Abstract
Many neural programs that shape behavior become established during adolescence. Adverse events at this age can have enduring consequences for both adolescent and adult mental health. Here we show that repeated social stress at different stages of adolescent development differentially affects rat behavior and neuronal activity. Early-adolescent (PND 28, EA), mid-adolescent (PND 42, MA), and adult (PND 63) rats were subjected to resident-intruder social stress (7 days) and behavior was examined 24–72 h later. In EA rats selectively, resident-intruder stress increased proactive responses in the defensive burying and forced swim tests. In adult rats, resident-intruder stress decreased burying behavior regardless of whether the animal was stressed as an adult or during early adolescence. As the locus coeruleus (LC)–norepinephrine system has been implicated in proactive defense behaviors, LC neuronal activity was quantified in separate cohorts. Stressed EA rats had elevated spontaneous LC discharge rates and diminished responses to sensory stimuli compared with controls. Microinjection of a CRF antagonist into the LC selectively inhibited neurons of stressed EA rats, suggesting that EA social stress induces tonic CRF release onto LC neurons, shifting the mode of discharge to an activated state that promotes active defensive behaviors. In all adult groups, resident-intruder stress resulted in an increased phasic response to sensory stimuli with no change in spontaneous rates. MA was a transition period during which social stress did not affect behavior or LC activity. The results suggest that social stress interacts with the brain norepinephrine system to regulate defensive strategies in an age-dependent manner.
Keywords: locus coeruleus, development, defensive burying, restraint, corticosterone, resident intruder
INTRODUCTION
Stress can shape future behavior and has been implicated in mood disorders, schizophrenia, and substance abuse (Nuechterlein and Dawson, 1984; Gold and Chrousos, 2002; Corcoran et al, 2003; Goeders, 2003; McEwen, 2003). Stress has complex interactions with development such that individuals may be especially vulnerable to stressors during specific developmental periods (Heim and Nemeroff, 2001; Casey et al, 2010). The prenatal and neonatal periods are critical windows in development during which stress can have enduring effects on behavior and endocrine function (Plotsky and Meaney, 1993; Plotsky et al, 2005; Cannizzaro et al, 2006; Cottrell and Seckl, 2009; Murgatroyd et al, 2009). Similarly, adolescence is a critical window in stress susceptibility as this is a time of substantial cerebral development and reorganization, as well as altered hypothalamic-pituitary-adrenal (HPA) function (Spear, 2000; Teicher et al, 2003; Romeo and McEwen, 2006; Danese et al, 2009; Casey et al, 2010).
Animal studies have shown unique effects of stress during adolescence on HPA activity and behavior (for review see McCormick and Mathews, 2010). For example, in many studies adolescent rats have prolonged or increased HPA responses to acute stressors compared with adults and fail to habituate to repeated stressors in the same manner as adults (Goldman et al, 1973; Romeo et al, 2006; McCormick et al, 2008; McCormick and Mathews, 2010; Weintraub et al, 2010). However, these effects are sex dependent and vary depending on the stressor (for review see McCormick and Mathews, 2010). Behaviorally, adolescent rats have been shown to have greater exploratory behavior in novel environments (Doremus et al, 2004; Stansfield and Kirstein, 2006). Stress during adolescence can have enduring effects that are expressed as increases in anxiogenic or depressive-like behaviors in animal models of psychopathology (Pohl et al, 2007; Toledo-Rodriguez and Sandi, 2007; Tsoory et al, 2007; McCormick et al, 2008; Toth et al, 2008; Weintraub et al, 2010). As end points of these animal models are often behavioral responses to a particular challenge (eg, elevated plus maze, a shock probe, swim stress), these studies show that stress during adolescence determines future behavioral strategies used to respond to subsequent stressors or environmental challenges.
Social stress has particular relevance to adolescents (Gladstone et al, 2006; Herrenkohl et al, 2009; Sebastian et al, 2010). Adolescence is characterized by an increase in child–parent conflict, search for autonomy, and a shift in social interaction from familial to peer relationships (Panksepp, 1981; Spear, 2000). With an increased importance of social signals and activity, there comes an increased potential for adverse social interactions to elicit a stress response. An ethologically relevant model used to study social stress in rats is the resident-intruder stressor (Miczek, 1979). In this model, a rat (intruder) is placed in the home cage of a larger, aggressive rat (resident) and subjected to repeated threatening encounters. Exposure of adult rats to this stressor alters HPA activity, produces anhedonia, promotes depressive-like behaviors, and increases the propensity to self-administer psychomotor stimulants (Heinrichs et al, 1992; Miczek et al, 2004; Buwalda et al, 2005; Covington et al, 2005; Rygula et al, 2005; Wood et al, 2010). In contrast to the numerous studies of adult resident-intruder stress, there are few studies of the effects of resident-intruder stress during adolescence. In hamsters, adolescent social stress altered patterns of aggression in a context-specific manner, increasing aggressive behavior toward smaller animals and decreasing it toward larger animals (Ferris, 2003; Wommack and Delville, 2003; Wommack et al, 2003). The few studies that examine adolescent social stress in rats suggest an increase in adult social anxiety (Vidal et al, 2007; Watt et al, 2009).
This study compared the behavioral effects of resident-intruder stress in rats during early adolescence, mid-adolescence, and adulthood. To determine whether the effects were selective to social stress, the effects of restraint stress on some of the behaviors were also examined. Plasma corticosterone levels were compared to assure that the manipulations elicited an endocrine stress response in all three groups. As some of the behavioral changes observed during these initial experiments have been linked to activation of the locus coeruleus (LC)–norepinephrine system, subsequent experiments investigated the effects of resident-intruder stress on LC discharge characteristics across adolescence (Detke et al, 1995; Bondi et al, 2007). Taken together, the behavioral and neurophysiological studies complemented each other to emphasize that early adolescence is a critical window during which social stress affects the brain norepinephrine system and regulates defensive behaviors.
MATERIALS AND METHODS
Animals
The subjects were male Sprague–Dawley rats (Taconic Farms, Germantown, NY) aged PND 28 (early-adolescence, EA), PND 42 (mid-adolescence, MA), or PND 63 (adulthood) (see Figure 1 for a timeline of ages and experiments). Ages were selected to span the social and physical stages of adolescence as described previously (Spear, 2000; McCormick and Mathews, 2010). PND 28 represents pre-pubertal peri-adolescence and is marked by a high degree of social play behavior. PND 42 represents a stage of MA when pubertal changes occur and male social behavior transitions to adult-like behavior. PND 63 represents early adulthood when male sexual and social behaviors are fully developed (Korenbrot et al, 1977; Sodersten et al, 1977; Panksepp, 1981; Primus and Kellogg, 1990). Rats were delivered to the animal facility approximately 1 week previous to experimental manipulations. Rats were initially housed 2–3 per cage in standard 26 × 46 cm2 polypropylene cages in a 12-h light/dark (lights on at 0600 hours), climate-controlled room with ad lib access to food and water. On the first experimental day, rats of both the control and experimental groups were transferred to new cages and individually housed to avoid the confounding and varied influence of cage mates on the effects of the resident-intruder stress (Ruis et al, 1999; Nakayasu and Ishii, 2008). Long–Evans retired breeder rats (Taconic Farms, Germantown, NY and Charles River, Wilmington, DE; >500 g, single-housed) were used as resident animals in resident-intruder experiments. Care and use of animals was approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia.
