Abstract
Appropriate animal models of attention deficit/hyperactivity disorder (ADHD) and drug reinforcement allow investigation of possible underlying biological bases of ADHD and its comorbidity with cocaine addiction. Toward this end, spontaneously hypertensive rats (SHRs) exhibiting an ADHD phenotype were compared with Wistar-Kyoto (WKY) and Wistar (WIS) rats. Initially, 1.5 mg/kg oral methylphenidate or vehicle was administered between postnatal days 28 and 55, and acquisition of visual discrimination learning was examined. After discontinuing adolescent treatments, adult rats were evaluated for cocaine self-administration and dopamine transporter (DAT) function in the prefrontal cortex (PFC) and striatum. During adolescence, SHRs showed deficits in visual discrimination relative to WKY and WIS rats when non-medicated. Methylphenidate improved visual discrimination only in SHRs. Compared with WKY and WIS rats, SHRs with previous methylphenidate treatment acquired cocaine self-administration faster, identified cocaine as a highly efficacious reinforcer by displaying an upward shift in the cocaine dose–response function, and showed the greatest motivation to self-administer cocaine by exhibiting the highest progressive ratio breakpoints. In the PFC, the maximal dopamine uptake (Vmax) at DAT was decreased in SHRs and increased in WKY and WIS rats by previous methylphenidate treatment. The affinity (Km) for dopamine at DAT in the PFC was not different between strains, nor was Vmax or Km altered in the striatum by previous methylphenidate treatment in any strain. Methylphenidate-induced decreases in dopamine clearance by DAT in the PFC may underlie increased cocaine self-administration in SHRs. These preclinical findings suggest that caution should be exercised when methylphenidate is prescribed for first-time treatment of ADHD in adolescent patients, as cocaine addiction vulnerability may be augmented.
Keywords: addiction, attention deficit/hyperactivity disorder, cocaine, dopamine transporter function, methylphenidate, spontaneously hypertensive rat
INTRODUCTION
Adults with a history of attention deficit hyperactivity disorder (ADHD) have double the risk of developing a substance use disorder than do adults without ADHD (Biederman et al, 1998). Although stimulant medications (such as methylphenidate) improve behavioral and neurocognitive deficits of ADHD (Mehta et al, 2004), disagreements remain regarding the use of stimulant medications and whether they make individuals more vulnerable or less vulnerable to later addiction to stimulant drugs, such as cocaine (Robbins, 2002). Compared with placebo treatment, studies have shown decreases (Biederman et al, 1999; Levin et al, 2007), increases (Barkley et al, 2003; Lambert and Hartsough, 1998), or no changes (Schubiner et al, 2002; Szobot et al, 2008) in cocaine use with methylphenidate treatment among individuals with ADHD. Contributing to these divergent findings may be differences in age at which stimulant treatment began and whether individuals were medicated when cocaine use was assessed.
On the basis of extensive meta-analysis (Wilens et al, 2003), it is quite clear that when stimulant treatment for ADHD is initiated during childhood, there is a decreased risk for developing a substance use disorder during adulthood. In contrast, evidence has linked the initiation of stimulant treatment for ADHD during adolescence to increased risk of non-medical stimulant use and behavior patterns consistent with the risk of developing a substance use disorder (Kollins, 2008). Prospective follow-up studies provide evidence for a positive relationship between age of stimulant treatment initiation for ADHD (treatment lasting 2–4 years) and later adult substance use disorder after treatment is discontinued (Mannuzza et al, 2008). Lifetime rates of substance use disorder (eg, cocaine) were significantly greater when methylphenidate treatment was initiated during late childhood/early adolescence (44%) relative to early childhood (27%) and to non-ADHD comparison subjects (29%). The latter two groups did not differ, supporting the view that methylphenidate treatment relatively early in childhood does not increase the risk for later substance use disorder. Further analyses indicated that the association between age at first treatment with methylphenidate and later substance abuse was mediated by the development of antisocial personality disorder. However, as there were no group differences in the severity of early conduct problems in the study by Mannuzza et al, initiation of methylphenidate treatment for ADHD during adolescence remains a relevant factor for increased risk of substance use disorder during adulthood after methylphenidate treatment is discontinued. Importantly, adolescence may represent a particularly sensitive period of vulnerability to insult from pharmacological agents due to a dramatic surge in the development of brain structure and function (Andersen, 2003), Clearly, additional research is urgently required to further examine the relationship between ADHD and methylphenidate treatment during adolescence and later vulnerability to cocaine addiction.
To address this issue at the preclinical level and to begin to understand the underlying neurochemical mechanisms, experiments were conducted using the spontaneously hypertensive rat (SHR) genetic model of ADHD (Kantak et al, 2008; Sagvolden et al, 1992; Wells et al, 2010). Adolescent SHRs were first evaluated for deficits in learning reflective of the prefrontal cortex (PFC) dysfunction and for determining whether methylphenidate could improve performance. Acquisition of two-choice visual discrimination was measured, as this is considered to be dependent on dopaminergic projections to the PFC (Crofts et al, 2001) and to reflect the development of an initial attentional set (Floresco et al, 2006; Kantak et al, 2008). After discontinuation of adolescent methylphenidate treatment, cocaine self-administration and dopamine transporter (DAT) function were evaluated during adulthood. Changes in cocaine self-administration and DAT function after discontinuation of adolescent methylphenidate treatment have never been investigated in rats with an ADHD phenotype, although some work on this topic has been conducted in outbred rat strains (Brandon et al, 2001; Thanos et al, 2007). The rationale for evaluating DAT function in this study is that both SHR and ADHD patients have a disturbance in the regulation of DAT translation (Sagvolden et al, 2005). In ADHD patients, the severity of hyperactive-impulsive symptoms of ADHD varies linearly with the number of alleles for the DAT-1 gene, which explains 1–4% of the overall variance in ADHD symptoms (Waldman et al, 1998). Animal studies show that SHRs have sequence changes in the DAT-1 gene, which may account for some of the behavioral differences between the SHR and the Wistar-Kyoto (WKY) comparator strains (Mill et al, 2005). Furthermore, cocaine binding at DAT has been extensively implicated in its reinforcing effects (Ramamoorthy et al, 2010). An approach that uses appropriate animal models of ADHD and drug reinforcement allows investigation of the possible underlying biological bases of ADHD and its comorbidity with cocaine addiction.
