Abstract
Although chemosensory signals generated by mouse pups may trigger maternal behavior of females, the mechanism for detection of these signals has not been fully defined. As some odorant receptors are coupled to the type 3 adenylyl cyclase (AC3), we evaluated the role of AC3 for maternal behavior using AC3−/− female mice. Here, we report that maternal behavior is impaired in virgin and postpartum AC3−/− mice. Female AC3−/− mice failed the pup retrieval assay, did not construct well-defined nests, and did not exhibit maternal aggression. Furthermore, AC3−/− females could not detect odorants or pup urine in the odorant habituation test and were unable to detect pups by chemoreception. In contrast to wild-type mice, AC activity in main olfactory epithelium (MOE) preparations from AC3−/− female mice was not stimulated by odorants or pheromones. Moreover, odorants and pheromones did not evoke electro-olfactogram (EOG) responses in the MOE of AC3−/− female mice. We hypothesize that the detection of chemical signals that trigger maternal behavior in female mice depends upon AC3 in the MOE.
Keywords: maternal behavior, type 3 adenylyl cyclase, cAMP
INTRODUCTION
Maternal behavior is the pattern of care given by mothers to their offspring. It increases the probability that offspring will reach maturity and is essential for the survival of mammalian newborns (Numan, 1988). As rodents are born deaf, blind, and immobile, maternal behaviors including nest building, gathering pups together in a nest, and keeping them warm are critical for survival. Interestingly, when exposed to newborns, virgin mice (Noirot, 1969b), virgin hamsters (Rowell, 1961), and virgin monkeys (Rowell et al, 1964) exhibit spontaneous maternal behaviors comparable with postpartum females. This indicates that there is a basic maternal responsiveness, which is not dependent upon hormones or sex for its arousal (Rosenblatt, 1967).
There is considerable evidence that maternal behavior in mice depends upon the detection of odorants and/or pheromones emanating from the pups (Noirot, 1969a). For example, female mice exhibit similar maternal behavior toward live and dead pups in the T-maze retrieval test, suggesting that body movements and vocalizations of the pups are not necessary to elicit retrieving behavior (Gandelman et al, 1970). Furthermore, in a modified Y-maze test, postpartum female mice retrieve young pups by chemosensory cues from the pups without the use of visual or auditory clues (Smotherman et al, 1974). Moreover, in the pup retrieval assay, female mice prefer fetuses treated with pup urine (Londei et al, 1989). The importance of chemoreception for maternal behaviors is also supported by studies showing that olfactory bulbectomy eliminates maternal behaviors (Gandelman et al, 1971, 1972; Zarrow et al, 1971). However, the underlying chemosensory mechanisms that mediate maternal behavior are not known.
Olfactory and pheromone signals in mammals are detected by sensory neurons at two locations: the MOE in the nasal cavity and the neuroepithelium of the vomeronasal organ (VNO). It was originally thought that volatile odorants are detected exclusively by the MOE, whereas pheromones are detected through the VNO. Accordingly, behaviors affected by pheromone signaling such as inter-male aggression, male sexual preference, puberty acceleration, maternal aggression, and pregnancy block were typically attributed to VNO control (Del Punta et al, 2002; Leypold et al, 2002; Stowers et al, 2002; Halpern and Martinez-Marcos, 2003; Norlin et al, 2003). However, recent evidence indicates that some pheromones may be detected through the MOE in mice (Mandiyan et al, 2005; Liberles and Buck, 2006; Wang et al, 2006).
Olfactory signal transduction in the MOE is mediated by second messenger cascades including the cGMP (Juilfs et al, 1997), as well as the cAMP signal transduction pathways (Ache and Zhainazarov, 1995; Ronnett and Payne, 1995; Restrepo et al, 1996), and is initiated by interactions of odorants with receptors encoded by a multigene family (Buck and Axel, 1991; Chess et al, 1992). The discovery of a unique G protein, Golf (Jones and Reed, 1989), AC3 (Bakalyar and Reed, 1990), and cyclic nucleotide gated (CNG) cation channels (Nakamura and Gold, 1987), all enriched in the olfactory cilia suggests an important role for cAMP in olfactory signaling. Furthermore, male AC3−/− mice cannot detect a number of odorants and show no EOG responses to odorants in the MOE, identifying cAMP as one of the major second messengers for signaling in the MOE of male mice (Wong et al, 2000). AC3 and other components of the receptor/cAMP signaling pathway including Golf and CNG are expressed in the MOE and not the VNO (Berghard et al, 1996).
To evaluate the role of AC3 and cAMP signaling for the detection of chemosensory signals mediating maternal behavior, we examined maternal behavior of female AC3−/− mice. Our data indicate that AC3 is required for maternal behavior and suggest that some chemosensory receptors contributing to maternal behavior may be coupled to AC3 in the MOE.
MATERIALS AND METHODS
Mice
AC3+/+ and AC3−/− female mice were bred from heterozygotes, and genotyped as previously reported (Wong et al, 2000). In the postpartum experiments, AC3−/− females were bred to AC3 +/+ males yielding litters, which were 100% AC3+/−. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. Adult mice used in this study were 3–6 months old unless indicated, and all the pups used were 1–3 days old unless indicated. Mice were maintained on a 12 h light/dark cycle and had access to food and water ad libitum. For olfactory behavioral tests, the animals were housed individually and handled daily for at least 1 week before experiments to minimize stress.
