This study provides the first evidence that LYVE-1 is expressed in the hyaloid vascular system in the eye. A macrophage lineage and the intimate association of the LYVE-1 expressing cells with the CD31 endothelial cells are also defined.
Abstract
Purpose.
The hyaloid vascular system (HVS) is a transient network nourishing developing eyes and has been widely used as a natural model to study blood vessel regression. Failure of its regression in humans leads to several blinding diseases. Lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1) is a recently defined lymphatic marker that is also expressed by a subpopulation of macrophages. To date, there is no report on its expression in the HVS. This study was conducted to investigate whether LYVE-1 is expressed in the HVS and how it is associated with the vascular structure and macrophage phenotype.
Methods.
Normal C57BL/6 mouse eyeballs were sampled from embryonic day (E) 10.5 to postnatal (P) and adult stages for immunofluorescent microscopic studies with antibodies against LYVE-1, CD31 (panendothelial cell marker), and F4/80 (macrophage marker). Additionally, Angiopoietin-2 (Ang-2) knockout mice with abnormally persistent HVS were examined.
Results.
The LYVE-1 expression was detected on normal HVS between E12.5 and P14. The LYVE-1+ cells were F4/80+ but CD31−, indicating a macrophage lineage. Additionally, LYVE-1+ cells bud on CD31+ vessels and constitute an integral part of the network in both normal developing and Ang-2 knockout mice.
Conclusions.
This study provides the first evidence that the HVS contains a LYVE-1+ cellular component in both physiological and pathologic conditions. This novel finding not only provides a new concept in defining the embryogenesis and pathogenesis of the HVS, it also leads to a completely natural model in which to study the functions of the LYVE-1 pathway, an important topic for lymphatic research as well.
The hyaloid vascular system (HVS) is a transient vascular network in developing mammal eyes. It is also one of few tissues in the body in which natural regression occurs. The HVS nourishes the intraocular components of the growing lens and the primary vitreous and undergoes spontaneous regression as these components mature in the eye. Anatomically, the HVS is composed of the following major parts1: the main hyaloid artery (HA), which arises from the dorsal ophthalmic artery and enters the eye cup through the embryonic fissures; the vasa hyaloidea propria (VHP), which branches from the HA into the primary vitreous; the tunica vasculosa lentis (TVL), which are terminal branches of the HA and include the posterior, lateral, and anterior portions surrounding the lens; and the papillary membrane (PM), which sprouts from the annular vessels of the TVL (Fig. 1A). The HVS normally diminishes around 2 weeks after birth in mice and before birth in humans. Failure of its regression in humans is associated with several serious blinding diseases, including persistent hyperplastic tunica vasculosa lentis (PHTVL), persistent hyperplastic primary vitreous (PHPV), and persistent prepupillary membrane (PPM).2,3 Because of its unique transient nature, the HVS has been widely used to study the formation and regression of blood vessels. To date, there is no report on any lymphatic component of this system.
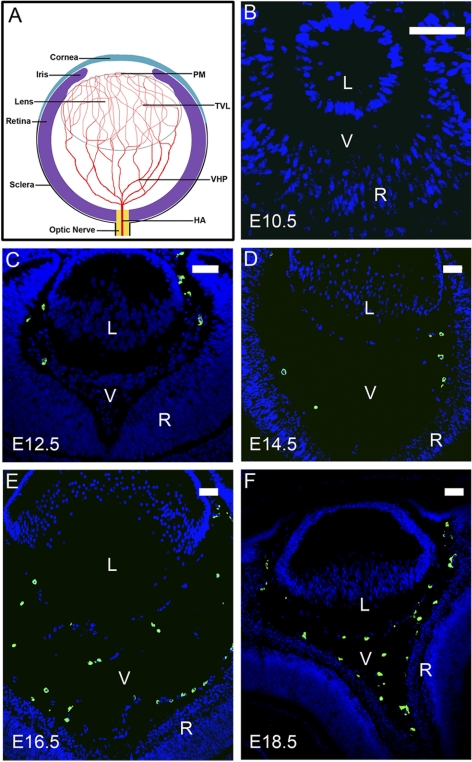
Figure 1.