MATERIALS AND METHODS
Subjects
Male rats of WKY/Cr, SHR/Cr, and Wistar (WIS)/Cr strains (Charles River Laboratories, Wilmington, MA) arrived on postnatal day 25 (P25) in cohorts of 2 per strain. WKY and WIS were used as inbred and outbred comparator strains, respectively. Housing and feeding details are included in Supplementary Methods. Policies stated in the Guide for the Care and Use of Laboratory Animals were followed. The Boston University and the University of Kentucky Institutional Animal Care and Use Committees approved the experimental protocols.
Apparatus
Visual discrimination was conducted in an eight-arm radial maze (Model ENV-538, Med Associates, Georgia, VT) that was configured as a T-maze. During the task, responses were monitored on a remote video screen connected to a ceiling-mounted video camera. A complete description of the maze environment was detailed previously (Kantak et al, 2001). Drug self-administration chambers were described previously (Kantak et al, 2002). [3H]dopamine (DA) uptake assays were conducted using an Avanti-J30I centrifuge (Beckman Coulter, Brea, CA), a Dubnoff incubator (Precision Scientific, Winchester, VA), a cell harvester (Biochemical Research and Development Laboratories, Gaithersburg, MD), and an 1600-TR scintillation spectrometer (Perkin-Elmer Life and Analytical Sciences, Downers Groove, IL).
Drugs
(±)-Methylphenidate hydrochloride (Sigma-Aldrich, St Louis, MO) was dissolved in tap water (1.5 mg/ml) and injected into an oyster cracker to attain a dose of 1.5 mg/kg for oral consumption. Oyster crackers containing tap water (1.0 ml/kg) were used for vehicle control. To mimic a clinical dosing schedule for ADHD treatment (American Academy of Pediatrics Committee on Children with Disabilities Committee on Drugs, 1996), groups from each strain were treated from Monday to Friday with vehicle or methylphenidate from P28 to P55. On days that overlapped with visual discrimination testing (experiment 1), rats were treated for 30 min before the start of sessions. The amount of time to consume daily oyster crackers averaged <3 min. A 1.5 mg/kg oral dose of methylphenidate ensured that therapeutically relevant plasma drug levels were achieved, and the 30-min pretreatment time ensured that plasma drug levels peaked before the start of behavioral sessions (Kuczenski and Segal, 2002).
Cocaine hydrochloride (NIDA, Bethesda, Maryland, USA) was dissolved in 0.9% sterile saline containing 3 IU heparin/ml. A cocaine unit dose of 0.3 mg/kg (0.8 mg/ml) was used for training. In addition, cocaine unit doses ranging from 0.003 to 1.0 mg/kg were used to determine dose–response curves. A constant drug delivery time of 1.2 s/100 g body weight was maintained by adjusting cocaine concentration for each dose.
For [3H]DA uptake assays, desipramine hydrochloride, paroxetine hydrochloride, nomifensine maleate, ethylenediaminetetraacetic acid, sucrose, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 3-hydroxytyramine (DA), sodium chloride, and magnesium sulfate were purchased from Sigma-Aldrich. [3H]DA (dihydroxyphenylethylamine,3,4-[7-3H] specific activity, 30.3 Ci/mmol) was purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA). α--Glucose, -ascorbic acid, and monobasic potassium phosphate were purchased from Aldrich Chemical (Milwaukee, WI), AnalaR-BHD (Poole, UK), and Mallinckrodt (St Louis, MO), respectively. All other chemicals in the assay buffer were purchased from Fisher Scientific (Pittsburgh, PA).
Surgery
In experiments 2–4, catheters were surgically implanted in the jugular vein as described previously (Kantak et al, 2000) on P70 under ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. Post-surgical care and catheter maintenance were as detailed previously (Harvey et al, 2009).
Experiment 1: Visual Discrimination
The purpose of this experiment was to determine whether adolescent SHRs have deficits in learning reflective of PFC dysfunction that could be improved by methylphenidate treatment. Acquisition of two-choice visual discrimination was examined in a T-maze in adolescent WKY rats, SHRs, and WIS rats treated chronically with methylphenidate or vehicle (n=6 per strain and treatment group). Habituation sessions began on P38 and were completed on P41. Rats then began visual discrimination training sessions for which a 45-mg chocolate-flavored food pellet (Research Diets, New Brunswick, NJ) was used to reinforce each correct turn from the start arm (ie, a turn into the arm containing a visual cue). The criterion was 10 consecutive correct training trials. Following criterion performance, rats were tested in a single probe trial that began in the north arm. Sessions for the visual discrimination task were completed by P46, although methylphenidate and vehicle treatments continued through P55. The number of training trials and probe trials required to reach criterion was recorded. A complete description of this task is given in Supplementary Methods.
Experiment 2: Acquisition of Cocaine Self-Administration
The purpose of this experiment was to determine the speed at which WKY rats, SHRs, and WIS rats acquired cocaine self-administration during adulthood, beginning 3 weeks after adolescent treatment with methylphenidate or vehicle was discontinued (n=7–8 per strain and treatment group). Groups consisted of 4–6 rats evaluated in experiment 1 plus an additional 1–3 rats that were treated identically to those used in experiment 1 but not tested for visual discrimination. On P77, rats were given the opportunity to press a lever (left or right, counterbalanced across rats) for delivery of 0.3 mg/kg cocaine under a fixed ratio 1 (FR1) schedule of reinforcement. A 20-s timeout followed each infusion to prevent accidental overdose. The acquisition of self-administration was evaluated during daily (Monday to Friday) 2-h sessions. No external inducements to respond on the active lever were used. Responses on the inactive lever were counted but had no consequences. The acquisition criterion was ⩾20 infusions for two consecutive sessions while exhibiting at least a 2 : 1 ratio of active to inactive lever responses (66.7 vs 33.3% of total responses, respectively). After reaching the acquisition criterion, self-administration sessions under the FR1 schedule continued with the 0.3 mg/kg training dose until a stable baseline rate of responding was achieved (<10% variation for 5 consecutive sessions). The number of sessions required to reach the acquisition criterion and the number of active and inactive lever responses and infusions earned at criterion were recorded.
