Abstract
Drug-metabolizing cytochrome P450 (CYPs) enzymes are expressed in the liver, as well as in extrahepatic tissues such as the brain. Here we show for the first time that drug metabolism by a CYP within the brain, illustrated using CYP2B and the anesthetic propofol (2, 6-diisopropylphenol, Diprivan), can meaningfully alter the pharmacological response to a CNS acting drug. CYP2B is expressed in the brains of animals and humans, and this CYP isoform is able to metabolize centrally acting substrates such as propofol, ecstasy, and serotonin. Rats were given intracerebroventricularly (i.c.v.) injections of vehicle, C8-xanthate, or 8-methoxypsoralen (CYP2B mechanism-based inhibitors) and then tested for sleep time following propofol (80 mg/kg intraperitoneally). Both inhibitors significantly increased sleep-time (1.8- to 2-fold) and brain propofol levels, while having no effect on plasma propofol levels. Seven days of nicotine treatment can induce the expression of brain, but not hepatic, CYP2B, and this induction reduced propofol sleep times by 2.5-fold. This reduction was reversed in a dose-dependent manner by i.c.v. injections of inhibitor. Sleep times correlated with brain (r=0.76, P=0.0009), but not plasma (r=0.24, P=0.39) propofol concentrations. Inhibitor treatments increased brain, but not plasma, propofol levels, and had no effect on hepatic enzyme activity. These data indicate that brain CYP2B can metabolize neuroactive substrates (eg, propofol) and can alter their pharmacological response. This has wider implications for localized CYP-mediated metabolism of drugs, neurotransmitters, and neurotoxins within the brain by this highly variable enzyme family and other CYP subfamilies expressed in the brain.
Keywords: cytochrome P450, brain, propofol, metabolism, sleep, anesthesia
INTRODUCTION
The liver is the primary site of cytochrome P450 (CYPs) enzyme-mediated drug metabolism (Lewis, 1996); however, several CYP isoforms have also been detected in the brain with variable distribution among different brain regions and expression in both neuronal and glial cell types. Cerebral expression of CYPs might be able to alter metabolism of drugs in a clinically relevant manner (Gervasini et al, 2004), but this has not been shown. Although these brain CYPs are functional in vitro (Albores et al, 2001; Miksys and Tyndale, 2009), it is unclear as to whether these enzymes have sufficient cofactors, coenzymes, and activity in the brain to meaningfully impact local drug metabolism and, by extension, central drug response. The clinical relevance of brain CYP-mediated drug metabolism has not been shown, in part, because of difficulty in determining the relative in vivo contribution of CNS metabolism in the presence of hepatic metabolism and the passage of peripheral metabolites into the brain. Recent advances in our ability to assess the expression and activity of extrahepatic CYPs indicate that rat brain CYPs are active in vivo and metabolism by these CYPs can be altered locally in the brain (Miksys and Tyndale, 2009). This enables us, for the first time, to investigate the impact of CNS CYPs on drug response.
CYP2Bs are a CYP subfamily, members of which are expressed in the brains of rats, mice, monkeys, and humans (Miksys and Tyndale, 2002). CYP2Bs metabolize a variety of CNS acting drugs such as propofol (2, 6-diisopropylphenol, Diprivan) and bupropion, and also play a role in the metabolism of neurochemicals and neurotoxins (Ekins et al, 2008). Rat brain, but not liver, CYP2B can be induced by 7-day nicotine treatment, with a return to baseline levels 7 days later (Khokhar et al, 2010). Both basal and induced CYP2B activity can be inhibited selectively in the brain by an injection of a mechanism-based inhibitor (MBI) also known as a suicide inhibitor (Miksys and Tyndale, 2009).
CYP2B is present in human brain and shows large interindividual variation in expression owing, in part, to genetic polymorphisms (Miksys et al, 2003). In addition, similar to the higher CYP2B brain levels found in nicotine-treated rats, human smokers have higher brain CYP2B levels than non-smokers (Miksys et al, 2003). Interindividual differences in brain CYP2B might alter the metabolism of propofol, thereby contributing to the great variability seen in the response to propofol (Iohom et al, 2007; Kanto and Gepts, 1989). Using a rat model of brain CYP2B manipulation, we investigated the functional consequences of inducing and inhibiting brain CYP2B-mediated metabolism of propofol on drug response. Propofol is a commonly used anesthetic and sedative because of its short duration of action, rapid onset, and preferable side effect and recovery profiles (Langley and Heel, 1988). Upon administration in humans, propofol distributes rapidly and is cleared by both glucuronidation and hydroxylation to the inactive 4-OH metabolite (Favetta et al, 2002). CYP2B6 is the major contributor to the interindividual differences in the rate of propofol hydroxylation (Court et al, 2001). In the rat, CYP-mediated hydroxylation is the primary route of metabolism for propofol (Le Guellec et al, 1995).
When the propofol dose was modeled to achieve a desired effect site (brain) concentration, it more accurately predicted the depth of anesthesia compared with modeling for plasma concentrations (Liu et al, 2009). In addition, a hysteresis exists between arterial propofol concentration and anesthetic effect, whereas brain propofol has a close relationship with cerebral blood flow and depth of anesthesia in a sheep model of propofol pharmacokinetics (Ludbrook et al, 1996). In the rat, brain propofol concentrations correlate with tail-flick latency after propofol administration (Shyr et al, 1995), which indicates that the local concentration of propofol in the brain contributes to the drug's effect. Propofol acts primarily on GABAA receptors (Altomare et al, 2003); it can also inhibit some subtypes of nicotinic acetylcholine receptors (nAChRs), but they are not the primary sites of action for the drug (Tassonyi et al, 2002). Repeated exposure to propofol does not result in tolerance (Fassoulaki et al, 1994), making this model suitable for multiple testing in a within-animal design.
Here, we use a rat model of propofol sedation to study the effects of altered brain, and not hepatic, CYP2B-mediated metabolism on propofol sleep time. More broadly, this study is a proof of concept for the contribution of central metabolism, in addition to hepatic metabolism, to altered response to CNS acting drugs. This mechanism could help clarify a disconnect which is often seen between plasma drug levels and central drug effects, as well as further our understanding of interindividual differences in drug response.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (250–300 g; Charles River, St-Constant, PQ, Canada) were housed in pairs and kept under a 12-h artificial light/dark cycle (lights on at 0600 hours). The animals were handled daily to habituate them to manipulation. All procedures were approved by the Animal Care Committee at the University of Toronto.
