Corneal transplants induce regulatory T cells (Tregs) that prevent graft rejection. Corneal cells injected into the anterior chamber also induce Tregs. This study shows that although each of these Treg populations promotes graft survival, they express distinctly different properties.
Abstract
Purpose.
To compare and contrast the T regulatory cells (Tregs) induced by anterior chamber (AC) injection of antigen with those induced by orthotopic corneal allografts.
Methods.
Anterior chamber–associated immune deviation (ACAID) Tregs were induced by injecting C57BL/6 spleen cells into the AC of BALB/c mice. Delayed-type hypersensitivity responses to C57BL/6 alloantigens were evaluated by a conventional ear swelling assay. Corneal allograft Tregs were induced by applying orthotopic C57BL/6 corneal allografts onto BALB/c hosts. The effects of anti-CD25, anti-CD8, anti-interferon-γ (IFN-γ), anti-IL-17A, or cyclophosphamide treatments on corneal allograft survival and ACAID were evaluated.
Results.
Administration of either anti-CD25 or anti-IFN-γ antibodies prevented the expression of ACAID and abolished the immune privilege of corneal allografts. By contrast, in vivo treatment with anti-CD8 antibody abrogated ACAID but had no effect on corneal allograft survival. Further discordance between ACAID and corneal allograft survival emerged in experiments in which the induction of allergic conjunctivitis or the administration of anti-IL-17A abolished the immune privilege of corneal allografts but had no effect on the induction or expression of ACAID.
Conclusions.
Although orthotopic corneal allografts are strategically located for the induction of ACAID by the sloughing of corneal cells into the AC, the results reported here indicate that the Tregs induced by orthotopic corneal allografts are remarkably different from the Tregs that are induced by AC injection of alloantigen. Although both of these Treg populations promote corneal allograft survival, they display distinctly different phenotypes.
Corneal transplantation has been performed successfully on humans for over 100 years and on animals since 1837.1,2 Corneal transplants are routinely performed without HLA typing or the use of systemic immunosuppressive drugs. Patients who require corneal transplants because of developmental anomalies of the cornea, which are not associated with inflammation of the ocular surface, have exceptionally high success rates that often reach 90%.3 This apparent defiance of the laws of transplantation was recognized over 50 years ago in animal studies by Billingham and Medawar.4,5 Medawar recognized the profound implications of these observations and coined the term “immune privilege” to reflect the unique immunologic properties of the anterior chamber (AC) and the cornea.5 The immune privilege of corneal allografts can be defined mathematically if one considers the fate of corneal allografts in rodents that receive corneal allografts that are mismatched at the entire major histocompatibility complex and multiple minor loci. In rat and mouse models of penetrating keratoplasty, 50% of such corneal allografts survive long term.6–8 By contrast, skin and heart allografts undergo 100% immune rejection in such hosts.
Three basic factors contribute to the immune privilege of corneal allografts: the blockade in the induction of the immune response to the alloantigens expressed on the corneal allograft, the generation of T regulatory cells (Tregs) that suppress the allodestructive immune responses against the donor alloantigens, and the expression of apoptosis-inducing molecules on the cell membranes of corneal cells that delete alloreactive T cells at the graft/host interface.
Antigens introduced into the AC elicit a unique form of systemic immune tolerance termed anterior chamber–associated immune deviation (ACAID), which culminates in the antigen-specific suppression of delayed-type hypersensitivity (DTH).9–11 Orthotopic corneal allografts are placed directly over the AC of the eye, and it has been proposed that this juxtapositioning of the orthotopic corneal allograft with the AC facilitates the sloughing or shedding of corneal alloantigens into the AC, which in turn would induce ACAID.10 Several observations support this hypothesis. Rodents with long-term clear corneal allografts display an antigen-specific suppression of DTH responses that resembles the suppression of DTH found in ACAID.10–12 Moreover, manipulations that inhibit the induction of ACAID, such as splenectomy, ablation of NK T cell or γδ T cell populations, invariably lead to an increased tempo and incidence of corneal allograft rejection.10,11,13–16 Injection of donor alloantigenic cells into the AC before corneal transplantation induces ACAID and results in a significant enhancement of corneal allograft survival in both the rat and mouse models of penetrating keratoplasty.17,18 With this in mind, we embarked on a series of experiments designed to compare and contrast maneuvers that affect ACAID with those that are known to influence the immune privilege of corneal allografts. The underlying hypothesis predicted that the Tregs that supported ACAID and corneal allograft survival were one in the same. However, the results indicate that two different forms of immune tolerance are involved in ACAID and corneal allograft survival.
Materials and Methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Taconic Farms (Germantown, NY). Animals used in grafting experiments were female, 8 to 12 weeks old. BALB/c nude mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals used in these experiments were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Orthotopic Corneal Transplantation
Full-thickness penetrating orthotopic corneal grafts were performed as described previously,19 with a few modifications. Mice were anesthetized systemically with an intraperitoneal (IP) injection of 115 mg/kg of ketamine HCl (Fort Dodge Laboratories, Fort Dodge, IA) and 5.6 mg/kg of xylazine (Bayer Corporation, Shawnee Mission, KS). Proparacaine HCl ophthalmic solution (USP 0.5%; Alcon Laboratories, Ft. Worth, TX) was used as a topical anesthetic. Donor grafts and recipient graft beds were scored with 2.0 mm trephines, and the corneas were excised with Vannas scissors. Donor grafts were sewn into place using running 11-0 nylon sutures (Ethicon, Sommerville, NJ), and sutures were removed on day 7 posttransplantation. Topical antibiotic (Akorn, Decatur, IL) was applied immediately after surgery as well as immediately after removal of sutures. No immunosuppressive drugs were used, either topically or systemically. Median survival times (MSTs) were calculated and used to determine the statistical significance of differences in the tempo of corneal allograft rejection between the experimental and control groups.
