The major molecular components of the lipids in normal human meibomian gland secretions were determined with shotgun electrospray ionization mass spectrometry and tandem mass spectrometry analysis.
Abstract
Purpose.
The purpose of this investigation was to determine the major molecular components of the lipids in normal human meibomian gland secretions (meibum).
Methods.
The meibum samples were studied by direct infusion electrospray ionization (ESI), quadrupole time-of-flight mass spectrometry, and tandem mass spectrometry (MS/MS) analysis, in both positive and negative detection modes.
Results.
Hundreds of peaks were detected, among which the molecular compositions and subclasses of approximately 160 major peaks were confidently identified. The compositions and subclasses of these peaks were determined from collision-induced dissociation fragmentation patterns, high-resolution and high-mass-accuracy spectra, and references of literature reports. The major peaks detected in positive mode were those of nonpolar lipids, including wax esters, cholesteryl esters, triacylglycerols, and diesters, whereas in negative mode, the major peaks detected were those of polar lipids, including free fatty acids and (O-acyl)-ω-hydroxy fatty acids.
Conclusions.
The analysis of intact lipids in meibum with direct infusion ESI-MS/MS analysis has the advantages of minimal sample preparation (no chromatography or pre-separation needed), mild experimental conditions, high throughput, and high sensitivity.
Lipids secreted from the meibomian glands (meibum) form the outmost layer of tear film covering the ocular surface. A primary function of this superficial lipid layer is to prevent evaporation of the aqueous component in the tear film.1 Changes in lipid profiles of meibum likely lead to ocular surface diseases such as dry eye disease, which is a prevalent condition affecting over 12 million Americans, or 6% to 27% of people worldwide.2 Therefore, it is important to know the identities of the components in meibum to better diagnose and manage ocular surface disease, with the additional goal of future therapeutic development.
The conventional methods applied to the analysis of meibum samples include thin layer chromatography (TLC), nuclear magnetic resonance spectroscopy, gas chromatography (GC), and gas chromatography-mass spectrometry (GC-MS).3 With these methods, progress has been made in identifying the lipid components in meibum. Previously, the major components of meibum have been reported to be wax esters (WEs),4–8 cholesteryl esters (CEs),4–8 diesters (DEs),4,5,7 and triacylglycerols (TGs).4–8 The major fatty acid and fatty alcohol moieties of these lipids have also been determined.7,9 However, limitations still exist with these methods, including low sensitivity, low resolution, possible degradation due to prolonged exposure time, low sample recovery due to derivatization, isomerization, and/or decomposition due to sustained high temperature analysis and long analysis time.3 Furthermore, these methods are mainly based on hydrolysis and derivatization followed by GC-MS analysis of all the meibum lipids or a certain subclasses; thus information of the fatty acid or fatty alcohol moieties only has been obtained and the more important qualitative and quantitative information of individual intact lipids was missing.
To circumvent these limitations, atmospheric pressure chemical ionization (APCI)-MS coupled with reversed-phase or normal phase high-performance liquid chromatography (HPLC) separation was recently applied for the direct analysis of the meibum lipids including WEs10,11 and CEs12 without derivatization. However, the high energy and the high temperature involved with APCI could cause the dissociation of some lipid species. For instance, saturated CE peaks are spontaneously fragmented with APCI-MS.12 This creates a challenge in providing accurate identification and quantitation. In addition, a large number of samples are required for APCI-MS analysis because only high flow rate (>0.2 mL/min) is compatible with this method. In contrast, electrospray ionization (ESI) is a “softer” ionization method13 and can be operated at low flow rate; thus it is more compatible for the analysis of unstable lipids as well as limited or small volume samples. The application of ESI-MS to meibum samples has been reported recently.11,14,15
In this work, direct ESI-MS and MS/MS analyses were performed for individual meibum samples collected by capillary tube after meibomian gland expression. After minimizing the interference peaks and increasing the signal-to-noise ratio, we confirmed the previously reported major lipid species, including WEs, CEs, DEs, TGs, and free fatty acids (FFAs) in meibum samples. More importantly, we also determined the major molecular components of these lipid species. To the best of our knowledge, this is the most detailed report of the major species in meibum on the molecular level. Furthermore, we also observed a set of peaks reported recently as those of (O-acyl)-ω-hydroxy fatty acids (OAHFAs).16 These OAHFAs may play important role in maintaining the stability of tear film.
Methods and Materials
Chemicals
Chloroform (HPLC grade, >99.9%, with amylene as the stabilizer, either Burdick & Jackson or Sigma brand), methanol (HPLC grade, >99.9%, Riedel-de Haen), ammonium acetate (>98%, Sigma), WE and CE standards, stearic acid, and triolein were purchased from Sigma-Aldrich (St. Louis, MO).
Sample Collection and Preparation
The study protocol was approved by the Ohio State University Institutional Review Board in accordance with the Declaration of Helsinki. For this investigation, 45 subjects donated meibum samples on up to five occasions per protocol. The subjects were predominantly female (68.9%) and white (91.1%), with an average age of 39.0 ± 14.4 years (range, 24 to 65).17 Samples were collected between May 2008 and November 2009.
To collect the meibum, the subject was positioned in the slit lamp, and the lid margin was viewed at ×16 magnification. The lower lid was palpated with the index finger or cotton swab, and direct pressure was used to express meibum from the meibomian glands. A 0.5 μL × 32 mm (0.0220″ OD, 0.00560″ ID) glass capillary (Drummond, Broomall, PA) was used to collect the expressed meibum by “tapping” the tube vertically into the meibum pool. Approximately 1 to 5 mm (volume of approximately 16 to 80 nL) was collected from each eye. Each capillary with meibum was put in a capped glass vial and stored under air at −20°C for up to several months.
The stock solutions were prepared by pushing meibum samples into a sample vial with a 0.00497″ OD stainless steel wire (Hamilton, Reno, NV) and dissolving in chloroform-methanol solvent mixture (2:1, v/v). The typical amount of the solvent mixture for dissolving each mm long meibum collected in the capillary was 100 μL unless otherwise indicated. The concentration of the stock solution was estimated to be 160 μg/mL assuming the density of meibum as 1 g/mL, similar to published densities of common oils and fats, which range from 0.86 to 0.97 g/mL.18 All samples were prepared with glass vials and glass syringes and without contact with any plastics. Stainless steel tubing was used to connect the syringe to the mass spectrometer. The analysis of these samples resulted in over 130 instrument analyses (approximately 100 in positive mode and 30 in negative mode). Depending on the analysis and instrument settings, some meibum samples were used for more than one run.
The stability of the samples prepared in the solvent mixture without additives was tested by comparing the spectra for the fresh samples and the same samples stored under air at 4°C for 6 months (data not shown). No significant difference was found, indicating the samples were quite stable even when prepared in the solvent mixture. The raw meibum samples stored in capillary at −20°C should be more stable. Indeed, no significant differences were found for the analysis of fresh meibum samples and those stored for several months.
