Abstract
Alcohol use disorders (AUDs) impact millions of individuals and there remain few effective treatment strategies. Despite evidence that neuronal nicotinic acetylcholine receptors (nAChRs) have a role in AUDs, it has not been established which subtypes of the nAChR are involved. Recent human genetic association studies have implicated the gene cluster CHRNA3–CHRNA5–CHRNB4 encoding the α3, α5, and β4 subunits of the nAChR in susceptibility to develop nicotine and alcohol dependence; however, their role in ethanol-mediated behaviors is unknown due to the lack of suitable and selective research tools. To determine the role of the α3, and β4 subunits of the nAChR in ethanol self-administration, we developed and characterized high-affinity partial agonists at α3β4 nAChRs, CP-601932, and PF-4575180. Both CP-601932 and PF-4575180 selectively decrease ethanol but not sucrose consumption and operant self-administration following long-term exposure. We show that the functional potencies of CP-601932 and PF-4575180 at α3β4 nAChRs correlate with their unbound rat brain concentrations, suggesting that the effects on ethanol self-administration are mediated via interaction with α3β4 nAChRs. Also varenicline, an approved smoking cessation aid previously shown to decrease ethanol consumption and seeking in rats and mice, reduces ethanol intake at unbound brain concentrations that allow functional interactions with α3β4 nAChRs. Furthermore, the selective α4β2* nAChR antagonist, DHβE, did not reduce ethanol intake. Together, these data provide further support for the human genetic association studies, implicating CHRNA3 and CHRNB4 genes in ethanol-mediated behaviors. CP-601932 has been shown to be safe in humans and may represent a potential novel treatment for AUDs.
Keywords: α3β4* nicotinic acetylcholine receptor, ethanol, drug abuse, addiction, rat
INTRODUCTION
Alcohol and nicotine addiction are often treated as separate disorders, although ∼60–80% of heavy drinkers smoke tobacco (Moss et al, 2007), and it has been suggested that common genes are involved in the susceptibility of both alcohol and nicotine dependence (Dani and Harris, 2005; de Fiebre and Collins, 1992; Joslyn et al, 2008; Kamens et al, 2010; Schlaepfer et al, 2008). Additionally, it has been shown that ethanol can directly or indirectly interact with neuronal nicotinic acetylcholine receptors (nAChRs) (Blomqvist et al, 1992; Davis and de Fiebre, 2006; Le et al, 2000), which have been identified as important therapeutic targets for the treatment of alcohol use disorders (AUDs) (Chatterjee and Bartlett, 2010).
The nAChRs are well-characterized pentameric ligand-gated ion channels consisting of different homomeric and heteromeric combinations of α2–α10 and β2–β4 subunits. The α4, β2, and α7 subunits of the nAChRs have been shown to be most widely expressed in the brain, forming functional receptor subtypes either as heteromeric (α4* and β2*) or homomeric (α7) receptors (* indicates the possibility of additional subunits). The α2, α3, α6, β2, β3, or β4 subunits can combine to form either simple subtypes, such as α3β4, or complex native nAChRs, such as α3β2β4* or α3β3β4* and are localized in different brain regions and have specific functions (Gotti et al, 2006, 2007).
The α4β2* nAChR is the predominant nAChR subtype in the brain, and it is now well established that it has an essential role in mediating nicotine's rewarding properties (Picciotto et al, 1998; Tapper et al, 2004). Consistent with an important role of α4β2 nAChRs in nicotine addiction, a recently introduced α4β2 nAChR partial agonist, varenicline (Coe et al, 2005a), has been demonstrated to be an efficacious smoking cessation aid in the clinic (Cahill et al, 2009). The subunit composition involved in ethanol's rewarding properties, however, remains controversial. It has been previously shown that varenicline reduces ethanol consumption and operant self-administration following long-term ethanol exposure in rats (Steensland et al, 2007) and mice (Kamens et al, 2010). This has since been supported by a clinical study showing that varenicline decreased ethanol consumption in heavy drinking smokers (McKee et al, 2009).
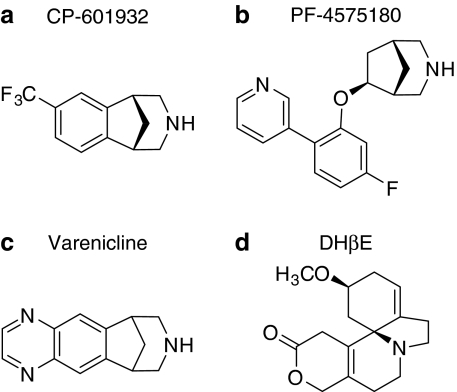
Recent human genetic association studies have identified a genetic locus, encoding for the α3(CHRNA3), α5(CHRNA5), and β4(CHRNB4) nAChR subunits in nicotine and alcohol-dependent subjects (Joslyn et al, 2008; Saccone et al, 2009; Wang et al, 2009), suggesting an involvement of these specific subunits in alcohol dependence. It has been difficult to determine the precise role of the individual subunits of the nAChR, as there have been few brain penetrant selective ligands that target the α3β4-containing nAChRs. In this study, we identified two nAChR ligands, CP-601932 and PF-4575180 (Figure 1), that are partial agonists at the α3β4 nAChR in order to determine the role of the α3β4 nAChRs in ethanol-mediated behaviors. CP-601932 has also high affinity for α4β2 nAChR and has been in development as a smoking cessation aid. It is an enantiomer derived from a racemic compound that was previously reported to be a potent, partial agonist at human α4β2 nAChRs expressed in oocytes (Coe et al, 2005b). The rat pharmacokinetic properties of CP-601932 have been described (Shaffer et al, 2009). PF-4575180 was synthesized during studies on [3.2.1]azabicyclic compounds that are selective for α3β4 and nAChRs comprising an α6/α4 chimera combined with the β4 subunit (α6/4β4), but have relatively low affinity for α7, α4β2, and α1-containing nAChRs (Lowe et al, 2010). The data presented here on these compounds and on varenicline suggest that α3β4* nAChRs could have an important role in ethanol-mediated behaviors.
Figure 1.
Structures of (a) CP-601932, (b) PF-4575180, (c) varenicline, and (d) dihydro-β-erythroidine.
MATERIALS AND METHODS
Animals and Housing
Adult, male Wistar rats (Harlan, Indianapolis, IN), a strain traditionally used for ethanol-intake studies, including in our previous publications (Simms et al, 2008; Steensland et al, 2007), were used for alcohol-intake studies at the Gallo Center. Adult male Sprague-Dawley rats (Charles River, Wilmington, MA), the same strain as used in previous pharmacokinetic and pharmacodynamic studies (Rollema et al, 2010; Shaffer et al, 2009), were used for pharmacokinetic studies at Pfizer Global Research and Development. Rats were individually housed in climate-controlled rooms and kept on a 12-h reversed light–dark cycle (lights off at 1000 hours), and food and water were available ad libitum. All procedures were preapproved by the Gallo Center and Pfizer Institutional Animal Care and Use Committees and were in accordance with the NIH guidelines for the Humane Care and Use of Laboratory Animals.
Pharmacodynamic Studies
In vitro binding affinity to nAChRs
Binding affinities of the test compounds to nAChR subtypes were determined as described (Rollema et al, 2007), using [3H] epibatidine to label α4β2 and α3β4 nAChRs expressed in HEK293 cells. Ki values were calculated according to Ki=IC50/(1+[3H] ligand)/Kd) and expressed as Ki.