Clinical Evaluation of Grafted Corneas
Corneal grafts were examined 2 to 3 times a week with a slit-lamp biomicroscope (Carl Zeiss, Oberkochen, Germany). Graft opacity was scored using a scale of 0–4. Degree of graft opacity was scored as follows: 0 = clear; 1+ = minimal superficial opacity; 2+ = mild deep stromal opacity with pupil margin and iris visible; 3+ = moderate stromal opacity with pupil margin visible, but iris structure obscured; and 4+ = complete opacity, with pupil and iris totally obscured. Corneal grafts were considered rejected on two successive scores of 3+.
AC Injection of Alloantigenic Cells
Mice were anesthetized as described above. A glass micropipette (approximately 80 μm diameter) was fitted onto a sterile infant feeding tube (no. 5 French, Professional Medical Products, Greenwood, SC) and mounted onto a 0.1 mL syringe (Hamilton Co., Whittier, CA). An automatic dispensing apparatus (Hamilton Co.) was used to inject plastic nonadherent C57BL/6 spleen cells (1 × 105 cells in 4 μL) into the AC of BALB/c mice.
DTH Assay
DTH was measured using a conventional ear swelling assay. An eliciting dose of 1 × 106 mitomycin C–treated (400 μg/mL) C57BL/6 spleen cells in 20 μL of Hanks' balanced salt solution (HBSS) was inoculated into the right ear. The left ear served as a negative control and was injected with 20 μL of HBSS without cells. Results were expressed as alloantigen-specific ear swelling response = (24 h measurement – 0 h measurement) for experimental ear – (24 h measurement – 0 h measurement) for negative control ear.
Cyclophosphamide Treatment
Administration of low doses of cyclophosphamide is known to inhibit Treg activity without producing global immunosuppression.20 Accordingly, mice were injected IP with cyclophosphamide (Sigma-Aldrich, St. Louis, MO) at a dose of 100 mg/kg the day before AC alloantigen or orthotopic corneal transplantation and at 7 day intervals thereafter.
Induction of Allergic Conjunctivitis
The protocol used to sensitize and challenge mice was modified from Magone et al.21 BALB/c mice were immunized with 50 μg of short ragweed (SRW) pollen (from Ambrosia artemisiifolia; International Biologicals, Piedmont, OK) in 5 mg alum (Imject; Pierce Chemical, Rockford, IL) by IP injection on day 0. Allergic conjunctivitis was induced by a “multi-hit” topical challenge method in which immunized mice were given topical 1.5 mg applications of SRW pollen in 10 μL PBS to the right eye once daily from days 10 to 16. Animals were examined clinically for signs of immediate hypersensitivity responses 20 minutes after each topical challenge with SRW pollen. A clinical scoring scheme, similar to that described by Magone et al.,21 was used and evaluated chemosis, conjunctival redness, lid edema, and tearing. Each parameter was graded on a scale ranging from 0 to 3+. Mice were challenged on day 17 with either a C57BL/6 corneal allograft or an AC injection of C57BL/6 spleen cells injected into the contralateral eye that was not exposed to SRW pollen.
Monoclonal Antibody Treatment
Anti-interferon-γ (IFN-γ) monoclonal antibody was isolated from cultures of R4–6A2 (ATCC, Rockville, MD). A rat anti-mouse IL-17A monoclonal antibody was prepared as described previously.22 Rat anti-mouse CD8 monoclonal antibody was purified from the YTS 169.4 hybridoma,23 and anti-mouse CD25 monoclonal antibody was purified from the PC 615.3 hybridoma (American Type Culture Collection; ATCC, Rockville, MD). Anti-CD25 and anti-CD8 antibodies were administered IP once weekly at a dose of 250 μg/injection. Anti-IFN-γ and anti-IL-17 antibodies were given twice weekly at a dose of 500 μg/injection.
Preparation of Antigen-Presenting Cells
Antigen-presenting cells (APCs) for local adoptive transfer (LAT) assays were generated using spleen cells from naive C57BL/6 mice. Briefly, cells were incubated with NH4Cl erythrocyte lysis solution, washed, and resuspended at 3 × 107 cells per ml of HBSS. The suspension was sonicated with 10 1-second pulsations. Lysates were frozen at −80°C for 10 minutes and thawed at 37°C for 5 minutes for two cycles. BALB/c APCs were isolated by incubating the cell suspension of splenocytes onto two 100 mm culture plates (Primaria; BD Labware, Franklin Lakes, NJ; 5 mL each plate) at 37°C for 1 hour. Nonadherent cells were removed by vigorous washing with PBS. Adherent APCs remaining in the plates were cultured in 4 mL of complete RPMI medium supplemented with 10% FBS and pulsed with the C57BL/6 cell lysate (1 mL). Cell cultures were incubated at 37°C overnight.