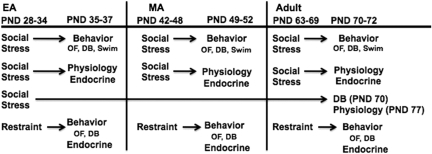
Figure 1.
Schematic depicting experimental groups. Rats aged postnatal days (PND) 28 (early-adolescent, EA), 42 (mid-adolescent, MA), or 63 (adult) were subjected to either resident-intruder stress or restraint stress for 1 week. On days 1, 2, and 3 following the last stress, rats were tested in the open field test (OF), defensive burying test (DB), and swim stress, respectively. Another cohort of rats likewise received 1 week of resident-intruder stress, but were subject to in vivo electrophysiology 24 h after the last resident-intruder stress. To investigate the endocrine response to repeated social or restraint stress, blood samples were taken from some of the animals in the electrophysiology cohorts of the resident-intruder group and the restraint group during the week of stress. Finally, to investigate the duration of the effects of resident-intruder stress, a cohort of animals were subject to resident-intruder stress as EA rats and allowed to grow to adulthood (PND 70) before behavioral and electrophysiological testing, which occurred on PND 70 and PND 77, respectively.
Stress
For social stress, an adaptation of the resident-intruder stress was used (Miczek, 1979). For 7 consecutive days, the experimental animals (intruders) were weighed and placed in the home cage of a novel Long–Evans retired breeder rat (resident) that was screened for proper aggressive behavior based on previous work (Bhatnagar and Vining, 2003; Bhatnagar et al, 2006). Residents that failed to consistently attack or that were overly aggressive and caused injury to intruders were not used. Each session began with a period of investigation and free interaction until one of three criteria was met: (1) the intruder exhibited a characteristic submissive defeat posture (frozen supine position for 2 s as described by Miczek, 1979); (2) the intruder was attacked five times; or (3) 15 min had elapsed. As soon as one of these criteria was met, rats were separated by a wire mesh barrier (1 cm weave), allowing continued olfactory and visual contact, but preventing physical contact for the duration of the 30-min session. Controls were weighed daily and then returned to their home cage. One exception to this was the controls for adult electrophysiological/endocrine studies that were placed behind a wire mesh barrier in novel cages without the resident to reproduce conditions of a previous study (Wood et al, 2010). When compared with the other control groups, this group had comparable corticosterone and LC electrophysiological characteristics as the other control groups (see Table 1 and Figure 5). Social stress occurred in a separate room from the controls to avoid potential stress-inducing effects of ultrasonic vocalizations on control rats. The 30 min resident-intruder encounters were repeated daily for 7 days with the intruders being exposed to a different resident on each day. For an additional comparison group, age-matched EA peers were used as residents and the protocol carried out as above for 7 consecutive days. The EA dyads were allowed to interact for 15 min before being separated as described above for an additional 15 min. They were then returned to home cages.
Table 1. Plasma Corticosterone Elicited by Resident-Intruder Stress.
| Age | Time |
Control (μg/dl) |
Stress (μg/dl) |
||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 7 | Day 1 | Day 4 | Day 7 | ||
| EA (8, 8) | 0 | 6.1±1.6 | 5.0±1.4 | 6.5±1.0 | 7.1±1.9 | 5.2±1.3 | 6.4±1.9 |
| 30 | 38.6±3.0 | 19.2±3.5 | 7.9±1.6# | 41.8±4.5 | 27.6±3.7 | 19.7±3.7*,# | |
| MA (8, 8) | 0 | 5.7±1.3 | 2.4±0.6 | 1.0±0.1 | 3.2±1.1 | 2.5±0.7 | 4.0±1.4 |
| 30 | 31.8±6.0 | 7.5±2.2 | 4.7±0.6# | 51.1±5.1 | 40.5±6.0 | 29.9±6.4*,# | |
| Adult (12, 12) | 0 | 0.9±0.2 | 1.6±0.5 | 4.3±1.7 | 1.1±0.1 | 2.2±0.5 | 4.3±1.4 |
| 30 | 22.2±5.1 | 19.9±4.9 | 6.2±2.5# | 51.9±4.3 | 42.3±5.4 | 42.6±3.7* | |
*p<0.05 compared with control.
#p<0.05 compared with day 1.
EA interactions: time by day (F2, 75=23; p<0.001); time by stress (F1, 75=5.6; p<0.05).
MA interactions: time by day (F2, 79=9.2; p<0.001; time by stress (F1, 79=35.6; p<0.001).
Adult interactions: time by day (F2, 117=4.3; p<0.05); time by stress (F1, 117=44.7; p<0.001).
Numbers within parentheses indicate the number of control and stressed rats.
Separate groups of rats were exposed to restraint stress. Restraint stressed rats and their matched controls were individually housed during the experimental days. Rats were restrained by placement in a flexible rodent restrainer (Decapicone; Braintree Scientific, Braintree, MA) for 120 min daily for 7 days. This was secured with tape around the base of the tail, leaving enough room for the animal to defecate. Controls were weighed daily and returned to their home cages.
Behavioral End Points
Behavior during each resident-intruder session was recorded as the number of days out of seven that the intruders were separated from residents as a result of meeting the criterion of (1) assuming the defeat posture (2) reaching the five attack limit before assuming the defeat posture, or (3) reaching the 15 min limit. Data were compared across ages using an ANOVA with Newman–Keuls pair-wise comparisons. The total number of attacks received was also compared between groups. These measures were further broken down by day to determine how they changed across the week of stress.
One group of rats was used to quantify behavioral consequences of stress. Twenty-four hours after the last exposure to stress (or control manipulation), open field behavior was recorded in the colony room during the light cycle. Lighting conditions were not altered from normal housing levels (300 lx). Rats were placed in a 70 × 70 × 30 cm3 black Plexiglas open field arena for 5 min and activity was videotaped and acquired using Roxio video acquisition software (Santa Clara, CA) and analyzed offline using the Noldus Ethovision software (Wageningen, the Netherlands). Time spent in each of four concentric square zones (sides=17.5, 35, 52.5, and 70 cm) and total distance traveled were compared between control and stress groups using a Student's t-test with significance at p<0.05.
Defensive burying behavior was measured 48 h after the last resident-intruder stress and 24 h after open field exposure as described previously (Howard et al, 2008) (see Supplementary Information for details). The latency to begin burying and bury duration was compared using a two-way ANOVA with stress and age as the main factors.
Behaviors during a 15-min swim stress were recorded 72 h after the last resident-intruder stress as described previously (Detke et al, 1995). Rats were placed in a cylindrical glass tank (46 cm high × 20 cm diameter) filled with water (25±1 °C) to a depth of 40 cm, allowing rats to swim or float without having their tails touch the bottom of the tank. The test was videotaped and analyzed offline by a treatment-blind individual. Behavior was scored using a time-sampling procedure identical to that reported previously (Detke et al, 1995) (see Supplementary Information for details). As an additional measure, the onset to the first 5 s bout of immobility was determined.
Electrophysiology
Separate groups of rats were exposed to resident-intruder stress (or control manipulation), and 24 h after the last experimental manipulation, single unit LC activity was recorded in the isoflurane-anesthetized state. Rats used in electrophysiological studies had not undergone behavioral testing, with the exception of rats stressed in early adolescence and tested in adulthood. This latter group was exposed to the defensive burying test as adults (PND 70) to assess the endurance of the behavioral effect of social stress and LC neuronal activity was recorded 1 week later.