Drug Treatment
Propofol (10 mg/ml Diprivan; AstraZeneca, Mississauga, ON, Canada) was injected intraperitoneally (i.p.) at 40–120 mg/kg for the dose–response experiments, 80 mg/kg for the inhibition and induction experiments, and 40 and 50 mg/kg for the dose–response shift experiments. C8-xanthate (C8-X) was purchased from Toronto Research Chemicals (Toronto, ON, Canada) and was dissolved in artificial cerebro-spinal fluid (ACSF), and 0.625–80 μg was given intracerebroventricularly (i.c.v.) in a 0.5–2 μl total volume. A dose of 40 μg radiolabeled MBI 8-methoxypsoralen (3H-8-MOP) (a gift from William F Trager) was dissolved in 0.5 μl ACSF and given i.c.v.. Nicotine bitartarate was purchased from Sigma-Aldrich Canada (Oakville, ON, Canada) and rats were injected subcutaneously (s.c.) once daily for 7 days with 1 mg nicotine base per kg body weight in sterile saline (pH 7.4).
Inhibition of Rat Brain CYP2B Activity Following I.C.V. Injection of a CYP2B MBI
The methods used were modified from previous studies (Khokhar et al, 2010; Miksys and Tyndale, 2009). Briefly, the rats were treated for 7 days with nicotine (1 mg/kg) or saline. The rats were anesthetized (isoflurane) and placed in the stereotaxic frame. The animals then received an i.c.v. injection into the right lateral ventricle (Bregma coordinates: dorsal–ventral, −3.6; anterior–posterior, −0.9; lateral, −1.4; Paxinos and Watson, 1986) of either 20 μg C8-X (CYP2B MBI; Yanev et al, 2000) in 0.5 μl ACSF or ACSF alone (vehicle). All i.c.v. injections were made over 2 min, and the Hamilton syringe was left in place for 3 min post-injection. At 20 h after the first i.c.v. injection, the rats received another i.c.v. injection of 40 μg of 3H-8-MOP in 0.5 μl sterile ACSF. The animals were killed 4 h after the 3H-8-MOP injection to assess the remaining rat brain CYP2B activity 24 h after i.c.v. injection of the inhibitor C8-X.
As described previously (Miksys and Tyndale, 2009), 400 μg brain membranes from animals killed as described above were incubated overnight with 100 μl of monoclonal antibody raised against rat CYP2B1/2 (Fitzgerald Industries, Concord, MA) in phosphate-buffered saline (PBS, pH 7.4) at 4°C. The antibody–CYP2B protein complex was then incubated with 400 μl of a 50% slurry of protein G immobilized on resin beads (Pierce, Rockford, IL) for 3 h on a rocker and then centrifuged for 1 min at 3000 g. The beads were washed twice with 500 μl PBS, centrifuged for 1 min at 3000 g, and then re-suspended in PBS. Aliquots of the beads and supernatants were counted. In vivo brain CYP2B activity is expressed as the percentage of radioactive counts bound to the beads relative to total counts detected in 400 μg of brain membranes. To further assess the specificity of the MBI for rat brain CYP2B, 100 μl of monoclonal antibodies raised against CYP2A, CYP2D, CYP2E, and CYP3A (BD Biosciences, Mississauga, ON, Canada) were also incubated with 400 μg of radiolabeled frontal cortex (FC) membranes as outlined above for the antibody raised against CYP2B.
Rat Hepatic CYP2B Activity Measured by In Vitro Nicotine Oxidation
Rat hepatic CYP2B activity was measured using in vitro nicotine oxidation, as this is a sensitive and selective assay for rat CYP2B (Nakayama et al, 1993). Rat liver microsomal proteins were prepared as done previously (Siu and Tyndale, 2008) from rats that received an i.c.v. injection of MBI (20 μg C8-X or 40 μg 8-MOP) or vehicle control (ACSF). As before (Siu and Tyndale, 2008), microsomes (0.5 mg/ml) were incubated with nicotine (a CYP2B substrate; 120 and 480 μM), 1 mM NADPH, and 1.5 mg/ml liver cytosol in 50 mM Tris-HCl buffer (pH 7.4) for 20 min at 37°C in a final volume of 0.5 ml. The reaction was stopped using 4% (v/v) Na2CO3. After incubation, the internal standard 5-methylcotinine (65 μg) was added with 50 μl of NaOH (10 M) and 4 ml of dichloromethane. The tubes were capped and shaken for 10 min, and then centrifuged at 3000 r.p.m. for 10 min. The organic fraction was added to 25 μl of 6 N HCl, evaporated under a nitrogen stream at 37°C, and dissolved in 105 μl of distilled water. A 90 μl aliquot was analyzed by HPLC-UV, with a limit of quantification of 5 ng/ml for both nicotine and cotinine.
Dose–Response Curve for Sleep Time Following an Intraperitoneal Injection of Propofol
Rats were given 40–120 mg/kg propofol i.p. and were then placed under a heat lamp and monitored for propofol-induced sedation. Sleep was defined as the loss of purposeful movement, with the end point being the initial moment of head lift (Dam et al, 1990). Blood draws to measure plasma propofol levels or killings were performed 2 h after propofol injection. The livers and brains were removed and frozen from killed animals for measurement of hepatic CYP2B activity and brain propofol levels; no perfusion was performed before killings to avoid differences in collection of blood for propofol analyses throughout the study. All experiments, unless otherwise indicated, were performed in a within-animal design.
Effect of I.C.V. MBI Injection on Propofol-Induced Sedation
Propofol-induced sleep times were measured at baseline and 24 h after an i.c.v. injection of a CYP2B MBI (20 μg C8-X or 40 μg 8-MOP) or the vehicle control (ACSF). C8-X and 8-MOP had similar effects, but C8-X has greater selectivity for CYP2B (Yanev et al, 1999), hence, C8-X was used for all further experiments.
Effects of Nicotine's Induction of Rat Brain CYP2B and nAChR Blockade on Propofol Response
Rats were tested for baseline propofol response and were then injected s.c. once daily for 7 days with nicotine bitartarate (1 mg/kg) or the vehicle (saline). The animals were tested for propofol response 24 h after 1 and 7 days of nicotine treatment. At this dose, nicotine is cleared from the plasma by 24 h and any acute effects of nicotine on nAChRs may have partially recovered, diminishing any potential confounding effects that the nicotine treatment could have on propofol's actions. CYP2B is still induced 24 h after 7-day nicotine treatment in the rat brain (Figure 1b).