Maternal Behaviors of Virgin Female Mice
Virgin wild-type mice spontaneously exhibit full maternal behaviors when exposed to foster mouse pups (Noirot, 1969a), a behavior which is triggered by pup odorants and/or pheromones. Virgin female mice were individually housed and were not exposed to young pups before testing, except for their own littermates. Maternal behaviors were assayed as described previously (Thomas and Palmiter, 1997) and a cotton nestlet was provided as nest material for each animal. On the test day, three 3-day old foster pups born to an AC3+/+ mother were placed into the three corners of the cage that did not contain the nest. Typical maternal behaviors of female mice toward pups includes pup retrieval, licking of pups, nest building, and crouching over the gathered pups in the nest in a ‘lactation-position' (Noirot, 1969b). Therefore, latency to retrieve the 1st, 2nd, and 3rd pups, duration of licking and crouching over pups, and duration of nest building behavior were observed during a 10-min period. Only pups brought into the nest completely were counted as retrieved. Full maternal behaviors were defined as retrieval of all the three pups into the nest and crouching over them during the 10 min test period. If an animal did not complete this behavior within 10 min the test was terminated, resulting in a latency of 600 s for any behaviors not yet observed. The latency to approach and explore the pups and the duration were also recorded during this period. All the pups were returned to their donor mothers after the 10-min test was completed. To test whether consecutive pup exposure improves maternal behavior of AC3−/− female mice, the pup retrieval test was performed daily on four consecutive days with AC3−/− virgin female mice and their AC3+/+ virgin littermates. We examined nest building behavior by placing a piece of cotton in the cage of a female AC3+/+ or AC3−/− mice and examining the nest, or lack thereof 24 h later. The number of animals used was 10 AC3+/+ and 10 AC3−/− mice.
Maternal Behavior of Postpartum Female Mice
Maternal behavior deficits caused by olfactory sensory deprivation can be modified by maternal experience in primiparous dams (Seegal and Denenberg, 1974). To test whether maternal experience, as well as the physiological changes accompanying pregnancy or parturition, improve maternal behavior of female AC3−/− mice, maternal behaviors were assayed daily in recently postpartum AC3−/− females as described previously with some modifications (Thomas and Palmiter, 1997; Jin et al, 2005). All female mice were separated from males and individually housed once visibly pregnant, and monitored each morning for pup birth. The date of birth was considered postpartum day zero. The retrieval assay was performed in the female's home cage during a 10-min period. On the test day, pups were separated from their mother for 1 h and kept warm. Pup retrieval was performed as described for virgin female mice. The mother was briefly removed from her home cage, and three of her own pups were placed in each corner of the cage except for the nest. The mother was then reintroduced into her cage facing the wall. The behavior of the female mice was recorded during a 10-min period as described for virgin females. Identical sessions were assayed daily for 4 consecutive days after parturition (P0–P3). As a majority of the AC3+/− pups born from AC3−/− dams were dead before postnatal day 2, pups born to AC3+/+ dams of the same age were provided for fostering by AC3−/− dams. Retrieval assays were performed with these fostered pups. The fact that postpartum AC3−/− females did not have their own pups during the pup retrieval assay, whereas the AC3+/+ mice did should not seriously impact interpretation of the data because intrastrain cross-fostering has been reported to have little effect on maternal care (van der Veen et al, 2007). Although starting testing for maternal behavior on postpartum day 0 may be stressful to the mothers, AC3+/+ and AC3−/− mice were subjected to the same protocol. The number of mice assayed was 11 AC3+/+ and 9 AC3−/− mice.
Maternal Aggression Test
Another indicator of mouse maternal behavior is maternal aggression, a behavior that protects young mice from infanticide (Wolff, 1985; Parmigiani, 1986; Paul, 1986). Female mice are usually not aggressive toward intruders. However, postpartum female mice display vigorous aggressive behaviors toward intruders, especially against intruders bearing novel scents compared with intruders soaked in either water or the resident females' urine (Lynds, 1976). This is taken as evidence that the chemosensory system of postpartum females has an important role in triggering maternal aggression. The aggressive behaviors of postpartum female mice were assayed as described previously (Norlin et al, 2003). At postpartum day 0 (P0; day of birth of litter), litter size was culled to a maximum of six pups, with at least two pups of each gender to decrease variability in maternal aggression (Maestripieri, 1990). Aggressive behaviors were observed at P4, P6, P8, and P10 daily during a 10-min period by introduction of an unfamiliar sexually naïve group-housed AC3+/+ male into the postpartum female's home cage. The females were tested on multiple days to maximize the chance that aggressive behaviors would be observed. The pups were removed from their cage 3 min before the male was introduced to avoid injury. Removal of the pups does not alter aggressive behavior of postpartum females (Svare et al, 1981). The latency to first attack, the number of aggressive bouts, and the duration of aggressive behavior were recorded during the 10-min period. Tail rattling, biting, chasing, and wrestling were considered aggressive behavior, and aggressive behavior separated by less than 5 s was considered as one bout. As the majority of pups born from AC3−/− dams were dead before postnatal day 2, pups born to AC3+/+ dams (>5 days old) were provided for fostering by AC3−/− dams once their own pups were found dead. For a given animal, data from the day that the animal showed peak aggression were used for group comparison and statistical analysis. The number of animals assayed was 11 AC3+/+ and 9 AC3−/− mice.