Experiment 3: Cocaine Dose–Response Function under the FR1 Schedule
The purpose of this experiment was to determine the efficacy of cocaine reinforcement in WKY rats, SHRs, and WIS rats after acquiring cocaine self-administration in experiment 2 (n=7–8 per strain and treatment group). Between P105 and P140, a full range of cocaine unit doses (0.003, 0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg) was substituted for the training dose in a random order. The 2-h dose substitution sessions were conducted twice each week (Tuesdays and Fridays), with 2-h sessions with the 0.3 mg/kg training dose on intervening days. After determination of cocaine dose–response functions, baseline responding was reestablished with 0.3 mg/kg under the FR1 schedule of reinforcement. The number of active and inactive lever responses and the number of cocaine infusions earned were recorded.
Experiment 4: PR Breakpoints for Self-Administered Cocaine
The purpose of this experiment was to determine progressive ratio (PR) breakpoints for various doses of self-administered cocaine in WKY rats, SHRs, and WIS rats after cocaine dose–response functions were established in experiment 3 (n=6–8 per strain and treatment group). PR breakpoint measures motivation to self-administer cocaine. The PR schedule developed previously (Roberts et al, 1989) was implemented between P147 and P168, and in effect daily (Monday to Friday) until rats failed to complete the current FR requirement within 60 min of the last cocaine infusion. The PR breakpoint was determined over 5 sessions for the 0.3 mg/kg cocaine training dose, and then additional cocaine unit doses (0.01, 0.1, and 1.0 mg/kg) were substituted in a descending order. The number of active and inactive lever responses and cocaine infusions earned, as well as the last FR completed were recorded.
Experiment 5: DAT Function in the PFC and Striatum
Separate groups of WKY rats, SHRs, and WIS rats were used to assess [3H]DA uptake (DAT function) in the PFC and striatum (n=6–8 per strain and treatment group). Rats were housed, fed, and treated with methylphenidate or vehicle as described previously. For each assay, the PFC and striata were obtained from one methylphenidate-treated and one vehicle-treated rat from the same strain and the [3H]DA uptake assay was conducted as described previously (Zhu et al, 2004) with minor modifications as detailed in Supplementary Methods.
Data Analyses
Dependent measures in behavioral studies initially were analyzed by three-factor (dose × strain × treatment) or two-factor (strain × treatment) analysis of variance (ANOVA), with repeated measures for dose. To deconstruct significant three-way interactions, two-factor (strain × treatment) ANOVAs were performed for each cocaine unit dose. For significant strain × treatment interactions, Tukey's post hoc test was used for multiple group comparisons. Non-significant strain × treatment interactions associated with significant main effects were followed by Dunnett's t-test to compare WKY and WIS with SHR for each treatment and with Bonferroni's t-test to compare vehicle with methylphenidate for each strain. Both procedures control for type I error and are appropriate for post hoc analysis of cell means when the F-value of the interaction is not significant (Winer et al, 1991). In functional assays, Vmax for methylphenidate treatment was normalized by expression as a percentage of Vmax for the vehicle-treated control. Outliers were eliminated using the Grubbs test (GraphPad Software; http://www.graphpad.com/quickcalcs/Grubbs1.cfm). Km and Vmax were analyzed by two-factor and one-factor ANOVAs, respectively, for differences followed by Tukey's post hoc test. In addition Vmax was analyzed using Student's one-sample t-tests for matched subjects (one sided) to compare the mean percentage methylphenidate value with the vehicle control value of 100% for each strain (Vadum and Rankin, 1998).
RESULTS
Experiment 1: Visual Discrimination
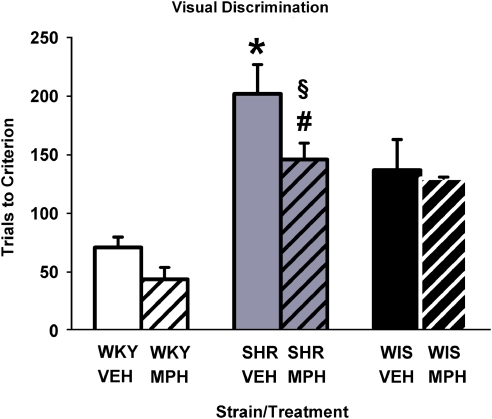
During adolescence, SHRs showed a deficit in the acquisition of learning in the visual discrimination task relative to WKY and WIS rats when non-medicated. Methylphenidate improved visual discrimination performance only in SHRs (Figure 1). Analysis of trials to criterion showed a main effect of strain (F[2, 30]=24.0, p⩽0.001) and treatment (F[1, 30]=4.5, p⩽0.04). Follow-up comparisons revealed that among vehicle-treated groups, SHRs required more trials than did WKY and WIS (p⩽0.04) to reach criterion. In methylphenidate-treated groups, SHRs required more trials than did WKY (p⩽0.001), but not WIS, to reach criterion. Relative to vehicle, methylphenidate treatment improved performance in SHRs (p⩽0.03), but not in WKY or WIS. The majority of rats in each group required a single probe trial after criterion was reached. There were no significant differences in the number of probe trials conducted across the three strains or between the two treatments (not shown).
Figure 1.
Effects of chronic methylphenidate and vehicle treatment on acquisition of visual discrimination conducted during adolescence in Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats. Values are mean±S.EM number of trials to reach criterion (n=6 per strain and treatment group). *p⩽0.04 compared with vehicle-treated WKY and WIS rats; #p⩽0.001 compared with methylphenidate-treated WKY rats; and §p⩽0.03 compared with vehicle-treated SHRs (see text for details).