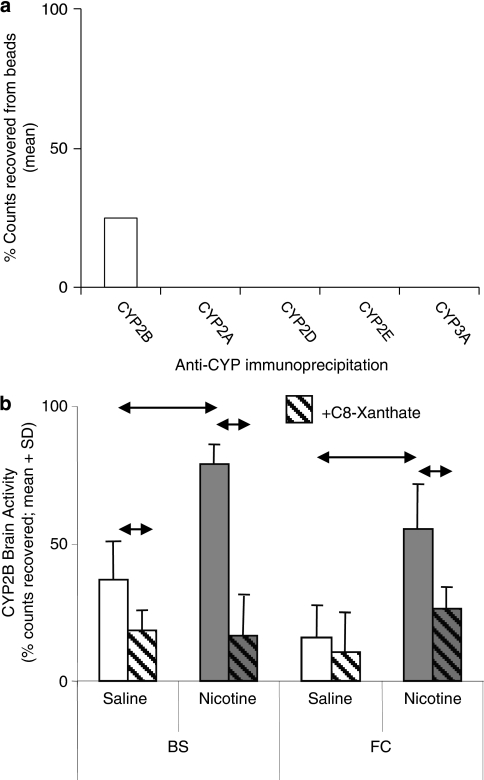
Figure 1.
Selective inhibition of rat brain CYP2B activity after intracerebroventricular (i.c.v.) injection of CYP2B mechanism-based inhibitor (MBI). (a) Radioactivity was detected following immunoprecipitation of rat brain frontal cortex tissue using antibodies specific to CYP2B, but not to CYP2A, CYP2D, CYP2E, or CYP3A, indicating a specificity of MBI activation and irreversible binding to CYP2B enzyme following i.c.v. injection of radiolabeled MBI. (b) Rat brain CYP2B activity was significantly increased by 7-day nicotine treatment in the brainstem (↔P=0.006). Both basal (↔P=0.05) and nicotine-induced CYP2B (↔P=0.03) activity in the brain were significantly reduced in animals pretreated with i.c.v. C8-xanthate (C8-X) compared with the i.c.v. artificial cerebro-spinal fluid (ACSF) pretreated animals in the brainstem. In the frontal cortex, which has low basal activity, 7-day nicotine treatment also increased CYP2B activity (↔P=0.04) and pretreatment with i.c.v. C8-X inhibited this (↔P=0.05).
After propofol testing post 7-day nicotine treatment, animals were either killed 2 h after propofol or received another nicotine injection. At 24 h after the eighth nicotine injection, animals received an i.c.v. injection of 20 μg C8-X. The animals then received a ninth injection of nicotine and were tested with propofol 24 h later.
As nAChRs are the primary site of action for nicotine in the brain and can be upregulated by chronic nicotine (Vallejo et al, 2005), we assessed whether nAChR blockade altered propofol response, both alone and following 7-day nicotine treatment to determine whether nAChR upregulation affects propofol response. We tested the effects of irreversible (chlorisondamine 10 mg/kg s.c.) or acute (mecamylamine 1 mg/kg s.c.) blockade of nAChRs on response to propofol. Chlorisondamine is a quasi-irreversible blocker of nAChRs and blocks nicotine-induced responses for up to 2 weeks (Clarke et al, 1994); chlorisondamine pretreatment does not alter CYP2B induction by nicotine (Khokhar et al, 2010). Propofol response was measured at baseline and after chlorisondamine pretreatment. The animals then received 7-day nicotine treatment and were tested with propofol 24 h after the last nicotine injection. The mecamylamine study was run similarly, but the animals received mecamylamine 30 min, as described previously (Bhargava and Saha, 2001), before every propofol test (except baseline measurements).
Dose-Dependent Reversal of Nicotine-Induced Sleep-Time Reductions by CYP2B C8-X I.C.V. Injections
Animals were tested with propofol: at baseline, after a 7-day nicotine treatment, and 24 h after receiving an eighth injection of nicotine and an i.c.v. injection (0–80 μg C8-X, n=4 per C8-X dose group, 12/ACSF group).
Rat Brain CYP2B Alteration Can Shift Propofol's Dose–Response Curve
Rats were tested with low doses of propofol (40 and 50 mg/kg) at baseline and after i.c.v. injection of C8-X (20 μg). The animals were tested again with propofol (50 mg/kg) after 7-day nicotine treatment (1 mg/kg s.c.) and after i.c.v. C8-X (20 μg).
Measurement of Plasma and Brain Propofol Levels
Plasma and brain levels of propofol were quantified by HPLC, essentially as before (Seno et al, 2002). Acetonitrile (100 μl) and 5 μl of thymol (100 μg/ml, internal standard) were added to 100 μl of plasma or brain homogenate (brain tissue homogenized in 1 : 2 (w/v) in 0.2 M phosphate buffer), and mixed by a vortex and centrifuged at 13 500 r.p.m. for 20 min (4°C). The supernatant (100 μl) was injected into the HPLC. The limit of quantification was 5 ng/100 μl and the assay was linear up to 5 μg/ml with an extraction efficiency of 95%. The HPLC-UV system (Agilent 1200 Separation Module) was set for detection at 270 nm and the propofol and internal standard were separated on an Agilent ZORBAX Bonus-RP Column (150 × 4.6 mm2 I.D.; particle size, 5 μm). The retention times were 6.2 min for thymol and 12.4 min for propofol. The mobile phase used was 40% methanol: acetonitrile (70 : 30 (v/v)), and the flow rate was 1.2 ml/min.
Statistical Analyses
For the within-region, between-treatment group comparisons of brain CYP2B in vivo activity, and the comparison of brain and plasma propofol levels between groups, a one-way ANOVA and Bonferroni correction were used. Pre- (baseline) and post-treatment mean sleep times were derived from animal sleep times; means were compared in a paired two-tailed t-test (owing to the within-animal design). The nAChR blockade experiment, dose-dependent inhibition experiment, and the shift in propofol dose–response curve experiment were all tested using repeated measures ANOVA and Bonferroni correction.
RESULTS
Selective Inhibition of Rat Brain CYP2B Activity after an Intracerebroventricular Injection of a CYP2B MBI
Using a technique that we have developed for establishing in vivo CYP activity in rat brain (Miksys and Tyndale, 2009), we assessed in situ CYP2B activity by measuring the binding of 3H-8-MOP (Koenigs and Trager, 1998) given i.c.v. to living animals. Radioactivity was detected following immunoprecipitation with antibodies specific to CYP2B, but not to CYP2A, CYP2D, CYP2E, or CYP3A, indicating specificity of radiolabeling for the CYP2B enzyme following i.c.v. injection of the radiolabeled MBI in this assay. CYP2B activity (radioactive counts from enzyme–inhibitor complex immunoprecipitated using CYP2B-specific antibody) was detectable in both brainstem (BS) and FC and was significantly increased by a 7-day nicotine treatment (P=0.005 and 0.04, respectively; Figure 1b), but no radioactive counts were detected in rat liver microsomes (data not shown). Basal and nicotine-induced CYP2B activity was reduced in animals pretreated with i.c.v. C8-X (another CYP2B MBI; Yanev et al, 1999) compared with the animals pretreated with i.c.v. ACSF. This indicates that the i.c.v.-delivered MBIs bound irreversibly to active CYP2B enzyme in disparate regions of the brain.
