Abstract
Purpose: To develop a computed tomography (CT) organ dose estimation method designed to readily provide organ doses in a reference adult male and female for different scan ranges to investigate the degree to which existing commercial programs can reasonably match organ doses defined in these more anatomically realistic adult hybrid phantoms
Methods: The x-ray fan beam in the SOMATOM Sensation 16 multidetector CT scanner was simulated within the Monte Carlo radiation transport code MCNPX2.6. The simulated CT scanner model was validated through comparison with experimentally measured lateral free-in-air dose profiles and computed tomography dose index (CTDI) values. The reference adult male and female hybrid phantoms were coupled with the established CT scanner model following arm removal to simulate clinical head and other body region scans. A set of organ dose matrices were calculated for a series of consecutive axial scans ranging from the top of the head to the bottom of the phantoms with a beam thickness of 10 mm and the tube potentials of 80, 100, and 120 kVp. The organ doses for head, chest, and abdomen∕pelvis examinations were calculated based on the organ dose matrices and compared to those obtained from two commercial programs, CT-EXPO and CTDOSIMETRY. Organ dose calculations were repeated for an adult stylized phantom by using the same simulation method used for the adult hybrid phantom.
Results: Comparisons of both lateral free-in-air dose profiles and CTDI values through experimental measurement with the Monte Carlo simulations showed good agreement to within 9%. Organ doses for head, chest, and abdomen∕pelvis scans reported in the commercial programs exceeded those from the Monte Carlo calculations in both the hybrid and stylized phantoms in this study, sometimes by orders of magnitude.
Conclusions: The organ dose estimation method and dose matrices established in this study readily provides organ doses for a reference adult male and female for different CT scan ranges and technical parameters. Organ doses from existing commercial programs do not reasonably match organ doses calculated for the hybrid phantoms due to differences in phantom anatomy, as well as differences in organ dose scaling parameters. The organ dose matrices developed in this study will be extended to cover different technical parameters, CT scanner models, and various age groups.
Keywords: computed tomography, organ dose, Monte Carlo transport, hybrid phantom
INTRODUCTION
From 1982 to 2006, the average per capita effective dose contributed from all radiation sources in the United States increased from 3.6 to 6.2 mSv.1 The major contribution to this increase was medical exposures, resulting in a change from 15% of total exposure in 1982 due to medical exposures to 48% of total exposure in 2006; importantly, 47% of the total medical exposure in 2006 was attributed from computed tomography (CT). The annual number of CT examinations has increased from 3.6×106 in 1980 to 72×106 in 2007.2 The dramatic increase of the CT examinations is of concern to the medical profession and to the radiation protection community because of the possible cancer risks associated with the increased use of CT scans.2, 3 In response to this concern, the U.S. Food and Drug Administration (USFDA) announced the “Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging,” which focuses on increasing the safety of using medical imaging devices, informed decision making about the use of CT, and increasing patient awareness.4 Quantification of the radiation exposure from CT scans should be accurate to correctly assess the collective risk and to provide patient-specific organ doses for use in retrospective epidemiologic or prospective risk estimation studies.
Obtaining organ dose distribution in a human body exposed to ionizing radiation from CT examinations presents several complex technical problems.5 One approach is by experimental measurement using a physical phantom of the human body with embedded dosimeters. Physical phantoms are often made of a real human skeleton with tissue equivalent material simulating the surrounding soft tissues and are constructed as vertically stackable slices with small holes in each slice for dosimeter placement. These phantoms are then scanned by the CT machine to determine representative dose measurements. Dosimeter readings provide the information on dose distributions within the human body for the specific CT system and examination. However, this approach has limitations. In particular, the process is typically laborious and time-consuming, the point doses measurements cannot accurately represent the average organ dose in cases of high dose gradients, and physical phantoms with reasonable variations in age, gender, and body dimensions are not available.
Organ dose calculation via the Monte Carlo simulation method has been reported to be the most sophisticated and reliable way to obtain accurate organ dose distributions within a human body exposed to radiation from CT imaging.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 The human body lying on a CT scanner table and helical fan beam x-ray generated from a CT scanner are fully simulated in the computer using the Monte Carlo method.
Two comprehensive organ dose databases based on the Monte Carlo simulation were introduced by different organizations in 1991 and revised in 1993 by the National Radiation Protection Board23 (NRPB) in the United Kingdom [currently the Health Protection Agency (HPA)] and the National Research Center for Environment and Health (GSF) in Germany.24, 25 The NRPB organ dose database was based on a hermaphrodite adult stylized phantom and the GSF database was developed based on male and female stylized phantoms, called ADAM and EVA, as well as two pediatric voxel phantoms. Those stylized phantoms were basically updated or modified from the original stylized phantoms developed at Oak Ridge National Laboratory (ORNL) in the 1960s. Graphical user interface-based software programs such as CT-EXPO, CTDOSIMETRY, CTDOSE, WINDOSE, and IMPACT have also been available, which are mostly based on the two organ dose databases of the NRPB and GSF mentioned above.
Although stylized phantoms have been utilized for external and internal dosimetry calculations worldwide, anatomic structures are represented by mathematical surface equations which are significantly limited in describing true and, for many organs, complicated human anatomy. To overcome the anatomical limitation of stylized phantoms, voxel phantoms which are segmented from patient tomographic images to represent more realistic anatomy have been developed and are widely utilized in medical physics as well as radiation protection research studies.26, 27, 28 More advanced formats of human computational phantoms, called hybrid phantoms, were developed by various investigators, included those at the University of Florida.19, 29, 30 In a hybrid phantom, more advanced mathematical descriptors such as nonuniform rational B-spline (NURBS) and polygon mesh (PM) surfaces were used, which are much more flexible than the conventional mathematical equations.31, 32, 33 The hybrid phantoms are also realistic as the basic anatomical frame is obtained from patient CT images. A comprehensive summary data on currently available computational phantoms are given in a review by Xu et al.34
This study was intended to (1) develop a CT organ dose estimation method designed to provide organ doses in a reference adult male and female for different scan ranges and (2) investigate if the existing commercial programs can reasonably match those organ doses.
MATERIALS AND METHODS
Modeling of x-ray source in the CT scanner
A SOMATOM Sensation 16 helical multislice CT scanner (Siemens Medical Solutions, Erlangen, Germany) was simulated within a general purpose Monte Carlo radiation transport code MCNPX2.6.35 The CT scanner has both inherent and external (i.e., bowtie filter) filtration. The material and thickness of the inherent filtrations for head and body filters was obtained from the manufacturer. Two different x-ray spectra for the inherent head and body filters were generated from a commercial x-ray spectrum generation program, called SPEC78, developed by the Institute of Physics and Engineering in Medicine (IPEM).36 The x-ray spectra were incorporated into the MCNPX input deck for energy sampling. X-ray spectra for inherent head filter were utilized for dose calculation in head scan and those for inherent body filter were used for chest and abdomen∕pelvis scans.