LAT Assay
The LAT assay used to compare the efferent suppression of corneal allograft–induced CD4+CD25+ and ACAID CD8+ Tregs has been described elsewhere.24 Spleen cells were harvested from BALB/c recipients with clear corneal allografts 3 weeks posttransplantation. CD4+CD25+ T cells were enriched using a mouse regulatory T cell isolation kit (Miltenyi Biotech, Inc., Auburn, CA) according to the manufacturer's protocol. To generate ACAID CD8+ Tregs, BALB/c mice were given an AC injection of nonadherent C57BL/6 spleen cells on day 0 and were immunized subcutaneously (SC) with 1 × 106 C57BL/6 spleen cells 7 days later. On day 14, spleen cells were collected, and CD8+ T cells were enriched by positive selection using rat anti-mouse CD8-conjugated magnetic microbeads (Miltenyi Biotec, Inc.). Effector CD4+ T cells from corneal allograft rejectors were isolated using rat anti-mouse CD4-conjugated magnetic microbeads (Miltenyi Biotec, Inc.). CD8+ T cells were mixed with BALB/c APCs pulsed with B6 splenocytes and effector CD4+ T cells from corneal allograft rejectors in a 1:1:1 ratio. Left and right ear pinnae of naive BALB/c mice were injected with 20 μL (1 × 106) of the mixed-cell population. The opposite ear was injected with HBSS as a negative control. Ear swelling was measured 24 hours later to assess DTH.
Statistical Analysis
MSTs and mean rejection times (MRTs) were calculated for the various corneal allografts. The Mann–Whitney U test determined the statistical significance in MSTs. Results for DTH assays were evaluated by Student's t-test. Differences in all experiments were considered to be statistically significant if P < 0.05.
Results
Effect of Anti-CD25 Antibody on ACAID and Corneal Allograft Survival
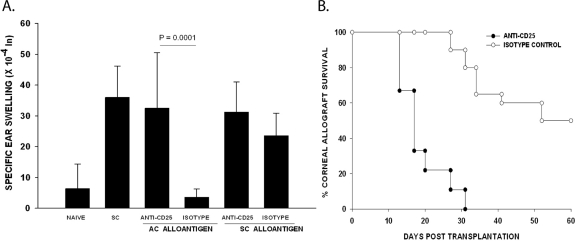
Previous studies have shown that the induction of ACAID requires the participation of two independent Treg populations.10,25 One population is CD4+ and acts at the afferent arm, whereas the other Treg population is CD8+ and acts at the efferent arm of the immune response to suppress DTH responses produced by previously sensitized T cells.26 Because many CD4+ Tregs also express CD25, we wished to determine whether in vivo treatment with a blocking anti-CD25 antibody would affect ACAID and corneal allograft survival. Mice were treated with either anti-CD25 antibody or an isotype control antibody 1 day before and at weekly intervals after administering either an AC injection with C57BL/6 spleen cells or an orthotopic corneal transplant. Mice injected in the AC with C57BL/6 spleen cells were immunized SC with C57BL/6 spleen cells 7 days later, and DTH was evaluated 7 days after the SC immunization. The results indicated that in vivo treatment with anti-CD25 antibody prevented the development of ACAID (Fig. 1A) and robbed the corneal allograft of its immune privilege (Fig. 1B).
Figure 1.
Effect of anti-CD25 on ACAID and corneal allograft survival. BALB/c mice were treated with anti-CD25 antibody or isotype control once before and once weekly after AC injection or corneal transplantation. (A) ACAID was induced on day 0 with C57BL/6 splenic nonadherent cells. A SC injection of C57BL/6 splenocytes was given on day +7. DTH challenge with mitomycin C–treated C57BL/6 cells was on day +14. Negative control animals received an ear challenge only (n = 5 for all groups). This experiment was performed twice with similar results. (B) C57BL/6 corneal allograft survival in BALB/c mice treated with anti-CD25 or rat IgG isotype control antibody. C57BL/6 corneal allografts underwent rejection in 50% of hosts treated with the isotype control IgG (n = 10) and had an MST of 52 days. C57BL/6 corneal allografts transplanted to BALB/c recipients treated with anti-CD25 were rejected in 100% of hosts with an MST of 26 days (n = 10). P < 0.05 between anti-CD25–treated group and rat IgG isotype control treated allograft recipients. The experiment was performed twice with similar results.
Effect of Low-Dose Cyclophosphamide on ACAID and the Immune Privilege of Corneal Allografts
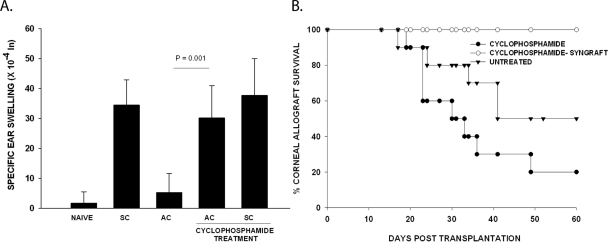
It has been reported that low-dose cyclophosphamide inhibits the activity of CD4+CD25+ Tregs without producing global immunosuppression.20 Accordingly, mice were treated with IP injections of cyclophosphamide (100 mg/injection) 1 day before either AC injection of C57BL/6 spleen cells or orthotopic transplantation of C57BL/6 corneal allografts and at 7 day intervals thereafter. Mice injected in the AC with C57BL/6 spleen cells were immunized SC with C57BL/6 spleen cells 7 days later, and DTH was evaluated 7 days after the SC immunization. Low-dose cyclophosphamide treatment prevented the development of ACAID (Fig. 2A) and promoted the accelerated rejection of C57BL/6 corneal allografts (Fig. 2B). Cyclophosphamide treatment alone did not produce an adjuvant effect, nor did it enhance the baseline DTH response in SC immunized mice, as the responses in SC immunized mice without cyclophosphamide treatment were identical with the responses of cyclophosphamide-treated mice that were immunized SC with C57BL/6 spleen cells (Fig. 2A). The effect of cyclophosphamide on the increased incidence of corneal allograft rejection was not due to a toxic effect, because BALB/c mice treated with cyclophosphamide did not reject syngeneic BALB/c corneal homografts (Fig. 2B).
Figure 2.