Electrospray Time-of-Flight Mass Spectrometry Analysis
A quadrupole time-of-flight mass spectrometer (Q-TOF II; Waters, Milford, MA) was used for all analyses. For positive mode, the working solutions were typically diluted 10-fold from meibum stock solutions (final concentration was ∼16 μg/mL). A solvent mixture containing either ammonium acetate (1 mM) or sodium iodide (typical concentration 8 μg/mL) was used as a solution additive. The working solutions were directly infused into the mass spectrometer at the flow rate of 10 to 40 μL/min. The spectra were acquired in the m/z range of 50–2000. The desolvation gas flow rate was 500 L/h. For positive mode, the cone voltage was set at either 15 V (for samples with ammonium acetate additive) or 50 V (for samples with sodium iodide additive). The source and desolvation temperatures were set at 100°C and 100–150°C, respectively. The capillary voltage was set at 3–3.2 kV. The instrument was calibrated with clustering ions of sodium iodide. After the calibration, the sodium iodide was washed away thoroughly. The MS analysis was performed with collision energy of 5 to 7 eV to increase resolution without causing significant fragmentation. The MS/MS analysis was performed with collision-induced dissociation (CID) of the isolated peaks of interest; high-mass and low-mass resolutions were set at 15; the collision energies were set depending on the species and ranged from 10 to 60 eV.
The major lipid peaks were identified from the MS/MS analysis of either sodiated peaks (for CEs and TGs) or ammoniated peaks (for WEs and DEs) by comparing the product ion pattern of standards or literature reports. The other related peaks were identified from high-resolution and high-mass accuracy MS spectra. The sodium iodide additive was found to increase signal-to-noise ratio significantly by converting almost all different forms of adducted peaks to sodiated forms. The simplified spectra were calibrated with the major peaks identified from MS/MS analysis. The lipid classes for the low-intensity peaks in MS spectra were determined within the mass accuracy of ± 5 ppm.
For negative ion mode, the mass spectrometer was first calibrated with sodium iodide. The working solutions were either the stock solutions (∼160 μg/mL) or its 10-fold diluted solution of meibum samples with 1 mM ammonium acetate or 0.1% ammonium hydroxide additive. The solutions were directly infused into the Q-TOF for MS and/or MS/MS analysis. The parameters for MS analysis were similar to those for positive mode except that the capillary voltage was set as 3.0 kV and the cone voltage was typically set at 50 V. The collision energy for MS/MS analysis was typically set as 30 eV. To get better mass accuracy for the major peaks, MS analysis was also performed with the addition of trace amount of sodium iodide (∼8 μg/mL) in the meibum samples. The spectra were internally calibrated with the clustering peaks of sodium iodide. Because of the suppression of signal by sodium iodide, only major peaks were observed with high signal-to-noise ratio. After these major peaks were confidently determined to be polar lipids, selected peaks along with the iodide anion peak (which might be from the environment or carryover that was difficult to wash away completely) were applied as the internal standards to calibrate spectra for samples with no sodium iodide added. The peaks in the calibrated spectra were identified based on accurate masses along with MS/MS analysis and literature reports. The mass accuracies for the peaks assigned in MS analysis were within ±5 ppm for positive mode and ±12 ppm for negative mode.
Although quantitative analysis was not the focus of this study, an estimate is provided of the percentages of the major lipid classes by comparison to available standards. Behenyl oleate, cholesteryl nervonate, triolein, and stearic acid were chosen as the representative standards for the lipid subclasses of WEs, CEs, TGs, and FFAs. Meibum samples were analyzed directly or with the spiking of these standards of known concentrations. For positive mode analysis, a mixture of these standards with the concentrations of 40 to 150 ng/mL was spiked in a typical meibum sample with the lipid concentration of 16 μg/mL. For negative mode analysis, 2.5 μg/mL stearic acid was spiked in the meibum sample with the concentration of 160 μg/mL. By comparing the intensity changes for these representative lipid species in the mass spectrometry spectra of the meibum sample with and without spiking, their percentages of total lipids in meibum were estimated. Assuming that the peak intensities for the lipid species of the same subclasses were proportional to their concentrations, the percentages of total lipids for these subclasses in meibum were also estimated.
Results and Discussion
The present findings indicated that for positive mode analysis, the MS spectra of the meibum samples contained less fragmentation when sodium iodide was applied as the sample additive. Different forms of adducted peaks were converted primarily to the sodiated form, which simplified the spectra. MS/MS analysis of sodiated CEs and TGs also showed many product ions consistent with those of standards and literature reports. However, for WEs and DEs, the sodiated peaks were often very stable and difficult to dissociate. In contrast, the MS/MS analysis of ammoniated WEs and DEs yielded more product ions for easier identification. MS/MS analysis of ammoniated TGs and CEs also generated many characteristic product ions. However, the product ions for CEs were quite weak except a high-intensity diagnostic peak m/z 369.4. For eye disease studies, meibum samples can be analyzed by MS with sodium iodide as the solution additive to find differentially expressed lipids. The identities of these lipids can be determined by MS/MS analysis of the same solution or a meibum sample prepared with ammonium acetate additive.
In contrast, for negative mode the MS spectra were less complicated because only the deprotonated form of lipids was observed.
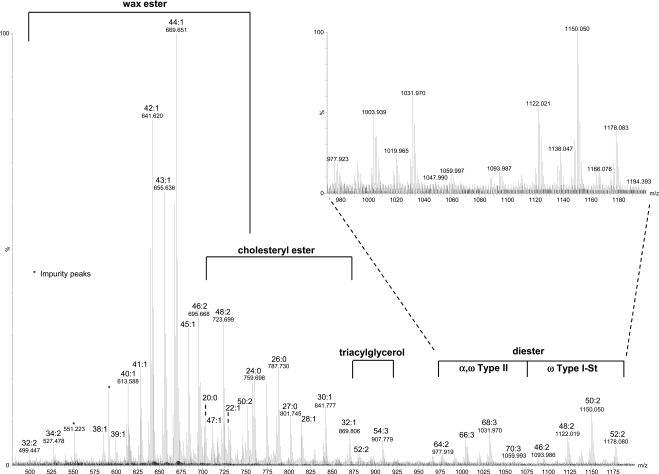
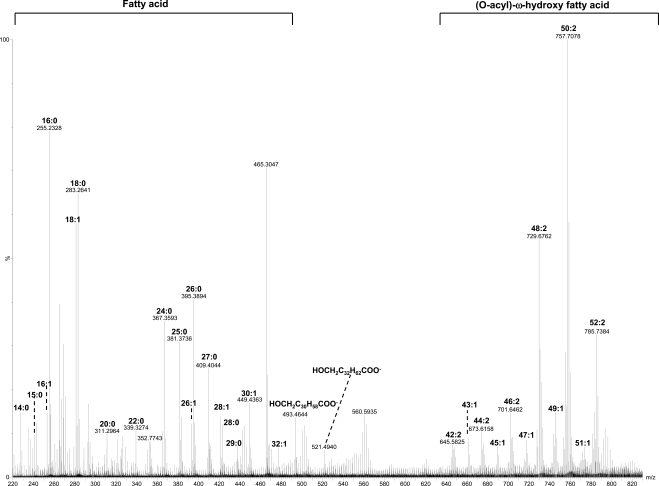
Table 1 lists the molecular components of major lipid peaks determined from the MS/MS and MS analyses. For positive mode MS analysis (Fig. 1), the relative intensities of all the signal peaks in the range of m/z 100 to 1200 were very similar between individual meibum samples. The relative intensities of the peaks above m/z 1200 may depend on several factors, such as lipid concentration, solvent, and temperature (data not shown). These peaks were primarily sodium ion- or proton-linked homo- or heterodimers of WEs, WEs and CEs, and possibly CEs. On the other hand, for negative mode MS analysis, the peaks were mainly in the range of m/z 200 to 800 (Fig. 2).
