Abstract
Continued gambling to recover losses—‘loss chasing'—is a prominent feature of social and pathological gambling. However, little is known about the neuromodulators that influence this behavior. In three separate experiments, we investigated the role of serotonin activity, D2/D3 receptor activity, and beta-adrenoceptor activity on the loss chasing of age and IQ-matched healthy adults randomized to treatment or an appropriate control/placebo. In Experiment 1, participants consumed amino-acid drinks that did or did not contain the serotonin precursor, tryptophan. In Experiment 2, participants received a single 176 μg dose of the D2/D3 receptor agonist, pramipexole, or placebo. In Experiment 3, participants received a single 80 mg dose of the beta-adrenoceptor blocker, propranolol, or placebo. Following treatment, participants completed a computerized loss-chasing game. Mood and heart rate were measured at baseline and following treatment. Tryptophan depletion significantly reduced the number of decisions made to chase losses, and the number of consecutive decisions to chase, in the absence of marked changes in mood. By contrast, pramipexole significantly increased the value of losses chased and diminished the value of losses surrendered. Propranolol markedly reduced heart rate, but produced no significant changes in loss-chasing behavior. Loss chasing can be thought of as an aversively motivated escape behavior controlled, in part, by the marginal value of continued gambling relative to the value of already accumulated losses. Serotonin and dopamine appear to play dissociable roles in the tendency of individuals to gamble to recover, or to seek to ‘escape' from, previous losses. Serotonergic activity seems to promote the availability of loss chasing as a behavioral option, whereas D2/D3 receptor activity produces complex changes in the value of losses judged worth chasing. Sympathetic arousal, at least as mediated by beta-adrenoceptors, does not play a major role in laboratory-based loss-chasing choices.
Keywords: serotonin, dopamine, loss chasing, gambling, persistence, value
INTRODUCTION
Gambling to recover losses, or loss chasing (Lesieur, 1977), is a central feature of human decision making (Kahneman and Tversky, 2000). However, in a clinical context, excessive loss chasing is also a prominent indicator of impaired control in a significant proportion of those individuals who report problems with their gambling behavior (Corless and Dickerson, 1989; McBride et al, 2010; Sacco et al, 2010). Left unchecked, loss chasing can produce a dangerous spiral of gambling involvement, increasing financial liabilities but diminishing resources, and, potentially, the serious adverse family, social, and occupational consequences of pathological gambling (Lesieur, 1979).
At a psychological level, loss chasing is complex and frequently involves conflicted motivational states, pitting the desire (or need) to keep playing against the dread of suffering even greater losses (Lesieur, 1977): powerful emotional states that are mediated by activity within dissociable neural circuits (Campbell-Meiklejohn et al, 2008). Gambling to recover losses is also associated with heightened states of arousal (see below) and a heightened preoccupation with gambling activities that is a prominent feature of the clinical presentation of gambling problems (Dickerson et al, 1987; McBride et al, 2010). Consequently, loss chasing may represent a salient target for the development of therapeutic interventions.
Despite its centrality to problem gambling, we know little about the way loss chasing is influenced by the activity of neurochemical systems. A small amount of clinical evidence suggests that pathological gambling is associated with serotonergic dysfunction as exemplified by (inconsistent) reports of reduced concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid in cerebrospinal fluid (Bergh et al, 1997; Roy et al, 1988) and by reports of increased prolactin release (and reports of a subjective ‘high') following acute challenge with the 5-HT2c receptor agonist, meta-chlorophenylpiperazine (Pallanti et al, 2006). Selective serotonin reuptake inhibitors have also shown some promise as a treatment of pathological gambling via their anticompulsive and anxiolytic effects (Grant and Potenza, 2006; Pallesen et al, 2007). Finally, serotonin exerts pronounced—albeit, complex—influences on impulsive behaviors (Winstanley et al, 2004), which both promote loss chasing (Breen and Zuckerman, 1999), and are exaggerated in problem gamblers (Blaszczynski et al, 1997).
The pathophysiology of problem gambling is also highly likely to involve dysfunction of the dopaminergic mid-brain, and its mesolimbic and prefrontal projections sites (Hewig et al, 2010; Potenza, 2008). Compared with matched healthy control subjects, pathological gamblers show reduced neuronal responses within mesostriatal nuclei while engaging in a simulated gambling behavior for monetary reward (Reuter et al, 2005). Administration of the psychostimulant, amphetamine, to pathological gamblers can prime cognitions about gambling (Zack and Poulos, 2004), whereas the D2 receptor antagonist, haloperidol, can enhance the rewarding properties of such behavior (Zack and Poulos, 2007). Finally, accumulating evidence indicates that dopaminergic treatments are associated with pathological gambling (and other impulse control problems) in a minority of patients with Parkinson's disease (Voon et al, 2007), presumably reflecting a disturbance of dopamine's wider role in reinforcement learning and the computation of action–value relationships (Dagher and Robbins, 2009; Voon et al, 2010). Thus, the extant evidence suggests that both serotonin and dopamine dysfunction mediate aspects of problematic gambling (Zeeb et al, 2009). However, to date, their role in the central feature of loss-chasing behavior has not yet been explored experimentally.
