The authors demonstrate that pretreatment of lacrimal gland acini with cAMP inhibits p42/p44 mitogen-activated protein kinase activity. This is the mechanism by which cAMP agonists potentiate protein secretion stimulated by cholinergic agonists and growth factors.
Abstract
Purpose.
The lacrimal gland is primarily responsible for the aqueous portion of the tear film. Simultaneous addition of cholinergic agonists or growth factors with cAMP-dependent agonists potentiates secretion. Recent investigations revealed that cAMP decreases p44/p42 mitogen-activated protein kinase (MAPK) activity stimulated by cholinergic agonists and growth factors that could account for this potentiation. In this study the authors identify the signal transduction pathway used by cAMP to inhibit MAPK activity.
Methods.
Rat lacrimal gland acini were incubated with H89, an inhibitor of protein kinase A, before the addition of dibutyryl cAMP (dbcAMP, 10−3 M) for 30 minutes. Basal MAPK and CREB activity and MAPK activity after stimulation with the cholinergic agonist carbachol (Cch) or epidermal growth factor (EGF) for 5 minutes was determined. The effect of dbcAMP on EGF receptor activity and basal and stimulated Ras, Raf-1, mitogen-activated protein kinase kinase (MEK), and MAPK activity was determined. The effect of a Rap-1 inhibitor, GGTI-298, on MAPK activity after the addition of dbcAMP was also determined.
Results.
H89 relieved the inhibition of cAMP on MAPK activity and inhibited CREB activity. Incubation with dbcAMP did not have any effect either on the EGF receptor or on Ras but significantly inhibited both basal and Raf-1 and MEK activity stimulated with Cch or EGF. GGTI-298 did not have any effect on cAMP-dependent decrease in MAPK activity.
Conclusions.
The authors conclude that cAMP mediates the inhibition of MAPK by PKA in a Raf-1–dependent manner.
The lacrimal gland (LG) is a tubuloacinar gland that is composed of acinar cells arranged in a pyramidal shape surrounding a central lumen. Acinar cells synthesize and secrete into the lumen proteins in the tear film as well as electrolytes and water. The lumen is lined with ductal cells that modify the primary secretory product by the secretion of electrolytes and water. The lumen coalesces with other ducts to form a main excretory duct that empties onto the ocular surface. The LG also contains a third major cell type, the myoepithelial cell. Myoepithelial cells are stellate-shaped cells that surround the acinar cells. Because these cells contain α-smooth muscle actin, it is possible that their contractions aid in expulsion of the secretory products from the acinar cells.1
The LG secretes a major portion of the aqueous portion of the tear film and includes water, electrolytes, and protein. Given that tears are necessary to provide a smooth refractive surface to ensure clear vision, secretion from the LG is tightly controlled because either an excess or a deficiency in tears can be harmful to vision. Sensory nerves in the cornea provide an efferent pathway by which stimuli to the ocular surface activate an afferent pathway by way of the parasympathetic and sympathetic nerves that innervate the LG to stimulate secretion.1
Parasympathetic nerves release the neurotransmitters acetylcholine (Ach) and vasoactive intestinal peptide (VIP). Ach, in the LG, binds to M3 muscarinic receptors on the basolateral membranes of acinar cells.2 Through a Gq/11α G-protein, M3 muscarinic receptors activate phospholipase Cβ to cleave phosphatidylinositol bisphosphate into inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG).3 IP3 diffuses to the endoplasmic reticulum, where it binds to its receptors to release Ca2+ into the cytosol from intracellular stores. The released Ca2+ is involved in the activation of kinases that are involved in secretion.3 The DAG that is released activates a family of proteins called protein kinase C (PKC). There are at least 10 PKC isoforms, of which four are present in the LG: PKCα, -δ, -ε, and -λ.4 Cholinergic agonists activate PKCα, -δ, and -ε to induce protein secretion.5
VIP binds to its receptors, VPAC1 and VPAC2, present on LG acinar cells to stimulate the Gsα subunit of G-proteins to activate adenylyl cyclase (AC).6 The LG contains at least three types of ACs, namely II, III, and IV.6 Activation of AC increases the intracellular levels of cAMP, which in turn activates protein kinase A (PKA). Activation of PKA stimulates protein secretion as the endogenous inhibitor of PKA—PKI—inhibits VIP-stimulated protein secretion.6
Growth factors are also stimuli of protein secretion from the LG. Epidermal growth factor (EGF) stimulates secretion through dimerization of the EGF receptor, leading to the activation of phospholipase Cγ.7 This leads to an increase in intracellular Ca2+ and the activation of PKCα and -δ to stimulate protein secretion.7
The Ca2+/PKC- and cAMP-dependent pathways are not separate, independent pathways but, in fact, can interact in many different tissues.8–10 In the LG, this was demonstrated by the observation that the addition of VIP and cholinergic agonists11 or VIP and EGF12 caused a potentiation of secretion, that is, a response greater than the sum of the individual responses. It was established that a potentiation of secretion was not a result of the potentiation of the increase in intracellular Ca2+, cAMP concentration, or PKC activity.13,14
One possible point at which these two pathways can interact is the activation of p42/p44 mitogen-activated protein kinase (MAPK, also known as ERK 1/2). Cholinergic agonists activate MAPK to attenuate stimulated protein secretion.15 Cholinergic agonists and EGF activate MAPK in different ways. Cholinergic agonists activate the nonreceptor tyrosine kinases Pyk2 and c-Src to activate the signaling cascade of Ras, Raf-1, MEK, and ultimately MAPK.15,16 EGF activates Shc, Grb2, and Sos to activate MAPK through Ras, Raf-1, and MEK.15
An additional pathway by which Raf-1, MEK, and MAPK can be activated is through the stimulation of Rap-1. Rap-1 is a small GTPase and a member of the Ras family. Rap-1 can be activated by a variety of stimuli, including compounds that increase intracellular cAMP concentrations, nerve growth factor, and epinephrine.17 In renal tubule epithelial cells, PKA has been shown to activate MAPK mediated by Rap-1.17–19 Activation of Rap-1 plays a role in cellular processes such as cell adhesion and proliferation.19
In support of the hypothesis that MAPK plays a role in the potentiation of secretion, we previously found that VIP through cAMP inhibits the activation of MAPK, preventing MAPK from attenuating secretion.12 Increasing cAMP concentrations within a cell through either the activation of adenylyl cyclase (using VIP or forskolin) or the inhibition of the phosphodiesterases, which degrade cAMP, inhibits the basal and stimulated activation of MAPK and causes the potentiation of protein secretion by blocking the MAPK-dependent inhibitory pathway.12
In the present study, we identify the components of the MAPK signal transduction pathway by which cAMP inhibits both basal- and agonist-stimulated MAPK activity.