Effect of cyclophosphamide on ACAID and corneal allograft survival. Cyclophosphamide treatments were performed 1 day before and once weekly after AC injection or corneal transplantation. (A) ACAID was induced on day 0 with C57BL/6 splenic nonadherent cells. A SC injection of C57BL/6 splenocytes was given on day +7. DTH challenge with mitomycin C-treated C57BL/6 cells was administered on day +14. Negative control animals received an ear challenge only. Positive and DTH control animals were immunized SC and received an ear challenge but were not injected in the AC. P = 0.001 for cyclophosphamide-treated versus untreated group in which ACAID was induced (n = 5). The experiment was performed twice with similar results. (B) C57BL/6 corneal allograft survival in BALB/c mice treated with cyclophosphamide. C57BL/6 corneal allografts underwent rejection in 50% of untreated recipients (n = 10) and had an MST of 52 days. BALB/c hosts treated with cyclophosphamide rejected 80% of grafts with an MST of 28 days (n = 10). P < 0.05 between cyclophosphamide-treated and untreated allograft recipients. No rejection was observed in the syngeneic recipient treated with cyclophosphamide.
Role of CD8+ T Cells in the Expression of ACAID and for the Immune Privilege of Corneal Allografts
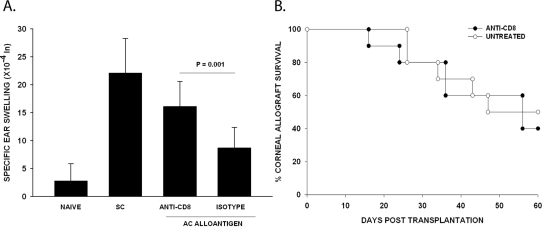
As stated earlier, a population of CD8+ Tregs is needed for the efferent suppression of DTH responses during ACAID. Experiments were performed to determine whether in vivo administration of anti-CD8 monoclonal antibody would influence the development of ACAID and affect the fate of corneal allografts. Mice were treated with either anti-CD8 antibody or an isotype control antibody on days −4 and −3 and at weekly intervals after either AC injection (day 0) with C57BL/6 spleen cells or orthotopic corneal transplantation. Mice injected in the AC with C57BL/6 spleen cells were immunized SC with C57BL/6 spleen cells 7 days later, and DTH was evaluated 7 days after the SC immunization. Although anti-CD8 antibody treatment prevented the expression of ACAID (Fig. 3A), it did not affect the immune privilege of corneal allografts (Fig. 3B). The tempo and incidence of corneal allograft rejection were virtually identical in the anti-CD8–treated mice and the untreated controls (MST = 52 days and 46 days, respectively; 60% rejection and 50% rejection, respectively; P > 0.05).
Figure 3.
Effect of anti-CD8 antibody treatment on ACAID and corneal allograft survival. (A) Mice were treated with 500 μg anti-CD8 or isotype control on days −3 and −4. ACAID was induced on day 0 with C57BL/6 splenic nonadherent cells. Subcutaneous injections of C57BL/6 splenocytes were given on day +7. DTH challenge with mitomycin C–treated C57BL/6 cells was on day +14. Negative control animals received an ear challenge only (n = 5 mice for each group). This experiment was performed 3 times with similar results. (B) BALB/c hosts were treated with 500 μg anti-CD8 1 week before and once weekly after allograft transplantation. Sixty percent of BALB/c hosts treated with anti-CD8 rejected their C57BL/6 corneal allografts with a MST of 52 days (n = 10). The incidence of C57BL/6 corneal allograft rejection in BALB/c mice treated isotype control was 50% (n = 10) and with an MST of 46 days (P > 0.05).
Effect of Allergic Conjunctivitis on ACAID and Corneal Allograft Survival
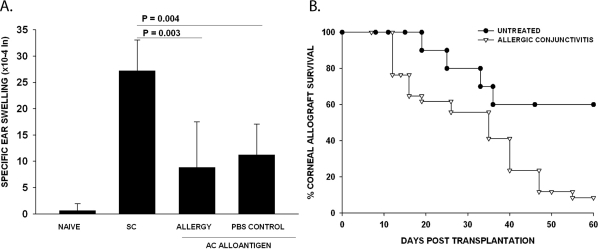
Other authors as well as this study have shown that allergic conjunctivitis abolishes the immune privilege of corneal allografts and leads to an increased incidence and tempo of rejection.27,28 The effect of allergy in exacerbating corneal allograft rejection is due to a systemic perturbation in the alloimmune response, because inducing allergic conjunctivitis in one eye still causes a steep increase in the immune rejection of corneal allografts placed into the contralateral eye that is neither challenged with allergen nor expresses any evidence of inflammation at the time of transplantation.27 Moreover, induction of airway hyperreactivity, which is a model for allergic asthma, also exacerbates corneal allograft rejection.29 Together, these findings indicate that allergic diseases disrupt immune privilege of corneal allografts. Because ACAID has been implicated in the immune privilege of corneal allografts, we examined the effect of an ongoing allergic reaction on the development and expression of ACAID. Accordingly, allergic conjunctivitis was induced through the topical application of SRW pollen in the right eyes of BALB/c mice before injecting C57BL/6 spleen cells in the left eyes. This design eliminates the possible confounding effect of a local ocular inflammatory response in evaluating the role of allergic conjunctivitis in the development of ACAID. The DTH responses in mice experiencing allergic conjunctivitis were the same as those in the PBS control mice, indicating that Th2-based inflammation did not affect the induction or expression of ACAID (Fig. 4A). By contrast, mice with allergic conjunctivitis displayed a steep reduction in corneal allograft survival (90% rejection vs. 40% rejection) and a reduced MST (31 days vs. 56 days) compared with untreated control mice (Fig. 4B).