LYVE-1 expression with the formation of the HVS at embryonic stages. (A) Schematic diagram of the hyaloid vascular system and its major components: HA, VHP, TVL, and PM. (B–F) Representative cross-sectional micrographs demonstrating LYVE-1 expression at embryonic (E) stages, which was detected at E12.5 (C) and thereafter (D–F). Green: LYVE-1; blue: DAPI nuclei staining. L, lens; V, vitreous; R, retina. Scale bars, 50 μm.
Lymphatic vessel endothelial hyaluronic acid receptor (LYVE-1) is a recently defined molecular marker for lymphatic vessels. It is a transmembrane protein discovered by searching the expressed sequence tag (EST) databases for sequences homologous to the hyaluronan receptor CD44, which is widely expressed on leukocytes, dendritic cells, and tumor cells.4 Despite the fact that the LYVE-1 is the most widely exploited marker to identify lymphatic vessels since its discovery in 1999, the physiological function of this important molecule remains largely unknown. In addition to lymphatic vessels, the expression of LYVE-1 was also reported on several cell populations, including the sinusoidal endothelial cells of the liver and spleen, and macrophages in normal and tumor tissues.4–9 Of note, to date, there is no report of its expression on the HVS in developing eyes. The purpose of this study was, therefore, to identify and characterize LYVE-1 expression in the HVS from its normal formation to regression. Additionally, LYVE-1 expression was examined in Angiopoietin-2 (Ang-2) knockout mice, which exhibit pathologic persistent HVS, as reported previously.10 Results from this study will not only provide new information on the embryogenesis and pathogenesis of the hyaloid vasculature but will also potentially reveal a completely natural model in which to study the functions of the LYVE-1 pathway.
Methods
Animals
Normal male and female C57BL/6 mice (Taconic Farms, Germantown, NY) were bred and maintained in the animal facilities of the University of Louisville in Kentucky and the University of Southern California. Ang-2 knockout mice of the C57BL/6 background (Regeneron Pharmaceuticals, Inc., Tarrytown, NY) were maintained in the animal facilities of the University of Arizona, as described previously.10 All mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the animal protocols approved by the Animal Care and Use Committees.
Tissue Preparation
In all timed pregnancies, the plug date was designed as embryonic day (E) 0.5, and the date of birth was defined as postnatal day (P) 0. For prenatal stages, normal embryos were sampled at E10.5, E12.5, E14.5, E16.5, and E18.5; for postnatal stages, normal ocular tissues were sampled at P2, P7, P14, and 6 weeks. Ocular tissues of Ang-2 homozygous knockout and control heterozygote mice were sampled at P14. For cross-sectional studies, the specimens were immersed in OCT compound (Tissue-Tek; Sakura Finechemical, Torrance, CA) and frozen at −80°C for further studies; for whole mount assays, the specimens were fixed in 4% paraformaldehyde overnight at 4°C for further studies.
Antibodies
The following primary antibodies were used: rabbit anti–mouse LYVE-1, rat anti–mouse F4/80 (Abcam, Cambridge, MA), and rat anti–mouse CD31/PECAM-1 (BD PharMingen, San Diego, California). The isotype controls included rat IgG1, normal rabbit IgG (Abcam), and purified rat IgG2a (BD PharMingen). The secondary antibodies were FITC-conjugated donkey-anti–rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), Alexa488-conjugated donkey anti–rat IgG, and Cy3-conjugated donkey anti–rabbit IgG (Jackson ImmunoResearch, West Grove, PA).