In vitro functional activity at α3β4 and α4β2 nAChRs expressed in HEK cells by FLIPR
Agonist and antagonist activities were assessed using HEK293 cell lines stably transfected with either human α3β4 or α4β2 nAChRs. Functional activity was measured as agonist-evoked calcium flux assay on a Fluorescence Imaging Plate Reader (FLIPR, Molecular Devices, Silicon Valley, CA), as previously described (Wishka et al, 2006). On the day of the assay, the cells were loaded with a 1 : 1 mixture of 2 μM Calcium Green-1, AM (Molecular Probes) dissolved in anhydrous DMSO, and 20% pluronic F-127 (Molecular Probes). This solution was added directly to the growth media of each well to achieve a final concentration of 2 μM of Calcium Green-1. Plates were then loaded onto the FLIPR and read at excitation and emission wavelengths of 488 and 516 nm, respectively. Agonist testing was done by measuring the effect of a single addition of test compound to the medium in comparison with the effect of 100 μM ACh. To assess inhibitory activities, test compounds were applied to cells for 2 min and subsequently challenged with 30 μM ACh. Inhibition for each concentration of the test compounds was calculated from the change in fluoresce (ΔF) evoked by 30 μM ACh in wells pretreated with the test compound relative to the ΔF of wells that were pretreated with vehicle. Each experiment was run in quadruplicate, and EC50 and IC50 values were estimated from the concentration-activation and concentration-inhibition curves. The fraction of activated α3β4 nAChRs was calculated from the compound's instantaneous activation and inhibition concentration response. This fraction was derived from the product of the equations for the fraction of ACh-evoked current: Emax × (C/(C+EC50) and for the fraction of receptors that are available to be activated (ie, the fraction not inhibited) at that concentration: 100−{100 × (C/(C+IC50)}.
Pharmacokinetic Studies
All pharmacokinetic and tissue binding studies were performed in Sprague-Dawley rats, or with tissues derived from them using standard procedures and a characterized LC–MS/MS assay to determine the estimates of unbound brain concentrations after each dose (details are given in Supplementary Information).
Ethanol-Intake Studies
Operant self-administration procedures
Operant self-administration testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA) as described previously (Steensland et al, 2007). In brief, rats were randomly divided into two groups each for CP-601932 (ethanol group: n=13; sucrose group: n=10) and PF-4575180 compound testing (ethanol group: n=8; sucrose group: n=14). Rats were trained to self-administer 10% ethanol (v/v) or 5% sucrose, on a fixed ratio 3 (three active lever presses required for 0.1 ml reward) schedule of reinforcement, daily (Monday through Friday) for 30 min. To evaluate the acute effects, CP-601932 (5 and 10 mg/kg subcutaneously (s.c.)) or vehicle (saline) were administered 30 min before the operant session (mean body weight before treatment: ethanol group: 550±11 g; sucrose group: 596±12 g; 11 weeks; ∼55 sessions). In a separate group of rats, PF-4575180 (1 and 10 mg/kg s.c.) or vehicle (saline) was administered 2 h before the operant session (mean body weight before treatment: ethanol group: 580±16g; sucrose group: 534±9 g; 10 weeks; ∼50 sessions). We found no effect of PF-4575180 when administered 30 min prior to the operant session and hence switched to administering 2 h prior. Each injection was given 7 days apart in a Latin square design, thus each animal served as its own control. Between the injection days, the rats were exposed to their normal schedule of reinforcement as described above, with no injections for the remaining days of that week.
To evaluate the effect of chronic/multiple administration of CP-601932 on operant self-administration of ethanol, rats were trained to self-administer 10% (v/v) ethanol as described above. CP-601932 (10 mg/kg s.c.) or vehicle (saline) was administered 30 min before the operant ethanol self-administration session on each of 5 consecutive days (Monday through Friday), and the number of lever presses was recorded each day. The post-treatment baseline active lever presses was recorded on the Saturday, 24 h following the last CP-601932 administration. It has previously been shown that the changing of the home cage results in decreased activity during the self-administration session (Balcombe et al, 2004). Thus, the data from the operant self-administration session following a cage change (day 3) were excluded from the analysis, as the results could not be attributed to the CP-601932 treatment.
Intermittent-access two-bottle-choice drinking paradigm
The intermittent-access 20% ethanol two-bottle-choice drinking paradigm has been described previously (Simms et al, 2008; Wise, 1973), and details are given in the Supplementary Information. For the intermittent-access 5% sucrose two-bottle-choice drinking paradigm, the rats were given access to 5% sucrose rather than 20% ethanol. Drug administration began after the rats had maintained stable baseline drinking levels of the 20% (v/v) ethanol solution or 5% sucrose. In the ethanol-intake studies, CP-601932 (1, 5, and 10 mg/kg s.c.) or vehicle (saline), dihydro-β-erythroidine (DHβE) (6 and 10 mg/kg s.c.) or vehicle (saline), PF-4575180 (1 and 10 mg/kg s.c.) or vehicle (saline) were administered to three separate groups of rats (CP-601932: n=12, 6±1 g/kg/24 h, 11 weeks; ∼33 drinking sessions; DHβE: n=12, 5.1±0.4 g/kg/24 h, 12 weeks; ∼36 sessions; PF-4575180: n=10, 6.1±0.3 g/kg/24 h, 9 weeks; ∼27 session). The mean body weight of the rats at the first CP-601932, DHβE, and PF-4575180 treatment were 539±18, 576±21, and 493±9 g, respectively. Administration of CP-601932 (1, 5, and 10 mg/kg s.c.) and PF-4575180 (1 and 10 mg/kg s.c.) for the sucrose consuming groups began once the animals had attained stable drinking levels (CP-601932 group: n=10, 25±2 g/kg/24 h, 8 weeks; 24 sessions; PF-4575180 group: n=10, 27±2 g/kg/24 h, 11 weeks; 33 sessions). The mean body weights of the rats at the first CP-601932 and PF-4575180 treatment in the sucrose groups were 444±16 and 486±20 g, respectively. All the treatments were administered 30 min before the presentation of either the water or 20% ethanol or 5% sucrose bottles. Each injection was given 7 days apart using a Latin square design, thus each animal served as its own control. Between the injection days, the rats were exposed to their normal drinking schedule of intermittent access as described above with no injections for the remaining days of that week.
Taste preference
To control for the modulation of taste sensitivity, CP-601932 (10 mg/kg s.c.) and PF-4575180 (10 mg/kg s.c.) were administered to two separate groups of rats that had access to water or to a solution containing saccharin (0.2%) with quinine (0.001%) (Cippitelli et al, 2010; Goodwin and Amit, 1998) instead of 20% ethanol in the two-bottle-choice intermittent-access drinking paradigm. The bottles were measured at 30 min, 6, and 24 h after the fluids were presented, and measurements were taken to the nearest gram. The taste preference of saccharin with quinine over water (the ratio of saccharin with quinine to total fluid intake) was calculated at each time point (CP-601932 group: n=5, 4±0.6 mls/30 min, 5 weeks; 15 sessions, average weight: 396±9 g; PF-4575180 group: n=5, 5.5±1 mls/30 min, 5 weeks; 15 sessions, average weight: 404±10 g). All treatments were administered 30 min before the presentation of either the water or saccharin/quinine bottles. Each injection was given 5 days apart using a Latin square design; thus, each animal served as its own control. Between the injection days, the rats were exposed to their normal drinking schedule of intermittent access as described above with no injections for the remaining days of that week.