Figure 4.
Effect of allergic conjunctivitis on ACAID and corneal allograft survival. (A) Allergic conjunctivitis was induced by IP immunization with SRW pollen followed by topical challenge with SRW pollen before AC injection of C57BL/6 splenocytes. ACAID was induced on day 0 with C57BL/6 splenic nonadherent cells. Subcutaneous injections of C57BL/6 splenocytes were given on day +7. DTH challenge with mitomycin C–treated C57BL/6 cells was on day +14. Negative control animals received an ear challenge only. This is representative of 2 independent experiments (n = 5/group). (B) Allergic conjunctivitis was induced by immunizing BABL/c mice IP with SRW pollen (day 0). Mice were challenged topically with SRW on days 10–16. C57BL/6 corneal allografts were applied on day +17. MSTs for allergic conjunctivitis group (n = 27) and untreated control group (n = 10) were significantly different (P = 0.003).
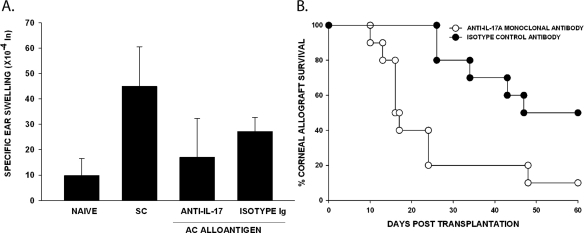
Differential Effects of IL-17A on ACAID and Corneal Allograft Survival
The recently discovered IL-17A–producing CD4+ Th17 cell has been implicated in the pathogenesis of a variety of autoimmune diseases that were previously believed to be mediated by Th1 cells.22,30–33 Accordingly, we examined the effect of in vivo neutralization of IL-17A on the induction of ACAID and the immune privilege of corneal allografts. Mice were treated with either monoclonal anti-IL-17A or an isotype control antibody on days −4 and −2 before either AC injection of C57BL/6 spleen cells or the application of an orthotopic C57BL/6 corneal allograft and twice weekly thereafter. ACAID was evaluated as before, and the fate of the corneal allografts was followed for 60 days. In each of several repeated experiments, we found that administration of this same monoclonal antibody did not affect the development of ACAID (Fig. 5A), yet it produced a profound increase in the incidence and tempo of corneal allograft rejection (Fig. 5B). Although IL-17A is not necessary for the generation of one form of ocular immune privilege (i.e., ACAID), it is absolutely required for the immune privilege of corneal allografts. Not only did neutralization of IL-17A abolish the immune privilege of corneal allografts, but it also led to accelerated graft rejection.
Figure 5.
Effect of anti-IL-17A on ACAID and corneal allograft survival. BALB/c mice were treated with anti-IL-17A antibody or isotype control on days −4 and −2 before and twice weekly after AC injection or corneal transplantation. (A) ACAID was induced on day 0 with C57BL/6 splenic nonadherent cells. Subcutaneous injections of C57BL/6 splenocytes were given on day +7. DTH challenge with mitomycin C–treated C57BL/6 cells was on day +14. Negative control animals received an ear challenge only. ACAID groups treated with anti-IL-17A or isotype control antibody were injected in the AC with C57BL/6 antigen, SC immunized, and ear challenged with mitomycin C–treated C57BL/6 splenocytes. P > 0.05 for the anti-IL-17A–treated versus isotype control-treated group (n = 5). This experiment was performed twice with similar results. (B) C57BL/6 corneal allograft survival in BALB/c mice treated with anti-IL-17A or a rat IgG isotype control antibody. C57BL/6 corneal allografts underwent rejection in 50% of hosts treated with the isotype control IgG (n = 10) and had an MST of 46 days. C57BL/6 corneal allografts transplanted to BALB/c recipients treated with anti-IL-17A were rejected in 90% of hosts with an MST of 14.5 days (n = 10). P < 0.05 between anti-IL-17A–treated group and rat IgG isotype control treated allograft recipients. The experiment was performed four times with similar results.
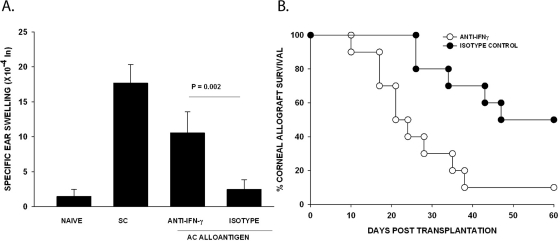
Influence of IFN-γ on the Development of ACAID and Corneal Allograft Survival
It was previously reported that lymph node (LN) cells from mice with ovalbumin (OVA)-induced ACAID produced significantly less IFN-γ but significantly more IL-4 and IL-10 compared with LN cells from mice that were immunized SC with OVA.34 This was interpreted by some investigators to be evidence that ACAID was the result of Th2 cross-regulation of Th1 responses. We have previously shown that corneal allografts undergo immune rejection in IFN-γ KO mice and in normal mice treated with anti-IFN-γ antibody.35 Because the long-term survival of corneal allografts correlates with the development of Tregs,36 we examined the effect of IFN-γ on the development of ACAID and the fate of corneal allografts. For corneal allograft survival experiments, mice were treated with either anti-IFN-γ antibody or an isotype control antibody on days −4 and −2 before corneal transplantation and twice weekly thereafter. For ACAID experiments, mice were treated with either anti-IFN-γ antibody or an isotype control antibody on days −1 and +7 relative to AC injection with C57BL/6 spleen cells (day 0) or orthotopic corneal transplantation. Mice injected in the AC with C57BL/6 spleen cells were immunized SC with C57BL/6 spleen cells 7 days later, and DTH was evaluated 7 days after the SC immunization. Mice treated with anti-IFN-γ antibody failed to develop ACAID (Fig. 6A), and in agreement with previous reports, depletion of IFN-γ led to accelerated corneal allograft rejection (Fig. 6B). Thus, both ACAID and corneal allograft survival require the presence of IFN-γ.
