Immune responses are commonly accompanied by localized changes in cytokine expression. To investigate possible immune activation associated with glaucoma, concentrations of multiple cytokines in the aqueous humor of glaucoma patients were determined by microparticle-based immunoassays. An inflammatory cytokine, interleukin-8, was found to be elevated in glaucomatous aqueous humor, consistent with immune activation.
Abstract
Purpose.
To test the hypothesis that immune activation occurs in glaucoma by comparing concentrations of multiple cytokines in aqueous humor (AH) from patients with primary open angle glaucoma (POAG) and from cataract patients without glaucoma as controls.
Methods.
Cytokine concentrations in AH obtained during surgery were measured using microparticle-based immunoassays. Localized expression of IL-8 protein was investigated by immunohistochemistry of human eyes.
Results.
Eight cytokines (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12, IFN-γ, and TNF-α) were below the limits of detection, and two cytokines (IL-18 and IL-15) were detected at low levels or in only a few patients. Although IL-6 was detected in 26 of 30 control patients (median, 2.7 pg/mL) and in 23 of 29 POAG patients (median, 1.6 pg/mL), the difference was not statistically significant. IL-8 was detected in 28 of 30 control patients (median, 1.8 pg/mL) and in all 29 POAG patients (median, 4.9 pg/mL). The higher IL-8 concentration in the AH of POAG patients was statistically significant (P < 0.001). In pairs of eyes from patients with asymmetric glaucomatous optic nerve damage, IL-8 concentration was higher in the AH of the more severely affected eye (P < 0.05). Patients with severe visual field defects had higher IL-8 concentrations in the AH than did patients with mild visual field defects. IL-8 protein expression was found in human retina and optic nerve.
Conclusions.
Concentration of the inflammatory cytokine IL-8 is significantly elevated in the AH of POAG patients, supporting the hypothesis that immune activation occurs in glaucoma.
POAG is a major public health problem, causing vision loss and blindness because of the death of retinal ganglion cells (RGCs); elevated intraocular pressure (IOP) is a major risk factor. Despite decades of research, the mechanisms leading to RGC death are not fully understood. Involvement of the immune system in the pathogenesis of glaucoma has been investigated in multiple recent studies.1–3 Evidence of immune responses associated with glaucoma includes increased autoantibodies in glaucoma patients,4 altered populations of T cells,5 glial cell activation in the optic nerve and retina,6 and activation of antigen presentation, seen as increased expression of major histocompatibility complex class II by lamina cribrosa astrocytes.7 Recently, the immune effector molecule complement factor C1q has been implicated in neuronal death in the DBA/2J mouse glaucoma model8 and has been shown to have increased expression in the glaucomatous retina.9 Increased synthesis and deposition of complement factors C1q and C3 within the retina have been shown to be induced by elevated IOP.10
Cytokines are secreted proteins that play central roles in modulating immunity, but they can also perform nonimmune functions in areas such as angiogenesis and development.11 Immune activation events such as autoimmune reactions, activation of antigen presentation, and neuroinflammation are caused by and result in localized changes in cytokine expression. In general, cytokines act in a paracrine or juxtacrine fashion, close to the sites of active immune responses. If immune activation is associated with glaucoma, changes in cytokine secretion within the eye might be detectable as changes in the concentration of cytokines in the aqueous humor (AH) of glaucoma patients.
Multiplex microparticle-based immunoassays,12 which allow for the detection of multiple cytokines in small volume clinical samples, have been used to determine cytokine expression profiles in the AH of patients who have experienced uveitis13–15 and corneal transplant rejection.16 To test for cytokine expression changes indicative of an immune response, we used this technique to measure multiple cytokines in the AH of POAG patients and patients without glaucoma as controls. We found significantly higher levels of one inflammatory cytokine, IL-8, in the AH of POAG patients. Our results are consistent with a neuroinflammatory process occurring in glaucoma, similar to other age-related chronic neurodegenerative diseases.
Patients and Methods
Patient Selection
All human samples were obtained with informed consent in adherence to the Declaration of Helsinki, under approval of the Institutional Review Board at Vanderbilt University. The optic nerve appearance of all cataract and glaucoma patients was evaluated through dilated pupils by the same glaucoma specialist (RWK) at Vanderbilt Eye Institute using a stereo fundus lens (Volk Super 66; Volk Opticals, Mentor, OH) and a calibrated slit lamp biomicroscope (Haag-Streit 900; Haag-Streit, Bern-Köniz, Switzerland). POAG diagnosis was based on an optic nerve appearance characteristic of glaucomatous damage, such as documented enlargement of cupping and focal thinning of the neuroretinal rim, and was supported by ancillary tests such as the Humphrey Visual Field (HVF) test (Carl Zeiss Meditec, Dublin, CA), optical coherence tomography (Stratus OCT; Carl Zeiss Meditec) measurement of retinal nerve fiber layer thickness, and stereoscopic optic nerve photographs (FF450plus; Carl Zeiss Meditec). All glaucoma patients had open iridocorneal angles based on gonioscopy examination. IOP was not used as a diagnostic criterion for glaucoma, although most glaucoma patients had a documented history of IOP >21 mm Hg before the initiation of antiglaucoma treatment. All possible secondary etiologies were ruled out except in two patients with exfoliation glaucoma. Cataract patients served as the control group and were free of glaucoma based on IOP <21 mm Hg and healthy appearance of the optic nerve. None of the participants had clinically detectable ocular inflammation, infection, diabetic retinopathy, or age-related macular degeneration. None of the patients in either group had previous intraocular surgeries within 1 year or laser procedures within 6 months before AH collection.
