-term spatial memory task. Caffeine was given daily (3 mg/kg, >12 h before trials), for 15 days before testing the effect of THC (see Methods). (a–d) Mice familiarized with the escape strategies during the first 4 tasks (training) and all groups showed improved performance in (a) the number of trials to reach criterion, (b) the escape latency, and (c) swim pathlength, whereas (d) average swim speed remained constant. THC (5 mg/kg), or vehicle, was tested in the absence (task 5) and in the presence (task 7) of DPCPX (1 mg/kg). A control (CTR) test in the absence of acute drugs was performed at task 6 to measure whether performance levels returned to baseline values. Subjects rested for one day off drug after each test task, to allow full metabolization of THC. The effect of WIN55,212-2 (WIN, 1 mg/kg), or vehicle, was tested at task 8. For clarity of comparison between groups, symbols were nudged in tasks 5–8. Note that chronic caffeine exacerbated the spatial memory deficits induced by acute THC and WIN. The effect of THC was prevented by the previous administration of DPCPX. All data represent mean±standard error of mean (SEM) of n=7. *p<0.05, **p<0.01, two-way analysis of variance, followed by Tukey's post hoc tests (see text for more details).
For the first set of experiments, the effect of THC (5 mg/kg), or vehicle, given at task 6 (30 min before first trial), as well as the modification of this effect by AM251 (3 mg/kg), given at task 7 (15 min before THC, or vehicle), are displayed in Figure 5a–d. After completion of task 6, each subject rested for 1 day to allow for the metabolic clearance of THC.
There was a significant effect of THC on ‘trials to criterion' (F(1, 25)=4.24, p=0.05), ‘latency' (F(1, 25)=6.98, p=0.01), and ‘pathlength' (F(1, 25)=7.18, p=0.01), but no significant effect on ‘average speed' (F(1, 25)<0.001, p=0.98). There were no effects of chronic caffeine treatment on all parameters, but a marginally significant ‘chronic caffeine × THC' interaction on ‘pathlength' (F(1,25)=3.11, p=0.09). Although acute THC injection (vs vehicle) did not induce significant effects in the control (vehicle-treated) group at any parameter, the effect of THC was exacerbated in the chronic caffeine group, on ‘latency' (p=0.03, Tukey's post hoc; Figure 5b) and ‘pathlength' (p=0.02, Tukey's post hoc; Figure 5c).
When mice received AM251 pretreatment, there were no significant effects of any treatment group on all parameters, indicating that the effects of THC were dependent on the activation of CB1 receptors. These results show that chronic caffeine exacerbates the CB1-dependent actions of THC in a short-term spatial memory task.
For the second set of experiments, the effect of THC (5 mg/kg), or vehicle, given at task 5 (30 min before first trial), the modification of this effect by DPCPX (1 mg/kg), given at task 7 (15 min before THC, or vehicle), as well as the effect of WIN55,212-2 (1 mg/kg), or vehicle, given at task 8 (30 min before first trial), are displayed in Figure 6a–d. After completion of each test task, subjects rested for one day off-drug to allow for metabolic clearance of THC. Task 6 was a control test in which no acute drug was given, to measure whether performance levels returned to baseline values 48 h after acute THC administration.
Consistent with the first set of experiments, there was a significant effect of THC on ‘trials to criterion' (F(1, 24)=6.34, p=0.02), ‘latency' (F(1, 24)=8.16, p=0.01), and ‘pathlength' (F(1, 24)=9.19, p=0.01), but no significant effect on ‘average speed' (F(1, 24)=2.09, p=0.16). On ‘pathlength' there was also a significant effect of chronic caffeine treatment (F(1, 24)=4.82, p=0.04), and a marginally significant ‘chronic caffeine × THC' interaction (F(1, 24)=3.29, p=0.08). Although acute THC injection (vs vehicle) did not induce significant effects in the control (vehicle-treated) group at any parameter, the effect of THC was exacerbated in the chronic caffeine group, on ‘latency' (p=0.02, Tukey's post hoc; Figure 6b) and ‘pathlength' (p=0.01, Tukey's post hoc; Figure 6c). There were also significant differences between THC on the chronic caffeine group vs THC in the vehicle group on ‘pathlength' (p=0.04), and between THC on the chronic caffeine group vs the vehicle control group on ‘trials to criterion' (p=0.05), ‘latency' (p=0.01), and ‘pathlength' (p=0.006).
When mice were tested 48 h after the last THC injection, there were no significant effects of any treatment group on all parameters, indicating that the effects of THC were not prevailing after this period. Importantly, when mice received a pretreatment of DPCPX, there were also no significant effects of any treatment group on all parameters, indicating that the effects of THC were reversed by the blockade of A1 receptors.