The methods for surgery and recording were as described previously (Curtis et al, 1997). LC spontaneous activity was recorded for at least 3 min, followed by a recording of LC sensory-evoked activity during a trial of sciatic nerve stimulation (50 stimuli, 3.0 mA, 0.5 ms duration, 0.2 Hz) from 1 to 6 neurons per subject. For experiments that tested the effect of the CRF antagonist, DPheCRF(12−41), double-barrel micropipettes were used to record neuronal activity and simultaneously microinfuse DPheCRF(12−41) (3 ng in 30 nl) by applying small pulses of pressure to the calibrated infusion pipette (15–25 psi, 10–30 ms in duration, Picospritzer; general valve) as described previously (Kreibich et al, 2008). LC activity was recorded for at least 3 min after the infusion. DPheCRF12−41 was only administered once to an individual rat.
Recording sites were marked by the iontophoresis of Pontamine sky blue (15 μA for 15 min). Brains were dissected out and 30 μm frozen sections were cut and stained with neutral red for localization of the recording site. Data were analyzed only from those neurons that were histologically identified as being within the nucleus LC.
LC activity during sciatic nerve stimulation was recorded as peri-stimulus time histograms (PSTHs) and analyzed as described previously (Valentino and Foote, 1987; Kreibich et al, 2008). Tonic discharge rate was the firing rate during the 500 ms before the stimulus and the signal-to-noise ratio was calculated as the evoked rate divided by the tonic rate (see Supplementary Information for details).
Plasma Corticosterone
In some of the subjects used for electrophysiological studies, blood (150–200 μl) was collected immediately before and after the resident-intruder sessions on days 1, 4, and 7 via tail vein nick into eppendorf tubes containing EDTA. Blood was also collected from restraint stressed rats and matched controls corresponding to times 0, 30, and 120 min of the restraint on days 1 and 7. Whole blood was centrifuged at 3000 r.p.m. for 15 min and the plasma was kept at −20°C until being assayed using an RIA kit from MP Biomedicals (Orangeburg, NY). Significance was determined using a three-way ANOVA across day, stress, and time.
Drugs
DPheCRF(12−41) (Jean Rivier, The Salk Institute, San Diego, CA) was dissolved in water at a concentration of 1 mg/ml and 10 μl aliquots were concentrated and stored at −20°C until the day of the experiment. Aliquots were then dissolved in artificial cerebrospinal fluid (10 μg/30 μl).
RESULTS
Age-Related Responses to Resident-Intruder Stress
As described previously, residents responded to adult intruders with an initial period of investigation followed by a show of aggression involving biting and pouncing attacks to the back, rump, or shoulders (Miczek, 1979). Adult intruders usually took a supine posture that signaled defeat or subordination (Tornatzky and Miczek, 1994) and were most often separated from the resident based on this criterion (Figure 2a). The mean number of days an adult intruder was separated from the resident based on the criterion of sustaining five attacks was 1.1±0.4 and no adult intruders reached the 15 min limit without taking the defeat posture or sustaining five attacks.
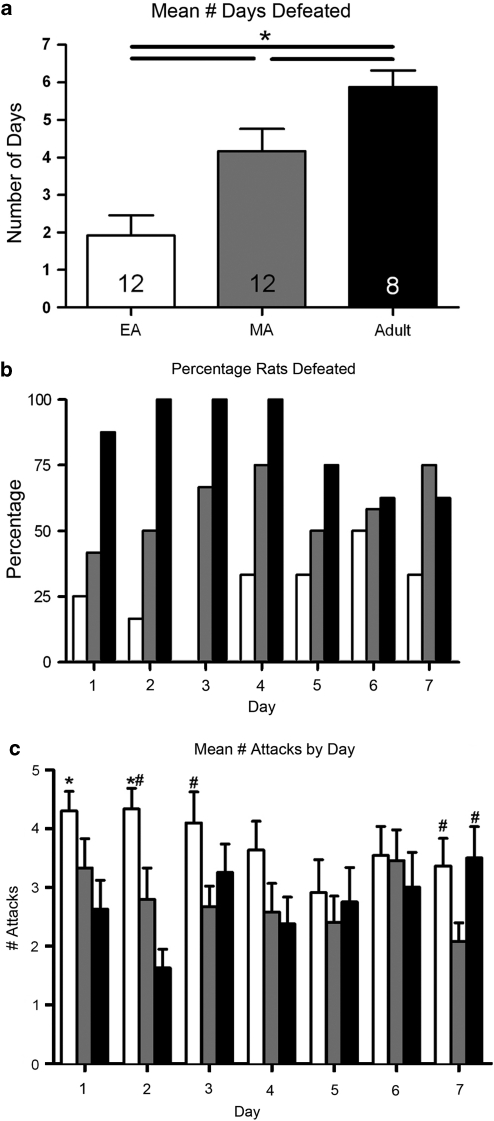
Figure 2.
Age-dependent expression of defeat behavior in response to resident-intruder stress. (a) Mean number of days intruder animals were separated from the resident because they exhibited defeat postures (possible maximum is 7). Bars are the mean of 12, 12, and 8 animals for the early-adolescent (EA) (white bars), mid-adolescent (MA) (gray bars), and adult (black bars) age groups, respectively. Vertical lines represent standard error of mean (SEM). *All pair-wise p<0.05 Student–Newman–Keuls. (b) Percentage of rats exhibiting defeat on each day. (c) Mean number of attacks/rat on each consecutive day of the stress for the same animals as in a. Vertical lines represent SEM. *p<0.05 compared with adult group; #p<0.05 compared with MA.
The placement of EA intruders into the cage also elicited a show of aggression from the resident characterized by biting and pouncing attacks directed at the back, head, and rump of the intruder. Often the resident would pounce on the intruder and repeatedly drive it into the bedding using rapid thrusting movements with the forepaws. In comparison to adult rats, EA rats exhibited the defeat posture less frequently and often remained in a crouched position in response to aggressive attacks (Figure 2a). Following each attack, the EA intruders would often remain motionless for a short period of time before reengaging social contact with the resident. When not taking the defeat posture, EA intruders were separated as a result of sustaining five attacks (3.3±0.4 days) or reaching the 15 min limit (1.8±0.3 days). The response of MA rats to resident-intruder stress was intermediate between EA and adult rats (Figure 2a). When MA intruders did not assume the defeat posture, they were more often separated as a result of the 15-min time limit (2.1±0.4 days) than as a result of sustaining five attacks (0.7±0.3).
Figure 2a shows that the expression of the defeat posture became more established as rats developed through adolescence as indicated by significant differences between age groups in the average number of days that an animal expressed the defeat posture over the entire week of stress (F(2, 31)=13.1, p<0.001) (Figure 2a). A day-by-day analysis indicated that the percentage of adult rats expressing defeat decreased with repeated exposure (Figure 2b). In contrast, the percentage of EA rats expressing the defeat posture increased as the week progressed and there was no obvious trend for MA rats (Figure 2b). The mean total number of attacks sustained by an intruder over the entire week was greater for the EA (24±1) compared with adult (19±1) or MA (17±1) rats (F2, 29=9.4, p<0.001). An day-by-day analysis of the mean number of attacks revealed an effect of age (F2, 187=10.6, p<0.002), but not day (F6, 187=1.03, p=0.4) or age-by-day interaction (F12, 187=1.38, p=0.18) (Figure 2c). Notably, although EA intruders received more attacks than adults, this was primarily owing to effects on the first two days of the stress (Figure 2c).