Materials and Methods
Materials
Sprague-Dawley rats were purchased from Taconic Farms (Germantown, NY). Dibutyryl adenosine 3′, 5′ cyclic monophosphate (dbcAMP) was purchased from Biomol (Plymouth Meeting, PA). EGF was from PeproTech (Rocky Hill, NJ), and H89 was from LC Laboratories (Waltham, MA). Collagenase type III was from Worthington Biochemicals (Lakewood, NJ). Antibodies directed against EGF receptor phosphorylated on Tyr1068, Raf-1 phosphorylated on Ser338, and MEK 1/2 phosphorylated on Ser217/221 and total EGF receptor, Raf-1, and MEK were from Cell Signaling (Danvers, MA), whereas antibodies against phosphorylated and total MAPK and secondary antibodies conjugated to horseradish peroxidase were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to total and phosphorylated cAMP response element binding protein (CREB) were obtained from Affinity BioReagents (Golden, CO). All other reagents were from Sigma Chemical Company (St. Louis, MO).
Methods
Isolation of LG Acini.
Male Sprague-Dawley rats (125–150 g) were anesthetized with CO2 for 1 minute and then decapitated. Both LGs were removed. All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use committee. LG acini were prepared as described previously.12 In brief, the pieces of the glands were incubated with collagenase (150 U/mL) in Krebs-Ringer bicarbonate buffer containing 119 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, and 25 mM NaHCO3 supplemented with 10 mM HEPES, 5.5 mM glucose, and 0.5% BSA (KRB-HEPES) for 30 minutes at 37°C. The preparation was filtered through a nylon mesh (150 μm), and the acini was pelleted by centrifugation at 500 g. Acini were washed twice by centrifugation through KRB-HEPES buffer containing 4% BSA. Dispersed acini were allowed to recover for 60 minutes in KRB-HEPES buffer at 37°C.
Western Blot Analysis.
LG acini were incubated for the indicated time with the indicated concentrations of agonists in the presence or absence of 10−3 M dbcAMP. The PKA inhibitor H89 was dissolved in DMSO and was added at the indicated concentrations 10 minutes before the addition of agonists. The concentration of DMSO was the same in all conditions (0.1%). After stimulation, the reaction was terminated by the addition of ice-cold KRB-HEPES buffer without BSA, and acini were centrifuged. RIPA buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 100 mg/mL PMSF, 0.2 U/mL aprotinin, and 1 mM Na3VO3) was added to the pellet, and cells were sonicated. After centrifugation at 10,000g for 30 minutes at 4°C, the proteins in the supernatant were separated by SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were blocked in 5% dried milk in Tris-buffered saline (40 mM Tris-HCl, pH 8, and 150 mM NaCl) with 0.1% Tween-20 (TBST). Activated proteins were detected using antibodies against the phosphorylated pools of enzymes. Antibodies to the total pool of enzymes were also used. The phosphorylated MAPK antibody was used at 1:100 dilution, whereas total MAPK antibody was used at 1:1000 dilution. Phosphorylated and total CREB and the EGF receptor antibodies were used at 1:1000 dilution. Phosphorylated Raf-1 and total Raf-1 were used at 1:2000 dilution and phosphorylated MEK at 1:1000. Antibodies were added either overnight at 4°C or 2 hours at room temperature. After washing with TBST, secondary antibodies were added at 1:1000 (mouse) or 1:2000 (rabbit) for 2 hours at room temperature. Immunoreactive bands were detected by the enhanced chemiluminescence method. Films were scanned and analyzed by ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Values for phosphorylated enzymes were normalized to the amount of total enzyme and were compared with the control value that was set to 1. Ras activity was measured by a pull-down assay according to the manufacturer's instructions (Cytoskeleton, Denver, CO).
Data Presentation and Statistical Analysis
Results are mean ± SEM. Data were analyzed using Student's t-test, and P < 0.05 was considered statistically significantly different.