Finally, there was a significant effect of WIN55,212-2 on ‘trials to criterion' (F(1, 24)=7.64, p=0.01), ‘latency' (F(1, 24)=7.50, p=0.01), and ‘pathlength' (F(1, 24)=7.74, p=0.01), but no significant effect on ‘average speed' (F(1, 24)=4.03, p=0.06). The acute injection of WIN55, 212-2 (vs vehicle) did not induce significant effects in the control (vehicle-treated) group at any parameter, but its effects were exacerbated in the chronic caffeine group, on ‘latency' (p=0.03, Tukey's post hoc; Figure 6b) and ‘pathlength' (p=0.03, Tukey's post hoc; Figure 6c). There were also significant differences between WIN55,212-2 on the chronic caffeine group vs the vehicle control group on ‘latency' (p=0.03) and ‘pathlength' (p=0.03). The lower effect of WIN55,212-2, compared with that of THC, could be owing to pharmacokinetic differences, as the penetration of WIN55,212-2 in the brain following intraperitoneal injection is much lower than that of THC (Petitet et al, 1999). Higher doses of WIN55,212-2 were not used to avoid nonspecific effects (Varvel and Lichtman, 2002). These findings show a significant chronic caffeine-induced, and A1 receptor-mediated, exacerbation of the CB1-dependent effects on short-term spatial memory.
Chronic Caffeine and A1 Receptor Number
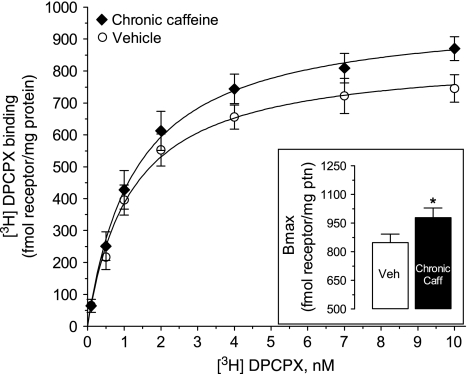
To quantify the influence of chronic caffeine administration upon A1 receptor number and affinity in cortico-hippocampal membranes, [3H]DPCPX (0.1–10 nM) binding assays were performed (Figure 7). In vehicle-treated mice, the total number of specific binding sites obtained by nonlinear regression analysis (Bmax) was 848±44 fmol/mg of protein, whereas the equilibrium dissociation constant (KD) was 1.20±0.21 nM. In the chronic caffeine group, the Bmax was increased to 980±50 fmol/mg of protein (p<0.05, n=6, vs vehicle group), but KD (1.31±0.23 nM) was not significantly (p>0.05) affected. Thus, animals under chronic caffeine had ∼16% higher density of A1 receptor without changes in affinity.
Figure 7.
Saturation analysis of specific [3H]DPCPX binding (0.1–10 nM) to cortico-hippocampal membranes (40 μg protein) from chronic caffeine- (3 mg/kg/day, for 22 days, ♦) and vehicle- (○) treated mice. Inset: Bmax obtained from nonlinear regression analysis. Nonspecific binding was determined at all [3H]DPCPX concentrations by the addition of 2 μM XAC. All points represent mean±standard error of mean (SEM) of n=6, and each saturation experiment was performed in duplicate. *p<0.05, vs control, calculated using the extra sum-of-squares F test.
Chronic Caffeine and CB1 Receptor Signaling in Mouse Cortico-Hippocampal Tissue
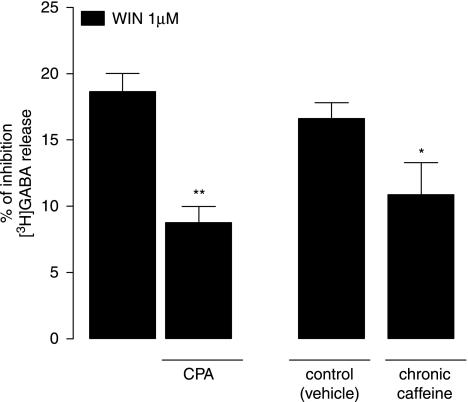
We first tested the consistency of our in vitro results between rats and mice by testing the effect of CPA (100 nM) on the WIN55,212-2-mediated inhibition of K+-evoked [3H]GABA release from cortico-hippocampal synaptosomes prepared from untreated mice (Figure 8). Consistent to previous observations in rats, the effect of 1 μM WIN55,212-2 alone was 19±1%, and it was significantly attenuated to 9±1% in the presence of CPA (p<0.01, n=4, paired Student's t-test; Figure 8). We then analyzed the influence of chronic caffeine administration upon the CB1 receptor-mediated inhibition of K+-evoked [3H]GABA release (Figure 8). In control (vehicle-treated) mice, 1 μM WIN55, 212-2 inhibited [3H]GABA release by 17±1%, whereas in the chronic caffeine group, the effect of WIN55,212-2 was significantly reduced to 11±2% (p<0.05, n=4, paired Student's t-test; Figure 8).
Figure 8.
Influence of the adenosine A1 receptor agonist, CPA (100 nM), and of chronic caffeine administration on the CB1-mediated inhibition of K+-evoked [3H]GABA release from mouse cortico-hippocampal synaptosomes. WIN55,212-2 (WIN, 1 μM) was tested in the absence and in the presence of CPA on synaptosomes prepared from untreated mice, as well as on synaptosomes prepared from chronic caffeine- (3 mg/kg/day, for 22 days) or vehicle-treated mice, as indicated below each column (see legend to Figure 1 for details). Note that the effect of WIN was significantly attenuated by CPA as well as by chronic caffeine consumption. Bars represent mean±SEM of four experiments, performed in duplicate. **p<0.01, compared with the effect of WIN alone; *p<0.05, compared with the effect of WIN in vehicle-treated mice (paired Student's t-test).