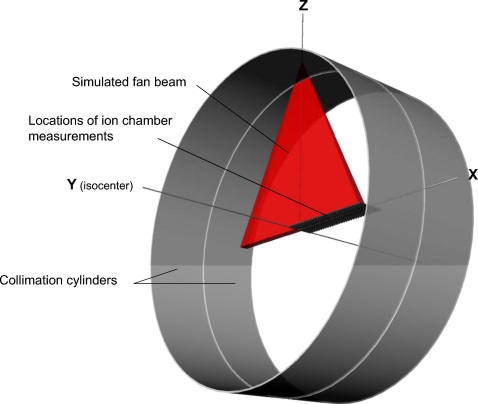
Explicit modeling of the external bowtie filter within MCNPX was not attempted because other previous researchers modeling CT scanners reported that a full Monte Carlo simulation does not change the resulting organ doses significantly.9, 17, 37 Instead, weighting factors were applied to angular sampling within a fan beam angle based on a lateral free-in-air dose profile measured by a pencil ion chamber. A 100 mm Radcal® ion chamber model 10×5-10.3CT and an MDH model 1015 electrometer (Radcal Corp., Monrovia, CA) were used to measure the lateral dose profile while the x-ray tube was fixed at the 12 o’clock position under service mode. The ion chamber was placed at isocenter perpendicular to patient table direction and then shifted up to 30 cm×1 cm interval as depicted in Fig. 1. The CT scanner has a fan beam angle of 52° with a focal-spot-to-axis distance of 57 cm so that the lateral profile covered by the half fan beam expands up to 28 cm from isocenter. The shape of the bowtie filter was exactly reflected in the lateral dose profile which was inserted within MCNPX input for angle sampling within the half fan beam angle from 0° to 26°. Collimators modeled by two cylindrical tubes with tiny gaps were placed to generate a fan-shaped x-ray beam as shown in Fig. 1. The importance of the collimators within MCNPX was set zero so that radiation particles entering the collimator volume were not transported further which reduced calculation time. This approach did not significantly change the organ doses when compared to the case where full radiations were transported after scattering from explicit collimators. The axial rotating beam was simulated by sampling different source starting positions along the circle ranging from 0° to 360°. A schematic diagram is given in Fig. 1 where the locations of the ion chamber measurements to obtain the lateral fan beam profile, the collimation cylinders, and the simulated fan beam are described.
Figure 1.
3D drawing of the CT beam simulation including the fan-shaped x-ray beam and collimation cylinders. The locations of the ion chamber for beam profile measurements were also depicted by using a series of pencil ion chambers.
The carbon-fiber patient table was modeled as two concentric cylinders of different radii. The concentric cylinders were truncated to a width of 40 cm corresponding to the width of the patient table. The thickness of the patient table was determined in an empirical fashion. Ion chamber measurements were performed at isocenter for two separate static beams fixed at 12 and 6 o’clock positions. The 12 o’clock beam reached the ion chamber without attenuation, but the 6 o’clock beam was attenuated by the patient table. The same static beams and ion chamber were simulated in MCNPX and the table thickness was varied until it matched the ratio of the ion chamber measurements between the 12 and 6 o’clock beams. The table was modeled using two concentric cylinders of radii 45 and 46 cm which provided 1 cm thickness.
Experimental validation of the CT beam model
Two different validations of the CT scanner model were undertaken by comparison with experimental measurements: Lateral dose profile and computed tomography dose index (CTDI). First, the lateral dose profile under a single rotation of the x-ray beam head was measured and compared to the simulated values obtained within the Monte Carlo calculation. Second, a set of (CTDI)100 data was measured for the collimation width of 10 mm. Head and body CTDI phantoms with diameters of 16 and 32 cm, respectively, were employed to measure central dose as well as three different peripheral doses (12, 3, and 6 o’clock positions) by using the ion chamber. Measured CTDI100 was compared to the simulated CTDI100. All measurement was made for the tube potential of 80, 100, and 120 kVp and small focal spot which are typical CT imaging condition.
To perform the second comparison, the CTDI phantoms were modeled as a cylinder having a diameter of 16 cm for head and 32 cm for body phantoms, with a length of 15 cm each. The material composition of the CTDI phantoms was simulated as polymethylmethacrylate with a density of 1.19 g cm−3. The Radcal ion chamber was modeled as three 10 cm long concentric cylinders. The innermost cylinder with a diameter of 6.7 mm defined the active air volume. The second cylinder with a diameter of 10.2 mm defined the chamber wall which is C552 air-equivalent material with a density of 1.76 g cm−3. The third cylinder with a diameter of 13.7 mm defined a build-up cap which was modeled as polyacetal plastic with a density of 1.43 g cm−3.
Since MCNPX provides dose per simulated photon, the number of photons per unit mA s, called the Monte Carlo normalization factor, should be multiplied to the MCNPX results to obtain organ doses normalized to current. The normalization factors were calculated based on the ratio of the ion chamber measurements in free-in-air (mGy∕mA s) to the simulated ion chamber doses in free-in-air (mGy∕photon). The normalization factors (photon∕mA s) were multiplied with the MCNPX dose results (mGy∕photon) in both the CTDI phantoms and the adult male hybrid phantom to obtain normalized doses (mGy∕mA s). Six different normalization factors for head and body filters and for the tube potentials of 80, 100, and 120 kVp under the collimation width of 10 mm were calculated and utilized to obtain absolute doses in this study. More detailed explanations about the normalization factors can be found in previous publications.17, 18, 19
Reference adult male and female hybrid phantoms
The adult male and female hybrid phantoms developed at the University of Florida were employed to model reference adult male and female individuals.30 Four different reference data sets are incorporated in the phantoms to represent reference individuals. First, the masses of organs and tissues are matched to the values in International Commission on Radiological Protection (ICRP) Publication 89.38 Second, the reference elemental compositions for organs and tissues provided by International Commission on Radiation Units and Measurements (ICRU) Report 46 (Ref. 39) and ICRP Publication 89 were used within the Monte Carlo calculations. Third, the body dimensions are matched to the reference anthropometric data of the United States (http:∕/www.cdc.gov∕nchs∕nhanes.htm). Finally, the dimensions of the alimentary tracts are matched to the data in ICRP Publication 100.40 The adult hybrid phantoms include more than 40 organs as well as 35 different skeletal sites. The skeleton is composed of cortical and spongiosa structures to facilitate active marrow dose calculation.
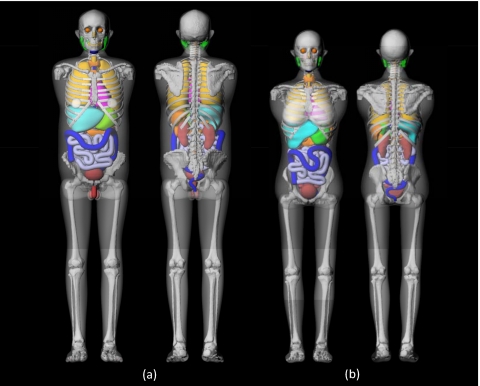
Arms were removed from the phantoms to more realistically simulate typical body postures in both head and torso CT scans except left and right humeral heads were included to accurately calculate their contribution to active marrow dose. The original hybrid phantoms were in both NURBS and PM formats. The phantoms were voxelized with the resolution of 2×2×2 mm3 to generate the voxel format which was actually utilized for Monte Carlo calculation. The frontal and rear 3D renderings of the adult male and female hybrid phantoms in NURBS∕PM format are shown in Fig. 2.
Figure 2.
3D frontal (left) and rear (right) views of hybrid adult (a) male and (b) female phantoms used in the Monte Carlo organ dose calculations in this study. Arms, including arm bones except the humeral head, were removed to more realistically simulate positioning of a patient during CT examination.
Organ and skeleton dose calculation
In this study, organ doses from helical scans were approximated by the summation of doses from multiple axial slices included in the given scan range of interest, which is the same approach used in the existing CT organ dose estimation programs.41 This approach provided flexibility to the CT scan dosimetry calculations in which one can assess organ doses for whatever CT scan coverage is applied by using the precalculated organ dose matrix without running time-consuming Monte Carlo calculation for each case. A series of organ dose calculations were performed for a single axial scan which started from the top of the head down to the bottom of the phantoms with an interval of 1 cm. In the case of the adult hybrid male and female phantoms, a total of 175 and 164 consecutive axial calculations, respectively, were performed. A total of 30 organs and tissues including active marrow were involved in the organ dose calculations under the tube potentials of 80, 100, and 120 kVp and the collimation width of 10 mm. Considering the energy range of x-ray spectrum, kinetic energy released in matter was evaluated in MCNPX by using F6 tally for the dose calculation instead of absorbed doses. No energy cutoff was implemented.
