Abstract
In yeast α cells the a cell-specific genes STE6 and BAR1 are packaged as gene-sized chromatin domains of positioned nucleosomes. Organized chromatin depends on Tup1p, a corepressor that interacts with the N–terminal regions of H3 and H4. If Tup1p functions to organize or stabilize a chromatin domain, the protein might be expected to be present at a level stoichiometric with nucleosomes. Chromatin immunoprecipitation assays using Tup1p antibodies showed Tup1p to be associated with the entire genomic STE6 coding region. To determine stoichiometry of Tup1p associated with the gene, a yeast plasmid containing varying lengths of the STE6 gene including flanking control regions and an Escherichia coli lac operator sequence was constructed. After assembly into chromatin in vivo in Saccharomyces cerevisiae, minichromosomes were isolated using an immobilized lac repressor. In these experiments, Tup1p was found to be specifically associated with repressed STE6 chromatin in vivo at a ratio of about two molecules of the corepressor per nucleosome. These observations strongly suggest a structural role for Tup1p in repression and constrain models for organized chromatin in repressive domains.
Keywords: chromatin/H3 and H4 tails/minichromosome/protein–nucleic acid affinity/transcriptional repression
Introduction
Saccharomyces cerevisiae exists as two haploid cell types: a and α (Herskowitz, 1989). Cell type is determined by the expression of master regulatory genes at the active mating type locus (MAT). In MATα cells, genes coding for Matα1p and Matα2p are transcribed. Matα1p is an activator for α cell-specific genes, and Matα2p is a repressor for a cell-specific genes. In MATa cells neither of these proteins are produced, resulting in the expression of the a cell-specific genes by default.
Repression of the five a cell-specific genes in MATα cells is mediated by the binding of a complex of proteins at the nearly symmetrical 31 bp α2 operator, a sequence present ∼200 bp upstream of each of these genes (Johnson and Herskowitz, 1985). The repressor complex is comprised of a homodimer of the homeodomain protein Matα2p, which binds to the operator in a cooperative manner with a homodimer of the MADS box protein, Mcm1p (Sauer et al., 1988; Acton et al., 1997). Mcm1p binds to the center of the operator while the homodimer of Matα2p makes contact with the operator sequences flanking the Mcm1p binding site (Sauer et al., 1988; Zhong and Vershon, 1997; Tan and Richmond, 1998). This configuration requires the Matα2p dimer to straddle the Mcm1p dimer. In addition to Matα2p and Mcm1p, full repression also requires the non-DNA-binding proteins Ssn6p (Schulz and Carlson, 1987) and Tup1p (Lemontt et al., 1980; Keleher et al., 1992), which have been identified as general repressors in yeast (Keleher et al., 1992).
It has been proposed that the role of Matα2p is to recruit Ssn6p and Tup1p, which in turn mediate repression of the a cell-specific genes (Keleher et al., 1992; Komachi et al., 1994; Tzamarias and Struhl, 1995). Both Ssn6p and Tup1p can mediate repression of transcription from a heterologous CYC1 promoter when targeted via a lexA–DNA-binding domain (Keleher et al., 1992; Tzamarias and Struhl, 1994). In these experiments, repression by Ssn6p was Tup1p dependent, whereas repression by Tup1p was found to occur in the absence of Ssn6p (Keleher et al., 1992; Tzamarias and Struhl, 1994). These data suggest that Tup1p may be the active repressor in the Ssn6p–Tup1p complex, and that Ssn6p may be required as a bridge between Tup1p multimers or Tup1p and other proteins.
Tup1p has a mol. wt of 78 kDa and belongs to a family of proteins that are characterized by a region of amino acid residues having highly conserved tryptophanyl and aspartyl residues positioned approximately every 40 residues, termed WD40 or β-transducin motifs (Fong et al., 1986). This motif is now known to exist in a number of S.cerevisiae proteins, as well as extra sex combs and groucho in Drosophila (Schultz and Carlson, 1987; Williams and Trumbly, 1990; Komachi et al., 1994; Gutjahr et al., 1995). Tup1p contains seven WD40 repeats in the C–terminal half of the protein (Lemontt et al., 1980; Komachi and Johnson, 1997), which are thought to be involved in mediating protein–protein interactions. Different combinations of these repeats are required for repression of different sets of genes (Carrico and Zitomer, 1998). As an example, WD40 repeats 1 and 2 of Tup1p have been shown to interact directly with Matα2p (Komachi et al., 1994).
