Abstract
The mechanisms underlying oligodendrocyte (OLG) loss and the precise roles played by OLG death in human demyelinating diseases such as multiple sclerosis (MS), and in the rodent model of MS, experimental autoimmune encephalomyelitis (EAE), remain to be elucidated. To clarify the involvement of OLG death in EAE, we have generated transgenic mice that express the baculovirus anti-apoptotic protein p35 in OLGs through the Cre-loxP system. OLGs from cre/p35 transgenic mice were resistant to tumor necrosis factor–α-, anti-Fas antibody- and interferon–γ-induced cell death. cre/p35 transgenic mice were resistant to EAE induction by immunization with the myelin oligodendrocyte glycoprotein. The numbers of infiltrating T cells and macrophages/microglia in the EAE lesions were significantly reduced, as were the numbers of apoptotic OLGs expressing the activated form of caspase–3. Thus, inhibition of apoptosis in OLGs by p35 expression alleviated the severity of the neurological manifestations observed in autoimmune demyelinating diseases.
Keywords: apoptosis/baculovirus p35/caspase/demyelination/oligodendrocyte
Introduction
Experimental autoimmune encephalomyelitis (EAE), which is induced in rodents, is an animal model for multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS) mediated in part by T cells. In both EAE and MS the myelin and myelin-forming cells, the oligodendrocytes (OLGs), are targets of autoimmune attack. Since the destruction of OLGs results in the loss of both myelin and neuronal function, considerable efforts have been made to identify the mechanisms responsible for this damage. Clinical observations and studies using animal models, and tissue and cell culture, have led to the proposal that pro-inflammatory cytokines such as tumor necrosis factor–α (TNF–α) and interferon–γ (IFN–γ) play an important role in mediating OLG death in MS and EAE (Ruddle et al., 1990; Simmons and Willenborg, 1990; D'Souza et al., 1996; Ledeen and Chakraborty, 1998). Markedly elevated Fas expression has been detected in OLGs within MS lesions (Selmaj and Raine, 1988; Selmaj et al., 1991; Louis et al., 1993; D'Souza et al., 1995) and infiltrating Th1 CD4+ cells express Fas ligand (FasL). In vitro, OLG death can be induced by TNF–α, IFN–γ, lymphotoxin (LT) and FasL (Selmaj and Raine, 1988; Selmaj et al., 1991; Louis et al., 1993; D'Souza et al., 1995; Hisahara et al., 1997).
OLGs represent ∼15% of the TUNEL-positive cells in acute MS plaques (60% are macrophages and microglia, 20% are astrocytes) and 25–40% of the dying cells in chronic MS plaques (Dowling et al., 1997). These reports support the concept that OLGs undergo apoptosis in MS and that their death plays an important role in the development and progression of this disease. However, some apparently conflicting results have also been reported. In MS lesions, the massive death of invading T cells, but not of OLGs is observed (for a review, see Raine, 1997). To clarify the precise role of OLG death in the progression of EAE, we generated transgenic mice with targeted expression of the baculovirus anti-apoptotic, caspase-inhibitory protein p35 (for a review, see Villa et al., 1997) in their OLGs and examined the susceptibility of these animals to a demyelinating form of EAE provoked by immunization with myelin oligodendrocyte glycoprotein (MOG). Since prevention of caspase activity in the early embryo could result in an embryonic lethal phenotype, we have generated mice carrying a p35 gene that can be expressed ubiquitously but is not expressed under normal conditions because its open reading frame (ORF) has been disrupted by the insertion of a DNA segment flanked by loxP sites, the Cre recognition sites. Expression of Cre, which mediates recombination of two loxP sites into a single site, with concomitant removal of the DNA segment they flank, restores the p35 ORF, thereby allowing production of p35. We have generated transgenic mice in which cre is expressed under the control of OLG specific promoter myelin basic protein (MBP) gene. By crossing p35 transgenic mice with cre transgenic mice, p35 can be expressed in OLGs. OLGs from cre/p35 transgenic mice were resistant to TNF–α-, anti-Fas antibody- and IFN–γ-induced cell death. When EAE is induced in mice by immunizing with MOG peptide, the numbers of infiltrating T cells and macrophages/microglia in the EAE lesions were significantly reduced, as were the numbers of apoptotic OLGs expressing the activated form of caspase–3 in cre/p35 transgenic mice. Consistent with these observations, the incidence and severity of the progression of EAE were significantly reduced in cre/p35 transgenic mice. Thus, inhibition of apoptosis in OLGs by p35 expression alleviated the severity of the neurological manifestations observed in EAE.
Results
Expression of anti-apoptotic protein p35 in oligodendrocytes
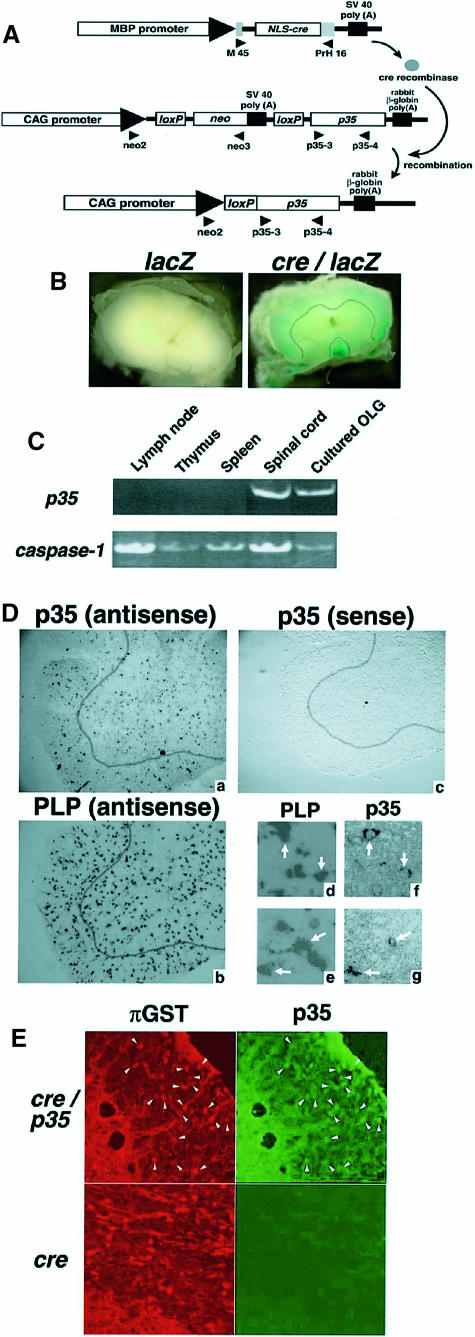
We have generated transgenic mice bearing a p35 gene whose ORF was disrupted by the insertion of a DNA segment flanked by loxP sites, which are Cre recognition sites. To express Cre recombinase in OLGs, we generated transgenic mice expressing Cre recombinase under the control of the MBP gene promoter, whose expression is OLG specific (Miura et al., 1989; Gow et al., 1992) (Figure 1A). The genetic background of both p35 and cre transgenic mice was C57BL/6. The cre transgenic mice (line #29) expressed cre only in the brain and testis, as determined by RT–PCR (data not shown). To visualize the site of recombination in vivo, cre transgenic mice were crossed with loxP-lacZ transgenic mice (Sakai and Miyazaki, 1997). In cre/lacZ transgenic mice, β–galactosidase activity was detected mainly in the white matter of the spinal cord (Figure 1B). After crossing cre and p35 transgenic mice, excision of the neo-stuffer sequence and OLG-specific p35 expression could occur in the cre/p35 transgenic mice. The mice expressing the cre/p35 transgenes showed no apparent developmental or behavioral abnormalities. We confirmed by RT–PCR that p35 mRNA was expressed in the spinal cord and cultured OLGs, but not in organs of the immune system, such as the lymph nodes, thymus and spleen derived from cre/p35 transgenic mice (Figure 1C). Next, we examined the expression of p35 in serial thin sections of the lumbar spinal cord of the cre/p35 transgenic mice by in situ hybridization, probing with p35 and proteolipid protein (PLP), an OLG marker. Many PLP-positive cells expressed p35 mRNA in the white matter of the spinal cord (Figure 1D). We also performed immunohistochemistry using anti–[pi] glutathione S–transferase (GST) antibody, a specific marker of the cell body of mature OLGs (Tansey and Cammer, 1991), and anti–p35 antibody. Most of the p35-expressing cells were also positive for [pi]GST in cre/p35 transgenic mice (Figure 1E). These results indicated that p35 was indeed expressed in the spinal cord of OLGs in the cre/p35 transgenic mice.