Sleep-Time Dose–Response Curve for Propofol
A dose response for sleep time was observed from 40 to 120 mg/kg propofol given i.p.. No sleep time was observed at the 40 mg/kg dose, whereas 120 mg/kg was fatal to 31% of the animals (four of 13 tested). At 80 mg/kg propofol, all of the animals slept, and they had an average sleep time of 30±8 min (mean±SD), allowing for measurement of both increases and decreases in sleep time.
I.C.V. Injection of CYP2B MBIs Extends Propofol-Induced Sleep Time
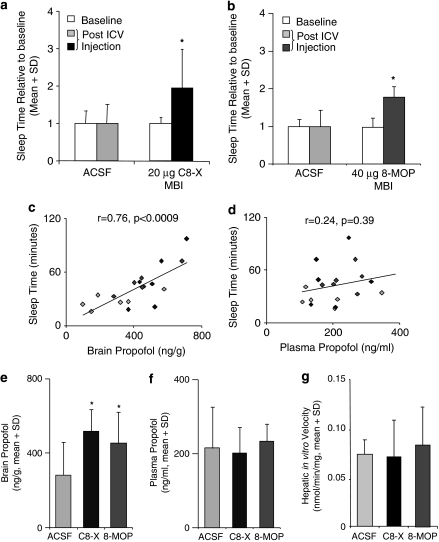
Repeating propofol tests (80 mg/kg i.p.) did not alter sleep time (during baseline trials), indicating no formation of tolerance as observed previously (Fassoulaki et al, 1994). I.C.V. injections of CYP2B MBIs (C8-X and 8-MOP) significantly (P=0.02 and 0.04; n=6/group) increased propofol sleep time, whereas the ASCF vehicle control did not (Figure 2a and b). The sleep times correlated with brain propofol levels (r=0.76, P=0.0009; Figure 2c), but not with plasma propofol levels (r=0.24, P=0.39; Figure 2d). This is consistent with the CNS metabolism, and resulting propofol levels, being responsible for the duration of sleep response. Brain propofol levels were significantly higher in the C8-X- (P=0.01) and 8-MOP-treated animals (P=0.04) compared with ACSF-treated controls (Figure 2e). The CNS MBI treatments did not alter plasma propofol concentrations (Figure 2f). There was no difference in the rates of hepatic CYP2B-mediated metabolism ex vivo between animals that received the vehicle (ACSF) or the i.c.v. MBIs C8-X and 8-MOP (Figure 2g). Taken together, the lack of impact of the CNS inhibitor injections on plasma propofol levels and on ex vivo hepatic CYP2B activity indicates that the MBIs given i.c.v. did not reach the liver at concentrations which altered hepatic CYP2B activity or propofol metabolism.
Figure 2.
Intracerebroventricular (i.c.v.) injection of CYP2B mechanism-based inhibitor (MBI) extends propofol-induced sleep time and increases brain propofol concentration. I.c.v. injection of the CYP2B MBIs, (a) 20 μg C8-xanthate (C8-X) (*P=0.02) and (b) 40 μg radiolabeled MBI 8-methoxypsoralen (8-MOP) (*P=0.04), significantly increased propofol sleep time, whereas i.c.v. injections of artificial cerebro-spinal fluid (ACSF) (vehicle control) did not alter sleep time. (c) Sleep times correlated with propofol levels in the brain (includes ACSF, C8-X, and 8-MOP animals), but not with (d) plasma propofol levels (gray: ACSF; black: C8-X; dark gray: 8-MOP). (e) Brain propofol levels were significantly higher in the C8-X (*P=0.01)- and 8-MOP (*P=0.04)-treated animals when compared with the ACSF controls. (f) No differences in plasma propofol concentrations were found following either MBI treatment. (g) There was no effect of either CYP2B MBI on in vitro hepatic CYP2B-mediated nicotine metabolism; there were no differences in ex vivo nicotine oxidation rates between i.c.v. ACSF and MBIs C8-X or 8-MOP (0.07±0.02, 0.07±0.04, and 0.08±0.04 nmol/min/mg, respectively), indicating no detectable effect of MBI injected i.c.v. on hepatic CYP2B metabolic activity.
Nicotine Treatment Induces CYP2B and Reduces Propofol-Induced Sleep Regardless of nAChR Blockade
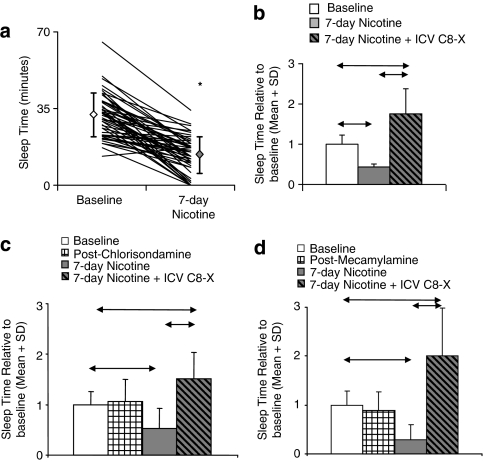
When animals were tested with propofol 24 h after a single nicotine injection, which does not induce CYP2B (Khokhar et al, 2010), there was no difference in sleep time compared with baseline (21±4 vs 23±3 min; P=0.42). After a 7-day nicotine treatment, which induces brain CYP2B (Figure 1b), there was a significant (P<0.0001) reduction in sleep time to ∼40% of baseline (n=52; Figure 3a). Rats were then given i.c.v. C8-X and the reduction in sleep time due to nicotine treatment was reversed and sleep time was extended to ∼180% of baseline (P=0.02; n=4; Figure 3b). Once again we saw a significant correlation between sleep-time and brain (r=0.72, P=0.02), but not plasma (r=0.24, P=0.38), propofol concentrations. To investigate whether alterations in the nAChRs following the 7-day nicotine treatments contributed to the reduced sleep times post-nicotine, we pretreated the animals with an irreversible nAChR blocker chlorisondamine or an acute antagonist mecamylamine. Neither pretreatment altered propofol sleep time compared with baseline (P>0.05) nor the degree of reduction seen following nicotine treatment (P>0.05); the i.c.v. C8-X was still able to reverse the reduction in sleep times (P<0.05; n=6/group; Figure 3c and d). This suggests that nAChR blockade does not alter propofol response and that the reduction in sleep time after chronic nicotine is not owing to alterations in nAChR status or interactions between the CYP2B, C8-X, and nAChRs.
Figure 3.