Olfactory Behavioral Test
In this assay, a cotton swab laced with 50 μl of water was introduced into the home cage of a virgin female mouse (Trinh and Storm, 2003; Wang et al, 2006). The number of times the mouse sniffed the target was recorded during a 2-min period. This trial was repeated several times with 1 min intervals until the animal was no longer interested in the object. Once the animal habituated to the object, a cotton swab laced with 50 μl of odorant or urine was introduced. We tested pup urine because mouse fetuses laced with pup urine stimulate greater frequency of maternal behaviors than fetuses treated with water, indicating that mouse pup urine contains odorants or pheromones that elicit maternal behavior (Londei et al, 1989). When a female mouse detected the new odorant, she sniffed the cotton swab a greater number of times than she did when the swab only contained water. The test was assayed in the female's home cage. The data are presented as a ratio of the number of times the mouse sniffed an odorant-laced cotton swab compared with the number of times it sniffed a water-laced cotton swab on the initial exposure. This is used as an indication of the ability of the female to detect a specific odorant. Adult male and pup urines were collected into containers by holding the animals by the scruff of the neck. Pup urines were collected from male and female at the age of 5–6 days. Urine was pooled and stored in aliquots at −80°C until use. All chemical odorants were diluted in water. The concentrations of citralva and 2-heptanone were 10 and 50 μM, respectively. The odorant habituation test was repeated three times on different days. The number of animals assayed was 10 AC3+/+ and 10 AC3−/− mice.
The pup odorant/pheromone preference test was performed as described previously with some modifications (Mak et al, 2007). A test chamber constructed with removable dividers to allow for tertiary compartmentalization was used in this assay. Dividers consisted of two perforated panels separated by 0.5 inches and offset, so that, when in place, physical and visual contact could not be established, only odors or volatile pheromones could be transferred between compartments. During habituation pre-training, a female mouse was placed in the central compartment of the test chamber and the sniffing duration of the animal on each side of the compartment was recorded during a 10-min period. The mouse could not see the contents of the two compartments adjacent to her. Identical sessions were repeated daily for 4 days to allow the animal to habituate to the test chamber. On the test day, three pups 5–6 days old were randomly placed on either side compartment with the other side empty. The pups were kept silent by injection of ketamine to eliminate the influence of ultrasounds produced by the pups. The female's sniffing of each side was recorded during the test time. A preference was defined by a statistically greater amount of time spent sniffing one side of the compartment vs the other. The number of mice assayed was eight AC3+/+ and eight AC3−/− mice.
AC Activity Assay
MOE membranes from virgin female mice were collected, homogenized, and processed as described previously (Wang et al, 2006). Protein concentrations were determined by the BCA assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. AC activity was measured in a buffer consisting of 1 mM cyclic AMP, 10 mM phosphocreatine, 0.5 unit of creatine phosphokinase, 5 μM GTP, 5 mM MgCl2, 0.2 mM EDTA, 50 mM Tris/HCl (pH 7.5), with [α-32P] ATP to 5 × 106 c.p.m. per reaction at 30°C for 15 min. The concentration of citralva and 2-heptanone, which was used as stimulus, was 100 μM. AC assays were carried out in triplicate in a final volume of 250 μl. The number of mice assayed was four AC3+/+ and four AC3−/− mice.
EOG Recording
EOG recording of the MOE of virgin female mice was performed as described previously (Wang et al, 2006). The MOE was dissected through the septum, and the EOG was recorded with an agar- and saline-filled glass microelectrode in contact with the apical surface of the MOE in the open circuit configuration. Odorant or pheromone solutions, which were diluted in 1 × ringer buffer, were puffed onto the exposed MOE for 2 s followed by a stream of moisturized oxygen. Traces were captured and digitized using a Digidata 1200A (Molecular Devices, Union City, CA) connected to a PC computer, low pass filtered at 30 Hz, and sampled at 100 Hz. The concentration of 2-heptanone and citralva for EOG recording was 50 μM. The concentration of farnesene used for EOG recording was 500 μM. The number of animals assayed was six AC3+/+ and five AC3−/− mice.
Statistical Analysis
All data were presented as mean±SEM, unless otherwise indicated. We analyzed the data by the Wilcoxon test for two-samples comparison and Friedman's test for multiple-sample comparisons, with p<0.05 considered as statistically significant. In all behavioral tests, the investigator was blind to the genotype of the animal.
RESULTS
Pup Retrieval is Impaired in Virgin AC3−/− Mice
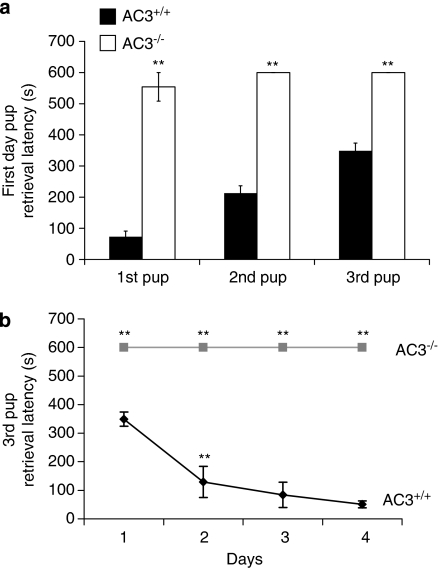
To evaluate the role of AC3 for maternal behavior, we examined virgin female AC3−/− mice and their wild-type littermates in the pup retrieval test as described in Materials and Methods. Foster pups were used with AC3+/+ and AC3−/− females. As expected, after approaching and investigating the foster pups, AC3+/+ female mice collected all three foster pups to their nests within 5 min (Figure 1a). Once the pups were brought to the nest, AC3+/+ female mice exhibited a number of additional maternal activities including licking of the pups, continued nest building and crouching over the pups. With the exception of one AC3−/− female, which retrieved one foster pup during the 10 min test period, all of the other AC3−/− females failed to retrieve any pups within the testing period (Figure 1a). In addition, the total amount of time that female mice were engaged in maternal activities including anogenital licking, nursing or nest building during the 10 min test period was monitored (Supplementary Figure S1a). Virgin AC3+/+ females (n=10) spent significantly more time engaged in maternal activities than AC3−/− females (n=10), p<0.01.