Fig. 1. (A) Schematic representation of OLG-specific p35 expression in the Cre-loxP ON/OFF system. The OLG-specific MBP promoter is located 5′ of the NLS-containing cre structural gene, followed by the SV40 polyadenylation signal. The neo gene is under the transcriptional control of the CAG promoter, and this gene is flanked with loxP sites. The baculovirus caspase inhibitory gene, p35, is placed 3′ of the loxP-neo cassette. After crossing these transgenic mice, excision of the neo-stuffer sequence and OLG-specific p35 expression occurred in the cre/p35 transgenic mice. The gray boxes indicate the β–globin exon. The location of each primer used for PCR and RT–PCR is indicated by an arrowhead. (B) β–galactosidase staining of the lumbar spinal cord from a lacZ and a cre/lacZ mouse. MBP-cre transgenic mice were crossed with CAG-loxP-CAT-loxP-lacZ homozygous mice. β–galactosidase staining was performed with 21–day-old cre/lacZ mice and their lacZ littermates. The spinal cord was sectioned axially at the lumbar level. A line has been drawn to separate the gray (outside) and white matter (inside) (right panel). (C) Tissue expression of p35 in a MOG-peptide-injected cre/p35 transgenic mouse. RT–PCR was performed using primers p35–3 and p35–4, indicated by the arrowheads in (A). We also observed the expression of caspase–1 as an internal control of cDNA synthesis. (D) p35 mRNA is expressed in PLP mRNA-positive cells in the cre/p35 mouse. In situ hybridization was performed with p35 and PLP probes in the lumbar spinal cord of a cre/p35 mouse. Many p35-positive and PLP-positive cells were located in the white matter. (a, f and g) p35 antisense; (b, d and e) PLP antisense. (d), (f), (e) and (g) are representative high-magnification views of the white matter (consecutive thin sections) indicating that p35 mRNA is expressed in PLP mRNA-positive cells (arrow). (c) sense probe of p35 in wild type. Lines have been drawn to separate the gray (outside) and white matter (inside) (a–c). Original magnification: (a–c) 100×; (d–g) 400×. (E) Immunohistochemical analysis of the lumbar spinal cords from a cre transgenic and a cre/p35 transgenic mouse. Most of the p35-expressing cells (green) were also [pi]GST-positive OLGs (red) in the white matter of the spinal cord in the cre/p35 transgenic mouse (arrowheads). p35 signal was not detected in the cre transgenic mouse. Original magnifications 400×.
Oligodendrocytes expressing p35 are resistant to TNF–α-, Fas- and IFN–γ-induced cell death
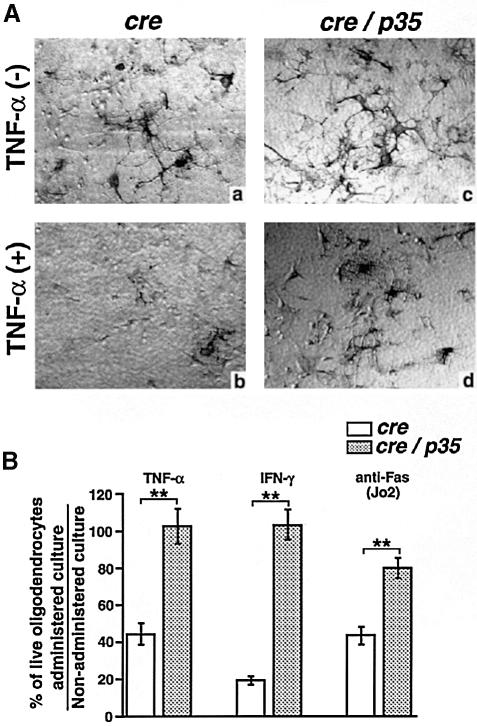
To examine the anti-apoptotic functions of the p35 transgene in OLGs, we tested the sensitivity of OLGs from cre/p35 transgenic mice to TNF–α and IFN–γ, because cytotoxic cytokines such as TNF–α and IFN–γ are thought to play a critical role in OLG apoptosis both in vitro and in EAE/MS (Sharief and Hentges, 1991; Stoll et al., 1993; Hisahara et al., 1997). OLGs from cre/p35 transgenic mice were significantly more resistant to TNF–α- and IFN–γ-induced cell death than OLGs from wild-type mice (Figure 2). It has also been reported that Fas is upregulated on OLGs in CNS tissue from MS lesions and that human CNS-derived OLGs are sensitive to FasL (D'Souza et al., 1996). To examine the effects of p35 on Fas-mediated OLG death, anti-Fas antibody (Jo2) was administered to the OLG culture. OLGs from cre/p35 mice were also significantly resistant to anti-Fas antibody-induced cell death (Figure 2B). These results suggest that p35 can prevent OLG death by various cytotoxic cytokines that may stimulate cell death in EAE/MS.