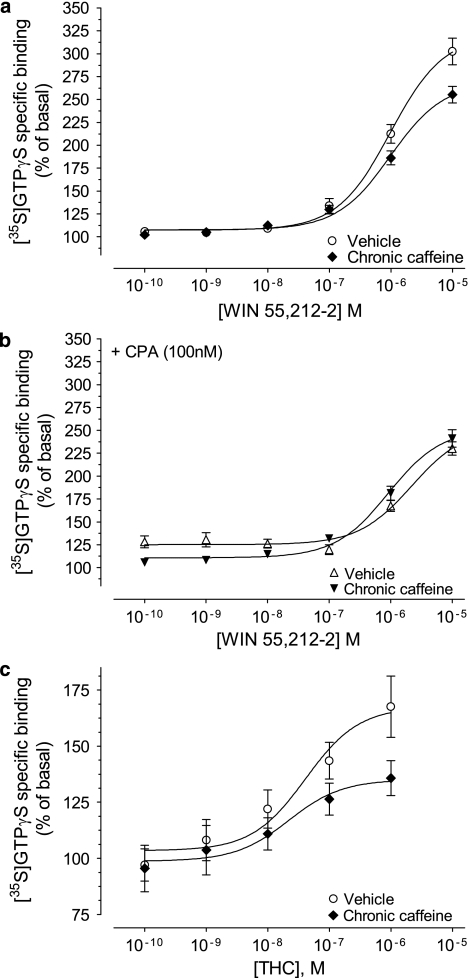
As Figure 9 and Table 2 show, in cortico-hippocampal membranes of vehicle-treated mice, WIN55,212-2 stimulated [35S]GTPγS binding (% of basal) with an EC50 ≈989 nM and Emax=321±11% (n=5; Figure 9a), whereas THC had an EC50 ≈41 nM and Emax=167±11% (n=4; Figure 9c). Chronic caffeine administration did not affect the EC50 of WIN55,212-2 or THC, but it significantly reduced the Emax of WIN55,212-2 to 269±8%, and of THC to 135±8% (p<0.05, n=4–5, extra sum-of-squares F test; Figure 9a and c). As observed in rats (Figure 4a), the co-application of 100 nM CPA in control mice significantly decreased the Emax of WIN55,212-2 (254±13%, p<0.05, n=5, extra sum-of-squares F test; Figure 9b and Table 2), but not the EC50. This reduction in WIN55,212-2-stimulated [35S]GTPγS binding, caused by CPA in membranes from control animals, was of similar magnitude as the decrease observed in the chronic caffeine-treated group in the absence of CPA (Table 2). In the chronic caffeine group, CPA did not induce a further decrease in the efficacy of WIN55,212-2 to stimulate G-protein activation (Emax=252±8% Figure 9a and b and Table 2), which may suggest that chronic caffeine treatment and A1 receptor activation do not have additive effects upon the modification of CB1 receptor signaling.
Figure 9.
Influence of chronic caffeine administration on CB1-induced stimulation of G proteins, as assayed by WIN55,212-2- or Δ9-tetrahydrocannabinol (THC)-induced [35S]GTPγS binding. Cortico-hippocampal membranes (10 μg protein) from chronic caffeine- (♦,▾) and vehicle- (○, ▵) treated mice were incubated for 30 min at 37 °C with 30 μM GDP, 0.1 nM [35S]GTPγS, and varying concentrations of WIN (0.1 nM–10 μM) in the absence (a) or in the presence of 100 nM CPA (b), or varying concentrations of THC (0.1 nM–1 μM) (c). Emax and log EC50 values are shown in Table 2. Data represent mean percentage of basal stimulation±standard error of mean (SEM) of n=5 (a, b) and n=4 (c), performed in duplicate. Non-visible error bars are within symbols.
Table 2. Emax and Log EC50 Values of WIN55,212-2 and THC-stimulated [35S]GTPγS Binding in Mouse Cortico-hippocampal Membranes.
| EC50 | Emax | |
|---|---|---|
| −log, M | % of stimulation | |
| Vehicle | ||
| WIN | −6.005±0.09 | 321±11 |
| WIN+CPA | −5.654±0.16 | 254±13a |
| THC | −7.389±0.37 | 167±11 |
| Chronic caffeine | ||
| WIN | −6.006±0.08 | 269±8a |
| WIN+CPA | −6.035±0.10 | 252±8a |
| THC | −7.660±0.56 | 135±8a |
Note that the Emax, but not the EC50, of WIN55,212-2 (WIN) and THC-stimulated [35S]GTPγS binding was significantly decreased in the chronic caffeine group (3 mg/kg/day, for 22 days), compared with control (vehicle-treated) mice. The A1 receptor agonist, CPA (100 nM), reduced the Emax of WIN55,212-2 in control mice, but did not further decrease the Emax of WIN55,212-2 in chronic caffeine-treated mice. The EC50 of WIN in either vehicle or chronic caffeine groups was not significantly affected by CPA (p>0.05, extra sum-of-squares F test). Data represent mean values±SEM (n=4–5) obtained from nonlinear regression analyses of the data shown in Figure 8.
p<0.05, versus corresponding control, calculated using the extra sum-of-squares F test.