AH Collection
AH samples (50–100 μL) were collected from 89 patients by a single surgeon (RWK) at the beginning of surgical procedures through limbal paracentesis using a 30-gauge needle attached to a TB syringe. Surgeries were performed between 8:00 AM and 2:00 PM. Care was taken to avoid forceful aspiration or touching of the iris or limbal blood vessels. AH samples were immediately frozen and stored at −80°C. Control patients underwent cataract surgery, which included treatment with standard mydriatic drops (1% cyclopentolate, 1% tropicamide, and 2.5% phenylephrine) 30 minutes before surgery. Glaucoma patients underwent glaucoma surgery (trabeculectomy, mini-glaucoma shunt [ExPress; Optonol Ltd., Neve Ilan, Israel], or glaucoma drainage device implantation), cataract surgery, or combined glaucoma and cataract surgery. If a glaucoma patient underwent combined glaucoma and cataract surgery, cataract extraction was always performed first. Glaucoma patients undergoing cataract surgery alone or cataract surgery combined with glaucoma surgery were treated with mydriatic eye drops, as were control patients. Glaucoma patients undergoing trabeculectomy only received miotic drops (1% pilocarpine) 30 minutes before surgery. Glaucoma patients undergoing mini-glaucoma shunt (ExPress; Optonol Ltd.) or glaucoma drainage device implantation did not receive mydriatic or miotic drops preoperatively. All procedures were performed under topical anesthesia with no retrobulbar block. Glaucoma patients did not discontinue their use of antiglaucoma medications in preparation for surgery. For patients from whom AH samples from both eyes were obtained, cytokine data from the left eye were used in Figures 1 and 2, and data from both eyes were included in Figure 3.
Figure 1.
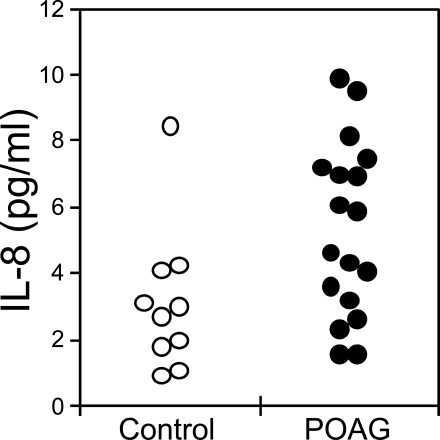
Concentrations of IL-6 (A) and IL-8 (B) in AH of patients without glaucoma (control, open circles, n = 30) and with POAG (POAG, closed circles, n = 29). Median concentrations for IL-6: control, 2.7 pg/mL; POAG, 1.6 pg/mL. Median concentrations for IL-8: control, 1.8 pg/mL; POAG, 4.9 pg/mL. IL-8 is significantly higher in POAG than in control patients (P < 0.001). Samples with cytokine concentration below the detection limit (0.5 pg/mL for IL-6 and 0.6 pg/mL for IL-8) are plotted on the bottom axis (0 pg/mL). Two control samples with IL-6 concentrations of 82 and 186 pg/mL are plotted on the top axis (>20 pg/mL).
Figure 2.
Concentrations of IL-8 in AH of patients without glaucoma (control, open circles, n = 10) and with POAG (POAG, closed circles, n = 18) undergoing cataract surgery. Median concentrations: control, 2.9 pg/mL; POAG, 5.2 pg/mL. IL-8 is significantly higher in POAG than in control patients (P < 0.05).
Figure 3.
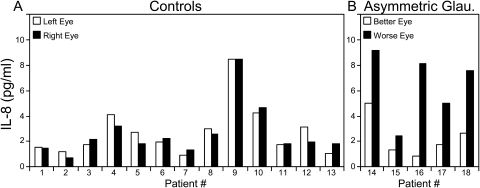
IL-8 in AH from pairs of eyes of individual patients. IL-8 concentrations from the left eye versus the right eye are shown for individual control patients (A) and from the less severely affected eye (better eye) versus the more severely affected eye (worse eye) for individual glaucoma patients with clearly defined asymmetric glaucoma (B). Patients 14, 15, and 16 have POAG, and patients 17 and 18 have exfoliation glaucoma (B). The absolute value of the difference in IL-8 concentration (4.1 ± 2.23 pg/mL [mean ± SD]) for pairs of asymmetric glaucoma eyes was significantly greater than for control pairs (0.5 ± 0.36 pg/mL; P < 0.01).