One way to start to understand the neurochemical substrates of the excessive loss chasing sometimes observed in problem gamblers is to investigate the roles of different neuromodulators in the chasing behavior of healthy adults with limited gambling experiences. Information gained from such experiments will assist in the formulation of hypotheses about how disturbances in the activity of neuromodulators mediate loss chasing in the pathological state. Here, in three separate experiments, we used a behavioral model of loss chasing developed in our laboratory and already validated with functional magnetic resonance imaging (Campbell-Meiklejohn et al, 2008) to compare the loss-chasing behavior of non-clinical healthy adults (who reported only very limited gambling involvement) following manipulations of serotonergic, dopaminergic (D2/D3) and beta-adrenoceptor activity.
In Experiment 1, we investigated the effects of tryptophan depletion on the tendency to continue gambling to recover losses and tested between two hypotheses with clearly divergent predictions. Serotonin is known to play a prominent role in the control of non-rewarded activity and the inhibition of behavior following the occurrence of punishing or aversive events (Soubrie, 1986). Furthermore, temporary reductions in central serotonin activity, achieved through tryptophan depletion, can diminish punishment-induced inhibition of ongoing behavior in healthy adults (Crockett et al, 2009). On this basis, we might expect that tryptophan depletion will increase the tendency to continue gambling in order to recover previous losses through a failure of serotonin-dependent behavioral inhibition.
On the other hand, serotonin also mediates learning about negative events (Bari et al, 2010; Daw et al, 2002; Deakin and Graeff, 1991; Evers et al, 2005). Dayan and Huys (2008) has proposed that failures of behavioral control following reductions in serotonin activity (experimental or clinical) can produce pervasive increases in the size of negative prediction errors that, in turn, engender negative affective states in vulnerable individuals (Dayan and Huys, 2008). Experimentally, tryptophan depletion can improve the accuracy of predictions of negative or punishing outcomes in healthy adults (Cools et al, 2008). Moreover, Evers et al (2005) showed that tryptophan depletion enhances neural activity in response to errors during reversal learning within the anterior cingulate region, an area that is activated while making decisions to stop chasing losses (Campbell-Meiklejohn et al, 2008). Thus, we might also predict that tryptophan depletion in healthy adults will enhance the salience of bad outcomes during a run of losing gambles, and diminish subsequent loss-chasing behavior.
In Experiment 2, we investigated the effects of a single dose of the non-ergoline D2/D3 receptor agonist, pramipexole (PPX). Alongside other dopaminergic treatments, treatment with PPX has been associated with gambling problems in a subset of Parkinson's disease patients (Voon et al, 2007). However, there has been no test of whether treatment with D2/D3 receptors agonists alter chasing behavior during a run of losing gambles.
PPX is significantly more selective for D3 than D2 receptors and binds to dopamine (autoreceptor and post-synaptic) receptors in mesolimbic reward pathways (Camacho-Ochoa et al, 1995) (see Supplementary Information). Single low doses of PPX (eg, 0.5 mg) can impair reinforcement learning in healthy adults (Pizzagalli et al, 2008), and increase risky choices in lottery-type games (Riba et al, 2008), possibly through blunted reward signalling of mesolimbic pathways (Riba et al, 2008; Santesso et al, 2009). In the light of this, and evidence that low doses of PPX, and other agents acting upon D2 receptors, impair the signalling of bad outcomes (‘negative prediction errors') (Frank and O'Reilly, 2006; Santesso et al, 2009; van Eimeren et al, 2009), we tested the hypothesis that single doses of PPX increase loss-chasing behavior and, perhaps, influence the value of losses that healthy individuals are prepared to chase.
Although it is unlikely that the findings we report in Experiments 1 and 2 reflect gross changes in subjective states associated with either tryptophan depletion or treatment with PPX, it is possible that our observations relate to changes in alerting or arousal, perhaps reflecting the relatively prolonged protocols of pharmacological experiments. For example, while tryptophan depletion typically does not modify state affect in adults who have been screened for affective disorders, it may attenuate physiological (cardiac) responses to negative performance feedback (van der Veen et al, 2008). Moreover, field studies indicate that commercial gambling is associated with the increase in sympathetic arousal (Anderson and Brown, 1984; Meyer et al, 2000). Therefore, it is unclear whether changes in arousal might increase or decrease the tendency to keep gambling to recover losses. Previously, we have found that single doses of the beta-adrenoceptor antagonist, propranolol, reduced decision-makers' attention toward punishment-related cues (Rogers et al, 2004), potentially releasing loss-chasing behavior. In Experiment 3, we tested whether changes in arousal, as reflected in the kind of reduced heart rate (HR) produced in healthy adults by a single dose of the beta-adrenoceptor antagonist propranolol would influence loss-chasing behavior.
MATERIALS AND METHODS
Participants and Designs
All participants provided written informed consent. Participants were given a clinical examination by an experienced psychiatrist, including a semi-structured SCID-I interview to ensure that none of the following exclusion criteria were met: (i) major physical illness; (ii) current or previous DSM-IV major mood or psychotic disorder; and (iii) current or previous DSM-IV substance abuse disorder. Participants were assessed with the South Oaks Gambling Screen (Lesieur and Blume, 1987); all scores were either 0 or 1, indicating no evidence of problem or pathological gambling.