The basal [35S]GTPγS binding in the absence of WIN55,212-2 (Figure 9a and b) or THC (Figure 9c) is represented as 100% in the ordinates, which corresponds to (fmol/mg protein): Figure 9a and b—143±10 (○, n=5), 154±15 (♦, n=5), 324±13 (▵, n=5), and 327±25 (▾, n=5); Figure 9c—53±9 (○, n=4) and 67±10 (♦, n=4). CPA (100 nM), by itself, enhanced [35S]GTPγS binding by 122±9% (over twofold net increase from basal binding) in membranes prepared from vehicle-treated subjects, and by 111±7% in the chronic caffeine group (p>0.05, n=5, Student's t-test, data not shown); hence, the ability of A1 receptors to activate G proteins is unaltered in chronic caffeine-treated mice. Accordingly, there were no statistically significant differences in the bottom of the nonlinear regression binding curves between chronic caffeine- and vehicle-treated animals, in the presence of CPA (p>0.05, n=5, extra sum-of-squares F test; Figure 9b).
Chronic Caffeine and CB1 Receptor Number
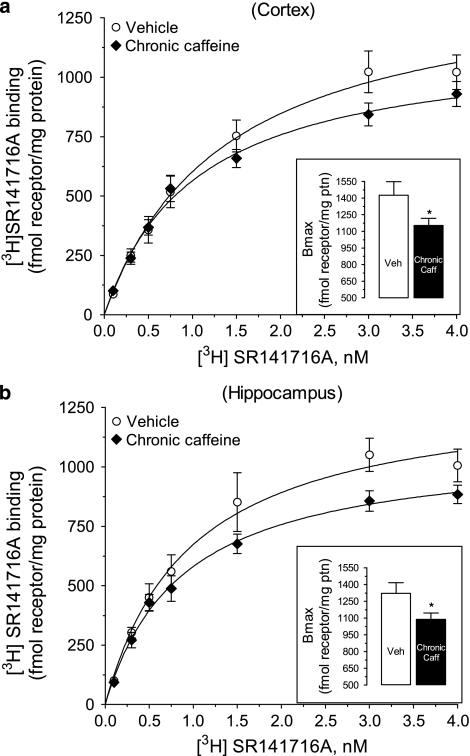
The effects of chronic caffeine administration upon CB1 signaling in vitro suggested that CB1 receptor number and/or affinity are decreased in these mice. We then directly analyzed the effect of chronic caffeine administration in CB1 receptor number and affinity by performing [3H]SR141716A (0.1–4 nM) saturation binding assays in tissue collected from mice used in the behavioral experiments. The nonlinear regression analysis of [3H]SR141716A binding to cortical membranes of vehicle-treated mice showed a Bmax=1425±123 fmol/mg of protein and a KD=1.4±0.3 nM (n=10; Figure 10a). In hippocampal membranes, the Bmax of [3H]SR141716A was 1322±97 fmol/mg of protein and the KD=1.0±0.2 nM (n=5; Figure 10b). In the chronic caffeine group, the Bmax of [3H]SR141716A binding was lower (p<0.05, compared with vehicle group), and this reduction was observed both in cortical membranes (Bmax=1151±65 fmol/mg of protein, n=10) and hippocampal membranes (Bmax=1089±57 fmol/mg of protein, n=5). There were no significant differences in affinity, as in the chronic caffeine group the KD values for [3H]SR141716A binding were 1.0±0.2 nM in the cortical and 0.9±0.1 nM in the hippocampal membranes.
Figure 10.
Saturation analysis of specific [3H]SR141716A binding (0.1–4 nM) to (a) cortical and (b) hippocampal membranes (50 μg protein) from chronic caffeine- (3 mg/kg/day, for 22 days) and vehicle-treated mice. Insets: Bmax values, obtained from nonlinear regression analysis. Nonspecific binding was determined at all [3H]SR141716A concentrations by the addition of 1 μM AM251. All points represent mean±standard error of mean (SEM) of 5–10 experiments, each performed in duplicate. *p<0.05, vs control, calculated using the extra sum-of-squares F test.
DISCUSSION
This study shows that adenosine A1 receptors located in GABAergic and glutamatergic nerve terminals of the hippocampus exert a negative modulatory effect on the cannabinoid CB1 receptor-mediated inhibition of GABA and glutamate release. CB1-mediated G-protein activation is also impaired by A1 receptor activation. In addition, chronic administration of caffeine leads to an A1 receptor-mediated enhancement of the CB1-dependent effects of THC upon short-term spatial memory, despite a reduction in CB1 receptor number and signaling. This provides first evidence for chronic caffeine-induced alterations in cannabinoid actions in the cortex and hippocampus.
The CB1–A1 receptor cross-talk might occur at the G-protein level, as A1 receptor activation with CPA reduced the efficacy of CB1 receptor agonists to stimulate [35S]GTPγS binding in the hippocampus. This is in accordance with a previous observation that simultaneous application of CB1 and A1 agonists produces less than additive stimulation of [35S]GTPγS binding in cerebellar membranes (Selley et al, 2004). Similarly to the A1 receptors, GABAB receptors couple to Gαi/o proteins and are expressed in the same interneuron populations as CB1 receptors (Neu et al, 2007; Sloviter et al, 1999). However, CB1 receptor-mediated signaling, assessed either as inhibition of [3H]GABA release or stimulation of [35S]GTPγS binding, was unaffected by GABAB receptor activation, which indicates that the modulation of CB1 receptor signaling by A1 receptors is not shared by all Gαi/o-coupled receptors.