Immunofluorescence Microscopic Studies
Five-micrometer frozen sections or whole mount tissues were used for immunofluorescence staining as described previously.7,11,12 Briefly, the samples were first incubated with PBS containing 3% bovine serum albumin (BSA), 3% normal donkey serum, and 0.3% Triton X-100 to reduce the staining background. Thereafter, for LYVE-1 single staining, the samples were incubated overnight at 4°C with rabbit anti–mouse LYVE-1 antibody followed by secondary antibody incubation. For sequential double-staining of LYVE-1 and CD31 or F4/80, the samples were incubated with rat anti–mouse CD31 or rat anti–mouse F4/80 antibody overnight at 4°C, followed by the incubation with secondary antibodies. These samples were then stained with rabbit anti–mouse LYVE-1 antibody at room temperature for 2 hours (sections) or at 4°C overnight (whole mounts), followed by another incubation with the secondary antibody. The samples were mounted with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA) with DAPI, observed with a fluorescence microscope (Carl Zeiss, München-Hallbergmoos, Germany), and photographed with a digital camera system (Axiocam; Carl Zeiss). For each antibody staining study, tissue samples from three to five mice were examined. For cross-sectional studies, at least five sections were analyzed for each tissue sample derived from each animal. For cell quantification analysis, cells that were F4/80+/LYVE-1+ or F4/80+/LYVE-1− were counted in the vitreous cavity for each section, and the results were presented in the percentage of the total number of F4/80+ cells.
Results
LYVE-1 Expression with the Formation of HVS
We first investigated whether LYVE-1 is expressed in the HVS and how this expression correlates with the normal onset of this structure. Given that HVS in mice starts to form at E10.5 and becomes full-developed by E13.5,3 we examined LYVE-1 expression in the cross-sections of the developing vitreous cavities from E10.5 to E18.5. Results from these studies clearly demonstrated that LYVE-1 was expressed in the embryonic vitreous at E12.5 (Fig. 1C) and thereafter (Figs. 1D–F) coincided with the developing phase of the HVS. Furthermore, LYVE-1 expression was aligned around the lens and widely scattered in the primary vitreous, which is also in accordance with the HVS locations. Therefore, both the time window and the spatial location of LYVE-1 expression synchronized with the onset of the HVS during mouse embryonic stages.
LYVE-1 Expression with the Regression of HVS
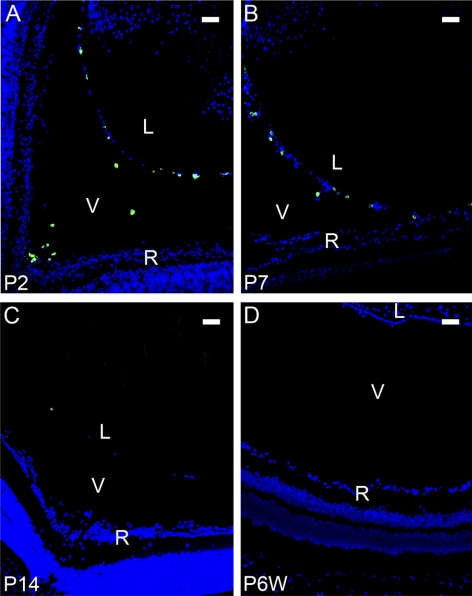
To further investigate whether LYVE-1 is also expressed during the regression phase of HVS, we next studied LYVE-1 expression in the cross-sections of the vitreous cavities from P2 to P14. It is known that most HVS vessels regress during the first 2 weeks after birth in mice.3,13–15 As a control, the adult vitreous cavity, which should be absent from the HVS structure, was also examined. Our results from these studies further demonstrated that LYVE-1 expression gradually diminished in the postnatal vitreous cavities as the HVS regressed. As shown in Figure 2, LYVE-1 expression was obvious in the vitreous cavity at P2 (Fig. 2A) and P7 (Fig. 2B), barely detectable at P14 (Fig. 2C), and completely disappeared in the adult (Fig. 2D). The expression of LYVE-1, hence, also synchronized with the regression of HVS.
Figure 2.
LYVE-1 expression with the regression of the HVS at postnatal stages. Representative cross-sectional micrographs demonstrating LYVE-1 expression at postnatal stages, which was obvious at P2 (A) and P7 (B), barely detectable at P14 (C), and completely absent in the adult (D). Green: LYVE-1; blue: DAPI nuclei staining. L, lens; V, vitreous; R, retina. Scale bars, 50 μm.