Results
Effect of PKA on cAMP-Mediated Inhibition of MAPK and CREB Activity
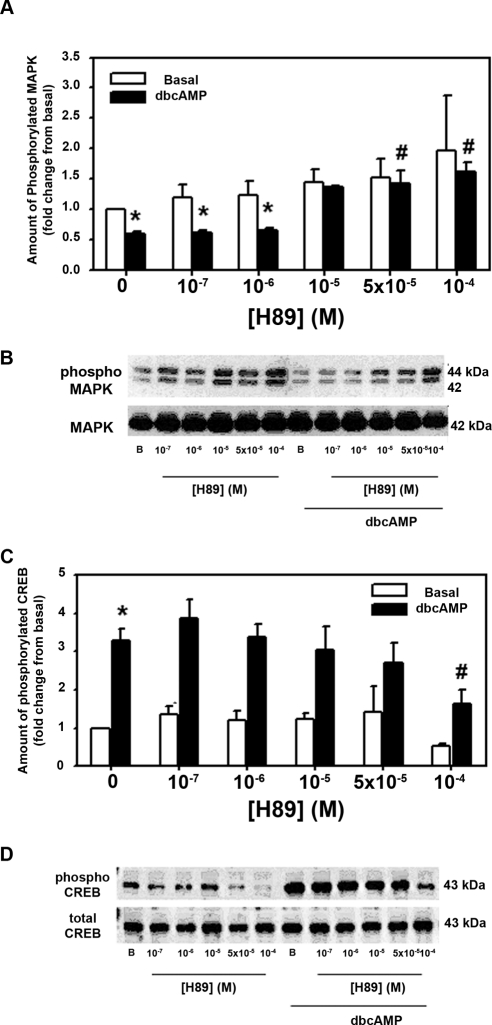
It is well established that cAMP is a ligand for PKA causing the dissociation of the regulatory from the catalytic subunit, allowing the enzyme to phosphorylate the appropriate target enzymes. To determine whether cAMP inhibits basal MAPK activity through the stimulation of PKA, we used H89, a selective inhibitor of PKA. LG acini were preincubated with H89 (10−7–10−4 M) for 10 minutes before 30-minute incubation with 10−3 M dbcAMP. H89 alone had no effect on the basal activity of MAPK that was set to 1. dbcAMP (10−3 M) alone significantly decreased basal MAPK activity to 0.60 ± 0.12 from 1 (Figs. 1A, 1B). In acini treated with dbcAMP and H89 at 10−5 and 10−4 M, MAPK activity was returned to basal levels and was significantly different from what it was in the presence of dbcAMP alone (Fig. 1A). Thus, H89 relieved the inhibition of cAMP on MAPK activity, indicating that the inhibition was mediated through PKA activation.
Figure 1.
Effect of inhibition of protein kinase A on dbcAMP-stimulated decrease in basal MAPK activity and increase in CREB activity. Lacrimal gland acini were incubated with the PKA inhibitor H89 (10−7–10−4 M) for 10 minutes before the addition of dbcAMP (10−3 M) for 30 minutes. Western blot analysis was performed using antibodies against phosphorylated p42/p44 MAPK (A, B) or CREB (C, D). Data are mean ± SEM from five MAPK (A) or five CREB (C) independent experiments. *Significant difference from basal. #Significant difference from dbcAMP alone. Representative blot of MAPK (B) and CREB (D).
It is well established that CREB is phosphorylated by PKA when PKA is activated by cAMP. To ensure that cellular concentration of cAMP was sufficient in cells incubated with dbcAMP to activate PKA and that H89 was inhibiting PKA,20 we determined the effect of H89 on CREB activation. Acini were preincubated with H89 (10−7–10−4 M) for 10 minutes before 30-minute incubation with 10−3 M dbcAMP. H89 alone had no effect on basal activity of CREB except at the highest concentration of H89 (10−4 M), where basal CREB activity was significantly decreased to 0.53 ± 0.07 times basal from no H89 (Figs. 1C, 1D). Addition of 10−3 M dbcAMP significantly increased the amount of phosphorylated CREB (Figs. 1C, 1D) to 3.28 ± 0.31-fold times basal. CREB phosphorylation decreased in a concentration-dependent manner, with H89 10−4 M significantly decreasing it to 1.63 ± 0.38-fold over basal. These data indicate that cAMP concentration within the cell was large enough to activate PKA, which in turn was inhibited, as expected, by H89.
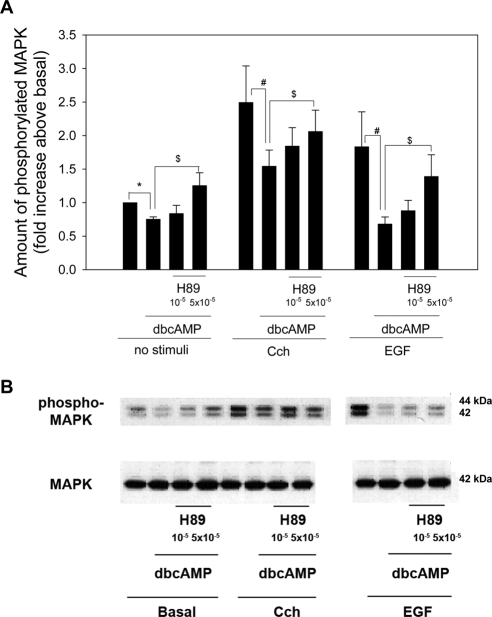
We previously showed that the increase in MAPK activity after stimulation with cholinergic agonists or growth factors was also decreased by cAMP.12 To determine whether the effects of dbcAMP on stimulated MAPK activity is also dependent on PKA, we measured MAPK activity in acini preincubated with H89 (5 × 10−5 and 10−5 M) and dbcAMP (10−3 M) for 30 minutes before stimulation with either the cholinergic agonist carbachol (10−4 M) or EGF (10−7 M) for 5 minutes. In the absence of agonists, dbcAMP significantly decreased MAPK activity to 0.75 ± 0.04 times basal (Figs. 2A, 2B). This was reversed by H89 at both concentrations, and values at 5 × 10−5 M were significantly greater than dbcAMP alone (Fig. 2A). Cch alone increased MAPK activity by 2.4 ± 0.08-fold above basal. Preincubation with dbcAMP significantly decreased Cch-stimulated MAPK activity to 1.54 ± 0.24-fold above basal. This was reversed by a 10-minute incubation of H89 to 1.84 ± 0.27-fold and 2.06 ± 0.31-fold times basal at H89 10−5 M and 5 × 10−5 M, respectively (Fig. 2A). The increase in MAPK activity in the presence of H89 at 5 × 10−5 M was significantly increased above activity in the absence of H89.