The CB1 receptor agonist WIN55,212-2 inhibited calcium-dependent [3H]GABA release with a maximum specific effect at 1 μM, in agreement with previous studies (Katona et al, 2000; Köfalvi et al, 2007). There are clear differences in the magnitude of the reported effects of WIN55,212-2 in studies using different methodologies. For example, we and others (Köfalvi et al, 2007) observed that 1 μM WIN55,212-2 induces 15–20% inhibition of K+-evoked [3H]GABA release from rat hippocampal synaptosomes, whereas several reports show that the same concentration of WIN55,212-2, by activating presynaptic CB1 receptors, inhibits GABAergic inhibitory postsynaptic currents (IPSCs) in rat hippocampal slices by ∼50% (eg, Hájos et al, 2000; Hoffman and Lupica, 2000; Wilson and Nicoll, 2001). These differences are likely owing to a combination of factors. The main reason possibly lies in the fact that [3H]GABA release assays provide a quantitative measurement of the amount of GABA released from the whole population of GABAergic nerve terminals at the hippocampus, whereas patch-clamp techniques provide a quantification of endogenous GABA release by measuring the post-synaptic responses of a single hippocampal pyramidal neuron. In addition, differences are likely owing to the type of stimulus used (electrical vs high K+), the time and length of WIN55,212-2 application, and to an amplifying effect of multiple afferents upon IPSC measurements. The effect of 1 μM WIN55,212-2 on the release of [3H]GABA from rat hippocampal slices (Katona et al, 1999) is also larger than in synaptosomes. Again, a longer exposure time (6 vs 18 min) to WIN55,212-2 and/or the amplification by intrinsic circuits in the slices is a likely explanation for these differences.
The CB1 receptor-mediated modulation of GABA release from hippocampal CCK-positive interneurons, which express large quantities of CB1 receptors, is a critical mechanism for spatial and episodic memory, as these interneurons regulate the temporal coordination of principal cell assemblies (Hájos et al, 2000; Robbe and Buzsáki, 2009; Robbe et al, 2006). However, the CCK-expressing interneuron populations mostly receive input from glutamatergic neurons (see Freund and Buzsáki, 1996; Freund and Katona, 2007), which also express CB1 (Katona et al, 2006; Kawamura et al, 2006) and A1 (Ochiishi et al, 1999) receptors. We found that A1 receptor activation also attenuates CB1 receptor-mediated inhibition of glutamate release from hippocampal synaptosomes. It was recently reported that endogenous adenosine, by activating A1 receptors, regulates CB1-mediated inhibition of glutamatergic synaptic transmission (Hoffman et al, 2010; but see Serpa et al, 2009). Thus, A1 and CB1 receptors also interact at glutamatergic neurons, which indicates that the inhibitory effect of A1 receptor activation upon the CB1-dependent stimulation of [35S]GTPγS binding might be derived from an A1–CB1 receptor interaction at both GABAergic and glutamatergic neurons. Interestingly, WIN55,212-2 did not attenuate the inhibitory action of CPA upon glutamate release, suggesting that the modulatory action of A1 receptors upon CB1 receptors is not reciprocal.
The relevance of the GABAergic circuitry for the CB1 receptor-mediated influences upon memory function became firmly established after the demonstration that intraperitoneal THC administration disrupts hippocampal-dependent memory through the activation of CB1 receptors (Wise et al, 2009) in GABAergic, but not glutamatergic, neurons (Puighermanal et al, 2009). We now show that chronic administration of a moderate dose of caffeine leads to increased levels of A1 receptors in the cortico-hippocampal membranes, and to an A1 receptor-mediated increase of the disruptive effects of acute THC in a hippocampal-dependent short-term spatial memory task. This finding points toward a significant functional relevance of the cross-talk between A1 and CB1 receptors in the hippocampus. Interestingly, the motor impairments induced by THC are enhanced by acute activation of A1 receptors (Dar, 2000). In contrast, acute administration of caffeine antagonizes THC-induced changes in cortico-hippocampal EEG wave recordings (Consroe et al, 1976). Several studies show that chronic exposure to adenosine receptor antagonists causes similar actions to acute agonist exposure (see Jacobson et al, 1996; Von Lubitz et al, 1993), whereas acute administration of caffeine is expected to have opposite effects to acute agonist exposure. The timing of caffeine administration and the presence of caffeine in the blood during testing must also be taken into account when comparing data from different studies. The behavioral tests now reported were performed in the absence of relevant plasma concentrations of caffeine (>12 h after caffeine injection), which was given 2 h after the last behavioral trial, to prevent effects on memory consolidation (Angelucci et al, 2002). It is therefore not surprising that acute caffeine administration prevents THC-induced effects (Consroe et al, 1976), whereas chronic caffeine exposure exacerbates the memory disruption induced by CB1 receptor agonists (present work). In a recent study, chronic administration of a high dose of caffeine (210 mg/kg/day) in rats was shown to potentiate CB1-dependent effects at striatal GABAergic, but not glutamatergic, synapses (Rossi et al, 2009). However, it is difficult to draw a comparison with this study, given the differences in the experimental approach, namely the dose of caffeine used, which is not adenosine receptor-selective and is more than about 70 times higher than the equivalent daily human intake. Exposure to high doses of caffeine (∼100 mg/kg/day) leads to altered brain levels of several receptors (Shi et al, 1993, 1994), inhibit phosphodiesterases, and may even block GABAA receptors, among others (see Daly and Fredholm, 1998).