Experiment 1
Thirty-four healthy adults participated. None had any history of mood disorder; there was no restriction on the phase of menstrual cycle in female participants. Seventeen participants (eight males) ingested an amino-acid drink that did not contain tryptophan (T−) and 17 participants (eight males) ingested an amino-acid drink that did contain tryptophan (T+). The T+ participants and T− participants were matched in terms of their gender (see Supplementary Table S1), age (F<1.00), and cognitive ability (Raven et al, 1998) (F(1, 30)<2.08).
Participants followed a low-protein diet (<2 g) the day before the study, and fasted overnight before attending the laboratory at 0830 hours on the day of the experiment. Measures of state-positive and -negative affect (Watson et al, 1988) were taken at this time along with 15 ml blood samples to obtain total plasma tryptophan concentrations. Participants then drank an amino-acid drink over a 60-min period. None of the participants reported side effects beyond transitory nausea. Participants were given a low-protein (<2 g) lunch at mid-day. Repeat state-positive and -negative affect measurements, and a second blood sample, were collected +5 h after consumption of the amino-acid drink, before completing the loss-chasing game.
Experiment 2
Thirty healthy adults were randomly assigned to receive 176 μg of PPX or placebo (placebo-PPX). Each group contained seven males. There were no significant differences between those participants who received placebo and those who received PPX in terms of their age or their cognitive ability (Supplementary Table S2) (both F's<1.00).
The 176 μg dose of PPX used in Experiment 2 is comparable to dosages shown to be clinically effective for restless leg syndrome (Manconi et al, 2007). There are good reasons to suppose that the subjective (Hamidovic et al, 2008) and behavioral (Pizzagalli et al, 2008; Riba et al, 2008; Santesso et al, 2009) effects of low doses of dopaminergic agents reflect pre-synaptic actions at the auto-receptors that regulate the activity of mid-brain dopaminergic neurons (Frank and O'Reilly, 2006; Grace, 1995). As described below, we replicate findings that single (1 mg) low doses of PPX reduce psychometric measurements of state-positive affect in healthy adults and that have been taken to suggest a pre-synaptic mode of action (Hamidovic et al, 2008). However, our 176 μg dose is also comparable to those shown to reduce serum prolactin over 2 h (Schilling et al, 1992), at least raising the possibility that our results also reflect some post-synaptic receptor activity (Ben-Jonathan, 1985).
Participants attended the laboratory at 0830 hours and completed baseline assessments of state-positive and -negative affect (Watson et al, 1988). Baseline measures of systolic/diastolic blood pressure (BP) and HR were collected. Following this, participants received a single 176 μg dose of PPX or a gelatine capsule containing lactose. After 2 h (+2 h), further measurements of systolic/diastolic BP and HR were taken. State-positive and -negative affect were also collected at this time, before completion of the loss-chasing game.
Experiment 3
Fourteen (seven males) participants were randomly assigned to receive 80 mg propranolol (placebo-PPL) and 14 participants (eight males) were randomly assigned to receive a lactose placebo (PLA-PPL). The two groups of participants were well matched in terms of their age (see Supplementary Table S4) (F<1) and their cognitive ability (F(1, 24)=1.87).
Participants attended the laboratory in the mornings having fasted for 2 h and without caffeine intake. State-positive and -negative affect (PANAS) (Watson et al, 1988), systolic BP, diastolic BP, and HR were assessed at baseline and then every 30 min thereafter. Participants completed the loss-chasing game +75 min following treatment.
Loss-Chasing Game
A version of our loss-chasing game suitable for functional magnetic resonance imaging has been described in detail elsewhere (Campbell-Meiklejohn et al, 2008). On each play, participants were required to choose between gambling to recover a loss (at the risk of doubling its size) or quitting (and sustaining a certain loss). Such dilemmas induce risky choices in a variety of social and economic contexts (Shafir and Tversky, 1995). Descriptive theories of choice (under uncertainty) attribute this behavior to the fact that losses fall on the convex part of a psychophysical function relating nominal value (eg, monetary outcomes) to subjective value or utility, such that the decreases in utility associated with chasing and suffering larger losses are proportionately smaller than the decreases in utility associated with certain but smaller losses (Kahneman and Tversky, 2000). Previously, we found that gambling to recover losses during our game is positively associated with psychometric measures of the tendency to chase losses in other gambling activities (Campbell-Meiklejohn et al, 2008).
At the start of the game, participants were told that they had a fictional £20 000 to play with, but that the participant with the most points at the end of the experiment would win a real prize of £70. On each ‘round' of the game, an initial £10, £20, £40, £80, or £160 was subtracted from their game total. This amount appeared below the choices: ‘Quit' and ‘Play' (Figure 1). At this point, participants could choose to ‘Quit', sustaining this loss and ending the round immediately (‘quit-loss' outcome), or they could choose to ‘Play', that is, chase the loss. Thus, they could gamble on recovering an amount equal to the loss, but at the risk of increasing their losses by the same amount. If the outcome of a decision to gamble were positive (‘chase-win' outcome), the loss was recovered and the round ended. If the outcome were negative (‘chase-loss' outcome), the loss was doubled and participants given another chance to quit or to chase in the next choice of the round. The options for each choice—‘Play' or ‘Quit'—appeared equally often on the left and right sides of the computer displays.
Figure 1.