Table 1.
Summary of the Major Molecular Lipid Components in Human Meibum*
| Species | Composition |
|---|---|
| Wax esters | CnH2nO2 (n = 39–41, 43), n: 0 |
| CnH2n−2O2 (n = 38–47), n: 1 | |
| CnH2n−4O2 (n = 34, 38–50), n: 2 | |
| CnH2n−6O2 (n = 40–50), n: 3 | |
| CnH2n−8O2 (n = 44), n: 4 | |
| CnH2n−10O2 (n = 44), n: 5 | |
| Cholesteryl esters | Cn−1H2n−1COOC27H45 (n = 20–29), n: 0 |
| Cn−1H2n−3COOC27H45 (n = 20–26, 28, 30, 32), n: 1 | |
| Cn−1H2n−5COOC27H45 (n = 20, 22, 24), n: 2 | |
| Diesters | |
| α, ω Type II | CnH2n−6O4 (n = 64–67), n: 2 |
| CnH2n−8O4 (n = 64, 66–68, 70), n: 3 | |
| CnH2n−10O4 (n = 66, 68, 70), n: 4 | |
| ω Type I-St | (CnH2n−5O3)OC27H45 (n = 46–51), n: 1 |
| (CnH2n−7O3)OC27H45 (n = 44, 46, 48–52), n: 2 | |
| (CnH2n−9O3)OC27H45 (n = 48, 50, 52), n: 3 | |
| Triacyglycerols | CnH2n−8O6 (n = 55, 57), n: 2 |
| CnH2n−10O6 (n = 55, 57), n: 3 | |
| Free fatty acids | CnH2nO2 (n = 12, 14–18, 20–29), n: 0 |
| CnH2n−2O2 (n = 16–18, 20, 22, 24, 26, 28, 30, 32), n: 1 | |
| CnH2n−4O2 (n = 18), n: 2 | |
| (O-acyl)-ω-hydroxy fatty acids | CnH2n−4O4 (n = 42–50), n: 1 |
| CnH2n−6O4 (n = 42, 44, 46, 48–52), n: 2 | |
| CnH2n−8O4 (n = 48, 50, 52), n: 3 | |
| CnH2n−10O4 (n = 50, 52), n: 4 |
The assignments were considered only when the corresponding mass accuracies were within ± 5 ppm for positive mode and within ± 12 ppm for negative mode (free fatty acids and (O-acyl)-ω-hydroxy fatty acids).
Figure 1.
MS spectrum acquired in positive mode for a meibum sample. The meibum sample (∼16 μg/mL) was dissolved in chloroform/methanol mixture (2:1) with 8 μg/mL sodium iodide additive. The lipid peaks were present in sodiated form. The m/z region corresponding to each major lipid subclass is indicated with the composition of the major peaks being labeled.
Figure 2.
MS spectrum acquired in negative mode for a meibum sample. The meibum sample (∼16 μg/mL) was dissolved in chloroform/methanol mixture (2:1) with 0.1% ammonia hydroxide additive. The m/z region corresponding to each major lipid subclass is indicated with the composition of the major peaks being labeled.
WEs
WEs have been reported in the literature as the lipid species with the highest percentage (13% to 68%) in meibum.4–8 The present MS analysis of meibum samples showed several sets of major peaks matching the composition of WEs within the range m/z 500–735. These sets of peaks in the order of relative intensities from high to low include CnH2n−2O2 (subset 1), CnH2n−4O2 (subset 2), CnH2nO2 (subset 3), CnH2n−6O2 (subset 4), CnH2n−8O2 (subset 5), and CnH2n−10O2 (subset 6) containing 1, 2, 0, 3, 4, and 5 double bonds, respectively (Table 1). Similar results have also been reported by Butovich et al.16
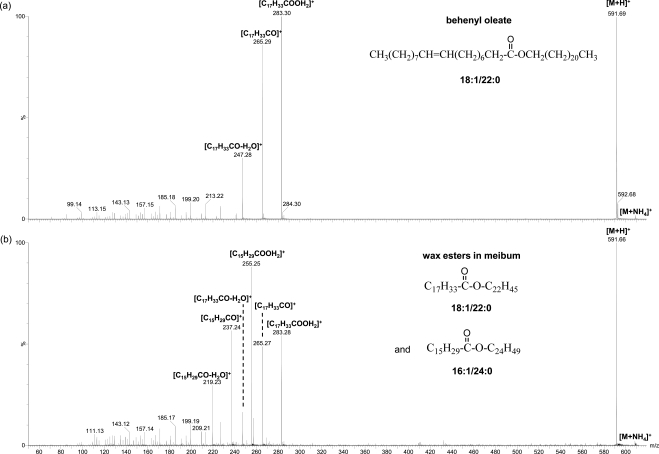
The identities of these peaks were confirmed by the similar MS/MS spectra patterns of most of these peaks with those of WE standards (Fig. 3). All the CID spectra of these unsaturated WEs showed the characteristic protonated unsaturated fatty acid peaks with one or two water molecule losses, similar to previous reports for unsaturated WEs with one or more double bonds in the fatty acid moiety.11,19 Some peaks were the mixture of more than one isobaric species. For instance, the example shown in Figure 3 suggests the peak m/z 591.7 is composed of two major species with the corresponding fatty acid/fatty alcohol moieties being 18:1/22:0 and 16:1/24:0. The fatty acid moieties for the other WEs were found to be mainly C18:1 but with a considerable amount of C16:1, which are consistent with their high percentages of total fatty acids (57.39% and 11.66%, respectively) in WEs reported by Nicolaides et al.,7 where the reported percentages of the fatty acid components were summed from those for different types of fatty acids including normal, iso, and anteiso with different double bond positions. However, the fatty acid C16:1-based WEs were not reported by Butovich et al.11,16 On the other hand, it was found in this study that the unsaturated WEs with two double bonds were primarily composed of at least two different species, with one mainly containing C18:1 fatty acid moiety, and the other containing C18:2 fatty acid moiety at a much smaller percentage. The corresponding fatty alcohol moieties for the high-intensity unsaturated WEs include C26:0, C24:0, C25:0, C26:1, C28:1, and C30:1, again consistent with the reported high percentages of these fatty alcohols in WEs (account for 23.35%, 18.59%, 15.04%, 5.94%, 4.33%, 5.13%, respectively, calculated in a similar manner as discussed above for the percentage of C18:1 and C16:1 fatty acid moieties in WEs).7 C32:1 fatty alcohol observed in this work was not reported by Nicolaides et al.,7 possibly because of the difficulty in detecting it with GC-MS.