Drugs and Chemicals
Ethanol and sucrose solutions were prepared in filtered water using 95% (v/v) ethanol (Gold Shield Chemical Ac., Hayward, CA) and sucrose (Fisher Scientific, Pittsburgh, PA), respectively. Radioligands were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA) CP-601932 [(1S,5R)-2,3,4,5-tetrahydro-7-(trifluoromethyl)-1,5-methano-1H-3-benzazepine] and PF-4575180 [rac-exo-[6-(2-(pyridin-3-yl)-5-fluorophenoxy))]-3-azabicyclo[3.2.1]octane dihydrochloride salt] were synthesized at Pfizer Global Research and Development, Groton, CT (for a description of the synthesis of PF-4575180, see Supplementary Information). DHβE was purchased from Sigma (St Louis, MO). All drugs were dissolved in saline and administered s.c. in a volume of 1 ml/kg. All drug solutions were prepared immediately before each injection.
Statistics
Statistical analysis was performed using Graph Pad Prism (Graph Pad, San Diego, CA) or Sigma Stat (Systat Software, San Jose). Behavioral data from acute treatment in the operant self-administration and two-bottle-choice paradigms were analyzed using repeated measures one-way ANOVA followed by Newman–Keuls post hoc analysis when a significant overall main effect was found (P<0.05). The taste preference data were analyzed using paired t–tests, with a significance level of P<0.05. The blood–ethanol concentration (BEC) and chronic treatment data were analyzed with two-way ANOVA followed by Newman–Keuls post hoc analysis when a significant overall main effect was found (P<0.05).
RESULTS
In Vitro Pharmacodynamics of CP-601932 and PF-4575180
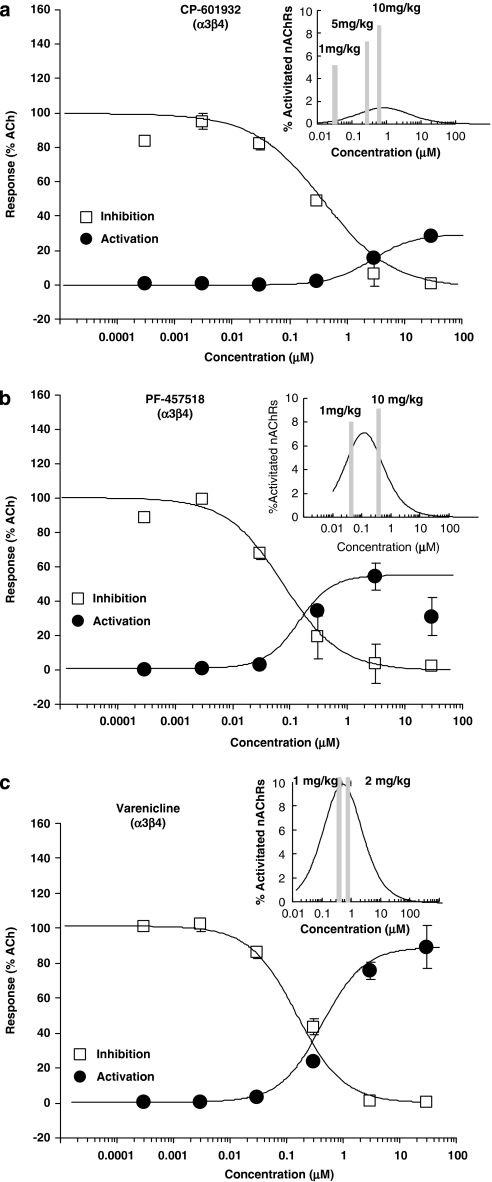
We measured binding affinities and functional activities of CP-601932 and PF-4575180 in HEK293 cells expressing either α3β4 or α4β2 nAChRs (Table 1). The averaged concentration-dependent inhibition and activation curves at α3β4 are shown in Figure 2 and for α4β2 nAChRs are shown in the Supplementary Information (Supplementary Figure S1), with inserts showing free brain concentrations and the fraction of the nAChR subtype that is inhibited and activated (see below). CP-601932 has the same high-binding affinity at α3β4 (Ki=21 nM) as at α4β2 nAChRs (Ki=21 nM) and an order of magnitude lower affinity for α6 and α7 nAChR subtypes (Ki>300 nM). Functional activity was measured using a Ca2+-sensitive dye in a cell-based fluorescence assay. Relative to ACh, CP-601932 is a partial agonist of α3β4 nAChRs, with an EC50 of about 3 μM and intrinsic efficacy of about 30% of that of ACh. CP-601932 produced no measurable change in Ca2+ fluorescence when applied to HEK293 cells expressing α4β2 nAChRs (Supplementary Figure S1A). When preapplied to cells expressing either α3β4 or α4β2 nAChRs, CP-601932 inhibited the Ca2+-signal evoked by a subsequent challenge with ACh, with IC50 values of 257 and 114 nM, respectively. A likely explanation for the inhibition of the ACh-evoked response is that, like other agonists of nAChRs, CP-601932 can stabilize the desensitized conformation of the receptors and thus prevent subsequent activation by ACh (for review, see Ochoa et al (1989)). However, further studies on the mechanism of inhibition of expressed receptors by the test compounds were not performed.
Table 1. Binding Affinities, Functional Potencies, and Intrinsic Efficacies vs Ach of CP-601932, PF-4575180, and Varenicline at Human α3β4 and α4β2 nAChRs Subtypes Expressed in HEK293 Cells.
| Compound | nAChR Subtype | Binding affinity |
Function potency and efficacy |
||
|---|---|---|---|---|---|
| Ki (nM) |
Activation |
Inhibition | |||
| EC50 (μM) | Efficacy (% vs ACh) | IC50 (nM) | |||
| CP-601932 | α3β4 | 21±28 (13) | 2.92±0.32 (3) | 30.2±3.4 (3) | 257±41 (3) |
| α4β2 | 21±19 (13) | >30 (3) | a | 114±29 (3) | |
| PF-4575180 | α3β4 | 4.2±0.9 (4) | 0.24±0.05 (4) | 52.4±3 (4) | 112±25 (4) |
| α4β2 | >1195±475 (4)b | >30 (4) | a | 733±190 (4) | |
| Varenicline | α3β4 | 74.7±6.2 (18) | 1.84±0.58 (5) | 93.5±1.4 (5) | 220±1.4 (5) |
| α4β2 | 0.3±0.02 (18) | 1.41±0.17 (5) | 32.4±1.1 (5) | 0.23±0.03 (5) | |
| DHβE | α3β4 | 0c | 23 000b | ||
| α4β2 | 0c | 370b | |||
Data are expressed as mean±SEM (n).
Intrinsic efficacy could not be determined due to insufficient signal amplitude at the highest test concentration.
Ki value of 1 of n=4 was determined to be >3.6 μM due to insufficient inhibition at the highest test concentration. This value was not included in the calculation of mean and SEM.
Antagonist, data are from Harvey et al (1996).
Figure 2.
Concentration-dependent activation and inhibition curves of α3β4 nAChRs expressed in HEK293 cells measured by FLIPR methodology. Activation data (filled circles) are expressed as fraction of the response evoked by 100 μM ACh. Inhibition data (open squares) were generated by applying 30 μM ACh in the presence of varying concentrations of the test compound, and the data are normalized to the response evoked by 30 μM ACh in the absence of test compound. The activation and inhibition curves are the curves of best fit through the data points. (Inserts) Concentration-dependent fraction of activated α3β4 nAChRs calculated from the fitted activation and inhibition curves. Vertical gray bars correspond to the estimated unbound rat brain concentrations (in nM) measured at 30 min after 5 and 10 mg/kg of CP-601932 (a), 1 and 10 mg/kg of PF-4575180 (b), and 1 and 2 mg/kg of varenicline (c).