Experiment 2: Acquisition of Cocaine Self-Administration
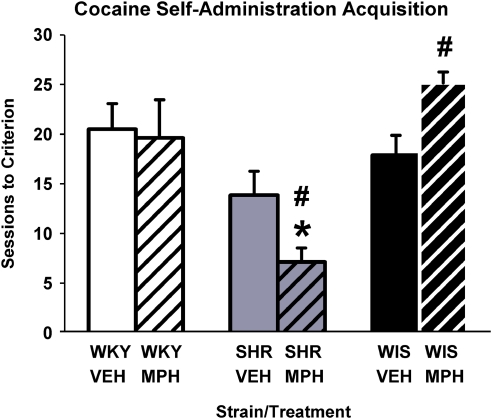
Cocaine self-administration during adulthood was acquired fastest in SHRs that received adolescent methylphenidate treatment (Figure 2). Analysis of the number of sessions to reach the infusion criterion showed a strain × treatment interaction (F[2, 40]=3.9, p⩽0.03). Follow-up comparisons revealed that in vehicle-treated groups, the speed to acquire cocaine self-administration in SHRs was not different from WKY and WIS; but in methylphenidate-treated groups, SHRs acquired cocaine self-administration faster than did WKY and WIS (p⩽0.009). Between-treatment comparisons revealed that after discontinuation of methylphenidate, the speed to acquire cocaine self-administration did not differ in WKY (p⩽0.99), was slower in WIS (p⩽0.01), and was faster in SHRs (p⩽0.04). The number of sessions required to discriminate the active from inactive lever preceded the achievement of the infusion criterion in each group (Supplementary Figure S1). In addition, self-administration behavior at criterion (infusions earned and active and inactive lever responses) was greatest in SHRs, although inactive lever responses were ⩽25% of total responses (Supplementary Figure S2 and Supplementary Table S1).
Figure 2.
Acquisition of cocaine self-administration in adult Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats after discontinuation of adolescent vehicle or methylphenidate treatment. Values are mean±SEM number of sessions to reach the acquisition criterion (n=7–8 per strain and treatment group). *p⩽0.009 compared with methylphenidate-treated WKY and WIS rats, and #p⩽0.04 compared with the corresponding vehicle-treated group (see text for details).
Experiment 3: Cocaine Dose–Response Function under the FR1 Schedule
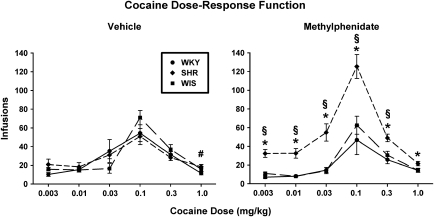
Cocaine self-administration under the FR1 schedule produced an inverted U-shaped dose–response function that was shifted upward in adult SHRs after discontinuation of adolescent methylphenidate treatment (Figure 3). Using the number of infusions earned as the dependent measure to assess dose–response functions, a three-factor ANOVA revealed a significant dose × strain × treatment interaction (F[10, 185]=4.6, p⩽0.001). Separate analyses for each cocaine unit dose showed a strain × treatment interaction (F[2, 37]=3.9–9.6, p⩽0.001 to p=0.003), except for the highest unit dose of 1.0 mg/kg, which showed a main effect of strain (F[2, 37]=6.0, p⩽0.01). Follow-up comparisons for 0.003–0.3 mg/kg unit doses revealed that SHRs with previous methylphenidate treatment self-administered more cocaine compared with WKY or WIS with previous methylphenidate treatment (p⩽0.001 to 0.01) and compared with SHRs with previous vehicle treatment (p⩽0.001 to 0.03). WKY and WIS groups with previous methylphenidate or vehicle treatment did not differ from each other or from SHRs with previous vehicle treatment.
Figure 3.
Dose–response functions for self-administered cocaine under a fixed ratio 1 schedule in adult Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats after discontinuation of adolescent vehicle (left panel) or methylphenidate (right panel) treatment. Values are mean±SEM number of infusions earned of cocaine unit doses ranging from 0.003 to 1.0 mg/kg (n=7–8 per strain and treatment group). *p⩽0.03 compared with methylphenidate-treated WKY and WIS rats; #p⩽0.02 compared with vehicle-treated WKY rats; and §p⩽0.03 compared with vehicle-treated SHRs (see text for details).
Follow-up comparisons for the 1.0 mg/kg unit dose revealed that SHRs with previous vehicle treatment earned a greater number of infusions than did WKY (p⩽0.02), but not WIS. In methylphenidate-treated groups, SHRs earned a greater number of infusions than did both WKY and WIS (p⩽0.03). Between-treatment comparisons for the 1.0 mg/kg unit dose revealed no differences between methylphenidate and vehicle treatments for each strain. Using active lever responses as the dependent measure resulted in a similar outcome as infusions earned, whereby the cocaine dose–response function was also shifted upward in SHRs after discontinuation of adolescent methylphenidate treatment (Supplementary Figure S3). Inactive lever responses are depicted in Supplementary Table S2, and were typically ⩽38% of total responses.
An exploratory study was conducted to determine whether the high rate of responding associated with 0.003 mg/kg cocaine (typically a non-reinforcing dose) in vehicle- and methylphenidate-treated SHRs was reflective of resistance to extinction vs enhanced motivation for cocaine reinforcement (for details, see Supplementary Methods). Relative to active lever responses emitted after the removal of cocaine reinforcement, responses maintained by the 0.003 mg/kg cocaine unit dose were proportionally higher in SHRs, but not in WKY or WIS rats, regardless of treatment (Supplementary Figure S4).