Fig. 2. (A) Primary cultures of OLGs from transgenic mice were incubated for 72 h with or without 200 ng/ml TNF–α, then fixed and stained with anti-MBP antibody. (a and c) No administration; (b and d) TNF–α administration. Many MBP-positive (live) OLGs from cre/p35 transgenic mice were observed, even in the presence of TNF–α (d). Original magnification 200×. (B) Proportions of live OLGs, calculated by counting MBP-positive OLGs. TNF–α (200 ng/ml), IFN–γ (200 U/ml) or anti-Fas antibody (Jo2) (200 ng/ml) were added to OLG cultures, then each culture was fixed and stained 72 h later. In each experiment, the OLGs collected from each independent well were counted, and relative viability was expressed as the mean ± SEM. Data were collected from three independent experiments. **p <0.01 (Student's t-test).
Progression of EAE is prevented in cre/p35 transgenic mice
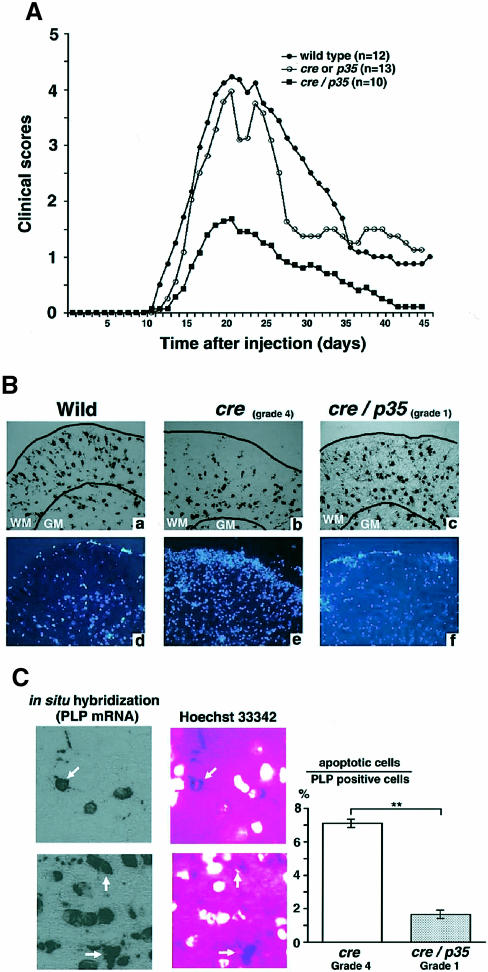
The EAE susceptibility of cre/p35 mice compared with their littermate controls (cre and p35) and wild-type mice was determined by immunizing them with the rat MOG35–55 peptide. Most of the cre, p35 or wild-type mice developed severe hind limb paralysis 11–20 days after immunization (Figure 3A). The incidence of EAE in control mice was ∼88% (11 out of 12 in wild-type and 11 out of 13 in cre or p35 mice, respectively), with a mean peak of clinical severity of 4.25 ± 0.281 in wild-type and 3.97 ± 0.402 in cre or p35 mice. In contrast, the incidence and severity of EAE in the cre/p35 transgenic mice (5 out of 10; 1.5 ± 0.641) were significantly reduced (p <0.01) compared with the control mice (Figure 3A).
Fig. 3. (A) Daily mean clinical course and severity of the EAE induced by MOG 35–55 peptide for each genotype. Clinical signs of the disease were monitored daily beginning on day 7 and were graded on a scale from 0 to 6. (B) Pattern of PLP mRNA expression (a–c) and Hoechst 33342 nuclear staining (d–f) in the lumbar spinal cord as detected by in situ hybridization in a wild-type mouse (a and d), a cre–transgenic mouse with grade 4 EAE (b and e), and a cre/p35 transgenic mouse with grade 1 EAE (c and f). The spinal cord of both transgenic mice was isolated at 20 days after MOG peptide injection. PLP mRNA was heavily expressed in the white matter (WM) and to a lesser extent in the gray matter (GM) in the wild-type mouse (a). Note that the PLP mRNA-expressing OLGs were decreased in the white matter of the grade 4 EAE mouse (b). Many infiltrating cells were found in these areas (e). The cre/p35 transgenic mouse did not show a decrease in PLP mRNA-expressing OLGs (c, f). Original magnifications 200×. (C) Nuclear staining with Hoechst 33342 in in situ hybridization samples in the cre transgenic mouse (grade 4 EAE). PLP mRNA-positive OLGs that showed condensed and fragmented chromatin (arrow). The right graph shows the ratio of apoptotic OLGs in lumbar spinal cord of EAE mice. Data are expressed as the percentage of apoptotic cells out of the total number of PLP-positive cells (mean ± SEM). Original magnifications 400×. **p <0.01 at each distance (Student's t-test).
Oligodendrocyte apoptosis is prevented in the EAE lesion of cre/p35 transgenic mice
To study changes in the number of OLGs in the spinal cord of EAE and control mice, we performed in situ hybridization using a PLP probe. The lumbar spinal cords of cre and cre/p35 transgenic mice 20 days after EAE induction, as well as of age- and sex-matched uninjected wild-type mice, were analyzed. In EAE cre mice, the number of PLP-positive OLGs was markedly reduced in the white matter of the spinal cord, compared with uninjected wild-type mice (Figure 3B, a and b). There were many infiltrating cells in the area where OLGs were decreasing, as revealed by Hoechst 33342 nuclear staining (Figure 3B, d and e). In contrast, in cre/p35 transgenic mice only a few infiltrating cells were observed in the white matter, and the number of OLGs was only slightly lower than in uninjected animals (Figure 3B, c and f). In addition, the number of apoptotic OLGs with fragmented and condensed nuclei was significantly lower in the white matter of the cre/p35 mice compared with cre mice (Figure 3C). The average number of apoptotic OLGs was <2% of the total PLP-positive OLG population in cre/p35 mice compared with ∼7% in cre mice. These results indicate that apoptosis and loss of OLGs are inhibited in cre/p35 mice.