Multiplex Cytokine Measurement
A microparticle (bead)-based multiplex cytokine assay12,17 (Bio-Plex Cytokine Assay; Bio-Rad Laboratories, Hercules, CA) was used to simultaneously measure multiple cytokines in AH, after recommended protocols from the manufacturer. AH samples were diluted 1:4, and serial 1:4 dilutions of cytokine standards were prepared using manufacturer-supplied diluent (Bio-Plex Human Serum Diluent; Bio-Rad). Quadruplicate samples for which sample diluent was substituted for AH were used to determine background fluorescence. A vacuum filtration system was used for all wash steps. In brief, cytokine standards or diluted AH samples were added to wells of a 96-well plate containing cytokine detection beads, which were coated with anti–cytokine antibodies, and incubated for 30 minutes. All incubations were carried out at room temperature with the 96-well plate sealed and placed on an orbital shaker (300 rpm or otherwise, as noted). After incubation, the plate was washed, secondary antibody was added, and the plate was incubated for 30 minutes. The plate was then washed, streptavidin-phycoerythrin detection reagent was added, and the plate was incubated for 10 minutes. The beads were then washed, resuspended in 125 μL wash buffer, and shaken for 30 seconds at 1100 rpm. The plate was read by a flow cytometry-based instrument (Bio-Plex Array Reader; Bio-Rad) that uses Luminex technology (Luminex Corporation, Austin, TX). Mean fluorescence intensity for each well was determined as the mean fluorescence of 200 beads for each cytokine. The lower limit of detection was defined by the lowest standard with fluorescence greater than background fluorescence +2 SD of the background fluorescence. Analysis software (Bio-Plex Manager 4.1; Bio-Rad) converted fluorescence readings to cytokine concentration by use of a calibration curve derived from a five-parameter logistic fit18 of fluorescence readings of the cytokine standards. No concentrations were extrapolated beyond the lower or upper range of the standard curve. Cross-reactivities of the IL-6 and IL-8 assays as determined by the manufacturer were less than 2%.
Immunohistochemistry of Human Eyes
Human cadaver eyes, free from glaucoma or other eye diseases, were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA) and were received in moist chambers within 48 hours of death. Ages of the seven female and seven male donors ranged from 58 to 93 years (75 ± 10.5 years [mean ± SD]). Whole eyes were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm–thick sections. Sections were placed on glass slides, deparaffinized, and rehydrated through graded alcohols. After treatment with 3% hydrogen peroxide and blocking with 5% normal rabbit serum (Jackson ImmunoResearch, West Grove, PA), sections were incubated overnight at 4°C with either goat anti–human IL-8 antibody (R&D Systems, Minneapolis, MN) or purified goat IgG (R&D Systems). Immunodetection was performed with biotinylated rabbit anti–goat secondary antibody and peroxidase-labeled streptavidin (Jackson ImmunoResearch), with red/brown chromogen (Nova Red; Vector Laboratories, Burlingame, CA). The slides were then counterstained in hematoxylin. Phase-contrast microscopy was performed with a microscope (model AX70; Olympus, Center Valley, PA) and a digital camera controlled with accompanying software (SPOT; Diagnostic Instruments, Sterling Heights, MD).
Statistical Analysis
To assess statistical significance, two-tailed P values were calculated by the nonparametric Mann-Whitney test using the VassarStats software package freely available on the internet (http://faculty.vassar.edu/lowry/VassarStats.html). When 12 cytokines were determined simultaneously, correction for multiple testing using the Bonferroni adjustment for 12 parameters was applied, and results with P < 0.0042 were considered significant. Results with P < 0.05 were considered significant for single-cytokine measurements.
Results
Measurement of Multiple Cytokines in AH
The concentrations of 12 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12(p70), IL-15, IL-18, IFN-γ, and TNF-α) were simultaneously determined in AH samples using a microparticle-based cytokine assay. To investigate possible abnormal immunity in glaucoma, comparisons of the concentrations of cytokines were made between AH samples of single eyes from 30 control patients undergoing cataract surgery and 29 POAG patients undergoing glaucoma surgery. The patient groups were well matched for race and sex, with no significant difference in age (Table 1). The concentrations of eight cytokines (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12(p70), IFN-γ, and TNF-α) were below the limits of detection (Table 2). Two other cytokines were detected at low levels or in only a few patients. IL-18 was detected near the lower limit of detection (0.8 pg/mL) in two control and five POAG patients (range, 0.9–1.5 pg/mL and 0.8–1.6 pg/mL, respectively). One POAG patient had an IL-15 concentration of 9.1 pg/mL above the lower limit of detection of 8.1 pg/mL.