Experiment 4: PR Breakpoints for Self-Administered Cocaine
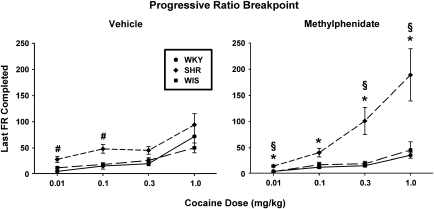
PR breakpoints for self-administered cocaine were highest in adult SHRs that received adolescent methylphenidate treatment (Figure 4). Using the last FR completed as the dependent measure for the PR breakpoint, a three-factor ANOVA revealed a significant dose × strain × treatment interaction (F[6, 108]=3.9, p⩽0.001). Separate analyses for each cocaine unit dose showed a strain × treatment interaction for 0.3 mg/kg (F[2, 36]=4.80, p⩽0.01) and 1.0 mg/kg (F[2, 36]=3.80, p⩽0.03) and a main effect of strain for 0.01 mg/kg (F[2, 36]=16.6, p⩽0.001) and 0.1 mg/kg (F[2, 36]=14.5, p⩽0.001). Follow-up comparisons for unit doses of 0.3 and 1.0 mg/kg revealed that SHRs with previous methylphenidate treatment exhibited higher PR breakpoints than did WKY or WIS rats with previous methylphenidate treatment (p⩽0.001) and than did SHRs with previous vehicle treatment (p⩽0.01). WKY and WIS groups with previous methylphenidate or vehicle treatment did not differ from each other or from SHRs with previous vehicle treatment.
Figure 4.
Progressive ratio (PR) breakpoints for self-administered cocaine in adult Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats after discontinuation of adolescent vehicle (left panel) or methylphenidate (right panel) treatment. Values are mean±SEM last fixed ratio (FR) completed for cocaine unit does ranging from 0.01 to 1.0 mg/kg (n=6–8 per strain and treatment group). *p⩽0.01 compared with methylphenidate-treated WKY rats; #p⩽0.01 compared with vehicle-treated WKY and/or WIS rats; and §p⩽0.002 compared with vehicle-treated SHRs (see text for details).
Follow-up comparisons for unit doses of 0.01 and 0.1 mg/kg revealed that SHRs with previous methylphenidate treatment exhibited higher PR breakpoints than did WKY and WIS rats with previous methylphenidate treatment (p⩽0.01). In vehicle-treated groups, SHRs exhibited higher PR breakpoints than did WKY and WIS for the 0.1 mg/kg unit dose (p⩽0.004) and than did WKY for the 0.01 mg/kg unit dose (p⩽0.002). Between-treatment comparisons revealed that PR breakpoints were higher in SHRs with previous vehicle than methylphenidate treatment for the 0.01 mg/kg unit dose (p⩽0.004), but not for the 0.1 mg/kg unit dose. Between-treatment comparisons for WKY and WIS revealed no differences in the PR breakpoint at these lower unit doses. Using other common indices of the PR breakpoint for self-administered cocaine (infusions earned and active lever responses) as the dependent measure resulted in a similar outcome as last FR completed, whereby PR breakpoints were highest in SHRs after discontinuation of adolescent methylphenidate treatment (Supplementary Figures S5 and S6). Inactive lever responses are depicted in Supplementary Table S3, and were ⩽27% of total responses.
Experiment 5: DAT Function in the PFC and Striatum
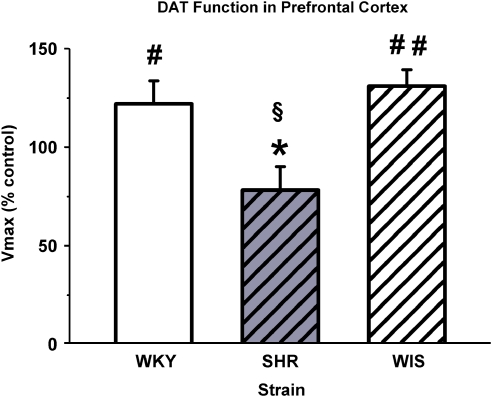
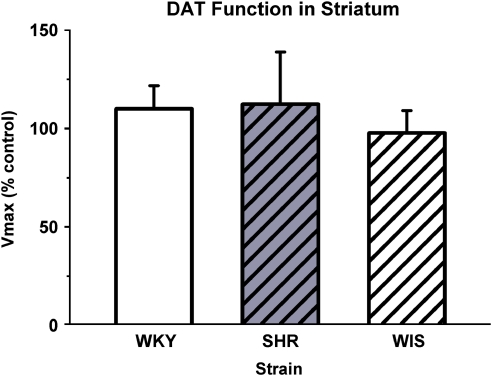
No strain or treatment differences were found for Km values in either the PFC or the striatum from adult WKY rats, SHRs, and WIS rats treated during adolescence with methylphenidate or vehicle (Table 1). Strain differences in the effects of previous methylphenidate treatment on Vmax in the PFC were found (F[2, 17]=5.8, p⩽0.05; Figure 5). In the PFC, Vmax for SHRs was less than that for WKY (p⩽0.05) and WIS (p⩽0.05), whereas WKY and WIS were not different. Comparison of mean percentage Vmax with the vehicle control value of 100% revealed that methylphenidate increased Vmax for WKY (t[6]=1.89, p⩽0.05) and WIS (t[5]=3.80, p⩽0.01), and tended to decrease Vmax for SHRs (t[5]=1.86, p⩽0.06) in the PFC. In contrast, no strain or treatment differences were found for Vmax in the striatum (Figure 6).
Table 1. Km for Dopamine Uptake at the Dopamine Transporter in the Prefrontal Cortex and Striatum of Adult Wistar-Kyoto (WKY), Spontaneously Hypertensive Rats (SHRs), and Wistar (WIS) Rats after Discontinuation of Adolescent Vehicle (VEH) or Methylphenidate (MPH) Treatment.
| Brain region |
WKY |
SHR |
WIS |
|||
|---|---|---|---|---|---|---|
| VEH | MPH | VEH | MPH | VEH | MPH | |
| Prefrontal cortexa | 0.032±0.007 | 0.035±0.007 | 0.030±0.004 | 0.027±0.007 | 0.025±0.007 | 0.019±0.003 |
| Striatumb | 0.026±0.006 | 0.025±0.005 | 0.040±0.009 | 0.031±0.007 | 0.031±0.007 | 0.024±0.004 |
Values (μM) are expressed as mean±SEM; n=6 for each of the groups, except WKY.
In the WKY group, n=8 in the prefrontal cortex.
In the WKY group, n=7 in the striatum.
