Abstract
Both iron deficiency and iron excess are detrimental in many organisms, and previous studies in both mice and humans suggest that genetic variation may influence iron status in mammals. However, these genetic factors are not well defined. To address this issue, we measured basal liver iron levels in 18 inbred strains of mice of both sexes on a defined iron diet and found ∼4-fold variation in liver iron in males (lowest 153 μg/g, highest 661 μg/g) and ∼3-fold variation in females (lowest 222 μg/g, highest 658 μg/g). We carried out a genome-wide association mapping to identify haplotypes underlying differences in liver iron and three other related traits (copper and zinc liver levels, and plasma diferric transferrin levels) in a subset of 14 inbred strains for which genotype information was available. We identified two putative quantitative trait loci (QTL) that contain genes with a known role in iron metabolism: Eif2ak1 and Igf2r. We also identified four putative QTL that reside in previously identified iron-related QTL and 22 novel putative QTL. The most promising putative QTL include a 0.22 Mb region on Chromosome 7 and a 0.32 Mb region on Chromosome 11 that both contain only one candidate gene, Adam12 and Gria1, respectively. Identified putative QTL are good candidates for further refinement and subsequent functional studies.
Keywords: genome-wide, haplotype association mapping, diferric transferrin, copper, zinc
iron is an essential metal that is involved in many processes requiring electron transport, with the carriage of oxygen being the most important. If present in excess, iron can catalyze the formation of highly reactive free radicals that are damaging to cellular components. Thus, tight regulation of body iron homeostasis is necessary. This occurs mainly at the level of absorption of dietary iron in the intestine as there is no regulated excretion mechanism for iron (27). Only 1–2 mg of iron is provided from the diet each day, and this balances the amount lost through the feces, urine, and dead skin cells (4). Much more iron is required for metabolic functions, so the rest is recycled from senescent red blood cells by macrophages. Iron in excess of immediate metabolic requirements is stored primarily as ferritin and hemosiderin in the liver, spleen, and bone marrow. Levels of body iron stores in healthy human adults can vary considerably. For example, serum ferritin levels, which usually reflect body iron stores, ranged from <15 μg/l to >1,000 μg/l in a multiethnic population (1). A change of 1 μg/l in the serum ferritin concentrations corresponds to a 7–7.5 mg change in iron stores (45). A study of 1,233 twin pairs found that 45% of the variation in serum ferritin could be explained by additive genetic factors (65). A similar study on 2,039 female twins found that genetic effects account for 47% of the variance in transferrin saturation and 41% of the variance in log-transformed serum ferritin concentration (66). Many individuals can compensate for reduced levels of iron in the diet by increasing the efficiency of their iron uptake and utilization, although there is variability in this capability that is also attributed to genetic factors (9). There are also differences in the prevalence of iron deficiency between different ethnic groups with similar dietary intake, suggesting that nondietary factors might be a source of the discrepancy between the groups (38, 54). Mutations in the genes encoding the hemochromatosis protein (HFE), hemojuvelin (HFE2), hepcidin (HAMP), transferrin receptor 2 (TFR2), ferroportin (SLC40A1), or rarely ceruloplasmin (CP) and transferrin (TF) lead to iron overload. The accumulation of iron in these diseases varies from gradual to rapid, but in all cases it ultimately leads to tissue damage (4). Similar to iron deficiency, multiple genetic factors likely contribute to the variation in the severity of these iron overload-related disorders. For example, in the case of HFE-associated hemochromatosis, there is variable biochemical and clinical penetrance with evidence of modifying genes involved (3, 14). All these studies suggest a significant genetic contribution to iron homeostasis that still needs to be fully defined.
Classical inbred mouse strains are extensively used as a model for different human traits, and they exhibit diversity of genetic and phenotypic differences (49). A 1995 study by Leboeuf et al. (33) demonstrated that iron stores are different between different mouse inbred strains. C57BL/6J and BALB/cJ mouse strains required 10 times more dietary iron to saturate their serum transferrin than DBA/2J and AKR/J mice (47). Several subsequent studies of iron metabolism indicated differences between inbred mice and/or supported a role of genetic control (13, 20, 25, 28, 61). A small number of these utilized a linkage analysis approach to map underlying genetic variation in intercross mice (25, 28). In this study, we describe use of a new approach, in silico mapping, to identify genetic regions underlying the variation in iron metabolism between inbred mouse strains. The creation of publically available dense single nucleotide polymorphism (SNP) data sets for number of inbred strains has enabled this in silico approach for mapping quantitative phenotypes (26). Compared with the linkage-based approaches commonly used in quantitative trait locus (QTL) mapping, the in silico method offers several advantages: 1) it is much faster since there is no need to create and genotype an intercross population; 2) it has a greater resolution due to denser SNP maps and a greater number of (historic) recombination events; and 3) multiple measurements on the same genetic background reduce the influence of measurement error and environmental effects (63). However, several concerns regarding the utility of this method have been expressed, including the number of strains needed to achieve statistical power, adequate control of false discovery rate (12, 16), population structure (44), and genome organization in inbred mice (15). Since then, a number of improved in silico mapping methods have been applied taking into account some of these concerns (11, 34–36, 40, 51, 64), but despite this, a few studies have reported a high number of false positives and have suggested that QTL discovery by in silico mapping should be verified by linkage studies (8, 39). Furthermore, several studies have pointed out that the value of in silico analysis increases in combination with other available information, such as known QTL mapped by classical approaches, or comparative genomic or gene expression data, in both mapping novel QTL as well as in narrowing down known QTL (7, 10, 11, 64). However, further studies are warranted to help better evaluate both advantages and limitations of an in silico approach.
In the present study, we measured basal liver iron levels in 18 inbred mouse strains and found substantial variation between the strains. Secondary phenotypes measured included the level of circulating diferric transferrin as another measure of iron status, and liver zinc and copper levels due to the known interactions between iron and these metals. We performed genome-wide haplotype association mapping (HAM) analysis of these traits in a subset of 14 inbred strains for which genotype information was available. Putative QTL significantly associated with any of the four traits were compared with previously identified QTL and descriptions of gene function were derived from the literature. We report the successful refinement of known QTL and the identification of novel putative QTL associated with these traits.
MATERIALS AND METHODS
Mouse Husbandry
Four-week-old mice were obtained from The Jackson Laboratory (Bar Harbor, ME). We used at least six male and six female animals (unless otherwise noted) of 17 inbred strains: 129S1/SvImJ, A/HeJ, A/J, AKR/J, B10.D2-Hc0H2dH2-T18c/oSnJ, BALB/cByJ, BALB/cJ, C3H/HeJ (nfemale = 5), C57BL/6J (nfemale = 10), CAST/EiJ (nmale = 7, nfemale = 11), DBA/2J (nmale = 12, nfemale = 12), LG/J, LP/J, MRL/MpJ, NZB/BlNJ (nmale = 10), SM/J (nfemale = 4), and SPRET/EiJ (nmale = 4). For an 18th strain, NZW/LacJ, female animals only were available (overview in Supplemental Table S1).1 Mice were fed ad libitum an AIN93G purified diet containing ∼35 ppm iron (Dyets) to 8 wk of age. At the time of death, mice were euthanized by carbon dioxide gas without fasting. Blood was collected by cardiac puncture, and at least 30 min later it was centrifuged at 1,000 g for 15 min at room temperature. Serum was aliquoted into microcentrifuge tubes, snap-frozen in liquid nitrogen, and stored at −70°C. Liver tissue was collected, snap-frozen in liquid nitrogen, and stored at −70°C. All experimental procedures were approved by the Office of Lab Animal Care at the University of California, Berkeley.
Liver Metal Content
Liver tissue was vacuum dried overnight using a freeze-drying system (Labconco), weighed, then digested in suprapure nitric acid using a microwave digestor (CEM microwave accelerated reaction system 5, CEM). Samples were diluted with metal-free water to give a final nitric acid concentration of ∼1 M of nitric acid and were used to assess the levels of iron, copper, and zinc by Vista AX CCD inductively coupled plasma-atomic emission spectrometry (ICP-AES, Varian). The metal content of the liver was expressed as micrograms per gram dry weight of tissue. All samples were measured three times.
Diferric Transferrin Levels
Levels of plasma diferric transferrin were determined by urea polyacrylamide gel electrophoresis (21). Western blotting was used to determine total transferrin levels in the same samples and was carried out as previously described (22) using a polyclonal antibody to human transferrin (1 in 1,000 dilution; Silenus Laboratories, Hawthorn, Australia) that cross-reacts with the mouse protein. Diferric transferrin was expressed as a percentage of total transferrin.