Figure 1.
Pup retrieval is impaired in virgin AC3−/− mice. (a) The retrieval latencies of three pups on the first test day of virgin female AC3+/+ and AC3−/− mice are shown. There were significant differences between AC3+/+ (n=10) and AC3−/− (n=10) mice in the retrieval latency of the first, second, and third pups (p<0.001 for each pair). Data are represented as means±SEM. (b) The third pup retrieval latency for virgin female AC3+/+ and AC3−/− mice on 4 consecutive days is reported. The retrieval latency for the third pup for each day (p<0.001 for each pair) was significantly different between AC3+/+ (n=10) and AC3−/− females (n=10). The retrieval latency of AC3+/+ mice, but not AC3−/− mice decreased with repeated tests. Data are represented as means±SEM. **p<0.001. If an animal did not complete this behavior within 10 min the test was terminated, resulting in a latency of 600 s for any behaviors not yet observed.
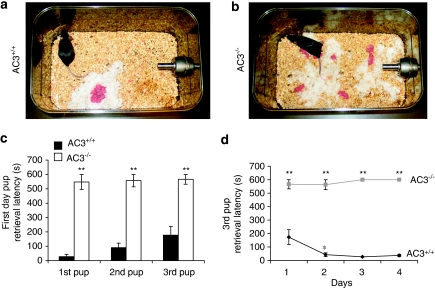
To test whether consecutive pup exposure improves maternal behavior of AC3−/− female mice, the pup retrieval test was performed daily on 4 consecutive days with AC3−/− virgin female mice and their AC3+/+ virgin female littermates as described in Materials and Methods. Exposure of AC3+/+ female mice to pups over four consecutive days significantly decreased the time for retrieval of all three pups (Figure 1b). In contrast, none of the AC3−/− virgin female mice retrieved pups even after 4 days of testing (Figure 1b). We conclude that AC3−/− mice lack pup retrieval behavior and that even consecutive exposure to pups over several days does not stimulate pup retrieval behavior. We also examined nest building behavior by placing a piece of cotton in the cage of a female AC3+/+ or AC3−/− mice and examining the nest 24 h later. Although most of the AC3+/+ female mice (8/10) constructed focused, circular nests within the first 24 h (Figure 2a), only 2 out of 10 AC3−/− females made well-defined nests (Figure 2b).
Figure 2.
Nest building and pup retrieval are impaired in recently postpartum AC3−/− females. (a and b) Representative nests and pup distribution for AC3+/+ and AC3−/− dams. Pictures were photographed ∼6 h following parturition. (c) The pup retrieval latencies of the first day following partition (P0) in AC3+/+ and AC3−/− dams are reported. There were significant differences between AC3+/+ (n=11) and AC3−/− (n=9) mice in the retrieval latency for the first, second, and third pups (p<0.001 for each pairs). Data are represented as means±SEM. (d) The retrieval latency of the third pup for AC3+/+ and AC3−/− dams on the 4 consecutive days following partition are shown. There were significant differences between AC3+/+ (n=11) and AC3−/− (n=9) dams for the retrieval latency of the third pup on each day (p<0.001 for each pairs). Data are represented as means±SEM. **p<0.001. If an animal did not complete this behavior within 10 min the test was terminated, resulting in a latency of 600 s for any behaviors not yet observed.
Pup Retrieval is Impaired in Postpartum Female AC3−/− Mice
Virgin female AC3+/+ or AC3−/− mice were housed with AC3+/+ males until pregnancy, after which time the females were housed individually. All of the AC3+/+ virgin female mice housed with AC3+/+ males (10 out of 10 females) and 9 out of 11 AC3−/− mice became pregnant, indicating that female AC3−/− mice are fertile. The pregnant female AC3−/− mice all delivered pups. The litter size and physical appearance of pups born to AC3−/− female at birth were indistinguishable from those of AC3+/+ mice. In addition, all of the pups born to AC3−/− female mice were cleaned by the mother indicating that AC3−/− females exhibit normal placentophagis. As pups born to AC3−/− female mice were scattered throughout the mother's cage (Figure 2a and b), we suspected that maternal behaviors of postpartum AC3−/− mice might also be impaired.