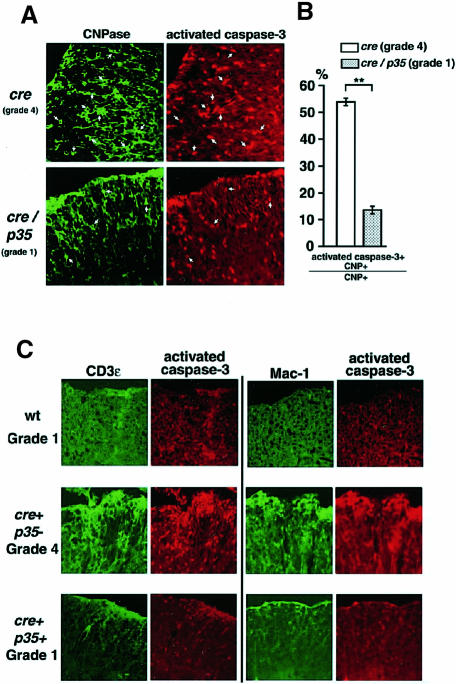
Since one of the hallmarks of apoptosis is the activation of caspase–3, we performed immunohistochemistry with an antibody that specifically recognizes activated caspase–3 (Kouroku et al., 1998) on the spinal cords from an EAE grade 4 cre mouse, an EAE grade 1 cre/p35 mouse and an EAE grade 1 wild-type mouse. We found that the number of OLGs expressing activated caspase–3 was reduced in the spinal cord of the cre/p35 mouse compared with the grade 4 EAE mouse (Figure 4A). Most nuclei of the OLGs expressing activated caspase–3 were fragmented and condensed, indicating that these cells were undergoing apoptosis (data not shown). While more than half of the OLGs expressed activated caspase–3 in the cre mouse, only 15% of the OLGs in the cre/p35 mouse expressed activated caspase–3 (Figure 4B). Moreover, the number of infiltrating cells such as macrophages/microglia and T cells was markedly decreased in the spinal cord of the cre/p35 mouse (Figure 4C). The degree of infiltration in the cre/p35 mouse was comparable to that observed in the wild-type mouse with grade 1 EAE. These results suggest that expression of p35 in OLGs resulted in the inhibition of OLG apoptosis, as well as of infiltration by T cells and macrophages in EAE.
Fig. 4. (A) Immunohistochemical analysis of the lumbar spinal cord from a cre transgenic (grade 4 EAE) and a cre/p35 transgenic (grade 1 EAE) mouse. Each panel shows sections stained with anti-CNPase (green), a specific marker of mature OLGs, and activated caspase–3 (red). Arrows indicate the OLGs that express both CNPase and activated caspase–3. Little expression of activated caspase–3 was detected in the lumbar spinal cord of the cre/p35 transgenic mouse. Original magnifications 400×. (B) Relative number of both CNPase and activated caspase–3-positive OLGs in the white matter of the lumbar spinal cord from both the cre and cre/p35 transgenic mouse. Data are expressed as the percentage of apoptotic cells out of the total number of PLP-positive cells (mean ± SEM). Note that double-stained OLGs are markedly decreased in the cre/p35 transgenic mouse. **p<0.01 at each distance (Student's t–test). (C) Double immunohistochemical staining with anti-CD3ɛ (a marker of T cells) and with anti-Mac–1 (a marker of macrophages/microglia) antibodies (green) and anti-activated caspase–3 antibody (red) in a wild-type (grade 1 EAE), cre transgenic (grade 4 EAE) and cre/p35 transgenic (grade 1 EAE) mouse. In the grade 4 EAE mouse, infiltrating T cells and macrophages/microglia in the EAE lesions of the lumbar spinal cord expressed high levels of activated caspase–3. There were fewer T cells and macrophages/microglia in wild-type and cre/p35 transgenic mice. Note that in wild-type mice with grade 1 EAE some of the cells that were negative for CD3ɛ and Mac–1 were positive for caspase–3. Fewer cells expressing caspase–3 could be detected in cre/p35 transgenic mice with the same grade of EAE. Original magnifications 400×.
Discussion
A broad-range inhibitor of the caspase family, baculovirus p35, blocks apoptosis induced by TNF, Fas, glucocorticoids, radiation, DNA-damaging agents and nerve growth factor withdrawal (for a review, see Villa et al., 1997), and inhibits caspases-1, -2, -3, -4, -6, -7, -8 and –10 in vitro (Bump et al., 1995; Zhou et al., 1998). In vivo, transgenic mice expressing p35 in their thymocytes show inhibition of the major histocompatibility complex (MHC) class II-restricted negative selection induced by staphylococcal enterotoxin B (SEB) superantigen (Izquierdo et al., 1999). These results support the idea that transgenic mice carrying p35 can be used to study the involvement of caspases in degenerative diseases including EAE/MS. There are some inherent difficulties in producing p35 transgenic mice. Inhibition of caspase activity in the embryo could prevent survival because the knockout of certain caspase family members (caspases -3, -8 and -9) causes embryonic lethality due to the CNS abnormality or cardiac failure (for a review, see Los et al., 1999). Thus, to address the role of effector or executioner caspases in OLGs on the induction of EAE, we specifically expressed p35 in OLGs using a Cre-loxP ON/OFF system in transgenic mice (Kanegae et al., 1995; Sakai and Miyazaki, 1997).
Pro-inflammatory cytokines such as TNF–α and IFN–γ, and Fas play an important role in mediating OLG death in MS and EAE (Selmaj and Raine, 1988; Ruddle et al., 1990; Simmons and Willenborg, 1990; Selmaj et al., 1991; Louis et al., 1993; D'Souza et al., 1995, 1996; Ledeen and Chakraborty, 1998). These agents exert their pro-apoptotic function through the activation of caspases. Activated TNF receptor I can bind to adapter molecule TRADD, which in turn binds to another adapter molecule, FADD. Activated Fas recruits FADD. In both cases, caspase–8 is recruited to FADD, then activated in this protein complex (for a review, see Nagata, 1997). In activated T cells, Bcl–2 provides little protection against Fas-induced cell death, but CrmA can prevent Fas-induced T cell death (Strasser et al., 1995). Bcl–2 has differential effects on Fas-induced cell death, which has been explained by the presence of two Fas signaling pathways (Scaffidi et al., 1998). In so-called type I cells, activated caspase–8 propagates the apoptotic signal by activating downstream caspases. In so-called type II cells, activated caspase–8 first cleaves BID, a pro-apoptotic member of the Bcl–2 family, then truncated BID translocates to the mitochondria, where it induces cytochrome c release (Li et al., 1998; Luo et al., 1998). Bcl–2 can prevent Fas-induced cell death in type II cells but not in type I cells (Scaffidi et al., 1998). These reports suggest that the inhibition of caspase activity by p35 may be more effective than Bcl–2 overexpression for preventing the cell death that occurs through the activation of death receptors. IFN–γ-induced cell death is mediated through the upregulation of caspase–1 (Chin et al., 1997). Our results also support the involvement of caspases in IFN–γ-induced OLG death. Taken together, these results indicate that p35 expression could efficiently prevent OLG death by various cytotoxic cytokines that may stimulate OLG injury in the induction of EAE/MS.