Figure 2.
Effect of inhibition of protein kinase A on dbcAMP-stimulated decrease in agonist-stimulated MAPK activity. Lacrimal gland acini were incubated with the PKA inhibitor H89 (10−5 and 5 × 10−5 M) for 10 minutes before the addition of dbcAMP (10−3 M) for 30 minutes. No stimulus, the cholinergic agonist carbachol (Cch 10−4 M), or the growth factor EGF (10−7 M) was then added for 5 minutes. Western blot analysis was performed using an antibody against phosphorylated p42/p44 MAPK. Data are mean ± SEM from three independent experiments (A). *Significant difference from basal. #Significant difference from MAPK activity in the presence of agonist alone. $Significant difference from MAPK activity in the presence of agonist and dbcAMP. Representative blot (B).
With the growth factor EGF, MAPK activity was increased to 1.84 ± 0.52-fold times basal. dcAMP decreased EGF-stimulated MAPK activity to 0.68 ± 0.10-fold times basal. This was reversed by a 10-minute incubation of H89 to 0.88 ± 0.15-fold and 1.39 ± 0.32-fold fold times basal at H89 10−5 M and 5 × 10−5 M, respectively (Figs. 2A, 2B). The increase in MAPK activity in the presence of H89 at 5 × 10−5 M was significantly increased above activity in the absence of H89. These data indicate that the decrease in agonist-stimulated MAPK activity by dcAMP is mediated by PKA.
Effect of Epac Activation on MAPK Activity
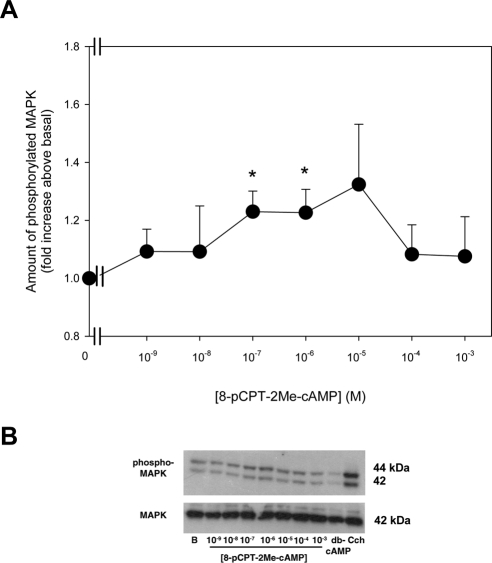
In addition to activating PKA, cAMP can also bind to and activate Epac.21 To determine whether a cAMP-mediated decrease in basal MAPK activity was also mediated by Epac, acini were incubated with the specific Epac activator 8pCPT-2Me cAMP (10−9–10−3 M) for 30 minutes. The basal activity of MAPK was then determined. 8pCPT-2Me cAMP significantly increased MAPK activity at 10−7 and 10−6 M to 1.2 ± 0.07-fold and 1.2 ± 0.08-fold above basal (Figs. 3A, 3B). In the same cells, dbcAMP (10−3 M) significantly decreased MAPK activity to 0.7 ± 0.06-fold above basal (data not shown). These data suggest that dbcAMP activates PKA but not Epac to inhibit MAPK activity in the LG.
Figure 3.
Effect of activation of Epac on MAPK activity. Lacrimal gland acini were incubated with the 8-pCPT-2Me-cAMP, a specific activator of Epac, (10−9–10−3 M) for 30 minutes. Western blot analysis was performed using an antibody against phosphorylated p42/p44 MAPK. Data are mean ± SEM of six independent experiments (A). *Significant difference from basal. Representative blot (B).
Effect of cAMP on Activation of the EGF Receptor
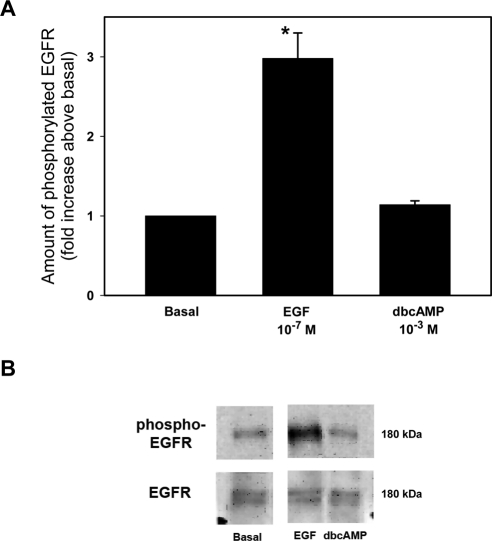
Dimerization of the EGF receptor results in its autophosphorylation causing the recruitment of adaptor proteins, which can lead to the activation of MAPK.22 As we previously showed that cAMP decreased the basal activity of MAPK, we sought to determine whether cAMP had an effect upstream of MAPK (i.e., through activation of the EGF receptor). Acini were incubated for 5 minutes with 10−3 M dbcAMP, a concentration and time that we previously showed inhibited MAPK activity or, as a positive control, EGF (10−7 M) for 5 minutes. The amount of phosphorylated (activated) and total EGF receptor were determined by Western blot analysis. A representative blot is shown in Figure 4B. When three independent experiments were analyzed, EGF significantly increased the amount of phosphorylated EGF receptor by 3.0 ± 0.3-fold above basal. In contrast, dbcAMP did not activate the EGF receptor (Fig. 4A). These data indicate that cAMP does not alter the basal activity of the EGF receptor to inhibit basal activity of MAPK.