As an end point to determine whether the resident-intruder stress elicited a stress response in all age groups, plasma corticosterone was assayed before and after the stress or control manipulation on days 1, 4, and 7. Social stress elevated plasma corticosterone in all groups compared with baseline. On the first day of the manipulation, plasma corticosterone levels at the 30-min time point were elevated in all rats, including controls and stressed rats of each age group. However, by the seventh day of manipulation, the plasma corticosterone levels in control rats had completely habituated to baseline levels, whereas the corticosterone levels in stressed rats remained elevated, although there was a degree of habituation in the stressed adolescent groups (Table 1).
Age-Related Behavioral Consequences of Resident-Intruder Stress
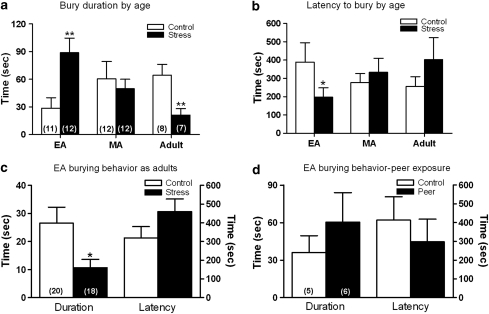
Repeated exposure to resident-intruder stress had no effect on open field activity at any age (Supplementary Information, Table 1). However, this stressor had distinct age-dependent effects on defensive burying behavior. Exposure to resident-intruder stress during early adolescence increased defensive burying behavior as indicated by an increased duration of burying and a decreased latency to begin burying compared with matched controls (Figure 3a and b). In contrast to its effects in EA rats, exposure to resident-intruder stress in adulthood decreased defensive burying duration compared with matched controls (Figure 3a). Exposure of MA rats to resident-intruder stress was without effect. A two-way ANOVA for burying duration revealed a significant stress by age interaction (F(2, 56)=6.53, p<0.01). There was no correlation between the total number of attacks received and burying duration for any age group; r2=0.0003, p=0.96, r2=0.09, p=0.35 and r2=0.0004, p=0.97 for EA, MA, and adult rats, respectively.
Figure 3.
Effects of resident-intruder stress or exposure to age-matched conspecific on defensive burying behavior. (a) Bars indicate the average burying duration of early-adolescent (EA) (n=11 control, n=12 stressed), mid-adolescent (MA) (n=12 control, n=12 stressed), or adult (n=8 control, n=7 stressed) rats. (b) Bars indicate the average latency to begin burying for the same animals as in (a). (c) Bars indicate the average burying duration (left bar pair) or latency (right bar pair) of EA rats that were tested as adults (n=20 control, n=18 stressed). Data from one control and two stressed rats were not used because they exhibited burying before an obvious shock occurred. (d) Bars indicate the average burying duration (left bar pair) or latency (right bar pair) of EA rats exposed to an age-matched peer (n=5 control, n=6 peer exposed). Vertical lines represent standard error of mean (SEM); *p<0.05; **p<0.01 post hoc Student's t-test.
The behavior of rats during swim stress was quantified as an additional measure of the behavioral strategy used in response to an environmental challenge. EA rats exposed to resident-intruder stress exhibited increased climbing behavior during swim stress compared with matched controls (Table 2). The effects were limited to climbing, as there were no significant effects on the incidence of either swimming or immobility behavior. The onset to the first 5 s bout of immobility was also not significantly affected in EA rats exposed to resident-intruder stress (35±10 vs 47±9 s, control vs stress groups, respectively). Exposure of MA or adult rats to social stress had no effect on the behavioral response to swim stress when compared with their matched controls.
Table 2. Age-Dependent Effects of Resident-Intruder Stress on Behavior in Response to Swim Stressa.
| Age |
Climbing |
Swimming |
Immobility |
|||
|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | Control | Stress | |
| EA (12, 11) | 8.3±1.6 | 13.9±1.8* | 23.8±5.0 | 29.6±6.3 | 147.9±6.3 | 136.5±7.9 |
| MA (12, 12) | 11.8±1.5 | 13.8±1.7 | 23.3±3.6 | 28.0±3.8 | 144.8±3.6 | 138.3±4.4 |
| Adult (8, 8) | 15.3±1.2 | 15.8±1.8 | 49.1±2.7 | 47.3±3.6 | 115.6±3.1 | 117.0±4.3 |
| EA peer (6, 6) | 16.5±2.7 | 22.7±3.6 | 42.7±3.5 | 40.7±5.0 | 120.8±2.5 | 116.7±7.0 |
Numbers are the mean incidence of each behavior±1 SEM recorded in 5 s intervals during a 15-min swim stress as described in detail in Supplementary Information.
*p<0.05.
Numbers within parentheses indicate the number of control and stressed groups.
Bold numbers indicates p<0.5.
To determine whether the increased active defensive behaviors expressed by EA stressed rats endured into adulthood, a separate cohort of EA rats were exposed to resident-intruder stress as adolescents and then allowed to grow to adulthood, at which time they were tested for defensive burying behavior (PND 70). A subset of these rats was also tested for defensive burying in early adolescence following the resident-intruder stress. As there was no effect of previous exposure to defensive burying on burying duration tested in adulthood, the groups were pooled (Supplementary Figure 1). Interestingly, the behavior of stressed EA rats tested in adulthood in the defensive burying test resembled that seen in rats that had been stressed as adults (Figure 3c). Thus, the burying duration of EA stressed rats tested in adulthood was decreased compared with matched controls (p<0.05).
To determine whether a non-aggressive social interaction would have similar effects as the resident-intruder stress on subsequent EA behavior, EA rats were introduced into the cage of an age-matched peer instead of an adult resident. This initiated a short period of investigation after which the rats would engage in rough and tumble play. This included chasing, nuzzling of the head and nape, and light pouncing. Dominant roles would reverse rapidly and often during the session such that the ‘attacker' became the ‘attacked.' These interactions never resulted in either rat adopting the classic defeat posture. In contrast to resident-intruder stress, EA exposure to an age-matched peer did not increase burying duration or decrease latency in the defensive burying test compared with matched controls (Figure 3d). In addition, there were no group differences in climbing behavior when peer-exposed EA rats were subjected to swim stress (Table 2).
Age-Related Behavioral and Endocrine Consequences of Restraint Stress
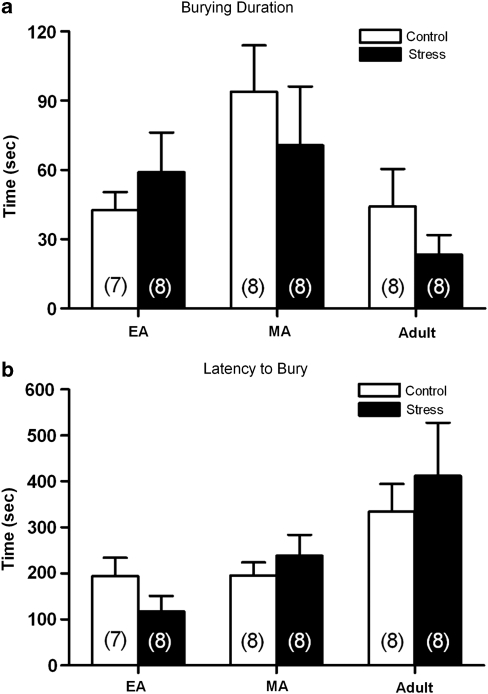
To determine whether the effects of resident-intruder stress during adolescence generalized to other stressors, the effects of repeated restraint stress on defensive burying behavior were compared across ages. Restraint stress increased plasma corticosterone in all age groups to values that were comparable to, or greater than, levels produced by social stress (Table 3). However, restraint did not affect defensive burying behavior of any group (Figure 4). There was no significant effect of restraint on burying duration (F1, 41=0.4; p=0.52) or latency (F1,41=0.1; p=0.77) or restraint by age interactions for burying duration (F(2, 41)=0.7; p=0.45) or latency (F(2, 41)=0.8; p=0.44).