To directly examine the maternal behaviors of postpartum AC3−/− females, the pup retrieval behavior of postpartum AC3+/+ and AC3−/− mice were assayed daily for 4 consecutive days. AC3+/+ recently postpartum females retrieved all three pups much faster than virgin female AC3+/+ mice (Figures 1a and 2c). Once the pups were retrieved into the nest, AC3+/+ females licked the pups, crouched over the pups and continued nest building. In contrast, only one out of nine postpartum AC3−/− females retrieved all three pups into her nest on the first test day. However, this mouse did not retrieve the pups on the third and fourth test days. Postpartum AC3−/− female mice did not lick pups, crouch over the pups or engage in nest building (Figure 2b). Moreover, there was no improvement in pup retrieval time of AC3−/− females over 4 days of testing (Figure 2d). The total amount of time that postpartum female mice were engaged in maternal activities including anogenital licking, nursing or nest building during the 10 min test period was monitored (Supplementary Figure S1b). Recently postpartum AC3+/+ females (n=11) spent significantly more time engaged in maternal activities than recently postpartum AC3−/− females (n=8, p<0.01). Although, mother–pup interactions may be affected by starting testing at postpartum day 0, both AC3−/− and AC3+/+ mice were separated from pups on day 0, controlling for any stress associated with separation from pups. Collectively, these data indicates that pup retrieval is disrupted in both virgin and postpartum AC3−/− female mice.
Maternal Aggression is Impaired in Recently Postpartum Female AC3−/− Mice
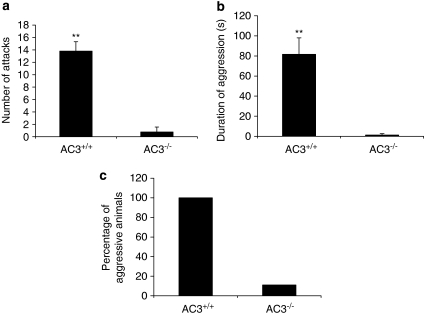
To assay maternal aggression, recently postpartum females were individually housed with their pups. The pups were removed 3 min before the introduction of a sexually naïve AC3+/+ male intruder into the home cage. The number of attacks, duration of aggression, and the percentage of aggressive animals were recorded during a 10-min period on postpartum days 4, 6, 8, and 10. As pups born to AC3−/− mothers died because of impaired maternal care, postpartum AC3−/− females were provided with foster pups. The use of foster pups with postpartum AC3−/− mice is appropriate because wild-type mice exhibit maternal aggression with their own pups or foster pups in their home cage (Ostermeyer and Elwood, 1983). All AC3+/+ postpartum female mice showed intense aggressive behaviors towards the intruder on at least one trial during the four trials (Figure 3). Postpartum female AC3−/− mice displayed little or no maternal aggression. The percentage of AC3−/− postpartum females displaying aggression, the average number of attacks against the intruder, and the total time spent attacking the intruder were significantly lower than AC3+/+ postpartum females (Figure 3). We conclude that AC3−/− postpartum female mice do not display maternal aggression, possibly because of defects in their chemosensory mechanisms.
Figure 3.
Maternal aggressive behaviors are impaired in recently postpartum AC3−/− mice. Maternal aggressive behaviors were assayed by introducing an unfamiliar male into the home cage of each female mouse during a 10-min period. (a) There were significant differences between postpartum AC3+/+ (n=11) and postpartum AC3−/− (n=9) dams in number of attacks (p<0.001), (b) duration of aggression (p<0.001) and (c) the percentage of dams that displayed aggressive behavior. Data are represented as means±SEM. **p<0.001.
Detection of Mouse Urine and Odorants is Impaired in Female AC3−/− Mice
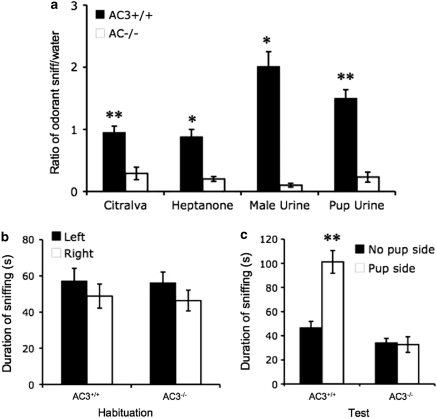
All of the virgin female AC3+/+ mice and none of the virgin AC3−/− females detected citralva, 2-heptanone, male mouse urine, or pup urine during the odorant habituation test (Figure 4a). This indicates that AC3−/− mice are unable to detect chemical signals present in adult male or pup urine, odorants or mouse pheromones.
Figure 4.
Virgin AC3−/− female mice fail to detect odorants and pup urine. (a) Odorant habituation data for AC3+/+ and AC3−/− virgin female mice are reported. Cotton swabs were laced with 50 μl of citralva (10 μM), 2-heptanone (50 μM), male urine (20-fold diluted), or pup urine (20-fold diluted). There were significant differences in the ability of AC3+/+ (n=10) and AC3−/− (n=10) mice to detect citralva (p<0.001), 2-heptanone (p<0.01), male urine (p<0.01), and pup urine (p<0.001). Data are represented as means±SEM. (b and c) Virgin female AC3+/+ mice but not virgin AC3−/− females detected anesthesized pups. Non-visual pup detection was assayed as described in Materials and Methods using anesthesized pups. (b) During context habituation, AC3+/+ (n=8) and AC3−/− (n=8) female mice sniffed each side chamber equally. However, during testing (c) AC3+/+ female mice, but not AC3−/− females, showed a strong preference for the side chamber containing the anesthesized pup (p<0.001). Data are presented as means±SEM. *p<0.01; **p<0.001.