Figure 4.
Effect of dibutyryl cAMP on EGF receptor activity. Lacrimal gland acini were incubated with EGF (10−7 M) or dbcAMP (10−3 M) for 5 minutes. Western blot analysis was performed using an antibody against phosphorylated EGF receptor. Data are mean ± SEM from five independent experiments (A). *Significant difference from basal. Representative blot (B).
Effect of cAMP on Basal Ras, Raf-1, MEK, and MAPK Activity
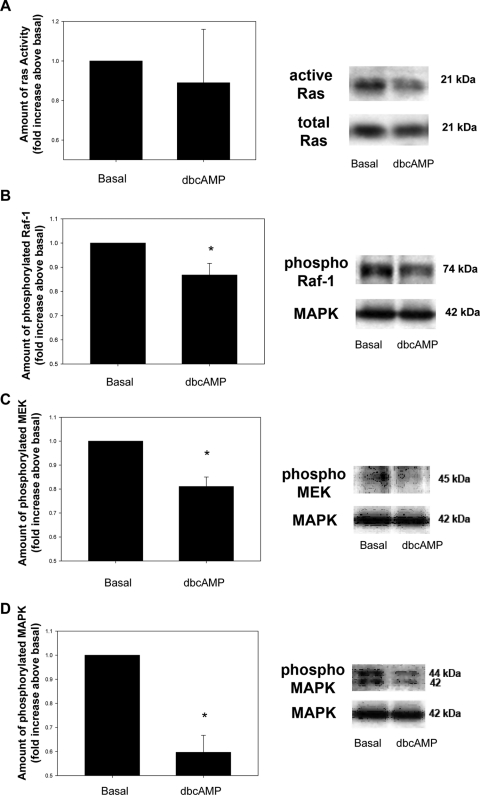
To determine which, if any, kinase in the cascade leading to the activation of MAPK was inhibited by cAMP, acini were incubated with dbcAMP (10−3 M) for 30 minutes, and the amounts of activated Ras, Raf-1, MEK, and MAPK were determined by either pull-down assay followed by Western blot analysis or Western blot analysis with antibodies to the phosphorylated (activated) form of each kinase. Incubation with dbcAMP did not alter the basal amount of activated Ras (Fig. 5A). However, activated Raf-1 significantly decreased in acini treated with dbcAMP to 0.87 ± 0.05-fold of basal (Fig. 5B). In addition, activated MEK was significantly decreased to 0.81 ± 0.04-fold of basal (Fig. 5C). As a control, MAPK activity was also measured and was also significantly decreased compared with basal to 0.6 ± 0.07-fold of basal (Fig. 5D).
Figure 5.
Effect of dbcAMP on basal Ras, Raf-1, MEK, and MAPK activity. Lacrimal gland acini were incubated with dbcAMP (10−3 M) for 30 minutes. Ras activity was measured by a pull-down assay (A). Western blot analysis was performed using antibodies against phosphorylated Raf-1 (B), MEK (C), and MAPK (D). Data are mean ± SEM of six (Ras), nine (Raf-1), or three (MEK and MAPK) independent experiments. *Significant difference from basal. Representative blots (right).
Effect of cAMP on Agonist-Stimulated Raf-1, MEK, and ERK Activity
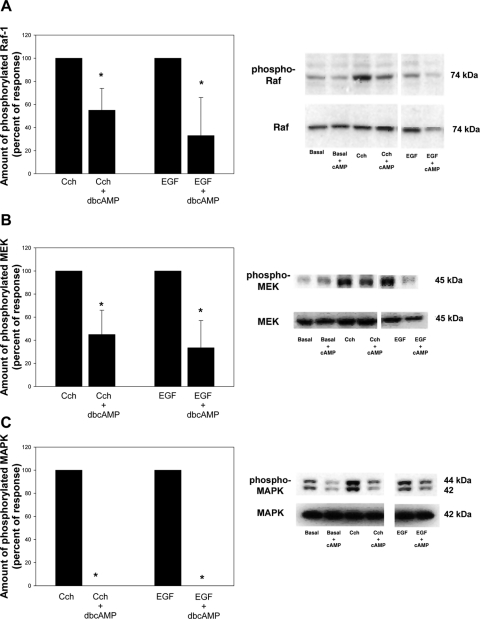
We previously showed that increasing the intracellular cAMP concentration by using either the cAMP analog dbcAMP or activating AC with VIP inhibited MAPK activity stimulated with agonists that increase Ca2+ and activate PKC.12 The agonists used were cholinergic and α1-adrenergic agonists and growth factors. Acini were preincubated with dbcAMP (10−3 M) for 30 minutes before the addition of Cch (10−4 M) or EGF (10−7 M) for 5 minutes. In the absence of dbcAMP, carbachol significantly increased Raf-1 to 2.2 ± 0.6-fold times basal. After preincubation with dbcAMP (10−3 M) for 30 minutes, Cch-stimulated Raf-1 activity was significantly decreased by 45.2% ± 19.3% in cells preincubated with dbcAMP (Fig. 6A). EGF also significantly increased Raf-1 activity to 1.8 ± 0.49-fold times basal. Preincubation with dbcAMP (10−3 M) for 30 minutes significantly altered EGF-stimulated Raf-1 activity by 66.7% ± 33.3% (Fig. 6A).