Table 1.
Patient Demographics
| Figure 1 |
Figure 2 |
|||
|---|---|---|---|---|
| Control (n = 30) | POAG (n = 29) | Control (n = 10) | POAG (n = 18) | |
| Age in years, mean ± SD | 68.0 ± 7.3 | 71.3 ± 9.9 | 66.7 ± 4.79 | 67.9 ± 9.17 |
| Female, % | 66.7 | 69.0 | 90.0 | 50.0 |
| Male, % | 33.3 | 31.0 | 10.0 | 50.0 |
| African American, % | 20.0 | 17.2 | 10.0 | 11.1 |
| Asian, % | 6.7 | 3.4 | 0.0 | 0.0 |
| Caucasian, % | 70.0 | 79.3 | 80.0 | 83.3 |
| Hispanic, % | 3.3 | 0.0 | 10 | 5.6 |
Table 2.
Lower Limits of Detection for Cytokines Not Detected in AH of POAG and Control Patients
| Cytokine | Detection Limit (pg/mL) |
|---|---|
| IL-1β | 0.7 |
| IL-2 | 2.0 |
| IL-4 | 0.1 |
| IL-5 | 0.8 |
| IL-10 | 2.1 |
| IL-12 (p70) | 0.7 |
| IFN-γ | 7.8 |
| TNF-α | 1.5 |
Control, n = 30 patients; POAG, n = 29 patients.
IL-6 was detected in 26 of 30 control patients and in 23 of 29 POAG patients (Fig. 1A). The lower limit of detection for IL-6 was 0.5 pg/mL. The median IL-6 concentration was 2.7 pg/mL for control and 1.6 pg/mL for POAG. Concentrations >8 pg/mL were detected in 10 of 30 control patients compared with only 1 of 29 POAG patients. Although the data suggest a possible trend toward lower IL-6 in glaucomatous AH, the difference was not statistically significant after correction for multiple testing using Bonferroni adjustment.
IL-8 was detected in the AH of 28 of 30 control patients and in all 29 POAG patients (Fig. 1B). The lower limit of detection for IL-8 was 0.6 pg/mL. The median IL-8 concentration in POAG eyes of 4.9 pg/mL was statistically greater than that in control eyes of 1.8 pg/mL (P < 0.001).
IL-8 Concentration in AH of POAG and Control Patients Undergoing Cataract Surgery
The initial study of multiple cytokines suggested that IL-8 is elevated in the AH of POAG patients undergoing glaucoma surgery compared with control patients undergoing cataract extraction. Approximately 30 minutes before cataract surgery, patients received mydriatic drops (1% cyclopentolate, 1% tropicamide, and 2.5% phenylephrine), whereas patients undergoing glaucoma surgery without cataract extraction did not. To control for the possible effects of mydriatic drops on cytokine concentrations, we compared another set of 10 eyes from 10 cataract patients with 18 eyes from 18 POAG patients undergoing cataract surgery at the time of AH collection, either alone or in combination with a glaucoma procedure. There was no significant difference in age between the two groups (Table 1; Fig. 2). The microparticle-based cytokine assay was used for the detection of IL-8 in the AH of only these patients. Similar to the previous results, IL-8 was higher in the AH of POAG patients (Fig. 2). The median IL-8 concentration was 2.9 pg/mL for control and 5.2 pg/mL for POAG. The increased IL-8 concentration in the AH of POAG patients was statistically significant (P < 0.05). Given that this set of POAG patients received presurgical mydriatic drops identical to those of the control group, these results support the contention that elevated IL-8 concentrations in the AH of POAG patients are not related to differences in preoperative pharmacologic treatment.
IL-8 Concentration in AH of Patients with Asymmetric Glaucomatous Optic Nerve Damage
An important difference between the control and POAG groups was that POAG patients were treated with topical antiglaucoma medications but control patients were not. To control for possible effects of medication on cytokines in AH, we compared IL-8 concentrations in pairs of eyes from glaucoma patients for whom one eye had more severe optic nerve damage than the fellow eye, but both eyes received identical antiglaucoma medications.
Patients with asymmetric glaucoma were selected using strict criteria based on optic nerve appearance and reliable HVF testing. Differences between pairs of eyes with optic nerve cup-to-disc ratios >0.2 were documented by optic nerve photographs viewed with a stereoscope. Asymmetry was also confirmed by OCT measurements of retinal nerve fiber layer thickness. In addition, patients with asymmetric glaucoma had to have mild to severe reliable HVF defects consistent with optic nerve appearance for the worse eye and entirely normal results for the better eye using the 24–2 Swedish Interactive Threshold Algorithm of the Humphrey Field Analyzer II 750. Because only three POAG patients met the criteria for asymmetric glaucoma, two additional patients with exfoliation glaucoma who met the asymmetry criterion were included in the analysis (Table 3).