As maternal behaviors may depend upon the ability of the female mouse to detect chemical signals or combinations thereof emanating from mouse pups, we examined the ability of AC3−/− mice to detect pups solely on the basis of chemoreception with no visual or auditory cues as described in Materials and Methods. During habituation when the adjacent compartments were empty, AC3−/− and AC3+/+ mice both sniffed the two sides equally, indicating no preference for either side (Figure 4b). During testing, three anesthesized pups were placed in one adjacent compartment; the other side was empty. As anticipated, AC3+/+ female mice spent more time sniffing the compartment containing pups than the opposite empty side (Figure 4c). However, AC3−/− female mice showed no preference for the compartment containing pups (Figure 4c). Similar results were obtained when dead pups were substituted for anesthesized pups (data not shown). These data confirm that AC3+/+ female mice can detect pups by chemoreception independent of visual or auditory cues, whereas AC3−/− females cannot detect these signals.
Odorants do not Stimulate AC Activity in MOE Preparations from Female AC3−/− Mice
The MOE expresses several ACs including the type 2, 3, and 4 ACs (Wong et al, 2000) and AC activity in mouse MOE preparations is stimulated by odorants (Pace et al, 1985; Wang et al, 2006). To determine if biochemical signaling in the MOE of virgin AC3−/− females is impaired, we examined odorant stimulation of AC activity in MOE preparations from female AC3+/+ and AC3−/− mice. The AC activity of MOE preparations from virgin AC3+/+ female mice but not AC3−/− mice was significantly stimulated by citralva and 2-heptanone (Figure 5). These data are consistent with the observation that AC3−/− females cannot detect odorants and mouse urine.
Figure 5.
Adenylyl cyclase activity in the MOE of female AC3+/+, but not AC3−/− mice is stimulated by odorants and pheromones. Citralva (100 μM, p<0.001) and 100 μM 2-heptanone (p<0.001) stimulated adenylyl cyclase activity in membranes from virgin AC3+/+ mice (n=4) but not virgin AC3−/− mice (n=4). Adenylyl cyclase activity in membrane preparations from the MOE was assayed in the absence or presence of citralva or 2-heptone as described in Materials and Methods. Data are represented as means±SEM. **p<0.001.
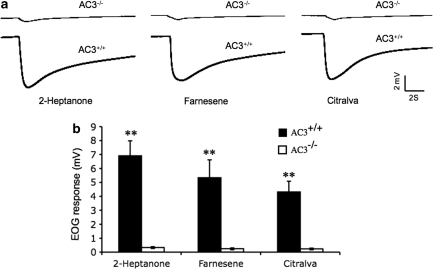
EOG Response to Pheromones and Odorants is Impaired in Female AC3−/− Mice
We monitored the EOG responses in the MOE of virgin AC3−/− and AC3+/+ mice to two pheromones, 2-hepatone, and farnesene, as well as the odorant citralva (Figure 6). Farnesene, 2-heptanone, and citralva evoked strong EOG responses in the MOE of AC3+/+ female mice (6.9±1.1, 5.3±0.2 and 4.3±0.2 mV, respectively). In contrast, the EOG responses evoked by these agents in the MOE from AC3−/− female mice was more than 20-fold lower than wild-type mice (0.3±0.1, 0.2±0.1, and 0.2±0.1 mV, respectively). These data are consistent with the hypothesis that AC3−/− female mice do not exhibit maternal behaviors because they cannot detect chemical signals that elicit these behaviors.
Figure 6.
EOG responses evoked by odorant and pheromone are ablated in the MOE of AC3−/− females. (a) Representative EOG responses evoked by 2-heptanone (50 μM), farnesene (500 μM), and citralva (50 μM), respectively, from the MOE of virgin AC3+/+ and AC3−/− females are shown. All agents were diluted in 1 × ringer buffer. (b) Summary of the mean EOG amplitudes in response to odorants and pheromones. Significantly greater EOG responses to all agents were exhibited in AC3+/+ (n=6) compared with AC3−/− (n=5) females (all agents p<0.001). Data are presented as mean±SEM. **p<0.001.
DISCUSSION
Common maternal behaviors exhibited by female mice include pup retrieval, licking of pups, nest building, crouching over grouped pups in a well-defined nest and maternal aggression (Noirot, 1969b). Pup retrieval is not modified by deafness, and visual cues seem to have little or no role in pup retrieval (Herrenkohl and Rosenberg, 1972; Herrenkohl and Sachs, 1972). However, accumulating evidence indicates that chemosensory cues from pups trigger female maternal behaviors (Fleming and Rosenblatt, 1974; Fleming et al, 1992). However, very little is know about the chemosensory mechanisms that mediate maternal behavior or the role of cAMP signaling and specific ACs in the detection of signals that evoke maternal behaviors. To evaluate the role of cAMP signaling in maternal behavior, we examined maternal behavior of female AC3−/− mice.
We discovered that postpartum and virgin AC3−/− females fail to exhibit several maternal behaviors including pup retrieval, licking of pups, crouching over pups in a nest, maternal aggression and nest building. Nest building is evolutionary conserved throughout the animal kingdom and is an important indicator of maternal care. In rodents, the nest insulates the altricial young and keeps the pups warm in the mother's absence (Numann et al, 1991; Deacon, 2006). Nest building is impaired in female mice with olfactory bulb removal (Zarrow et al, 1971), indicating that nest building may depend on chemosensory mechanisms, a hypothesis that is supported by the fact that anosmic AC3−/− female mice showed impaired nest building.