Figure 5.
Vmax of dopamine uptake by the dopamine transporter (DAT) in the prefrontal cortex (PFC) of adult Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats after discontinuation of adolescent vehicle or methylphenidate treatment. Values are mean±SEM for Vmax following methylphenidate treatment expressed as a percentage of vehicle control treatment; n=6 for each group, except WKY in which n=8. Vmax values were 3.20±0.56, 3.24±0.63, and 1.41±0.16 pmol/mg per min for vehicle control of WKY, SHR, and WIS groups, respectively, and were not significantly different from each other. *p⩽0.05 compared with WKY and WIS. #p⩽0.05, ##p⩽0.01, and §p⩽0.06 compared with the control value of 100%.
Figure 6.
Vmax of dopamine uptake by the dopamine transporter (DAT) in the striatum of adult Wistar-Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs), and Wistar (WIS) rats after discontinuation of adolescent vehicle or methylphenidate treatment. Values are mean±SEM for Vmax following methylphenidate treatment expressed as a percentage of vehicle control treatment; n=6 for each group, except WKY in which n=7. Vmax values were 12.8±2.0, 12.8±2.1, and 17.1±2.1 pmol/mg per min for the vehicle control WKY, SHR, and WIS groups, respectively, and were not significantly different from each other.
DISCUSSION
SHR as an Animal Model for ADHD and Substance Abuse Risk
Few studies have focused on behavioral traits of adolescent SHRs, instead evaluating this strain during adulthood (Russell, 2007). In vehicle-treated adolescent rats used in this study, SHRs had deficits in acquisition of visual discrimination learning, shown by requiring more trials than did WKY and WIS rats to reach a criterion level of performance. Earlier studies have observed other ADHD-like traits in adolescent SHRs relative to comparator strains, including hyperactivity (Pandolfo et al, 2007) and impulsivity (Adriani et al, 2003). However, it is important to keep in mind that the SHR is not a comprehensive model of ADHD, but it does provide a good simulation of ADHD in many aspects (Sagvolden et al, 2005).
As adults with a history of ADHD have double the risk of developing a substance use disorder than do adults without ADHD (Biederman et al, 1998), one question of interest was to determine whether SHRs, when non-medicated, would exhibit an increased vulnerability to cocaine addiction during adulthood. Increased vulnerability to cocaine addiction in rats is reflected by faster acquisition of self-administration, a vertical shift in the dose–response function, and higher PR breakpoints, among other indices (Deroche-Gamonet et al, 2004; Piazza et al, 2000). Although acquisition of cocaine self-administration and the overall reinforcing efficacy of cocaine were not significantly different in vehicle-treated WKY rats, SHRs, and WIS rats, vehicle-treated SHRs were more motivated to self-administer cocaine (had higher PR breakpoints) at lower unit doses than were vehicle-treated WKY or WIS rats. With only one of three criteria exhibited, it is not clear whether the higher PR breakpoints in non-medicated SHRs reflect increased cocaine addiction vulnerability or some other factor. One possibility is that the higher PR breakpoints reflect greater impulsivity for lever pressing. However, research has demonstrated that high- and low-impulsive rats exhibit similar PR breakpoints across a range of cocaine doses (Anker et al, 2009). Another possibility is that hyperactivity is responsible for greater lever responding in non-medicated SHRs, which could result in higher PR breakpoints. Along these lines, SHRs emitted more inter-trial interval lever responses and displayed more locomotor activity than did WKY rats during an operant task measuring visual stimulus position discrimination (Thanos et al, 2010). Given the nature of the PR schedule used in this study, hyperactive SHRs may have been more prone than normoactive WKY or WIS rats to continue lever pressing during the progressively increasing inter-infusion intervals. Nonetheless, increased cocaine addiction vulnerability is the most likely interpretation for higher PR breakpoints because SHRs show an exaggerated reaction to other drugs of abuse. With regard to other drugs of abuse, SHRs exhibit greater cannabinoid-induced conditioned place preference (Pandolfo et al, 2009) and greater analgesic sensitivity to morphine (Hoffmann et al, 1998) than do WKY rats. In addition, SHRs were found to consume more ethanol than Lewis rats (Da Silva et al, 2005). Collectively, these findings suggest that the SHR genetic model captures several important aspects of ADHD such as visual discrimination deficits, hyperactivity, and impulsivity before adulthood and increased risk of developing a substance use disorder during adulthood.
Methylphenidate in Adolescent SHRs: Increased Cocaine Addiction Vulnerability
Similar to a previous report showing that low-dose oral methylphenidate improved attentional set shifting in SHRs but not in WKY (Kantak et al, 2008), this study demonstrated that methylphenidate improved visual discrimination only in SHRs. It is possible that improvement was more easily obtained in SHRs relative to WKY and WIS because of poorer performance in SHRs under vehicle conditions. In support of this, visual attention is improved by methylphenidate in healthy volunteers only when pre-drug baseline performance is low (Finke et al, 2010). Nonetheless, the visual discrimination task has relevance because the results establish the validity of the ADHD model in adolescent SHRs (deficits in non-medicated SHRs and improvement in medicated SHRs during adolescence). Adding to the relevance of the visual discrimination task are findings showing that although spatial working memory and planning are improved after methylphenidate in healthy volunteers, attentional set shifting and verbal fluency are not (Elliott et al, 1997). These findings suggest that in an animal model of ADHD, not all neurocognitive measures showing improvement after methylphenidate treatment in rats with an ADHD phenotype need be enhanced in rats without an ADHD phenotype.
