Abstract
The regulation of body weight and composition is complex, simultaneously affected by genetic architecture, the environment, and their interactions. We sought to analyze the complex phenotypic relationships between voluntary exercise, food consumption, and changes in body weight and composition and simultaneously localize quantitative trait loci (QTL) controlling these traits. A large (n = 815) murine advanced intercross line (G4) was created from a reciprocal cross between a high-running line and the inbred strain C57BL/6J. Body weight and composition (% fat, % lean) were measured at 4, 6, and 8 wk of age. After measurements at 8 wk of age, mice were given access to running wheels, during which food consumption was quantified and after which body weight and composition were assessed to evaluate exercise-induced changes. Phenotypic correlations indicated that the relationship between exercise and overall change in weight and adiposity depended on body composition before the initiation of exercise. Interval mapping revealed QTL for body weight, % fat, and % lean at 4, 6, and 8 wk of age. Furthermore, QTL were observed for food consumption and changes in weight, % fat, and % lean in response to short-term exercise. Here we provide some clarity for the relationship between weight loss, reduction in adiposity, food consumption, and exercise. Simultaneously, we reinforce the genetic basis for body weight and composition with some independent loci controlling growth at different ages. Finally, we present unique QTL providing insight regarding variation in weight loss and reduction in adiposity in response to exercise.
Keywords: artificial selection, exercise physiology, quantitative trait loci, voluntary wheel running
the regulation of body weight and composition is complex, simultaneously affected by genetic architecture, the environment, and their interactions (38). Constancy of body weight and composition is maintained by a balance between energy intake and expenditure, and reductions in weight and alterations in composition are most commonly sought through elevated expenditure and/or reduced intake. Elevated energy expenditure is most commonly achieved through an increase in voluntary physical activity via general exercise participation (14, 42). Metabolically induced changes resulting from physical activity have been extensively characterized and include reduction in triglyceride and LDL levels, increased HDL, enhanced insulin sensitivity, weight loss, and reduced adiposity (33, 55). Although the evidence for these physiological modifications is consistent, changes resulting from exercise remain considerably variable within populations of both humans and rodents (18, 52), especially with regard to weight and adiposity.
Individual variation in human weight loss and reduction in adiposity, in response to voluntary exercise, has in part been attributed to the frequency, duration, and intensity of the activity engaged in (6, 9, 34), although results remain inconsistent (16, 17, 44). However, even when exercise doses and the resulting energy expenditure are tightly controlled, changes in weight remain variable (see Fig. 2 in Ref. 9). Another source of individual variation in weight loss and reduction in adiposity is the relationship between food intake and exercise. Some recent studies of humans (9, 22, 57) have indicated that increases in exercise are positively associated with increased food intake, which may potentially mitigate weight loss (see also review in Ref. 14). However, this association has been shown to be complex, with compensation varying with the intensity and duration of exercise, with sex (13), and among individuals (“compensators” and “noncompensators”) (2). Regardless, when energy expenditure and intake were both tightly controlled, Bouchard et al. (3) found that reductions in body weight still varied substantially, from 5% to 12% of initial body weight.
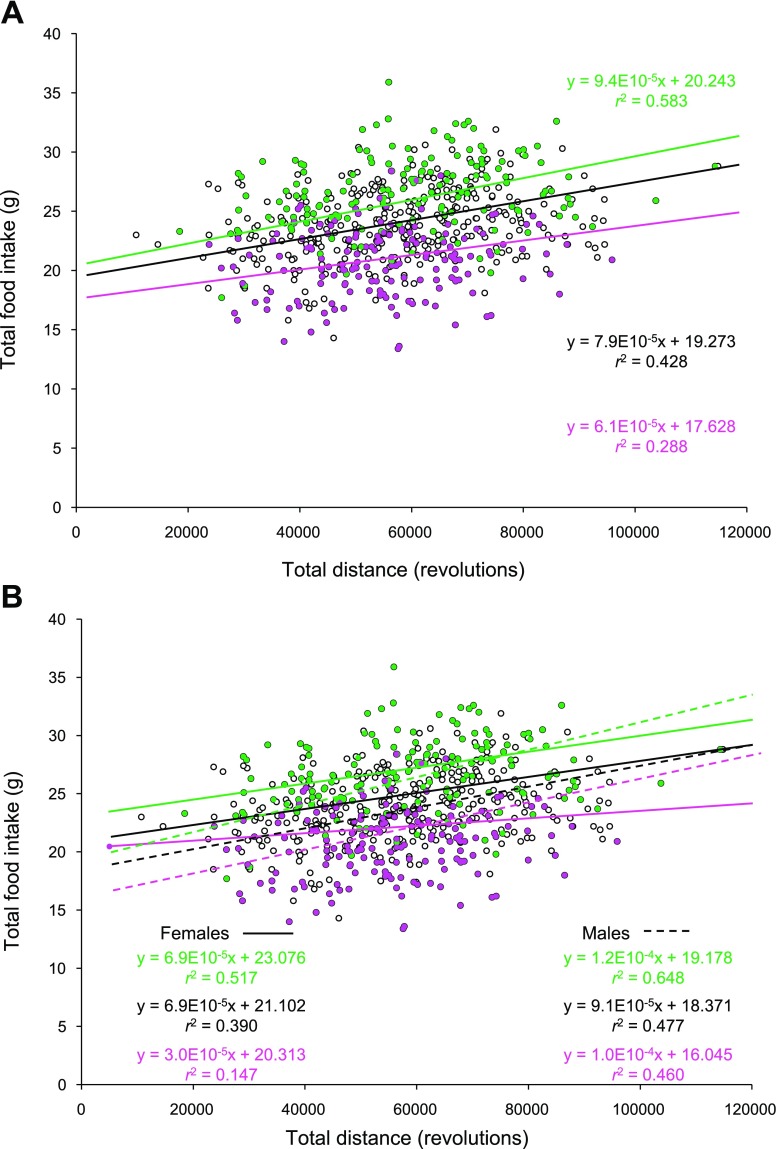
Fig. 2.
A: regression analyses between total food consumption and total running distance during the 6-day wheel exposure for the entire population (black), the 25% leanest Pre wheel access (green), and the 25% fattest Pre wheel access (pink). B: sex-specific results; the dashed regression lines characterize the females in each of 3 subpopulations, while the solid lines represent the males. Conditional slopes and r2 were controlled for sex, parent of origin, and mean fat and lean mass [(Pre exercise + Post exercise)/2]. Running wheel circumference was 1.1 m.
Although the genetic basis for physical activity and body weight continues to be characterized in mice (21, 37) and humans (12, 41), the importance of genetics in regulating the change in weight and adiposity in response to exercise is also becoming increasingly clear (4, 25, 30, 31). Previously, we generated a moderately (G4) advanced intercross line (AIL) originating from mice selectively bred for high voluntary wheel running and the inbred strain C57BL/6J (hereafter referred to as B6) (20). The random and sequential intercrossing over four generations resulted in a threefold expansion of the genetic map, providing increased quantitative trait locus (QTL) mapping resolution and reductions in confidence intervals relative to an F2 population. Utilizing this AIL, we identified several QTL for voluntary exercise traits, including daily wheel running (distance, duration, average speed, and maximum speed), running values averaged across days, and the running trajectory (slope and intercept) across 6 days of wheel access (21).
The high-runner (HR) line used to generate the AIL originated from a long-term, replicated artificial selection experiment for high voluntary wheel-running behavior on days 5 and 6 of a 6-day wheel exposure (50). In response to selection, HR mice have diverged from control lines with a 2.5- to 3.0-fold increase in revolutions per day. HR mice have been extensively characterized with regard to morphological, physiological, and behavioral alterations (reviewed in Refs. 43, 51). HR mice have increased home cage activity when housed without access to wheels (29), reduced body mass (53), less body fat (52), lower leptin levels (15), and increased levels of adiponectin (56).
In this report, we examine locations and magnitude of QTL controlling body weight and composition (% fat and % lean mass) in 4-, 6-, and 8-wk-old G4 mice. We also investigated potential QTL responsible for changes in body weight and composition in response to 6 days of wheel access, as well as for food consumption during exercise. Finally, we analyzed the complex phenotypic relationships between exercise (distance, duration, and intensity), food consumption, and changes in body weight and composition.
MATERIALS AND METHODS
Phenotypes.
A complete description of the creation, phenotyping, and single nucleotide polymorphism (SNP) marker genotyping of the G4 population has previously been provided (20, 21). Only methods relevant to specific phenotypes examined here and the corresponding statistical analyses are described below.