Naïve virgin female rats do not spontaneously exhibit maternal responsiveness to young pups. However, they show maternal behaviors comparable with postpartum female rats following repeated exposure to newborns for several days (Rosenblatt, 1967). This induction of maternal behavior is not dependent upon hormones or sex, because ovariectomized and hypophysectomized virgin females also exhibit maternal behaviors after repeated exposure to young pups. It has also been reported that the latency to express full maternal behavior by female mice is reduced following 2 consecutive days of the pup retrieval test (Noirot, 1969a; Larsen et al, 2008). Nevertheless, exposure of AC3−/− females to pups repeatedly over a period of days did not induce pup retrieval behavior. These defects are most likely attributable to chemosensory defects as AC3−/− females were unable to detect pups, pup urine, urine from adult male mice, pheromones or odorants. In contrast to wild-type mice, AC activity in MOE preparations from AC3−/− females was not stimulated by pheromones or odorants and EOG responses to odorant and pheromones were also nonexistent. These data support the hypothesis that chemical signals from mouse pups trigger maternal behavior by activating receptors coupled to AC3 in the MOE of female mice.
Maternal behavior can be affected by behavior of the mother and/or the pups used in the assay. This study only addressed the contribution of the female AC3−/− mice to maternal behavior and not the AC3−/− pups as foster pups were used for both virgin AC3+/+ and AC3−/− female mice in the pup retrieval assay. In contrast, pups born to postpartum AC3+/+ females were used in the pup retrieval assay, whereas AC3−/− females were tested with their own pups on day 1 followed by foster pups in subsequent days. However, the use of foster pups should not seriously compromise the pup retrieval assays because intrastrain cross-fostering has been reported to have minimal effects on maternal behavior (van der Veen et al, 2007). For example, heterozygote pups born to AC3−/− females survive when they are cross-fostered to AC3+/+ female mice (data not shown).
As AC3 and other components of the receptor/cAMP signaling pathway including Golf and CNG ion channels are expressed in the MOE and not the VNO (Berghard et al, 1996), our data support the general hypothesis that the MOE of mice is important for the detection of chemical signals that initiate maternal behavior. As the MOE can detect both odorants and pheromones (Mandiyan et al, 2005; Liberles and Buck, 2006; Wang et al, 2006), we cannot readily define the nature of the chemical signals that trigger pup retrieval. Although we cannot rule out a role for the VNO in maternal behavior, it is interesting that disruption of genes specifically in the VNO, including V1r cluster (Del Punta et al, 2002) and Gαi2 (Norlin et al, 2003) does not compromise pup retrieval behavior. On the other hand, transgenic mice lacking TrpC2, a channel specifically expressed in the VNO, exhibit deficits in nest building and time on the nest suggesting that the VNO does have a role in these maternal behaviors (Hasen and Gammie, 2009; Kimchi et al, 2007).
In contrast to pup retrieval behavior of female mice, maternal aggression depends on the function of the VNO and is disrupted by removal of the VNO (Bean and Wysocki, 1989). Furthermore, disruption of genes only expressed in the VNO such as TrpC2 (Leypold et al, 2002; Hasen and Gammie, 2009), Gαi2 (Norlin et al, 2003), and V1r cluster (Del Punta et al, 2002) blocks maternal aggression of postpartum females. As AC3−/− females also lack maternal aggression and AC3 is expressed in the MOE and not the VNO, this suggests that maternal aggression also depends on signaling through the MOE. We conclude that chemosensory signaling through AC3 in the MOE is necessary but not sufficient for eliciting maternal aggression and that maternal aggression may depend upon the MOE and VNO.
AC3 is not specific to the olfactory epithelium and according to the Allen Brain Atlas is expressed in several areas of brain including the hippocampus, cortex, amygdala, medial preoptic nucleus, and suprachiasmatic nucleus. As the AC3−/− mouse strain used in this study was a global knockout, we cannot conclude with certainty that the defects in maternal behavior shown by the AC3−/− are solely because of the absence of the enzyme in the olfactory epithelium. For example, the olfactory and vomeronasal chemosensory inputs are integrated in the medial amygdala, the bed nucleus of the stria terminalis and hypothalamus (Keller et al, 2009; Kang et al, 2009; Martel and Baum, 2009; Baum and Kelliher, 2009), raising the possibility that the deficits of maternal behaviors exhibited by AC3−/− mice might be caused by amygdala or other integrative areas of brain. Nevertheless, the EOG data illustrating defective signaling through the MOE, the loss of odorant-stimulated AC activity in the MOE, and the behavioral data showing that AC3−/− females cannot detect pups by chemosensory signals support the hypothesis that disruption of signaling in the MOE caused by ablation of AC3 contributes to the defects in maternal behavior.
In conclusion, the maternal behavior of mice depends upon AC3 activity. These data support the hypothesis that chemical signals emanating from pups activate receptors in the MOE coupled to AC3 through Golf and are consistent with data showing that Golf−/− mice also fail to exhibit maternal behavior (Belluscio et al, 1998). This suggests that cAMP generated by AC3 is a major second messenger used in the detection of chemical signals that trigger maternal behavior.