Figure 3.
MS/MS spectra of ammoniated WE peaks: (a) a standard (behenyl oleate 18:1/22:0, ∼30 μg/mL) and (b) a WE mixture in meibum (total lipid concentration ∼16 μg/mL). The solutions were prepared in chloroform/methanol mixture (2:1, v/v) with 1 mM ammonium acetate additive. The WE mixtures in meibum are composed of species with the composition of fatty acid/fatty alcohol moieties being 18:1/22:0 and 16:1/24:0.
There were also some peaks with lower intensities matching the theoretical m/z of saturated WEs. The fragmentation patterns for these putative saturated WEs in meibum were similar to those of standards. Several isobaric species were often found for a single peak. For example, fragmentation of protonated saturated WE C41:0 shows that it is composed of at least three species with the fatty acid/fatty alcohol moieties as C17:0/C24:0, C16:0/C25:0, and C15:0/C26:0 (data not shown). In most cases, the highest intensity fatty acid moiety from the fragmentation of saturated WEs is C17:0. With the decrease of the molecular weights of the saturated WEs, the relative intensities of the fragment peaks m/z 257.3 (C16:0) and m/z 243.3 (C15:0) increased. It is noteworthy that of the total lipids in WEs, the percentages of C17:0, C16:0, and C15:0 fatty alcohol moieties previously reported as 10.43%, 6.17%, and 1.48%, respectively,7 were consistent with the present observations.
Behenyl oleate (m/z 613.6) was estimated to account for 0.8% of the total lipids in meibum. From the ratio of the intensity for behenyl oleate to the sum of the intensities for all WEs (Fig. 1), WEs were estimated to account for 28% of total lipids in meibum.
CEs
Sterol esters have been reported as the lipid species with the second highest percentage (8% to 39%) in meibum samples.4–8 The dominant sterol was suggested to be cholesterol from GC-MS analysis of hydrolyzed human meibomian lipids.20 The only other sterol detected in that report was lathosterol, an isomer of cholesterol with almost the same structure as cholesterol except the different position of the double bond. The amount of lathosterol was only approximately 2.5% of cholesterol.20 It is beyond the scope of this study to examine each peak to find out if they are CEs or lathosteryl esters or some other related sterol esters. In simplicity, only CEs are discussed in this work.
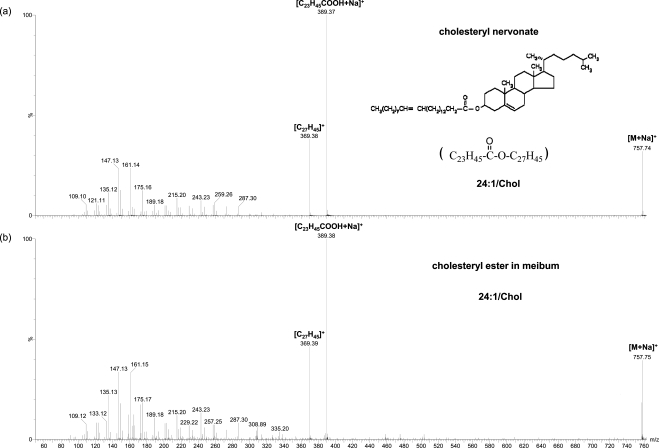
As presented here, in the region m/z 670–900, the major peaks other than those determined to be WEs match sodiated CEs, corresponding to the composition of Cn−1H2n−1COOC27H45 (subset 1), Cn−1H2n−3COOC27H45 (subset 2), and Cn−1H2n−5COOC27H45 (subset 3) with zero to two double bonds, respectively (Table 1). The identities of the CEs were further confirmed with CID. The initial analysis based on ammoniated CEs showed few product ions except the peak m/z 369.4, which is the water loss cholesterol peak. The CID of sodiated CEs were found to provide more product ions for the identification of CEs. The putative sodiated CE peaks in meibum samples showed a similar pattern to CE standards including the diagnostic cholesterol water loss peak (m/z 369.4) and its complementary fragments (Fig. 4).
Figure 4.
MS/MS spectra of CE peaks: (a) a standard (cholesteryl nervonate) and (b) a CE in meibum. The two working solutions were prepared from diluting the stock solutions. The final concentrations of cholesteryl nervonate in (a) and the total lipids in (b) were 2 to 3 μg/mL. The final solutions contained 96:4 (v/v) methanol/chloroform with 7 to 8 μg/mL sodium iodide additives.
For all the samples analyzed, CE peaks were always observed, consistent with Butovich;12 however, this is different from the observation of cholesteryl absent sterol esters in some normal samples reported by Shine and McCulley.21 The highest intensity CEs detected in the present studies contain the fatty acid moieties C24:0, C25:0, and C26:0, consistent with the classic work by Nicolaides et al.,7 where the same top three fatty acid moieties with the greatest frequency were determined in the sterol esters in human meibum. These high-intensity peaks are different from the peaks claimed as C26:1, C25:0, and C28:1 by Butovich,12 likely because of the challenge in detecting intact saturated CEs with their methods.
Cholesteryl nervonate (m/z 757.7) was estimated to account for 1% of the total lipids in meibum. From the ratio of the intensity for cholesteryl nervonate to the sum of the intensities for all CEs (Fig. 1), CEs were estimated to account for 13% of total lipids in meibum.
TGs
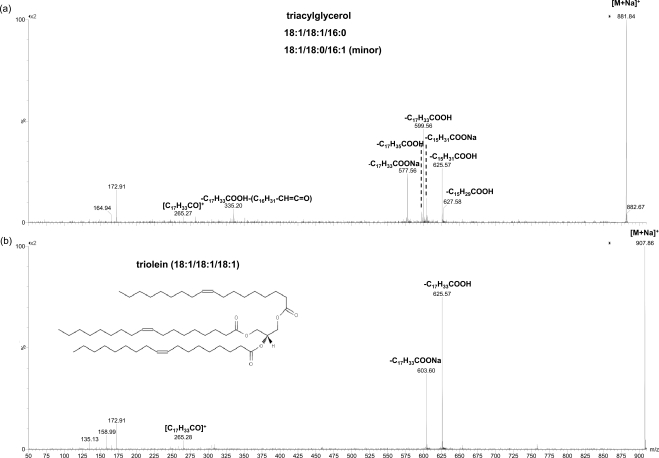
TGs have been reported to account for 2% to 6%4–7 or 11% to 43%8 of all the lipids in meibum, with the former range more consistent between different analyses. In the present work, the peaks including m/z 907.777 match the composition of TGs with two or three double bonds (Table 1). The identities of the TGs were further confirmed with CID (Fig. 5), which show similar fragmentation patterns to previous reports of sodiated22 and ammoniated23,24 TGs. Based on the fragmentation of these peaks, the most abundant TG is likely triolein, with the composition of C54:3. The fatty acid chains contained in other TGs were primarily C18:1, C16:0, C16:1, C17:0, and C18:2. These fatty acid moieties are consistent with previously reported high percentages of total fatty acids in TGs found in human meibum: 44.74%, 15.34%, 9.57%, 3.85%, and 2.27%, respectively.7 A high-intensity C18:1 fatty acid moiety (44.33%) in TGs in normal human meibum has also been reported.25
Figure 5.