Non-Fas- and non-TNF-dependent mechanisms might also be involved in OLG injury in EAE. Cytotoxic T lymphocytes (CTL) rapidly kill target cells using both the granule exocytosis and Fas–Fas ligand pathways. The Fas–Fas ligand-mediated cell death pathway is dependent on caspases (for a review, see Nagata, 1997). Granzyme B can also activate several caspases including caspase–3 (Darmon et al., 1995), and granule-dependent CTL-mediated cell death may be the direct result of granzyme B-mediated caspase–3 activation. While granzyme B seems to initiate cell death via caspase–3 activation, there have been reports that CTL granule exocytosis may be independent of caspases (Sarin et al., 1997; Trapani et al., 1998). Perforin knockout mice show more severe EAE compared with wild type, but there are no major differences in the extent of demyelination and OLG death in the spinal cord in the acute phase of the disease (Malipiero et al., 1997). The regulatory role of CD8+ T cells in EAE involves a perforin-dependent pathway that may operate in the killing of antigen-presenting cells. However, the expression of MHC molecules has not been detected in OLGs in situ (Lee and Raine, 1989). Thus, CTL granule cytolysis does not seem to play a direct role in promoting OLG death in situ.
Caspase–1 plays crucial roles in the cleavage and secretion of pro interleukin–1β (IL–1β) and interferon–γ-inducing factor (IGIF) (Cerretti et al., 1992; Thornberry et al., 1992; Ghayur et al., 1997; Gu et al., 1997). The production of IGIF and IFN–γ in OLGs has not been reported, but IL–1β is constitutively expressed in rat OLGs (Blasi et al., 1999). The major sources of these cytokines in EAE are inflammatory cells, activated microglia and astrocytes (Bauer et al., 1993; Stoll et al., 1993; Wildbaum et al., 1998), not OLGs. It is possible that the expression of p35 prevents IL–1β production in OLGs, but the amount of OLG-derived IL–1β relative to the total IL–1β observed in EAE lesions must be quite low. The expression of p35 in OLGs reduced OLG death and the infiltration of inflammatory cells in the spinal cord. Thus, the expression of p35 in OLGs could also affect the secretion of various cytokines from inflammatory cells in EAE indirectly.
Our study revealed that the inhibition of OLG death by p35 expression results in a significant reduction in both cellular infiltration and demyelination following the induction of EAE by MOG. Interestingly, the clinical severity of EAE was not completely inhibited in all cre/p35 mice. There are several possible explanations for this observation. First, the inhibition of caspase activity depends on p35 expression, and the p35 expression level in the OLGs of these mice may have been insufficient to block the caspase activity completely. Secondly, the activation of caspase family members may be dose dependent. By increasing the antigen peptide dose, it is conceivable that a member of this family could overwhelm the activity of the p35.
Whereas OLGs are a target of the inflammatory response in EAE, the contribution of the OLGs has been unclear. Some apparently conflicting results have been reported. In MS lesions, the massive death of invading T cells, but not of OLGs is observed (for a review, see Raine, 1997). In contrast, apoptotic OLGs were reported in the spinal cords of EAE lesions (Pender et al., 1991). OLGs comprise 15% of the TUNEL-positive cells (60% are macrophages and microglia, 20% are astrocytes) in the acute plaques of MS (Dowling et al., 1997). In the rat EAE model, 64% of the apoptotic cells are T lymphocytes and 9% are OLGs (Gold et al., 1997). In our study, few healthy mature 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)-positive OLGs were detected in the acute plaques of EAE, which were filled with infiltrating cells such as T lymphocytes, macrophages and microglia (S.Hisahara and M.Miura, unpublished data). Apoptotic OLGs could be identified in the regions surrounding the plaques. These OLGs expressed activated caspase–3 and showed nuclear fragmentation and condensation (S.Hisahara and M.Miura, unpublished data). Our results suggest that OLGs located around the plaques undergo apoptosis due to exposure to cytotoxic cytokines secreted by the infiltrating cells. The reduction in the number of PLP mRNA-positive OLGs and the increase in the number of apoptotic OLGs in the regions surrounding the plaques also support the idea that OLG death is tightly associated with the pathology of EAE. These results indicate that OLG death may be partly responsible for the progression of EAE. Our findings also support the idea that decreasing the antigenic myelin fraction by inhibiting OLG death may weaken T cell activation and turn off the ensuing vicious circles. We propose that the inhibition of caspases in target cells might eventually be applicable to reducing the progression of autoimmune diseases, including MS, in humans.
A number of cre transgenic mice or cre knock-in mice have been reported to express Cre in certain tissues, e.g. the insulin promoter for pancreatic β–cells, the myosin light chain 2v locus for cardiac ventricular muscle, and the Ca2+/calmodulin-dependent protein kinase IIa promoter for the pyramidal cells of the hippocampal CA1 region (Tsien et al., 1996; Hirota et al., 1999; Kulkarni et al., 1999). By crossing these mice with p35 mice, we can express p35 in many tissues of interest. Our p35 transgenic mice could provide a useful model for examining the involvement of caspases in development and/or in various models of degenerative diseases in vivo.
Materials and methods
Transgenic mice
pMPV302, a plasmid containing the MBP promoter, was a kind gift from Dr M.Kimura (Kimura et al., 1989). The MBP 5′ region (HindIII fragment, 5 kb) was isolated from cosBJAB5 (Okano et al., 1991), then cloned into the HindIII site of pMPV302. The resulting plasmid was named pM138. NLS-cre/pBlue II, a nuclear localizing signal (NLS)–Cre recombinase gene in the pBluescript II vector (a kind gift from Dr M.Hashimoto), was digested with XbaI, blunt-ended with Klenow, then an EcoRI linker was added to both ends. The fragment was digested with EcoRI and inserted into the EcoRI site of pM138. The resulting plasmid was named pH80. The transgene containing 6.3 kb of the MBP promoter, NLS-cre, and a polyadenylation signal was isolated from pH80 by digestion with KpnI and was used to generate transgenic mice. Anti-apoptotic p35 gene was a kind gift from Dr V.M.Dixit (Beidler et al., 1995), and was cloned into the SwaI site of pCALNLw (a kind gift from Dr I.Saito) (Kanegae et al., 1995). A fragment containing the CAG promoter, loxP-neo-loxP, p35 coding sequence and a polyadenylation signal was cloned into pBluescript and named pA23. This fragment was used to generate p35 transgenic mice. Each transgene was injected into fertilized C57BL/6 eggs. DNA from each transgenic mouse was extracted from tail biopsies as described (Laird et al., 1991) and genotyped by Southern blotting. Genomic DNA was digested with EcoRI and probed with the EcoRI fragment of pH80 for cre transgenic mice, and probed with the SpeI–HindIII fragment of pA23 for p35 transgenic mice. The CAG-loxP-CAT-loxP-lacZ mouse has been described previously (Sakai and Miyazaki, 1997).