Figure 6.
Effect of anti-IFN-γ on ACAID and corneal allograft survival. (A) Mice were treated with 500 μg anti-IFN-γ or isotype control antibody on days −1 and +7. ACAID was induced with nonadherent C57BL/6 spleen cells on day 0, followed by SC immunization with C57BL/6 splenocytes on day +7. DTH challenge was performed with mitomycin C-treated C57BL/6 cells on day 14. P = 0.002 between anti-IFN-γ and isotype control (n = 5). This experiment was performed 2 additional times with similar results. (B) BALB/c animals were treated with anti-IFN-γ antibody or isotype control on days −4 and −2 and twice weekly after corneal transplantation. Ninety percent of BALB/c hosts treated with anti-IFN-γ rejected their C57BL/6 corneal allografts with a MST of 19 days (n = 10). The incidence of C57BL/6 corneal allograft rejection in BALB/c mice treated with an isotype control was 50% (n = 10) and with a MST of 46 days (P < 0.05).
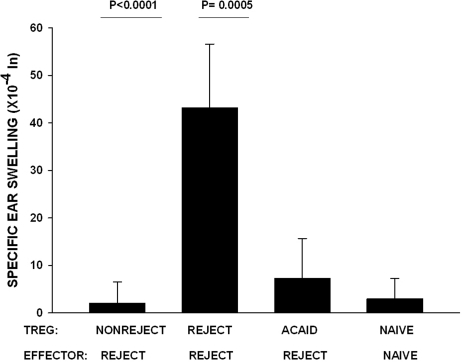
Suppression of the Efferent Immune Response by Tregs from ACAID and Corneal Allograft Acceptor Mice
The original hypothesis for this study proposed that orthotopic corneal transplantation was tantamount to an AC injection of alloantigens and that the Tregs in mice with long-term surviving corneal allografts were, in fact, ACAID Tregs. Experiments were performed to compare ACAID Tregs induced by AC injection of C57BL/6 alloantigenic cells with the Tregs that were present in mice with long-term surviving corneal allografts. The rationale for this experiment is based on the following observations: corneal allograft rejection is accompanied by a reduction in Treg activity and increased DTH responses to the alloantigens expressed by the corneal allograft donor36; the presence of Tregs correlates with corneal allograft survival36; ACAID correlates with the generation of CD4+ afferent Tregs and CD8+ efferent-acting Tregs26; and suppression of alloantigen-sensitized immune cells (i.e., the efferent arm of the alloimmune response) correlates with corneal allograft survival.12 It was important to test the efficacy of ACAID Tregs and Tregs from corneal allograft acceptors to suppress DTH mediated by CD4+ T cells from mice that had rejected their C57BL/6 corneal allografts (i.e., CD4+ T cells that are known to mediate corneal allograft rejection).37 Accordingly, CD4+ effector T cells were isolated from the spleens of corneal allograft rejector mice and were mixed with either CD4+CD8−CD25+ Tregs from corneal allograft acceptor mice or CD4−CD8+ Tregs from mice primed in the AC with C57BL/6 spleen cells (i.e., ACAID Tregs). BALB/c APCs pulsed with C57BL/6 alloantigens in vitro were added to each culture. The positive control consisted of CD4+ T cells isolated from rejector mice, and the negative control consisted of naive CD4+ effector cells cultured with naive CD4+CD25+ natural Tregs. Admixed cell cultures were injected in the ear pinnae of naive BALB/c mice, and DTH was measured 24 hours later. The results demonstrate that CD4+CD8−CD25+ Tregs from corneal allograft acceptor mice and CD4−CD8+ Tregs from ACAID mice suppressed DTH responses mediated by CD4+ effector cells isolated from corneal allograft rejector mice (Fig. 7). Thus, the Tregs induced by AC injection of alloantigens (i.e., ACAID Tregs) and Tregs induced in corneal allograft acceptor mice represent different populations based on their expression of CD8 and CD4, respectively, yet both are capable of suppressing DTH responses by previously sensitized CD4+ effector T cells.
Figure 7.
Efferent Tregs in ACAID and corneal allograft acceptor hosts. CD4+CD8−CD25+ Tregs were isolated from nonrejector mice 3 weeks posttransplantation. ACAID CD4−CD8 + Tregs were isolated on day 14 after AC priming with nonadherent C57BL/6 spleen cells. Effector CD4+ T cells were isolated from corneal allograft rejector mice and mixed with the Tregs. DTH-positive control animals received rejector CD4+CD25+ Tregs and CD4+ rejector effectors, and negative control animals received naive CD4+CD25+ Tregs and naive effectors. BALB/c APCs were pulsed with C57BL/6 alloantigens in vitro and added to all cell cultures. P < 0.0001 for nonrejector versus rejector CD4+CD25+ Tregs recipients; P = 0.0005 for ACAID CD8+ Tregs versus rejector CD4+CD25+ Tregs recipients (n = 5 per group). This experiment was performed twice with similar results.