SNP Data
The “SNPster” genotype file contained genotypic information for 16 of the phenotyped strains (A/HeJ and B10.D2-Hc0H2dH2-T18c/oSnJ had no genotype data available). Wild-derived strains, CAST/EiJ and SPRET/EiJ, were omitted for in silico analysis since the presence of unique haplotypes in these strains compromises characterization of haplotype blocks (63). The dataset from the remaining 14 strains contained allele calls for 137,868 SNPs (including high-confidence imputed calls) distributed over 20 chromosomes. SNP density averaged ∼19 kb per SNP across the genome. All SNP locations were mapped to the National Center for Biotechnology Information mouse genome map build 36.1.
HAM
For in silico QTL analysis we used the web-based program “SNPster” (at http://snpster.gnf.org), which incorporates an HAM approach over inferred three SNP haplotypes (40, 41, 51). Briefly, sliding three SNP windows were used to infer haplotypes across strains. The F-statistic was calculated for a given phenotype and inferred haplotype groups in one-factor ANOVA. The significance of the F-statistic was estimated from background distribution simulated nonparametrically using 1 × 106 bootstraps of the phenotypic values. The resulting P value was −log(10) transformed to produce an association score (AS). To account for possible population structure, a “weighted” bootstrap was used, where one strain phenotype served as substitute for the other strain phenotype based on values of a weighted genetic similarity matrix. In brief, a genetic similarity matrix was calculated for all pair-wise strain combinations by dividing the number of genotypes in common between two strains with the total number of genotypes. The ratios obtained were then raised to the exponent of an assigned weight factor. A weight factor of zero corresponds to the unweighted case where all strains are equally likely to be chosen as a substitute. The higher the weight factor, the higher the likelihood of choosing a substitution strain that is genetically and phenotypically similar. As a result, the significance and association scores of those associations that arise due to population structure were decreased. For all analyses, a default weight factor of 3 was used. All data were log transformed prior to analysis. We report results for putative QTL above a threshold set at the fifth percentile of the best 1,000 results for each trait. For every putative QTL corresponding QTL locations (Mb) are calculated by expanding each QTL to the left and right for as long as the association score remained >2 (or by at least 100 kb). If two putative QTL overlapped or were <1 Mb apart they were considered to be one signal. For the comparison of our findings with those from linkage studies, centimorgan (cM) to megabase (Mb) conversions were performed using Mouse Map Converter at http://cgd.jax.org. We used “Gene Relationships Across Implicated Loci” (GRAIL) software to examine relationships between genes in putative QTL and known genes influencing a given trait (56).
Statistical Analysis
Phenotype data are shown as means (± SD). An unequal variance t-test was used to compare the groups. The Pearson product-moment correlation coefficient (r) was used to calculate the phenotypic correlation between strain mean values for iron, zinc, copper, and diferric transferrin levels in males and females (17 and 18 strains, respectively). The significance level of the correlation coefficient is distributed approximately as t with N − 2 degrees of freedom, where N is the number of strains. To evaluate the extent of linkage disequilibrium (LD), we calculated the r2 between all pairs of SNPs in a given region, using the program Goldsurfer 2 (50).
Heritability
The proportion of total phenotypic variation that is due to the additive effects of genes, narrow-sense heritability (h2), was estimated as described in Koch and Britton (32). Briefly,
where MSB is the mean square between strains and MSW is the mean square within strains, as estimated from ANOVA, and n is number of animals in each strain. Since the number of animals is not the same for each strain, the average n was calculated as
where a is the number of strains and ni is the number of animals of each strain. Heritability was estimated for males and females of all strains separately.
RESULTS
Liver Iron, Zinc, and Copper Levels
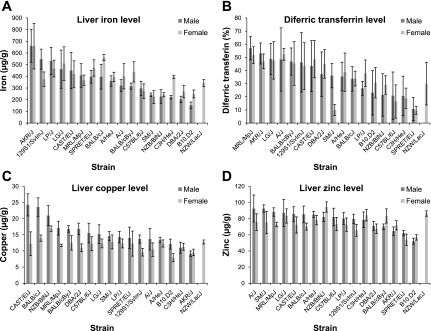
The liver iron level in males varied from 153.2 μg/g (27.2) in the B10.D2-Hc0H2dH2-T18c/oSnJ strain to 661.1 μg/g (141.5) in AKR/J mice. In females, hepatic iron varied from 222.1 μg/g (55.4) in the SM/J strain to 658.7 μg/g (194.9) in the AKR/J strain (Fig. 1A, Supplemental Table S1). The differences in iron level between the strains with the highest and lowest values were found to be highly significant both in males and females (P = 0.0002 and P = 0.0019, respectively). In males, copper and zinc levels varied from 9.2 μg/g (0.8) in AKR/J to 24.3 μg/g (3.4) in CAST/EiJ, and from 52.1 μg/g (3.7) in B10.D2-Hc0H2dH2-T18c/oSnJ to 92.6 μg/g (16.7) in A/J, respectively. Copper and zinc values in females varied from 7.9 μg/g (1.3) in B10.D2-Hc0H2dH2-T18c/oSnJ to 16.9 μg/g (0.8) in NZB/BlNJ, and from 53.8 μg/g (7.1) in SPRET/EiJ strain to 94.4 μg/g (6.3) in NZB/BlNJ, respectively (Fig. 1, C and D; Supplemental Table S1). The differences between the strains with the highest and lowest values were also found to be highly significant both for copper (P = 1.15 × 10−5 and P = 2.2 × 10−7, for males and females respectively) and zinc (P = 0.0016 and P = 1.12 × 10−6, for males and females, respectively) levels. No correlations were seen between iron and copper or between iron and zinc in either sex. However, there was a significant correlation in females between copper and zinc (Table 1).
Fig. 1.
Distribution of liver iron (A), plasma diferric transferrin levels (B), liver copper (C), and liver zinc levels (D) in male and female animals of 18 inbred strains. For the NZW/LacJ strain there were no male animals available. All data are expressed as means (± SD) and ordered by decreasing values of the mean in males.
Table 1.
Phenotypic correlations between iron, diferric transferrin, copper, and zinc in males and females of 17 and 18 strains, respectively
| Male |
Female |
||||
|---|---|---|---|---|---|
| Phenotype 1 | Phenotype 2 | r | P Value | r | P Value |
| Iron | Fe2TF | 0.467 | 0.0587 | 0.364 | 0.1376 |
| Iron | copper | −0.099 | 0.7058 | −0.107 | 0.6718 |
| Iron | zinc | 0.079 | 0.7631 | −0.090 | 0.7210 |
| Copper | zinc | 0.374 | 0.1396 | 0.608 | 0.0074 |
Pearson product-moment correlation coefficients (r) were calculated for each phenotype combination using mean strain values for each trait. The significance level (P value) is approximately t distributed. Significant/highly suggestive results are marked in boldface. Fe2TF, diferric transferrin levels.
Diferric Transferrin Levels
Mean diferric transferrin values varied from 10.7% (8.9) in SPRET/EiJ to 57.0% (8.9) in MRL/MpJ in males, and from 9.7% (3.0) in SPRET/EiJ strain to 52.9% (4.5) in A/J in females (Fig. 1B, Supplemental Table S1). Strains with the highest values for diferric transferrin significantly differed from strains with the lowest values, both in males and females (P = 0.0001 and P = 2.04 × 10−8, respectively). The correlation between iron and diferric transferrin was found to be highly suggestive in females (Table 1).
Heritability
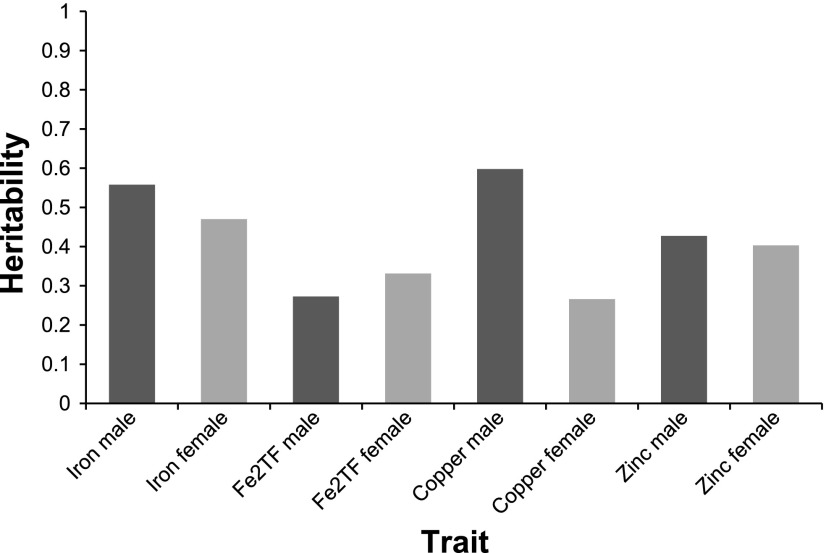
For all traits, heritability (h2) estimates were moderate to high, ranging from 0.27 to 0.60 (Fig. 2). In general, heritability was higher in males for all traits except for diferric transferrin levels. However, this difference was not significant (P = 0.3).