Table 3.
Comparison of Visual Field Testing Results between Both Eyes of Five Glaucoma Patients with Asymmetric Optic Nerve Damage
| Patient 14 POAG |
Patient 15 POAG |
Patient 16 POAG |
Patient 17 Exfoliation Glaucoma |
Patient 18 Exfoliation Glaucoma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Worse | Better | Worse | Better | Worse | Better | Worse | Better | Worse | Better | |
| FL | 2/14 | 1/14 | 1/16 | 0/14 | 2/16 | 5/15 | 1/17 | 0/14 | 7/16 | 5/14 |
| FPE, % | 10 | 8 | 1 | 1 | 0 | 0 | 2 | 5 | 5 | 0 |
| FNE, % | 2 | 0 | 8 | 0 | 0 | 0 | 0 | 9 | 26 | 0 |
| MD (%) | −2.53 (<5) | −0.16 | −7.16 (<0.5) | −0.72 | −17.09 (<0.5) | 0.59 | −7.93 (<0.5) | −0.32 | −18.97 (<0.5) | 1.51 |
| PSD (%) | 2.58 (<2) | 1.75 | 8.53 (<0.5) | 1.30 | 13.88 (<0.5) | 1.67 | 10.02 (<0.5) | 2.10 (<5) | 10.8 (<0.5) | 2.08 |
FL, fixation loss; FPE, false-positive error; FNE, false-negative error; MD, mean deviation; PSD, pattern standard deviation.
The microparticle-based cytokine assay was used to detect only IL-8 in the AH of the five pairs of eyes with asymmetric glaucoma and 13 pairs of eyes of control patients. In the 13 control patients, the IL-8 concentration was similar in each eye (Fig. 3A), with left eyes not significantly different from right eyes (P = 0.96). The absolute value of the difference in IL-8 concentration between pairs of control eyes was 0.5 ± 0.36 pg/mL (mean ± SD). As shown in Figure 3B, IL-8 concentration was higher in the more severely affected eyes of the three patients with asymmetric POAG (patients 14, 15, and 16) and the two patients with asymmetric exfoliation glaucoma (patients 17 and 18). In patients with asymmetric glaucoma, the more severely affected eye had a significantly higher IL-8 concentration than did the less severely affected fellow eye (P < 0.05). The absolute value of the difference in IL-8 concentration was 4.1 ± 2.23 pg/mL (mean ± SD) for pairs of asymmetric glaucoma eyes and was significantly greater than for control pairs (P < 0.01). Because both eyes of each glaucoma patient were treated with identical antiglaucoma medication before AH collection, the higher IL-8 concentrations in the more severely affected eyes were not artifacts of medication and were likely related to glaucoma severity.
Correlation of IL-8 with Severity of Visual Field Defect
To further investigate the correlation between glaucoma severity and IL-8, we compared all POAG patients with mild visual field defects (mean deviation no worse than −6 dB; n = 15) with all those with severe visual field defects (mean deviation worse than −12 dB; n = 15),19 as demonstrated by reliable HVF test results. As can be seen in Figure 4, patients with severe visual field defects had higher AH concentrations of IL-8 than did those with mild visual field defects (median, 7.3 vs. 4.7 pg/mL; P < 0.05), supporting a correlation between elevation of IL-8 and severity of POAG.
Figure 4.
IL-8 in AH of POAG patients with mild versus severe visual field defects. Concentrations of IL-8 in AH of patients with reliable HVF test results showing either mild (mild, open circles, n = 15) or severe (severe, closed circles, n = 15) visual field defects were compared. Median concentrations: mild, 4.7 pg/mL; severe, 7.3 pg/mL. IL-8 was significantly higher in patients with severe visual field defects than in patients with mild visual field defects (P < 0.05). Visual field defects were defined as mild for patients with mean deviations no worse than −6 dB and severe for mean deviations worse than −12 dB.