Discussion
Orthotopic corneal allografts are placed over the AC of the eye, and it is reasonable to expect that corneal cells and alloantigens sloughed from the transplanted cornea would find their way into the AC. Thus, an orthotopic corneal allograft might have the same effect as an AC injection of alloantigens: that is, it would induce ACAID. A significant body of evidence supports this hypothesis. For example, hosts that have received AC injections and mice with long-term surviving corneal allografts have suppressed DTH responses to donor alloantigens.12 Moreover, maneuvers that abrogate the induction of ACAID, such as splenectomy or deletion of NK T cells or γδ T cells, greatly increase corneal allograft rejection.13–15,17 The present study tested the hypothesis that conditions that affect the generation or function ACAID Tregs would also have a similar impact on the fate of orthotopic corneal allografts. However, the results revealed that in some cases the opposite occurred.
In vivo treatment with anti-CD25 antibody prevented the induction of ACAID and robbed the corneal allograft of its immune privilege, which is consistent with previous findings suggesting that the presence of CD25+ Tregs correlates with corneal allograft survival.36 By contrast, in vivo treatment with anti-CD8 antibody prevented the induction of ACAID and parallels previous reports indicating that CD8+ spleen cells from ACAID mice suppress DTH responses when these Tregs are coinjected with immune cells and antigen in a conventional LAT assay for detecting efferent suppression of DTH by ACAID Tregs.38 However, the same anti-CD8 antibody treatment did not affect the immune privilege of orthotopic corneal allografts. A similar disconnect was found in mice with SRW pollen-induced allergic conjunctivitis. SRW pollen-induced allergic conjunctivitis abolished the immune privilege of corneal allografts yet did not affect the development of ACAID.
CD4+ T regs are needed for the induction of ACAID and the survival of corneal allografts, yet they appear to function in different capacities. ACAID CD4+ T regs act in a contact-independent manner through their elaboration of IL-10, which suggests that they do not mediate their regulatory function by an FasL-dependent pathway.39 Moreover, ACAID CD4+ T regs do not directly suppress DTH responses to the donor's alloantigens, but instead are required for the generation of CD8+ T regs that act as end-stage T regs that inhibit the expression of DTH by sensitized T cells.39 By contrast, CD4+ T regs induced by orthotopic corneal allografts do not require the participation of CD8+ T cells, but act at the efferent stage of the immune response to suppress T cell proliferation36 and DTH (Cunnusamy K, Chen P, Niederkorn J, manuscript in preparation).
Th17 cells are believed to play a crucial role in a variety of immune-mediated diseases, including ocular autoimmune diseases such as experimental dry eye disease and experimental autoimmune uveitis.22,30,33 Moreover, IL-17A has been implicated in the immune rejection of lung and cardiac allografts.40–42 However, the present results indicate that instead of reducing corneal allograft rejection, neutralization of IL-17A produced a dramatic exacerbation of rejection. A similar mitigating effect of IL-17A has been noted in other forms of T cell–mediated inflammation such as dextran sulfate-induced and TNBS-induced colitis43,44 and allergic asthma.45 Under many conditions, IL-17A is a proinflammatory cytokine, and thus one might expect that in vivo neutralization of IL-17A would affect the development of ACAID and also have an impact on corneal allograft survival. However, neutralization of IL-17A did not affect the development of ACAID, but, as stated earlier, had a profound effect on the survival of corneal allografts. We consistently observed >90% rejection of corneal allografts in anti-IL-17-treated mice. Thus, the T regs that are induced in ACAID are distinctly different from the T reg population that is induced by keratoplasty and supports the long-term survival of corneal allografts. In some regards, IFN-γ resembles IL-17A in its pleiotropic effects. It was widely believed that IFN-γ acts as a proinflammatory cytokine and is involved in the pathogenesis of a variety of Th1-mediated autoimmune diseases, yet there is a growing body of evidence that IFN-γ is required for the maximal expression of some Th2-based inflammatory diseases, such as allergic conjunctivitis and asthma.46–48 It was previously reported that lymph node cells from mice with ovalbumin (OVA)-induced ACAID produced significantly less IFN-γ and simultaneously upregulated IL-4 and IL-10 production.34 This was interpreted by some investigators to be evidence that ACAID was the result of Th2 cross-regulation of Th1 responses. However, the same study also reported that spleen cells from the same mice with OVA-induced ACAID produced the same quantities of IFN-γ that were produced by spleen cells from mice immunized SC with OVA. Subsequent studies by Cone and co-workers indicated that IFN-γ was required for the suppressive function of CD8+ Tregs in ACAID.38 Although CD8+ Tregs induced during ACAID did not need to produce IFN-γ, they could exert their suppressive effects on DTH only if they expressed the IFN-γ receptor and were capable of responding to IFN-γ. The present findings are in agreement with the work of Cone et al. and indicate that IFN-γ is needed for the expression ACAID Treg activity. The current results also demonstrate that the CD4+CD25+ Tregs that support corneal allograft survival also require IFN-γ. Recently, it has become clear that the absence of IFN-γ exacerbates experimental autoimmune encephalitis (EAE).49–51 Interestingly, IFN-γ is necessary for the generation of CD4+CD25+Foxp3+ Tregs that mitigate EAE.52 In both human and murine systems, IFN-γ treatment leads to conversion of CD4+CD25− T cells to CD4+CD25+ Tregs, an increased expression of Foxp3, and heightened suppressive activity. It remains to be determined if IFN-γ has a similar effect in the induction of CD4+CD25+ Treg recipients of corneal allografts, but the present results and previous findings by Chauhan et al.,36 which indicated that Foxp3 expression on CD4+CD25+ T cells correlated with corneal allograft survival, are consistent with this hypothesis.