G4 mice (n = 815) were weighed (±0.1 g) and body composition (% fat tissue and % lean tissue) was assessed (EchoMRI-100, Echo Medical Systems, Houston, TX) at 4, 6, and 8 wk of age. After body mass and composition measurements at 8 wk of age, mice were individually housed with access to a running wheel (model 80850, circumference = 1.1 m; Lafayette Instruments, Lafayette, IN). Daily distance (total revolutions), time spent running (cumulative 1-min intervals in which at least 1 revolution was recorded), average speed (total revolutions/time spent running), and maximum speed (highest number of revolutions in any 1-min interval within a 24-h period) were recorded. After 6 days of wheel access, during which mice could voluntarily run or not, body mass and composition were immediately measured. Six days of wheel access was chosen, as opposed to a longer exposure, as it mimics the selection protocol under which the HR line was generated. At all time points (4 wk, 6 wk, 8 wk, and Post exercise), percent fat (and lean) was calculated as (fat mass/body mass) × 100. Percent change, in response to 6 days of voluntary wheel running, in body mass was calculated as [(Pre wheel mass − Post wheel mass)/Pre wheel mass] × 100. Percent change (after wheel access) in percent body fat (and lean) was calculated as [(% Post wheel access − % Pre wheel access)/% Pre wheel access] × 100. Additionally, food consumption during the 6-day exercise period was calculated by weighing (±0.1 g) food before and after wheel access. To account for wasting (24), cages were examined and food fragments weighed. All procedures were approved by and are in accordance with guidelines set forth by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Descriptive statistics.
Descriptive statistics for body mass, composition, and food consumption traits are presented in Table 1. Partial phenotypic correlations were performed in SAS (version 9.1; SAS Institute, Cary, NC) and are presented in Supplemental Tables S1 and S2.1 For simplicity, correlations were first performed for body weight and composition at 4, 6, and 8 (Pre exercise) wk of age (Supplemental Table S2). Next, correlations were performed among Pre (∼8 wk of age) and Post (∼9 wk of age) exercise traits (Supplemental Table S1). These values consisted of body weight and composition traits before and after exercise; the percent change in body weight, % fat, and % lean in response to exercise; and food consumption across the entire 6-day wheel access period. Correlations were adjusted for parent of origin [whether a G4 individual was descended from a progenitor (F0) cross of HR♀ × B6♂ or B6♀ × HR♂, coded as 1 or 0, respectively], sex, and parity (order of litters from individual F3 dams). Adjustments for multiple comparisons were performed in SAS with the false discovery rate procedure controlling the overall type I error rate at 5% (11).
Table 1.
Descriptive statistics for weight and body composition at different ages and in response to exercise
| Trait | Whole Population |
Females Only |
Males Only |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Range | n | Mean | SD | Range | n | Mean | SD | Range | |
| ∼4 wk of age | ||||||||||||
| Body mass, g | 796 | 20.6 | 2.9 | 12.2–29.0 | 395 | 18.7 | 1.8 | 13.6–23.9 | 401 | 22.4 | 2.6 | 12.2–29.0 |
| % Fat | 796 | 14.2 | 3.6 | 7.2–29.8 | 395 | 16.3 | 3.4 | 9.4–29.8 | 401 | 12.1 | 2.4 | 7.2–22.4 |
| % Lean | 796 | 82.5 | 3.9 | 66.8–91.1 | 395 | 80.7 | 3.9 | 66.8–91.1 | 401 | 84.3 | 2.9 | 68.4–90.6 |
| ∼6 wk of age | ||||||||||||
| Body mass, g | 793 | 24.1 | 3.9 | 15.0–35.4 | 395 | 21.1 | 2.1 | 15.6–27.0 | 398 | 27.2 | 2.7 | 15.0–35.4 |
| % Fat | 793 | 14.4 | 4.4 | 4.0–30.4 | 395 | 15.8 | 4.8 | 5.6–30.4 | 398 | 12.9 | 3.4 | 4.0–29.2 |
| % Lean | 792 | 79.9 | 4.3 | 62.8–97.0 | 395 | 79.2 | 4.8 | 62.8–90.1 | 397 | 80.5 | 3.5 | 65.6–97.0 |
| ∼8 wk of age | ||||||||||||
| Body mass, g | 797 | 26.0 | 4.7 | 16.3–39.3 | 395 | 22.2 | 2.4 | 16.3–31.4 | 402 | 29.8 | 3.1 | 17.1–39.3 |
| % Fat | 797 | 14.4 | 4.4 | 4.6–34.1 | 395 | 15.1 | 4.7 | 6.0–30.0 | 402 | 13.6 | 3.9 | 4.6–34.1 |
| % Lean | 797 | 78.7 | 4.3 | 59.9–97.2 | 395 | 78.8 | 4.7 | 63.3–97.2 | 402 | 78.6 | 3.9 | 59.9–88.3 |
| Post exercise | ||||||||||||
| Body mass, g | 797 | 24.5 | 3.9 | 14.1–34.9 | 395 | 21.2 | 1.8 | 14.1–27.3 | 402 | 27.8 | 2.4 | 18.3–34.9 |
| % Fat | 797 | 9.4 | 3.0 | 3.7–23.3 | 395 | 10.3 | 3.2 | 4.4–23.3 | 402 | 8.5 | 2.5 | 3.7–22.4 |
| % Lean | 797 | 83.9 | 3.2 | 70.5–91.3 | 395 | 84.0 | 3.6 | 71.6–91.2 | 402 | 83.8 | 2.9 | 70.5–91.3 |
| % Change in body mass | 797 | −5.3 | 5.2 | −22.9–23.6 | 395 | −4.4 | 5.7 | −22.9–23.6 | 402 | −6.3 | 4.5 | −18.1–10.0 |
| % Change in % fat | 797 | −32.2 | 19.4 | −69.1–88.3 | 395 | −29.1 | 20.4 | −69.1–58.0 | 402 | −35.3 | 17.7 | −66.6–88.3 |
| % Change in % lean | 797 | 6.8 | 5.2 | −17.2–26.3 | 395 | 6.8 | 5.9 | −17.2–26.3 | 402 | 6.8 | 4.4 | −9.7–20.5 |
| Food intake, g | 798 | 23.6 | 3.6 | 11.8–35.9 | 395 | 22.6 | 3.4 | 11.8–30.3 | 403 | 24.6 | 3.6 | 15.6–35.9 |
| Food intake/mass | 795 | 1.0 | 0.2 | 0.5–1.4 | 394 | 1.1 | 0.2 | 0.6–1.4 | 401 | 0.9 | 0.1 | 0.5–1.3 |
Beginning at 8 wk of age body composition measurements were taken immediately before (Pre) and after (Post) 6 days of wheel access. Food intake was quantified as the amount eaten over the entire 6-day access to running wheels, and values are presented as raw values and per gram of body mass [(Pre wheel access + Post wheel access)/2]. Percent body fat (and lean) was calculated as (fat mass/body mass) × 100. Percent change variables were calculated as [(Post − Pre)/Pre] × 100.
Phenotypic relationships.
First, we investigated the phenotypic relationships between exercise (distance, duration, and intensity), food consumption during exercise, and changes in body weight and composition as a result of exercise. Percent change in body mass, percent fat mass, and percent lean mass have previously been shown to depend on sex and parent of origin (20). Here, utilizing paired t-tests, we examined the effects of exercise on changes in weight, adiposity, and lean mass. Second, utilizing multiple regression analyses, we examined the effects of exercise on food consumption and body composition at the level of the individual. In other words, did the farther, longer, or faster an individual ran influence how much it ate or the response to change in weight, adiposity, and lean mass? The conditional slopes and partial correlations resulting from regression analyses between body composition and exercise traits were controlled for sex, parent of origin, and food consumption by use of multiple regressions. The conditional slopes and partial correlations resulting from regression analyses between total food consumption and total running distance were controlled for sex, parent of origin, and mean fat and lean mass [(Pre exercise + Post exercise)/2]. We initially performed these analyses for the entire G4 population, and subsequently, to better approximate human populations with variable adiposity, we subdivided the G4 population into the 25% leanest (n = 199) and 25% fattest (n = 199) mice, based on Pre exercise body composition measurements, and performed regressions on these two groups independently. The mean (±SD) percent body fat in the subdivided groups was 7.2 ± 1.0% (range = 4.6–8.3%, n = 199) for the 25% leanest and 20.1 ± 3.1% (range = 16.7–34.1%, n = 199) for the 25% fattest. Additionally, because running and body composition traits have been previously been shown to depend on sex in this mapping population (20), we performed an identical set of regression analyses on each sex separately.
A univariate GLM ANOVA (SPSS v18, Chicago, IL) was performed for food consumption and each of the body composition traits to identify statistical differences in the conditional slopes between the three groups (entire population, leanest 25%, fattest 25%). In addition to the interaction term, the models consisted of the same factors as the multiple regression analyses described above with the exception of the total food consumption ANOVA, for which mean fat was excluded. If a significant interaction was observed, then a post hoc analysis of the leanest and fattest groups was also performed. To examine whether the conditional slopes of the three groups differed as a function of sex, an additional three-way interaction term was added to the ANOVA model.
Genetic architecture.
In total, we evaluated 18 quantitative traits related to body mass, composition, and food consumption. QTL analyses were performed within R/qtl (5), for the R environment (v. 2.8.1) (40), with the multiple imputation method (47). The final set of SNPs (n = 530, with an average spacing of 4.7 Mb) used for QTL analyses is provided elsewhere (21). Statistical models included parent of origin type, sex, and parity, with the exception of food consumption, where parity was excluded because these factors have known effects on the variables of interest. The X chromosome was treated as an autosome since the statistical model assumes an F2 population and requires the identity of the parental grandmother (coded as 0, 1).