PCR and RT–PCR
To detect the p35 transgene in mice, we performed PCR with primers of neo2 and neo3 or p35–3 and p35–4 as follows: forward (neo2) 5′–TCTGACTGACCGCGTTACTC–3′; reverse (neo3) 5′–TATTCGGCAAGCAGGCATCG–3′; forward (p35–3) 5′–TGGATGGATTCCACGATAGC–3′; reverse (p35–4) 5′–TGCACACTCTCCACGTAAGC–3′. DNA was denatured at 94°C for 2 min, annealed at 55°C for 1.5 min and elongated at 72°C for 1 min with 30 cycles. The primers used for PCR and RT–PCR to detect cre expression in MBP-NLS-cre mouse were as follows: forward (M45) 5′–GGATCCTGAGAACTTCAGG–3′; reverse (PrH16) 5′–GGGAATTCACTATCCAGGTTACGGAT–3′. Total RNAs were isolated from thymus, spleen, lymph nodes and spinal cord of cre/p35 transgenic mouse 20 days after EAE induction. Total RNA samples (1 μg) were treated with 1 U of DNase I (Gibco–BRL) at 25°C for 1 h. We confirmed the complete digestion of genomic DNA in each RNA solution by PCR using p35–3 and p35–4 primers (data not shown), then cDNAs were synthesized as described (Hisahara et al., 1997). RT–PCR (25 cycles) using p35–3 and p35–4 primers was performed to detect p35 expression. We also investigated caspase–1 expression by RT–PCR (25 cycles) with specific primers as follows: forward (M47) 5′–TCCAGGAGGGAATATGTGG–3′; reverse (M48) 5′–CTTGTTTCTCTCCACGGCA–3′. To detect the CAG-loxP-CAT-loxP-lacZ transgene by PCR, we used CAT 1 and CAT 2 primers (Sakai and Miyazaki, 1997).
β–galactosidase staining
The spinal cords were dissected from 8–week-old male cre/lacZ mice and from their male littermates after the animals were killed under diethyl ether and perfused with 2% paraformaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline (PBS). The lumbar cord was cut into pieces ∼1–2 mm in length and incubated with 15% sucrose in PBS at 4°C overnight. These samples were washed with PBS three times, then incubated with β–galactosidase staining solution (0.08 M sodium phosphate, 8 mM KCl, 1 μM MgCl2, 3 mM K4[Fe(CN)6], 3 mM K3[Fe(CN)6], 0.1% Triton X–100, 0.05% X–gal) at 37°C for 14–18 h. Specimens were examined by microscopy (Olympus IX70).
In situ hybridization
A cre/p35 mouse and one of its littermate cre mice were perfused under anesthesia through the left ventricle with 4% paraformaldehyde in PBS, then the cervical/thoracic and lumbar spinal cords were dissected. For in situ hybridization, cryostat sections (7 μm) were cut and affixed to glass slides pre-coated with 3–aminopropyltriethoxysilane (APS). Slides were post-fixed with 3.7% formaldehyde in PBS pH 7.4 for 5 min, acetylated in 0.25% acetic anhydride in 0.1 M triethanolamine–HCl pH 8.0 for 10 min, and dehydrated in graded alcohol solutions. Mouse PLP cDNA was a kind gift from Dr Ikenaka (Kagawa et al., 1994). Antisense and sense RNA probes were prepared by in vitro transcription with SP6 (sense)/T7 (antisense) RNA polymerase for the PLP probes and T7 (sense)/SP6 (antisense) RNA polymerase for p35. Hybridization was performed with 80 ng/ml RNA probes at 68°C in hybridization buffer (50% formamide, 5× SSPE, 5% SDS, 1 mg/ml yeast tRNA) overnight. The next day, the slides were washed with three incubations in washing solution (50% formamide, 2× SSC) at 65°C for 30 min, then incubated with blocking solution [1% skimmed milk in TBST (25 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.2% Tween)] at room temperature for 1 h. The samples were then incubated with anti-digoxigenin antibody conjugated with alkaline phosphatase (ALP) (1:1000 dilution) (Boehringer Manheim, Mannheim, Germany) overnight at 4°C. After washing with TBST, signals bound to the antibody were detected with an ALP buffer (0.1 M Tris–HCl pH 9.5, 0.1 M NaCl, 0.1% polyoxyethylene sorbitan monolaurate, 0.05 M MgCl2) containing bromo-chloro-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT) at room temperature for 3–12 h. Nuclear staining was performed with Hoechst 33342 (10 μM) simultaneously. PLP mRNA-positive OLGs were examined with a fluorescence microscope (Zeiss Axiophot2). The proportion of apoptotic PLP mRNA-positive OLGs was determined by counting the number of cells. Data were collected in three independent visual fields in a blind fashion.
Primary cultures
The methods for OLG primary culture and analysis of cell death with cytotoxic cytokines were described previously (Hisahara et al., 1997). Briefly, embryonic day 17–18 pups were decapitated and their brains were minced and placed in 25–cm2 delta (Δ)-treated flasks (Nunc, Roskilde, Denmark) in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS). After 7–9 days in culture, the flask was shaken by hand. Cells that became detached were collected and plated in poly-l–lysine-coated dishes in DMEM containing 2% FBS and Bottenstein and Sato's (BS) supplement (Hall et al., 1996). Mature OLGs were observed after several days, at which time 200 ng/ml TNF–α, 200 U/ml IFN–γ (Genzyme) and 200 ng/ml anti-Fas antibody (Jo2) (Pharmingen) were added. After a 72 h incubation, cells were fixed with 4% paraformaldehyde in PBS for 10 min. Samples were incubated with rabbit polyclonal anti-MBP antibody and ALP-conjugated anti-rabbit IgG antibody (Dako). Proteins were detected with an ALP buffer containing BCIP and NBT as substrates. We performed three independent experiments. In each, the number of live OLGs collected from each independent well was counted, and the relative viability was expressed as the mean ± SEM. Total RNA of cultured OLGs for RT–PCR in cre/p35 mouse was isolated as described previously (Hisahara et al., 1997).
Induction and assessment of EAE
The Animal Welfare Guidelines of Osaka University Medical School were followed for all studies and experiments with mice. The procedure for inducing EAE was as described previously (Liu et al., 1998). Briefly, rat oligodendrocyte glycoprotein (MOG) 35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide was synthesized by Biologica, Japan. Eight-week-old transgenic and wild-type mice received a subcutaneous injection into the footpads of bilateral hind feet of 100 μg of peptide in 0.1 ml of sterilized PBS emulsified with an equal volume of complete Freund's adjuvant (CFA) containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco). Each mouse also received 300 ng of pertussis toxin (List Biological Laboratories) in 0.25 ml of PBS, intravenously, immediately and 48 h after the immunization. Clinical signs of disease were graded on a scale of 0–6, with 0.5 points for immediate clinical findings as follows: 0 = normal; 1 = weakness of the tail; 2 = complete loss of tail tonicity or abnormal gait; 3 = partial hind limb paralysis; 4 = complete hind limb paralysis; 5 = forelimb paralysis or moribund; 6 = death. To eliminate any diagnostic bias, genotyping of transgenic mice was performed 30–40 days after injection.