The conclusion that A1 receptors are involved in the chronic caffeine-induced exacerbation of the effects of THC presently reported is reinforced by the finding that A1 receptor blockade with DPCPX fully prevented the effects of THC in the chronic caffeine group. DPCPX was administered at a dose that occupies A1 receptors (Baumgold et al, 1992; Hooper et al, 1996) while not affecting motor activity (present work; Von Lubitz et al, 1993). In addition, DPCPX by itself had no effects in the absence of THC, which suggests that A1 receptors do not directly influence short-term spatial memory. Furthermore, acute application of DPCPX did not influence the action of THC in vehicle-treated animals, which further supports previous evidence (see above) that chronic and acute blockade of A1 receptors have different functional consequences. The effects of DPCPX also exclude the involvement of A2A receptors, which are known to modulate the actions of CB1 receptors in the striatum (Carriba et al, 2007; Tebano et al, 2009). A1 and A2A receptors have similar affinities for caffeine (Fredholm et al, 1994), yet the expression of A2A receptors in the hippocampus and cortex is much lower than that of A1 receptors (reviewed by Ribeiro et al, 2002). Furthermore, chronic caffeine exposure does not alter the expression of A2A receptors (Jacobson et al, 1996).
The increase in A1 receptor expression caused by moderate doses of chronically administered caffeine results from prevention of tonic adenosine-mediated receptor downregulation (see Fredholm et al, 1999). The dose of caffeine we have administered to mice is equivalent to the estimated US average human daily caffeine consumption (Barone and Roberts, 1996) and, in addition to the expected increase in A1 receptor levels, it also caused a decrease of cortical and hippocampal CB1 receptors. Accordingly, in chronic caffeine-treated mice there was a reduction in the CB1 receptor-mediated inhibition of GABA release and stimulation of G-protein activation. As tonic activation of A1 receptors was prevented through elimination of endogenous adenosine by vertical superfusion in the [3H]GABA release assays, and by ADA in the [35S]GTPγS binding assays, it is unlikely that chronic caffeine-induced A1 receptor upregulation could be responsible for reduction of CB1-dependent actions in the in vitro assays. Most probably, chronic caffeine intake, by inducing an imbalance in adenosinergic signaling, disturbs the A1–CB1 cross-talk, which reflects in CB1 receptor downregulation. Independently of the exact mechanisms involved, it is clear that CB1 receptors are affected after chronic caffeine exposure.
Given that chronic caffeine decreases CB1 and increases A1 receptor levels, and that activation of A1 receptors inhibited the CB1-mediated actions in the in vitro assays, it was somewhat surprising that the memory impairment caused by the CB1 receptor agonists was exacerbated by chronic caffeine intake. It is therefore evident that changes observed in vitro do not necessarily reflect, in a linear way, the effects upon the integrated hippocampal circuitry in vivo. An imbalance in GABAergic transmission resulting from the chronic caffeine-induced alterations of A1 and CB1 levels may have occurred, leading to some adaptive changes in the pyramidal cells and/or in the parvalbumin-expressing (PV) GABAergic neurons, which do not express CB1 receptors (Katona et al, 1999). Interestingly, the blockade of GABAergic transmission was shown to reverse the cognitive effects of acute THC in vivo (Varvel et al, 2005). A critical imbalance in the temporal coordination of pyramidal cell firing could have become evident when THC was administered, if there was an enhanced sensitivity to the fast spiking activity of PV cells; hence, leading to increased inhibition of pyramidal cell firing.
In summary, this work highlights two relevant factors influencing cannabinoid CB1 signaling in the hippocampus: the activity of A1 receptors and the chronic consumption of caffeine. This A1–CB1 receptor interaction therefore points toward the possibility that the pathophysiological or therapeutically relevant actions operated by CB1 receptors can be significantly affected by interference with A1 receptor activity, as is the case of chronic caffeine intake.
Acknowledgments
We thank Dr Stephanie Daumas and Dr Bruno da Silva (University of Edinburgh) for helpful assistance in the experimental design of the behavioral tests. We acknowledge the Institute of Physiology at the Faculty of Medicine (University of Lisbon) for the animal house facility, Prof. Alexandre de Mendonça (University of Lisbon) and Dr Attila Kofalvi (University of Coimbra) for useful scientific discussions, and Dr João Maroco (Instituto Superior de Psicologia Aplicada, ISPA) for valuable advice with statistical analysis. This work was supported by grants from Fundação para a Ciência e Tecnologia (FCT, VCS: SFRH/BD/21359/2005 and NAL: SFRH/BD/21374/2005), and the European Union (European Cooperation in Science and Technology (COST) COST B30 concerted action, Neural Regeneration and Plasticity (NEREPLAS)).