Appropriate locus-specific P values and genomewide significance thresholds, given the multigeneration breeding protocol and resulting G4 family structure, were obtained by the Genome Reshuffling for Advanced Intercross Permutation (GRAIP) procedure. This statistical method, and accompanying statistical software, has been detailed previously (35, 36). Modifications to the GRAIP procedure, specific to this G4 population, have also been described elsewhere (21). In brief, parental (F3) genotypes were estimated and a permutation scheme was used to simulate sets of F3 progenitors. From these progenitor sets, simulated recombination and inheritance was used to create “randomized” G4 populations (n = 50,000) that respected the original family structure while removing any association between genotype and phenotype. QTL analyses, as described above, were then performed with the original population and the GRAIP-permuted populations.
Locus-specific P values were calculated by utilizing the output from R/qtl as previously described (21, 35, 36). It is important to note that genomewide adjusted significance thresholds were generated with 50,000 permutations, and thus for the GRAIP output a minimum possible P value with 50,000 permutations is 0.00002 (1/50,000) with a corresponding maximum −log P of 4.7. Loci that met or exceeded 95th (P ≥ 0.05) and 90th (P ≥ 0.1) percentiles were deemed significant and suggestive, respectively. Approximate confidence intervals (90–95%) were determined by one logarithm of odds (LOD)-drop intervals (Mb) relative to the GRAIP-permuted LOD score. The percent variation and additive [a = (μBB − μAA)/2] and dominance [d = μAB − (μAA + μBB)/2] effects of each QTL were estimated in R/qtl.
In additional analyses, to test for possible covariate interactions with a QTL (i.e., the effect of the QTL varying with the covariate), we included QTL × group in the model. These additional analyses were performed for percent change in body mass, percent fat, and percent lean with group representing preexercise subpopulations: 25% leanest, 50% middle, and 25% fattest (coded as 0, 1, 2, respectively). Significant interactions were identified when LODFull − LODAdditive = LODInteraction ≥ 3.0 (47).
RESULTS
Phenotypic relationships.
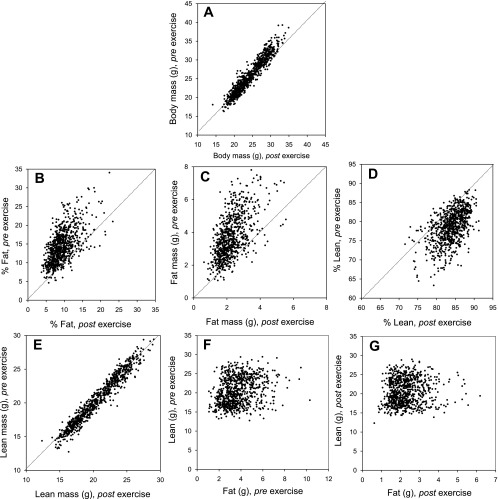
Body mass, percent fat, and percent lean measures were significantly correlated before and after wheel access (Supplemental Table S1), and paired t-tests revealed exercise in general reduced body mass (P < 0.001), reduced % body fat (P < 0.001), and increased % lean mass (P < 0.0001) (Fig. 1).
Fig. 1.
Body composition measures before (Pre) and after (Post) 6 days of voluntary wheel running. A: body mass (g). B: % fat. C: fat mass (g). D: % lean. E: lean mass (g). F: lean vs. fat (Pre exercise). G: lean vs. fat (Post exercise). The dotted line represents a 1-to-1 relation and demonstrates that 6 days of wheel access consistently reduced body mass (paired t-test, P < 0.001), reduced % body fat (paired t-test, P < 0.001), and increased % lean mass (paired t-test, P < 0.0001).
When the entire population was examined, total food consumption was significantly correlated with total running distance (cumulative across all 6 days) after controlling for parent of origin, sex, and mean fat and lean mass [(Pre exercise + Post exercise)/2] (r2 = 0.428, P < 0.0001). Additionally, the conditional slope indicated that for a 1-revolution increase in running there was a corresponding 0.000079-g increase in food consumption with an intercept of 19.273. When only the 25% leanest animals (defined by Pre exercise measures) were examined, regression analyses revealed a higher partial correlation (r2 = 0.583, P < 0.0001), slope (for 1-revolution increase, 0.000094-g increase in food consumption), and intercept compared with the entire population (above) or the 25% fattest individuals (r2 = 0.288, P < 0.0001, slope = 0.000061) (Fig. 2A). An ANOVA comparing the slopes of all three groups revealed that the group-by-total revolutions interaction was not significant (P = 0.541). However, the interaction between sex and total revolutions was significant (P = 0.013), and the three-way interaction term between group, sex, and total revolutions approached statistical significance (P = 0.082). These results indicate that the cost of running (as revealed by the relationship between total food consumption and total running distance) is significantly different between sexes and varies substantially by sex even among the leanest and fattest segments of this population of mice (Fig. 2B).
When the entire population was examined, controlling for parent of origin, sex, and food consumption, increasing running distance was associated with significant reductions in body mass (r2 = −0.222, P < 0.0001; Fig. 3A) and adiposity (r2 = −0.203, P < 0.0001; Fig. 4A). Increases in running duration were significantly associated with reductions in body mass (r2 = −0.274, P < 0.0001; Fig. 3B) and adiposity (r2 = −0.320, P < 0.0001; Fig. 4B). Conversely, increasing running speeds were not significantly associated with changes in mass (r2 = −0.070, P = 0.053; Fig. 3C) or adiposity (r2 = 0.021, P = 0.563; Fig. 4C).
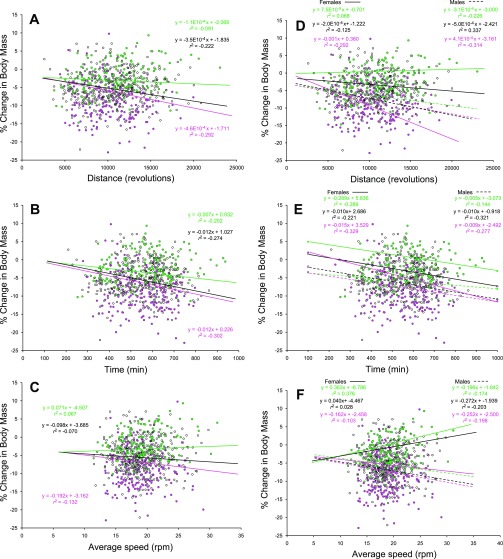
Fig. 3.
Regression analyses between % change in body mass [(Post − Pre)/Pre] × 100 and exercise. Values for the entire population (black), the leanest 25% (green), and the fattest 25% (pink) (based on Pre exercise values) are shown. Exercise traits were mean running distance (revolutions/day), duration (i.e., cumulative 1-min intervals in which at least 1 revolution was recorded), and average speed (total revolutions/time spent running) on days 5 and 6 of a 6-day test. In A–C the sexes are pooled in each of the 3 groups, while in D–F the regression results are sex specific (dashed line denotes females, solid line denotes males). Conditional slopes and r2 were controlled for sex, parent of origin, and food consumption. Individuals with a positive % change gained mass as a result of 6 days of wheel access. Running wheel circumference was 1.1 m.
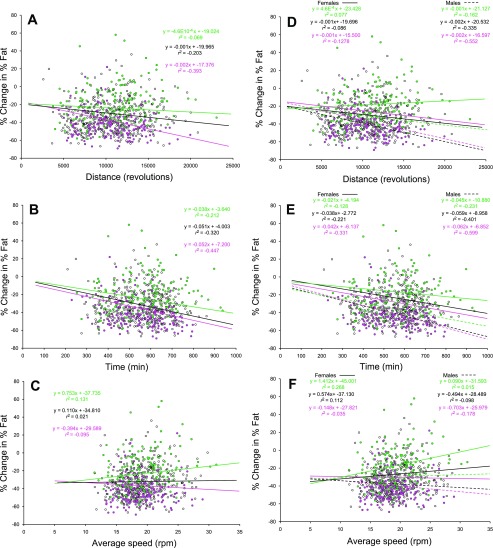
Fig. 4.
Regression analyses between % change [(Post − Pre)/Pre] × 100 in % fat mass and mean running traits on days 5 and 6 of a 6-day test. Running distance (revolutions/day), duration (i.e., cumulative 1-min intervals in which at least 1 revolution was recorded), and average speed (total revolutions/time spent running) for the entire population (black), the leanest 25% (green), and the fattest 25% (pink) are shown. In A–C the sexes are pooled in each of the 3 groups, while in D–F the regression results are sex specific (dashed line denotes females, solid line denotes males). Conditional slopes and r2 were controlled for sex, parent of origin, and food consumption. Individuals with a positive % change in % mass increased adiposity as a result of 6 days of wheel access. Running wheel circumference was 1.1 m.
After the population was subdivided, increasing running distance was correlated with a general overall reduction in body mass (r2 = −0.292, P < 0.0001) and adiposity (r2 = −0.393, P < 0.0001) for the fattest 25% of individuals, while for the leanest 25% there was no relationship with changes in body (r2 = −0.091, P = 0.214) or fat (r2 = −0.069, P = 0.346) mass (Figs. 3A, 4A). An ANOVA comparing the slopes of all three groups revealed that the slopes did not differ statistically for changes in mass (P = 0.362) or adiposity (P = 0.123). However, an additional three-way interaction term (group × sex × running distance) was significant for both percent change in body mass (P < 0.001) and percent change in percent fat (P = 0.001). These significant three-way interaction terms indicate that the relationships between changes in body mass and body fat in relation to running distance are dependent on sex and adiposity (measured immediately before wheel running) (Figs. 3D, 4D).