Nicotine-induced CYP2B reduces propofol-induced sleep time, with no effect of nicotinic acetylcholine receptors (nAChR) blockade on this response. (a) Seven-day nicotine treatment significantly reduced mean sleep time by 60% compared with the animals' sleep time at baseline (n=52, *P<0.0001). Diamonds represent mean sleep time±standard deviation. (b) An intracerebroventricular (i.c.v.) injection of C8-xanthate (C8-X) reversed the reduction in sleep time owing to nicotine treatment and sleep time was extended beyond the baseline sleep times (P=0.02; n=4). (c) Pretreatments with the nAChR blocker chlorisondamine and (d) the nAChR antagonist mecamylamine did not alter propofol sleep at baseline or the reduction in sleep time following nicotine treatment, and i.c.v. C8-X reversed the reduction in both groups (↔P<0.05; n=6/group).
I.C.V. Injections of C8-X Dose Dependently Reverse Nicotine-Induced Reductions in Sleep Time
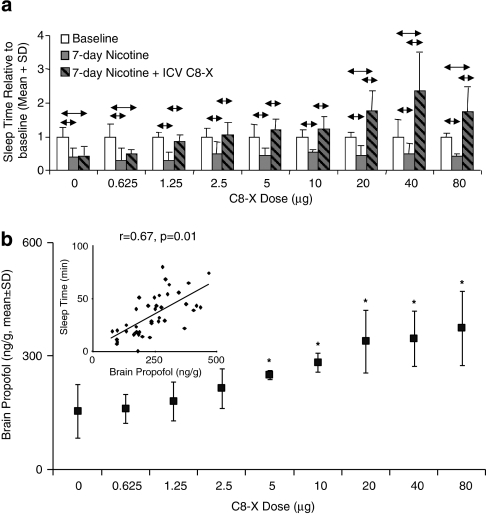
Low doses of i.c.v. C8-X (1.25–10 μg) reversed the effects of nicotine treatments on propofol sleep times, while 0.625 μg had no effect (Figure 4a). Doses between 20 and 80 μg not only reversed the reduction in sleep times caused by the 7-day nicotine treatment, but also significantly extended it beyond baseline sleep times (P<0.05; n=4/C8-X group, n=12 ACSF); in vitro hepatic CYP2B nicotine metabolism was not altered by these doses of i.c.v. C8-X (0.65±0.04, 0.62±0.08, 0.69±0.06, and 0.70±0.09 nmol/min/mg for 0, 20, 40, and 80 μg C8-X, respectively). There was a dose-dependent effect of i.c.v. C8-X on propofol levels in the rat brain with i.c.v. C8-X between 5 and 80 μg, resulting in significantly higher brain propofol levels than the ACSF control (P=0.02; Figure 4b). In addition, the mean brain propofol levels in the ACSF-treated animals post-nicotine were significantly lower (P=0.04) than animals that did not receive nicotine (Figure 2e), consistent with the reduced sleep times seen after nicotine treatment. Once again, the sleep times correlated with brain (r=0.67, P=0.01; Figure 4b, inset), but not plasma (r=−0.03, P=0.84) propofol levels. No differences in daily sleeping or feeding behaviors were seen after either nicotine or i.c.v. inhibitor treatments.
Figure 4.
Rat brain CYP2B inhibition dose dependently reverses nicotine-induced sleep-time reduction. (a) A low dose of C8-xanthate (C8-X) (0.625 μg) did not reverse nicotine-induced reduction in sleep times, whereas 1.25–10 μg of C8-X given intracerebroventricularly (i.c.v.) reversed the effects of nicotine treatment returning the sleep times to those seen at baseline. Doses of C8-X between 20 and 80 μg not only reversed the reduction in sleep time after 7-day nicotine treatment, but also significantly extended it beyond baseline sleep times (n=4/group per C8-X dose, n=12/ACSF group; ↔P<0.05). (b) CYP2B inhibition dose dependently increased rat brain propofol concentration with doses between 5 and 80 μg of C8-X, resulting in significantly higher brain propofol levels compared with the animals that received 0 μg C8-X (*P<0.05). Inset: Sleep times were correlated with brain propofol levels and not with plasma propofol levels (r=−0.03, P=0.84).
Inhibition or Induction of Rat Brain CYP2B can Shift Propofol's Dose–Response Curve
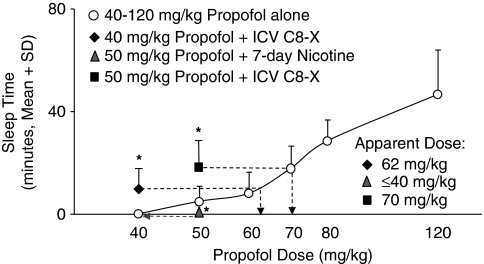
A low dose of propofol, 40 mg/kg, was inactive in all animals with a mean sleep time of 0 min (Figure 5), but was made active after an i.c.v. C8-X injection. The animals displayed significantly (P=0.009) longer sleep times, with a mean sleep time of 9±10 min (apparent dose: 64 mg/kg). A dose of 50 mg/kg resulted in only five of nine animals sleeping at baseline, with a mean sleep time of 5±6 min (Figure 5). Following i.c.v. C8-X, the animals displayed significantly (P=0.02) longer sleep times (19±12 min) and seven of nine animals slept (Figure 5). To assess whether this active dose of 50 mg/kg propofol can be made inactive by increasing brain CYP2B, we treated these animals with nicotine for 7 days to induce brain CYP2B. This treatment abolished the propofol sleep response completely, with no animals exhibiting sleep resulting in an average sleep time of 0 min (P=0.04; Figure 5). An i.c.v. injection of C8-X following nicotine's induction of brain CYP2B reversed the reduction in sleep time and resulted in significantly greater sleep times (23±14 min, eight of nine animals slept) compared with the sleep times at baseline and after nicotine treatment (P=0.01). The sleep times resulting from 50 mg/kg propofol following nicotine treatment and i.c.v. C8-X were equivalent to propofol doses of 40 and 70–75 mg, respectively. Inhibition or induction of rat brain CYP2B can make doses of propofol active or inactive, respectively, indicating that altered CNS CYP2B activity can cause significant shifts in the apparent dose response for propofol.
Figure 5.
Rat brain CYP2B manipulation shifts the propofol sleep-time dose–response curve. A dose of 40 mg/kg was inactive at baseline—none of the animals slept (sleep time: 0 min). An intracerebroventricular (i.c.v.) injection of C8-xanthate (C8-X) significantly increased sleep time consequently rendering the 40 mg/kg dose active (sleep time: 9 min; apparent dose: 62 mg/kg; P=0.009; n=9). Upon administration of a 50 mg/kg dose of propofol, the animals slept an average of ∼5 min at baseline. Induction of brain CYP2B activity by 7-day nicotine treatment abolished the propofol response—none of the animals slept (sleep time: 0 min; apparent dose: 40 mg/kg; P=0.02; n=9). After i.c.v. C8-X, the rats displayed significantly longer sleep times (sleep time: 19 min; apparent dose: 70 mg/kg; P=0.04; n=9). Dashed arrows indicate apparent dose (graph is plotted on a log x axis, but values on x axis are actual doses).