Acknowledgments
This work was supported by National Institutes of Health Grant DC04156. We are grateful for Dr Jae H Lim and Trongha Phan for excellent technical assistances. We thank members of the Storm laboratory for critical reading of this paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ache BW, Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Current Opin In Neurobiol. 1995;5:461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Bean NJ, Wysocki CJ. Vomeronasal organ removal and female mouse aggression: the role of experience. Physiol Behav. 1989;45:875–882. doi: 10.1016/0031-9384(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Belluscio I, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Berghard A, Buck LB, Liman ER. Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. Proc Natl Acad Sci USA. 1996;93:2365–2369. doi: 10.1073/pnas.93.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chess A, Buck L, Dowling MM, Axel R, Ngai J. Molecular biology of smell: expression of the multigene family encoding putative odorant receptors. Cold Spring Harb Symp Quant Biol. 1992;57:505–516. doi: 10.1101/sqb.1992.057.01.056. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nature protocols. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Gavarth K, Sarker J. Effects of transections to the vomeronasal nerves or to the main olfactory bulbs on the initiation and long-term retention of maternal behavior in primiparous rats. Behav Neural Biology. 1992;57:177–188. doi: 10.1016/0163-1047(92)90122-k. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86:221–232. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow M, Denenberg V. Maternal behavior: differences between mother and virgin mice as a function of the testing procedure. Dev Psychobiology. 1970;3:207–214. doi: 10.1002/dev.420030308. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH. Reproductive and maternal performance in the mouse following removal of the olfactory bulbs. J Reprod Fertil. 1972;28:453–456. doi: 10.1530/jrf.0.0280453. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH, Myers M. Olfactory bulb removal eliminates maternal behavior in the mouse. Science. 1971;171:210–211. doi: 10.1126/science.171.3967.210. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Trpc2 gene impacts on maternal aggression, accessory olfactory bulb anatomy and brain activity. Genes, Brain, Behav. 2009;8:639–649. doi: 10.1111/j.1601-183X.2009.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenkohl LR, Rosenberg PA. Exteroceptive stimulation of maternal behavior in the naive rat. Physiol Behav. 1972;8:595–598. doi: 10.1016/0031-9384(72)90080-7. [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR, Sachs BD. Proceedings: Sensory regulation of maternal behavior in mammals. Physiol Behav. 1972;9:689–692. doi: 10.1016/0031-9384(72)90034-0. [DOI] [PubMed] [Google Scholar]
- Jin SH, Blendy JA, Thomas SA. Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience. 2005;133:647–655. doi: 10.1016/j.neuroscience.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behvior in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Kokay IC, Grattan DR. Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm Behav. 2008;53:509–517. doi: 10.1016/j.yhbeh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Leypold B, Yu C, Leinders-Zufall T, Kim M, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Londei T, Segala P, Leone VG. Mouse pup urine as an infant signal. Physiol Behav. 1989;45:579–583. doi: 10.1016/0031-9384(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Lynds PG. Olfactory control of aggression in lactating female housemice. Physiol Behav. 1976;17:157–159. doi: 10.1016/0031-9384(76)90285-7. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal aggression and litter size in the female house mouse. Ethology. 1990;84:27–34. [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, et al. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2009;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Noirot E. Changes in responsiveness to young in the adult mouse. V. Priming. Anim Behav. 1969a;17:542–546. doi: 10.1016/0003-3472(69)90161-4. [DOI] [PubMed] [Google Scholar]
- Noirot E. Serial order of maternal responses in mice. Anim Behav. 1969b;17:547–550. doi: 10.1016/0003-3472(69)90162-6. [DOI] [PubMed] [Google Scholar]
- Norlin E, Gussing F, Berghard A. Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr Biol. 2003;13:1214–1219. doi: 10.1016/s0960-9822(03)00452-4. [DOI] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Ostermeyer MC, Elwood RW. Pup recognition in Mus musculus: parental discrimination between their own and alien young. Dev Psychobiol. 1983;16:75–82. doi: 10.1002/dev.420160202. [DOI] [PubMed] [Google Scholar]
- Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Parmigiani S. Rank order in pairs of communally nursing female mice (Mus musculus domesticus) and maternal aggression towards conspecific introders of differing sex. Aggressive Behav. 1986;12:377–386. [Google Scholar]
- Paul L. Infanticide and maternal aggression: synchrony of male and female reproductive strategies in mice. Aggressive Behav. 1986;12:1–12. [Google Scholar]
- Restrepo D, Teeter JH, Schild D. Second messenger signaling in olfactory transduction. J Neurobiol. 1996;30:37–48. doi: 10.1002/(SICI)1097-4695(199605)30:1<37::AID-NEU4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Payne R. A tale of two senses. Neuron. 1995;15:11–16. doi: 10.1016/0896-6273(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rowell TE. Maternal behaviour in nonlactating golden hamsters. Anim Behav. 1961;9:11–15. [Google Scholar]
- Rowell TE, Hinde RA, Spencer-Booth Y. ‘Aunt'-infant interaction in captive rhesus monkeys. Anim Behav. 1964;12:219–226. [Google Scholar]
- Seegal RF, Denenberg VH. Maternal experience prevents pup-killing in mice induced by peripheral anosmia. Physiol Behav. 1974;13:339–341. doi: 10.1016/0031-9384(74)90056-0. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiol Behav. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Thomas S, Palmiter R. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Abrous DN, de Kloet ER, Piazza PV, Koehl M. Impact of intra -and interstrain cross-fostering on mouse maternal care. Genes, Brain, Behav. 2007;7:184–192. doi: 10.1111/j.1601-183X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JO. Maternal aggression as a deterrent to infanticide in Peromiscus leucopus and Peromiscus maniculatus. Anim Behav. 1985;33:117–123. [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Gandelman R, Denenberg VH. Lack of nest building and maternal behavior in the mouse following olfactory bulb removal. Horm Behav. 1971;2:227–238. doi: 10.1126/science.171.3967.210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.