Similar correlations were observed for increased running duration and reductions in mass (r2 = −0.302, P < 0.0001) and adiposity (r2 = −0.447, P < 0.0001; Fig. 4B) for the fattest 25% of individuals, with more modest effects for body (r2 = −0.202, P = 0.005) or fat (r2 = −0.212, P = 0.003) mass for the leanest 25% of individuals (Figs. 3B, 4B). Again, an ANOVA revealed significant three-way interactions (P < 0.001), indicating that the relationship between changes in body mass and body fat in relation to running duration was partly a function of sex and adiposity (measured before wheel running) (Figs. 3E, 4E).
Higher average running speeds were positively, but not significantly, correlated with an overall increase in mass (r2 = 0.067, P = 0.357) and adiposity (r2 = 0.131, P = 0.072) among the 25% leanest individuals, while the reverse was generally true among the fattest 25% of the population for either body (r2 = −0.132, P = 0.074) or fat (r2 = −0.095, P = 0.199) mass (Figs. 3C, 4C). Similar to the results reported above, an ANOVA revealed significant three-way interactions (P < 0.001) indicating that the relationship between change in body mass and body fat in relation to running speed was partly a function of sex and adiposity (measured before wheel running) (Figs. 3F, 4F).
Genetic architecture.
Results for all significant or suggestive GRAIP-adjusted QTL are presented in Table 2. Given the relatively short number of intercrosses (G4 as opposed to G20), it is possible that the GRAIP-adjusted LOD scores are overly conservative for our population, and we thus additionally present, but do not concentrate on, the naive or unadjusted significant LOD scores from the simple mapping output (Supplemental Table S3).
Table 2.
QTL detected and respective statistics for body composition traits and food consumption
| Trait | Nearest Marker | MMU | Peak Position, Mb | Naive LOD | GRAIP LOD | CI, Mb | % Var | Additive ± SE | Dominance ± SE |
|---|---|---|---|---|---|---|---|---|---|
| ∼4 wk of age | |||||||||
| Body mass, g | JAX00005495 | 1 | 77 | 7.7 | 3.7 | 74–82 | 1.9 | 0.54 ± 0.15 | 0.41 ± 0.21 |
| JAX00155508 | 7 | 109 | 8.0 | 4.2* | 95–113 | 3.2 | 0.59 ± 0.14† | 0.49 ± 0.21 | |
| % Fat | JAX00005495 | 1 | 77 | 17.5 | 4.7* | 75–79‡ | 6.7 | 1.30 ± 0.17† | 0.25 ± 0.25 |
| JAX00010715 | 1 | 148 | 16.3 | 4.7* | 144–150‡ | 5.6 | 1.06 ± 0.16† | −0.52 ± 0.25 | |
| JAX00107680 | 3 | 56 | 9.5 | 4.7* | 40–59 | 5.9 | −1.22 ± 0.17† | −0.06 ± 0.26 | |
| JAX00154099 | 7 | 90 | 7.9 | 3.6 | 75–99 | 2.7 | −0.41 ± 0.17 | 1.03 ± 0.26 | |
| JAX00190351 | 8 | 89 | 20.4 | 4.7* | 82–93‡ | 8.8 | 1.39 ± 0.17† | −0.81 ± 0.24 | |
| JAX00033353 | 12 | 12 | 10.4 | 4.2* | −22 | 3.7 | −0.96 ± 0.17† | 0.16 ± 0.25 | |
| % Lean | JAX00005495 | 1 | 77 | 12.1 | 4.7* | 74–91 | 5.2 | −1.24 ± 0.19† | −0.34 ± 0.27 |
| JAX00010715 | 1 | 148 | 16.1 | 4.7* | 134–172 | 6.0 | −1.12 ± 0.17† | −0.80 ± 0.27 | |
| JAX00107680 | 3 | 56 | 8.1 | 4.0* | 39–59 | 6.8 | 1.37 ± 0.19† | −0.17 ± 0.27 | |
| JAX00675742 | 8 | 92 | 22.0 | 4.7* | 83–95‡ | 10.0 | −1.66 ± 0.18† | 0.66 ± 0.26 | |
| ∼6 wk of age | |||||||||
| Body mass, g | JAX00139789 | 6 | 36 | 10.2 | 4.1* | 25–43 | 1.5 | 0.63 ± 0.18 | 0.004 ± 0.278 |
| % Fat | JAX00005495 | 1 | 77 | 6.9 | 3.9* | 74–89 | 3.4 | 1.12 ± 0.22† | 0.09 ± 0.31 |
| JAX00010715 | 1 | 149 | 7.6 | 4.5* | 146–152 | 3.2 | 1.01 ± 0.20† | −0.27 ± 0.31 | |
| JAX00675742 | 8 | 92 | 15.5 | 4.7* | 86–94‡ | 8.9 | 1.78 ± 0.21† | −0.68 ± 0.31 | |
| % Lean | JAX00010715 | 1 | 149 | 6.2 | 3.5 | 143–158 | 2.6 | −0.85 ± 0.20 | 0.59 ± 0.32 |
| JAX00675742 | 8 | 92 | 12.3 | 4.7* | 68–99 | 8.3 | −1.76 ± 0.21† | 0.48 ± 0.31 | |
| JAX00391461 | 14 | 120 | 5.3 | 3.5 | 109– | 3.0 | 1.07 ± 0.22† | −0.53 ± 0.31 | |
| ∼8 wk of age | |||||||||
| Body mass, g | JAX00263199 | 1 | 116 | 6.9 | 3.5 | 95–141 | 1.1 | 0.65 ± 0.22 | 0.37 ± 0.33 |
| JAX00127022 | 5 | 11 | 9.4 | 4.7* | −16 | 1.8 | −0.62 ± 0.24 | −0.37 ± 0.33 | |
| JAX00139789 | 6 | 36 | 10.6 | 4.7* | 25–40 | 1.0 | 0.63 ± 0.22 | −0.08 ± 0.33 | |
| JAX00415862 | 16 | 24 | 7.3 | 3.5 | 11–28 | 1.5 | −0.73 ± 0.23 | 0.33 ± 0.33 | |
| % Fat | JAX00160567 | 8 | 37 | 10.7 | 4.7* | 21–52 | 6.1 | 1.56 ± 0.22† | −0.17 ± 0.30 |
| JAX00695061 | 9 | 57 | 6.0 | 3.6 | 50–70 | 2.1 | 0.90 ± 0.22 | 0.38 ± 0.32 | |
| % Lean | JAX00160567 | 8 | 37 | 9.9 | 4.7* | 27–49 | 6.3 | −1.54 ± 0.21† | −0.04 ± 0.30 |
| JAX00190133 | 7 | 80 | 5.9 | 3.6 | 74–87 | 5.2 | 0.75 ± 0.21 | −1.7 ± 0.30† | |
| Post exercise | |||||||||
| Body mass, g | JAX00247128 | 1 | 39 | 8.6 | 4.6* | 35–42 | 1.3 | 0.48 ± 0.20 | −0.60 ± 0.28 |
| JAX00261568 | 1 | 106 | 8.4 | 4.2* | 89–141 | 1.0 | 0.52 ± 0.19 | 0.21 ± 0.28 | |
| JAX00127022 | 5 | 1 | 9.5 | 4.4* | −19 | 0.8 | −0.46 ± 0.20 | −0.23 ± 0.28 | |
| JAX00139789 | 6 | 36 | 8.1 | 3.5 | 24–41 | 0.5 | 0.37 ± 0.19 | 0.02 ± 0.28 | |
| % Fat | JAX00011133 | 1 | 155 | 11.8 | 4.7* | 130–183 | 4.7 | 0.90 ± 0.15† | −0.08 ± 0.21 |
| JAX00105078 | 3 | 21 | 6.8 | 3.7 | 15–25 | 3.6 | −0.09 ± 0.14 | 1.13 ± 0.21† | |
| JAX00131820 | 5 | 77 | 8.0 | 4.5* | 73–91 | 3.7 | −0.82 ± 0.15† | −0.21 ± 0.21 | |
| JAX00190133 | 7 | 80 | 7.2 | 4.4* | 77–83 | 5.6 | −0.56 ± 0.15 | 1.23 ± 0.21† | |
| JAX00675742 | 8 | 93 | 10.4 | 4.7* | 64–96 | 6.6 | 1.06 ± 0.15† | −0.35 ± 0.21 | |
| JAX00391461 | 14 | 120 | 7.7 | 4.7* | 78– | 3.8 | −0.82 ± 0.15† | 0.37 ± 0.21 | |
| % Lean | JAX00268776 | 1 | 140 | 10.3 | 4.7* | 131–170 | 4.3 | −0.90 ± 0.16† | 0.16 ± 0.23 |
| JAX00131820 | 5 | 77 | 9.0 | 4.7* | 67–92 | 5.4 | 1.02 ± 0.16† | 0.47 ± 0.22 | |
| JAX00190133 | 7 | 80 | 7.2 | 4.7* | 77–82 | 6.5 | 0.64 ± 0.16 | −1.43 ± 0.22† | |
| JAX00675742 | 8 | 93 | 12.4 | 4.7* | 65–97 | 8.6 | −1.31 ± 0.15† | 0.41 ± 0.23 | |
| JAX00391461 | 14 | 120 | 7.4 | 4.7* | 104– | 4.4 | 0.96 ± 0.16† | −0.30 ± 0.23 | |
| % Change in body mass | JAX00031382 | 11 | 105 | 4.6 | 3.5 | 102–110 | 2.3 | −1.17 ± 0.27† | −0.06 ± 0.37 |
| % Change in % fat | JAX00277411 | 1 | 178 | 5.5 | 3.8 | 170–183 | 3.2 | 4.56 ± 0.93† | 2.03 ± 1.36 |
| JAX00132785 | 5 | 90 | 8.5 | 4.7* | 48–100 | 4.1 | −5.35 ± 0.96† | −2.27 ± 1.34 | |
| % Change in % lean | JAX00133006 | 5 | 93 | 6.0 | 4.1* | 71–103 | 3.5 | 1.47 ± 0.27† | 0.20 ± 0.37 |
| Food intake, g | JAX00155961 | 7 | 115 | 10.8 | 4.7 | 112–117‡ | 7.8 | 1.4 ± 0.17† | 0.002 ± 0.25 |
| Food intake/mass | JAX00612506 | 6 | 67 | 6.4 | 4.6* | 59–73 | 4.0 | −0.04 ± 0.01† | −0.05 ± 0.01 |
| JAX00645408 | 7 | 83 | 6.7 | 4.2* | 78–92 | 3.4 | 0.03 ± 0.01 | −0.05 ± 0.01† | |