Fig. 2.
Heritability of liver iron, plasma diferric transferrin, liver copper, and liver zinc in male and female animals (17 and 18 strains, respectively). Heritability was calculated and expressed as ratios of between strain variance and total variance, estimated by ANOVA. Fe2TF, diferric transferrin level.
In Silico Mapping
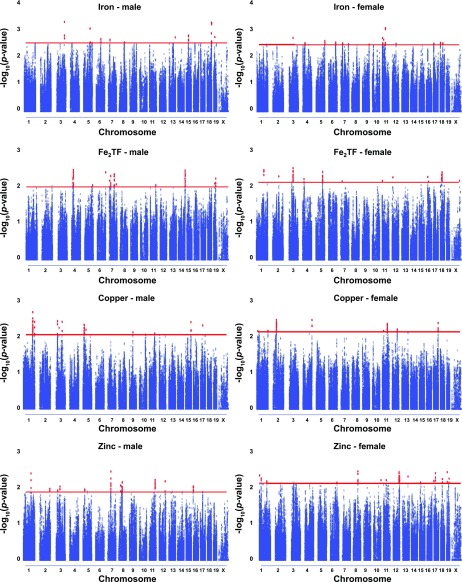
Liver iron.
Genome-wide in silico QTL analysis identified 12 putative QTL over 9 chromosomes influencing hepatic iron levels in males, and 16 putative QTL over 10 chromosomes in females (Table 2, Fig. 3). Two of these putative QTL contain genes recently reported to play a role in iron homeostasis: a 0.35 Mb region on Chromosome 5 containing Eif2ak1, eukaryotic translation initiation factor 2-α kinase 1, which plays a role in hemoglobin production, the maturation of macrophages and the inflammatory response, and a 0.48 Mb region on Chromosome 17 containing Igf2r, insulin-like growth factor 2 receptor, which has a role in iron sensing. In addition, GRAIL analysis identified Eif2ak1 as significantly similar to the Hmox1 and Tfrc genes (P = 0.007).
Table 2.
Results of haplotype association mapping of liver iron levels in male and female animals of 13 and 14 inbred mouse strains, respectively
| Chr | QTL Location, Mb | QTL Size, Mb | Sex | Genes |
|---|---|---|---|---|
| 3 | 66.19–66.66 | 0.47 | F | Veph1,Ptx3 |
| 3 | 130.66–131.31 | 0.65 | M | Lef1, Hadh, Cyp2u1, Ostc, Rpl34 |
| 4 | 38.30–40.25 | 1.95 | F | |
| 5 | 112.35–112.62 | 0.27 | M | Miat, Cryba4, Crybb1, Tpst2, Tfip11, Srrd, Gm6583, Hps4 |
| 5 | 114.73–114.95 | 0.22 | M | Trpv4, Gltp |
| 5 | 144.01–144.36 | 0.35 | M | Eif2ak1, Aimp2, Pms2, Ocm, Lmtk2 |
| 5 | 149.63–151.50 | 1.87 | F | 4930434E21Rik, Hsph1, B3 galtl, Lgr8, Fry, Gm5, Brca2, N4bp2l1, N4bp2l2, Aprin, Kl, Stard13 |
| 6 | 81.83–82.2 | 0.37 | M | AW146020, Mrpl19, Fam176a |
| 6 | 120.35–122.69 | 2.34 | F | Kdm5a, Il17ra, Cecr6, Cecr5, Cecr2, Slc25a18, Atp6v1e1, Bcl2l13, Bid, Mical3, Pex26, Tuba8, Usp18, Slc6a13, Slc6a12, Iqsec3, A2m, Mug1, Cpamd8, Gm10319, Mug-ps1, Klrg1, M6pr, Phc1, Rimklb, Mfap5, Aicda, Apobec1, Gdf3, Dppa3, Nanog |
| 7 | 33.92–34.21 | 0.29 | M | Gpi1, Lsm14a |
| 7 | 35.96–36.50 | 0.54 | F | Tshz3 |
| 7 | 133.93–134.15 | 0.22 | F | Adam12 |
| 8 | 89.22–89.86 | 0.64 | M | Abcc12, Lonp2, Siah1a, Gm10638, N4bp1 |
| 9 | 117.48–118.17 | 0.69 | F | Azi2, Cmc1 |
| 11 | 7.80–9.88 | 2.08 | F | Tns3, Hus1, Sunc1, Upp1, Abca13 |
| 11 | 11.72–12.23 | 0.51 | F | Ddc, Grb10, Cobl |
| 11 | 13.79–14.43 | 0.64 | F | |
| 11 | 26.25–26.49 | 0.24 | F | Fancl, Vrk2 |
| 11 | 56.70–57.02 | 0.32 | F | Gria1 |
| 12 | 69.73–70.05 | 0.32 | F | |
| 13 | 108.40–108.76 | 0.36 | M | Apoo-ps |
| 15 | 50.67–50.88 | 0.21 | M | Trps1 |
| 17 | 12.16–12.64 | 0.48 | F | Map3k4, Plg, Slc22a3, Slc22a2, Slc22a1, Igf2r, Airn |
| 18 | 14.56–15.35 | 0.79 | F | Ss18, Psma8, Taf4b, Kctd1 |
| 18 | 53.80–54.02 | 0.22 | F | Cep120, Csnk1 g3 |
| 18 | 78.88–79.09 | 0.21 | M | Setbp1 |
| 18 | 80.15–81.41 | 1.26 | M | Pard6 g, Adnp2, Txnl4a, Hsbp1l1, Pqlc1, Ctdp1, Gm2176, Nfatc1, Atp9b, Sall3 |
| 19 | 29.81–30.15 | 0.34 | M | Ranbp6, Il33, Trpd52l3, Uhrf2 |
QTL, quantitative trait locus. Sex, male (M), female (F).
Fig. 3.
Results of genome-wide haplotype association mapping in male (13 strains) and female (14 strains) animals of inbred mice for liver iron, diferric transferrin, copper and zinc. The association score threshold of 5th percentile is marked red, as well as all the results exceeding it.
Four putative QTL were found to be in previously reported iron-related QTL in mice (Table 3). First, a 0.29 Mb putative QTL (at ∼34 Mb) on Chromosome 7 associated with iron levels in males in our study, overlaps with a 2.2 cM QTL affecting the severity of hepatic iron loading (6) and ventral midbrain iron content (29) in mice. Second, a 0.22 Mb putative QTL (at ∼134 Mb) on Chromosome 7 in females, overlaps with a 31 cM QTL for basal liver iron in both males and males/females combined (25), and is also close to a reported QTL for ventral midbrain iron content (29). The only validated gene in this QTL is Adam12, previously identified in a separate in silico mapping study as a single candidate for fat mass after 8 wk on an atherogenic diet (35). Third, a 0.64 Mb putative QTL on Chromosome 8 in males overlaps with a 19 cM QTL with effect on iron status in females (25). Fourth, a 0.32 Mb putative QTL on Chromosome 11 in females overlaps with a 39 cM QTL affecting basal liver iron status in females (25) and is close to the 3 cM QTL associated with hepatic iron loading reported by Bensaid et al. (6). It also borders a ventral midbrain iron content QTL (29). Our in silico results refine these four QTL from ∼12 Mb to 0.28 Mb and from ∼60 Mb to 0.22 Mb for the first and second QTL on Chromosome 7, respectively, from ∼51 Mb to 0.64 Mb for the third QTL on Chromosome 8, and from ∼49 Mb to 0.32 Mb for the fourth QTL on Chromosome 11.
Table 3.
Comparison of QTL regions associated with liver iron levels in inbred mice (mapped by an “in silico” approach) to previously identified iron-related QTL regions (mapped by a linkage approach)
| “In Silico” Mapping |
Linkage Mapping |
||||||
|---|---|---|---|---|---|---|---|
| Chr | QTL Location, Mb | Sex | Trait | Marker | QTL Location, Mb | Sex | Reference |
| 7 | 33.92–34.21 | M | hepatic iron loading, ventral midbrain iron content | D7Mit246 | 25.80–37.21 | Bensaid et al. (6), Jones et al. (29) | |
| 7* | 133.93–134.15 | F | iron status | D7Mit71 | 90.88–151.79 | C, M | Grant et al. (25) |
| 8 | 89.22–89.86 | M | iron status | D8Mit195 | 46.54–97.42 | F | Grant et al. (25) |
| 11 | 56.70–57.02 | F | iron status | D11Mit36 | 53.98–103.24 | F | Grant et al. (25) |
Sex: male (M), female (F), or combined (C).