Despite an improvement in the visual discrimination task by methylphenidate in adolescent SHR, vulnerability to cocaine addiction was further enhanced in this strain during adulthood after methylphenidate treatment was discontinued. In particular, methylphenidate-treated SHRs acquired cocaine self-administration faster, displayed an upward shift in the cocaine dose–response function, and exhibited the highest PR breakpoints. This profile suggests that methylphenidate-treated SHRs identified cocaine as a highly efficacious reinforcer and were exceedingly motivated to self-administer cocaine across a range of unit doses (Deroche-Gamonet et al, 2004; Piazza et al, 2000). These effects were not related to methylphenidate treatment per se in that vulnerability to cocaine addiction was not enhanced by previous methylphenidate treatment in WKY and WIS. Notably, WIS acquired cocaine self-administration more slowly after methylphenidate than vehicle treatment, suggesting some degree of reduced vulnerability to cocaine addiction after adolescent methylphenidate treatment in a rat strain that typically does not display an ADHD phenotype (Sagvolden et al, 2009). These findings in WIS and WKY are consistent with a body of evidence collected from outbred rat strains. Earlier studies in outbred rats showed decreased or unchanged cocaine self-administration, and decreased or unchanged cocaine-conditioned place preference during adulthood after discontinuation of repeated methylphenidate treatment that began during adolescence (Adriani et al, 2006; Andersen et al, 2002; Carlezon et al, 2003; Ferguson and Boctor, 2010; Thanos et al, 2007), although Brandon et al (2001) showed increased cocaine self-administration in outbred rats.
An important consideration for interpreting these findings is whether lever responding in SHRs reflects a greater degree of non-specific and/or indiscriminant responding in general rather than a greater motivation for cocaine reinforcement. An earlier study reported that SHRs exhibited extinction deficits (greater responding than did WKY), particularly during the initial transition from scheduled water reinforcement under a fixed-interval schedule to extinction, irrespective of whether the conditioned stimulus (light cue) was present during extinction training (Johansen and Sagvolden, 2004). In this study, responding maintained by low doses of cocaine in SHRs cannot be explained by an extinction deficit, as responding maintained by 0.003 mg/kg in SHRs (a non-reinforcing dose in WKY and WIS) was proportionally greater than responding during initial extinction training. Notably, with few exceptions, even SHRs with previous methylphenidate treatment maintained at least a 2 : 1 ratio of active-to-inactive lever responses throughout the study, suggesting that their behavior was goal directed. Previous work using a food foraging task demonstrated that SHRs do not have deficits in goal-directed behavior relative to WKY (Wells et al, 2010). Thus, SHRs seem to have greater motivation for cocaine reinforcement than do WKY or WIS rats, especially after discontinuation of adolescent methylphenidate treatment. Moreover, the graded ascending and descending limbs of the FR dose–response curve in methylphenidate-treated SHRs indicate that these rats were discriminating among the different doses of cocaine and support the view that they were responding in a goal-directed manner.
Collectively, these findings illustrate that a rat strain exhibiting an ADHD phenotype is required for addressing a key controversial question regarding the impact of adolescent methylphenidate treatment on later cocaine addiction vulnerability. Although the current findings support an increased vulnerability, it should be noted that in an earlier study, methylphenidate-treated SHRs had attenuated cocaine-conditioned place preference compared with vehicle-treated SHRs (Augustyniak et al, 2006). Complicating this issue further are findings showing that methylphenidate-treated SHRs consumed less ethanol than did methylphenidate-treated WKY rats under home cage, unlimited access conditions (Soeters et al, 2008). Considering that drug self-administration differs conceptually from conditioned place preference and home cage drug consumption and also that drug self-administration is the strongest predictor of abuse liability of drugs (Carter and Griffiths, 2009), the conclusion that adolescent methylphenidate treatment increased later vulnerability to cocaine addiction is supported empirically by the current results. Additional work using the self-administration method is required to confirm whether this outcome with cocaine in methylphenidate-treated SHRs extends to other drugs (such as marijuana, ethanol, nicotine) commonly used by individuals with ADHD (Biederman et al, 1998, 1999).
Methylphenidate, DAT Function, and Cocaine Addiction Vulnerability in SHRs
The current kinetic analysis of DA uptake at DAT in the striatum showing no strain or treatment differences in Km or Vmax support the idea that the PFC is the primary site of action for methylphenidate at low therapeutically relevant doses (Berridge et al, 2006; Devilbiss and Berridge, 2008). Previous research has shown greater striatal DAT density in non-medicated SHRs than in WKY and WIS rats, with significant decreases in density in all three strains during adulthood after discontinuation of adolescent treatment with chronic low-dose oral methylphenidate (Moll et al, 2001; Roessner et al, 2010). However, the relationship between striatal DAT density and ADHD is uncertain (Volkow et al, 2007). Moreover, the similar influence of adolescent methylphenidate on DAT density in the striatum reported in adult WKY rats, SHRs, and WIS rats (Moll et al, 2001; Roessner et al, 2010) suggests that the enhanced cocaine self-administration observed in this study only in methylphenidate-treated SHRs is not related to potential striatal DAT density changes. In support of this, the study by Ramamoorthy et al (2010) has demonstrated that habitual patterns of cocaine-seeking behavior are not associated with DAT density changes in the striatum.
The Vmax for DA uptake at DAT in the PFC was decreased in SHRs several weeks after discontinuation of adolescent methylphenidate treatment. One possibility is that methylphenidate-induced decreases in DA clearance in the PFC of SHRs may underlie their increased vulnerability to cocaine addiction. Methylphenidate-induced increases in DA clearance in the PFC of WKY and WIS rats may have been protective in this regard. Although DAT protein density has been reported to have a lower (∼50%) expression in the PFC than in the striatum (Sesack et al, 1998), DAT function in the PFC (DA uptake in the presence of a norepinephrine transporter (NET) inhibitor) is ∼25% of that in the striatum, consistent with previous work using Sprague Dawley rats (Zhu et al, 2009). When evaluating DA uptake in the PFC, NET is a factor as DA is transported by NET in this region (Carboni et al, 1990; Mundorf et al, 2001; Moron et al, 2002; Shen et al, 2004), and NET is expressed at a higher density relative to DAT in this region (Giros et al, 1994). Furthermore, in vivo microdialysis studies show that NET is predominant over DAT in DA clearance in the PFC (Masana et al, 2010). However, a caveat of using microdialysis for measuring DAT function in the PFC is that DAT expression is not homogeneous in this region. In contrast, in vitro methods used in this study allow for regional determination of maximal velocity of DA uptake by DAT in isolation by inclusion of desipramine in the assay. Therefore, the kinetic parameter changes in Vmax for DA uptake at DAT in the PFC after discontinuation of 1.5 mg/kg oral methylphenidate may be particularly meaningful. First, low-dose oral methylphenidate has been shown to improve PFC-related neurocognitive function and to increase DA efflux in the PFC, but not in other sites (Berridge et al, 2006). Second, an increase in synaptic concentration of DA in the PFC is reinforcing as rats robustly self-administer cocaine directly into this site (Goeders and Smith, 1983, 1986). Thus, if cocaine-induced DA efflux in the PFC (Ikegami and Duvauchelle, 2004; Pum et al, 2007) undergoes lower clearance by DAT after discontinuation of adolescent methylphenidate treatment in SHRs, then cocaine may serve as a more highly efficacious reinforcer and further enhance motivation to maintain self-administration.