| Energy balance | JAX00160567 | 8 | 37 | 5.4 | 4.1* | 31–44 | 4.4 | −5.94 ± 1.12† | −0.33 ± 1.57 |
Beginning at 8 wk of age body composition measures were taken immediately before (Pre) and after (Post) 6 days of wheel access. Food consumption was quantified as the amount eaten over the entire 6-day access to running wheels, and values are presented per gram of body mass [(Pre wheel access + Post wheel access)/2]. Percent body fat (and lean) was calculated as (fat mass/body mass) × 100. Percent change variables were calculated as [(Post − Pre)/Pre] × 100. Logarithm of odds (LOD) exceeding the 95% (P ≤ 0.05, LOD ≥3.9) permutation threshold are denoted by
; other quantitative trait loci (QTL) exceeded the 90% (P ≤ 0.1, LOD ≥3.5) threshold. Confidence intervals (CIs) for QTL positions were obtained with a 1.0-LOD drop in Mb. CIs are relative to the Genome Reshuffling for Advanced Intercross Permutation (GRAIP)-permuted LOD score with the exception of those denoted by
, which are relative to the naive LOD score. Percentage of phenotypic variance accounted for by the QTL effect: for additive and dominance effects, positive values indicate increasing effect of the high runner (HR) allele or increasing effect of the heterozygote, respectively.
Additive and dominance effects were statistically significant at P < 0.05. Body mass QTL at ∼8 wk of age have been previously published in Kelly et al. (21) and are simply reproduced here for completeness.
At 4 wk of age, GRAIP-adjusted mapping revealed 10 significant (P ≤ 0.05, LOD ≥ 3.9) and 2 suggestive (P ≤ 0.1, LOD ≥ 3.5) QTL representing body mass, percent fat mass, and percent lean mass. Body mass QTL individually accounted for 1.9% (MMU1) and 3.2% (MMU7) of the total phenotypic variation. Both mass QTL had increasing effects resulting from the HR allele, with relatively large average additive (significant for QTL on MMU7) and dominance effects. QTL observed for percent fat mass at 4 wk of age individually accounted for 3.7–8.8% of the total phenotypic variation, and increasing effects as a result of the HR and B6 alleles varied depending on the locus. Average additive effects were frequently significant, and dominance effects were especially large for loci observed on MMU7. Results for percent lean mass at 4 wk of age were similar to those observed for percent fat with QTL colocalizing on MMU1, MMU3, and MMU8. Allelic effects for these colocalizing QTL were in the opposite direction of those observed for percent fat mass, and significant additive effect were always observed.
At 6 wk of age, we observed five significant and two suggestive QTL (GRAIP adjusted) representing body mass and composition traits. For body mass a QTL, accounting for 1.45% of the phenotypic variation, was observed on MMU6 with increasing values resulting from the HR allele. Average additive effects were relatively large, while dominance effects were small. This body mass QTL was independent of the loci observed at 4 wk of age. QTL observed for percent fat and lean mass on MMU1 and MMU8 overlapped with those observed at 4 wk of age with similar allelic, additive, and dominance effects. An additional QTL for percent lean mass was observed on MMU14, with increasing effects resulting from the HR allele.
At 8 wk of age (Pre exercise), we identified, in total, four significant and four suggestive QTL for body, percent fat, and percent lean mass. With the exception of the loci identified on MMU6, the QTL for body mass did not colocalize with those observed at 4 and 6 wk of age and collectively accounted for 5.4% of the total phenotypic variation. Additive and dominance effects both played a substantial role, with allelic effects varying depending on the loci. One significant (MMU8) and 1 suggestive (MMU9) QTL were observed for percent fat, and, again, these QTL did not colocalize with those observed at 4 or 6 wk of age. The percent fat QTL, collectively, accounted for 8.2% of the phenotypic variation, with large additive effects (significance noted for the QTL on MMU8) and smaller dominance effects.
After 6 days of exercise (Post), QTL were identified representing body mass, percent fat, and percent lean, as well as percent change (in mass, % fat, % lean) and additionally food consumption. Post exercise body mass QTL largely colocalized with those identified at 8 wk of age (Pre exercise). One suggestive QTL, on MMU16, was observed for Pre exercise body mass but not for Post exercise body mass. Collectively, the Post exercise body mass QTL explained 3.6% of the total phenotypic variation, a smaller percentage than was observed at 8 wk of age (Pre exercise). Allelic effects varied among the Post exercise body mass QTL, and additive and dominance effects were reasonably consistent and large.
Analysis of Post exercise percent fat revealed five significant (MMU1, 5, 7, 8, 14) and one suggestive (MMU3) QTL. QTL collectively accounted for 28% of the phenotypic variation in percent fat. Allelic effects depended on the locus, average additive effects remained important, and dominance effects had particularly highly significant values for QTL on MMU3 and MMU7 (both for Post exercise fat percentage). QTL identified after exercise did not colocalize with those identified at 8 wk of age (Pre exercise) and collectively explained a much larger percentage of the phenotypic variation.
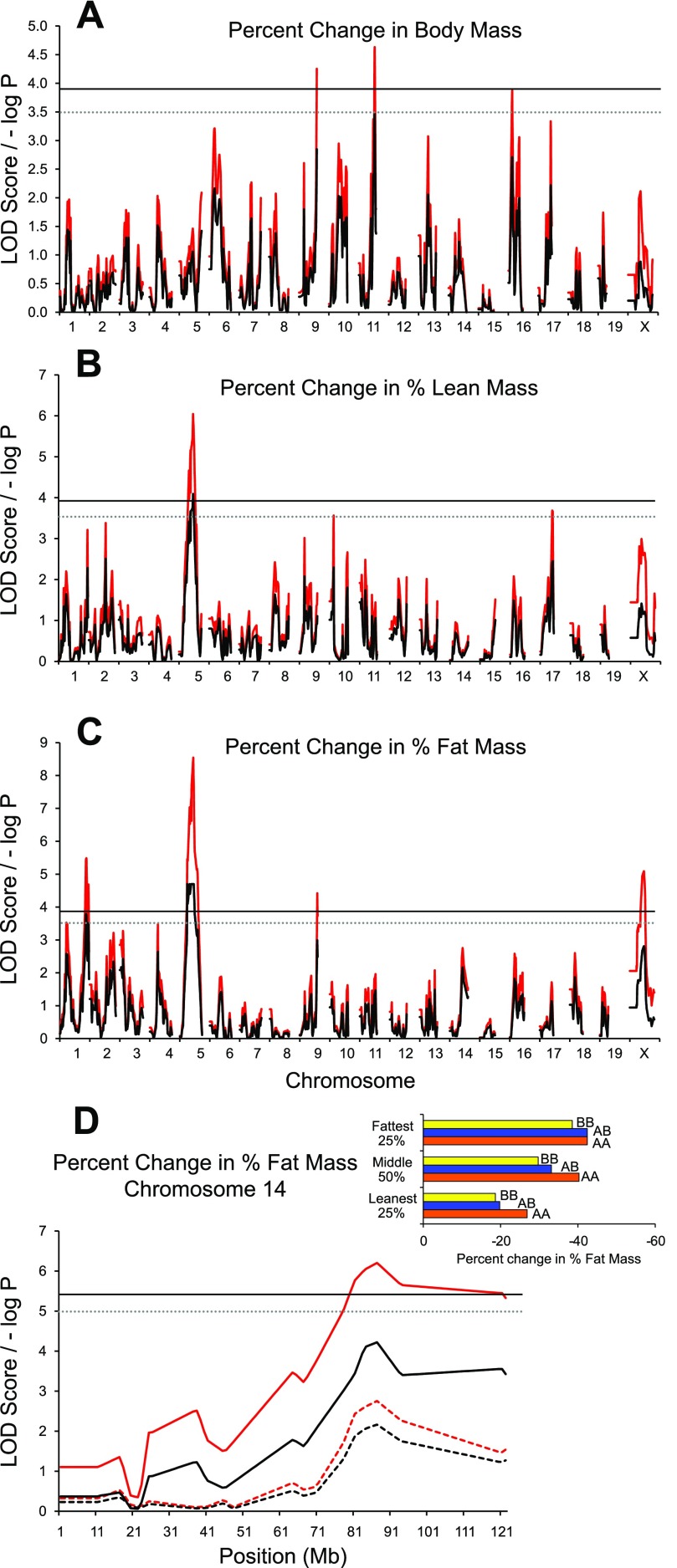
Analysis of the percent change in body mass as a result of 6 days of voluntary wheel running revealed a QTL on MMU11 with a naive LOD score of 4.6 and a GRAIP-adjusted LOD score of 3.5 (Fig. 5A). This QTL accounted for 2.3% of the phenotypic variation and had increasing effects resulting from the B6 allele, and average additive effects were large with little dominance. QTL observed for the percent change in percent fat as a result of 6 days of wheel running were identified on MMU1 and MMU5 (Fig. 5C). Both of these loci colocalized with those observed for Post exercise percent fat. These QTL accounted for 3.2% and 4.1% of the total phenotypic variation, and HR alleles had both positive and negative additive effects depending upon the locus. Additional analyses examining QTL × group interactions revealed a significant QTL for percent change in percent fat on MMU14 (86 Mb) (Fig. 5C). This QTL had an effect in all groups except the 25% fattest individuals in the population.