Collectively, the present results indicate the existence of two separate populations of Tregs that are capable of supporting corneal allograft survival. One population is induced by the corneal allograft, CD4+CD25+, and acts at the efferent arm of the immune response to suppress DTH responses by previously sensitized allospecific T cells. The second population is induced artificially by AC injection of alloantigens, is CD8+, and also suppresses at the efferent phase of the immune response. These findings suggest that optimizing the induction of each of these Treg populations may have a beneficial impact in promoting corneal allograft survival, especially in hosts that have preexisting conditions that create a high risk for corneal allograft rejection.
Acknowledgments
The authors thank Elizabeth Mayhew, Jessamee Mellon, and Christina Stevens for technical assistance.
Footnotes
Supported by NIH Grant EY007641 and Core Grant, and an unrestricted grant from Research to Prevent Blindness, New York, New York.
Disclosure: K. Cunnusamy, None; K. Paunicka, None; N. Reyes, None; W. Yang, None; P.W. Chen, None; J.Y. Niederkorn, None
References
- 1. Bigger SL. An inquiry into the possibility of transplanting the cornea with the view of relieving blindness (hitherto deemed incurable) caused by several diseases of that structure. Dublin J Med Sci. 1837;11:408–447 [Google Scholar]
- 2. Zirm E. Eine erfolgreiche totale Keratoplastik. Albrecht von Graefes Arch Ophthalmol. 1906;64:580–593 [Google Scholar]
- 3. Collaborative Corneal Transplantation Studies Research Group The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403 [PubMed] [Google Scholar]
- 4. Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci. 1953;141:392–406 [DOI] [PubMed] [Google Scholar]
- 5. Medawar PB. Immunity to homologous grafted skin, III: the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69 [PMC free article] [PubMed] [Google Scholar]
- 6. Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–1508 [DOI] [PubMed] [Google Scholar]
- 7. Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74:167–171 [DOI] [PubMed] [Google Scholar]
- 8. Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005–1016 [DOI] [PubMed] [Google Scholar]
- 9. Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359 [DOI] [PubMed] [Google Scholar]
- 10. Niederkorn JY. Anterior chamber-associated immune deviation and its impact on corneal allograft survival. Curr Opin Organ Transplant. 2006;11:360–365 [Google Scholar]
- 11. Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889 [DOI] [PubMed] [Google Scholar]
- 12. Sonoda Y, Streilein JW. Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J Immunol. 1993;150:1727–1734 [PubMed] [Google Scholar]
- 13. Plskova J, Duncan L, Holan V, Filipec M, Kraal G, Forrester JV. The immune response to corneal allograft requires a site-specific draining lymph node. Transplantation. 2002;73:210–215 [DOI] [PubMed] [Google Scholar]
- 14. Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333 [DOI] [PubMed] [Google Scholar]
- 15. Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168:2028–2034 [DOI] [PubMed] [Google Scholar]
- 16. Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42:1293–1298 [PubMed] [Google Scholar]
- 17. Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest Ophthalmol Vis Sci. 1996;37:2700–2707 [PubMed] [Google Scholar]
- 18. She SC, Steahly LP, Moticka EJ. Intracameral injection of allogeneic lymphocytes enhances corneal graft survival. Invest Ophthalmol Vis Sci. 1990;31:1950–1956 [PubMed] [Google Scholar]
- 19. He Y, Mellon J, Apte R, Niederkorn JY. Effect of LFA-1 and ICAM-1 antibody treatment on murine corneal allograft survival. Invest Ophthalmol Vis Sci. 1994;35:3218–3225 [PubMed] [Google Scholar]
- 20. Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868 [DOI] [PubMed] [Google Scholar]
- 21. Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998;87:75–84 [DOI] [PubMed] [Google Scholar]
- 22. De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551 [DOI] [PubMed] [Google Scholar]
- 24. D'Orazio TJ, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID). Immunology. 1998;95:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22:13–46 [PubMed] [Google Scholar]
- 26. Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:383–389 [PMC free article] [PubMed] [Google Scholar]
- 27. Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174:6577–6581 [DOI] [PubMed] [Google Scholar]
- 28. Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48:4044–4049 [DOI] [PubMed] [Google Scholar]
- 29. Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. Am J Transplant. 2009;9:1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2:375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748 [DOI] [PubMed] [Google Scholar]
- 32. Hirota K, Hashimoto M, Yoshitomi H, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kosiewicz MM, Alard P, Streilein JW. Alterations in cytokine production following intraocular injection of soluble protein antigen: impairment in IFN-gamma and induction of TGF- beta and IL-4 production. J Immunol. 1998;161:5382–5390 [PubMed] [Google Scholar]
- 35. Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193 [DOI] [PubMed] [Google Scholar]
- 36. Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79:23–31 [DOI] [PubMed] [Google Scholar]
- 38. Cone RE, Li X, Sharafieh R, O'Rourke J, Vella AT. The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires IFN-120:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skelsey ME, Mayhew E, Niederkorn JY. CD25+, interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology. 2003;110:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332 [DOI] [PubMed] [Google Scholar]
- 41. Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14:326–331 [DOI] [PubMed] [Google Scholar]
- 42. Yoshida S, Haque A, Mizobuchi T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735 [DOI] [PubMed] [Google Scholar]
- 43. O'Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62 [DOI] [PubMed] [Google Scholar]
- 45. Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343 [DOI] [PubMed] [Google Scholar]
- 47. Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stern ME, Siemasko K, Gao J, et al. Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2005;46:3239–3246 [DOI] [PubMed] [Google Scholar]
- 49. Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7 [PubMed] [Google Scholar]
- 50. Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646 [DOI] [PubMed] [Google Scholar]
- 51. Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227 [PubMed] [Google Scholar]
- 52. Wang Z, Hong J, Sun W, et al. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]