This QTL was identified by separate “in silico” study as associated with percent of fat mass after 6 wk on atherogenic diet (Ref. 35).
Our mapping study also identified 22 novel putative QTL influencing iron levels in the liver (Table 2). Several of these putative QTL contain candidate genes which are reported to play roles in different disorders, e.g., the 0.65 Mb region on Chromosome 3 containing Lef1 and Cyp2u1, the 0.37 Mb region on Chromosome 6 with Mrpl19 and Fam176a, a 0.24 Mb region on Chromosome 11 containing only the Fancl and Vrk2 genes, and a 0.21 Mb region on Chromosome 15 with Trps1 as the only candidate gene. The average size of each putative QTL is 0.67, with only five QTL exceeding 1 Mb in size (Table 2).
Two of the putative QTL exceeding 1 Mb in size contain several interesting candidate genes (the Chromosome 6 QTL contains Slc25a18 and Gdf3 and that on Chromosome 18 contains Nfatc1 and Atp9b, among others). To further dissect the putative QTL on Chromosomes 6 and 18, we calculated pair-wise linkage disequilibrium for all SNPs used in the analysis. Between 120.35 and 122.69 Mb on Chromosome 6, 95 SNPs form three distinct LD blocks (Supplemental Fig. S1A). The most significant results by in silico analysis are all located in the first two LD blocks containing around half of the genes located in the whole 2.34 Mb region. The results on Chromosome 18 between 80.15 and 81.41 Mb using 114 SNPs show the presence of substantial LD throughout the region (Supplemental Fig. S1B). The haplotypes reaching highest significance on Chromosome 18 (AS >2.45) are mostly contained in the third and fourth LD blocks, which harbor only two validated genes, Atp9b and Sall3, and three predicted genes, LOC665115, LOC665124, and LOC665133. However, further dissection of both putative QTL is necessary to obtain higher resolution and distinguish between one or possibly more tightly linked QTL affecting liver iron levels.
Diferric transferrin levels.
QTL analysis identified 11 putative QTL over 7 chromosomes influencing diferric transferrin in males, and 13 putative QTL over 11 chromosomes in females (Table 4, Fig. 3). Previous reports on QTL influencing transferrin saturation did not overlap with our results (28). Liver iron levels and diferric transferrin levels shared one common putative QTL, on Chromosome 5 at ∼114.8 Mb, with Trpv4 and Gltp in the overlapping section. Some of the novel putative QTL contain interesting candidate genes, e.g., GRAIL identified Ramp3 as significantly similar to the Tfrc and Tf genes (P = 0.04). Also, Ankrd6 on Chromosome 4, Hnf1a on Chromosome 5, Nfe2l1 on Chromosome 11 and Apc on Chromosome 18 are interesting candidate genes in corresponding putative QTL. The average putative QTL size is 0.81, with only five QTL exceeding 1 Mb in size (Table 4).
Table 4.
Results of haplotype association mapping of diferric transferrin levels in male and female animals of 13 and 14 inbred mouse strains, respectively
| Chr | QTL Location, Mb | QTL Size, Mb | Sex | Genes |
|---|---|---|---|---|
| 1 | 128.39–128.63 | 0.24 | F | Nckap5 |
| 2 | 104.36–105.08 | 0.72 | F | Cstf3, Tcp11l1, Pin1l, Depdc7, Qser1, Prrg4, Ccdc73, Eif3m, Wt1, 0610012H03Rik |
| 3 | 69.76–71.20 | 1.44 | F | Nmd3, 1110032A04Rik, Gm414 |
| 4 | 23.61–25.92 | 2.31 | M, F | F730047E07Rik, Klhl32, Ndufaf4, Gpr63, Fhl5, 1810074P20Rik, Fut9 |
| 4 | 32.61–33.49 | 0.88 | M | Bach2, Gja10, Casp8ap2, Mdn1, Lyrm2, Ankrd6, Rragd, Ube2j1, Gabrr2, Gabrr1 |
| 5 | 114.54–116.09 | 1.55 | F | Kctd10, Ube3b, Mmab, Mvk, Trpv4, Gltp, Tchp, Git2, 4930515G01Rik, Ankrd13a, 2610524H06Rik, 4930519G04Rik, Oasl2, Oasl1, Hnf1a, Sppl3, Acads, Unc119b, Mlec, Cabp1, Pop5, Rnf10, Coq5, Dynll1, Sfrs9, Gatc, Triap1, Cox6a1, Msi1, Pla2 g1b, Sirt4, Pxn, Rplp0, Gcn1l1, 1110006O24Rik, Rab35, Ccdc64 |
| 5 | 134.23–134.70 | 0.47 | M | Gatsl2, Wbscr16, Gtf2ird2, Ncf1, Gtf2i, Gtf2ird1 |
| 6 | 147.66–147.93 | 0.27 | M | |
| 7 | 13.27–13.61 | 0.34 | M | |
| 7 | 29.92–30.56 | 0.64 | M | Tbcb, Polr2i, Wdr62, Clip3, Alkbh6, AI428936, Sdhaf1, Lrfn3, Tyrobp, Hcst, Nfkbid, Aplp1, Kirrel2, Nphs1,Prodh2, Snx26, Hspb6, Lin37, Psenen, U2af1l4, Wbp7, Zbtb32, Upk1a, Cox6b1, Etv2, Rbm42, Haus5, 4930479M11Rik, 2200002J24Rik, Atp4a, Tmem147, Sbsn, Dmkn, Gapdhs, Krtdap, Ffar2 |
| 7 | 30.96–31.32 | 0.36 | F | Apbh, Abpe |
| 7 | 90.09–91.30 | 1.21 | M | Ccdc83, Sytl2, Ccdc89, Crebzf, Tmem126a, Tmem126b, Dlg2 |
| 11 | 6.49–6.83 | 0.34 | F | Ccm2, Nacad, Tbrg4, Wap, Ramp3 |
| 11 | 96.59–97.02 | 0.43 | M | Snx11, Cbx1, Nfe2l1, Copz2, Cdk5rap3, Atad4, Pnpo, Sp2, Sp6, Scrn2, Lrrc46, Mrpl10, Osbpl7, Tbx21, Tbkbp1, Kpnb1 |
| 12 | 17.26–17.75 | 0.49 | F | Pdia6, Atp6v1c2, Nol10, Odc1, Hpcal1 |
| 14 | 120.68–124.00 | 3.32 | M | Dock9, Gpr18, Gpr183, Timm8a2, Tm9 sf2, Clybl, Zic5, Zic2, Pcca, A2ld1, Tmtc4, Nalcn, Itgbl1, Fgf14 |
| 16 | 29.33–30.18 | 0.85 | F | Atp13a4, Opa1, Hes1, Cpn2 |
| 16 | 41.00–41.39 | 0.39 | F | |
| 18 | 33.79–34.40 | 0.61 | F | Epb4.1l4a, Apc |
| 18 | 40.85–41.18 | 0.33 | F | |
| 19 | 28.49–28.79 | 0.30 | M | Glis3 |
| 19 | 42.40–43.36 | 0.96 | M | Crtac1, D19Ertd386e, Loxl4, Pyroxd2, Hps1, Hpse2 |
| X | 135.91–136.12 | 0.21 | F | Mid2 |
Liver copper and zinc.