Fig. 5.
G4 quantitative trait locus (QTL) maps of % change [(Post − Pre)/Pre] × 100 in body composition following exercise. A: body mass. B: % lean mass. C: % fat mass as a result of 6 days of running wheel exposure. Red traces are the simple mapping output, and black traces are Genome Reshuffling for Advanced Intercross Permutation (GRAIP) permutation output. The solid and dotted lines represent the permuted 95% [logarithm of odds (LOD) ≥ 3.9, P ≤ 0.05] and 90% (LOD ≥ 3.5, P ≤ 0.1) LOD thresholds, respectively. D: follow-up analysis of results illustrated in C, depicting a QTL × group (Pre exercise; leanest 25%, middle 50%, fattest 25%) interaction. Dashed lines represent analysis without interaction term in the model.
Large QTL congruencies were generally observed between the percent body fat and percent lean mass traits. These congruencies are in accordance with the negative correlations between these sets of traits (Supplemental Tables S1, S2). Allelic effects for these congruent QTL were generally in opposing directions. Notably, for percent change in percent lean mass only the QTL on MMU5 was shared with percent change in percent fat mass (Fig. 5B).
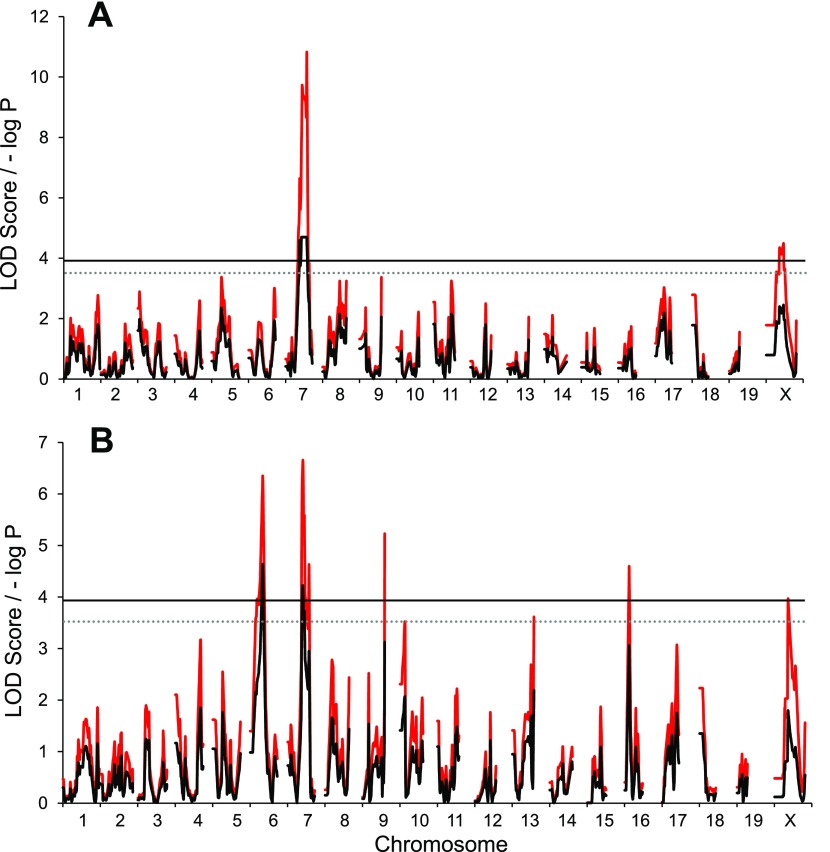
For food consumption, we identified a QTL on MMU7 that colocalized with previously (21) identified running distance QTL on MMU7 (Fig. 6A). Alternatively, we mapped food consumption (on a per gram basis) and observed significant QTL on MMU6 and MMU7 (Fig. 6B). The QTL on MMU7 colocalized with loci on MMU7 for Post exercise percent fat and percent lean and was reasonably close to previously identified running QTL (21).
Fig. 6.
G4 QTL map of food consumption independent of body mass (A) and per gram of body mass (B) for the entire 6-day wheel access period. Red traces are the simple mapping output, and black traces are GRAIP permutation output. Solid and dotted lines represent the permuted 95% (LOD ≥ 3.9, P ≤ 0.05) and 90% (LOD ≥ 3.5, P ≤ 0.1) LOD thresholds, respectively.
We also mapped a measure of the change in overall energy balance (intake − expenditure) as a result of food consumption (intake), exercise (expenditure), and fat loss (indirect expenditure). First, we calculated overall energy intake by multiplying total food consumption (g) by the energy content in a gram of food (3.9 kcal). Next, we determined the energy expended through wheel running by multiplying the total revolutions over a 6-day period by 0.0003081 kcal. This kcal fraction represents the cost of an increase in 1 wheel revolution as determined by the conditional slope of food consumption as a function of total revolutions. As we used total food consumption and running across the entire 6 days, it is important to note that the incremental cost of 1 wheel revolution is inclusive of any changes in body mass and composition during the exercise period. Therefore, as an additional potential measure of energy expenditure, we estimated the energy yield from complete oxidation [if lost; fat lost (g) × 9 (kcal/g)] or storage [if gained; fat gained (g) × −13.2 (kcal/g)] of fat during the exercise period (39, 46). We also estimated the energy yield from the gain [lean gained (g) × −2.2 (kcal/g)] or loss [lean lost (g) × 1.1 (kcal/g)] in lean mass accordingly (39, 46). We detected a significant GRAIP-adjusted peak on MMU8 with a LOD score of 4.1 (Table 2).
DISCUSSION
The genetic basis of individual variation in body weight and voluntary activity has been investigated in a variety of mouse populations (19, 27, 32). This emergence of interest has primarily been driven by a desire to better understand variation in human exercise behavior and how this disparity may interact with disease from both proximate and ultimate perspectives (7). Although exercise behavior has conclusively been demonstrated to be associated with positive human health outcomes, often these outcomes are dependent upon the reduction in weight and adiposity in response to exercise regimens. Therefore, the present investigation is useful for several reasons. To begin with, it represents a large examination of changes in body composition in response to exercise. Second, we have provided some interesting insights with regard to the complex interplay between weight loss, reduction in adiposity, food consumption, and exercise, in particular with regard to relatively lean versus obese individuals at the outset of an exercise program (in the context of the present population). Third, we have presented unique QTL that provide insight into the individual variation in change of weight and adiposity in response to exercise. Finally, we have reinforced the evidence concerning the genetic basis for body weight and composition at 4, 6, and 8 wk of age in laboratory mice, with identification of some independent loci controlling growth at different ages (specifically with regard to body weight).
Phenotypic relationships.
At the level of the population, engaging in voluntary exercise decreased overall body mass, decreased adiposity, and increased lean mass. Additionally, wheel running was significantly positively correlated with food consumption, as is found typically but not always in studies of rodents (14). However, there was large individual variability in both of the above effects. This variation may be partially attributable to the initial variation in adiposity before wheel access and/or the effects of sex. The mean (±SD) percent body fat in the G4 population before the initiation of physical activity was 14.4 ± 4.4%, with a range of 4.6–34.1%. Given that a large majority of human investigations examine the relationship between exercise and change in weight and adiposity in the subset of overweight or obese individuals rather than an entire population (9), we subdivided the G4 population into the 25% leanest and 25% fattest, based on Pre exercise body composition measures, and, given our large sample size, we were further able to test for the effects of sex within these two subpopulations.