Display sequences for the loss-chasing game. At the beginning of each round of the game, a loss was imposed and a decision made either to play (gamble further) or quit (to accept the loss), and end the round. Consecutive losses and decisions occurred until a maximum round loss of £640 was incurred, participants won a gamble and cleared their losses, or participants chose to quit, at which point the round ended.
Outcome displays (see Figure 1) indicated whether participants had won a gamble and that no money was lost (‘chase-win'); whether they had lost a gamble and the amount lost (‘chase-loss'); or the amount lost if participants chose to quit the round (‘quit-loss'). At the end of each round, participants were also informed of their final losses in a ‘round-loss' display. This display indicated the total cumulative losses for that round, in red text if the losses were greater than 0, but in green text if 0. Rounds of the loss-chasing game began with losses of £10, £20, £40, £80, or £160. If participants continued losing, losses kept doubling until they reached £640, at which point the round ended, having incurred the maximum loss.
All participants played 20 rounds of the loss-chasing game. Chase-win outcomes were positioned randomly within each round such that winning outcomes occurred equally often after any number of (between 0 and 5) consecutive losses. The outcomes of the loss-chasing game were distributed such that 14 rounds returned all losses if participants decided to play on every choice of the game. However, six rounds resulted in the maximum loss of £640.
Participants were not told anything about the probabilities of good vs bad outcomes so that their decisions were made under conditions of ‘ambiguity' (Camerer and Weber, 1992). In order to discourage participants from adopting conservative strategies by which they quit early to preserve as much of their play money as possible, no information was provided about their cumulative game total of play money during the game. Participants were also informed that they would not achieve the best possible score by exclusively playing or quitting.
To summarize, participants were confronted with a series of dilemmas involving a choice between gambling to recover a loss of at the risk of doubling its size, or sustaining the loss and ending the chase, whereas at the same time preserving as many resources as possible (Campbell-Meiklejohn et al, 2008). The value of these resources (experimenter-defined points) was provided by the context of an inter-participant competition requiring participants to retain as many points as possible. This mixture of nominal and actual rewards have been used in behavioral economics to show behavior qualitatively and quantitatively similar to that observed outside the laboratory (Cubitt et al, 1998).
Statistical Analysis
Dependent measures included the proportion of choices to gamble (or chase) out of all choices made during the game, and the mean number of consecutive losses chased per round. We analyzed the magnitude (or value) of losses chased and the magnitude (or value) of losses surrendered during the game. These values were expressed as ratios to the mean values of all losses encountered during the game (see Supplementary Information for more details).
Demographic, subjective, and loss-chasing measures for the three experiments were tested using one-way analysis of variance (ANOVA) with the between-subjects factors of treatment (T+ vs T−, PPX vs placebo, or propranolol vs placebo) and gender.
RESULTS
Experiment 1: Tryptophan Depletion
Physiological and subjective effects
Consumption of the amino-acid drink without tryptophan (in the T− treatment) produced a significant reduction in total plasma concentration+5 h later compared with the control drink (in the T+ treatment) (see Supplementary Table S1). However, the T− treatment did not produce any marked changes in either state-positive or -negative affect compared with the T+ treatment (Supplementary Table S1) (all F(1, 30)'s<2.29).
Loss chasing
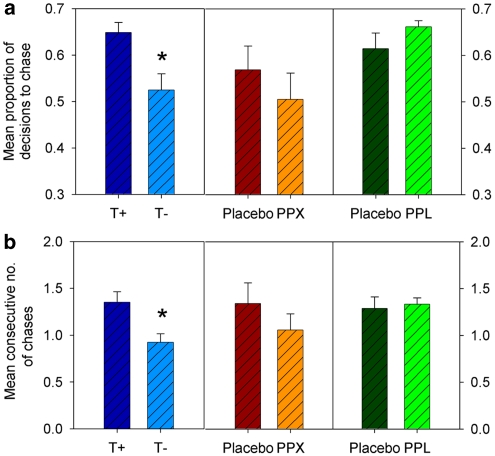
Participants who received the T− treatment showed a marked and significant reduction in the proportion of decisions to chase losses compared with participants who received the T+ treatment (Figure 2a) (F(1, 30)=8.43, p<0.01). The number of consecutive decisions to chase in a run of losing gambles was also reliably reduced following tryptophan depletion (Figure 2b) (F(1, 30)=8.06, p<0.01).
Figure 2.
Persistence of loss-chasing behavior in three samples of healthy, non-clinical adult participants following tryptophan depletion (vs a control amino-acid drink), a single 176 μg of the D2/D3 receptor agonist, pramipexole (PPX vs placebo), and a single 80 mg dose of the beta-adrenoceptor antagonist, propranolol (vs placebo). (a) Mean proportion of decisions to chase losses during the loss-chasing game. (b) Mean consecutive number of decisions to chase losses per round of the loss-chasing game. *p<0.05.