DISCUSSION
This is the first study to show that localized metabolism of a centrally active drug in the brain can alter its pharmacological effect. We manipulated the activity of rat brain CYP2B by local enzyme inhibition or induction, which increased or decreased propofol-induced sleep times accordingly. We also showed that the concentrations of propofol in the brain correlated with the duration of sleep time. Our findings clearly show that CYP2B enzymatic activity in the rat brain significantly contributes to the metabolism of propofol and the resulting effect of the drug.
Some factors contributing to our inability to previously establish the role of CYP-mediated metabolism in the brain include: our inability to distinguish hepatic metabolism from that in the brain, lack of techniques to selectively alter brain metabolism alone, and the heterogeneity of the brain cellular and regional environments. This is further compounded by the great variability across these brain regions and cell types in CYP expression and inducibility (Miksys and Tyndale, 2004). To assess the contribution of brain CYPs to local drug metabolism and drug effect, we needed to establish a model where CYP2B activity could be altered across the entire brain without altering hepatic activity. Using an i.c.v. injection of a CYP2B MBI, we were able to inhibit basal and induced rat brain CYP2B activity in two anatomically and functionally distinct brain regions, BS and FC, respectively, using a specific radiolabeling assay. These brain regions have significant decreases in brain glucose metabolism (a proxy measure for brain activity) during propofol anesthesia (Cavazzuti et al, 1991). The increase in brain, but not plasma, propofol concentrations as well as the strong correlation between brain levels and sleep response after an i.c.v. injection of a CYP2B MBI provide strong evidence that the altered propofol response is owing to the inhibition of brain, and not hepatic, CYP2B-mediated metabolism. In addition, the lack of effect of the i.c.v. injection of the CYP2B MBI on hepatic metabolism by CYP2B is further evidence for the selective inhibition of brain CYP2B. Genetically slow metabolizers for CYP2B, or individuals exposed to CYP2B inhibitors, might have higher levels of CYP2B in the brain, and would require lower levels to maintain anesthesia, as evidenced by the left-ward shift in the propofol response curve in the inhibited animals.
We have previously detected CYP2B in the human brain and found higher levels of CYP2B in the brains of human smokers (Miksys et al, 2003). Consistent with a role for induced rat brain CYP2B in reducing propofol response, there are also some case reports suggesting that smokers require higher doses of propofol to achieve loss of consciousness (Lysakowski et al, 2006), and that fewer smokers reported symptoms of postoperative nausea and vomiting (Chimbira and Sweeney, 2000). Alcoholics also have higher brain CYP2B levels compared with non-alcoholics (Miksys et al, 2003). Alcoholics required higher induction doses to achieve anesthesia compared with non-alcoholics, but plasma propofol levels did not differ significantly between the two groups at loss of consciousness (Fassoulaki et al, 1993). This provides indirect evidence to suggest that people (or animals) with higher brain CYP2B may require greater doses of propofol to achieve anesthesia, consistent with induced rat brain CYP2B shifting the propofol dose–response curve to the right. Similar to the lack of effect of chlorisondamine or mecamylamine in this study, varenicline, used in smoking cessation, and other nAChR blockers should not affect propofol response. The dose-dependent reversal of the reduction in sleep time by C8-X and the subsequent increases in brain propofol concentrations (which correlate with sleep time) also suggest that the effects seen here are owing to changes in local propofol concentrations, and are unlikely to be affected by altered receptor-mediated responses. Although the effects of induction and inhibition of brain CYP2B on propofol response were robust, and were unaffected by nAChR blockade, it is possible that in addition to binding to CYP2B, the two structurally diverse MBIs may have additional pharmacological targets, which were not examined in this study.
Our previous investigation of rat brain CYP2B showed a diurnal pattern of expression of rat brain CYP2B with higher levels during the night phase (Khokhar et al, 2010). Consistent with this finding, rats receiving an i.p. injection of propofol at night-time sleep for a shorter duration compared with animals tested with propofol during the daytime, which could possibly be due to faster propofol metabolism (Challet et al, 2007). Thus, variation in brain CYP2B levels may introduce noise or error into rat studies, if the drugs tested are CYP2B substrates and the experiments vary in the time of day. It is not known if there is a diurnal pattern of human brain CYP2B6 expression.
Our study employed an i.p. route of administration for propofol in contrast with the commonly used i.v. infusion route that is used in humans (Sebel and Lowdon, 1989). This route was chosen owing to its ease of use, and compared with an i.v. infusion, it is a less stressful and invasive procedure for the animals. One advantage of this method of administration is that the drug is susceptible to first-pass metabolism by the liver (Lukas et al, 1971); thus, if we show a role for CNS metabolism in pharmacological response using i.p. administration, it suggests this may also be the case for other drugs delivered orally. Even with a larger contribution of hepatic metabolism owing to the i.p. route of administration, alterations in brain CYP2B levels affected local propofol concentrations and response, indicating that these CYPs in the brain can act in addition to those in the liver, and alter drug response.
Although it is not clear if these findings have direct clinical relevance to human propofol anesthesia, they do suggest that CYPs in the rat brain are active and can alter drug effects through local brain metabolism. A variety of drug-metabolizing CYPs, including CYP2B, CYP2D, CYP2E1, CYP3A, and CYP4, have been detected in the brain (Meyer and Gehlhaus, 2010; Strobel et al, 2001) and their substrates include a wide range of centrally active drugs including opiate, antipsychotic, and antidepressant medications; thus, the impact of brain CYPs may be an important determinant of drug response. Local CYP-mediated metabolism of these CNS acting drugs could have a direct impact on drug efficacy and could also contribute to drug tolerance, as seen from the shifts in propofol dose–response curve following selective brain CYP2B manipulation. The ability of CYPs to metabolize endogenous neurosubstrates, such as serotonin (Fradette et al, 2004) and dopamine (Bromek et al, 2010), suggests that these CYPs have a role in normal brain function as well as in xenobiotic metabolism. Associations between genetic variation in CYP2D6 and resting brain activity (Kirchheiner et al, 2010) and personality scores (Bijl et al, 2009) support this possibility. CYPs inactivate and activate neurotoxins and procarcinogens, including chlorpyrifos and a variety of tobacco-specific nitrosamines (Ekins et al, 2008). Combined with the region-specific regulation of CYPs by commonly used drugs such as alcohol and nicotine, exposure to, and subsequent metabolism of, these toxins could contribute to local region-specific neurotoxicities or carcinomas. Phenobarbital, which can induce both hepatic and brain CYP2B, potentiated the neurotoxicity resulting from 9-methoxy-N-2-methylellipticinium acetate (Upadhya et al, 2002). Recently, we have shown in a human neuronal cell line that toxicity following 1-methyl-4-phenylpyridinum was increased with the inhibition of CYP2D, an enzyme that can metabolically inactive this potent neurotoxin (Mann and Tyndale, 2010). Recent findings have also suggested a role for brain CYPs in altering the drug–hormone cross-talk in the brain, and found that neuroactive drugs that induce brain CYPs are associated with alterations in the side-effect profiles of these drugs and, in some cases exacerbate, the disease condition (Meyer and Gehlhaus, 2010).