Table 3. Plasma Corticosterone Elicited by Restraint Stress.
| Age | Time |
Control (μg/dl) |
Stress (μg/dl) |
||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | ||
| EA | 0 | 5.5±0.4 | 2.9±0.7# | 4.3±1.2 | 3.7±0.7 |
| 30 | 48.0±7.8 | 16.8±2.8# | 59.5±5.4 | 40.5±8.1* | |
| 120 | 18.4±5.4 | 7.5±2.0 | 57.7±13.4 | 37.3±8.7* | |
| MA | 0 | 1.6±0.4 | 1.5±0.6 | 1.9±0.6 | 3.5±0.7 |
| 30 | 27.7±4.1 | 13.6±2.4# | 61.5±3.9 | 45.4±3.6*,# | |
| 120 | 5.0±2.1 | 6.4±2.0 | 46.8±7.4 | 19.1±5.9*,# | |
| Adult | 0 | 6.7±1.4 | 5.0±1.2 | 1.2±0.4 | 2.0±0.5 |
| 30 | 24.4±4.6 | 9.1±2.4# | 62.1±3.6 | 52.0±5.6*,# | |
| 120 | 16.7±4.0 | 10.5±2.9 | 52.4±5.1 | 8.3±2.9# | |
*p<0.05 compared with corresponding control.
#p<0.05 compared with day 1.
EA interactions: time by day (F2, 73=4.3; p<0.05) and time by stress (F2, 73=9.3; p<0.001).
MA interaction: day by stress by time (F2, 80=5.8; p<0.01).
Adult interaction: day by stress by time (F2, 82=25.5; p<0.001).
N=6–8.
Figure 4.
Effects of restraint stress on burying behavior. (a) Bars represent the mean burying duration for early-adolescent (EA) (n=7 control, n=8 restraint), mid-adolescent (MA) (n=8 control, n=8 restraint), and adult (n=8 control, n=8 restraint) rats. Vertical lines represent standard error of mean (SEM). (b) Bars represent the mean latency to bury for the same EA, MA, and adult rats as in (a). Vertical lines represent SEM.
Age-Related Effects of Resident-Intruder Stress on LC Neuronal Activity
As the LC–norepinephrine system has been implicated in the active coping behaviors that were elevated in EA rats exposed to social stress (ie, defensive burying and climbing), LC neuronal activity was compared in EA, MA, and adult rats exposed to resident-intruder stress and their matched controls (Detke et al, 1995; Bondi et al, 2007; Howard et al, 2008). Both spontaneous discharge rate and sensory-evoked activity during trials of repeated sciatic nerve stimulation were compared (Figure 5).
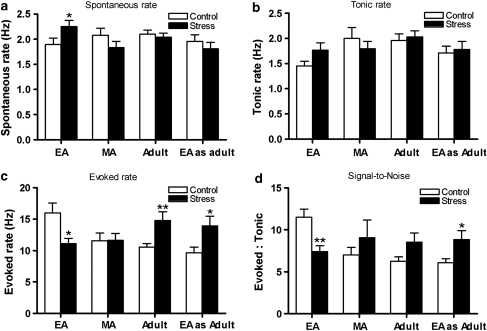
Figure 5.
Age-dependent effects of social stress on locus coeruleus (LC) discharge characteristics. (a) Bars indicate the mean LC spontaneous discharge rate determined in early-adolescent (EA) control rats (103 cells, 32 rats), EA stressed rats (103 cells, 29 rats), mid-adolescent (MA) control rats (41 cells, 8 rats), MA stressed rats (43 cells, 8 rats), adult control rats (110 cells, 42 rats), adult stressed rats (105 cells, 33 rats), EA tested as adult control rats (36 cells, 7 rats), and EA tested as adult stressed rats (38 cells, 7 rats). (b) Bars indicate the mean tonic LC firing rate during trials of sciatic nerve stimulation for EA control rats (42 cells, 25 rats), EA stressed rats (37 cells, 23 rats), MA control rats (26 cells, 8 rats), MA stressed rats (29 cells, 8 rats), adult control rats (50 cells, 31 rats), adult stressed rats (51 cells, 41 rats), EA as adult control rats (25 cells, 7 rats), and EA as adult stressed rats (24 cells, 7 rats). (c) Bars indicate the mean evoked LC firing rate during trials of sciatic nerve stimulation for same cells as in (b). (d) Bars indicate the mean signal-to-noise ratio of the LC sensory response for same cells as in (b). *p<0.05, **p<0.01 test vs control. Vertical lines represent standard error for mean (SEM).
EA rats exposed to resident-intruder stress had elevated LC spontaneous discharge rates compared to their matched controls and this effect was only apparent in the EA group (Figure 5a). During the trial of sensory stimulation, LC-evoked discharge rate was lower in stressed EA rats compared with matched controls and coupled with a nonsignificant change in tonic discharge rate, this resulted in a decreased signal-to-noise ratio (Figure 5b–d). Interestingly, when LC activity of EA rats exposed to resident-intruder stress was examined in adulthood, the profile was different. There was no change in spontaneous discharge rate, an increase in evoked LC discharge, and an increased signal-to-noise ratio compared with matched controls (Figure 5). This was similar to the effects produced by social stress in adulthood (Figure 5). For adults exposed to resident-intruder stress, evoked activity was increased and the signal-to-noise ratio tended to be greater compared with matched controls, although this did not reach statistical significance (p<0.07). Exposure of MA rats to resident-intruder stress did not alter any parameter of LC discharge compared with matched controls. Analysis across EA, MA, and adult rats using two-way ANOVA's revealed significant age by stress interactions for spontaneous rate (F(2, 499)=3.02 p<0.05), evoked rate (F(2, 207)=7.72, p<0.001), and signal-to-noise ratio (F(2, 206)=5.42, p<0.01).