Intimate Association between the LYVE-1+ and CD31+ Cells in HVS
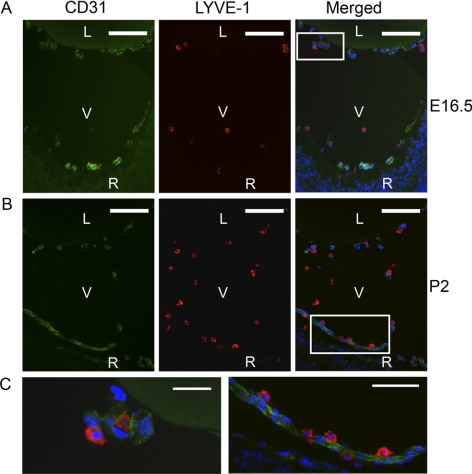
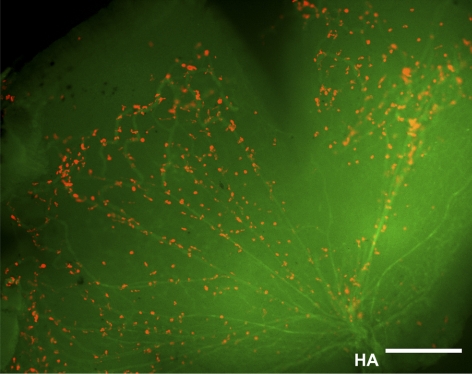
We and other researchers7,8,16 have reported previously that in addition to lymphatic vessels, LYVE-1 is also expressed in some isolated cells in normal adult ocular tissues. To further investigate whether the LYVE-1 signals we detected in the developing vitreous cavities are from the HVS vasculatures or single isolated cells, we next performed a series of double-staining assays using the specific antibodies against LYVE-1 and CD31, a pan-endothelial cell marker. Both embryonic and postnatal tissues were examined. These studies further revealed that the LYVE-1–expressing cells were CD31− at embryonic (Fig. 3A) and postnatal (Fig. 3B) stages. However, some LYVE-1+ cells appeared, abutting the CD31+ cells (Fig. 3C, left) or linearly arranged along the CD31+ vasculatures (Fig 3C, right), indicating an intimate association between these two cellular types in the hyaloid network. To further examine the exact dimensional arrangement of these two cell populations, we next performed a whole mount assay to circumvent technical limitations of the cross-sectional study. It was discovered that the LYVE-1+ cells were indeed attached to the CD31+ branches and formed an integral part of the system (Fig 4), a unique phenomenon that might be termed “buds on branches.”
Figure 3.
Intimate association of LYVE-1+ and CD31+ cells in the HVS. (A–C) Representative cross-sectional micrographs demonstrating the intimate link between LYVE-1+ and CD31+ cells at both embryonic (A) and postnatal (B) stages. (C) Higher magnification views of the boxed areas in (A) and (B), respectively. LYVE-1+ cells appeared abutting the CD31+ cells (C, left) or linearly arranged along the CD31+ branches (C, right). Red: LYVE-1; green: CD31; blue: DAPI nuclei staining. L, lens; V, vitreous; R, retina. Scale bars: 100 μm (A, B); 25 μm (C).
Figure 4.
LYVE-1 cells as an integral part of the HVS. Whole-mount micrograph showing the LYVE-1+ cells attached to the hyaloid vasculatures. Red: LYVE-1; green: CD31. HA, hyaloid artery. Scale bar, 50 μm.
LYVE-1+ Cells of the Macrophage Lineage
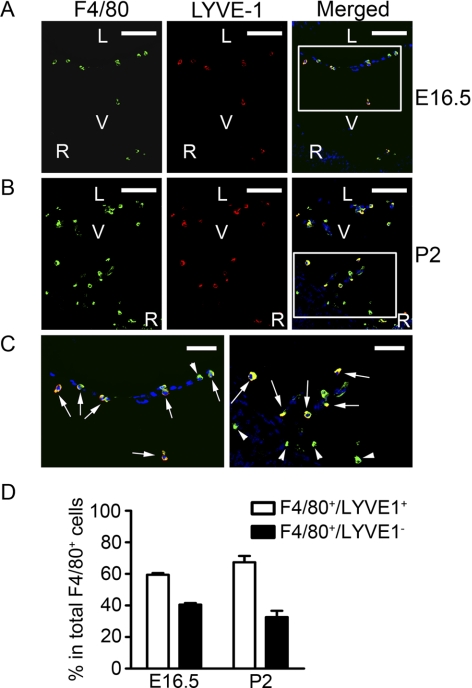
It has been shown previously that macrophages are present in the developing vitreous cavities.1 To further study the relationship between the LYVE-1+ cells and the macrophages in the HVS, we next performed a series of double-staining assays using the specific antibodies against LYVE-1 and F4/80, a macrophage marker. Both embryonic and postnatal tissues were examined. Our results from these studies further demonstrated that all the LYVE-1+ cells in the HVS expressed F4/80 (Fig. 5C, arrows), denoting their macrophage lineage. An additional macrophage population that was F4/80+ positive, but LYVE-1− was also observed in these vitreous cavities (Fig. 5C, arrowheads). Summarized data for these two cell populations are presented in Figure 5D.