PF-4575180 is one of a series of compounds that bind to α3* and α6* nAChRs, but have very low affinity for α7 nAChR (Lowe et al, 2010). PF-4575180 was found to have 10 times higher affinity for α3β4 nAChRs (Ki=4.2 nM) than for α6/4β4 nAChRs (Ki=45 nM) and very low affinity for α4β2 nAChRs (Ki>1.2 μM). The intrinsic agonist efficacy of PF-4575180 is 52% relative to ACh at α3β4 nAChRs, but PF-4575180 produces no measurable activation of α4β2 nAChRs as measured by Ca2+-evoked fluorescence (Supplementary Figure S1B). PF-4575180 is also more potent at inhibiting α3β4 (IC50=112 nM) than α4β2 nAChRs (IC50=733 nM). For comparison, binding and functional data for varenicline at α3β4 and α4β2 nAChRs (Rollema et al, 2007), as well as reported IC50 values of DHβE for antagonism of α4β2 and α3β4 nAChRs (Harvey et al, 1996), are also included in Table 1.
In Vivo Pharmacokinetics of CP-601932 and PF-4575180
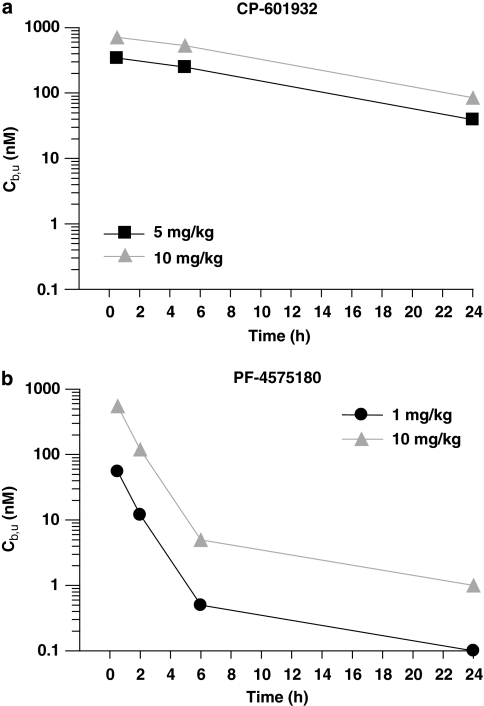
Time courses of unbound brain compound concentrations (Cb,u) (see Supplementary Information) after s.c. doses that have been used for the behavioral studies are shown in Figure 3. CP-601932 readily penetrates the CNS and at 30 min reaches maximal Cb,u values of 340 nM after 5 mg/kg and 710 nM after 10 mg/kg. Brain concentrations of CP-601932 decline very slowly and levels stay relatively high, eg, 530 nM at 5 h and 85 nM at 24 h after 10 mg/kg (Figure 3a). PF-4571580 also rapidly reaches its highest Cb,u of 55 and 550 nM after 1 and 10 mg/kg, respectively, but levels decline rapidly and are <1 and 5 nM at 6 h after 1 and 10 mg/kg, respectively (Figure 3b).
Figure 3.
Time courses of unbound CP-601932 and PF-4575180 concentrations in rat brain (Cb,u) after s.c. administration of 5 mg/kg (▪-▪) and 10 mg/kg (▴-▴)CP-601932 (a) and 1 mg/kg (•-•) and 10 mg/kg (▴-▴) PF-4575180 (b). Data are expressed as nM (n=2).
In Vivo Ethanol-Intake Studies
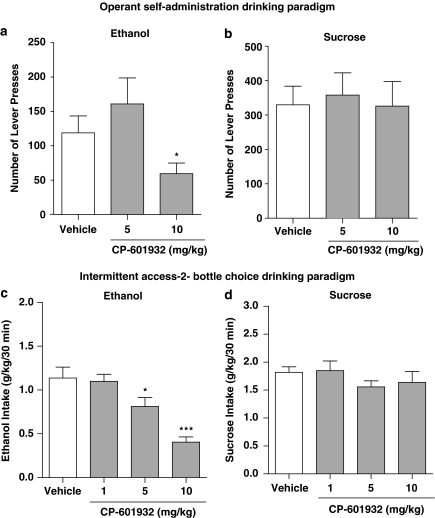
To evaluate the effect of CP-601932 on ethanol self-administration, the compound was given to a group of Wistar rats that were trained to self-administer 10% ethanol as previously described (Steensland et al, 2007). CP-601932 (5 and 10 mg/kg s.c.) or vehicle was administered 30 min before the start of the operant session. CP-601932 treatment had an overall main effect on operant self-administration of 10% ethanol (F(2,12)=9.1, P<0.01). Post hoc analysis revealed that the highest dose of CP-601932 (10 mg/kg) significantly decreased the number of presses on the active lever and inhibited 10% ethanol self-administration compared with active lever presses for vehicle treatment (Figure 4a), with no overall main effect on the number of inactive lever presses, see Supplementary Table S1 (Supplementary Information). To determine whether CP-601932's effect in decreasing ethanol self-administration behavior was specific, CP-601932 was given to a separate group of rats trained to self-administer 5% sucrose (see Materials and Methods). CP-601932 treatment did not have an overall main effect on 5% sucrose self-administration (F(2,9)=0.19, n.s.; Figure 4b). We also found no effect on the number of presses on the inactive lever, see Supplementary Table S1. These results suggest that CP-601932 selectively decreases ethanol self-administration by modulating the reinforcing properties of ethanol without affecting natural reward seeking behavior.
Figure 4.
Acute administration of CP-601932 decreases ethanol but not sucrose consumption and seeking. CP-601932 (10 mg/kg) decreased active lever presses for (a) 10% ethanol, but not (b) 5% sucrose in the operant self-administration paradigm. CP-601932 (5 and 10 mg/kg) significantly decreased high voluntary ethanol consumption (c) but not sucrose consumption (d) at 30 min after the onset of drinking in rats using the intermittent-access two-bottle-choice drinking paradigm. All values are expressed as mean number of active lever presses±SEM (a, b) and mean ethanol or sucrose intake (g/kg)±SEM (c, d) (repeated measures ANOVA followed by Newman–Keuls post hoc test. *P<0.05 compared with vehicle, n=10–13 for the operant paradigm and *P<0.05, ***P<0.001 compared with vehicle, n=10–12 for the two-bottle-choice paradigm.
We have previously shown that the 20% ethanol intermittent-access two-bottle-choice drinking paradigm induces high voluntary ethanol intake in rats (Simms et al, 2008; Steensland et al, 2007). We examined the effect of CP-601932 (1, 5, and 10 mg/kg s.c.) or vehicle using this paradigm once the rats had maintained stable baseline drinking (see Materials and Methods). There was an overall main effect of CP-601932 treatment on ethanol consumption at all time points examined separately (30 min: F(3,11)=14, P<0.001; 6 h: F(3,11)=19.8, P<0.001; and 24 h: F(3,11)=17.3, P<0.001). Post hoc analysis shows that both 5 and 10 mg/kg CP-601932 significantly decreased ethanol consumption at 30 min (Figure 4c), but only the highest CP-601932 dose (10 mg/kg) decreased ethanol consumption at the 6-h (Supplementary Table S2) and 24-h (Supplementary Table S2) time points. Furthermore, there was an overall main effect of CP-601932 on the preference of ethanol over water at all time points (30 min: F(3,11)=7.6, P<0.001 (data not shown); 6 h: F(3,11)=7.8, P<0.001; 24 h: F(3,11)=11.5, P<0.0001; Supplementary Table S2). Post hoc analysis shows that the highest dose of CP-601932 decreased the preference for ethanol over water at all time points (Supplementary Table S2). In contrast, CP-601932 treatment did not have an overall main effect on water consumption or the total fluid intake at any time point (Supplementary Table S2). To further evaluate the specificity of its effect on high ethanol intake, CP-601932 was administered to a group of rats consuming high amounts of 5% sucrose using the intermittent-access drinking paradigm (see Materials and Methods). CP-601932 treatment did not have an overall main effect on sucrose consumption at any of the measured time points examined separately (30 min: F(3,10)=1, n.s.; Figure 4d; 6 h: F(3,10)=3, n.s.; Supplementary Table S2; and 24 h: F(3,10)=1, n.s.; Supplementary Table S2). Similarly, there was no overall main effect of CP-601932 on the preference of sucrose over water (Supplementary Table S2; Supplementary Information). These results show that CP-601932 selectively reduces ethanol consumption without having any significant effect on water or sucrose consumption in rats. We also examined the possibility that CP-601932 decreases ethanol intake by affecting BEC (Supplementary Table S3; Supplementary Information). We found no difference in the BECs between a vehicle and CP-601932-treated group, suggesting that CP-601932 is reducing ethanol consumption by directly affecting the reinforcing properties of ethanol.