The N–terminal 72 amino acid residues of Tup1p have been shown to interact with Ssn6p, and to be required for multimerization, but are not sufficient to repress transcription (Tzamarias and Struhl, 1994). Amino acids 73–386 have been defined as the Tup1p repression domain (Tzamarias and Struhl, 1994); this same part of the protein interacts with the N-terminal regions of histones H3 and H4 (Edmondson et al., 1996). Recently, Tup1p has been shown to exist as a trimer or a tetramer in complexes with one molecule of Ssn6p (Varanasi et al., 1996; Redd et al., 1997), which may be the functional oligomeric state of these proteins in the cell.
Despite what is known about Tup1p, it is still unclear how it mediates repression of genes in S.cerevisiae. Given that Tup1p is involved in repression of a diverse group of genes, it appears likely that it interacts with a component common to many different promoters. Obvious potential targets include the basal transcription machinery and histones. There is evidence in support of both possibilities. Herschbach et al. (1994) observed modest repression of a naked DNA reporter construct in vitro after incubation with purified, recombinant Matα2p and cell extracts prepared from a yeast strain that overexpressed Ssn6p and Tup1p. From this result, these authors concluded that Tup1p may be mediating repression via interaction with the basal transcription machinery, as no chromatin assembly was likely to have occurred in the assay. A functional relationship has also been proposed between Ssn3p–Ssn8p, a part of the polymerase II holoenzyme mediator complex, and the Ssn6p–Tup1p complex (Kuchin and Carlson, 1998). Other experiments, however, suggest that Tup1p repression may be mediated through the organization of repressive chromatin.
Previous work in our laboratory has shown that the binding of the Matα2p–Mcm1p complex to the α2 operator initiates the establishment of a chromatin domain starting at the α2 operator. In this chromatin domain, nucleosomes are positioned precisely over essential promoter elements and the entire coding region of the genes (Shimizu et al., 1991; Roth et al., 1992; Patterton and Simpson, 1994). The absence of this organized chromatin in a cells suggests that it is required for, or a result of, the gene being repressed in α cells. α cells expressing N-tail mutations in histone H4 show partial derepression of the a cell-specific genes (Roth et al., 1992). In these cells, the positions of nucleosomes are less well defined (Roth et al., 1992), again suggesting a relationship between organized chromatin and repression of the a cell-specific genes. Deletion of Tup1p results in the derepression of the a cell-specific genes and disruption of the positioned nucleosomes adjacent to the α2 operator. This perturbation of the chromatin structure does not appear to be due solely to the transcriptional activity of the derepressed gene (Cooper et al., 1994).
One caveat to the chromatin organization hypothesis is the observation that the haploid-specific genes do not appear to show the extent of chromatin organization seen with other genes repressed in a Tup1p-dependent manner. This may reflect the ability of Tup1p to confer repression via multiple mechanisms, as is suggested by the two lines of evidence presented above. However, repression of these genes is clearly chromatin dependent despite the apparent lack of an organized chromatin domain (Huang et al., 1997).
To elucidate further the precise mechanism by which Tup1p mediates repression of the a cell-specific genes, we have determined the extent of spreading and stoichiometry of Tup1p with the in vivo assembled chromatin of STE6. We reason that if the role of Tup1p is solely to mediate interactions between the basal transcription machinery and the promoter, then the protein will be present in a limited copy number at the promoter. However, if Tup1p is involved in either establishing or maintaining a repressive chromatin structure by interacting with the histone tails, then it will be present in multiple copies possibly spread along the entire nucleosomal domain of the gene. In order to distinguish between these two possibilities, we have used chromatin immunoprecipitation techniques to investigate the extent of Tup1p spreading. To determine the stoichiometry of Tup1p to STE6 chromatin we have developed a minichromosome affinity purification (MAP) technique, which allows the isolation of the gene as in vivo packaged chromatin.