IL-8 Expression in Ocular Tissues
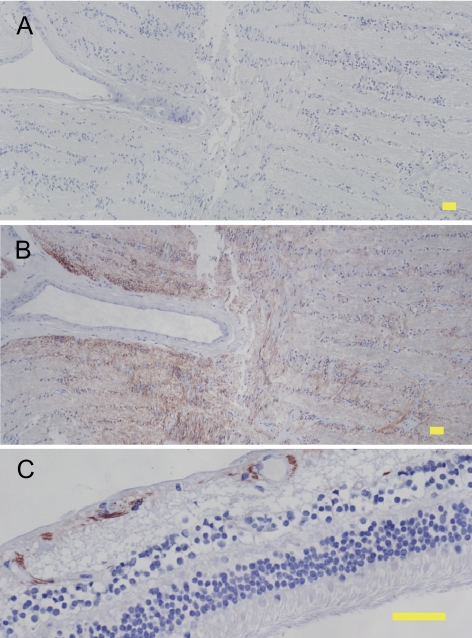
To determine whether the IL-8 detected in AH could be produced locally, IL-8 protein expression in the eye was investigated. Human cadaver eyes free of ocular diseases, including glaucoma, were fixed in paraformaldehyde within 48 hours of death, embedded in paraffin, and sectioned for immunohistochemistry. Representative results of staining of 28 donor eyes are shown in Figure 5. Immunostaining with normal goat IgG as a negative control resulted in no immunoreactivity (Fig. 5A). Immunostaining using an affinity-purified goat IgG raised against human IL-8 resulted in positive immunoreactivity (reddish/brown color) in the optic nerve (Fig. 5B) and the inner retina (Fig. 5C). In other ocular tissue, anti–IL-8 immunoreactivity was not observed. These results suggest that IL-8 is produced by ocular tissues, and this local source may contribute to the IL-8 detected in AH.
Figure 5.
IL-8 protein expression in human cadaver eyes. Immunohistochemistry was performed on sections of paraformaldehyde-fixed human cadaver eyes using negative control IgG (A) or anti–IL-8 antibody (B, C) and detection with Nova Red substrate followed by hematoxylin counterstain. Reddish-brown: positive immunoreactivity. Blue: hematoxylin counterstain. Staining with negative control IgG resulted in no detectable immunoreactivity (A). IL-8 expression was found in the optic nerve (B) and inner retina (C). Yellow scale bar, 50 μm.
Discussion
Our overall aim was to test the hypothesis that immune activation is associated with glaucoma. Given that eight of the 12 cytokines investigated by multiplex analysis were below detection limits and another two cytokines were detected at low levels in a few patients, our data do not provide evidence of widespread or large changes in cytokine expression in POAG. Results from previous studies suggested that some cytokines might have increased expression in glaucoma. TNFα was previously shown to be increased in the optic nerve20 and retina21 of human glaucomatous eyes, although Sawada et al.22 recently reported no elevation of TNFα in the AH of POAG patients. IL-1 was suggested to be elevated in glaucoma, possibly by increased expression by trabecular meshwork cells.23 IL-18 was reported to be increased in the AH of the DBA/2J mouse glaucoma model.24 We did not detect TNFα or IL-1, and IL-18 was detected at low concentrations in a small number of patients, with no differences between control and POAG. Lack of detection of these cytokines in our study of AH does not support the elevation of IL-18, TNFα, or IL-1 in glaucoma, but it does not disprove these possibilities either because the amount or location of any increased expression might not have resulted in detectable levels in the AH. We did find that the inflammatory cytokine IL-8 is elevated in the AH of glaucoma patients, consistent with a body of evidence1–4,6 suggesting that glaucoma may be accompanied by immune activation.
In our initial experiments (Fig. 1), patients in the POAG group underwent glaucoma surgery without cataract extraction. These POAG patients did not receive mydriatic drops, as did the cataract control group, and those who underwent trabeculectomy received miotic drops (pilocarpine) before surgery. In addition, lens status between the POAG and control groups differed because some POAG patients were pseudophakic, although none had undergone previous intraocular surgery within 1 year of AH collection. To address these differences, we compared IL-8 AH concentrations in another set of POAG patients who underwent cataract extraction with another set of control patients (Fig. 2) and again found that IL-8 was significantly higher in the POAG patients. These results ruled out the possibility that higher IL-8 concentration was attributed to differences in treatment with mydriatic drops or lens status before surgery.
A complication in the glaucoma patient samples was that nearly all patients were treated with topical antiglaucoma medications, which could have affected cytokine production.25,26 To address this, patients with clearly defined asymmetric glaucomatous optic nerve damage were investigated. Because both eyes of glaucoma patients receive identical medications, the fellow eyes served as internal controls. We found that IL-8 concentration was significantly higher in the more severely affected eyes of the patients with asymmetric glaucoma. Our results suggest that elevated IL-8 concentration in the AH correlates with glaucoma severity and is not an artifact of antiglaucoma medication because both eyes of each patient received identical antiglaucoma medication. A correlation between IL-8 and glaucoma severity is further supported by our results that IL-8 concentration was higher in patients with severe visual field defects than in patients with mild visual field defects.