Absorbed doses to active marrow was estimated by using fluence-to-dose response function developed at the University of Florida,42 which is the updated version over the one developed at ORNL.43 The fluence-to-dose response functions relate the absorbed dose in a skeletal target region to the photon fluence in a skeletal source region and are primarily implemented using fluence estimates provided by a Monte Carlo transport code. Photon fluence crossing the spongiosa region of each bone site was calculated for 25 energy bins ranging from 0.01 to 10 MeV for each axial scan within the Monte Carlo simulation. Active marrow doses in each bone site were then calculated by multiplying the photon fluence by the dose response function prepared for active marrow. Total absorbed doses for active marrow were calculated by weighting site-specific doses by active marrow distribution in a reference adult male.38
Typical scan ranges for the three CT examinations (head, chest, and abdomen∕pelvis) were obtained from the CT scan protocol which is utilized at the Radiology Department of the University of Florida Shands Hospital (http:∕/xray.ufl.edu∕protocols∕snips∕procedures∕display). The head examination covers from the top of the head to the second cervical vertebra which included the first 18 slices, from 0 to 18 cm, of the adult male and female phantoms. The chest examination represents a scan from the clavicle to the middle of liver which included the slices from 27 to 54 cm and the slices from 27 to 50 cm in the adult male and female phantoms, respectively. The abdomen∕pelvis examination covers the anatomy ranging from the top of liver to the midfemoral head which is equivalent to the range from 54 to 87 cm and from 50 to 82 cm in the adult male and female phantoms, respectively. The distances were measured from the top of the head. Mass-weighted average doses were calculated for the separated∕paired organs to provide a single dose. For instance, absorbed doses to three different parts of salivary glands (parotid, submandible, and sublingual) were averaged with being weighted by mass as a single salivary gland dose. The same approach was applied for other separated∕paired organs such as lens, eye balls, lung, breast, adrenal, kidney, and testes.
Organ dose comparison with the commercial programs
Organ doses for head, chest, and abdomen∕pelvis examinations were obtained from two commercial programs, CT-EXPO v1.6 (Ref. 41) and CTDOSIMETRY v1.0.2 (http:∕/www.impactscan.org∕ctdosimetry.htm), for the adult male by using the technical parameters used in the simulation and compared to those from the hybrid male phantom. CT-EXPO is based on the organ dose database developed at the GSF in Germany which was calculated by using male and female stylized phantoms, called ADAM and EVA.24, 25CTDOSIMETRY is based on the organ dose database calculated at the NRPB in the United Kingdom. The database is based on the hermaphrodite adult stylized phantom where male and female bodies are combined.23
Scan ranges for head, chest, and abdomen∕pelvis studies were defined in the commercial programs as from the head top to the bottom of the cranium (from 0 to 16 cm), from the top of the trunk to the bottom of the ribs (from 24 to 58 cm), and from the top of the liver to the bottom of the pelvis (from 51 to 94 cm), respectively. The distances were measured from the top of the head. The adult male was selected in CT-EXPO but only the hermaphrodite adult was available in CTDOSIMETRY. The Siemens Sensation 16 CT scanner was selected with the tube potential of 120 kVp under axial scan mode. Although the scan ranges were defined within both the stylized phantom in the commercial programs and the hybrid phantoms by using the identical anatomical landmarks, different portions of organs were included in a given scan range because of the less realistic organ shapes and locations in the stylized phantom.
The hybrid phantoms are based on patient CT images but the stylized phantoms used in the two computer programs are based on simplified mathematical surfaces. To investigate the possible effect of the anatomical differences on organ doses, the Monte Carlo calculations used for the hybrid phantom were repeated by using adult male ORNL stylized phantom.43 The organ dose results were also included in the organ dose comparison study.
RESULTS AND DISCUSSIONS
Experimental validations
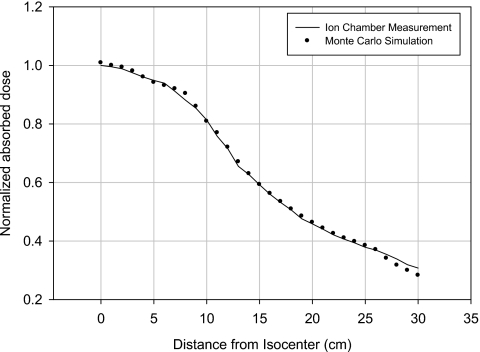
First, the lateral free-in-air dose profile within the half fan beam was measured from the isocenter up to 30 cm for the tube potentials of 80, 100, and 120 kVp by using ion chamber shown in Fig. 1. The measurements were simulated by our Monte Carlo calculations by using the simulated CT x-ray beam and the ion chamber. The comparison for the tube potential of 120 kVp is given in Fig. 3, where the simulated doses overall agreed with the measured values within 3% except in the peripheral region between 27 and 30 cm with an error in that case of just under 8%.
Figure 3.
Comparison of lateral dose profile from isocenter up to 30 cm obtained from ion chamber measurements with the values from Monte Carlo simulations for the tube potential of 120 kVp.
Four different point doses (central dose and doses at 12, 3, and 6 o’clock positions) were measured within the CTDI head and body phantoms by using the ion chamber with the collimation of 10 mm under the three tube potentials of 80, 100, and 120 kVp. The CTDI phantoms and the ion chamber were simulated within the Monte Carlo calculation including the patient table. The CTDI100 comparison was performed for different tube potentials (80, 100, and 120 kVp) and 10 mm collimation as presented in Table 1. The simulated doses agreed with the measured ones within 8.6% for all tube potentials. These errors were comparable with those given in other published studies.17, 21, 44
Table 1.
Comparison of CTDI100 (mGy∕100 mA s) obtained from measurements and simulations for head and body CTDI phantoms and 10 mm collimation for the tube potentials of 80, 100, and 120 kVp.
| Collimation width (mm) | Phantoms | Measurement position in CTDI phantoms | Measured CTDI100 (mGy∕100 mA s) | Simulated CTDI100 (mGy∕100 mA s) | Percent error (%) |
|---|---|---|---|---|---|
| 80 kVp | Head phantom | Center | 5.3 | 5.1 | −4.1 |
| 12 o’clock | 6.9 | 6.6 | −3.3 | ||
| 3 o’clock | 6.5 | 6.3 | −3.4 | ||
| 6 o’clock | 5.7 | 5.7 | −0.3 | ||
| Body phantom | Center | 1.0 | 0.9 | −5.7 | |
| 12 o’clock | 2.7 | 2.5 | −6.2 | ||
| 3 o’clock | 2.4 | 2.4 | 1.1 | ||
| 6 o’clock | 2.1 | 2.1 | 0.4 | ||
| 100 kVp | Head phantom | Center | 9.7 | 9.2 | −5.2 |
| 12 o’clock | 11.8 | 11.4 | −3.7 | ||
| 3 o’clock | 11.3 | 10.8 | −4.3 | ||
| 6 o’clock | 9.9 | 9.9 | −0.3 | ||
| Body phantom | Center | 2.1 | 2.0 | −6.5 | |
| 12 o’clock | 5.0 | 4.6 | −7.2 | ||
| 3 o’clock | 4.6 | 4.6 | 0.1 | ||
| 6 o’clock | 4.0 | 4.0 | 0.0 | ||
| 120 kVp | Head phantom | Center | 13.8 | 13.1 | −5.2 |
| 12 o’clock | 16.8 | 15.6 | −7.2 | ||
| 3 o’clock | 15.6 | 14.9 | −4.6 | ||
| 6 o’clock | 13.6 | 13.6 | 0.1 | ||
| Body phantom | Center | 3.4 | 3.2 | −7.9 | |
| 12 o’clock | 7.4 | 6.7 | −8.6 | ||
| 3 o’clock | 6.8 | 6.7 | −2.0 | ||
| 6 o’clock | 6.0 | 5.9 | −1.0 |
Organ dose calculations in the adult male and female hybrid phantoms
Organ absorbed doses for 30 organs and tissues were calculated for a total of 175 and 164 axial scans for the adult male and female, respectively, ranging from the top of the head to the bottom of the phantoms with an interval of 1 cm. Organ doses for three typical CT studies including head, chest, and abdomen∕pelvis were calculated by the summation method and are presented in Tables 2, 3 for the adult male and female, respectively. A total of 108 particle histories were used within the Monte Carlo calculations to decrease relative errors to less than 2% for the major organs. Organ doses less than 0.1 mGy∕100 mA s were removed from the tables. In case of the tube potential of 120 kVp, the eye lens received the highest dose (13.6 mGy∕100 mA s for male and 13.3 mGy∕100mA s for female) in the head scan. The eye lens received 70% and 40% of the dose at 120 kVp when the tube potential decreased to 100 and 80 kVp, respectively, in both male and female phantoms. The thymus and the lung received relatively high doses in both male and female phantoms in the chest study. Both the thymus and the lung in the male phantom received 6.8 mGy∕100 mA s at 120 kVp. The thymus and the lung in the female phantom received 7.4 and 7.3 mGy∕100 mA s, respectively, at the tube potential of 120 kVp. The lung doses decreased to 65% and 30% of the dose at 120 kVp when the tube potential was lowered to 100 and 80 kVp, respectively. The kidneys, small intestine wall, and colon wall in the male and female phantoms received relatively high doses which were greater than 6 and 7 mGy∕100 mA s, respectively, in the abdomen∕pelvis study. The active marrow in the male and female phantoms received the highest dose of 2.2 and 2.0 mGy∕100mA s at 120 kVp, respectively, in the abdomen∕pelvis scan compared to the head and chest studies, which was attributed to the greater fraction of the active marrow in the pelvis compared to other bone sites included in the head and chest regions.