Between these two groups we observed unique relationships at the level of the individual. With regard to food consumption, we observed a positive relationship between food consumption and running distance. However, the relationships were considerably stronger in the leanest group of animals. Additionally, when the slopes among the subgroups were compared, our results indicated that the cost of running (as revealed by the relationship between total food consumption and total running distance) was significantly different between sexes and varied substantially by sex even among the leanest 25%, middle 50%, and fattest segments of this population of mice. A similar observation (pooled across sexes) was previously made in selectively bred fat and lean mice originating from an initial cross between two inbred (CBA, JU) and one outbred (CFLP) strain (48, 49). The similarity in results between the study of Simoncic et al. (49) and the present study is striking, especially given the difference in exercise exposures. Here, we only provided the G4 population 6 days of wheel access, while Simoncic et al. (49) provided mice access to running wheels for 42 days. In human investigations, acute exposures to exercise only result in loose associations with a compensatory increase in hunger, and compensation for energy expenditure is only partial (compensation for ∼30% of energy expended) up until ∼16 days of activity (2). However, in the case of humans, a longer-term negative energy balance as a result of physical activity has been demonstrated to result in compensatory increases in hunger, but this compensation was dependent on adiposity levels at the outset of the exercise regimen (Ref. 28; see also Fig. 3 in Ref. 2).
Our results suggest that, in mice, tolerating a negative energy balance as a result of short (present study)- or long (Ref. 49)-term physical activity is more plausible for mice that have larger fat depots at the outset of exercise, and may be dependent on sex as well. And, for the leanest mice, in the present study and that of Simoncic et al. (49) increases in compensatory hunger in association with physical activity are more immediate and stronger, and again may be potentially affected by sex. This is most likely due to their low initial energy stores and a more precarious overall energy balance.
However, the disparity in the relationship between food consumption and exercise (between lean and fat individuals and between males and females in each of these populations) does not completely explain the differences we observed in the correlations between the amount, duration, and intensity of exercise and change in weight and adiposity. Although among the leanest individuals there was generally a negative relation between running distance and duration and the change in weight and adiposity, among the fattest 25% of individuals these correlations were more strongly negative. That is, if you were among the fattest 25% of individuals before exercise, then the more you exercised, the greater the reduction in overall body weight and adiposity. Even though there was a weaker relationship between exercise and a reduction in weight or adiposity among the leanest of G4 individuals, it is possible that potential benefits of cardiovascular exercise were realized but went unmeasured in the present study. Again, the relationship between changes in body weight and composition as a result of exercise were dependent on adiposity at the outset of wheel running, but these changes were also dependent upon complex interactions between the sexes. These interactions may potentially be a result of the difference in the regulation of sex hormones; however, we did not quantify estrogen/testosterone levels in the present study. Regardless, we agree with the conclusions of King et al. (23) that “from a public health perspective, exercise should be encouraged and the emphasis on weight loss reduced”; this appears to be an especially paramount recommendation among moderately lean individuals as weight loss may be more difficult to achieve.
We acknowledge that we have used relatively young, potentially developing, and quite lean mice. And it is important to stress that the meaningfulness of our findings is only one piece when placed in a much larger context. When we chose to create an AIL useful for the investigation of the genetic architecture of not only body composition-related traits but also exercise-related traits, we faced limitations. In so doing, we tried to closely match the exercise paradigm that most reflected the selection protocol under which the HR line was created and the design that previous growth studies have employed (see Refs. 8 and 45). Despite these limitations, we feel that we have presented compelling findings in the context of the strains and protocol utilized, but caution should be taken when extrapolating these findings generally.
Genetic architecture.
With regard to body mass, independent loci often controlled weight at different ages, with average additive, dominance, and variance QTL effects generally coinciding. In only one case did a QTL colocalize at more than one age: at 6 and 8 wk of age a significant body mass QTL was observed on MMU6 (36 Mb). The general independence of loci controlling body weight across different ages was not surprising, given previous work on the genetic architecture of growth in mouse populations (1, 8, 10, 45). Cheverud et al. (8) identified numerous independent QTL for body weight at multiple ages, while Rocha et al. (45) identified a comparable number of growth QTL with map locations corresponding generally well with those of Cheverud et al. (8). Although we identified substantially fewer QTL than Cheverud et al. (8) or Rocha et al. (45), we employed lines of mice that were both, in general, relatively small and lean [see Nehrenberg et al. (31)]. In contrast, both Cheverud et al. (8) and Rocha et al. (45) crossed lines that were highly divergent for body weight and/or fat.
Unlike body weight, fat and lean mass generally shared more common QTL across ages, and loci identified for fat and lean mass frequently coincided at the same genomic location. Additionally, fat and lean mass QTL generally explained a much larger percentage of the total phenotypic variation of the respective trait than the loci observed for body mass. As was observed for body mass QTL, average additive effects were frequently significant, with generally large concurrent dominance effects for most loci. We are currently unaware of any other mapping study that has examined body composition (percent fat and lean) across multiple ages. Importantly, these results may suggest that while independent loci control overall body mass at different ages, the control of body composition (percent fat and lean) may generally be more genetically stable.
To our knowledge, only two other studies have examined the genetic architecture of weight change in response to physical activity (25, 26), and neither examined the change in adiposity or lean mass. The QTL for percent change in body mass did not directly overlap with any physical activity QTL previously identified (21). Presumably, given that the QTL on MMU11 did not colocalize with any other body composition QTL, this genomic region affects the amount of weight change in response to exercise independently of other mass loci identified in the present population. Leamy et al. (25) previously identified five QTL associated with weight change in response to exercise that in total explained 13% of the phenotypic variation and did not, in general, overlap with activity QTL identified in the same population (27). The weight change QTL identified here, found on MMU11, did not overlap with those identified by Leamy et al. (25). This lack of congruence is not surprising, given that the F2 mapping population utilized by Leamy et al. (25) originated from different strains (C57L/J and C3H/HeJ) than those utilized here. Additionally, Leamy and colleagues examined weight change as a result of 3 wk of exercise, while we examined change over a significantly shorter activity period (6 days). Furthermore, differences in running wheel type and circumference, diet composition, animal facility environment, and many other methodological differences, cannot be discounted when comparing the results of the two studies. These are all important distinctions, especially given the large number of examples (10) of independent loci controlling growth at different ages in mice. Therefore, we find it perfectly plausible that largely independent loci may govern the regulation of weight change at various stages of exercise training.
We previously (21) identified numerous exercise-related loci across a 6-day exposure to running wheels. Individual days generally shared common QTL, but the initial exposure to wheels (days 1 and 2) revealed unique genomic regions for running distance and duration. These loci were observed on MMU1, MMU5, and MMU6, and we previously hypothesized that the temporal differences in loci may be related to anxiety- or fear-related behavioral differences between HR and B6 mice upon initial exposure to a new environment (i.e., individual housing, introduction to a running wheel). Here, we also hypothesize that the loci identified in the present experiment underlying the change in weight, adiposity, and lean mass may also be related to acute effects of anxiety- or fear-related behavioral differences between HR and B6 mice. This is particularly important given the observation of a QTL on MMU1 at 178 Mb for percent change in percent fat. While this QTL does not colocalize with the formerly identified running loci on days 1 and 2, it is found in a region previously implicated in open-field behavior in mice (a measure frequently used to assess anxiety- or fear-related behavioral differences) (see Ref. 21 and references therein). Accordingly, we can preliminarily conclude that anxiety (or lack thereof) may be playing some role in the change in adiposity with exposure to a novel environment. However, at this time we cannot conclude whether this variation in anxiety directly contributes to alterations in body composition or acts via increases/decreases in exercise behavior, which in turn cause changes in body composition.
Unique to the present investigation are the identification of QTL for percent change in percent fat and percent lean mass in response to 6 days of voluntary wheel running. A likely factor in the locus-specific allelic effects observed for percent change in percent fat and lean is the body composition of the parental strains used to create the G4 population. HR mice tend to weigh more than B6 but have a lower percentage of body fat and a higher percentage of lean mass in the absence of a running wheel (31). And, as described above, body composition before the initiation of exercise is a critical factor in determining the relationship between food consumption and body composition in response to exercise. Moreover, we have demonstrated in one case that the genetic architecture for percent change in percent fat also depends on the level of adiposity before the initiation of exercise. In this case, the QTL identified had decreasing effects on fat loss with increasing levels of adiposity before physical activity.
Concluding remarks.