MS/MS spectra of sodiated TG peaks in a meibum sample. The precursor ion in (a) is composed of at least two TG species with the compositions of 18:1/18:1/16:0 and 18:1/18:0/16:1 (the order may not be necessarily corresponding to the actual compositions), while that in (b) is a triolein peak. The meibum solution was the same as that in Figure 4.
Triolein (m/z 907.8) was estimated to account for 0.01% of the total lipids in meibum. From the ratio of the intensity for triolein to the sum of the intensities for all TGs (Fig. 1), TGs were estimated to account for 0.05% of total lipids in meibum.
DEs
DEs have been reported previously to make up 2% to 18% of lipids in meibum4,5,7; however, they were not detected by two other groups.6,8 The identification of DEs in these reports were based on ratios of different forms of moieties for each DE band, determined after hydrolysis, derivatization, and GC-MS analysis.9
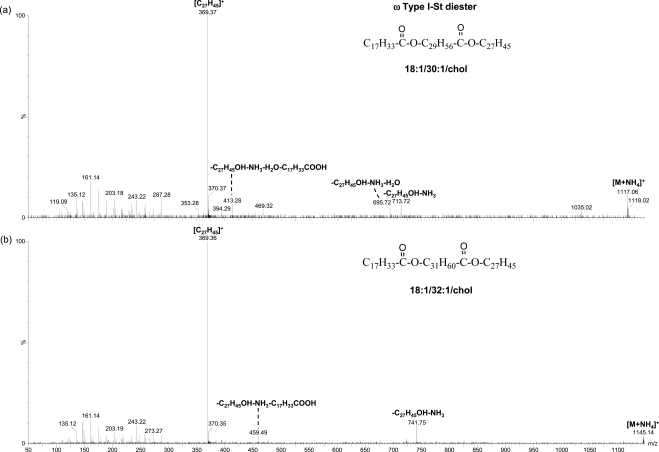
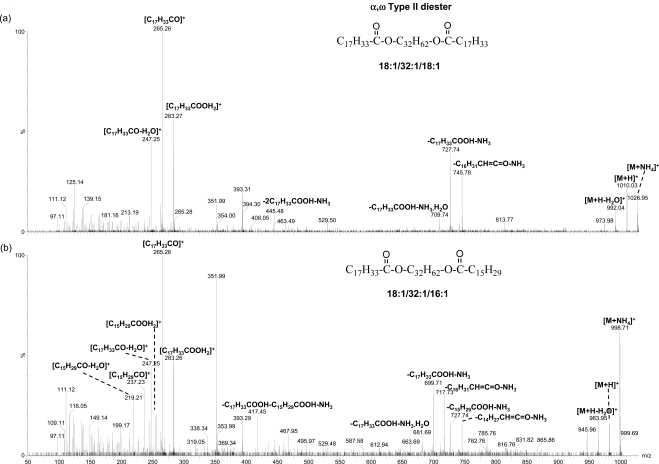
In this study, the peaks in the ranges of m/z 1075 to 1200 and m/z 975 to 1075 matched the composition of two types of DEs: ω type I-St and α,ω type II, respectively9 (Table 1). The former is composed of a cholesteryl group, a ω-hydroxy fatty acid moiety, and a fatty acid moiety, and the latter is composed of two fatty acid moieties and one α,ω diol moiety. The compositions for the putative ω type I-St DE peaks correspond to (CnH2n−5O3)OC27H45, (CnH2n−7O3)OC27H45, and (CnH2n−9O3)OC27H45, containing one to three double bonds, respectively. On the other hand, the compositions for the putative α,ω type II DE peaks correspond to CnH2n−6O4, CnH2n−8O4, and CnH2n−10O4, containing two to four double bonds, respectively.
CID analysis of the major peaks of each type of DEs further supports their identities. No DE standards are commercially available at this time. However, the composition of the DEs can be inferred from the fragmentation patterns. For the putative ω type I-St DE peaks, the MS/MS analysis showed dominant fragments corresponding to the cholesterol water loss peak and the complementary cholesteryl group loss peak (Fig. 6). The molecules of the three dominant peaks (ammoniated, m/z 1117.07, m/z 1145.10, and m/z 1173.14) for this type of DEs are likely composed of a C18:1 fatty acid moiety and a C30:1, C32:1, or C34:1 ω-hydroxy fatty acid moiety, in addition to the cholesteryl moiety. It is consistent that C30:1, C32:1, and C34:1 ω-hydroxy fatty acids are among the most abundant ω-hydroxy fatty acids, reported earlier for steer meibum constituting 6.9%, 40.3%, and 23.6% of the total ω hydroxyl fatty acids, respectively.26 The C18:1 and C16:1 fatty acids have also been reported to be the highest content (the sum was 56.2% to 59.5%) of fatty acids in ω type I-St DEs9 in steer meibum. The corresponding information of ω-hydroxy fatty acids is not available for human meibum. However, the lipid profile for steer meibum is quite similar to human meibum. The identities of the α,ω type II DE peaks were also confirmed with CID analysis (Fig. 7) in the meibum samples. The composition of the two most abundant DEs are proposed to contain a C32:1 α,ω diol moiety, consistent with the high abundance of this diol moiety (54.4% of the total α,ω diols) reported earlier in human meibum DEs.27 The C18:1 and C16:1 are the main fatty acid moieties observed in DEs, which is also consistent with the two fatty acid moieties reported as the most abundant (the sum was 41.7% to 65.8%) in α,ω type II DEs in steer meibum.9
Figure 6.
MS/MS spectra of ammoniated ω type I-St DE peaks. The proposed composition for the two precursor ions are shown. The meibum solution was the same as that in Figure 3.
Figure 7.
MS/MS spectra of ammoniated α,ω type II DE peaks. The proposed composition for the two precursor ions is shown. The meibum solution was the same as that in Figure 3.
Free Sterols
Free sterols have been reported to account for 0% to 5% of total lipids in human meibum.4,5,7,8 In this study, for samples with ammonium acetate additive or without any additive, a peak m/z 369.4 was often seen corresponding to the molecular weight of protonated cholesterol water loss peak. However, when sodium iodide was applied as the additive, the peak was small (data not shown). The results indicate that most of the cholesterol is present in the form of CEs with very little amount of free cholesterol. The protonated and ammoniated CE peaks easily dissociate and generate the m/z 369.4 peak, while sodiated CE peaks are very stable and are difficult to dissociate under typical MS conditions. Cholesterol and CE standards showed similar ionization efficiency during ESI-MS analysis, which suggests the amount of free cholesterol in meibum is probably less than previously reported. Some of the free sterols demonstrated in previous reports could be from dissociation during sample processing.