The authors declare that, except for income received from primary employers, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal holdings that could be perceived as constituting a potential conflict of interest.
References
- Angelucci MEM, Cesário C, Hiroi RH, Rosalen PL, Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Braz J Med Biol Res. 2002;35:1201–1208. doi: 10.1590/s0100-879x2002001000013. [DOI] [PubMed] [Google Scholar]
- Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Chao MV, Sebastião AM. Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation. J Neurosci. 2010;30:8468–8480. doi: 10.1523/JNEUROSCI.5695-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Baumgold J, Nikodijevic O, Jacobson KA. Penetration of adenosine antagonists into mouse brain as determined by ex vivo binding. Biochem Pharmacol. 1992;43:889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J Biol Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, D'Amico M, Preziosi P, Martire M. Presynaptic effects of anandamide and WIN55,212-2 on glutamatergic nerve endings isolated from rat hippocampus. Neurochem Int. 2006;48:159–165. doi: 10.1016/j.neuint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol Biochem Behav. 1995;50:521–526. doi: 10.1016/0091-3057(94)00331-9. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Cinar R, Freund TF, Katona I, Mackie K, Szucs M. Reciprocal inhibition of G-protein signaling is induced by CB(1) cannabinoid and GABA(B) receptor interactions in rat hippocampal membranes. Neurochem Int. 2008;52:1402–1409. doi: 10.1016/j.neuint.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Consroe P, Jones B, Laird H. EEG and behavioral effects of delta9-tetrahydrocannabinol in combination with stimulant drugs in rabbits. Psychopharmacology. 1976;50:47–52. doi: 10.1007/BF00634153. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Ribeiro JA. Inhibition of [3H] gamma-aminobutyric acid release by kainate receptor activation in rat hippocampal synaptosomes. Eur J Pharmacol. 1997;323:167–172. doi: 10.1016/s0014-2999(97)00043-5. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Constantino MD, Ribeiro JA. G protein coupling of CGS 21680 binding sites in the rat hippocampus and cortex is different from that of adenosine A1 and striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:295–302. doi: 10.1007/pl00005355. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA. Purinergic modulation of [(3)H]GABA release from rat hippocampal nerve terminals. Neuropharmacology. 2000;39:1156–1167. doi: 10.1016/s0028-3908(99)00237-3. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Ribeiro JA, Sebastião AM. A1 and A2A receptor activation by endogenous adenosine is required for VIP enhancement of K+-evoked [3H]-GABA release from rat hippocampal nerve terminals. Neurosci Lett. 2008;430:207–212. doi: 10.1016/j.neulet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- D'Amico M, Cannizzaro C, Preziosi P, Martire M. Inhibition by anandamide and synthetic cannabimimetics of the release of [3H]-aspartate and [3H]GABA from synaptosomes isolated from the rat hippocampus. Neurochem Res. 2004;29:1553–1561. doi: 10.1023/b:nere.0000029569.20266.3f. [DOI] [PubMed] [Google Scholar]
- Daly JW, Fredholm BB. Caffeine—an atypical drug of dependence. Drug Alcohol Depend. 1998;51:199–206. doi: 10.1016/s0376-8716(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Dar MS. Cerebellar CB(1) receptor mediation of Delta(9)-THC-induced motor incoordination and its potentiation by ethanol and modulation by the cerebellar adenosinergic A(1) receptor in the mouse. Brain Res. 2000;864:186–194. doi: 10.1016/s0006-8993(00)02103-x. [DOI] [PubMed] [Google Scholar]
- Dar MS, Mustafa SJ. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 2002;957:53–60. doi: 10.1016/s0006-8993(02)03599-0. [DOI] [PubMed] [Google Scholar]
- Daumas S, Betourne A, Halley H, Wolfer DP, Lipp H-P, Lassalle J-M, et al. Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem. 2007;88:94–103. doi: 10.1016/j.nlm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- DeSanty KP, Dar MS. Involvement of the cerebellar adenosine A(1) receptor in cannabinoid-induced motor incoordination in the acute and tolerant state in mice. Brain Res. 2001;905:178–187. doi: 10.1016/s0006-8993(01)02533-1. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989;249:31–37. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmisén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 2000;295:1101–1111. [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Little M, Johnson M, Melvin L, de Costa B, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N, Fraser C, Stone TW. Effects of purine analogues on spontaneous alternation in mice. Psychopharmacology (Berl) 1996;123:250–257. doi: 10.1007/BF02246579. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H-J, Jang I-S, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89:1214–1222. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Barnes C, Wertheim CE, Pappas LA, Goldberg SR, et al. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology. 2009;203:355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Maglóczky Z, Sántha E, Köfalvi A, Czirják S, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung K-M, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi2008Alternative interacting sites and novel receptors for cannabinoid ligandsIn: Köfalvi A (ed). Cannabinoids and the Brain Springer: New York; pp 131–160. [Google Scholar]