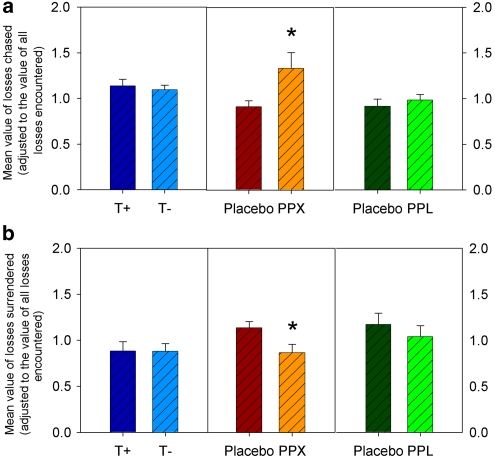
In contrast to the effects on the proportion of gambles to recover losses, there was no significant change in the value of losses that the tryptophan-depleted participants decided to chase (expressed as ratios to the mean values of all losses encountered during the game; see Supplementary Information) (Figure 3a) compared with the participants who received the control procedure (F's<1). Neither was there any significant change in the value of losses surrendered when deciding to quit (Figure 3b) (F's<1).
Figure 3.
The use of value information in the loss-chasing behavior in three samples of healthy, non-clinical participants following tryptophan depletion (vs a control amino-acid drink), a single 176 μg of the D2/D3 receptor agonist, pramipexole (PPX vs placebo), and a single 80 mg dose of the beta-adrenoceptor antagonist, propranolol (vs placebo). (a) Mean value of losses chased (adjusted to the value of all losses encountered). (b) Mean value of losses surrendered (adjusted to the value of all losses encountered during the loss-chasing game). *p<0.05.
Experiment 2: PPX
Physiological and subjective effects
Systolic BP, diastolic BP, and HR were not significantly altered following treatment with PPX compared to treatment with placebo (see Supplementary Information and Supplementary Table S3) (all F(1, 25)'s<1.86).
Treatment with PPX significantly reduced state-positive affect in comparison to placebo across the +2 h following treatment (F(1, 26)=10.05, p<0.005) (Supplementary Table S2). Specifically, while positive affect tended to increase following treatment with placebo (F(1, 13)=3.53, p=0.08), it was significantly decreased following treatment with PPX (F(1, 13)=6.84, p<0.05). At +2 h, when completing the loss-chasing game, participants who received PPX reported lower positive affect than those who received placebo (F(1, 26)=8.34, p<0.01). PPX did not alter state-negative affect compared with placebo (all F's<1).
Loss chasing
PPX slightly reduced the number of decisions to chase, and the number of consecutive decisions to chase, during a run of losing gambles compared with placebo (Figure 2); however, neither of these effects were statistically significant (F's<1). By contrast, PPX significantly increased the value of losses that participants decided to gamble to recover (Figure 3a) (F(1, 26)=4.94, p<0.05), and also significantly reduced the value of losses participants surrendered (Figure 3b) (F(1, 26)=5.87, p<0.05). These changes in the value of losses chased and surrendered remained significant when positive affect at +2 h was entered as a covariate (F(1, 25)=4.48, p<0.05 and F(1, 25)=4.39, p<0.05, respectively). They were also broadly unaltered when the statistical analysis was performed on the unadjusted value of losses chased or values surrendered (see Supplementary Information for full details).
Experiment 3: Propranolol
Physiological and subjective effects
Propranolol did not produce significantly larger or smaller changes in systolic or diastolic BP compared with placebo (all F's<1). HR diminished over the +75 min following treatment (73.64±10.82 vs 62.04±7.68 b.p.m.) (F(1, 24)=60.30, p<0.0001). However, this reduction was significantly greater following propranolol compared with placebo (Supplementary Table S5) (F(1, 24)=4.98, p<0.05). As baseline HR tended to be greater in participants treated with propranolol compared to participants treated with placebo (F(1, 24)=2.64), we also examined the treatment effects on the proportionate change in participants' HR. This confirmed that propranolol produced a significantly larger reduction in HR compared with placebo (18.64±8.45 vs 11.08±11.38%) (F(1, 24)=4.64, p<0.05).
State-positive and -negative affect were not substantially different following treatment with propranolol compared to treatment with placebo (see Supplementary Information and Supplementary Table S4) (F<1.00 and F(1, 24)=1.61, respectively). There were no significant treatment-related differences in either measure at +75 min when the loss-chasing game was completed.
Loss chasing
There were no significant differences between propranolol and placebo in terms of the number of decisions to chase, number of consecutive decisions to chase (Figure 2), or the value of losses chased and the value of losses surrendered (Figure 3) (all F's<1).
DISCUSSION
Our findings suggest that serotonin and dopamine play complementary roles in the tendency to keep gambling to recover losses. Serotonin activity appears to play a role in sustaining loss-chasing behavior, whereas dopamine activity, involving at least the D2/D3 receptor system, appears to regulate the magnitude of losses chased or surrendered. By contrast, both these aspects of loss chasing are broadly independent of changes in sympathetic arousal, at least as mediated by beta-adrenoceptor activity. Our data highlight novel hypotheses about the monoaminergic mechanisms that promote the expression of this central, but poorly understood, aspect of gambling behavior.