Table 2.
Organ doses (mGy∕100 mA s) obtained from the adult male hybrid phantom for head, chest, and abdomen∕pelvis examinations for the tube potentials of 80, 100, and 120 kVp. Scan coverage (the distance from the top of head) and the corresponding anatomical landmarks are also included in rows 2 and 3, respectively.
| CT study type | Head | Chest | Abdomen∕pelvis | ||||||
| Scan coverage (cm) | 0 –18 cm | 27–54 cm | 54–87 cm | ||||||
| Anatomical landmark | From the top of the head to second cervical vertebra | From clavicle to midliver | From the top of the liver to the bottom of the pelvis | ||||||
| Tube potential (kVp) | 80 | 100 | 120 | 80 | 100 | 120 | 80 | 100 | 120 |
| Brain | 3.3 | 6.6 | 10.0 | ⋯ | ⋯ | 0.1 | ⋯ | ⋯ | ⋯ |
| Pituitary gland | 2.9 | 6.0 | 9.2 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Lensa | 5.5 | 9.6 | 13.6 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Eye ballsa | 4.9 | 8.8 | 12.6 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Salivary glandsa | 1.9 | 5.1 | 5.1 | 0.2 | 0.3 | 0.6 | ⋯ | ⋯ | ⋯ |
| Oral cavity layer | 2.0 | 5.2 | 6.3 | 0.1 | 0.2 | 0.5 | ⋯ | ⋯ | ⋯ |
| Spinal cord | ⋯ | 0.4 | 0.3 | 0.6 | 1.5 | 2.3 | 0.7 | 1.4 | 2.5 |
| Thyroid | ⋯ | 0.2 | 0.3 | 2.9 | 5.1 | 6.2 | ⋯ | ⋯ | ⋯ |
| Esophagus | ⋯ | ⋯ | ⋯ | 1.5 | 3.4 | 5.4 | 0.2 | 0.3 | 0.7 |
| Trachea | ⋯ | 0.1 | 0.2 | 1.9 | 3.9 | 6.4 | ⋯ | ⋯ | ⋯ |
| Thymus | ⋯ | ⋯ | ⋯ | 2.0 | 4.3 | 6.8 | ⋯ | ⋯ | ⋯ |
| Lunga | ⋯ | ⋯ | ⋯ | 2.1 | 4.4 | 6.8 | 0.1 | 0.2 | 0.4 |
| Breasta | ⋯ | ⋯ | ⋯ | 2.1 | 4.4 | 6.7 | ⋯ | ⋯ | 0.1 |
| Heart wall | ⋯ | ⋯ | ⋯ | 2.1 | 4.6 | 7.1 | 0.2 | 0.3 | 0.7 |
| Stomach wall | ⋯ | ⋯ | ⋯ | 0.9 | 2.2 | 3.0 | 1.3 | 2.6 | 4.6 |
| Liver | ⋯ | ⋯ | ⋯ | 1.1 | 2.7 | 3.7 | 1.2 | 2.2 | 4.0 |
| Gall bladder wall | ⋯ | ⋯ | ⋯ | 0.3 | 0.7 | 1.0 | 1.5 | 3.2 | 5.3 |
| Adrenala | ⋯ | ⋯ | ⋯ | 0.4 | 1.1 | 1.4 | 1.4 | 2.9 | 4.9 |
| Spleen | ⋯ | ⋯ | ⋯ | 1.0 | 2.7 | 3.4 | 1.4 | 2.4 | 4.6 |
| Pancreas | ⋯ | ⋯ | ⋯ | 0.2 | 0.5 | 0.6 | 1.6 | 3.4 | 5.6 |
| Kidneya | ⋯ | ⋯ | ⋯ | 0.1 | 0.4 | 0.6 | 2.0 | 4.3 | 6.8 |
| Small intestine wall | ⋯ | ⋯ | ⋯ | ⋯ | 0.1 | 0.2 | 1.8 | 3.9 | 6.2 |
| Colon wall | ⋯ | ⋯ | ⋯ | ⋯ | 0.2 | 0.2 | 2.3 | 4.7 | 7.3 |
| Rectosigmoid wall | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.3 | 3.0 | 4.7 |
| Urinary bladder wall | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.5 | 3.5 | 5.3 |
| Prostate | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 0.6 | 2.0 | 2.2 |
| Testesa | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 0.2 | 0.5 | 0.7 |
| Skin | 0.3 | 0.6 | 0.9 | 0.5 | 1.0 | 1.5 | 0.6 | 1.2 | 1.8 |
| Muscle | 0.1 | 0.2 | 0.2 | 0.5 | 1.1 | 1.7 | 0.8 | 1.7 | 2.6 |
| Active marrow | 0.2 | 0.4 | 0.6 | 0.5 | 1.2 | 1.9 | 0.5 | 1.3 | 2.2 |
Doses to separated suborgans were averaged to provide a single dose with mass weighting.
Table 3.
Organ doses (mGy∕100 mA s) obtained from the adult female hybrid phantom for head, chest, and abdomen∕pelvis examinations for the tube potentials of 80, 100, and 120 kVp. Scan coverage (the distance from the top of head) and the corresponding anatomical landmarks are also included in rows 2 and 3, respectively.