Figure 6.
Effect of dbcAMP on agonist-stimulated decrease in stimulated Raf-1, MEK, and MAPK activity. Lacrimal gland acini were incubated with dbcAMP (10−3 M) for 30 minutes. The cholinergic agonist carbachol (Cch 10−4 M) or the growth factor EGF (10−7 M) was then added for 5 minutes. Western blot analysis was performed using an antibody against phosphorylated Raf-1 (A), MEK (B), and MAPK (C). Data are mean ± SEM of five independent experiments. *Significant difference from agonist alone. Representative blots (right).
When the same samples were analyzed for MEK activity, basal MEK activity was significantly decreased by dbcAMP to 0.88 ± 0.09-fold times basal (data not shown). Without dbcAMP, Cch significantly increased MEK activity to 1.81 ± 0.48-fold times basal. Incubation with dbcAMP significantly decreased this activity by 65.6% ± 21.5% (Fig. 6B). Similarly, EGF alone significantly increased MEK activity by 1.85 ± 0.77-fold times basal. This was also significantly decreased 66.5% ± 23.6% (Fig. 6B).
These same samples were again analyzed for MAPK activity. Incubation with dbcAMP for 30 minutes significantly decreased basal MAPK activity to 0.69 ± 0.04-fold times basal (data not shown). Cch significantly increased MAPK activity to 1.74 ± 0.21-fold times basal. This was completely inhibited by incubation with dbcAMP (Fig. 6C). EGF significantly increased MAPK activity 1.36 ± 0.12-fold times basal, which was completely inhibited by incubation with cAMP (Fig. 6C).
Effect of Rap-1 on cAMP-Decreased MAPK Activity
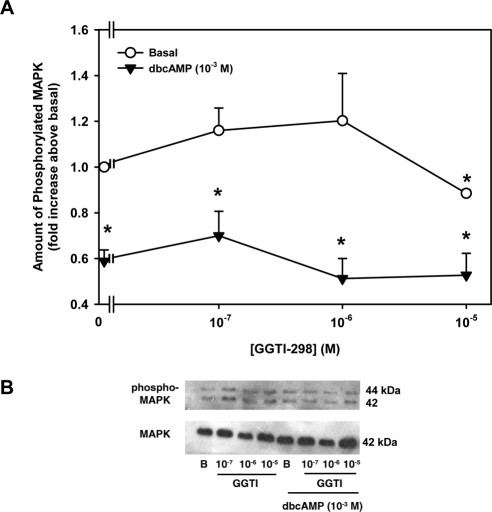
Activation of Rap-1 is known in many cell types to lead to activation of the Ras/MEK/MAPK pathway.23 To determine whether PKA interacts with Rap-1, acini were incubated with increasing concentrations of the Rap-1 inhibitor GGTI-298 and with or without 10−3 M dbcAMP. The amount of MAPK activity was then measured by Western blot analysis. GGTI-298 alone had no significant effect on basal MAPK activity except at the highest concentration (10−5 M; Fig. 7A). In the absence of GGTI-298, dbcAMP significantly decreased MAPK activity to 0.59 ± 0.05-fold times basal (Figs. 7A, 7B). In the presence of dbcAMP, GGTI-298 did not reverse the inhibition of MAPK activity caused by dbcAMP. These data indicate that PKA does not interact with Rap-1 to inhibit MAPK.
Figure 7.
Effect of inhibition of Rap-1 on dbcAMP-stimulated decrease in basal MAPK activity. Lacrimal gland acini were incubated with the Rap-1 inhibitor GGTI-298 (10−7–10−5 M) for 10 minutes before the addition of dbcAMP (10−3 M) for 30 minutes. Western blot analysis was performed using antibodies against phosphorylated p42/p44 MAPK (A). Data are mean ± SEM of four independent experiments. *Significant difference from basal. Representative blot of MAPK (B).
Discussion
We previously showed that cAMP decreased basal and stimulated MAPK activity in rat LG.12 In this study we demonstrate that cAMP activates PKA, but not Epac, to inhibit both basal and stimulated MAPK. Further investigation into the pathway that activates MAPK shows that cAMP does not affect Ras activity but does inhibit basal- and agonist-stimulated Raf-1 and MEK activity (Fig. 8).
Figure 8.