In Experiment 1, we investigated the effects of tryptophan depletion to test whether central serotonin activity mediates loss-chasing behavior. This might have been manifested in at least two ways. First, several lines of evidence suggest that serotonin mediates the inhibition of non-rewarded or punished behavior (Crockett et al, 2009; Dayan and Huys, 2008; Soubrie, 1986). So, tryptophan depletion, leading to a reduction in serotonin activity, might have been expected to increase gambling to recover losses in our healthy adult participants. By contrast, serotonin activity also plays a significant role in learning from, and coping with, aversive events (Bari et al, 2010; Daw et al, 2002; Deakin and Graeff, 1991; Evers et al, 2005). Given that tryptophan depletion can also improve the prediction of punishing outcomes (Cools et al, 2008), and enhance neural responses to punishing outcomes within the anterior cingulate cortex (Evers et al, 2005), we also anticipated that tryptophan depletion might increase the salience of bad outcomes and diminish loss-chasing behavior. In fact, while producing no marked changes in healthy adults' state affect, tryptophan depletion significantly reduced the proportion of decisions participants made to chase losses, and reduced the number of consecutive decisions to chase, during a run of losing gambles. This suggests that, in this instance at least, serotonin activity helps to sustain loss chasing rather than inhibit it.
Descriptive theories of choice under uncertainty attribute loss-chasing behavior to the idea that the prospective reductions in subjective value or utility associated with chasing and suffering larger losses still are proportionately smaller than the reductions in utility associated with the smaller losses already incurred (Kahneman and Tversky, 2000). Under these conditions, it makes sense for gamblers to continue to play, so long as the necessary resources are available. From this perspective, loss chasing can be viewed as an aversively motivated escape behavior, but one controlled, at least in part, by the marginal utility of continued play relative to its cessation. Our finding that tryptophan depletion reduced our behavioral model of loss chasing suggests that, in this instance at least, diminished central serotonin activity reduced the marginal utility of continued play by increasing the salience of future bad outcomes across the range of values encountered during the game (Cools et al, 2008; Deakin and Graeff, 1991).
Further experiments will be needed to establish the relationship between serotonin activity and gambling to recover losses. However, given serotonin's complex contribution to impulse control, we should not assume that this relationship will be simple or linear (Winstanley et al, 2004). Our finding that tryptophan depletion reduced loss chasing is in line with other observations, obtained using simple elicitation procedures to measure risk attitudes, that carriers of the 10-repeat allele of the STin2 gene (that results in higher serotonin tone) show increased risk-seeking choices for losses (Zhong et al, 2009). By contrast, our data are apparently inconsistent with findings that 2 weeks treatment with tryptophan, as a dietary substrate, reduced shifts between risk-averse choices when making single decisions between certain gains and uncertain larger or smaller gains, and risk-seeking choices when making single decisions between certain losses and uncertain larger or smaller losses (Murphy et al, 2009). Collectively, these data indicate that serotonin's influence upon gambling to recover losses may vary depending on a number of psychological and pharmacological factors, including whether the experimental situation involves single or multiple consecutive choices to recover losses and whether there is a context of other choices that involve positive expected values.
The effects of a single 176 μg dose of PPX were quite different. This treatment did not increase the proportion of decisions to chase losses or the number of consecutive decisions to chase during a run of losing gambles; however, PPX did significantly increase the value of losses that participants were willing to chase and, at the same time, reduce the value of losses that participants were willing to surrender when quitting. Thus, a single dose of PPX induced a preference for chasing larger losses at the expense of smaller losses.
We acknowledge that the mode of action of the single 176 μg dose of PPX used in Experiment 2 remains uncertain. Although the behavioral effects of low doses of dopaminergic drugs may reflect pre-synaptic action at the auto-receptors of dopamine neurones within the mid-brain (Frank and O'Reilly, 2006; Santesso et al, 2009), single doses of 100 and 200 μg PPX may also reduce serum prolactin, suggesting a post-synaptic action of the drug at dopamine receptors in the anterior pituitary (Schilling et al, 1992). Here, replicating previous findings, we note that our dose of 176 μg PPX also significantly reduced participants' positive state affect (Hamidovic et al, 2008). This suggests that, in this experiment at least, doses of PPX influenced the performance of our loss-chasing game via activity at D2/D3 dopamine auto-receptors
D2 and D3 receptors are predominantly expressed within reinforcement pathways in the nucleus accumbens and amygdala (Camacho-Ochoa et al, 1995), in which both appear to influence the reinforcement value of stimulant drugs such as cocaine (Caine et al, 1997; Thiel et al, 2010). At the current time, we have no way of knowing which of these receptor subtypes makes the larger contribution to the loss-chasing behavior observed. Previous experiments have suggested that activity at D2 receptors can impair learning from the bad outcomes of risky decisions (‘no-go learning') by impairing the expression of dips in mid-brain dopamine activity that signal negative prediction errors (Frank and O'Reilly, 2006; Frank et al, 2007a,2007b, 2009). However, our data suggest that this insensitivity to losing outcomes associated with D2/D3 receptor activity produces more complex changes in risky choices than a simple failure to learn from negative events. Rather, we speculate that impairments in the detection of dips in dopamine activity following bad outcomes produced a straight failure to register small losses, thus increasing the number of PPX-treated participants' decisions to quit for small stakes. However, the reduced sensitivity to losing outcomes associated with D2/D3 activity also further diminished the negative change in subjective value associated with larger losses, increasing the marginal value of continued play; thus promoting decisions to chase for larger value losses compared with placebo.