The α3β4* nAChR Partial Agonist PF-4575180, Selectively Decreases Ethanol Self-administration and Voluntary Ethanol Consumption
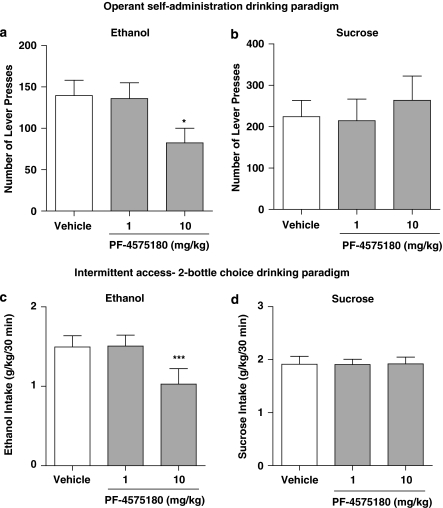
As CP-601932 has a similar affinity at α3β4 and α4β2 nAChRs, a partial agonist at α3β4 nAChRs, PF-4575180, was used to distinguish between the role of α3β4* and α4β2* nAChRs in ethanol-mediated effects. PF-4575180 is a partial agonist at α3β4 nAChRs (Table 1) and is tested using the self-administration and intermittent-access two-bottle-choice drinking paradigms. Unlike CP-601932, PF-4575180 (1 and 10 mg/kg s.c.) or vehicle was given 2 h and not 30 min before the start of the operant session (see Materials and Methods). We found no effect when administering 30 min prior to the operant session. We speculate that it could be due to complex PK/PD relationships in vivo, such as a possible active metabolite; however, this remains to be investigated. PF-4575180 treatment had an overall main effect on operant self-administration of 10% ethanol (F(2,7)=4.6, P<0.05). Post hoc analysis revealed that the highest dose of PF-4575180 (10 mg/kg) significantly decreased the number of presses on the active lever and inhibited 10% ethanol self-administration compared with active lever presses for vehicle treatment (Figure 5a). Furthermore, PF-4575180 had no overall main effect on the number of presses on the inactive lever, see Supplementary Table S1 (Supplementary Information). To evaluate the specificity of PF-4575180 in decreasing ethanol administration, it was given to another group of rats self-administering 5% sucrose (see Materials and Methods). PF-4575180 treatment did not have an overall main effect on 5% sucrose self-administration (F(2,13)=0.22, n.s.; Figure 5b) or have any effect on the number of presses on the inactive lever (Supplementary Table S1). These results indicate that PF-4575180, similar to CP-601932, selectively decreases ethanol seeking without affecting sucrose-seeking behavior.
Figure 5.
PF-4575180, an α3β4 partial agonist, decreased ethanol but not sucrose consumption and seeking. PF-4575180 (10 mg/kg s.c.) treatment decreased active lever presses for ethanol (a) but not for 5% sucrose self-administration (b) in the operant drinking paradigm. PF-4575180 (10 mg/kg s.c.) treatment decreased voluntary ethanol consumption (c) but not sucrose consumption (d) 30 min after the onset of drinking in the intermittent-access two-bottle-choice drinking paradigm. The values are expressed as mean number of active lever presses±SEM (a, b) or ethanol or sucrose intake (g/kg) ±SEM (c, d) (repeated measures ANOVA followed by Newman–Keuls post hoc test). *P<0.05, compared with vehicle, n=8–14 for the operant paradigm and ***P<0.001 compared with vehicle, n=10 for the two-bottle-choice paradigm.
We also evaluated the effect of PF-4575180 on voluntary excessive ethanol consumption using the intermittent-access two-bottle-choice paradigm as previously described (Simms et al, 2008). PF-4575180 (1 and 10 mg/kg) or vehicle (saline) was given when the rats had attained stable baseline drinking levels. There was an overall main effect of PF-4575180 treatment on ethanol consumption at all time points examined separately (30 min: F(2,9)=13.8, P<0.0001; 6 h: F(2,9)=64.6, P<0.0001; and 24 h: F(2,9)=29, P<0.0001). Post hoc analysis shows that only the highest dose of PF-4575180 (10 mg/kg s.c.) decreased ethanol consumption at 30 min (Figure 5c), whereas both doses of PF-4575180 (1 and 10 mg/kg s.c.) decreased ethanol consumption at the 6-h (Supplementary Table S4) and 24-h time points (Supplementary Table S4). Furthermore, there was an overall main effect of PF-4575180 on the preference of ethanol over water at all time points (30 min: F(2,9)=5.9, P<0.05; 6 h: F(2,9)=33, P<0.0001; and 24 h: F(2,9)=31, P<0.0001). Post hoc analysis shows that the highest dose of PF-4575180 (10 mg/kg) decreased the preference for ethanol over water at the 30-min time point and 24-h time point, whereas both doses 1 and 10 mg/kg of PF-4575180 decreased the preference at the 6-h time point (Supplementary Table S4). In contrast to CP-601932, PF-4575180 treatment showed an overall main effect on water consumption at the 6- and 24-h time points (30 min: F(2,9)=2.7, n.s.; 6 h: F(2,9)=4.6, P<0.05, Supplementary Table S4; and 24 h: F(2,9)=13.4, P<0.001). Post hoc analysis revealed that only the highest dose of PF-4575180 (10 mg/kg) increased water consumption at the 6- and 24-h time points (Supplementary Table S4). However, PF-4575180's increase in water consumption does not affect the total fluid consumption at any time point (Supplementary Table S4). Similar to CP-601932, we evaluated the selectivity of PF-4575180 in decreasing ethanol consumption using 5% sucrose intermittent-access paradigm. PF-4575180 treatment did not have an overall main effect on sucrose consumption at any of the measured time points examined separately (30 min: F(2,9)=0.0009, n.s.; Figure 5d; 6 h: F(2,9)=1.6, n.s.; Supplementary Table S4; and 24 h: F(2,9)=0.27, n.s.; Supplementary Table S4). Similarly, there was no overall main effect of PF-4575180 on the preference of sucrose over water (Supplementary Table S4). These results show that PF-4575180 with preference for the α3β4 nAChR selectively reduces ethanol consumption without having any significant effect on sucrose consumption in rats. However, in contrast to CP-601932 treatment, PF-4575180 increases water consumption but without affecting the total fluid intake.