Conclusions
This study has implications for the decisions of physicians and parents in determining the time course of ADHD medication. These preclinical findings suggest that caution should be exercised when methylphenidate is prescribed for first-time treatment of ADHD in adolescent patients, as cocaine addiction vulnerability may be augmented.
Acknowledgments
This study was funded by grant R01 DA011617. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse. Experiments adhered to the Institutional Animal Care and Use Committee guidelines for animal research. We thank David Tassin and Jonathan Woodson for assistance with data collection.
Over the past 3 years, Dr Kantak reports consulting fees and stock options from Yaupon Therapeutics and Dr Dwoskin reports stock and stock options from Yaupon Therapeutics, as Founder and Vice President. Ms Harvey, Ms Sen, and Ms Deaciuc have no financial interests or conflicts of interest to disclose.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, et al. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Children with Disabilities Committee on Drugs Medication for children with attentional disorders. Pediatrics. 1996;98 (2 Pt 1:301–304. [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustyniak PN, Kourrich S, Rezazadeh SM, Stewart J, Arvanitogiannis A. Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2006;167:379–382. doi: 10.1016/j.bbr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence. Biol Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105 (Suppl 1:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Da Silva GE, Vendruscolo LF, Takahashi RN. Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci. 2005;77:693–706. doi: 10.1016/j.lfs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Boctor SY. Cocaine responsiveness or anhedonia in rats treated with methylphenidate during adolescence. Neurotoxicol Teratol. 2010;32:432–442. doi: 10.1016/j.ntt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, et al. Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 2010;210:317–329. doi: 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Giros B, Wang YM, Suter S, McLeskey SB, Pifl C, Caron MG. Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem. 1994;269:15985–15988. [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Reinforcing properties of cocaine in the medical prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav. 1986;25:191–199. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology (Berl) 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O, Plesan A, Wiesenfeld-Hallin Z. Genetic differences in morphine sensitivity, tolerance and withdrawal in rats. Brain Res. 1998;806:232–237. doi: 10.1016/s0006-8993(98)00768-9. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Nucleus accumbens and medial prefrontal cortex dopaminergic response to self-administered cocaine in naive rats. Neurosci Lett. 2004;354:205–208. doi: 10.1016/j.neulet.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T. Response disinhibition may be explained as an extinction deficit in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2004;149:183–196. doi: 10.1016/s0166-4328(03)00229-8. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, et al. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24:1345–1357. doi: 10.1185/030079908x280707. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, III, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masana M, Bortolozzi A, Artigas F.2010Selective enhacement of mesocortical dopaminergic transmission by noradrenergic drugs: therapeutic opportunities in schizophrenia Int J Neuropsychopharmacolprint copy in press (originally published online 12 August 2010 at http://www.journals.cambridge.org/). [DOI] [PubMed]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1 in SHR and WKY rat strains. Behav Brain Funct. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Pamplona FA, Prediger RD, Takahashi RN. Increased sensitivity of adolescent spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder, to the locomotor stimulation induced by the cannabinoid receptor agonist WIN 55 212-2. Eur J Pharmacol. 2007;563:141–148. doi: 10.1016/j.ejphar.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat-an animal model of attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2009;205:319–326. doi: 10.1007/s00213-009-1542-3. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, Muller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 2007;193:375–390. doi: 10.1007/s00213-007-0791-2. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. ADHD and addiction. Nat Med. 2002;8:24–25. doi: 10.1038/nm0102-24. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, et al. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods. 2007;161:185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Woien G, Walaas SI, Storm-Mathisen J, Bergersen LH, et al. The spontaneously hypertensive rat model of ADHD–the importance of selecting the appropriate reference strain. Neuropharmacology. 2009;57:619–626. doi: 10.1016/j.neuropharm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Soeters HS, Howells FM, Russell VA. Methylphenidate does not increase ethanol consumption in a rat model for attention-deficit hyperactivity disorder-the spontaneously hypertensive rat. Metab Brain Dis. 2008;23:303–314. doi: 10.1007/s11011-008-9098-1. [DOI] [PubMed] [Google Scholar]
- Szobot CM, Rohde LA, Katz B, Ruaro P, Schaefer T, Walcher M, et al. A randomized crossover clinical study showing that methylphenidate-SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz J Med Biol Res. 2008;41:250–257. doi: 10.1590/s0100-879x2008005000011. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang GJ, Swanson JM, et al. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention∣, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol Biochem Behav. 2010;94:374–379. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadum A, Rankin NM. Psychological Research: Methods for Discovery and Validation. McGraw-Hill: New York; 1998. [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Janes AC, Liu X, Deschepper CF, Kaufman MJ, Kantak KM. Medial temporal lobe functioning and structure in the spontaneously hypertensive rat: comparison with Wistar-Kyoto normotensive and Wistar-Kyoto hypertensive strains. Hippocampus. 2010;20:787–797. doi: 10.1002/hipo.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown BD, Michels KM.1991Statistical Principles in Experimental Design3rd edn. McGraw-Hill: New York [Google Scholar]
- Zhu J, Apparsundaram S, Dwoskin LP. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther. 2009;328:931–939. doi: 10.1124/jpet.108.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.