Changes in reinforcement learning following treatment with PPX (Pizzagalli et al, 2008) are associated with altered signalling within the anterior cingulate region following bad outcomes (Santesso et al, 2009) and blunted signalling within the striatum following good outcomes (Riba et al, 2008). Previously, we have observed that attenuated neural responses to bad gambling outcomes within the anterior cingulate sulcus is also associated with continued chasing behavior during performance of our loss-chasing game (Campbell-Meiklejohn et al, 2008). This is consistent with recent electrophysiological evidence that the reward-related functions of the anterior cingulate and mid-line structures may be disrupted in pathological gamblers (Hewig et al, 2010). Therefore, the findings of Experiment 2 raise the possibility that single doses of PPX increase the value of losses judged worth chasing via altered reinforcement signalling within a distributed neural circuit encompassing the anterior cingulate region and its afferent ventral striatal targets (Nakano et al, 2000).
Finally, the results of Experiment 3 indicate that while a single dose of 80 mg propranolol significantly reduced HR compared with placebo, it did not significantly alter the number of decisions to chase losses, the value of losses chased, or the value of losses surrendered. These findings suggest that the cognitive and emotional aspects of loss-chasing modelled by our game—although obviously not the excitement associated with commercial gaming activities (Anderson and Brown, 1984)—are not influenced by manipulations of beta-adrenoceptor activity. They also provide some reassurance that the effects of tryptophan depletion and PPX we observed in Experiments 1 and 2 cannot be attributed to undetected changes in sympathetic and/or peripheral arousal. However, loss-chasing behavior might well be influenced by other aspects of noradrenaline function, including activity of alpha2-adrenoceptors that influence the activity of the ascending innervation of the locus coeruleus and modulate the processing of negative decision outcomes (or action errors) in the cingulate area (Riba et al, 2005).
Several limitations to our findings need to be addressed in future investigations. First, while our loss-chasing game captures the essential behavior of continued play that brings mounting losses, this necessarily limits our ability to isolate the specific psychological mechanisms that might be influenced by serotonin and D2/D3 activity to influence gambling to recover losses. Tryptophan depletion and single low doses of PPX produced distinct behavioral changes in gambling to recover losses, but additional experiments are needed to establish how these changes relate to what we already know about serotonin's role in avoidance or punishment-induced inhibition (Crockett et al, 2009; Soubrie, 1986) and what we know about the role of D2 receptors in learning from negative outcomes (Frank, 2006). Second, the clinical implications of these findings need to be explored by examining the effects of serotonergic and dopaminergic treatments on the performance of our loss-chasing game in samples of pathological gamblers, as well as testing loss chasing as a model of impaired control in other addictions (Rogers et al, 2010). We might also examine the role of other neurotransmitters, such as the opiate and glutamate systems, which may sustain gambling problems (Grant et al, 2007, 2008).
Pathological gambling is a source of enormous personal and family distress and represents a significant public health issue (Shaffer and Korn, 2002). Yet, we know very little about the biological factors that confer vulnerability for gambling problems, with no licensed pharmacological treatments currently available to clinicians. The experiments presented here indicate one way to start to tackle these issues empirically; namely, by investigating the neural and pharmacological basis of the cognitive and behavioral biases evident in the individuals who present at the clinic. These findings suggest that the general persistence of gamblers in playing to recover losses is modulated by serotonin activity, whereas the evaluation of losses that gamblers judge worth chasing is mediated by the activity of the D2/D3 receptor system.
Acknowledgments
This research was funded by a Medical Research Council studentship to Daniel Campbell-Meiklejohn and by an independent award from the Biotechnology and Biological Sciences Research Council (BBSRC) to Robert Rogers. We would also like to thank Michael Frank for helpful suggestions about an earlier version of this manuscript.
We report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Anderson G, Brown RI. Real and laboratory gambling, sensation-seeking and arousal. Br J Psychol. 1984;75 (Part 3:401–410. doi: 10.1111/j.2044-8295.1984.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. 2010Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats Neuropsychopharmacology 351290–1301.(E-pub ahead of print 27 January 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bergh C, Eklund T, Sodersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–475. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Steel Z, McConaghy N. Impulsivity in pathological gambling: the antisocial impulsivist. Addiction. 1997;92:75–87. [PubMed] [Google Scholar]
- Breen R-B, Zuckerman M. Chasing in gambling behavior: personality and cognitive determinants. Person Individ Differ. 1999;92:1097–1111. [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Camacho-Ochoa M, Walker EL, Evans DL, Piercey MF. Rat brain binding sites for pramipexole, a clinically useful D3-preferring dopamine agonist. Neurosci Lett. 1995;196:97–100. doi: 10.1016/0304-3940(95)11857-s. [DOI] [PubMed] [Google Scholar]
- Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain. 1992;5:325–370. [Google Scholar]
- Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD. Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry. 2008;63:293–300. doi: 10.1016/j.biopsych.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Cools R, Robinson OJ, Sahakian B. Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology. 2008;33:2291–2299. doi: 10.1038/sj.npp.1301598. [DOI] [PubMed] [Google Scholar]