Immunohistochemistry
Tissue preparation was as described above. Specimens were incubated with anti–[pi]GST antibody (1:200 dilution; MBL, Nagoya, Japan), a marker of the mature cell body of OLGs, and polyclonal anti-p35 antibody (1:50 dilution). Polyclonal antibody against p35 was produced by injecting a recombinant GST–p35 fusion protein into mice. We confirmed the specificity of this antibody by Western blotting and immunocytochemistry using COS7 cells expressing p35 (data not shown). The fluorescent signal of p35 was enhanced using a Tyramide Signal Amplification (TSA™-Direct) kit (NEN Life Science Products). For investigation of caspase family expression in the spinal cord lesion, samples were incubated with a rabbit polyclonal anti-activated caspase–3 antibody that recognizes only the processed form of caspase–3 (Kouroku et al., 1998). Samples were also incubated with anti-CNPase antibody (1:100 dilution; Sigma), a marker of mature OLGs, anti-Mac–1 antibody (1:100 dilution; Boehringer Mannheim), a marker of macrophages/microglia, or with an anti-CD–3ɛ antibody (1:100 dilution; Pharmingen), a marker of T cells. The specimens were examined with a fluorescence microscope (Zeiss LSM–510). Counting and analysis of the proportion of activated caspase–3-positive OLGs were performed in three independent visual fields at a magnification of 400×, in a blind fashion.
Statistical methods
Results were expressed as the mean ± SEM. The probability of statistical differences between experimental groups was determined by Student's t-test, as indicated.
Acknowledgments
Acknowledgements
We are grateful to Drs Izumu Saito at the University of Tokyo, Japan, Minoru Kimura at Tokai University Medical School, Japan, Mitsuhiro Hashimoto at Brain Science Institute, RIKEN, Japan, Vishva M.Dixit at Genentech, USA, and Kazuhiro Ikenaka at Okazaki National Institutes, Japan, for providing us with recombinant adenovirus AxCALNLw, pMPV302, NLS-cre/pBlue II, pcDNA–p35 and PLP cDNA, respectively. We thank Drs Ralf Gold at Julius-Maximilians-University, Germany, and Saburou Sakoda and Yoshinobu Okuda at Osaka University, Japan, for teaching us how to induce EAE. This work is supported by grants from the Human Frontier Science Program to H.O. and grants-in-aid from the Ministry of Education, Science and Culture to M.M. and H.O. This work is also supported by CREST of the Japan Science and Technology Corporation.
References
- Bauer J., Berkenbosch, F., Van Dam, A.M. and Dijkstra, C.D. (1993) Demonstration of interleukin–1β in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultrastructural level. J. Neuroimmunol., 48, 13–21. [DOI] [PubMed] [Google Scholar]
- Beidler D.R., Tewari, M., Friesen, P.D., Poirier, G. and Dixit, V.M. (1995) The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J. Biol. Chem., 270, 16526–16528. [DOI] [PubMed] [Google Scholar]
- Blasi F. et al. (1999) Constitutive expression of interleukin–1β (IL–1β) in rat oligodendrocytes. Biol. Chem., 380, 259–264. [DOI] [PubMed] [Google Scholar]
- Bump N.J. et al. (1995) Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science, 269, 1885–1888. [DOI] [PubMed] [Google Scholar]
- Cerretti D.P. et al. (1992) Molecular cloning of the interleukin–1β converting enzyme. Science, 256, 97–100. [DOI] [PubMed] [Google Scholar]
- Chin Y.E., Kitagawa, M., Kuida, K., Flavell, R.A. and Fu, X.Y. (1997) Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol., 17, 5328–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon A.J., Nicholson, D.W. and Bleackley, R.C. (1995) Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature, 377, 446–448. [DOI] [PubMed] [Google Scholar]
- Dowling P., Husar, W., Menonna, J., Donnenfeld, H., Cook, S. and Sidhu, M. (1997) Cell death and birth in multiple sclerosis brain. J. Neurol. Sci., 149, 1–11. [DOI] [PubMed] [Google Scholar]
- D'Souza S., Alinauskas, K., McCrea, E., Goodyer, C. and Antel, J.P. (1995) Differential susceptibility of human CNS-derived cell populations to TNF–dependent and independent immune-mediated injury. J. Neurosci., 15, 7293–7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S.D., Bonetti, B., Balasingam, V., Cashman, N.R., Barker, P.A., Troutt, A.B., Raine, C.S. and Antel, J.P. (1996) Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J. Exp. Med., 184, 2361–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayur T. et al. (1997) Caspase–1 processes IFN–γ-inducing factor and regulates LPS-induced IFN–γ production. Nature, 386, 619–623. [DOI] [PubMed] [Google Scholar]
- Gold R., Hartung, H.P. and Lassmann, H. (1997) T-cell apoptosis in autoimmune diseases: termination of inflammation in the nervous system and other sites with specialized immune-defense mechanisms. Trends Neurosci., 20, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A., Friedrich, V.L.,Jr and Lazzarini, R.A. (1992) Myelin basic protein gene contains separate enhancers for oligodendrocytes and Schwann cell expression. J. Cell Biol., 119, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. et al. (1997) Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science, 275, 206–209. [DOI] [PubMed] [Google Scholar]
- Hall A., Giese, N.A. and Richardson, W.D. (1996) Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGFα-receptors. Development, 122, 4085–4094. [DOI] [PubMed] [Google Scholar]
- Hirota H., Chen, J., Betz, U.A., Rajewsky, K., Gu, Y., Ross, J.,Jr, Muller, W. and Chien, K.R. (1999) Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell, 97, 189–198. [DOI] [PubMed] [Google Scholar]
- Hisahara S., Shoji, S., Okano, H. and Miura, M. (1997) ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J. Neurochem., 69, 10–20. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Grandien, A., Criado, L.M., Robles, S., Leonardo, E., Albar, J.P., de Buitrago, G.G. and Martinez, A.C. (1999) Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO J., 18, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T. et al. (1994) Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron, 13, 427–442. [DOI] [PubMed] [Google Scholar]
- Kanegae Y., Lee, G., Sato, Y., Tanaka, M., Nakai, M., Sakaki, T., Sugano, S. and Saito, I. (1995) Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res., 23, 3816–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Sato, M., Akatsuka, A., Nozawa-Kimura, S., Takahashi, R., Yokoyama, M., Nomura, T. and Katsuki, M. (1989) Restoration of myelin formation by a single type of myelin basic protein in transgenic shiverer mice. Proc. Natl Acad. Sci. USA, 86, 5661–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroku Y., Urase, K., Fujita, E., Isahara, K., Ohsawa, Y., Uchiyama, Y., Momoi, M.Y. and Momoi, T. (1998) Detection of activated caspase–3 by a cleavage site-directed antiserum during naturally occurring DRG neurons apoptosis. Biochem. Biophys. Res. Commun., 247, 780–784. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.N., Bruning, J.C., Winnay, J.N., Postic, C., Magnuson, M.A. and Kahn, C.R. (1999) Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell, 96, 329–339. [DOI] [PubMed] [Google Scholar]