FFAs
The percentages of reported FFAs in meibum varies widely with values including 2.1%,7 0% to 24%,8 10.4%,4 1.0%,6 2.8%,5 and 0.52%.28 In the present work, a series of peaks was observed in negative mode with the m/z corresponding to FFA peaks. The MS/MS analysis of the major peaks of this series showed the characteristic water loss peak as previously reported (data not shown).29 Most of these FFAs are saturated (Fig. 2). With the addition of trace amount of sodium iodide as the additive for the internal calibration, the major FFA peaks were determined from accurate masses. The most intense FFA peaks found were C18:0, C16:0, and C18:1 in all cases. Part of the unsaturated fatty acids including C18:1 and C16:1 could be from the in-source dissociation of a series peaks around m/z 757, as discussed below in the section on polar lipids. The same three types of fatty acids were also reported to be of the highest quantity (26.17%, 19.43%, and 27.69% of total FFAs) in meibum.7 The high percentage of C18:1 was also reported by Dougherry et al.28 The reported relatively high amount of C18:1 could result from the hydrolysis of the lipids corresponding to the series of peaks around m/z 757 during their sample preparation.
The spectra for samples with sodium iodide additive often show suppressed signal for low-intensity peaks. To circumvent this problem, samples were analyzed with no intentional addition of sodium iodide, and the spectra were then calibrated with a series of peaks with known compositions: the iodide peak, the peaks corresponding to FFAs C18:0, C18:1; and the three major OAHFA peaks around m/z 757 (see discussion of polar lipids for details). In this way, more FFA peaks were determined with the mass accuracy within ±10 ppm (Tables 1 and 2).
Table 2.
Major Free Fatty Acids in Meibum Detected in Negative Mode
| Composition | Theoretical m/z | Experimental m/z | Mass Error (ppm) |
|---|---|---|---|
| C12H23O2 (12:0) | 199.1699 | 0.5 | |
| C14H27O2 (14:0) | 227.2011 | 227.1997 | −6.2 |
| C15H29O2 (15:0) | 241.2168 | 241.2155 | −5.4 |
| C16H29O2 (16:1) | 253.2168 | 253.2164 | −1.6 |
| C16H31O2 (16:0) | 255.2324 | 255.2328 | 1.6 |
| C17H31O2 (17:1) | 267.2324 | 267.231 | −5.2 |
| C17H33O2 (17:0) | 269.2481 | 269.2474 | −2.6 |
| C18H31O2 (18:2) | 279.2324 | 279.2328 | 1.4 |
| C18H33O2 (18:1) | 281.2481 | 281.2484 | 1.1 |
| C18H35O2 (18:0) | 283.2637 | 283.2641 | 1.4 |
| C20H37O2 (20:1) | 309.2794 | 309.2789 | −1.6 |
| C20H39O2 (20:0) | 311.2950 | 311.2964 | 4.5 |
| C21H41O2 (21:0) | 325.3107 | 325.3111 | 1.2 |
| C22H41O2 (22:1) | 337.3107 | 337.3136 | 8.6 |
| C22H43O2 (22:0) | 339.3263 | 339.3274 | 3.2 |
| C23H45O2 (23:0) | 353.3420 | 353.3445 | 7.1 |
| C24H45O2 (24:1) | 365.3420 | 365.3459 | 10.7 |
| C24H47O2 (24:0) | 367.3576 | 367.3593 | 4.6 |
| C25H49O2 (25:0) | 381.3733 | 381.3736 | 0.8 |
| C26H49O2 (26:1) | 393.3733 | 393.3734 | 0.3 |
| C26H51O2 (26:0) | 395.3889 | 395.3894 | 1.3 |
| C27H53O2 (27:0) | 409.4046 | 409.4044 | −0.5 |
| C28H53O2 (28:1) | 421.4046 | 421.4058 | 2.8 |
| C28H55O2 (28:0) | 423.4202 | 423.4225 | 5.4 |
| C29H57O2 (29:0) | 437.4359 | 437.4308 | −11.7 |
| C30H57O2 (30:1) | 449.4359 | 449.4363 | 0.9 |
| C32H61O2 (32:1) | 477.4672 | 477.4713 | 8.6 |
McCulley and Shine30 hypothesized the FFAs were from the degradation of sterol esters, WEs, and TGs by lipases derived from bacteria, lysosomes, and peroxisomes. However, compared to the relatively low percentage of saturated fatty acid moiety reported in all lipids as being only 51.63%7 or 44.8%,31 the greater amount of saturated FFAs observed in both this study and the work by Nicolaides et al.7 (62.96% of total FFAs) suggested that a small amount of FFAs probably do exist in meibum.
Stearic acid (m/z 907.8) was estimated to account for 0.4% of the total lipids in meibum. From the ratio of the intensity for stearic acid to the sum of the intensities for all FFAs (Fig. 2), FFAs were estimated to account for 3% of total lipids in meibum.
Polar Lipids
The presence of polar lipids in human meibum is controversial. Nicolaides et al.7 reported that the polar lipids account for up to 16% of the total lipids in meibum. The specific subclass(es) for these polar lipids was not mentioned in their work. Nicolaides32 did briefly hypothesize the presence of polar WEs and polar wax DEs in meibum. Tiffany8 observed some lipids in meibum that did not migrate on a TLC plate and claimed them as phospholipids. The relative amounts of phospholipids quantified in four samples were 0%, 5%, 0.8%, and 1.1% of the total lipids, respectively. Tiffany noted that the components of the spot included phospholipids, glycolipids, and possibly some protein. The detection method and the large variation of the amount detected for the phospholipids cast doubts on the reliability of the identification and quantitation. Several more recent reports on polar lipids were reported by McCulley and Shine.6,33,34 In their work, the polar lipid fraction from TLC was removed and separated with normal phase HPLC; phospholipids and sphingolipids were confirmed based on an earlier report.35,36 A detection wavelength of 220 nm, different from the original designed 205 nm in literature,35 has been used by those authors. The change shifts the absorbance from maximum to close to the baseline of the absorbance peak.37 This may introduce uncertainty in the detection of phospholipids and sphingolipids. In fact, no discernable presence of common phospholipids has been observed by Butovich et al.,11 including the lack of the diagnostic peak of m/z 184.1 based on the comparison of CID experiments of meibum with phospholipid standards.
In this article, an attempt has also been made to identify polar lipids in human meibum samples with advanced mass spectrometry technology. In positive mode, most of the major peaks found were those of nonpolar lipids, and no phospholipid and sphingolipid peaks were detected with high confidence, which is consistent with the observation by Butovich et al.11 The meibomian gland secrets through a holocrine mechanism; therefore it is expected to observe all cell contents including phospholipids.1 The apparent small amount of phospholipids in meibum could be due to recycling during the disintegration of the cells, similar as proposed for seibum.38 There are several factors that can impact the detection of phospholipids, including the efficiency of generating ions (dependent on the polar head group, the chain length, and saturation). Ion-suppression effects make it very difficult to detect low abundant species in a complex mixture, especially in the presence of high abundant species. It is possible that recent mass spectrometry techniques have not been optimized appropriately to detect small amounts of phospholipids.