Although previous studies have shown that CYP enzymes are active (Miksys and Tyndale, 2009) and inducible (Miksys and Tyndale, 2004) in the brain, there was no indication of whether their levels in the brain were sufficient to alter local drug metabolism and resulting drug effects. Here we provide strong evidence supporting a role for local drug metabolism by brain CYPs in altering the pharmacological actions of drugs. Centrally acting drugs can show large interindividual variability in response, which often does not correlate with plasma drug levels (Michels and Marzuk, 1993). Thus, localized CNS metabolism, which does not alter plasma levels as shown here, could contribute to variation in central drug response and potentially adverse drug reactions.
Acknowledgments
We would like to thank Drs Philip G Williams, Hiromi Morimoto, and William F Trager for generously providing us the radiolabeled 8-methoxypsoralen and Dr Howard Kaplan for his statistical guidance. We would also like to thank Fariba Baghai Wadji and Dr Bin Zhao for their technical assistance and Dr Sharon Miksys for her scientific consultation. This work was funded by the Centre for Addiction and Mental Health, CIHR MOP 97751, and a Canada Research Chair to Rachel F Tyndale.
Dr Rachel F Tyndale is a shareholder in Nicogen Research, a company focused on the development of novel smoking cessation treatment approaches. None of the data contained in this manuscript alters or improves any commercial aspect of Nicogen and no Nicogen funds were used in this work. The manuscript was not reviewed by anyone else associated with Nicogen. Jibran Y Khokhar has no conflict of interest to declare.
References
- Albores A, Ortega-Mantilla G, Sierra-Santoyo A, Cebrian ME, Munoz-Sanchez JL, Calderon-Salinas JV, et al. Cytochrome P450 2B (CYP2B)-mediated activation of methyl-parathion in rat brain extracts. Toxicol Lett. 2001;124:1–10. doi: 10.1016/s0378-4274(01)00382-4. [DOI] [PubMed] [Google Scholar]
- Altomare C, Trapani G, Latrofa A, Serra M, Sanna E, Biggio G, et al. Highly water-soluble derivatives of the anesthetic agent propofol: in vitro and in vivo evaluation of cyclic amino acid esters. Eur J Pharm Sci. 2003;20:17–26. doi: 10.1016/s0928-0987(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Bhargava VK, Saha L. Cholinergic mechanism in imipramine and morphine antinoception. Boll Chim Farm. 2001;140:201–204. [PubMed] [Google Scholar]
- Bijl MJ, Luijendijk HJ, van den Berg JF, Visser LE, van Schaik RH, Hofman A, et al. Association between the CYP2D6*4 polymorphism and depression or anxiety in the elderly. Pharmacogenomics. 2009;10:541–547. doi: 10.2217/pgs.09.9. [DOI] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Daniel WA. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: an in vitro study. Eur J Pharmacol. 2010;626:171–178. doi: 10.1016/j.ejphar.2009.09.062. [DOI] [PubMed] [Google Scholar]
- Cavazzuti M, Porro CA, Barbieri A, Galetti A. Brain and spinal cord metabolic activity during propofol anaesthesia. Br J Anaesth. 1991;66:490–495. doi: 10.1093/bja/66.4.490. [DOI] [PubMed] [Google Scholar]
- Challet E, Gourmelen S, Pevet P, Oberling P, Pain L. Reciprocal relationships between general (propofol) anesthesia and circadian time in rats. Neuropsychopharmacology. 2007;32:728–735. doi: 10.1038/sj.npp.1301081. [DOI] [PubMed] [Google Scholar]
- Chimbira W, Sweeney BP. The effect of smoking on postoperative nausea and vomiting. Anaesthesia. 2000;55:540–544. doi: 10.1046/j.1365-2044.2000.01474.x. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Chaudieu I, el-Bizri H, Boksa P, Quik M, Esplin BA, et al. The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol. 1994;111:397–405. doi: 10.1111/j.1476-5381.1994.tb14748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court MH, Duan SX, Hesse LM, Venkatakrishnan K, Greenblatt DJ. Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology. 2001;94:110–119. doi: 10.1097/00000542-200101000-00021. [DOI] [PubMed] [Google Scholar]
- Dam M, Ori C, Pizzolato G, Ricchieri GL, Pellegrini A, Giron GP, et al. The effects of propofol anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1990;73:499–505. doi: 10.1097/00000542-199009000-00021. [DOI] [PubMed] [Google Scholar]
- Ekins S, Iyer M, Krasowski MD, Kharasch ED. Molecular characterization of CYP2B6 substrates. Curr Drug Metab. 2008;9:363–373. doi: 10.2174/138920008784746346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassoulaki A, Farinotti R, Mantz J, Desmonts JM. Does tolerance develop to the anaesthetic effects of propofol in rats. Br J Anaesth. 1994;72:127–128. doi: 10.1093/bja/72.1.127. [DOI] [PubMed] [Google Scholar]
- Fassoulaki A, Farinotti R, Servin F, Desmonts JM. Chronic alcoholism increases the induction dose of propofol in humans. Anesth Analg. 1993;77:553–556. doi: 10.1213/00000539-199309000-00021. [DOI] [PubMed] [Google Scholar]
- Favetta P, Degoute CS, Perdrix JP, Dufresne C, Boulieu R, Guitton J. Propofol metabolites in man following propofol induction and maintenance. Br J Anaesth. 2002;88:653–658. doi: 10.1093/bja/88.5.653. [DOI] [PubMed] [Google Scholar]
- Fradette C, Yamaguchi N, Du Souich P. 5-Hydroxytryptamine is biotransformed by CYP2C9, 2C19 and 2B6 to hydroxylamine, which is converted into nitric oxide. Br J Pharmacol. 2004;141:407–414. doi: 10.1038/sj.bjp.0705632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasini G, Carrillo JA, Benitez J. Potential role of cerebral cytochrome P450 in clinical pharmacokinetics: modulation by endogenous compounds. Clin Pharmacokinet. 2004;43:693–706. doi: 10.2165/00003088-200443110-00001. [DOI] [PubMed] [Google Scholar]
- Iohom G, Ni Chonghaile M, O′Brien JK, Cunningham AJ, Fitzgerald DF, Shields DC. An investigation of potential genetic determinants of propofol requirements and recovery from anaesthesia. Eur J Anaesthesiol. 2007;24:912–919. doi: 10.1017/S0265021507000476. [DOI] [PubMed] [Google Scholar]