In silico analysis identified 13 putative QTL over 8 chromosomes influencing copper levels in males, and 8 putative QTL over 6 chromosomes in females (Table 5, Fig. 3). For zinc levels, we identified 13 putative QTL over 9 chromosomes in males and 17 putative QTL s over 12 chromosomes in females (Table 5, Fig. 3). The putative QTL on Chromosome 11 influencing liver copper levels in females (at ∼87 Mb) and in males (at ∼95 Mb) overlaps with a QTL previously reported to affect liver copper concentration and stores in male rats (18). This QTL, between D10Rat27-D10Rat98 in the rat, corresponds to 80.02–97.17 Mb on mouse Chromosome 11 (Rat Genome Database at http://rgd.mcw.edu). We have identified a putative QTL close to a QTL influencing prefrontal cortex copper at marker D17Mit49 on Chromosome 17 and zinc at marker rs6260196 on Chromosome 3 in males and a QTL influencing nucleus accumbens zinc at marker rs3663761 on Chromosome 8 in females (30). However, this comparison is difficult to make with certainty since only QTL peaks were reported in the previous study. Liver iron and zinc levels shared one common putative QTL, on Chromosome 3 at ∼66 Mb, with Veph1 and Ptx3 in an overlapping section. GRAIL analysis indicated that several genes, Cox7a2l and Rer1 (P = 0.01), Itga3 (P = 0.02), Ap4b1, Phtf1, and Mpo (P = 0.03), and Pmpcb (P = 0.05) are significantly similar to known genes of copper metabolism including Cox(-6b1, -11, -17, and -19), Cop(-a, -b1, and -z1), Itgb2, Soc1, and Sod1. For zinc, GRAIL found Ptx3 (P = 0.004), Serpina6 (P = 0.009), Il23a and Tff1 (P = 0.01), Crip1 and Cyp24a1 (P = 0.02), Mta1 and Tff2 (P = 0.03), Bcas1, Spz1, and Sorcs1 (P = 0.04), and Serinc5 and Mefv (0.05) to be significantly similar to known genes of zinc metabolism including members of the Slc30 and Slc39 families, Mt1(-a, -e, and g), Hspa1b, Tnf, and Il6. Novel putative QTL include only a few with interesting candidate genes. For example, the 0.34 Mb putative copper QTL on Chromosome 3 contains Ptpn22, the 1.09 Mb putative zinc QTL on Chromosome 7 contains Gabrb3, and the 0.5 Mb putative zinc QTL on Chromosome 17 contains the Abcg1 gene (Table 5). The average putative QTL size is 0.81 for copper, with five exceeding 1 Mb, and 0.64 for zinc, with four exceeding 1 Mb (Table 5).
Table 5.
Results of haplotype association mapping of liver copper and zinc levels in male and female animals of 13 and 14 inbred mouse strains, respectively
| Chr | Trait | QTL Location, Mb | QTL Size, Mb | Sex | Genes |
|---|---|---|---|---|---|
| 1 | zinc | 5.93–6.15 | 0.22 | F | |
| 1 | copper | 21.59–21.81 | 0.22 | F | Kcnq5 |
| 1 | zinc | 34.67–34.92 | 0.25 | F | Arhgef4, Fam168b, Plekhb2 |
| 1 | zinc | 129.89–130.39 | 0.50 | F, M | Zranb3, R3 hdm1, Ubxn4, Lct, Mcm6, Dars |
| 1 | copper | 158.67–160.36 | 1.69 | M | Angptl1, Ralgps2, Sec16b, Fam5b, Astn1 |
| 1 | copper | 190.85–191.78 | 0.93 | F, M | Kcnk2, Cenpf, Ptpn14, Smyd2 |
| 2 | copper | 56.22–58.36 | 2.14 | F | Nr4a2, Gpd2, Galnt5, Ermn, Cytip, Acvr1c, Acvr1 |
| 2 | zinc | 170.00–170.38 | 0.38 | M | Bcas1, Cyp24a1, Pfdn4, 4930470P17Rik |
| 3 | copper | 25.97–26.53 | 0.56 | M | Nlgn1 |
| 3 | zinc | 30.75–31.00 | 0.25 | M | Arpm1, Mynn, Lrriq4, Samd7, Sec62 |
| 3 | zinc | 45.75–46.45 | 0.70 | F | |
| 3 | copper | 52.69–52.93 | 0.24 | M | |
| 3 | zinc | 65.91–67.73 | 1.82 | M | Lekr1, Ccnl1, Veph1, Ptx3, Shox2, Rsrc1, Mlf1, Gfm1, Lxn, Rarres1, Mfsd1 |
| 3 | copper | 103.68–104.20 | 0.52 | M | Syt6, Olfml3, Hipk1, Dclre1b, Ap4b1, Bcl2l15, Ptpn22, Rsbn1, Phtf1, Magi3 |
| 4 | copper | 153.21–154.16 | 0.95 | F | Prdm16, Actrt2, Ttc34, Mmel1, Tnfrsf14, Hes5, Pank4, Plch2, Pex10, Rer1, Morn1, Ski, Prkcz |
| 5 | copper | 20.98–22.46 | 1.48 | M | Fam185a, Fbxl13, Armc10, Napepld, Pmpcb, Dnajc2, Psmc2, Slc26a5, Reln, Orc5l, Lhfpl3 |
| 5 | copper | 24.08–24.69 | 0.61 | M | Abcf2, Smarcd3, Nub1, Wdr86, Crygn, Rheb, Prkag2, 2900005J15Rik |
| 5 | zinc | 33.38–33.73 | 0.35 | M | Slc5a1, Spon2, Ctbp1, Maea, 4933407H18Rik |
| 5 | copper | 52.93–53.20 | 0.27 | M | Sepsecs, Pi4k2b, Zcchc4, Anapc4 |
| 6 | zinc | 114.58–114.80 | 0.22 | F | Atg7 |
| 7 | zinc | 57.01–58.69 | 1.68 | M | Gabrg3, Gabra5, Gabrb3, Atp10a |
| 8 | zinc | 33.28–33.49 | 0.21 | M | Nrg1 |
| 8 | zinc | 52.73–53.37 | 0.64 | M | |
| 8 | zinc | 55.54–56.01 | 0.47 | M | Vegfc |
| 8 | zinc | 58.79–61.94 | 3.15 | M | Adam29, Glra3, Hpgd, Fbxo8, Hand2, Scrg1, Sap30, Hmgb2, Galnt7, Galntl6 |
| 8 | zinc | 80.63–81.22 | 0.59 | F | Ttc29 |
| 9 | copper | 63.21–63.41 | 0.20 | M | Iqch, Aagab |
| 10 | copper | 103.82–104.48 | 0.66 | M | |
| 10 | zinc | 127.51–127.73 | 0.22 | F | Baz2a, Rbms2, Gls2, Spryd4, Mip, Timeless, Apon, Apof, Stat2, Il23a, Usp52, Cnpy2 |
| 11 | copper | 22.99–23.32 | 0.33 | F | Xpo1 |
| 11 | zinc | 51.21–51.54 | 0.33 | F | Col23a1, Agxt2l2, Hnrnpab, Nhp2, Rmnd5b, C330016O10Rik, D930048N14Rik, Sec24a |
| 11 | copper | 86.21–88.35 | 2.14 | F | Rps6kb1, Tubd1, Tmem49, Ptrh2, Cltc, Dhx40, Ypel2, Gdpd1, 1200011M11Rik, Prr11, Fam33a, Trim37, Ppm1e, Rad51c, Rnu3b4, Tex14, Sept4, Mtmr4, Hsf5, Rnf43, Gm3258, Supt4 h1, Bzrap1, Mpo, Lpo, Mks1, Epx, Olfr462, Olfr463, Olfr464, Dynll2, Sfrs1, Vezf1, Cuedc1, Mrps23, 1700106J16Rik, Msi2 h |
| 11 | copper | 94.85–95.63 | 0.78 | M | Pdk2, Itga3, Dlx3, Dlx4, Tac4, Myst2, Fam117a, Slc35b1, Spop, Nxph3, Ngfr, Phb, Zfp652 |
| 11 | zinc | 101.69–102.14 | 0.45 | M | Meox1, Sost, Dusp3, 1700006E09Rik, Mpp3, Cd300lg, Mpp2, Ppy, Pyy, Nags, Tmem101, Lsm12, G6pc3, Hdac5, BC030867, Asb16, Tmub2, Atxn7l3, Ubtf |
| 12 | copper | 88.78–89.36 | 0.58 | F | Gm5662, Gm5039, Adck1 |
| 12 | zinc | 103.28–104.86 | 1.58 | M, F | Cox8c, Unc79, Prima1, Asb2, Otub2, Ddx24, Ifi27l1, Ifi27l2a, Ifi27l2b, Ppp4r4, Serpina (10, 6, 1f, 1b, 1d, 1a, 1c, 11, 9, 4-ps1, 5, 3a, 3b, 3c, 3f, 3 g, 3 h, 3k, 3m, 3n), Gm4931 |
| 12 | zinc | 112.89–113.69 | 0.80 | F | Tmem179, 2610204M08Rik, Adssl1, Siva1, Akt1, Zbtb42, AW555464, Pld4, Cdca4, Gpr132, Jag2, Nudt14, Btbd6, Brf1, Pacs2, Tex22, Mta1, Crip2, Crip1, 4930427A07Rik, Tmem121 |
| 13 | zinc | 93.59–93.88 | 0.29 | F | Zfyve16, Spz1, Serinc5, Thbs4 |
| 15 | zinc | 24.83–25.45 | 0.62 | F | Basp1, Gm5468 |
| 15 | copper | 95.43–96.13 | 0.70 | M | Dbx2, Ano6, D030018L15Rik, Gm4371 |
| 16 | zinc | 3.55–4.10 | 0.55 | M | Mefv, Zfp263, Olfr15, Zfp174, Zfp597, Nat15, 1700037C18Rik, Cluap1, Btbd12, Dnase1, Trap1, Crebbp |
| 17 | zinc | 4.08–4.30 | 0.22 | F | |
| 17 | zinc | 30.70–31.20 | 0.50 | F | Umodl1, Abcg1, Tff3, Tff2, Tff1, Tmprss3, Ubash3a, Rsph1, Slc37a1, Pde9a |
| 17 | copper | 42.41–42.65 | 0.24 | M | Tnfrsf21 |
| 17 | copper | 82.84–83.84 | 1.00 | F | Pkdcc, Eml4, Cox7a2l, Kcng3, Mta3, Haao |
| 18 | zinc | 37.97–38.49 | 0.52 | F | Diap1, Hdac3, Rell2, Fchsd1, Arap3, Pcdh1, 0610009O20Rik, Pcdh12, Rnf14, Gnpda1 |
| 19 | zinc | 19.87–20.10 | 0.23 | F | |
| 19 | zinc | 50.56–50.77 | 0.21 | F | Sorcs1 |
DISCUSSION
In this study we have measured liver iron levels in 18 strains of inbred mouse and found a substantial variation between different strains. This finding is in concordance with previously published studies (13, 25, 28, 33, 47) and strengthens the case for the role of genetic factors in iron homeostasis. Likewise, variation between different strains in copper, zinc, and diferric transferrin levels supports a polygenic influence on these traits as well. Moderate to high heritability (27–60%) in all traits also suggests substantial genetic contribution to the phenotypic variance and corresponds well with reported heritability estimates of known polygenic traits, such as body weight (40–70%, Ref. 43) for example. The significant positive correlation between liver iron and diferric transferrin levels in males and suggestive correlation in females imply possible co-regulation of these traits. The lack of correlations between iron and copper as well as iron and zinc suggests that iron homeostasis in the liver might be regulated independently from copper and zinc metabolism. However, the highly significant positive correlation between copper and zinc in females and the suggestion of a correlation in males support possible co-regulation of these two traits.