IL-8, also called CXCL8, is a member of the CXC family of chemokines and is known to have both immune and vascular functions.27,28 Our immunohistochemistry experiments using normal eye tissue showed that IL-8 is endogenously produced by retina and optic nerve, potentially local sources of IL-8 that could contribute to the IL-8 detected in AH. Although IL-8 was not detected by immunohistochemistry in the lens or trabecular meshwork (TM), these are potential local sources of IL-8 given that lens epithelial cells29 and TM cells30 in culture have been shown to constitutively secrete IL-8. Localized responses to IL-8 are likely because receptors for IL-8 (CXCR1 and CXCR2) are widely expressed in neurons and glial cells and have been demonstrated to be expressed throughout the retina.31 Because IL-8 has been shown to be a neurotoxin,32 the overproduction of IL-8 in the retina and optic nerve could contribute to RGC death and axonal damage in glaucoma.
Multiple studies have demonstrated the upregulation of IL-8 under suboptimal oxygenation conditions,33–35 suggesting possible mechanisms for the increased IL-8 found in our study of POAG patients. In the eye, IL-8 expression has been shown to be stimulated by ischemia-reperfusion.36 Jo et al.36 demonstrated the upregulation of IL-8 expression in the retinal vasculature and in the ganglion cell layer and inner nuclear layer of the retina 6 hours after reperfusion in a rat model of ischemia-reperfusion injury. Two conditions that can cause reperfusion injury, sleep apnea37–39 and vascular dysregulation,40,41 have been linked to glaucoma. It is possible that suboptimal oxygenation could induce higher expression of IL-8 in some patients.
IL-8 induction in the specific context of glaucoma has also been investigated. Wang et al.42 found that serum amyloid A (SAA), a protein that plays a role in inflammation and tissue repair, is expressed at high levels in glaucomatous trabecular meshwork (TM) compared with normal cells. When exogenous SAA was added to perfused anterior chambers, elevated IOP was observed, suggesting SAA plays a role in glaucoma pathogenesis.42 Importantly, in the context of our findings, they also found that the addition of recombinant SAA to cultured TM cells potently stimulated the secretion of IL-8.42 It is possible that elevated expression of SAA could have caused the increased IL-8 concentrations found in the AH of POAG patients in our study.
It has been suggested that neuroinflammation may contribute to glaucoma pathogenesis.43 Neuroinflammation refers to chronic inflammation-like responses in the central nervous system that may participate in neurodegeneration.44 Although mediated by cytokines, neuroinflammatory processes do not produce classic leukocyte infiltrates as in the periphery. Neuroinflammation may be involved in other age-related chronic neurodegenerative disorders, such as Parkinson's disease45 and Alzheimer's disease.46 Glaucoma is also an age-related chronic neurodegenerative disorder and has features common to neuroinflammation, such as glial cell and complement cascade activation. Elevated IL-8 in POAG patients found in our study would be consistent with a neuroinflammatory process in glaucoma.
An immune response associated with glaucoma could be protective or pathogenic. If the immune system plays a pathogenic role, patients with immune-related disease could potentially have altered susceptibility to glaucoma. There is limited evidence in the literature to support this. Cartwright et al.47 looked for an association between immune-related disease and glaucoma by comparing 67 patients with normal tension glaucoma with 67 age- and sex-matched patients with ocular hypertension (IOP>22 mm Hg). Their retrospective study showed that patients with normal tension glaucoma were more likely to have immune-related diseases than patients with ocular hypertension without glaucoma, suggesting that susceptibility to glaucomatous damage might be related to immunoreactive tendencies.47
In conclusion, we found that the concentration of IL-8, a proinflammatory cytokine, is elevated in the AH of glaucoma patients, suggesting that immune activation may be associated with glaucoma. Further study is needed to investigate potential pathogenic roles for IL-8 and immunity in glaucoma.
Footnotes
Presented in part at the annual meeting of the International Congress of Eye Research, Beijing, China, September 2008, and at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2009.
Supported by National Eye Institute Grant P30-EY008126 (Core Grant in Vision Research), National Eye Institute Grant R01-EY007892 (PS), and an Unrestricted Challenge Grant (Vanderbilt Eye Institute) and a Career Development Award (RWK) from Research to Prevent Blindness, Inc.