Figure 5.
LYVE-1+ cells of the macrophage lineage. Representative cross-sectional micrographs demonstrating the coexpression of LYVE-1 and F4/80, a macrophage marker, on cells at both embryonic (A) and postnatal (B) stages. (C) Higher magnification views of the boxed areas in (A) and (B), respectively. Arrows: LYVE-1 and F4/80 double-positive cells. Arrowheads: LYVE-1− but F4/80+ cells. Red: LYVE-1; green: F4/80; blue: DAPI nuclei staining. L, lens; V, vitreous; R, retina. Scale bars: 100 μm (A, B); 50 μm (C). (D) Summarized data on the two populations of F4/80+/LYVE-1+ and F4/80+/LYVE-1− cells.
LYVE-1+ Cells in the Persistent HVS of Ang-2 Knockout Mice
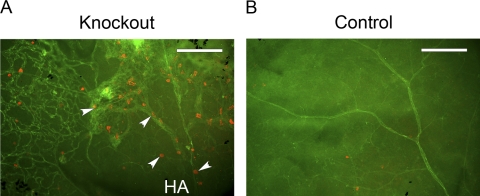
Finally, to investigate whether LYVE-1+ cells also accompany the HVS under a pathologic situation, such as persistent HVS, we also examined the expression of LYVE-1 in Ang-2 knockout mice. It has been reported that Ang-2 deficiency leads to a defect in HVS regression, resembling persistent fetal vasculature (PFA) in humans.17 Whole mount tissues from both Ang-2 knockout and control mice were sampled at P14 and were double stained with CD31 and LYVE-1 antibodies. As shown in Figure 6, abundant LYVE-1+ cells (Fig. 6A, arrowheads) were observed in the remnant CD31+ hyaloid vasculatures in Ang-2 knockout mice. As a comparison, no hyaloid structures were detected in the control mouse sample in which retinal vessels were well developed with sparsely distributed LYVE-1+ cells, as reported previously.8 These results further confirm that LYVE-1+ cells constitute an integral part of HVS under pathologic conditions as well.
Figure 6.
LYVE-1+ cells in the persistent HVS of Ang-2 knockout mice. Representative whole-mount micrographs demonstrating abundant LYVE-1+ cells (A, arrowheads) detected in CD31+ remnant hyaloid vasculatures of the Ang-2 knockout mouse. (B) The control mouse exhibited no hyaloid vasculatures with the normal presence of retinal vessels and a few LYVE-1+ cells. HA, hyaloid artery. Red: LYVE-1; green: CD31. Scale bars, 200 μm.
Discussion
In this study, we provide the first evidence that LYVE-1 is expressed in mouse HVS under both physiological and pathologic conditions. We have also demonstrated that LYVE-1–expressing cells are of the macrophage lineage and have an intimate association with CD31+ endothelial cells. Further investigation on this interesting phenomenon may provide novel information on the mechanisms and treatment of HVS diseases. Allied to this prediction are results from some earlier studies demonstrating that macrophages of the HVS are involved in the programmed vascular regression of the HVS.18,19
The expression of LYVE-1 on single cells has been reported previously.5–8,16 Our finding that LYVE-1 is expressed on the macrophage-lineage cells in the HVS is new but consistent with previous findings on other ocular7,8,16 and nonocular tissues.5,6,9 However, compared with other tissues in which LYVE-1 is expressed on single and isolated cells that are not part of any vascular structures, the hyaloid system is unique in that its LYVE-1+ macrophages are attached to the vessels and form an integral part of the network. Further studies are required to investigate whether the LYVE-1+ network is involved in the metabolism of hyaloid acid or contributes to the temporary drainage of the intraocular components in developing eyes.