| CT study type | Head | Chest | Abdomen∕pelvis | ||||||
| Scan coverage (cm) | 0–18 cm | 27–50 cm | 54–82 cm | ||||||
| Anatomical landmark | From the top of the head to 2nd cervical vertebra | From clavicle to midliver | From the top of the liver to the bottom of the pelvis | ||||||
| Tube potential (kVp) | 80 | 100 | 120 | 80 | 100 | 120 | 80 | 100 | 120 |
| Brain | 3.6 | 7.2 | 10.7 | ⋯ | ⋯ | 0.1 | ⋯ | ⋯ | ⋯ |
| Pituitary gland | 3.1 | 6.3 | 9.6 | ⋯ | ⋯ | 0.1 | ⋯ | ⋯ | ⋯ |
| Lensa | 5.4 | 9.5 | 13.3 | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| Eye ballsa | 5.1 | 9.1 | 13.0 | ⋯ | ⋯ | 0.1 | ⋯ | ⋯ | ⋯ |
| Salivary Glandsa | 1.8 | 3.3 | 4.9 | 0.2 | 0.4 | 0.7 | ⋯ | ⋯ | ⋯ |
| Oral cavity layer | 2.6 | 5.2 | 8.0 | 0.1 | 0.3 | 0.4 | ⋯ | ⋯ | ⋯ |
| Spinal cord | 0.2 | 0.3 | 0.5 | 0.8 | 1.8 | 2.8 | 0.9 | 2.0 | 3.2 |
| Thyroid | 0.1 | 0.3 | 0.5 | 1.9 | 3.9 | 5.9 | ⋯ | ⋯ | ⋯ |
| Esophagus | ⋯ | ⋯ | 0.1 | 1.7 | 3.7 | 5.8 | 0.2 | 0.5 | 0.8 |
| Trachea | ⋯ | 0.2 | 0.2 | 2.0 | 4.3 | 6.8 | ⋯ | ⋯ | 0.1 |
| Thymus | ⋯ | 0.1 | 0.2 | 2.2 | 4.7 | 7.4 | ⋯ | ⋯ | 0.1 |
| Lunga | ⋯ | ⋯ | ⋯ | 2.2 | 4.7 | 7.3 | 0.2 | 0.4 | 0.6 |
| Breasta | ⋯ | ⋯ | ⋯ | 1.9 | 3.8 | 5.9 | 0.1 | 0.3 | 0.5 |
| Heart wall | ⋯ | ⋯ | ⋯ | 2.1 | 4.6 | 7.1 | 0.2 | 0.5 | 0.8 |
| Stomach wall | ⋯ | ⋯ | ⋯ | 0.7 | 1.6 | 2.6 | 1.9 | 4.0 | 6.1 |
| Liver | ⋯ | ⋯ | ⋯ | 1.1 | 2.3 | 3.6 | 1.6 | 3.3 | 5.2 |
| Gall bladder wall | ⋯ | ⋯ | ⋯ | 0.4 | 0.9 | 1.4 | 1.8 | 3.9 | 6.2 |
| Adrenala | ⋯ | ⋯ | ⋯ | 0.3 | 0.7 | 1.2 | 1.8 | 3.9 | 6.2 |
| Spleen | ⋯ | ⋯ | ⋯ | 0.8 | 1.7 | 2.6 | 2.2 | 4.4 | 6.7 |
| Pancreas | ⋯ | ⋯ | ⋯ | 0.2 | 0.6 | 0.9 | 2.0 | 4.3 | 6.8 |
| Kidneya | ⋯ | ⋯ | ⋯ | 0.1 | 0.4 | 0.6 | 2.8 | 5.9 | 9.0 |
| Small Intestine wall | ⋯ | ⋯ | ⋯ | ⋯ | 0.1 | 0.2 | 2.2 | 4.7 | 7.3 |
| Colon wall | ⋯ | ⋯ | ⋯ | ⋯ | 0.1 | 0.2 | 2.5 | 5.4 | 8.2 |
| Rectosigmoid wall | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.4 | 3.1 | 4.9 |
| Urinary bladder wall | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.5 | 3.1 | 4.9 |
| Prostate | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.1 | 2.6 | 4.2 |
| Testesa | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | ⋯ | 1.6 | 3.6 | 5.6 |
| Skin | 0.4 | 0.7 | 1.0 | 0.5 | 1.0 | 1.4 | 0.7 | 1.3 | 2.0 |
| Muscle | 0.1 | 0.2 | 0.3 | 0.5 | 1.0 | 1.6 | 0.9 | 1.8 | 2.8 |
| Active marrow | 0.6 | 1.1 | 1.8 | 0.5 | 1.1 | 1.7 | 0.5 | 1.2 | 2.0 |
Doses to separated suborgans were averaged to provide a single dose with mass weighting.
Comparison of organ doses with commercial programs
Organ doses in adult male for head, chest, and abdomen∕pelvis examinations were calculated by using CT-EXPO and CTDOSIMETRY and compared to the values calculated in the hybrid adult male phantom. The organ doses calculated from ORNL adult male stylized phantom by using the same method used for the hybrid phantom were also added to the dose comparison.
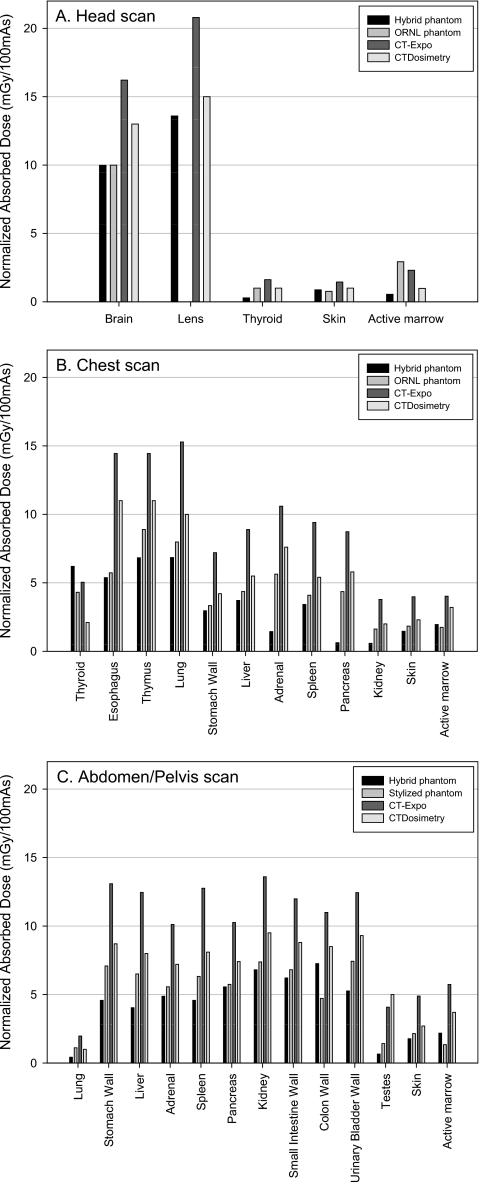
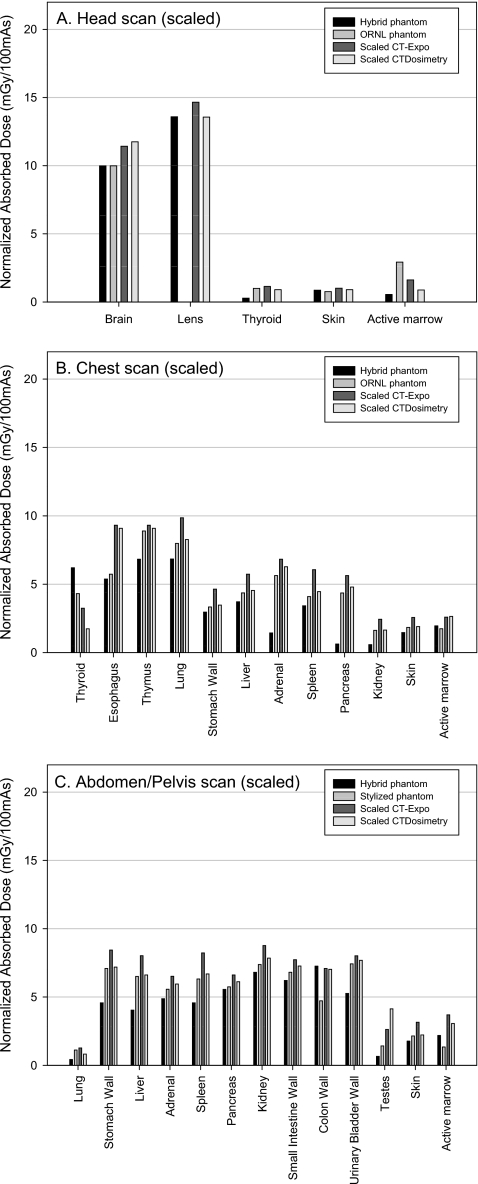
Figure 4a shows the comparison of organ doses in head scan. The eye lens was not available in the ORNL stylized phantom. The thyroid in CT-EXPO and CTDOSIMETRY received up to 5.6 and 3.5 times greater dose than the hybrid male phantom, respectively. The active marrow doses from the CT-EXPO and CTDOSIMETRY were 4.2 and 1.8 times greater than the hybrid phantom, respectively. The organ doses from the ORNL phantom are overall comparable to those from the hybrid phantom except in the case of the thyroid and active marrow dose. The difference in the thyroid doses is caused by the different anatomies in the hybrid and ORNL phantoms which will be discussed later in detail. The active marrow doses are different because different dose response functions were used in the hybrid and ORNL phantoms. The dose response function documented in the ORNL∕TM-8381 report was used in the stylized phantom, whereas the updated values were used in the hybrid phantom as mentioned in Sec. 2D.