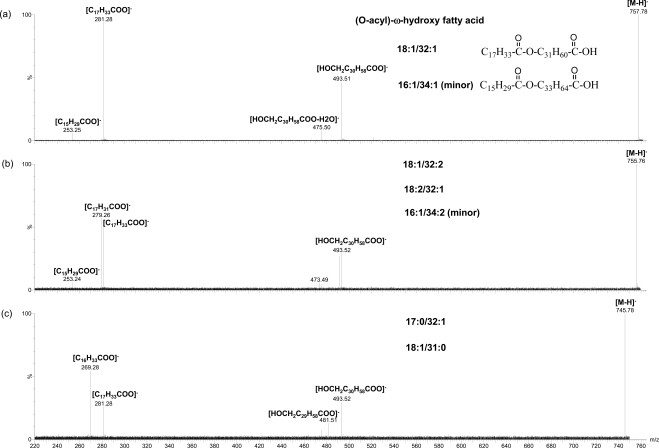
On the other hand, in negative mode a series of peaks around m/z 757 match the compositions of OAHFA16 (Fig. 2). By adding sodium iodide additive in the meibum sample as an internal calibration standard, the accurate m/z values determined for the major peaks of this series include 757.7040, 755.6925, 729.6736, 701.6447, and 785.7322. These values match the following compositions: C50H93O4 (−4.5 ppm), C50H91O4 (1.1 ppm), C48H89O4 (−3.4 ppm), C46H85O4 (−0.1 ppm), and C52H97O4 (−8.3 ppm), respectively. Similar set of peaks was also reported by Butovich et al.16 and assigned as OAHFA based on their fragmentation pattern, which appeared similar to that of a synthesized (O-oleoyl)-16-hydroxypalmitic acid. As addressed in the discussion of FFAs, a series of peaks were applied as the internal standards to calibrate spectra for samples with no internal standard additive. With the same calibration, the major peaks and other related ones around theoretical m/z 757 were found to match the composition of CnH2n−5O4, CnH2n−7O4, CnH2n−9O4, and CnH2n−11O4 with high mass accuracy (Tables 1 and 3).
Table 3.
Major (O-Acyl)-ω-Hydroxy Fatty Acids in Meibum Detected in Negative Mode
| Composition | Theoretical m/z | Experimental m/z | Mass Error (ppm) |
|---|---|---|---|
| C42H77O4 (42:2) | 645.5822 | 645.5825 | 0.5 |
| C42H79O4 (42:1) | 647.5978 | 647.5988 | 1.5 |
| C43H81O4 (43:1) | 661.6135 | 661.6115 | −3.0 |
| C44H81O4 (44:2) | 673.6135 | 673.6158 | 3.4 |
| C44H83O4 (44:1) | 675.6291 | 675.6322 | 4.6 |
| C45H85O4 (45:1) | 689.6448 | 689.6437 | −1.6 |
| C46H85O4 (46:2) | 701.6448 | 701.6462 | 2.0 |
| C46H87O4 (46:1) | 703.6604 | 703.6592 | −1.7 |
| C47H89O4 (47:1) | 717.6761 | 717.676 | −0.1 |
| C48H87O4 (48:3) | 727.6604 | 727.6647 | 5.9 |
| C48H89O4 (48:2) | 729.6761 | 729.6762 | 0.1 |
| C48H91O4 (48:1) | 731.6917 | 731.6877 | −5.5 |
| C49H91O4 (49:2) | 743.6917 | 743.6918 | 0.1 |
| C49H93O4 (49:1) | 745.7074 | 745.7062 | −1.6 |
| C50H89O4 (50:4) | 753.6761 | 753.6766 | 0.7 |
| C50H91O4 (50:3) | 755.6917 | 755.6908 | −1.2 |
| C50H93O4 (50:2) | 757.7074 | 757.7078 | 0.5 |
| C50H95O4 (50:1) | 759.723 | 759.7161 | −9.1 |
| C51H95O4 (51:2) | 771.723 | 771.7155 | −9.7 |
| C52H93O4 (52:4) | 781.7074 | 781.7084 | 1.3 |
| C52H95O4 (52:3) | 783.723 | 783.7222 | −1.0 |
| C52H97O4 (52:2) | 785.7387 | 785.7384 | −0.4 |
MS/MS analysis was performed for the peaks corresponding to the compositions of OAHFAs C50:2, C48:2, C52:2, C49:1, and C50:3 (Fig. 8). All the major peaks of this series are composed of more than one species based on the MS/MS analysis. The same strong product ion corresponding to fatty acid C18:1 was observed for all the major peaks, similar to earlier studies.16 The other product ions include the ones corresponding to fatty acid C16:1 (for OAHFAs C50:2, C50:3, C48:2), C17:0 (for OAHFA C49:1), C18:2 (for OAHFA C50:3), or C20:1 (for OAHFA C52:2) and the corresponding ω-hydroxy fatty acids. The peaks m/z 493.46 and m/z 521.49 shown in Figure 2 were likely generated from the in-source dissociation of this set of peaks.
Figure 8.
MS/MS spectra of three (O-acyl)-ω-hydroxy fatty acid peaks in negative mode. The experimental condition is the same as described in Figure 2.
In addition to FFAs and OAHFAs shown in Figure 2, a peak m/z 465.30 also showed high intensity. The MS/MS of this peak showed only one dominant product ion m/z 96.97. The identity of this peak has not been determined at this time.
Polar lipids have been shown to conduct the initial phase of the lipid spread on an aqueous phase by in vitro studies,36,39–43 which could also be true for the formation of tear film. In an early model of the tear film, polar lipids including several types of phospholipids and sphingolipids were incorporated in the structure.6 Although the recent reports and the present work cast doubt on the significant presence of these types of polar lipids reported,33,34 the newly found polar lipids discussed above could instead play an important function in maintaining the structure of tear film in a way similar as proposed by McCulley and Shine.6
Conclusions
This study reported that ESI-MS and MS/MS are excellent tools for lipidomics studies of meibum samples. One advantage of this technology is that it can keep labile lipids intact. For instance, saturated CE peaks can be easily observed in this work in contrast to APCI.12 It was also demonstrated that various types of lipids can be detected under the same condition, which makes it easier to compare the relative changes between different samples and to save time in optimizing the experimental conditions. In contrast, the previous identifications of different subclasses of lipids with APCI could be realized only with different conditions.11,12 Furthermore, a very small amount of analyte is needed for ESI, which makes ESI-MS the preferred choice for studies with limited lipid sample volume.
With direct infusion ESI-MS and MS/MS analysis of intact lipids in meibum samples it was possible to identify the major species therein. The molecular components identified in this work are generally consistent with the classical work by Nicolaides et al.7,9,26,27 However, Nicolaides et al. identified the building blocks of only each subclass and hypothesized that the esterification of fatty acids by fatty alcohols was a random process and no one of the molecules is more important than any of the others.7,9 In contrast, the present results showed that the esterification of fatty acids actually prefers certain types of fatty alcohols, and thus certain types of molecules are much more abundant than the others. For instance, contrasted to the expectation that 2760 species of WEs were of equal importance in the composition of meibum based on the types of fatty acid and fatty alcohol moieties,9 this study observed only approximately 20 WEs to be abundant. This suggests that studying the intact lipids in meibum instead of the building blocks could make it easier to find differentially expressed lipids.