PF-4575180 and CP-601932 do not Affect Taste Preference for Saccharin with Quinine
As PF-4575180 treatment decreased ethanol intake while increasing water consumption, we evaluated whether this effect could be due to a shift in the preference from ethanol to water by interacting with the taste of ethanol. Hence, we assessed the effect of both PF-4575180 (10 mg/kg) and CP-601932 (10 mg/kg) in two separate groups of rats that were voluntarily consuming a solution of 0.2% saccharin with 0.001% quinine in the intermittent-access drinking paradigm (see Materials and Methods). There was no effect of PF-4575180 treatment on saccharin/quinine taste preference and consumption measured at all the time points, examined separately using t-test (saccharin/quinine preference: 30 min: t=0.4, df=4, P=n.s.; 6 h: t=1.6, df=4, P=n.s.; and 24 h: t=1.6, df=4, P=n.s. (Table 2); consumption (mls): 30 min: t=0.02, df=4, P=n.s.; 6 h: t=0.3, df=4, P=n.s.; and 24 h: t=0.79, df=4, P=n.s. (Table 2)). CP-601932 treatment had no effect on the taste preference of saccharin/quinine at any time point (saccharin/quinine preference: 30 min: t=0.6, df=4, P=n.s.; 6 h: t=1.2, df=4, P=n.s.; and 24 h: t=0.97, df=4, P=n.s. (Table 2)). It had no effect on the saccharin/quinine consumption at both 30 min and 6 h, but had an effect only at the 24-h time point (saccharin/quinine consumption (mls): 30 min: t=1.5, df=4, P=n.s.; 6 h: t=2.6, df=4, P=n.s.; and 24 h: t=5, df=4, P<0.01 (Table 2)). Both CP-601932 and PF-4575180 do not modulate the taste preference for saccharin/quinine solution. Hence, although PF-4575180 increases water consumption, it most likely does not shift the preference of ethanol to water.
Table 2. CP-601932 and PF-4575180 Treatment has no Effect on Taste Preference of Saccharin/Quinine Solution Using the Intermittent Access Two-Bottle-Choice Drinking Paradigm.
|
30 min |
6 h |
24 h |
||||||
|---|---|---|---|---|---|---|---|---|
| S+Q (ml) | Preference | S+Q (ml) | Water (ml) | Preference | S+Q (ml) | Water (ml) | Preference | |
| CP-601932 (mg/kg) | ||||||||
| 0 | 5±1 | 80±9 | 27±6 | 4±1 | 83±7 | 52±10 | 8±2 | 87±6 |
| 10 | 3±1 | 83±5 | 15±2 | 4±2 | 78±10 | 41±9*** | 7±3 | 85±7 |
| PF-4575180 (mg/kg) | ||||||||
| 0 | 6±1 | 81±9 | 32±5 | 3±1 | 90±3 | 65±10 | 7±2 | 90±3 |
| 10 | 6±2 | 75±11 | 33±10 | 4±1 | 84±6 | 62±13 | 10±4 | 83±7 |
Data are expressed as mean±SEM (paired t-test). ***P<0.01.
Effect of Repeated Administrations of CP-601932 on Ethanol Intake
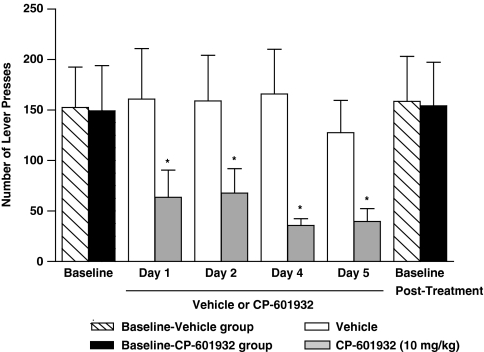
CP-601932 has been shown to be safe in humans, and as it could have beneficial effects in human subjects with AUDs using a chronic treatment regimen, we evaluated whether repeated administration of CP-601932 would also suppress ethanol consumption without any subsequent rebound increase in drinking in rats. Vehicle or CP-601932 (10 mg/kg s.c.) were given for 5 consecutive days, once per day 30 min before the self-administration session in rats using the operant self-administration paradigm (see Materials and Methods). Once the rats had attained stable baseline active lever presses, they were randomly divided into two groups, CP-601932 (10 mg/kg s.c.) and vehicle (saline). We found that CP-601932, but not vehicle treatment, decreased ethanol operant self-administration on each of the 5 days. Two-way ANOVA analysis of active lever presses revealed a significant effect of treatment (F(1,64)=25, P<0.001) but no effect of day (F(4,64)=0.761, n.s.) and no interaction between treatment × day (F(4,64)=0.263, n.s.). Post hoc analysis showed that CP-601932 significantly reduced the number of active lever presses during the treatment period (Figure 6). There was no rebound increase in active lever presses in comparison to baseline when the chronic CP-601932 treatment was terminated (F(3,22)=0.008, n.s.). Furthermore, there was no overall main effect on the number of inactive lever responding of treatment (F(1,64)=0.3, n.s.), or day (F(4,64)=0.06, n.s.), or the interaction of treatment × day (F(4,64)=0.08, n.s.). These results show that both acute and multiple administrations of CP-601932 selectively reduce ethanol self-administration in chronically exposed ethanol consuming rats without inducing a rebound increase in the ethanol self-administration after the cessation of the treatment. In addition, the active lever presses returned to pretreatment baseline drinking levels 24 h following the end of the treatment period.
Figure 6.
Multiple repeated administration of CP-601932 decreased ethanol consumption using the operant self-administration paradigm. Chronic administration of CP-601932 (10 mg/kg s.c.) but not vehicle decreased active lever presses for 10% ethanol. CP-601932 (10 mg/kg s.c.) or vehicle (saline s.c.) was administered on each of 5 consecutive days, 30 min before the start of the operant session. The values are expressed as ethanol intake (g/kg) ±SEM (two-way ANOVA followed by Newman–Keuls post hoc test). *P<0.05 compared with vehicle, n=6–7.
The α4β2*nAChR Antagonist DHβE, does not Decrease Heavy Ethanol Consumption
Considering the difference in in vitro activity of CP-601932 and PF-4575180 at nAChRs and the finding that both compounds effectively decrease ethanol consumption, it appears that α4β2* nAChRs have a minor role in ethanol-mediated behaviors. To confirm this hypothesis, the α4β2* nAChR antagonist, DHβE, previously shown (Le et al, 2000) to have no effect in other ethanol-intake models, was examined in long-term drinking rats, using the intermittent-access two-bottle-choice paradigm model. We found that DHβE does not affect ethanol consumption or preference in long-term heavy drinking rats (Supplementary Figure S2; Supplementary Table S5; Supplementary Information). The fact that both CP-601932 and PF-4575180, but not DHβE, reduced ethanol consumption suggests that α3β4*, rather than α4β2* nAChRs, have a primary role in the modulation of ethanol consumption in long-term heavy drinking rats.
Correlation Between Effects on Ethanol Intake and nAChR Interactions
To assess the functional interactions of PF-4575180 and CP-601932 with α3β4* nAChRs after doses administered in the ethanol-intake studies, we compared their Cb,u values at 30 min after each dose with effects in the voluntary ethanol consumption model. By superimposing Cb,u (bars in Figure 2 inserts) over the in vitro inhibition- and activation-concentration curves of α3β4 nAChRs (Figure 2), the extent of inhibition/activation of the α3β4 subtype can be estimated for each dose. The 5 and 10 mg/kg doses of CP-601932, which both decrease ethanol intake, result in Cb,u values that are close to the area of overlap between the α3β4 inhibition and activation curves, thought to represent a pharmacologically active range (Hogg and Bertrand, 2004). Taking into account that the majority of receptors will be inactivated, presumably via receptor desensitization, both doses are expected to result in sustained activation of a small fraction of α3β4 nAChRs (insert, Figure 2a). PF-4575180 achieves Cb,u values at 30 min after 1 and 10 mg/kg that can functionally interact with α3β4 nAChRs and both doses are predicted to produce sustained activation of a small portion of the α3β4 subtype (Figure 2b, insert). The finding that 10 mg/kg, but not 1 mg/kg PF-4575180 was effective in reducing ethanol consumption 30 min after dosing was therefore unexpected and will be discussed below. At these Cb,u, CP-601932 and PF-4575180 can partially inhibit, but not activate α4β2 nAChRs: CP-601932 acts as an α4β2 nAChR antagonist and PF-4575180 has relatively low affinity for α4β2 nAChRs (Supplementary Figure S1A and B).