Figure 4.
Comparison of the organ doses obtained from the hybrid adult male phantom and the ORNL phantom using the established CT scanner model and the commercial programs, CT-EXPO and CTDOSIMETRY, for (a) head, (b) chest, and (c) abdomen∕pelvis examinations for the tube potential of 120 kVp.
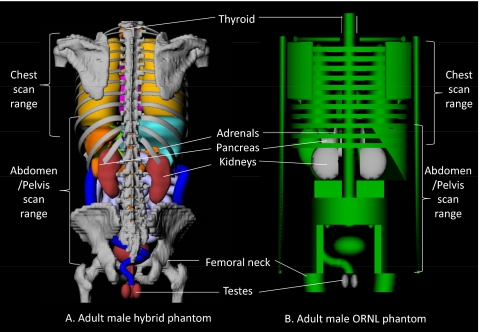
A comparison of organ doses in the simulated chest scan is depicted in Fig. 4b. Organ doses from the hybrid male phantom were, overall, less than the values from CT-EXPO and CTDOSIMETRY except in the case of the thyroid. The thyroid dose discrepancy is again caused by an unrealistic vertical placement of the thyroid in the stylized phantoms used within the CT-EXPO and CTDOSIMETRY codes, an observation reported previously.18, 45 The thyroid in the stylized phantoms is included solely within the neck and is excluded from the torso region as shown in Fig. 5. In the real human anatomy, however, the thyroid is partially included in the torso region and thus partially irradiated by CT x rays for a chest CT examination. Higher thyroid position in the stylized phantom also caused greater thyroid dose in CT-EXPO and CTDOSIMETRY in the head scan, which was mentioned in Sec. 3B. Pancreas, adrenals, and kidneys in CT-EXPO received significantly greater doses (more than tenfold in pancreas) than those in the hybrid phantom. These same organs in CTDOSIMETRY also received significantly higher doses by factors of 9.3, 5.3, and 3.4, respectively, than the organs in the hybrid adult male phantom. These organ dose discrepancies are caused by the unrealistic positions of the adrenals, pancreas, and kidneys in the ORNL stylized phantom compared to the real human anatomy as shown in Fig. 5. Although the chest scan ranges were defined within both the stylized phantom and the hybrid phantoms by using the identical anatomical landmarks (from clavicle to the middle of liver), a larger portion of the adrenals, pancreas, and kidneys are included in the scan range in the stylized phantom, which is not the case for the adult male hybrid phantom. The analysis is also supported by the fact that the same trend of dose differences in adrenals, pancreas, and kidneys are also shown between the hybrid phantom and the ORNL phantom. Although the similar stylized phantoms were utilized in the both CT-EXPO and CTDOSIMETRY, the results from CT-EXPO were significantly higher than the values from CTDOSIMETRY, more than twofold in case of thyroid.
Figure 5.
Comparison of the positions of thyroid, adrenals, pancreas, kidneys, and testes in the rear views of (a) the adult male hybrid phantom and (b) adult male stylized phantom. The skin and muscle are made transparent to better view the internal structure. The scan ranges of chest scan (from clavicle to the middle of liver) and abdomen/pelvis scan (from the top of liver to the midfemoral head) are indicated in both phantoms.
Figure 4c shows the organ dose comparison for abdomen∕pelvis examinations. The organ doses from the commercial programs were up to 7.6-fold (testes in CTDOSIMETRY) greater than the value from the hybrid phantom. The difference is attributed to the anatomical difference between the hybrid and the stylized phantoms. As shown in Fig. 5, the scan range for the abdomen∕pelvis study stops at the midfemoral neck which is higher than the level of the testes in the hybrid adult male phantom. However, the femoral head is at the similar level of the testes in the stylized phantom so the testes in CT-EXPO and CTDOSIMETRY received greater dose than the hybrid phantom in the abdomen∕pelvis scan.
The unrealistic anatomy in the stylized phantoms also caused the discrepancy in the scan lengths especially for the chest and abdomen∕pelvis scans between the stylized and the hybrid phantoms even when the identical anatomical landmarks were utilized to define scan coverage. The scan lengths used for the CT-Expo and CTDosimetry were about 1.3-fold greater than those used for the hybrid phantoms in chest and abdomen∕pelvis scans. The greater scan lengths may also contribute to the higher organ doses in the CT-EXPO and CTDOSIMETRY compared to the hybrid phantoms.
Another parameter causing the dose differences between our results and the commercial programs might be the scanner matching methods used by the programs. Both programs are using CTDI values to scale organ doses to match different CT scanner models. CT-EXPO and CTDOSIMETRY are using the weighted CTDI (CTDIw), defined as the summation of one-third of CTDIcenter and two-thirds of CTDIperiphery, to scale organ doses to match different CT scanner models. CTDIcenter and CTDIperiphery were measured for the tube potential of 120 kVp, current-time product of 100 mA s and 10 mm collimation width in the Siemens SOMATOM Sensation 16 CT scanner and then CTDIw data were obtained as 15.0 and 5.7 mGy for head and body phantoms, respectively, in this study. However, the CT-EXPO is using the CTDIw data of 21.3 and 8.8 mGy for the same CT scanner model at the same technical settings for head and body modes, respectively. CTDIw data in CTDOSIMETRY are 16.6 and 6.9 mGy for head and body phantoms, respectively, derived from the CTDI table included in the program, which in part explains the relatively greater doses in CTDosimetry than the results from this study.
Consequently, the organ doses obtained from CT-EXPO and CTDOSIMETRY were rescaled by using the ratio of the actual CTDIw measured in this study to the default CTDIw values used in the computer programs and were then compared to those in this study as shown in Fig. 6. The scaled organ doses from CT-EXPO and CTDOSIMETRY in the head scan agreed with the values from the hybrid phantom within 18% as shown in Fig. 6a except for the thyroid and the active marrow of which differences were not attributed to the differences in CTDIw data. Although overall dose differences between the hybrid phantom and the commercial programs decreased in chest scan as shown in Fig. 6b, the organs unrealistically located in the stylized phantom still show the dose difference greater than 800% (pancreas in CT-EXPO). As for abdomen∕pelvis scan in Fig. 6c, the dose differences shown in the Fig. 5c substantially decreased except for dose to the testes, which is still 500% (in CTDOSIMETRY) greater than the value from the hybrid phantom.
Figure 6.
Comparison of the organ doses from the hybrid adult male phantom and the ORNL stylized phantom with the scaled organ doses from the commercial programs, CT-EXPO and CTDOSIMETRY, for (a) head, (b) chest, and (c) abdomen∕pelvis examinations.
CONCLUSIONS
An organ dose estimation method and dose matrices for reference adult male and female undergoing CT examinations was developed by using Monte Carlo simulations of CT scanner coupled with the UF adult male and female hybrid phantoms. After multistep experimental validations of the simulated CT beams, 30×175 and 30×164 organ dose matrices for the adult male and female, respectively, were calculated for 30 organs∕tissues and 175 (adult male) and 164 (adult female) slices ranging from the top of head and the bottom of the phantoms for the tube potentials of 80, 100, and 120 kVp and the collimation width of 10 mm. Organ doses for head, chest, and abdomen∕pelvis examinations were calculated from the dose matrices and compared to the values from the commercial programs. This comparison indicated significant differences in estimated doses which can be attributed to two main reasons.