Direct infusion ESI-MS and MS/MS analysis is a good method for the initial study of meibum samples. However, considering the normal, iso, and anteiso isomers of fatty acids/fatty alcohols and the position of double bonds, it is challenging to fully identify and quantify the various types of lipids in meibum. To better understand the lipid compositions of meibum samples, the ESI-MS and MS/MS analysis may eventually need to be coupled with HPLC separation. Various types of lipid isoforms with little difference in their fragmentation patterns may be identified with the additional HPLC retention time information, and good HPLC separation may also increase the quantitation reliability by decreasing the ion suppression effect. Further lipidomic studies in patients with ocular surface disease are warranted for comparison to meibum from normal patients presented here.
Acknowledgments
The authors thank Kathy Weibel for meibum collection and Nan Kleinholz for assistance on the instrument.
Footnotes
Supported by National Institutes of Health Grant NEI R01EY015519.
Disclosure: J. Chen, None; K.B. Green-Church, None; K.K. Nichols, None
References
- 1. McCulley JP, Shine WE. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res. 2004;78:361–365 [DOI] [PubMed] [Google Scholar]
- 2. Epidemiology Subcommittee of the International Dry Eye WorkShop The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:93–107 [DOI] [PubMed] [Google Scholar]
- 3. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids—a review. Curr Eye Res. 2008;33:405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27 [DOI] [PubMed] [Google Scholar]
- 5. Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360 [DOI] [PubMed] [Google Scholar]
- 6. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88; discussion 88–93 [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536 [PubMed] [Google Scholar]
- 8. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300 [DOI] [PubMed] [Google Scholar]
- 9. Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467 [DOI] [PubMed] [Google Scholar]
- 10. Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776 [DOI] [PubMed] [Google Scholar]
- 11. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235 [DOI] [PubMed] [Google Scholar]
- 12. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaikin VG, Halket JM. Derivatization in mass spectrometry, 8: soft ionization mass spectrometry of small molecules. Eur J Mass Spectrom. 2006;12:79–115 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699 [DOI] [PubMed] [Google Scholar]
- 15. Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292 [DOI] [PubMed] [Google Scholar]
- 16. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 44:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lide DR. ed. Composition and properties of common oils and fat. In: CRC Handbook of Chemistry and Physics. 79th (Internet version 2009) Boca Raton, FL: CRC Press/Taylor and Francis; 2009:7-9–13 [Google Scholar]
- 19. Krank JL, Murphy RC. Analysis of wax ester molecular species by liquid chromatography tandem mass spectrometry. In: Proceedings of the 53rd ASMS Conference on Mass Spectrometry and Allied Topics San Antonio, TX; 2005:239 (in Session: Lipids; Structural Analysis) [Google Scholar]
- 20. Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987;414:253–263 [DOI] [PubMed] [Google Scholar]
- 21. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280 [PubMed] [Google Scholar]
- 22. Segall SD, Artz WE, Raslan DS, Ferraz VP, Takahashi JA. Triacylglycerol analysis of pequi (Caryocar brasiliensis Camb.) oil by electrospray and tandem mass spectrometry. J Sci Food Agric. 2006;86:445–452 [Google Scholar]
- 23. Duffin KL, Henion JD, Shieh JJ. Electrospray and tandem mass spectrometric characterization of acylglycerol mixtures that are dissolved in nonpolar solvents. Anal Chem. 1991;63:1781–1788 [DOI] [PubMed] [Google Scholar]
- 24. McAnoy AM, Wu CC, Murphy RC. Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap. J Am Soc Mass Spectrom. 2005;16:1498–1509 [DOI] [PubMed] [Google Scholar]
- 25. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346 [DOI] [PubMed] [Google Scholar]
- 26. Nicolaides N, Santos EC, Papadakis K. Double-bond patterns of fatty acids and alcohols in steer and human meibomian gland lipids. Lipids. 1984;19:264–277 [DOI] [PubMed] [Google Scholar]
- 27. Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984;19:990–993 [DOI] [PubMed] [Google Scholar]
- 28. Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:52–56 [PubMed] [Google Scholar]
- 29. Kerwin JL, Wiens AM, Ericsson LH. Identification of fatty acids by electrospray mass spectrometry and tandem mass spectrometry. J Mass Spectrom. 1996;31:184–192 [DOI] [PubMed] [Google Scholar]
- 30. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106 [DOI] [PubMed] [Google Scholar]
- 31. Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119 [DOI] [PubMed] [Google Scholar]
- 32. Nicolaides N. Recent findings on the chemical composition of steer and human meibomian glands. In: Holly FJ. ed. The Preocular Tear Film in Health, Disease, and Contact Lens Wear. Lubbock, TX: Dry Eye Institute; 1986:570–576 [Google Scholar]
- 33. Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94 [DOI] [PubMed] [Google Scholar]
- 34. Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783 [DOI] [PubMed] [Google Scholar]
- 35. Bernhard W, Linck M, Creutzburg H, et al. High-performance liquid chromatographic analysis of phospholipids from different sources with combined fluorescence and ultraviolet detection. Anal Biochem. 1994;220:172–180 [DOI] [PubMed] [Google Scholar]
- 36. Bernhard W, Postle AD, Linck M, Sewing KF. Composition of phospholipid classes and phosphatidylcholine molecular species of gastric mucosa and mucus. Biochim Biophys Acta. 1995;1255:99–104 [DOI] [PubMed] [Google Scholar]
- 37. McHowat J, Jones JH, Creer MH. Quantitation of individual phospholipid molecular species by UV absorption measurements. J Lipid Res. 1996;37:2450–2460 [PubMed] [Google Scholar]
- 38. Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004;123:1–12 [DOI] [PubMed] [Google Scholar]
- 39. Jendrasiak GL, Smith RL, Shaw W. The water adsorption characteristics of charged phospholipids. Biochim Biophys Acta. 1996;1279:63–69 [DOI] [PubMed] [Google Scholar]
- 40. Nibu Y, Inoue T, Motoda I. Effect of headgroup type on the miscibility of homologous phospholipids with different acyl chain lengths in hydrated bilayer. Biophys Chem. 1995;56:273–280 [DOI] [PubMed] [Google Scholar]
- 41. Gong DH, Turner B, Bhaskar KR, Lamont JT. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am J Physiol. 1990;259:G681–686 [DOI] [PubMed] [Google Scholar]
- 42. Lichtenberger LM, Graziani LA, Dial EJ, Butler BD, Hills BA. Role of surface-active phospholipids in gastric cytoprotection. Science. 1983;219:1327–1329 [DOI] [PubMed] [Google Scholar]
- 43. Slomiany BL, Murty VL, Sarosiek J, Piotrowski J, Slomiany A. Role of associated and covalently bound lipids in salivary mucin hydrophobicity: effect of proteolysis and disulfide bridge reduction. Biochem Biophys Res Commun. 1988;151:1046–1053 [DOI] [PubMed] [Google Scholar]