Using 14 inbred mouse strains and a haplotype-based approach to perform a search at the genome-wide level, we were able to identify 28 putative QTL influencing basal iron levels in liver using a threshold set at the 5th percentile of the best 1,000 results for each trait. Two of these putative QTL contain genes with a role in iron homeostasis, four overlap known iron-related QTL in mice and 22 are novel. In interpreting these results several factors need to be considered. First, since we used a relatively small number of strains, only loci with stronger genetic effects are picked up by our analysis (63). Secondly, the success of in silico analysis depends not only on the number of strains used, but also on the complexity of the trait itself. More complex traits will mostly be influenced by many loci, each with a small genetic effect, which in turn reduces their likelihood of being detected (62). Thirdly, the HAM approach uses sliding three SNP windows to infer haplotypes across strains. Inferred haplotypes are divided into haplotype groups and singleton haplotypes are left out of the analysis. Some regions of the genome, such as regions of low polymorphism, high haplotype diversity or high recombination rate, as well as new and acquired mutations in the haplotype, can increase the number of singletons and decrease the power of the haplotype analysis (62). This effect, however, is reduced when using three SNP windows compared with four or more SNP windows (51). In summary, the number of strains analyzed, the number of inferred haplotype groups in a specific region and the genetic effect of that region will all have an influence on the statistical power of in silico mapping. Furthermore, the choice of strains plays a role in the success of the in silico approach. Wild-derived strains make inferring haplotypes more difficult, although this is the subject of debate (12, 35, 62, 63). More importantly, the genealogy of inbred strains, which are derived from a small number of founder animals (23), inflates false positive rates due to population structure between the strains. In practice this means that some regions of the genome will have a potential to show spurious association with the trait due solely to genetic relatedness. Even though the HAM method we used corrects for population structure, if the genetic background effect is large it could still lead to spurious associations. Finally, the number of putative QTL identified is dependent on the significance threshold used. Commonly used procedures for correcting for multiple testing may be too stringent for in silico mapping studies with a limited number of strains. However, if not applied, false positive findings may result (62). As a compromise, we used relaxed significance criteria and focused our discussion only on six putative QTL for which corroborating data have been obtained [in the form of previously reported QTL influencing that specific trait, literature reports on the function of specific gene(s) in the putative QTL, or similarity to genes known to influence that trait, as predicted by GRAIL analysis]. Other in silico studies have utilized a similar approach (e.g., Ref. 64). Despite problems facing in silico analysis when relatively small number of strains is used, in practice such numbers are usual. Taking into account cost, space, and work requirements pertaining to every animal in the experiment it is likely that studies focused on certain phenotype will continue using rather small selected sets of strains. Therefore, our methods and results offer some insight for the future studies.
Two identified putative QTL contain genes recently reported to play a role in iron metabolism: Eif2ak1 on Chromosome 5 and Igf2r on Chromosome 17. Eif2ak1 plays an important role in hemoglobin production and red blood cell formation and is important for maturation of macrophages, their capacity for erythrophagocytosis, and the inflammatory response (37). It also plays a protective role in anemias of iron deficiency, erythropoietic protoporphyria, and β-thalassemia (37). In vitro studies suggest Igf2r is a ligand for Hfe, with a possible role in sensing body iron levels; however, these findings need to be confirmed in vivo (58).
Approximately 45 QTL affecting iron status have been identified in mice by classic intercross approaches (2, 6, 25, 28, 29), although the true number is somewhat difficult to ascertain. Comprehensive comparison is prevented because most studies only report QTL peaks. Since QTL size/confidence intervals are not always reported, unless the signal was on the same marker or on a marker <1 Mb away, we counted two QTL as distinct signals. However, we strongly believe that the true number of QTL influencing iron metabolism is significantly smaller. We applied the same criteria to assess overlap between known iron-related QTL and the putative QTL identified by our study. Four putative QTL identified by us reside in, and substantially refine, previously identified QTL with an effect on iron metabolism and are therefore of particular interest. However, due to the difficulties outlined above, we strongly believe that the number of overlapping QTL is likely to be higher.
Out of four putative QTL, those on Chromosome 7 (at ∼34 Mb) and 11 (at ∼57 Mb) each contain only a single well characterized candidate gene. The in silico mapping allowed for a significant refinement of the QTL on Chromosome 7 from 60 Mb to 0.22 Mb. The only gene in the region, Adam12, is a metalloproteinase that is upregulated in liver fibrogenesis and has been implicated in modulating signaling via c-SRC and the TGF-β type II receptor (5, 59). Other members of the TGF-β receptor family, the BMP type I and type II receptors, which transduce their signal via SMAD proteins, are intimately involved in liver iron homeostasis (17). We speculate that Adam12 may play a role in modulating the BMP response. In addition, ADAM12 has a role in adipogenesis (31), and it was also identified as a single candidate for fat mass QTL in a separate in silico study (35). Obesity can result in alterations of iron metabolism in humans, but further studies are needed to better understand their relationship and its clinical relevance (67). Likewise, a study in BSB mice showed that liver iron levels and iron transport genes are correlated with obesity (19). In this context, Adam12 represents interesting candidate for further functional studies.
The putative QTL for iron status on Chromosome 11 overlaps a QTL affecting basal liver iron status in females (25) and borders a QTL associated with hepatic iron loading (6) and ventral midbrain iron content (29). Our analysis significantly reduced the QTL from 49 Mb to 0.32 Mb. The only characterized gene in this region, Gria1, codes for a neurotransmitter receptor in the brain that binds to glutamate. A recent study in retina showed regulation of glutamate production by iron and emphasized that both glutamate and iron are known to be affected in many retinal degeneration and neurological disorders (42). Another study in human cell lines reported a connection between the H63D HFE variant and glutamate excitotoxicity, which might play a role in adult-onset neurodegenerative diseases (46). As noted by McGahan et al. (42), glutamatergic systems are found in many nonneural tissues, and glutamate and iron interactions in the liver, as suggested by our studies and previous linkage findings, seem plausible.
We identified 22 novel putative QTL that did not contain genes previously implicated in iron metabolism. The most plausible candidates, based on the proposed functions of the proteins encoded by the genes underlying putative QTL, are listed in Supplemental Table S2. However, further work is necessary to distinguish between real putative QTL and false positives.