Disclosure: J. Kuchtey, None; K.A. Rezaei, None; P. Jaru-Ampornpan, None; P. Sternberg, Jr, None; R.W. Kuchtey, None
References
- 1. Tezel G, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004;15:80–84 [DOI] [PubMed] [Google Scholar]
- 2. Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009;88:825–830 [DOI] [PubMed] [Google Scholar]
- 3. Tezel G. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1001–1012 [DOI] [PubMed] [Google Scholar]
- 4. Grus FH, Joachim SC, Wuenschig D, Rieck J, Pfeiffer N. Autoimmunity and glaucoma. J Glaucoma. 2008;17:79–84 [DOI] [PubMed] [Google Scholar]
- 5. Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001;131:421–426 [DOI] [PubMed] [Google Scholar]
- 6. Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009;18:341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 2001;42:365–371 [PubMed] [Google Scholar]
- 8. Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178 [DOI] [PubMed] [Google Scholar]
- 9. Stasi K, Nagel D, Yang X, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47:1024–1029 [DOI] [PubMed] [Google Scholar]
- 10. Kuehn MH, Kim CY, Ostojic J, et al. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp Eye Res. 2006;83:620–628 [DOI] [PubMed] [Google Scholar]
- 11. Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(suppl 1):S34–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255 [DOI] [PubMed] [Google Scholar]
- 13. Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561 [DOI] [PubMed] [Google Scholar]
- 14. Ooi KG, Galatowicz G, Towler HM, Lightman SL, Calder VL. Multiplex cytokine detection versus ELISA for aqueous humor: IL-5, IL-10, and IFNγ profiles in uveitis. Invest Ophthalmol Vis Sci. 2006;47:272–277 [DOI] [PubMed] [Google Scholar]
- 15. Curnow SJ, Falciani F, Durrani OM, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251–4259 [DOI] [PubMed] [Google Scholar]
- 16. Funding M, Hansen TK, Gjedsted J, Ehlers N. Simultaneous quantification of 17 immune mediators in aqueous humour from patients with corneal rejection. Acta Ophthalmol Scand. 2006;84:759–765 [DOI] [PubMed] [Google Scholar]
- 17. Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999;227:41–52 [DOI] [PubMed] [Google Scholar]
- 18. Nix B, Wild D. Calibration curve fitting. In: Wild D. ed. The Immunoassay Handbook. New York: Nature Publishing Group; 2001:198–210 [Google Scholar]
- 19. Cello KE, Nelson-Quigg JM, Johnson CA. Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol. 2000;129:314–322 [DOI] [PubMed] [Google Scholar]
- 20. Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50 [PubMed] [Google Scholar]
- 21. Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794 [PubMed] [Google Scholar]
- 22. Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 51:903–906 [DOI] [PubMed] [Google Scholar]
- 23. Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005;280:31240–31248 [DOI] [PubMed] [Google Scholar]
- 25. Malvitte L, Montange T, Vejux A, et al. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91:29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111:2186–2192 [DOI] [PubMed] [Google Scholar]
- 27. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621 [DOI] [PubMed] [Google Scholar]
- 28. Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishi O, Nishi K, Wada K, Ohmoto Y. Expression of transforming growth factor (TGF)-alpha, TGF-beta(2) and interleukin 8 messenger RNA in postsurgical and cultured lens epithelial cells obtained from patients with senile cataracts. Graefes Arch Clin Exp Ophthalmol. 1999;237:806–811 [DOI] [PubMed] [Google Scholar]
- 30. Shifera AS, Trivedi S, Chau P, Bonnemaison LH, Iguchi R, Alvarado JA. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010;91:42–47 [DOI] [PubMed] [Google Scholar]
- 31. Goczalik I, Ulbricht E, Hollborn M, et al. Expression of CXCL8, CXCR1, and CXCR2 in neurons and glial cells of the human and rabbit retina. Invest Ophthalmol Vis Sci. 2008;49:4578–4589 [DOI] [PubMed] [Google Scholar]
- 32. Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11:305–311 [DOI] [PubMed] [Google Scholar]
- 33. Lutz J, Luong le A, Strobl M, et al. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med. 2008;86:1329–1339 [DOI] [PubMed] [Google Scholar]
- 34. Patel N, Gonsalves CS, Malik P, Kalra VK. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood. 2008;112:856–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9:119–126 [DOI] [PubMed] [Google Scholar]
- 36. Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:4054–4060 [DOI] [PubMed] [Google Scholar]
- 37. Mojon DS, Hess CW, Goldblum D, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106:1009–1012 [DOI] [PubMed] [Google Scholar]
- 38. Kiekens S, Veva De G, Coeckelbergh T, et al. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49:934–940 [DOI] [PubMed] [Google Scholar]
- 39. Sergi M, Salerno DE, Rizzi M, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16:42–46 [DOI] [PubMed] [Google Scholar]
- 40. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393 [DOI] [PubMed] [Google Scholar]
- 41. Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008;115:246–252 [DOI] [PubMed] [Google Scholar]
- 42. Wang WH, McNatt LG, Pang IH, et al. Increased expression of serum amyloid A in glaucoma and its effect on intraocular pressure. Invest Ophthalmol Vis Sci. 2008;49:1916–1923 [DOI] [PubMed] [Google Scholar]
- 43. Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258 [DOI] [PubMed] [Google Scholar]
- 44. Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(suppl 3):S49–S58, discussion S58–S60 [DOI] [PubMed] [Google Scholar]
- 46. Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91 [DOI] [PubMed] [Google Scholar]
- 47. Cartwright MJ, Grajewski AL, Friedberg ML, Anderson DR, Richards DW. Immune-related disease and normal-tension glaucoma: a case-control study. Arch Ophthalmol. 1992;110:500–502 [DOI] [PubMed] [Google Scholar]