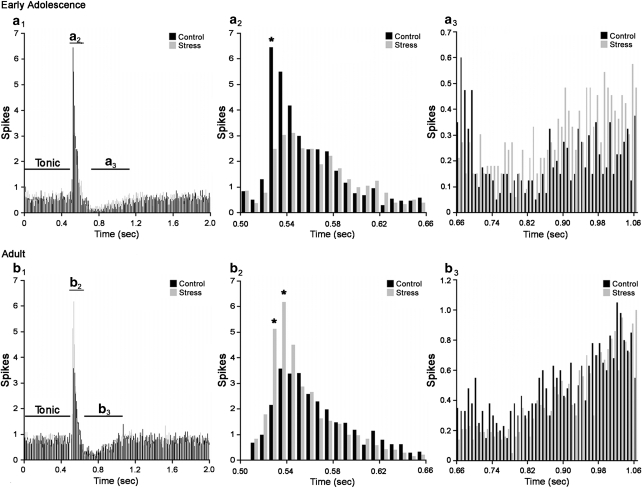
Figure 6 shows the averaged PSTH generated from all LC neurons of control and stressed rats for EA and adult groups. Components of the histograms containing the evoked response (Figure 6a2 and b2) and post-stimulus inhibition (Figure 6a3 and b3) were isolated and compared using repeated measure ANOVAs. This analysis showed that the excitatory component of the PSTH was blunted over time in stressed EA rats (time by stress interaction (F(12, 852)=2.5; p<0.01)). Bonferroni pairwise analysis by bin indicated a significant difference between control and stress in the bin corresponding to 520 ms (*p<0.05). There was also a faster recovery from post-stimulus inhibition in adolescent animals (Figure 6a3). A two-way repeated measure ANOVA indicated a significant time by stress interaction (F(49, 3479)=1.56; p<0.05).
Figure 6.
Age-dependent effects of social stress on sensory-evoked locus coeruleus (LC) discharge as compared using an averaged peri-stimulus time histograms (PSTH). (a1) and (b1) represent the entire average PSTH of all cells from early-adolescent (EA) and adult rats, respectively. Bars indicate average spikes per 8 ms bin for control (black) or stress (gray) rats. In (a2–b2) and (a3–b3), the components of the PSTH are broken down and compared between experimental groups. (a2–b2) Average evoked phase (from 520 to 624 ms) plus several bins on either side for reference. (a3–b3) Average post-stimulus inhibitory phase (from 656 to 1048 ms) plus several bins for reference. *p<0.05 Bonferroni post hoc stress vs control.
Consistent with the results shown in Figure 5, analysis of the mean excitatory component of the PSTH in adult animals showed an opposing effect of social stress on LC-evoked discharge in adult compared with EA rats, with stress resulting in an enhanced sensory response (time by stress interaction (F(12, 972)=4.3; p<0.0001)). Bonferroni pairwise analysis by bin also indicated a significant difference between control and stressed rats in the bins corresponding to 520 and 528 ms (*p<0.05). Similar effects of social stress on the average PSTH components were noted in EA rats tested as adults (data not shown).
Potential Role of CRF in the Neuronal Changes Produced by EA Exposure to Resident-Intruder Stress
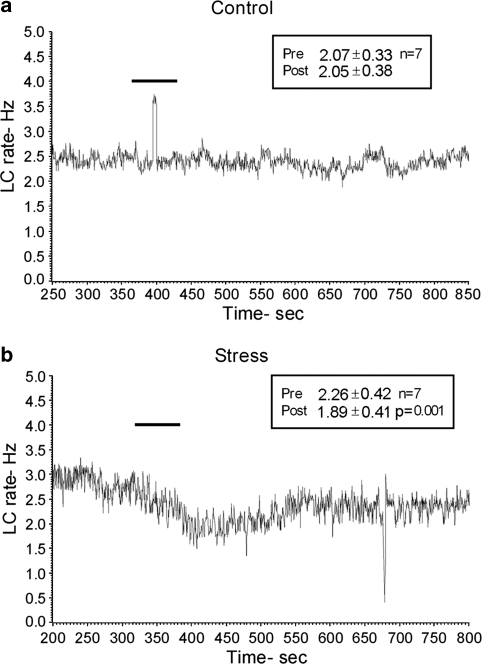
The increase in LC spontaneous activity and decrease in sensory-evoked activity in EA rats exposed to social stress were reminiscent of the effects of CRF or of stressors that release CRF in the LC (Valentino and Foote, 1987, 1988; Valentino and Wehby, 1988). To determine whether CRF release in the LC is involved in the effects of social stress on neuronal activity, LC firing rate was recorded before and after microinfusion of the CRF antagonist, DPheCRF(12−41), into the LC of stressed EA rats and controls. DPheCRF12−41 decreased LC spontaneous discharge rates of EA exposed to social stress but not controls (Figure 7).
Figure 7.
Representative rate meter records from a single locus coeruleus (LC) neuron in (a) a control and (b) a stressed early-adolescent (EA) rat before and after DPheCRF12−41 (10 ng/30 nl) microinfusion into the LC (indicated by bar above the trace). The mean discharge rates for 7 cells in each group before (Pre) and after (Post) DPheCRF12−41 administration are indicated in the upper right corner of the graphs. The corticotropin-releasing factor (CRF) antagonist decreased LC spontaneous discharge rates of stressed but not control rats, p<0.001 Student's paired t-test.
DISCUSSION
This study showed that resident-intruder stress interacts with adolescent development to influence LC neuronal activity and defensive behavioral strategy. The expression of the defeat posture developed through early adolescence to adulthood. Temporally associated with this, the effects of resident-intruder stress on the behavioral response to subsequent stressors shifted from facilitation to inhibition of proactive defense responses. Early adolescence was a developmental window during which resident-intruder stress promoted proactive defensive behaviors. Consistent with this, resident-intruder stress at this age shifted the discharge mode of LC neurons to a state of higher spontaneous activity and lower sensory activation that has been associated with hyperarousal and behavioral flexibility (Aston-Jones and Cohen, 2005), and this was mediated in part by CRF release. Early adolescent social stress continued to affect behavior and LC activity into adulthood, although the effect was differentially expressed as an inhibition of proactive behavior and a shift toward increased phasic LC activity, identical to the pattern seen in socially stressed adults. Finally, the effects of resident-intruder stress did not generalize to a non-social stressor or a non-threatening social interaction. Taken together, the findings suggest that social stress at different ages has distinct neuronal and behavioral consequences, perhaps by affecting common neural substrates that are at different stages of development.
Relationship to Previous Studies
Aversive social interactions, such as the resident-intruder stress, model a common stressor encountered by adolescent human beings (Bjorkqvist, 2001; Huhman, 2006). Although there are many reports on the consequences of resident-intruder stress in adult rats, studies using adolescent animals are relatively rare. The few studies that have been carried out showed that adolescent rats exposed to resident-intruder stress exhibit inhibited social interactions when tested as adults (Vidal et al, 2007; Watt et al, 2009). This study significantly expands on previous work by systematically analyzing the behavioral and neuronal effects of agonistic social stress from the pubertal transition to early adulthood in rats.
One methodological consideration relates to the condition of isolation housing during the course of the stressor. This was necessary because group housing attenuates the impact of resident-intruder stress (Ruis et al, 1999; Nakayasu and Ishii, 2008). In addition, group-housed experimental rats have different cage mates, unique social interactions, and are likely to be in different hierarchal positions in their home cages, making it difficult to standardize conditions between subjects. Although social isolation can be a stressor, particularly in EA rats (Weiss et al, 2004; Leussis and Andersen, 2008), it is unlikely to have contributed to the effects seen in this study for several reasons. The matched controls that served as comparisons to stressed rats were also singly housed. Housing conditions were comparable for the resident-intruder stress, the restraint stress, and the exposure to peer experiments, yet the behavioral effects were only observed in the resident-intruder stress group. Most importantly, social isolation of EA and adult rats has effects on defensive burying that are opposite to those produced by the resident-intruder stress in this study (Arakawa, 2007a, 2007b). Thus, isolation housing of EA rats inhibited defensive burying and isolation housing of adults increased defensive burying, and that these reported effects of isolation housing are opposite to those produced by the resident-intruder stress in this study support the interpretation that exposure to the resident-intruder stress was the primary determinant of subsequent behavior.