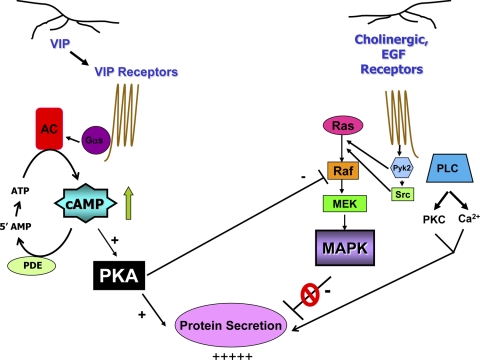
Schematic diagram of the mechanism of cAMP inhibition of MAPK activity. Upon stimulation in lacrimal gland acini, both VIP and the cholinergic agonist acetylcholine are released from parasympathetic nerves, which bind to their receptors and stimulate secretion by increasing [Ca2+]i and activity of PKC. The VIP receptor activates adenylate cyclase (AC) through the G protein Gαs to increase intracellular [cAMP]. cAMP, in turn, stimulates PKA activity, which leads to protein secretion. cAMP is quickly broken down to AMP by phosphodiesterase (PDE). After binding to their receptors, cholinergic agonists activate the tyrosine kinases Pyk2 and Src to stimulate the kinase cascade of Ras, Raf-1, MEK, and MAPK. EGF also activates this cascade in a similar manner. Activation of MAPK attenuates protein secretion. In addition to stimulating protein secretion, PKA inhibits Raf-1 activity, which, in turn, decreases the activity of MEK and MAPK, relieving the inhibition of secretion by MAPK and thereby potentiating secretion.
The role of MAPK in LG protein secretion is dependent on the signaling pathway activated. In cholinergic and EGF-stimulated pathways, MAPK is activated. Interestingly this activation attenuates protein secretion such that protein secretion is increased if MAPK is inhibited. In contrast, increasing cAMP concentrations through the use of cell-permeable cAMP analogs, inhibiting phosphodiesterases that break down cAMP, or increasing cAMP concentrations through agonists such as VIP that increase intracellular cAMP levels decreases MAPK activity. It has been known for many years that simultaneous addition of a cAMP-dependent stimulus with a Ca2+/PKC-dependent pathway or a growth factor-stimulated pathway results in the potentiation of secretion in the LG. It has been demonstrated that the potentiation did not occur at the level of the receptors, production of cAMP, or increase in intracellular [Ca2+].11–13 We recently identified the possible mechanism of potentiation, which is cAMP-mediated inhibition of MAPK activity,12 and showed that this occurs through the activation of PKA, Raf-1, and MEK1/2 in a Ras-independent manner.
The effects of cAMP on MAPK activity is cell dependent.23 cAMP inhibits cell proliferation in fibroblasts and smooth muscle cells while stimulating proliferation in Swiss 3T3 and thyrocytes.23,24 In addition, cAMP can have opposing effects in the same cell type. Parathyroid hormone-related protein, which is known to work through PKA, causes the inhibition of MAPK in differentiated, but not undifferentiated, MC4 cells.25 Our laboratory has now shown that the LG is the only tissue studied to date in which MAPK inhibits protein secretion.12 Relieving this inhibition results in an increase in secretion.
The MAPK and cAMP pathways are two pathways in which multiple potential cross-talk events can occur. One such method is through the activation of Raf-1. Raf-1 is regulated by a variety of proteins, including kinases, phosphatases, and scaffolding proteins.23,26 In Swiss 3T3 cells and thyrocytes, it was shown that PKA can block EGF-stimulated MAPK activation by inhibiting Raf-1 phosphorylation on Ser338. This phosphorylation is at least partially a result of a direct interaction between PKA and Raf-1. It is also involves p21- activated kinase (PAK). PKA phosphorylates and inhibits PAK, and PAK phosphorylates Raf-1 on Ser338.26 In this study, we used an antibody directed against Raf-1 when it was phosphorylated on Ser338 but did not study the role of PAK in the LG. It has also been reported that PKA can either directly or through AKT phosphorylate Raf-1, leading to its inability to bind to Ras.27 In the present study, PKA blocked basal- and agonist-stimulated MAPK activity by inhibiting Raf-1, though we did not investigate the mechanism by which Raf-1 activity was decreased.
The effects of cAMP are controlled by anchoring complexes known as A-kinase-anchoring proteins (AKAPs).28 These complexes permit the compartmentalization of receptors, cAMP, phosphodiesterases, substrates, and PKA to allow for specificity and termination of the reaction.28 Dodge-Kafka et al.29 demonstrated that muscle AKAP (mAKAP) complexes PKA, Epac, ERK5, and phosphodiesterase 4D3. ERK5 phosphorylates PDE4D3 to decrease its activity, resulting in an increase in local cAMP concentrations. This increase in cAMP stimulates PKA to activate Epac. PKA also phosphorylates PDE4D3 to increase its activity and activates ERK5.29 It is likely that a similar situation exists in the LG, where an AKAP complexes PKA, Raf-1, MEK, and MAPK but not Epac.
In addition to PKA, cAMP can also activate Epac.21 The involvement of Epac in activating MAPK appears to be cell specific. 8-pCPT-2OMe-cAMP, the Epac-specific cAMP isoform, has been shown to increase the phosphorylation of MAPK, leading to cell proliferation in a Ras-independent but Rap-1–dependent manner in human umbilical vein endothelial and human melanoma cells.30,31 In contrast, this same agonist has no effect on MAPK phosphorylation in Chinese hamster ovary, HEK293T, or PC12 cells.32 In the LG, Epac does not appear to play a role in MAPK attenuation of protein secretion.
We conclude that cAMP-mediated inhibition of MAPK occurs through the activation of PKA, but not Epac. PKA interacts with Raf-1 to decrease its activity and subsequent MEK and MAPK activity. This mechanism occurs under both basal and conditions in which the cells are stimulated with cholinergic agonists and growth factors.
Footnotes
Supported by National Institutes of Health Grant EY06177.