- Laird P.W., Zijderveld, A., Linders, K., Rudnicki, M.A., Jaenisch, R. and Berns, A. (1991) Simplified mammalian DNA isolation procedure. Nucleic Acids Res., 19, 4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R.W. and Chakraborty, G. (1998) Cytokines, signal transduction and inflammatory demyelination: review and hypothesis. Neurochem. Res., 23, 277–289. [DOI] [PubMed] [Google Scholar]
- Lee S.C. and Raine, C.S. (1989) Multiple sclerosis: oligodendrocytes in active lesions do not express class II major histocompatibility complex molecules. J. Neuroimmunol., 25, 261–266. [DOI] [PubMed] [Google Scholar]
- Li H., Zhu, H., Xu, C.J. and Yuan, J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell, 94, 491–501. [DOI] [PubMed] [Google Scholar]
- Liu J., Marino, M.W., Wong, G., Grail, D., Dunn, A., Bettadapura, J., Slavin, A.J., Old, L. and Bernard, C.C. (1998) TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nature Med., 4, 78–83. [DOI] [PubMed] [Google Scholar]
- Los M., Wesselborg, S. and Schulze-Osthoff, K. (1999) The role of caspases in development, immunity and apoptotic signal transduction: lessons from knockout mice. Immunity, 10, 629–639. [DOI] [PubMed] [Google Scholar]
- Louis J.C., Magal, E., Takayama, S. and Varon, S. (1993) CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science, 259, 689–692. [DOI] [PubMed] [Google Scholar]
- Luo X., Budihardjo, I., Zou, H., Slaughter, C. and Wang, X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell, 94, 481–490. [DOI] [PubMed] [Google Scholar]
- Malipiero U., Frei, K., Spanaus, K.S., Agresti, C., Lassmann, H., Hahne, M., Tschopp, J., Eugster, H.P. and Fontana, A. (1997) Myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis is chronic/relapsing in perforin knockout mice, but monophasic in Fas- and Fas ligand-deficient lpr and gld mice. Eur. J. Immunol., 27, 3151–3160. [DOI] [PubMed] [Google Scholar]
- Miura M., Tamura, T., Aoyama, A. and Mikoshiba, K. (1989) The promoter elements of the mouse myelin basic protein gene function efficiently in NG108-15 neuronal/glial cells. Gene, 75, 31–38. [DOI] [PubMed] [Google Scholar]
- Nagata S. (1997) Apoptosis by death factor. Cell, 88, 355–365. [DOI] [PubMed] [Google Scholar]
- Okano H., Aruga, J., Nakagawa, T., Shiota, C. and Mikoshiba, K. (1991) Myelin basic protein gene and the function of antisense RNA in its repression in myelin-deficient mutant mouse. J. Neurochem., 56, 560–567. [DOI] [PubMed] [Google Scholar]
- Pender M.P., Nguyen, K.B., McCombe, P.A. and Kerr, J.F. (1991) Apoptosis in the nervous system in experimental allergic encephalomyelitis. J. Neurol. Sci., 104, 81–87. [DOI] [PubMed] [Google Scholar]
- Raine C.S. (1997) The Norton Lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J. Neuroimmunol., 77, 135–152. [DOI] [PubMed] [Google Scholar]
- Ruddle N.H., Bergman, C.M., McGrath, K.M., Lingenheld, E.G., Grunnet, M.L., Padula, S.J. and Clark, R.B. (1990) An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med., 172, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. and Miyazaki, J. (1997) A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem. Biophys. Res. Commun., 237, 318–324. [DOI] [PubMed] [Google Scholar]
- Sarin A., Williams, M.S., Alexander-Miller, M.A., Berzofsky, J.A., Zacharchuk, C.M. and Henkart, P.A. (1997) Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity, 6, 209–215. [DOI] [PubMed] [Google Scholar]
- Scaffidi C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K.J., Debatin, K.M., Krammer, P.H. and Peter, M.E. (1998) Two CD95 (APO–1/Fas) signaling pathways. EMBO J., 17, 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K.W. and Raine, C.S. (1988) Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol., 23, 339–346. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine, C.S., Cannella, B. and Brosnan, C.F. (1991) Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Invest., 87, 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief M.K. and Hentges, R. (1991) Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N. Engl. J. Med., 325, 467–472. [DOI] [PubMed] [Google Scholar]
- Simmons R.D. and Willenborg, D.O. (1990) Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J. Neurol. Sci., 100, 37–42. [DOI] [PubMed] [Google Scholar]
- Stoll G., Muller, S., Schmidt, B., van der Meide, P., Jung, S., Toyka, K.V. and Hartung, H.P. (1993) Localization of interferon-γ and Ia-antigen in T cell line-mediated experimental autoimmune encephalomyelitis. Am. J. Pathol., 142, 1866–1875. [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris, A.W., Huang, D.C., Krammer, P.H. and Cory, S. (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J., 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey F.A. and Cammer, W. (1991) A [pi] form of glutathione-S-transferase is a myelin- and oligodendrocyte-associated enzyme in mouse brain. J. Neurochem., 57, 95–102. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. et al. (1992) A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature, 356, 768–774. [DOI] [PubMed] [Google Scholar]
- Trapani J.A., Jans, D.A., Jans, P.J., Smyth, M.J., Browne, K.A. and Sutton, V.R. (1998) Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J. Biol. Chem., 273, 27934–27938. [DOI] [PubMed] [Google Scholar]
- Tsien J.Z., Chen, D.F., Gerber, D., Tom, C., Mercer, E.H., Anderson, D.J., Mayford, M., Kandel, E.R. and Tonegawa, S. (1996) Subregion- and cell type-restricted gene knockout in mouse brain. Cell, 87, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Villa P., Kaufmann, S.H. and Earnshaw, W.C. (1997) Caspases and caspase inhibitors. Trends Biochem. Sci., 22, 388–393. [DOI] [PubMed] [Google Scholar]
- Wildbaum G., Youssef, S., Grabie, N. and Karin, N. (1998) Neutralizing antibodies to IFN–γ-inducing factor prevent experimental autoimmune encephalomyelitis. J. Immunol., 161, 6368–6374. [PubMed] [Google Scholar]
- Zhou Q., Krebs, J.F., Snipas, S.J., Price, A., Alnemri, E.S., Tomaselli, K.J. and Salvesen, G.S. (1998) Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics and characterization of the caspase/p35 complex. Biochemistry, 37, 10757–10765. [DOI] [PubMed] [Google Scholar]