There is only one common putative QTL for liver iron levels and diferric transferrin levels on Chromosome 5 at ∼114.8 Mb, with Trpv4 and Gltp in an overlapping section. There is no published information linking these genes to iron homeostasis. Several other interesting genes reside in putative QTL influencing diferric transferrin. Ramp3 was found to be significantly similar to Tfrc and Tf in GRAIL analysis. Ramp3 and Tfrc are both known to be Wnt target genes (57). Several other genes located in identified putative QTL belong to Wnt pathway: Ankrd6 on Chromosome 4, Hnf1a on Chromosome 5 and Apc on Chromosome 18. Mutations in genes of this pathway lead to a wide range of developmental defects and a variety of diseases, including cancer (48). No common putative QTL were found between iron and copper, or diferric transferrin levels and zinc or copper. Interestingly, there were no shared putative QTL for liver copper and zinc in spite of significant correlation between these two traits. On the contrary, even though there is no evidence of correlation between them, liver iron and zinc levels share one common putative QTL on Chromosome 3 at ∼66 Mb, with Veph1 and Ptx3 in overlapping section. Ptx3 is also indicated by GRAIL analysis as the most similar to known genes of zinc metabolism. It is suggested that Ptx3 plays role in innate immunity, inflammation, female fertility (24) and Parkinson's disease (55), all of which could involve iron and/or zinc. Another interesting gene with similarity to zinc metabolism genes was Cyp24a1 in the putative QTL on Chromosome 2. Cyp24a1 has an important role in vitamin D metabolism (53), and an evaluation of zinc supplement intake has shown that zinc levels increase significantly when zinc is given together with vitamins A and D (52).
There are no previously reported QTL that overlap with those we identified for zinc levels, and there is only one putative QTL overlap previously reported QTL for copper, a QTL influencing hepatic copper in rats. In silico analysis reduces this QTL from 17.15 Mb to 9.42 Mb, but there are still 50 genes in this region. Two of them, Itga3 and Mpo, are marked by GRAIL analysis as similar to known genes of copper metabolism. However, there is no published information that connects these two genes to copper homeostasis. Few of the novel putative QTL have genes with obvious potential links to diferric transferrin, copper or zinc status (Supplemental Table S2). Once again, however, further work is necessary to distinguish between real putative QTL and false positives.
In conclusion, in silico mapping offers a time- and cost-efficient tool for detection of putative QTL underlying complex traits. Our results suggest that in silico mapping is useful even when a relatively small number of strains is used. We were able to identify two genes with a known role in iron metabolism, as well as several QTL involved in iron homeostasis previously mapped by linkage studies. More importantly, we were able to greatly reduce the size of known QTL by in silico mapping to a size that, in general, contains only a small number of candidate genes. Together these serve as important positive controls for the in silico approach. However, the use of a small number of strains causes uncertainty when it comes to newly identified putative QTL, and further work is needed to separate real from false associations. Carefully chosen additional mouse strains would increase the statistical power of this approach and facilitate further dissection of identified regions. They would also facilitate detection of loci that have been missed with the current set of strains. The use of newly developed resources such as Collaborative Cross (60) would further enhance the search for genes with roles in iron, copper, and zinc metabolism. Finally, strategic crosses between strains that carry certain haplotype-phenotype combination would bring unequivocal confirmation of the loci in question.
In addition to the work on newly identified QTL, more work is needed to better characterize confirmed QTL to better understand the genes they contain and their relationship with the trait of interest. Underlying polymorphism(s) might be located in coding, noncoding, or regulatory regions affecting function, splicing, expression, mRNA stability, or microRNA regulation of the gene. Also, translating these findings to humans can potentially give more information about the influence of given variation(s) in affected individuals. The relationships of given variants with the trait will provide insights into possible genetic contributions to susceptibility or resistance to many clinical disorders in which significant morbidity and mortality are associated with perturbations of metabolism of the micronutrients investigated, especially iron. Future research should focus on the development of innovative prevention and treatment strategies tailored to the individual and the present study can help inform such an approach.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-05637 and a Faculty Research Grant both to C. D. Vulpe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank our undergraduate student researchers Su-Fan Vanessa Lin, Ji A. Kim, and Emily Su for contributions to experimental part of the study.
Present addresses: S.-M. Lee, Dept. of Biological Sciences, 815 Fairchild Center, M.C. 2461, Columbia Univ., 1212 Amsterdam Ave., New York, NY 10027; P. L. Hawthorne, Australian Equine Genetics Research Centre, Level 7, Bldg. 68 (Chemistry), Univ. of Queensland, Queensland, 4072, Australia.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, Leiendecker-Foster C, Speechley M, Snively BM, Holup JL, Thomson E, Sholinsky P. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352: 1769–1778, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Ajioka RS, LeBoeuf RC, Gillespie RR, Amon LM, Kushner JP. Mapping genes responsible for strain-specific iron phenotypes in murine chromosome substitution strains. Blood Cells Mol Dis 39: 199–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 358: 221–230, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Anderson GJ. Mechanisms of iron loading and toxicity. Am J Hematol 82: 1128–1131, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Atfi A, Dumont E, Colland F, Bonnier D, L'Helgoualc'h A, Prunier C, Ferrand N, Clement B, Wewer UM, Theret N. The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor. J Cell Biol 178: 201–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bensaid M, Fruchon S, Mazeres C, Bahram S, Roth MP, Coppin H. Multigenic control of hepatic iron loading in a murine model of hemochromatosis. Gastroenterology 126: 1400–1408, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Burgess-Herbert SL, Cox A, Tsaih SW, Paigen B. Practical applications of the bioinformatics toolbox for narrowing quantitative trait loci. Genetics 180: 2227–2235, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burgess-Herbert SL, Tsaih SW, Stylianou IM, Walsh K, Cox AJ, Paigen B. An experimental assessment of in silico haplotype association mapping in laboratory mice. BMC Genet 10: 81, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burke W, Imperatore G, Reyes M. Iron deficiency and iron overload: effects of diet and genes. Proc Nutr Soc 60: 73–80, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Cervino AC, Darvasi A, Fallahi M, Mader CC, Tsinoremas NF. An integrated in silico gene mapping strategy in inbred mice. Genetics 175: 321–333, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cervino AC, Li G, Edwards S, Zhu J, Laurie C, Tokiwa G, Lum PY, Wang S, Castellani LW, Lusis AJ, Carlson S, Sachs AB, Schadt EE. Integrating QTL and high-density SNP analyses in mice to identify Insig2 as a susceptibility gene for plasma cholesterol levels. Genomics 86: 505–517, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Chesler EJ, Rodriguez-Zas SL, Mogil JS. In silico mapping of mouse quantitative trait loci. Science 294: 2423, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Clothier B, Robinson S, Akhtar RA, Francis JE, Peters TJ, Raja K, Smith AG. Genetic variation of basal iron status, ferritin and iron regulatory protein in mice: potential for modulation of oxidative stress. Biochem Pharmacol 59: 115–122, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Constantine CC, Anderson GJ, Vulpe CD, McLaren CE, Bahlo M, Yeap HL, Gertig DM, Osborne NJ, Bertalli NA, Beckman KB, Chen V, Matak P, McKie AT, Delatycki MB, Olynyk JK, English DR, Southey MC, Giles GG, Hopper JL, Allen KJ, Gurrin LC. A novel association between a SNP in CYBRD1 and serum ferritin levels in a cohort study of HFE hereditary haemochromatosis. Br J Haematol 147: 140–149, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuppen E. Haplotype-based genetics in mice and rats. Trends Genet 21: 318–322, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Darvasi A. In silico mapping of mouse quantitative trait loci. Science 294: 2423, 2001. [PubMed] [Google Scholar]