First, it is difficult to define a given scan range in the stylized phantom equivalent to real human anatomy because of the simplified organ shape and their unrealistic locations in the former phantom. Doses for the organs especially at the border of the scan range were very sensitive to the position of the organs with respect to the scan coverage, which was most evident in chest scan in this study. The hybrid phantoms, which have more realistic anatomy compared to the stylized phantom, can be assumed to have provided more accurate organ doses for a given scan coverage.
Second, variations in CTDIw might cause the organ dose difference when matching different CT scanner models. The CTDIw for measured in this study was 35% smaller than the value from CT-EXPO. However, this is not surprising because the significant variations in the CTDIw among different clinical sites have been reported with the same CT scanner model and scanning protocols.46, 47, 48 If the organ doses are scaled by the actual measurement of the CTDIw under a CT scanner of interest, it will substantially improve the accuracy of the resulting organ doses. However, the measurement is not feasible in most cases of the organ dose reconstruction for patients examined in the past (as in radiation epidemiology studies). The user should provide the appropriate CTDIw for the scanner of interest or should obtain survey values from the manufacturers while keeping in mind possible dose uncertainties.
The organ dose estimation method and dose matrices developed in this study will be extended to cover different technical parameters, CT scanner models, and various age groups. In this study, the organ doses for pitch other than unity must be derived by dividing the doses by a given pitch,49 which will be verified by comparing with explicit helical CT scan models in future studies. A library of phantoms with different body sizes which is feasible using hybrid phantoms also will be incorporated into the database to more individualize the organ dose calculation.50 The results of this study will be also compared to those from other computational human phantoms including ICRP reference adult voxel phantoms.27
References
- NCRP, “Ionizing radiation exposure of the population of the United States,” NCRP Report No. 160 (National Council on Radiation Protection and Measurement, Bethesda, MD, 2009).
- de Gonzalez A., Kim K., and Samet J., “Radiation-induced cancer risk from annual computed tomography for patients with cystic fibrosis,” Am. J. Respir. Crit. Care Med. 176, 970–973 (2007). 10.1164/rccm.200704-591OC [DOI] [PubMed] [Google Scholar]
- Brenner D. J. and Hall E. J., “Current concepts—Computed tomography—An increasing source of radiation exposure,” N. Engl. J. Med. 357, 2277–2284 (2007). 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- USFDA, “FDA unveils initiative to reduce unnecessary radiation exposure from medical imaging.” See http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm200085.htm.
- Chodick G., Kim K., Shwarz M., Horev G., Shalev V., and Ron E., “Radiation risks from pediatric computed tomography scanning,” Pediatr. Endocrinol. Rev. 7, 29–36 (2009). [PMC free article] [PubMed] [Google Scholar]
- Angel E., Wellnitz C. V., Goodsitt M. M., Yaghmai N., DeMarco J. J., Cagnon C. H., Sayre J. W., Cody D. D., Stevens D. M., Primak A. N., McCollough C. H., and McNitt-Gray M. F., “Radiation dose to the fetus for pregnant patients undergoing multidetector CT imaging: Monte Carlo simulations estimating fetal dose for a range of gestational age and patient size,” Radiology 249, 220–227 (2008). 10.1148/radiol.2491071665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel E., Yaghmai N., Jude C. M., DeMarco J. J., Cagnon C. H., Goldin J. G., McCollough C. H., Primak A. N., Cody D. D., Stevens D. M., and McNitt-Gray M. F., “Dose to radiosensitive organs during routine chest CT: Effects of tube current modulation,” AJR, Am. J. Roentgenol. 193, 1340–1345 (2009). 10.2214/AJR.09.2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel E., Yaghmai N., Jude C. M., Demarco J. J., Cagnon C. H., Goldin J. G., Primak A. N., Stevens D. M., Cody D. D., McCollough C. H., and McNitt-Gray M. F., “Monte Carlo simulations to assess the effects of tube current modulation on breast dose for multidetector CT,” Phys. Med. Biol. 54, 497–512 (2009). 10.1088/0031-9155/54/3/003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caon M., Bibbo G., and Pattison J., “A comparison of radiation dose measured in CT dosimetry phantoms with calculations using EGS4 and voxel-based computational models,” Phys. Med. Biol. 42, 219–229 (1997). 10.1088/0031-9155/42/1/014 [DOI] [PubMed] [Google Scholar]
- Caon M., Bibbo G., and Pattison J., “An EGS4-ready tomographic computational model of a 14-year-old female torso for calculating organ doses from CT examinations,” Phys. Med. Biol. 44, 2213–2225 (1999). 10.1088/0031-9155/44/9/309 [DOI] [PubMed] [Google Scholar]
- Caon M., Bibbo G., and Pattison J., “Monte Carlo calculated effective dose to teenage girls from computed tomography examinations,” Radiat. Prot. Dosim. 90, 445–448 (2000). [Google Scholar]
- Deak P., van Straten M., Shrimpton P. C., Zankl M., and Kalender W. A., “Validation of a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography,” Eur. Radiol. 18, 759–772 (2008). 10.1007/s00330-007-0815-7 [DOI] [PubMed] [Google Scholar]
- DeMarco J. J., Cagnon C. H., Cody D. D., Stevens D. M., McCollough C. H., O’Daniel J., and McNitt-Gray M. F., “A Monte Carlo based method to estimate radiation dose from multidetector CT (MDCT): Cylindrical and anthropomorphic phantoms,” Phys. Med. Biol. 50, 3989–4004 (2005). 10.1088/0031-9155/50/17/005 [DOI] [PubMed] [Google Scholar]
- DeMarco J. J., Cagnon C. H., Cody D. D., Stevens D. M., McCollough C. H., Zankl M., Angel E., and McNitt-Gray M. F., “Estimating radiation doses from multidetector CT using Monte Carlo simulations: Effects of different size voxelized patient models on magnitudes of organ and effective dose,” Phys. Med. Biol. 52, 2583–2597 (2007). 10.1088/0031-9155/52/9/017 [DOI] [PubMed] [Google Scholar]
- DeMarco J. J., Solberg T. D., and Smathers J. B., “A CT-based Monte Carlo simulation tool for dosimetry planning and analysis,” Med. Phys. 25, 1–11 (1998). 10.1118/1.598167 [DOI] [PubMed] [Google Scholar]
- Hausleiter J., Meyer T., Hadamitzky M., Huber E., Zankl M., Martinoff S., Kastrati A., and Schomig A., “Radiation dose estimates from cardiac multislice computed tomography in daily practice: Impact of different scanning protocols on effective dose estimates,” Circulation 113, 1305–1310 (2006). 10.1161/CIRCULATIONAHA.105.602490 [DOI] [PubMed] [Google Scholar]
- Jarry G., DeMarco J. J., Beifuss U., Cagnon C. H., and McNitt-Gray M. F., “A Monte Carlo-based method to estimate radiation dose from spiral CT: From phantom testing to patient-specific models,” Phys. Med. Biol. 48, 2645–2663 (2003). 10.1088/0031-9155/48/16/306 [DOI] [PubMed] [Google Scholar]
- Lee C., Lee C., Staton R. J., Hintenlang D. E., Arreola M. M., Williams J. L., and Bolch W. E., “Organ and effective doses in pediatric patients undergoing helical multislice computed tomography examination,” Med. Phys. 34, 1858–1873 (2007). 10.1118/1.2723885 [DOI] [PubMed] [Google Scholar]