Finally, from a broader perspective, this study also identifies a novel and completely natural model in which to study the functions of the LYVE-1 pathway, which may also be involved in other physiological and pathologic processes outside the eye. The LYVE-1 is a defined and the most widely used marker to identify lymphatic vessels that penetrate most tissues in the body. Lymphatic dysfunctions have been found in a wide array of disorders, including cancer metastasis, autoimmune diseases, transplant rejection, and lymphedema.16,20–24 To date, there is still little effective treatment for lymphatic diseases. It is hoped that continued studies on the LYVE-1 pathway using the unique hyaloid system may indulge novel mechanisms and therapeutic factors to combat currently untreatable diseases inside and outside the eye.
Acknowledgments
The authors thank Jeffrey LeDue (University of California at Berkeley) for his excellent technical assistance with the immunofluorescence microscopic studies.
Footnotes
Supported in part by research grants from the National Institutes of Health, Department of Defense, the University of California at Berkeley (LC), and the National Natural Science Foundation of China (HZ).
Disclosure: H. Zhang, None; J. Tse, None; X. Hu, None; M. Witte, None; M. Bernas, None; J. Kang, None; F. Tilahun, None; Y.-K. Hong, None; M. Qiu, None; L. Chen, None
References
- 1. Zhu M, Provis JM, Penfold PL. The human hyaloid system: cellular phenotypes and inter-relationships. Exp Eye Res. 1999;68:553–563 [DOI] [PubMed] [Google Scholar]
- 2. Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058 [DOI] [PubMed] [Google Scholar]
- 3. Smith RS, John SWM, Nishina PM, Sundberg JP. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods. Boca Raton, FL: CRC Press; 2002 [Google Scholar]
- 4. Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084 [PubMed] [Google Scholar]
- 6. Schledzewski K, Falkowski M, Moldenhauer G, et al. Lymphatic endothelium- specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77 [DOI] [PubMed] [Google Scholar]
- 7. Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46:4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu H, Chen M, Reid DM, Forrester JV. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci. 2007;48:2162–2171 [DOI] [PubMed] [Google Scholar]
- 9. Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914 [DOI] [PubMed] [Google Scholar]
- 10. Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, Huq S, Gardner H, de Fougerolles AR, Barabino S, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007;125:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Hara M, Seki K, Fukuda K, Nishida T. Eyelid fusion and epithelial differentiation at the ocular surface during mouse embryonic development. Jpn J Ophthalmol. 2005;49:195–204 [DOI] [PubMed] [Google Scholar]
- 13. Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol (Berl). 1999;200:403–411 [DOI] [PubMed] [Google Scholar]
- 14. Brown AS, Zhang M, Cucevic V, Pavlin CJ, Foster FS. In vivo assessment of postnatal murine ocular development by ultrasound biomicroscopy. Curr Eye Res. 2005;30:45–51 [DOI] [PubMed] [Google Scholar]
- 15. Albe E, Escalona E, Rajagopal R, Javier JA, Chang JH, Azar DT. Proteomic identification of activin receptor-like kinase-1 as a differentially expressed protein during hyaloid vascular system regression. FEBS Lett. 2005;579:5481–5486 [DOI] [PubMed] [Google Scholar]
- 16. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009;42:66–76 [PMC free article] [PubMed] [Google Scholar]
- 17. Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187 [DOI] [PubMed] [Google Scholar]
- 18. Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999;126:2141–2147 [DOI] [PubMed] [Google Scholar]
- 19. Lobov IB, Rao S, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953 [DOI] [PubMed] [Google Scholar]
- 21. Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436:456–458 [DOI] [PubMed] [Google Scholar]
- 22. Folkman J, Kaipainen A. Genes tell lymphatics to sprout or not. Nat Immunol. 2004;5:11–12 [DOI] [PubMed] [Google Scholar]
- 23. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145 [DOI] [PubMed] [Google Scholar]