The present study demonstrates the genetic complexity of both growth and changes in body composition in response to exercise in laboratory house mice. Undoubtedly, genetic polymorphisms contribute to body weight and composition, and these loci often act independently at different ages. Furthermore, genomic regions responsible for changes in weight, adiposity, and lean mass in response to exercise do not appear to colocalize with one another, thus indicating that regulation of each of these changes may be genetically distinct. Although the magnitude of change in weight, adiposity, and lean body mass is dependent on exercise distance, duration, and intensity, these effects are also contingent on the level of adiposity before the initiation of exercise, as is the positive relation between food consumption and wheel running. Taken together, our results are demonstrative of the complexity of weight regulation and the relationships between genetics, body composition, exercise, and food consumption. Body composition, exercise, and food consumption each have their own complex underlying genetic architectures, but they clearly interact in a complex way, making it, in our opinion, imperative to begin to unify isolated investigations of each of these traits. Comprehensive studies, such as the present investigation, are critical in guiding clinicians and future public health recommendations in their efforts to curb weight disregulation and accompanying comorbidities.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-076050 to D. Pomp. S. A. Kelly was supported through a National Institute of Mental Health-funded (5T32-MH-075854-04) Interdisciplinary Obesity Training (IDOT) program. Phenotypes were collected with the Animal Metabolism Phenotyping core facility within UNC's Clinical Nutrition Research Center (funded by NIDDK Grant DK-056350).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Z. Yun for assistance with animal care and data collection. We thank Chris Wiesen at UNC's Odum Institute for Research in Social Science for statistical consultation.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Allan MF, Eisen EJ, Pomp D. Genomic mapping of direct and correlated responses to long-term selection for rapid growth rate in mice. Genetics 170: 1863–1877, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc 62: 651–661, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Bouchard C, Tremblay A, Nadeau A, Dussault J, Despres JP, Theriault G, Lupien PJ, Serresse O, Boulay MR, Fournier G. Long-term exercise training with constant energy intake. 1. Effect on body composition and selected metabolic variables. Int J Obes 14: 57–73, 1990. [PubMed] [Google Scholar]
- 4. Bray MS, Hagberg JM, Perusse L, Rankinen T, Roth SM, Wolfarth B, Bouchard C. The human gene map for performance and health related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exerc 41: 34–42, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Bryner RW, Toffle RC, Ulrich IH, Yeater RA. The effect of exercise intensity on body composition, weight loss, and dietary composition in women. J Am Coll Nutr 16: 68–73, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Chakravarthy M, Booth F. Eating, exercise, and “thrift” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol 96: 3–10, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Cheverud JM, Routman EJ, Duarte FAM, van Swinderen B, Cothran K, Perel C. Quantitative trait loci for murine growth. Genetics 142: 1305–1319, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS ONE 4: e4515, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corva PM, Medrano JF. Quantitative trait loci (QTLs) mapping for growth traits in the mouse: a review. Genet Sel Evol 33: 105–132, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 279: R1–R8, 2000. [DOI] [PubMed] [Google Scholar]
- 12. De Moor MH, Liu YJ, Boomsma DI, Hamilton JJ, Hottenga JJ, Levy S, Liu XG, Pei YF, Posthuma D, Recker RR, Sullivan PF, Wang L, Willemsen G, Yan H, Geus EJ DE, Deng HW. Genome-wide association study of exercise behavior in Dutch and American adults. Med Sci Sports Exerc 41: 1887–1895, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev 33: 169–174, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity, and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206–229, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girard I, Rezende EL, Garland T., Jr Leptin levels and body composition of mice selectively bred for high voluntary activity. Physiol Biochem Zool 80: 568–579, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Grediagin A, Cody M, Rupp J, Benardot D, Shern R. Exercise does not effect body composition in untrained, moderately overfat women. J Am Diet Assoc 95: 661–665, 1995. [DOI] [PubMed] [Google Scholar]
- 17. Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 290: 1323–1330, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Karavirta L, Hakkinen K, Kauhanen A, Arija-Blazquez A, Sillanpaa E, Rinkinen N, Hakkinen A. Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc (September 24, 2010). doi:10.1249/MSS.0b013e3181f1bf0d. [DOI] [PubMed] [Google Scholar]
- 19. Kas MJ, de Mooij-van Malsen JG, de Krom M, van Gassen KLI, van Lith HA, Olivier B, Oppelaar H, Hendriks J, de Wit M, Groot Loerkamp MJ, Holstege FC, van Oost BA, de Graan PN. High-resolution genetic mapping of mammalian motor activity levels in mice. Genes Brain Behav 8: 13–22, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Kelly SA, Nehrenberg DL, Hua K, Gordon RR, Garland T, Jr, Pomp D. Parent-of-origin effects on voluntary exercise levels and body composition in mice. Physiol Genomics 40: 111–120, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly SA, Nehrenberg DL, Peirce JL, Hua K, Steffy BM, Wiltshire T, Pardo-Manuel de Villena F, Garland T, Jr, Pomp D. Genetic architecture of voluntary exercise in an advanced intercross line of mice. Physiol Genomics 42: 190–200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes 32: 177–184, 2008. [DOI] [PubMed] [Google Scholar]
- 23. King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Beneficial effects of exercise: shifting the focus from body weight to other markers of health. Br J Sports Med 43: 924–927, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Koteja P, Carter PA, Swallow JG, Garland T., Jr Food wasting in house mice: variation among individuals, families, and genetic lines. Physiol Behav 80: 375–383, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Leamy LJ, Pomp D, Lightfoot JT. Genetic variation for body weight change in mice in response to physical exercise. BMC Genet 10: 58, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leamy LJ, Pomp D, Lightfoot JT. Genetic variation in the pleiotropic association between physical activity and body weight in mice. Genet Sel Evol 41: 41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR, Leamy LJ. Quantitative trait loci (QTL) for physical activity traits in mice. Physiol Genomics 32: 401–408, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim CL, Lee LK. The effects of 20 weeks basic military training program on body composition, VO2max and aerobic fitness of obese recruits. J Sports Med Phys Fitness 34: 271–278, 1994. [PubMed] [Google Scholar]
- 29. Malisch JL, Breuner CW, Kolb Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T., Jr Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet 39: 192–201, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell JA, Church TS, Rankinen T, Earnest CP, Sui X, Blair SN. FTO genotype and the weight loss benefits of moderate intensity exercise. Obesity (Silver Spring) 18: 641–643, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nehrenberg DL, Hua K, Estrada-Smith D, Garland T, Jr, Pomp D. Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity (Silver Spring) 17: 1402–1409, 2009. [DOI] [PubMed] [Google Scholar]
- 32. Nehrenberg DL, Wang S, Hannon RM, Garland T, Jr, Pomp D. QTL underlying voluntary exercise in mice: interactions with the “mini-muscle” locus and sex. J Hered 101: 42–53, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. NHLBI Obesity Task Force. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res 2: 51S–209S, 1998. [PubMed] [Google Scholar]
- 34. Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes 31: 1786–1797, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Peirce JL, Broman KW, Lu L, Chesler EJ, Zhou G, Airey DC, Birmingham AE, Williams RW. Genome reshuffling for advanced intercross permutation (GRAIP): simulation and permutation for advanced intercross population analysis. PLoS ONE 3: e1977, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peirce JL, Broman KW, Lu L, Williams RW. A simple method for combining genetic mapping data from multiple crosses and experimental designs. PLoS ONE 2: e1036, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pomp D, Allan MF, Wesolowski SR. Quantitative genomics: exploring the genetic architecture of complex trait predisposition. J Anim Sci 82E: 300–312, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Pomp D, Nehrenberg D, Estrada-Smith D. Complex genetics of obesity in mouse models. Annu Rev Nutr 28: 331–345, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pullar JD, Webster AJ. The energy cost of fat and protein deposition in the rat. Br J Nutr 37: 355–363, 1977. [DOI] [PubMed] [Google Scholar]
- 40. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. (www.r-project.org). [Google Scholar]
- 41. Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14: 529–644, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr 49: 968–975, 1989. [DOI] [PubMed] [Google Scholar]
- 43. Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol 45: 438–455, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Ritt M, Lechleitner M. Effect of exercise intensity on body composition. JAMA 290: 3069, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Rocha JL, Eisen EJ, Van Vleck LD, Pomp D. A large-sample QTL study in mice. I. Growth. Mamm Genome 15: 83–99, 2004. [DOI] [PubMed] [Google Scholar]
- 46. Schulz LO, Alger S, Harper I, Wilmore JH, Ravussin E. Energy expenditure of elite female runners measured by respiratory chamber and doubly labeled water. J Appl Physiol 72: 23–28, 1992. [DOI] [PubMed] [Google Scholar]
- 47. Sen S, Churchill GA. A statistical framework for quantitative trait mapping. Genetics 159: 371–387, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharp GL, Hill WG, Robertson A. Effects of selection on growth, body composition and food intake in mice. Responses in selected traits. Genet Res 43: 75–92, 1984. [DOI] [PubMed] [Google Scholar]
- 49. Simoncic M, Horvat S, Stevenson PL, Bunger L, Holmes MC, Kenyon CJ, Speakman JR, Morton NM. Divergent physical activity and novel alternative responses to high fat feeding in polygenic fat and lean mice. Behav Genet 38: 292–300, 2008. [DOI] [PubMed] [Google Scholar]
- 50. Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28: 227–237, 1998. [DOI] [PubMed] [Google Scholar]
- 51. Swallow JG, Hayes JP, Koteja P, Garland T., Jr Selection experiments and experimental evolution of performance and physiology. In: Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments, edited by Garland T, Jr, Rose MR. Berkeley, CA: Univ. of California Press, 2009. [Google Scholar]
- 52. Swallow JG, Koteja P, Carter PA, Garland T., Jr Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol [B] 171: 651–659, 2001. [DOI] [PubMed] [Google Scholar]
- 53. Swallow JG, Koteja P, Carter PA, Garland T., Jr Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J Exp Biol 202: 2513–2520, 1999. [DOI] [PubMed] [Google Scholar]
- 54. Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol (October 28, 2010). doi:10.1152/japplphysiol.00934.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. US Department of Health and Human Services. Physical Activity and Health: a Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 1996. [Google Scholar]
- 56. Vaanholt LM, Meerlo P, Garland T, Jr, Visser GH, van Dijk G. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per se. Horm Metab Res 39: 377–383, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev 68: 148–154, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.