- Lee C., Lodwick D., Williams J. L., and Bolch W. E., “Hybrid computational phantoms of the 15-year male and female adolescent: Applications to CT organ dosimetry for patients of variable morphometry,” Med. Phys. 35, 2366–2382 (2008). 10.1118/1.2912178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Gu J., Caracappa P. F., and Xu X. G., “Comparison of two types of adult phantoms in terms of organ doses from diagnostic CT procedures,” Phys. Med. Biol. 55, 1441–1451 (2010). 10.1088/0031-9155/55/5/012 [DOI] [PubMed] [Google Scholar]
- Staton R. J., Lee C., Lee C., Williams M. D., Hintenlang D. E., Arreola M. M., Williams J. L., and Bolch W. E., “Organ and effective doses in newborn patients during helical multislice computed tomography examination,” Phys. Med. Biol. 51, 5151–5166 (2006). 10.1088/0031-9155/51/20/005 [DOI] [PubMed] [Google Scholar]
- Turner A. C., Zhang D., Kim H. J., DeMarco J. J., Cagnon C. H., Angel E., Cody D. D., Stevens D. M., Primak A. N., McCollough C. H., and McNitt-Gray M. F., “A method to generate equivalent energy spectra and filtration models based on measurement for multidetector CT Monte Carlo dosimetry simulations,” Med. Phys. 36, 2154–2164 (2009). 10.1118/1.3117683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. and Britain G., Survey of CT practice in the UK, Part 3: Normalised Organ Doses Calculated Using the Monte Carlo Techniques (National Radiological Protection Board, Chilton, 1991). [Google Scholar]
- Zankl M., Panzer W., and Drexler G., The Calculation of Dose from External Photon Exposures Using Reference Human Phantoms and Monte Carlo Methods, Part VI: Organ Doses From Computed Tomographic Examinations (Gesellschaft für Strahlen und Umweltforschung mbH, Neuherberg, 1991). [Google Scholar]
- Zankl M., Panzer W., and Drexler G., Tomographic Anthropomorphic Models, Part II: Organ Doses From Computed Tomographic Examinations in Paediatric Radiology (Gesellschaft für Strahlen und Umweltforschung mbH, Neuherberg, 1993). [Google Scholar]
- Caon M., “Voxel-based computational models of real human anatomy: A review,” Radiat. Environ. Biophys. 42, 229–235 (2004). 10.1007/s00411-003-0221-8 [DOI] [PubMed] [Google Scholar]
- ICRP, Adult Reference Computational Phantoms, ICRP Publication 110 (Pergamon, Oxford, 2010). [Google Scholar]
- Zaidi H. and Xu X. G., “Computational anthropomorphic models of the human anatomy: The path to realistic Monte Carlo modeling in radiological sciences,” Annu. Rev. Biomed. Eng. 9, 471–500 (2007). 10.1146/annurev.bioeng.9.060906.151934 [DOI] [PubMed] [Google Scholar]
- Lee C., Lodwick D., Hasenauer D., Williams J. L., Lee C., and Bolch W. E., “Hybrid computational phantoms of the male and female newborn patient: NURBS-based whole-body models,” Phys. Med. Biol. 52, 3309–3333 (2007). 10.1088/0031-9155/52/12/001 [DOI] [PubMed] [Google Scholar]
- Lee C., Lodwick D., Hurtado J. L., Pafundi D. H., Williams J. L., and Bolch W. E., “The UF family of reference hybrid phantoms for computational radiation dosimetry,” Phys. Med. Biol. 55, 339–363 (2010). 10.1088/0031-9155/55/2/002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola V., de Melo Lima V., Kramer R., and Khoury H., “FASH and MASH: Female and male adult human phantoms based on polygon mesh surfaces: I. Development of the anatomy,” Phys. Med. Biol. 55, 133–162 (2010). 10.1088/0031-9155/55/1/009 [DOI] [PubMed] [Google Scholar]
- Xu X. G., Taranenko V., Zhang J., and Shi C., “A boundary-representation method for designing whole-body radiation dosimetry models: Pregnant females at the ends of three gestational periods—RPI-P3, -P6 and -P9,” Phys. Med. Biol. 52, 7023–7044 (2007). 10.1088/0031-9155/52/23/017 [DOI] [PubMed] [Google Scholar]
- Segars W., Sturgeon G., Mendonca S., Grimes J., and Tsui B., “4D XCAT phantom for multimodality imaging research,” Med. Phys. 37, 4902–4902 (2010). 10.1118/1.3480985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. G. and Eckerman K. F., Handbook of Anatomical Models for Radiation Dosimetry (Taylor & Francis, London, 2009). [Google Scholar]
- Pelowitz D. B., “MCNPX user’s manual version 2.6.0,” LANL Report No. LA-CP-05-0369 (Los Alamos National Laboratory, 2008).
- Cranley K., Gilmore B., Fogarty G., and Desponds L., “Catalogue of diagnostic x-ray spectra and other data,” IPEM Report No. 78 (The Institute of Physics and Engineering in Medicine, York, 1997).
- Shrimpton P. and Jones D., “Normalised organ doses for x ray computed tomography calculated using Monte Carlo techniques and a mathematical anthropomorphic phantom,” Radiat. Prot. Dosim. 49, 241–241 (1993). [Google Scholar]
- ICRP, Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values, ICRP Publication 89 (International Commission on Radiological Protection, Oxford, 2003). [Google Scholar]
- ICRU, “Photon, electron, proton and neutron interaction data for body tissues,” ICRU Report No. 46 (International Commission on Radiation Unit and Measurement, Bethesda, MD, 1992).
- ICRP, “Human alimentary tract model for radiological protection. ICRP Publication 100. A report of The International Commission on Radiological Protection,” Ann. ICRP 36, 25–327 (2006). 10.1016/j.icrp.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Stamm G. and Nagel H. D., “CT-expo—A novel program for dose evaluation in CT,” RoeFo, Fortschr. Geb. Roentgenstr. Nuklearmed. 174, 1570 (2002). 10.1055/s-2002-35937 [DOI] [PubMed] [Google Scholar]
- Johnson P., Bahadori A., Jokisch D., Rajon D., Lee C., and Bolch W., “Skeletal photon dose response functions: A comprehensive method for evaluating absorbed dose to active marrow and endosteum from photon irradiation,” Phys. Med. Biol. (submitted).
- Cristy M. and Eckerman K. F., “Specific absorbed fractions of energy at various ages from internal photon sources,” ORNL Report No. ORNL/TM-8381, Vols. 1–7 (Oak Ridge National Laboratory, Oak Ridge, TN, 1987).
- Chang K., Lee W., Choo D., Lee C., and Kim Y., “Evaluation of dose reduction for eye, thyroid, and breast using bismuth shielding during CT examinations,” Radiat. Prot. Dosim. 138, 382–388 (2010). 10.1093/rpd/ncp278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Lee J., and Lee C., “The effect of unrealistic thyroid vertical position on thyroid dose in the MIRD phantom,” Med. Phys. 31, 2038–2041 (2004). 10.1118/1.1764702 [DOI] [PubMed] [Google Scholar]
- Clarke J., Cranley K., Robinson J., Smith P., and Workman A., “Application of draft European Commission reference levels to a regional CT dose survey,” Br. J. Radiol. 73, 43–50 (2000). [DOI] [PubMed] [Google Scholar]
- Hiles P., Brennen S., Scott S., and Davies J., “A survey of patient dose and image quality for computed tomography scanners in Wales,” J. Radiol. Prot. 21, 345–354 (2001). 10.1088/0952-4746/21/4/302 [DOI] [PubMed] [Google Scholar]
- Koller C., Eatough J., and Bettridge A., “Variations in radiation dose between the same model of multislice CT scanner at different hospitals,” Br. J. Radiol. 76, 798–802 (2003). 10.1259/bjr/33117342 [DOI] [PubMed] [Google Scholar]
- Bushberg J., Seibert A., Leidholt E., and Boone J., The Essential Physics of Medical Imaging (Lippincott/Williams and Wilkins, New York/Baltimore, 2001). [Google Scholar]
- Johnson P. B., Whalen S. R., Wayson M., Juneja B., Lee C., and Bolch W. E., “Hybrid patient-dependent phantoms covering statistical distributions of body morphometry in the US adult and pediatric population,” Proc. IEEE 97, 2060–2075 (2009). 10.1109/JPROC.2009.2032855 [DOI] [Google Scholar]