- Kanto J, Gepts E. Pharmacokinetic implications for the clinical use of propofol. Clin Pharmacokinet. 1989;17:308–326. doi: 10.2165/00003088-198917050-00002. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Miksys SL, Tyndale RF. Rat brain CYP2B induction by nicotine is persistent and does not involve nicotinic acetylcholine receptors. Brain Res. 2010;1348:1–9. doi: 10.1016/j.brainres.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Seeringer A, Godoy AL, Ohmle B, Maier C, Beschoner P, et al. 2010CYP2D6 in the brain: genotype effects on resting brain perfusion Mol Psychiatrye-pub ahead of print 6 April 2010; doi: 10.138/mp.2010.42 [DOI] [PubMed]
- Koenigs LL, Trager WF. Mechanism-based inactivation of cytochrome P450 2B1 by 8-methoxypsoralen and several other furanocoumarins. Biochemistry. 1998;37:13184–13193. doi: 10.1021/bi981198r. [DOI] [PubMed] [Google Scholar]
- Langley MS, Heel RC. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs. 1988;35:334–372. doi: 10.2165/00003495-198835040-00002. [DOI] [PubMed] [Google Scholar]
- Le Guellec C, Lacarelle B, Villard PH, Point H, Catalin J, Durand A. Glucuronidation of propofol in microsomal fractions from various tissues and species including humans: effect of different drugs. Anesth Analg. 1995;81:855–861. doi: 10.1097/00000539-199510000-00034. [DOI] [PubMed] [Google Scholar]
- Lewis D.1996Cytochromes P450. Structure, Function and Mechanism Taylor & Francis: Bristol; pp 122–123. [Google Scholar]
- Liu SH, Wei W, Ding GN, Ke JD, Hong FX, Tian M. Relationship between depth of anesthesia and effect–site concentration of propofol during induction with the target-controlled infusion technique in elderly patients. Chin Med J (Engl) 2009;122:935–940. [PubMed] [Google Scholar]
- Ludbrook GL, Upton RN, Grant C, Gray EC. Brain and blood concentrations of propofol after rapid intravenous injection in sheep, and their relationships to cerebral effects. Anaesth Intens Care. 1996;24:445–452. doi: 10.1177/0310057X9602400406. [DOI] [PubMed] [Google Scholar]
- Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178:562–564. [PubMed] [Google Scholar]
- Lysakowski C, Dumont L, Czarnetzki C, Bertrand D, Tassonyi E, Tramer MR. The effect of cigarette smoking on the hypnotic efficacy of propofol. Anaesthesia. 2006;61:826–831. doi: 10.1111/j.1365-2044.2006.04747.x. [DOI] [PubMed] [Google Scholar]
- Mann A, Tyndale RF. Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci. 2010;31:1185–1193. doi: 10.1111/j.1460-9568.2010.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RP, Gehlhaus M. A role for CYP in the drug–hormone crosstalk of the brain. Expert Opin Drug Metab Toxicol. 2010;6:675–687. doi: 10.1517/17425251003680791. [DOI] [PubMed] [Google Scholar]
- Michels R, Marzuk PM. Progress in psychiatry (1) N Engl J Med. 1993;329:552–560. doi: 10.1056/NEJM199308193290808. [DOI] [PubMed] [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. The unique regulation of brain cytochrome P450 2 (CYP2) family enzymes by drugs and genetics. Drug Metab Rev. 2004;36:313–333. doi: 10.1081/dmr-120034149. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. Brain drug-metabolizing cytochrome P450 enzymes are active in vivo, demonstrated by mechanism-based enzyme inhibition. Neuropsychopharmacology. 2009;34:634–640. doi: 10.1038/npp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksys SL, Tyndale RF. Drug-metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci. 2002;27:406–415. [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. Nicotine metabolism by rat hepatic cytochrome P450s. Biochem Pharmacol. 1993;45:2554–2556. doi: 10.1016/0006-2952(93)90238-r. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.1986The Rat Brain in Stereotaxic Coordinates2nd edn.Academic Press: San Diego [Google Scholar]
- Sebel PS, Lowdon JD. Propofol: a new intravenous anesthetic. Anesthesiology. 1989;71:260–277. [PubMed] [Google Scholar]
- Seno H, He YL, Tashiro C, Ueyama H, Mashimo T. Simple high-performance liquid chromatographic assay of propofol in human and rat plasma and various rat tissues. J Anesth. 2002;16:87–89. doi: 10.1007/s540-002-8101-8. [DOI] [PubMed] [Google Scholar]
- Shyr MH, Tsai TH, Tan PP, Chen CF, Chan SH. Concentration and regional distribution of propofol in brain and spinal cord during propofol anesthesia in the rat. Neurosci Lett. 1995;184:212–215. doi: 10.1016/0304-3940(94)11209-2. [DOI] [PubMed] [Google Scholar]
- Siu EC, Tyndale RF. Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice. J Pharmacol Exp Ther. 2008;324:992–999. doi: 10.1124/jpet.107.133900. [DOI] [PubMed] [Google Scholar]
- Strobel HW, Thompson CM, Antonovic L. Cytochromes P450 in brain: function and significance. Curr Drug Metab. 2001;2:199–214. doi: 10.2174/1389200013338577. [DOI] [PubMed] [Google Scholar]
- Tassonyi E, Charpantier E, Muller D, Dumont L, Bertrand D. The role of nicotinic acetylcholine receptors in the mechanisms of anesthesia. Brain Res Bull. 2002;57:133–150. doi: 10.1016/s0361-9230(01)00740-7. [DOI] [PubMed] [Google Scholar]
- Upadhya SC, Chinta SJ, Pai HV, Boyd MR, Ravindranath V. Toxicological consequences of differential regulation of cytochrome p450 isoforms in rat brain regions by phenobarbital. Arch Biochem Biophys. 2002;399:56–65. doi: 10.1006/abbi.2001.2727. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanev S, Kent UM, Pandova B, Hollenberg PF. Selective mechanism-based inactivation of cytochromes P-450 2B1 and P-450 2B6 by a series of xanthates. Drug Metab Dispos. 1999;27:600–604. [PubMed] [Google Scholar]
- Yanev SG, Kent UM, Roberts ES, Ballou DP, Hollenberg PF. Mechanistic studies of cytochrome P450 2B1 inactivation by xanthates. Arch Biochem Biophys. 2000;378:157–166. doi: 10.1006/abbi.2000.1807. [DOI] [PubMed] [Google Scholar]