A comparison of varenicline rat neuropharmacokinetic data (Rollema et al, 2009) with its functional potencies at α3β4 nAChRs (Table 1), demonstrates that doses of 1 and 2 mg/kg that were previously found to significantly reduce ethanol intake (Steensland et al, 2007), are associated with a sufficiently high Cb,u to produce robust activation, as well as inhibition of α3β4 nAChRs (Figure 2c). As shown previously (Rollema et al, 2007, 2010), varenicline can potently interact with α4β2 nAChRs and at the Cb,u achieved after 1 and 2 mg/kg it will mainly inhibit this subtype (Supplementary Figure S1C).
These results suggest that PF-4575180, CP-601932, and varenicline can reduce ethanol intake via partial inhibition of α3β4* nAChRs and α4β2* nAChRs and/or by sustained modest activation of α3β4* nAChRs. Given the lack of effect of the α4β2* nAChR antagonist DHβE, inhibition of α4β2* nAChRs alone is apparently not sufficient to suppress alcohol intake, which further supports a role for α3β4 nAChRs. Finally, an interesting finding is that all compounds are still active at 24 h after single doses and still reduce voluntary alcohol consumption at a time point when drug exposures have declined significantly.
DISCUSSION
Recent human genetic association studies suggest that the gene cluster CHRNA3–CHRNA5–CHRNB4, encoding the α3, α5, and β4 subunits, is associated with both alcohol and nicotine dependence (Chen et al, 2009; Freathy et al, 2009; Joslyn et al, 2008). To investigate the role of α3β4* nAChRs in ethanol-mediated behaviors, we characterized the effects of two compounds, CP-601932 and PF-4575180, that are partial agonist at α3β4 nAChRs. CP-601932 is also a low-efficacy α4β2 nAChR partial agonist that has been clinically tested as a smoking cessation aid. It was generally well tolerated, but not further developed because of a moderate two fold increase in abstinence rate that was not statistically significant (Pfizer, data on file). PF-4575180 is a [3.2.1]azabicyclic analog (Lowe et al, 2010) that was found to bind with an order of magnitude higher affinity to α3β4 (Ki=4.2 nM) than to α6* nAChRs and to have very low affinity for α4β2 nAChRs (Ki>1.2 μM). We report here that both CP-601932 and PF-4575180 reduce ethanol self-administration and consumption and have a long-lasting effect on ethanol-mediated behaviors, reducing the preference for ethanol consumption for up to 24 h with no effect on sucrose intake. PF-4575180, but not CP-601932, was found to also increase water consumption, in other words to shift the preference from ethanol to water. However, neither compound modulated the taste preference for saccharin–quinine solutions and hence do not interact with the taste of ethanol.
Active doses of CP-601932 result in Cb,u that are predicted to activate, but not completely inhibit nAChRs and that represent a pharmacologically relevant concentration range (Hogg and Bertrand, 2004). Based on in vitro functional data Cb,u of CP-601932 at active doses, it is predicted that a substantial portion of both α4β2 and α3β4 nAChRs would be inhibited and that a small portion of α3β4 nAChRs would also be persistently activated. The α4β2 nAChRs are predicted not to be activated in vivo, due to the very low (undetectable) intrinsic activity of CP-601932 at α4β2 nAChRs in vitro. It is important to note that this is also the case for the effects of the α4β2 nAChR partial agonist varenicline on ethanol-mediated behaviors. After doses of 1 and 2 mg/kg s.c., previously shown to significantly reduce ethanol consumption and self-administration in rats (Steensland et al, 2007), its rat Cb,u is sufficiently high to also interact functionally with α3β4 nAChRs and to both partially activate and partially inhibit this subtype. We hypothesize that this combination of activities would lead to a low level of persistent channel activation in the brain at active doses. As expected, at these doses, varenicline almost completely inhibits its main target, the α4β2 nAChR subtype. Effects of the preferential α3β4 nAChR partial agonist PF-4575180 on ethanol consumption are probably predominantly mediated by activation and inhibition of α3β4* nAChRs, as its low-binding affinity for α4β2 nAChRs results in minimal inhibition of that subtype at the highest PF-4575180 dose.
One caveat is, however, that the in vitro pharmacology used in this assessment was determined with expressed human receptors, whereas the behavioral experiments were conducted in rats. In addition, the cells express an undefined stoichiometry of α and β subunits, which can also influence receptor pharmacology (Nelson et al, 2003; Zhou et al, 2003). Therefore, although the conclusions described above are aligned with the in vitro pharmacology described in this work, potency differences between the in vitro systems used and native rat brain receptors could significantly influence the interpretation. Although both the 1 and 10 mg/kg doses are associated with initial PF-4575180 brain concentrations that can desensitize a large portion and activate a small fraction of α3β4* nAChRs, only 10 mg/kg PF-4575180 was found to be effective in reducing ethanol consumption at 30 min post-dose. Although it is conceivable that shortly after 1 mg/kg, the Cb,u of PF-4575180 may be insufficient to produce a behavioral effect, it was surprising that the 1-mg/kg dose did show activity at later time points, ie, at 24 h, when Cb,u values are significantly lower than at 30 min. Interestingly, a similar phenomenon was also observed for the other compounds that still displayed behavioral activity 24 h post-dose at much lower Cb,u than after 30 min. Further studies are required to examine possible underlying mechanisms, as this type of hysteresis could suggest an indirect exposure-response relationship. In that scenario, initial drug levels in the pharmacological relevant concentration range could trigger an α3β4 nAChR-mediated event that has longer-lasting downstream consequences, eg on mesolimbic dopamine. It is clear that the long-lasting effects of CP-601932 and varenicline in rats cannot be attributed to active metabolites, as these are not formed in vivo (Obach et al, 2006; Shaffer et al, 2009), but that possibility has not been investigated for PF-4575180. Finally, although PF-4575180 is more potent than CP-601932 in vitro, it is less efficacious in decreasing ethanol consumption in the animal model, because of lower Cb,u that reduce in vivo potency. However, free Cb,u of CP-601932 decline much more slowly than that of PF-4575180, which may account, at least in part, for this apparent discrepancy. Although the correlation of in vitro binding affinities and behavioral potencies need to be better understood, a consistent finding is that both compounds selectively decrease ethanol and not sucrose consumption.