Development of the Defeat Posture
The adoption of the defeat posture in response to agonistic social stress developed through the progression of adolescence to become prominent in adulthood. The gradual increase in the percentage of EA animals expressing defeat behavior as the week progressed could indicate the development of circuits underlying the defeat posture from PND 28–35 or that the rats were learning to assume the posture with repeated exposures. As the incidence of MA rats expressing the defeat posture was intermediate between EA and adult rats and the percentage of MA rats exhibiting defeat did not change systematically as the week progressed, it is more likely that the gradual increase in EA rats assuming the defeat posture from the first to the last day of stress represents developmental as opposed to learned changes. In addition, in adults, expression of the defeat posture decreases with repeated exposure to the resident-intruder stress as indicated by the decreased percentage of adults rats assuming the defeat posture (this study) and the longer onset to express the posture (Wood et al, 2010). These findings suggest that the increased incidence of EA rats to defeat with repeated exposures represents a developmental change rather than learning. Notably, the average number of daily attacks did not change across the course of the week for any age, indicating that shifts in defeat behavior are not owing to changes in resident aggression.
In this study, every attempt was made to select resident rats that would reliably attack the adolescent intruders as well as adults. However, it is possible that differences in the nature of the interaction between the resident rats and adolescent compared with adult intruders contributed to the age-related differences in social behavior. To verify that the social encounter was stressful to EA rats, plasma corticosterone levels in response to the encounter were quantified. Although the corticosterone response of the EA rats habituated to a degree, the overall corticosterone levels remained elevated for this group throughout the week. It is also worth noting that, in the long term, stressed EA rats had a similar behavioral and electrophysiological profile to stressed adult rats, further indicating that the early adolescent resident-intruder stress had a behavioral and physiological impact.
Developmentally Distinct Consequences of Social Stress
This study is the first to analyze the effects of adolescent exposure to resident-intruder stress on defensive burying and responses to swim stress. Although these tests are often used in animal models of affective disorders, in this study the manipulations were employed to examine behavioral responses used to master environmental challenges or ‘coping strategies' (Koolhaas et al, 1999). Coping strategies are categorized as being proactive or reactive. In rats proactive coping is characterized by aggression, defensive burying, and active escape, whereas reactive coping is characterized by conservation of energy, withdrawal, and immobility (Koolhaas et al, 1999). As coping strategies in human beings have been linked to disease vulnerability, the question of how stress during development may influence these strategies has clinical relevance (Rohde et al, 1990; Ravindran et al, 1995; Bardwell et al, 2001; Mao et al, 2003; Matheson and Anisman, 2003).
A significant behavioral finding of this study was that EA resident-intruder stress increased proactive responding in both the defensive burying and swim test. This effect did not generalize to restraint stress. The finding that substituting the larger, aggressive resident rat with an EA peer did not reproduce the effect of resident-intruder stress suggests that this is specific to agonistic social interactions. Facilitation of the proactive response of burying may be considered to be a positive adaptation because it decreases plasma corticosterone (Korte et al, 1992; De Boer and Koolhaas, 2003). On the other hand, propensity to bury correlates with social aggression (Koolhaas et al, 1999). Thus, these rodent findings are relevant to studies showing that social stress in human adolescents increases the risk for hyperactivity, conduct disorder, and violence (Pelcovitz et al, 1994; Kaplan et al, 1998; Duke et al, 2010).
In contrast to early adolescence, resident-intruder stress occurring in adulthood decreased defensive burying, suggesting that agonistic social stress in adulthood inhibits proactive strategies. That this effect was not reproduced by restraint indicates that it is selective to social stress. The findings are consistent with studies showing that group housing adult male rats, which involves the establishment of social hierarchies, decreases defensive burying compared with isolation housing (Arakawa, 2007a, 2007b). The interpretation of those findings was that social stress decreased proactive defensive behaviors and increased reactive behaviors. The current results would agree with this interpretation. Interestingly, MA appeared to be a time of transition both in the development of the defeat posture and in the effect of social stress on defensive behaviors in response to other challenges.
Early adolescent exposure to resident-intruder stress had enduring behavioral effects in adulthood. However, its consequences were expressed differently in adulthood, in the form of decreased burying. Notably, this was similar to the consequences of resident-intruder exposure in adulthood. The results suggest that social stress occurring at different ages converges on certain common neural substrates that regulate defensive behavior. As these substrates are at different stages of development in early adolescence compared with adulthood responses to subsequent challenges may be differentially expressed.
Social Stress and LC Activity
The LC housed norepinephrine system is a potential substrate for the effects of social stress reported here because it is activated by stressors and has been implicated in both defensive burying behavior and climbing behavior. For example, defensive burying is associated with increased plasma and brain norepinephrine levels (Korte et al, 1992; Bondi et al, 2007). Increases in forebrain norepinephrine facilitate defensive burying, whereas noradrenergic receptor antagonists and selective LC lesion inhibit it (Bondi et al, 2007; Howard et al, 2008). Similarly, climbing in response to swim stress is enhanced by agents that increase extracellular levels of norepinephrine (Detke et al, 1995). Norepinephrine projections arising from the LC form a vast network that innervates the entire neuroaxis (Swanson, 1976; Swanson and Hartman, 1976). Through this broad projection system, different patterns of LC discharge activity modulate states of arousal, attention, and cognitive flexibility (Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005; Bouret and Sara, 2005). Acute stressors, or exposure of LC neurons to CRF, shift the mode of discharge from a phasic state to a state of higher spontaneous activity that is associated with increased arousal, blunted responses to discrete sensory stimuli, and behavioral flexibility (Valentino and Foote, 1987, 1988; Valentino and Wehby, 1988; Aston-Jones and Cohen, 2005). Consistent with this, in this present study, social stress in EA rats shifted the mode of LC discharge toward a state of higher spontaneous activity in which phasic responses to discrete sensory stimuli were diminished compared with controls. The ability of the CRF antagonist to selectively inhibit LC neurons of stressed EA rats and decrease discharge rates to the level of controls suggests that this shift was mediated in part by tonic CRF release within the LC. Given that CRF elicits burying behavior that requires the LC–norepinephrine system, CRF antagonists attenuate defensive burying, and that CRF microinfusion into the LC increases active responses to swim stress, tonic CRF release in the LC could account for the promotion of these proactive behaviors seen in EA rats exposed to social stress (Butler et al, 1990; Korte et al, 1994; Howard et al, 2008). As CRF antagonists attenuate defensive burying behavior in unstressed subjects, they cannot be used as tools to directly test the link between increased CRF in the LC and increased burying behavior in EA rats exposed to social stress. Future studies directed at identifying and manipulating sources of CRF that are involved in this response may be required to directly test this hypothesis. Nonetheless, these findings, taken with literature suggesting that burying is an end point of CRF activation of the LC, make a compelling argument that one mechanism by which EA social stress increases proactive behaviors is via CRF activation of LC neurons.