- Köfalvi A, Pereira MF, Rebola N, Rodrigues RJ, Oliveira CR, Cunha RA. Anandamide and NADA bi-directionally modulate presynaptic Ca2+ levels and transmitter release in the hippocampus. Br J Pharmacol. 2007;151:551–563. doi: 10.1038/sj.bjp.0707252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova M, Kelley B, Shen M, Thayer SA. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol Pharmacol. 2002;61:477–485. doi: 10.1124/mol.61.3.477. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Teyler TJ. Adenosine depresses excitatory but not fast inhibitory synaptic transmission in area CA1 of the rat hippocampus. Neurosci Lett. 1991;122:50–52. doi: 10.1016/0304-3940(91)90190-5. [DOI] [PubMed] [Google Scholar]
- Leggett T. A review of the world cannabis situation. Bull Narc. 2006;58]}:1–155. [PubMed] [Google Scholar]
- Li H, Henry JL. Adenosine action on interneurons and synaptic transmission onto interneurons in rat hippocampus in vitro. Eur J Pharmacol. 2000;407:237–244. doi: 10.1016/s0014-2999(00)00661-0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, Martin BR. Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition. Neuroscience. 2002;112:319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Foran P, Dolly JO, Verhage M, Wiegant VM, Nicholls DG. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes. Clues to the locus of action. J Biol Chem. 1992;267:21338–21343. [PubMed] [Google Scholar]
- Németh B, Ledent C, Freund TF, Hájos N. CB1 receptor-dependent and -independent inhibition of excitatory postsynaptic currents in the hippocampus by WIN 55,212-2. Neuropharmacology. 2008;54:51–57. doi: 10.1016/j.neuropharm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu A, Földy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578 (Part 1:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiishi T, Chen L, Yukawa A, Saitoh Y, Sekino Y, Arai T, et al. Cellular localization of adenosine A1 receptors in rat forebrain: immunohistochemical analysis using adenosine A1 receptor-specific monoclonal antibody. J Comp Neurol. 1999;411:301–316. [PubMed] [Google Scholar]
- Ohno M, Watanabe S. Working memory failure by stimulation of hippocampal adenosine A1 receptors in rats. Neuroreport. 1996;7:3013–3016. doi: 10.1097/00001756-199611250-00043. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Elrick DB, Mechoulam R, Corbett AD. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol. 1992;105:980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol. 1999;374:417–421. doi: 10.1016/s0014-2999(99)00189-2. [DOI] [PubMed] [Google Scholar]
- Phelan P, Gordon-Weeks PR.1997Isolation of synaptosomes, growth cones, and their subcellular componentsIn: Turner AJ, Bachelard HS (eds).Neurochemistry, A Practical Approach2nd edn.Oxford University Press: Oxford; 1–36. [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M. Synaptosomes still viable after 25 years of superfusion. Neurochem Res. 2000;25:1265–1274. doi: 10.1023/a:1007648229795. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Angelini F, Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol. 1974;25:411–414. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20 (Suppl 1:S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Progr Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Robbe D, Buzsáki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J Neurosci. 2009;29:12597–12605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, Mcnaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Rossi S, Chiara VD, Musella A, Mataluni G, Sacchetti L, Siracusano A, et al. Caffeine drinking potentiates cannabinoid transmission in the striatum: interaction with stress effects. Neuropharmacology. 2009;56:590–597. doi: 10.1016/j.neuropharm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Stone TW, Ribeiro JA. The inhibitory adenosine receptor at the neuromuscular junction and hippocampus of the rat: antagonism by 1,3,8-substituted xanthines. Br J Pharmacol. 1990;101:453–459. doi: 10.1111/j.1476-5381.1990.tb12729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ. Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol. 2004;66:1275–1284. doi: 10.1124/mol.104.000604. [DOI] [PubMed] [Google Scholar]
- Serpa A, Ribeiro JA, Sebastião AM. Cannabinoid CB(1) and adenosine A(1) receptors independently inhibit hippocampal synaptic transmission. Eur J Pharmacol. 2009;623:41–46. doi: 10.1016/j.ejphar.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- Shi D, Nikodijevic O, Jacobson KA, Daly JW. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell Mol Neurobiol. 1993;13:247–261. doi: 10.1007/BF00733753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Nikodijevic O, Jacobson KA, Daly JW. Effects of chronic caffeine on adenosine, dopamine and acetylcholine systems in mice. Arch Int Pharmacodyn Ther. 1994;328:261–287. [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Straiker AJ, Borden CR, Sullivan JM. G-protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses. J Neurosci. 2002;22:2460–2468. doi: 10.1523/JNEUROSCI.22-07-02460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Chiodi V, Pepponi R, Ferrante A, Domenici MR, et al. Adenosine A2A receptors enable the synaptic effects of cannabinoid CB1 receptors in the rodent striatum. J Neurochem. 2009;110:1921–1930. doi: 10.1111/j.1471-4159.2009.06282.x. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH. Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology. 2005;178:317–327. doi: 10.1007/s00213-004-1988-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DK, Paul IA, Bartus RT, Jacobson KA. Effects of chronic administration of adenosine A1 receptor agonist and antagonist on spatial learning and memory. Eur J Pharmacol. 1993;249:271–280. doi: 10.1016/0014-2999(93)90522-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Rothman SM. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J Neurosci. 1991;11:1375–1380. doi: 10.1523/JNEUROSCI.11-05-01375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]