- 17. De Domenico I, Ward DM, Kaplan J. Hepcidin regulation: ironing out the details. J Clin Invest 117: 1755–1758, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Wolf ID, Bonne AC, Fielmich-Bouman XM, van Oost BA, Beynen AC, van Zutphen LF, van Lith HA. Quantitative trait loci influencing hepatic copper in rats. Exp Biol Med (Maywood) 227: 529–534, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Farahani P, Chiu S, Bowlus CL, Boffelli D, Lee E, Fisler JS, Krauss RM, Warden CH. Obesity in BSB mice is correlated with expression of genes for iron homeostasis and leptin. Obes Res 12: 191–204, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, Bacon BR, Roopenian DC, Sly WS. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA 98: 2707–2711, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frazer DM, Inglis HR, Wilkins SJ, Millard KN, Steele TM, McLaren GD, McKie AT, Vulpe CD, Anderson GJ. Delayed hepcidin response explains the lag period in iron absorption following a stimulus to increase erythropoiesis. Gut 53: 1509–1515, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frazer DM, Vulpe CD, McKie AT, Wilkins SJ, Trinder D, Cleghorn GJ, Anderson GJ. Cloning and gastrointestinal expression of rat hephaestin: relationship to other iron transport proteins. Am J Physiol Gastrointest Liver Physiol 281: G931–G939, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448: 1050–1053, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 23: 337–366, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Grant GR, Robinson SW, Edwards RE, Clothier B, Davies R, Judah DJ, Broman KW, Smith AG. Multiple polymorphic loci determine basal hepatic and splenic iron status in mice. Hepatology 44: 174–185, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G. In silico mapping of complex disease-related traits in mice. Science 292: 1915–1918, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Jones BC, Beard JL, Gibson JN, Unger EL, Allen RP, McCarthy KA, Earley CJ. Systems genetic analysis of peripheral iron parameters in the mouse. Am J Physiol Regul Integr Comp Physiol 293: R116–R124, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Jones BC, Reed CL, Hitzemann R, Wiesinger JA, McCarthy KA, Buwen JP, Beard JL. Quantitative genetic analysis of ventral midbrain and liver iron in BXD recombinant inbred mice. Nutr Neurosci 6: 369–377, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Jones LC, McCarthy KA, Beard JL, Keen CL, Jones BC. Quantitative genetic analysis of brain copper and zinc in BXD recombinant inbred mice. Nutr Neurosci 9: 81–92, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Kawaguchi N, Xu X, Tajima R, Kronqvist P, Sundberg C, Loechel F, Albrechtsen R, Wewer UM. ADAM 12 protease induces adipogenesis in transgenic mice. Am J Pathol 160: 1895–1903, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koch LG, Britton SL. Genetic component of sensorimotor capacity in rats. Physiol Genomics 13: 241–247, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Leboeuf RC, Tolson D, Heinecke JW. Dissociation between tissue iron concentrations and transferrin saturation among inbred mouse strains. J Lab Clin Med 126: 128–136, 1995. [PubMed] [Google Scholar]
- 34. Liao G, Wang J, Guo J, Allard J, Cheng J, Ng A, Shafer S, Puech A, McPherson JD, Foernzler D, Peltz G, Usuka J. In silico genetics: identification of a functional element regulating H2-Ealpha gene expression. Science 306: 690–695, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Liu P, Vikis H, Lu Y, Wang D, You M. Large-scale in silico mapping of complex quantitative traits in inbred mice. PLoS One 2: e651, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet 38: 888–895, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Liu S, Suragani RN, Wang F, Han A, Zhao W, Andrews NC, Chen JJ. The function of heme-regulated eIF2alpha kinase in murine iron homeostasis and macrophage maturation. J Clin Invest 117: 3296–3305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 277: 973–976, 1997. [DOI] [PubMed] [Google Scholar]
- 39. Manenti G, Galvan A, Pettinicchio A, Trincucci G, Spada E, Zolin A, Milani S, Gonzalez-Neira A, Dragani TA. Mouse genome-wide association mapping needs linkage analysis to avoid false-positive loci. PLoS Genet 5: e1000331, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McClurg P, Janes J, Wu C, Delano DL, Walker JR, Batalov S, Takahashi JS, Shimomura K, Kohsaka A, Bass J, Wiltshire T, Su AI. Genomewide association analysis in diverse inbred mice: power and population structure. Genetics 176: 675–683, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McClurg P, Pletcher MT, Wiltshire T, Su AI. Comparative analysis of haplotype association mapping algorithms. BMC Bioinformatics 7: 61, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGahan MC, Harned J, Mukunnemkeril M, Goralska M, Fleisher L, Ferrell JB. Iron alters glutamate secretion by regulating cytosolic aconitase activity. Am J Physiol Cell Physiol 288: C1117–C1124, 2005. [DOI] [PubMed] [Google Scholar]
- 43. McPherson R. Genetic contributors to obesity. Can J Cardiol 23, Suppl A: 23A–27A, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mhyre TR, Chesler EJ, Thiruchelvam M, Lungu C, Cory-Slechta DA, Fry JD, Richfield EK. Heritability, correlations and in silico mapping of locomotor behavior and neurochemistry in inbred strains of mice. Genes Brain Behav 4: 209–228, 2005. [DOI] [PubMed] [Google Scholar]
- 45. Milman N, Byg KE, Ovesen L. Iron status in Danes 1994. II: Prevalence of iron deficiency and iron overload in 1319 Danish women aged 40–70 years. Influence of blood donation, alcohol intake and iron supplementation. Ann Hematol 79: 612–621, 2000. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell RM, Lee SY, Simmons Z, Connor JR. HFE polymorphisms affect cellular glutamate regulation. Neurobiol Aging [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47. Morse AC, Beard JL, Jones BC. A genetic developmental model of iron deficiency: biological aspects. Proc Soc Exp Biol Med 220: 147–152, 1999. [DOI] [PubMed] [Google Scholar]
- 48. Nusse R. Wnt signaling in disease and in development. Cell Res 15: 28–32, 2005. [DOI] [PubMed] [Google Scholar]
- 49. Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet 8: 58–69, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Pettersson F, Morris AP, Barnes MR, Cardon LR. Goldsurfer2 (Gs2): a comprehensive tool for the analysis and visualization of genome wide association studies. BMC Bioinformatics 9: 138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, Lagler E, Korstanje R, Wang X, Nusskern D, Bogue MA, Mural RJ, Paigen B, Wiltshire T. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS Biol 2: e393, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Potocnik FC, van Rensburg SJ, Hon D, Emsley RA, Moodie IM, Erasmus RT. Oral zinc augmentation with vitamins A and D increases plasma zinc concentration: implications for burden of disease. Metab Brain Dis 21: 139–147, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29: 664–673, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Ramakrishnan U, Frith-Terhune A, Cogswell M, KettelKhan L. Dietary intake does not account for differences in low iron stores among Mexican American and non-Hispanic white women: Third National Health and Nutrition Examination Survey, 1988–1994. J Nutr 132: 996–1001, 2002. [DOI] [PubMed] [Google Scholar]
- 55. Ramsden DB, Parsons RB, Ho SL, Waring RH. The aetiology of idiopathic Parkinson's disease. Mol Pathol 54: 369–380, 2001. [PMC free article] [PubMed] [Google Scholar]
- 56. Raychaudhuri S, Plenge RM, Rossin EJ, Ng AC, Purcell SM, Sklar P, Scolnick EM, Xavier RJ, Altshuler D, Daly MJ. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet 5: e1000534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rohrs S, Kutzner N, Vlad A, Grunwald T, Ziegler S, Muller O. Chronological expression of Wnt target genes Ccnd1, Myc, Cdkn1a, Tfrc, Plf1 and Ramp3. Cell Biol Int 33: 501–508, 2009. [DOI] [PubMed] [Google Scholar]
- 58. Schimanski LM, Drakesmith H, Sweetland E, Bastin J, Rezgui D, Edelmann M, Kessler B, Merryweather-Clarke AT, Robson KJ, Townsend AR. In vitro binding of HFE to the cation-independent mannose-6 phosphate receptor. Blood Cells Mol Dis 43: 180–193, 2009. [DOI] [PubMed] [Google Scholar]
- 59. Stautz D, Sanjay A, Hansen MT, Albrechtsen R, Wewer UM, Kveiborg M. ADAM12 localizes with c-Src to actin-rich structures at the cell periphery and regulates Src kinase activity. Exp Cell Res 316: 55–67, 2009. [DOI] [PubMed] [Google Scholar]
- 60. Threadgill DW, Hunter KW, Williams RW. Genetic dissection of complex and quantitative traits: from fantasy to reality via a community effort. Mamm Genome 13: 175–178, 2002. [DOI] [PubMed] [Google Scholar]
- 61. Tolosano E, Fagoonee S, Garuti C, Valli L, Andrews NC, Altruda F, Pietrangelo A. Haptoglobin modifies the hemochromatosis phenotype in mice. Blood 105: 3353–3355, 2005. [DOI] [PubMed] [Google Scholar]
- 62. Tsaih SW, Korstanje R. Haplotype association mapping in mice. Methods Mol Biol 573: 213–222, 2009. [DOI] [PubMed] [Google Scholar]
- 63. Wang J, Liao G, Usuka J, Peltz G. Computational genetics: from mouse to human? Trends Genet 21: 526–532, 2005. [DOI] [PubMed] [Google Scholar]
- 64. Webb BT, McClay JL, Vargas-Irwin C, York TP, van den Oord EJ. In silico whole genome association scan for murine prepulse inhibition. PLoS One 4: e5246, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitfield JB, Cullen LM, Jazwinska EC, Powell LW, Heath AC, Zhu G, Duffy DL, Martin NG. Effects of HFE C282Y and H63D polymorphisms and polygenic background on iron stores in a large community sample of twins. Am J Hum Genet 66: 1246–1258, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whitfield JB, Treloar S, Zhu G, Powell LW, Martin NG. Relative importance of female-specific and non-female-specific effects on variation in iron stores between women. Br J Haematol 120: 860–866, 2003. [DOI] [PubMed] [Google Scholar]
- 67. Zafon C, Lecube A, Simo R. Iron in obesity. An ancient micronutrient for a modern disease. Obes Rev 11: 322–328, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.