Similar to the effects of adult social stress on behavior, the consequences of adult social stress on LC activity contrasted with those produced in early adolescence. The finding that LC spontaneous activity was not altered in adults exposed to social stress is consistent with other studies in adult rats with a history of shock or swim stress (Curtis et al, 1995, 1999). In addition, there is no evidence for tonic CRF release in the LC in adult animals regardless of stress history as CRF antagonists locally injected into the LC are without effect (Curtis et al, 1994, 1995; Pavcovich and Valentino, 1997). It is possible that CRF afferents to the LC become more highly regulated with age so that previous stress is less likely to cause a long-term perturbation from baseline. This form of adaptation could also account for the lack of change in LC spontaneous discharge in EA stress rats when examined during adulthood. Interestingly, social stress is somewhat unique in increasing LC sensory-evoked responses in adulthood. Neither previous exposure to shock or swim stress affected LC sensory-evoked discharge, although this was reportedly enhanced by chronic cold stress (Curtis et al, 1995, 1999; Mana and Grace, 1997). Given the role of excitatory amino-acid afferents in LC sensory-evoked responses, enhancement of glutamatergic transmission in the LC could account for this effect of social stress in adulthood (Ennis et al, 1992).
The MA stage of development was unique in that social stress at this time had no effect on either the behaviors examined or on LC neuronal activity. This may represent a transition stage that may even be resilient to stressors. Relevant to this, resident-intruder stress altered adult monoamine levels in a region-specific manner when it occurred in early adolescence, but not in middle or late adolescence (Vidal et al, 2007; Watt et al, 2009). It is possible that monoamine systems are buffered during middle and late adolescence.
Early Adolescent Social Stress and Mental Health
A critical question raised by these data is why different coping strategies should be favored depending on when in development resident-intruder stress occurs. We speculate that by favoring proactive strategies early in life, agonistic social stress prepares the individual for a future in which it plays a major role or may occur frequently. On the other hand, when it occurs in an adult with no previous history of social stress or if the social stress does not continue past the early adolescent stage, inhibition of proactive defenses may be a strategy that incurs less risk. These rodent studies may translate to human behavior in that adolescent social stress is reported to increase the risk for externalizing disorders (eg, conduct disorder, aggression) in adolescence while simultaneously increasing the risk for depression in adulthood (Dodge et al, 1990; Pine et al, 2002). Thus, early social stress may be a common root cause of certain adolescent and adult behavioral disorders that have different symptomatology. The data presented here implicate the brain norepinephrine system as one potential system that social stress interacts with to contribute to these disorders.
Acknowledgments
This work was supported by PHS Grants MH52850, MH40008 (RJV), and N00014-03-10311 (SGB).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Arakawa H. Ontogeny of sex differences in defensive burying behavior in rats: effect of social isolation. Aggress Behav. 2007a;33:38–47. doi: 10.1002/ab.20165. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Ontogenetic interaction between social relationships and defensive burying behavior in the rat. Physiol Behav. 2007b;90:751–759. doi: 10.1016/j.physbeh.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bardwell WA, Ancoli-Israel S, Dimsdale JE. Types of coping strategies are associated with increased depressive symptoms in patients with obstructive sleep apnea. Sleep. 2001;24:905–909. doi: 10.1093/sleep/24.8.905. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the LC. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, et al. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: interaction with a brief, daily maternal separation. Behav Brain Res. 2006;169:128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, et al. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long term regulation of locus coeruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289:1211–1219. [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Curtis AL, Grigoradis D, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994;268:359–365. [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- Duke NN, Pettingell SL, McMorris BJ, Borowsky IW. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125:e778–e786. doi: 10.1542/peds.2009-0597. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G, Shiekhattar R. Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res. 1992;598:185–195. doi: 10.1016/0006-8993(92)90182-9. [DOI] [PubMed] [Google Scholar]
- Ferris CF. Using an animal model to assess the long-term behavioral and biological consequences of adolescent abuse and exposure to alcohol. Ann N Y Acad Sci. 2003;1008:69–78. doi: 10.1196/annals.1301.008. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Malhi GS. Do bullied children become anxious and depressed adults? a cross-sectional investigation of the correlates of bullying and anxious depression. J Nerv Ment Dis. 2006;194:201–208. doi: 10.1097/01.nmd.0000202491.99719.c3. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psych. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary–adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Herrenkohl TI, Kosterman R, Hawkins JD, Mason WA. Effects of growth in family conflict in adolescence on adult depressive symptoms: mediating and moderating effects of stress and school bonding. J Adolesc Health. 2009;44:146–152. doi: 10.1016/j.jadohealth.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology. Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kaplan SJ, Pelcovitz D, Salzinger S, Weiner M, Mandel FS, Lesser ML, et al. Adolescent physical abuse: risk for adolescent psychiatric disorders. Am J Psychiatry. 1998;155:954–959. doi: 10.1176/ajp.155.7.954. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: effects of ipsapirone. Physiol Behav. 1992;52:355–361. doi: 10.1016/0031-9384(92)90284-9. [DOI] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Bohus B, Koob GF. Effect of corticotropin-releasing factor antagonist on behavioral and neuroendocrine responses during exposure to defensive burying paradigm in rats. Physiol Behav. 1994;56:115–120. doi: 10.1016/0031-9384(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, et al. Presynaptic inhibition of diverse afferents to the locus coeruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- Mao WC, Bardwell WA, Major JM, Dimsdale JE. Coping strategies, hostility, and depressive symptoms: a path model. Int J Behav Med. 2003;10:331–342. doi: 10.1207/s15327558ijbm1004_4. [DOI] [PubMed] [Google Scholar]
- Matheson K, Anisman H. Systems of coping associated with dysphoria, anxiety and depressive illness: a multivariate profile perspective. Stress. 2003;6:223–234. doi: 10.1080/10253890310001594487. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, III, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nakayasu T, Ishii K. Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav Processes. 2008;78:477–480. doi: 10.1016/j.beproc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull. 1984;10:300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Valentino RJ. Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci. 1997;17:401–408. doi: 10.1523/JNEUROSCI.17-01-00401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelcovitz D, Kaplan S, Goldenberg B, Mandel F, Lehane J, Guarrera J. Post-traumatic stress disorder in physically abused adolescents. J Am Acad Child Adolesc Psychiatry. 1994;33:305–312. doi: 10.1097/00004583-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Johnson JG, Brook JS. Adolescent life events as predictors of adult depression. J Affect Disord. 2002;68:49–57. doi: 10.1016/s0165-0327(00)00331-1. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Griffiths J, Waddell C, Anisman H. Stressful life events and coping styles in relation to dysthymia and major depressive disorder: variations associated with alleviation of symptoms following pharmacotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:637–653. doi: 10.1016/0278-5846(95)00108-8. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Tilson M, Seeley JR. Dimensionality of coping and its relation to depression. J Pers Soc Psychol. 1990;58:499–511. doi: 10.1037//0022-3514.58.3.499. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Damassa DA, Smith ER. Sexual behavior in developing male rats. Horm Behav. 1977;8:320–341. doi: 10.1016/0018-506x(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J Comp Neurol. 1976;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology (Berl) 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Repeated social stress and the development of agonistic behavior: individual differences in coping responses in male golden hamsters. Physiol Behav. 2003;80:303–308. doi: 10.1016/j.physbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Taravosh-Lahn K, David JT, Delville Y. Repeated exposure to social stress alters the development of agonistic behavior in male golden hamsters. Horm Behav. 2003;43:229–236. doi: 10.1016/s0018-506x(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.