Disclosure: C. Funaki, None; R.R. Hodges, None; D.A. Dartt, None
References
- 1. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lemullois M, Rossignol B, Mauduit P. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86(2–3):175–181 [DOI] [PubMed] [Google Scholar]
- 3. Dartt DA, Dicker DM, Ronco LV, Kjeldsen IM, Hodges RR, Murphy SA. Lacrimal gland inositol trisphosphate isomer and inositol tetrakisphosphate production. Am J Physiol. 1990;259(2 pt 1):G274–G281 [DOI] [PubMed] [Google Scholar]
- 4. Zoukhri D, Hodges RR, Willert S, Dartt DA. Immunolocalization of lacrimal gland PKC isoforms: effect of phorbol esters and cholinergic agonists on their cellular distribution. J Membr Biol. 1997;157(2):169–175 [DOI] [PubMed] [Google Scholar]
- 5. Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272(1 pt 1):C263–C269 [DOI] [PubMed] [Google Scholar]
- 6. Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997;38(3):610–619 [PubMed] [Google Scholar]
- 7. Tepavcevic V, Hodges RR, Zoukhri D, Dartt DA. Signal transduction pathways used by EGF to stimulate protein secretion in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2003;44(3):1075–1081 [DOI] [PubMed] [Google Scholar]
- 8. Schmidt R, Baumann O, Walz B. cAMP potentiates InsP3-induced Ca2+ release from the endoplasmic reticulum in blowfly salivary glands. BMC Physiol. 2008;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu M, Simon MI. Regulation by cAMP-dependent protein kinase of a G-protein-mediated phospholipase C. Nature. 1996;382(6586): 83–87 [DOI] [PubMed] [Google Scholar]
- 10. Romano D, Magalon K, Ciampini A, Talet C, Enjalbert A, Gerard C. Differential involvement of the Ras and Rap1 small GTPases in vasoactive intestinal and pituitary adenylyl cyclase activating polypeptides control of the prolactin gene. J Biol Chem. 2003;278(51):51386–51394 [DOI] [PubMed] [Google Scholar]
- 11. Dartt DA, Baker AK, Vaillant C, Rose PE. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am J Physiol. 1984;247(5 pt 1):G502–G509 [DOI] [PubMed] [Google Scholar]
- 12. Funaki C, Hodges RR, Dartt DA. Role of cAMP inhibition of p44/p42 mitogen-activated protein kinase in potentiation of protein secretion in rat lacrimal gland. Am J Physiol Cell Physiol. 2007;293(5):C1551–C1560 [DOI] [PubMed] [Google Scholar]
- 13. Dartt DA, Baker AK, Rose PE, Murphy SA, Ronco LV, Unser MF. Role of cyclic AMP and Ca2+ in potentiation of rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 1988;29(11):1732–1738 [PubMed] [Google Scholar]
- 14. Dartt DA, Ronco LV, Murphy SA, Unser MF. Effect of phorbol esters on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 1988;29(11):1726–1731 [PubMed] [Google Scholar]
- 15. Ota I, Zoukhri D, Hodges RR, et al. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol. 2003;284(1):C168–C178 [DOI] [PubMed] [Google Scholar]
- 16. Hodges RR, Rios JD, Vrouvlianis J, Ota I, Zoukhri D, Dartt DA. Roles of protein kinase C, Ca2+, Pyk2, and c-Src in agonist activation of rat lacrimal gland p42/p44 MAPK. Invest Ophthalmol Vis Sci. 2006;47(8):3352–3359 [DOI] [PubMed] [Google Scholar]
- 17. Stork PJ. Does Rap1 deserve a bad Rap? Trends Biochem Sci. 2003;28(5):267–275 [DOI] [PubMed] [Google Scholar]
- 18. Nagao S, Yamaguchi T, Kusaka M, et al. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;63(2):427–437 [DOI] [PubMed] [Google Scholar]
- 19. Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17(2):123–128 [DOI] [PubMed] [Google Scholar]
- 20. Hagiwara M, Alberts A, Brindle P, et al. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70(1):105–113 [DOI] [PubMed] [Google Scholar]
- 21. Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158(1):70–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dartt DA. Interaction of EGF family growth factors and neurotransmitters in regulating lacrimal gland secretion. Exp Eye Res. 2004;78(3):337–345 [DOI] [PubMed] [Google Scholar]
- 23. Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell Signal. 2008;20(9):1592–1607 [DOI] [PubMed] [Google Scholar]
- 24. Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262(5136):1069–1072 [DOI] [PubMed] [Google Scholar]
- 25. Chen C, Koh AJ, Datta NS, et al. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem. 2004;279(28):29121–29129 [DOI] [PubMed] [Google Scholar]
- 26. Edin ML, Juliano RL. Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol Cell Biol. 2005;25(11):4466–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12(6):258–266 [DOI] [PubMed] [Google Scholar]
- 28. Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007;35(pt 5):931–937 [DOI] [PubMed] [Google Scholar]
- 29. Dodge-Kafka KL, Soughayer J, Pare GC, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437(7058):574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang Y, Olah ME. Cyclic AMP-dependent, protein kinase A-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: role of exchange protein activated by cAMP 1 (Epac1). J Pharmacol Exp Ther. 2007;322(3):1189–2000 [DOI] [PubMed] [Google Scholar]
- 31. Gao L, Feng Y, Bowers R, et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66(16):7880–7888 [DOI] [PubMed] [Google Scholar]
- 32. Enserink JM, Christensen AE, de Rooij J, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4(11):901–906 [DOI] [PubMed] [Google Scholar]