The present data on the α3β4 nAChR partial agonists CP-601932, PF-4575180, and varenicline, as well as previously published data on the selective α3β4* nAChR desensitizing agent 18-methoxycoronaridine (18-MC) (Rezvani et al, 1997), suggest that effects on ethanol-mediated behaviors are mediated via inhibition and/or activation of α3β4* nAChRs, but do not exclude a potential role of inhibition of α4β2* nAChRs, at least after CP-601932 and varenicline treatment. To explore this further, we showed that α4β2* nAChR antagonist, DHβE, which lacks activity at α3β4* nAChRs (Harvey et al, 1996) does not decrease ethanol consumption in long-term heavy drinking rats, further supporting the findings of other investigators in animals that consume smaller amounts of ethanol (Larsson et al, 2002; Le et al, 2000). In studies involving short-term drinking mice (10–15 days), varenicline's effect has been shown to be mediated by the α4 and not the β2 or α7 subunit of the nAChR in genetically modified mice (Hendrickson et al, 2010; Kamens et al, 2010). It is important to note that our studies were conducted in animals with a longer drinking history of at least 8 weeks. PF-4575180 has good affinity for α6/4β4 nAChRs (Ki=45 nM), but given the inability of the α6* selective αCtxPIA analogue to antagonize ethanol-induced locomotor stimulation and dopamine release (Jerlhag et al, 2006), it seems unlikely that the α6 subunit is involved in the effects of ethanol. The α3 subunit of the nAChR has been shown to be important for acute locomotor responses to ethanol in mice (Kamens et al, 2008). Taken together, with the at least 10-fold higher affinity of CP-601932 and PF-4575180 for α3β4 than for α6/4β4 nAChRs, our data suggest that α3β4* nAChRs may have a predominant role in mediating ethanol-induced behaviors in long-term drinking animals.
The α4β2* nAChRs are one of the major subtypes in the brain and are expressed in the ventral tegmental area (VTA) and nucleus accumbens (NAcc) and have been shown to modulate dopamine release (Duvoisin et al, 1989; Gotti et al, 1997; Klink et al, 2001). In contrast, the α3β4* nAChRs are more highly expressed outside the VTA and NAcc in regions such as the medial habenula, nucleus interpeduncularis, dorsal medulla, and pineal gland (Fonck et al, 2009; Han et al, 2000; Quick et al, 1999; Wada et al, 1989; Zoli et al, 1998). The habenula complex has been shown to indirectly modulate dopamine release in the NAcc via the VTA (Matsumoto and Hikosaka, 2007; Ullsperger and von Cramon, 2003). We hypothesize that partial agonists at α3β4* nAChRs decrease ethanol self-administration and consumption by indirectly modulating the mesolimbic dopaminergic system. Data on 18-MC, the only other compound that has been reported to have activity at α3β4* nAChRs and to reduce ethanol-seeking (Rezvani et al, 1997), as well as nicotine-seeking behavior in rats (Glick et al, 2002), are in agreement with this hypothesis. 18-MC has no effect at α4β2 nAChRs and binds with micromolar affinities to other receptors (Glick et al, 2000), but is thought to act by stabilizing the desensitized state of the α3β4 nAChR with IC50 values ranging from 0.75 to 0.90 μM (Pace et al, 2004). CP-601932 and PF-4571580 are also expected to inhibit α3β4 nAChRs, but are more potent with IC50 values of 0.1–0.3 μM, while their partial agonist properties will also permit some receptor activation.
Ligands with affinity for α3* nAChRs have generally been avoided in drug discovery programs, as α3* nAChRs have a role in ganglionic transmission and modulation of this receptor is believed to underlie nicotinic adverse effects, such as constipation, urinary retention, hypertension, and mydriasis. However, a growing number of studies have indicated the presence of nAChRs that contain subunits in numerous configurations with accessory subunits that would not only change the pharmacology and kinetics of nAChRs but also differ in expression in the CNS and periphery (see review (Gotti et al, 2007)). Indeed, CP-601932 has been examined in a clinical trial as a potential smoking cessation aid and did not show side effects indicative of ganglion blocking effects. CP-601932 has been shown after oral administration to be well absorbed, to have a long half-life, to undergo renal and metabolic clearance equally, and to have extensive brain penetration in rats, which are key attributes when considering new medicinal agents (Shaffer et al, 2009). Hence, we hypothesize that CP-601932 could present a pharmacotherapeutic option to reduce ethanol consumption via central α3β4* nAChRs and suggest that a clinical CP-601932 study in subjects with AUDS could evaluate its efficacy and potential benefits.
In summary, our data suggest that α3β4* nAChRs may be important therapeutic target for the treatment of AUDs. Furthermore, as α4β2* nAChRs have been strongly implicated in nicotine dependence and as CP-601932 is equipotent at α3β4 and α4β2 nAChRs, this compound has also the potential to reduce smoking in alcoholics with co-morbid nicotine dependence.
Acknowledgments
We thank Brian Medina, Haley Pierson, Jade Bito-Onon, and Rui Li (Gallo Research Institute), and Shari L DeNinno, Alka Shrikhande, Sarah M Osgood, Mary MacDougall, Laura McDowell, and JianHua Liu (Pfizer Global Research and Development) for excellent technical assistance. This work was supported by funding from the NIH, 1R01AA017924-01 (to SEB), DOD, #W81XWH-08-1-0016 (to SEB), and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (to SEB). The Foundation BLANCEFLOR Boncompagni-Ludovisi née Bildt (to PS), the Sweden-America Foundation (to PS), and Insamlingsstiftelsen Hjärnfonden/The Swedish Brain Foundation (to PS).
JWC, RSH, CLS, JL, and HR are employees of Pfizer, the manufacturer of varenicline. The other authors declare that there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. SEB has received financial support for research on an unrelated clinical study on varenicline, but has not received compensation from any individual or corporate entity over the past 3 years for research or professional service.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors. Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead L, Lancaster T. A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf. 2009;32:119–135. doi: 10.2165/00002018-200932020-00005. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M. Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology (Berl) 2010;211:367–375. doi: 10.1007/s00213-010-1891-y. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005a;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Wirtz MC, Bashore CG, Bianco KE, Vetelino MG, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett. 2005b;15:4889–4897. doi: 10.1016/j.bmcl.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, Collins AC. Classical genetic analyses of responses to nicotine and ethanol in crosses derived from long- and short-sleep mice. J Pharmacol Exp Ther. 1992;261:173–180. [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Fonck C, Nashmi R, Salas R, Zhou C, Huang Q, De Biasi M, et al. Demonstration of functional alpha4-containing nicotinic receptors in the medial habenula. Neuropharmacology. 2009;56:247–253. doi: 10.1016/j.neuropharm.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann N Y Acad Sci. 2000;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Amit Z. Do taste factors contribute to the mediation of ethanol intake? Ethanol and saccharin-quinine intake in three rat strains. Alcohol Clin Exp Res. 1998;22:837–844. [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA, Jr, Champtiaux N, Changeux JP. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Bertrand D. Nicotinic acetylcholine receptors as drug targets. Curr Drug Targets CNS Neurol Disord. 2004;3:123–130. doi: 10.2174/1568007043482507. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol. 2006;41:486–493. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, et al. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci USA. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology. 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The alpha3 subunit of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes Brain Behav. 2008;6:600–609. doi: 10.1111/j.1601-183X.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lowe JA, III, DeNinno SL, Coe JW, Zhang L, Mente S, Hurst RS, et al. A novel series of [3.2.1] azabicyclic biaryl ethers as alpha3beta4 and alpha6/4beta4 nicotinic receptor agonists. Bioorg Med Chem Lett. 2010;20:4749–4752. doi: 10.1016/j.bmcl.2010.06.142. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Ochoa EL, Chattopadhyay A, McNamee MG. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989;9:141–178. doi: 10.1007/BF00713026. [DOI] [PubMed] [Google Scholar]
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, et al. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Eur J Pharmacol. 2004;492:159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, et al. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, III, Coe JW, O'Neill BT, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer CL, Ryder TF, Venkatakrishnan K, Henne IK, O'Connell TN. Biotransformation of an alpha4beta2 nicotinic acetylcholine receptor partial agonist at Sprague-Dawley rats and the dispositional characterization of its n-carbamoyl glucuronide metabolit. Drug Metab Dispos. 2009;37:1480–1489. doi: 10.1124/dmd.109.027037. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure–activity relationship. J Med Chem. 2006;49:4425–4436. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.