- Corless T, Dickerson M. Gamblers' self-perceptions of the determinants of impaired control. Br J Addict. 1989;84:1527–1537. doi: 10.1111/j.1360-0443.1989.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt R, Starmer C, Sugden R. On the validity of the random lottery incentive system. Exp Econ. 1998;1:115–131. [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson's disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS Comput Biol. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Dickerson M, Hinchy J, Fabre J. Chasing, arousal and sensation seeking in off-course gamblers. Br J Addict. 1987;82:673–680. doi: 10.1111/j.1360-0443.1987.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, et al. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O'Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA. 2007a;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007b;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN. Escitalopram treatment of pathological gambling with co-occurring anxiety: an open-label pilot study with double-blind discontinuation. Int Clin Psychopharmacol. 2006;21:203–209. doi: 10.1097/00004850-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl) 2008;200:521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MG, Holroyd CB, et al. Hypersensitivity to reward in problem gamblers. Biol Psychiatry. 2010;67:781–783. doi: 10.1016/j.biopsych.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Choices Values and Frames. Cambridge University Press: Cambridge, UK; 2000. [Google Scholar]
- Lesieur H.1977The Chase: Career of the Compuslive Gambler1st edn.Anchor Press/Doubleday: Garden City, NY [Google Scholar]
- Lesieur HR. The compulsive gambler's spiral of options and involvement. Psychiatry. 1979;42:79–87. doi: 10.1080/00332747.1979.11024008. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Zucconi M, Oldani A, Fantini ML, Castronovo V, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–497. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- McBride O, Adamson G, Shevlin M. A latent class analysis of DSM-IV pathological gambling criteria in a nationally representative British sample. Psychiatry Res. 2010;178:401–407. doi: 10.1016/j.psychres.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Meyer G, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, Exton MS. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biol Psychiatry. 2000;48:948–953. doi: 10.1016/s0006-3223(00)00888-x. [DOI] [PubMed] [Google Scholar]
- Murphy S, Longhitano C, Ayres R, Cowen P, Harmer C, Rogers R. The role of serotonin in non-normative risky choice: the effects of tryptophan supplements on the ‘reflection effect' in healthy adult volunteers. J Cogn Neurosci. 2009;21:1709–1719. doi: 10.1162/jocn.2009.21122. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247V1 (Suppl 5:15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Bernardi S, Quercioli L, DeCaria C, Hollander E. Serotonin dysfunction in pathological gamblers: increased prolactin response to oral m-CPP versus placebo. CNS Spectr. 2006;11:956–964. doi: 10.1017/s1092852900015145. [DOI] [PubMed] [Google Scholar]
- Pallesen S, Molde H, Arnestad HM, Laberg JC, Skutle A, Iversen E, et al. Outcome of pharmacological treatments of pathological gambling: a review and meta-analysis. J Clin Psychopharmacol. 2007;27:357–364. doi: 10.1097/jcp.013e3180dcc304d. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond Ser B. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court HJ, Raven J. Manual for Raven's Progressive Matrices and Vocabulary Scales. Harcourt Assessment: San Antonio, TX; 1998. [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriguez-Fornells A, Morte A, Munte TF, Barbanoj MJ. Noradrenergic stimulation enhances human action monitoring. J Neurosci. 2005;25:4370–4374. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Kramer UM, Heldmann M, Richter S, Munte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoceptor blockade on components of human decision-making. Psychopharmacology (Berl) 2004;172:157–164. doi: 10.1007/s00213-003-1641-5. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health and disorders: implications for alcoholism. Alcohol Clini Exp Res. 2010;34:1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, et al. Pathological gambling. A psychobiological study. Arch Gen Psychiatry. 1988;45:369–373. doi: 10.1001/archpsyc.1988.01800280085011. [DOI] [PubMed] [Google Scholar]
- Sacco P, Torres LR, Cunningham-Williams RM, Woods C, Unick GJ. Differential item functioning of pathological gambling criteria: an examination of gender, race/ethnicity, and age. J Gambl Stud. 2010. [DOI] [PMC free article] [PubMed]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal–cortical function. Hum Brain Mapp. 2009;30:1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JC, Adamus WS, Palluk R. Neuroendocrine and side effect profile of pramipexole, a new dopamine receptor agonist, in humans. Clin Pharmacol Therapeut. 1992;51:541–548. doi: 10.1038/clpt.1992.60. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Korn DA. Gambling and related mental disorders: a public health analysis. Annu Rev Public Health. 2002;23:171–212. doi: 10.1146/annurev.publhealth.23.100901.140532. [DOI] [PubMed] [Google Scholar]
- Shafir E, Tversky A.1995Decision MakingIn: Smith EE, Oscherson DN (eds).Thinking MIT Press: Cambridge, MA; 77–100. [Google Scholar]
- Soubrie P. Serotonergic neurons and behavior. J Pharmacol. 1986;17:107–112. [PubMed] [Google Scholar]
- Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL.2010Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior Behav Brain Res 214386–394.(E-pub ahead of print 19 June 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen FM, Mies GW, van der Molen MW, Evers EA. Acute tryptophan depletion in healthy males attenuates phasic cardiac slowing but does not affect electro-cortical response to negative feedback. Psychopharmacology (Berl) 2008;199:255–263. doi: 10.1007/s00213-008-1176-x. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease. Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007;64:212–216. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX.2007A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers Neuropsychopharmacology 321678–1686.(E-pub ahead of print 3 January 2007). [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zhong S, Israel S, Xue H, Sham PC, Ebstein RP, Chew SH. A neurochemical approach to valuation sensitivity over gains and losses. Proc Biol Sci. 2009;276:4181–4188. doi: 10.1098/rspb.2009.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.