Central to the MAP technique is the fact that episomal DNA in S.cerevisiae is stably packaged and maintained as chromatin. The resulting minichromosomes provide a unique model system for studying the relationship between gene regulation and chromatin. Yeast minichromosomes have been used to study how nucleosome positioning affects the function of specific cis-acting elements (Simpson, 1990; Patterton and Simpson, 1994), chromatin remodeling upon transcription factor binding (Xu et al., 1998), and chromatin structure effects on DNA repair (Smerdon and Thoma, 1990; Smerdon et al., 1990; Bedoyan et al., 1992; Suter et al., 1997). Minichromosomes have also been used to study the interactions of trans-acting factors with DNA in chromatin (Morse et al., 1992; Morse, 1993), as well as twisting constraints on DNA in nucleosomes (Morse et al., 1987). Yeast minichromosomes have also been used extensively to study the factors involved in repression of the a cell-specific genes in α cells (Roth et al., 1990, 1992; Shimizu et al., 1991; Cooper et al., 1994; Patterton and Simpson, 1994).
Chromatin immunoprecipitation experiments using Tup1p antibodies show Tup1p to be associated with the STE6 chromatin along the entire coding region of the gene. To investigate the stoichiometric relationship between Tup1p and the STE6 nucleosomal array we employed the MAP technique. Using this technique we isolated three minichromosome constructs containing different fragments of the STE6 gene, and determined the stoichiometric relationship between Tup1p and the positioned nucleosomes present on these constructs in vivo. The results of these experiments led us to propose that Tup1p may exert its repressive effect on the STE6 gene in α cells by forming a scaffold along the length of the chromatin domain associated with the gene.
Results
Tup1p spreads over the entire STE6 chromatin domain
The currently accepted model for repression of the a cell-specific genes in α cells proposes that Tup1p initiates repression after being recruited to the gene by the DNA-binding protein Matα2p (Keleher et al., 1992; Tzamarias and Struhl, 1994, 1995). Edmondson et al. (1996) have demonstrated that the repression domain (Tzamarias and Struhl, 1994) of Tup1p interacts with the tails of histones H3 and H4 in vitro, suggesting a direct connection between Tup1p repression and chromatin organization. To this end Cooper et al. (1994) have shown that a TUP1 deletion has a disorganizing effect on the chromatin of the a cell-specific gene STE6 in α cells. These findings all suggest that Tup1p may facilitate repression by interacting with chromatin directly.
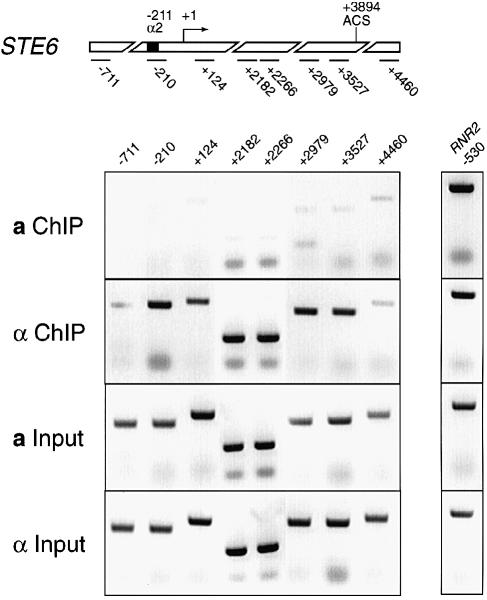
To address this possibility chromatin immunoprecipitations (ChIP) were performed. Tup1p antibodies were used to immunoprecipitate formaldehyde-cross-linked, sonicated chromatin from wild-type a and α cells. The precipitated DNA was visualized by PCR (Figure 1). At the top of Figure 1 is a schematic diagram of the STE6 gene showing the positions of the fragments amplified in the PCRs. Each fragment is 150–200 bp long and identified as the position of the 5′ base of each fragment relative to the start site of transcription. The first panel shows the STE6 fragments amplified from a cell ChIPed material. Comparing this signal to the input for this cell type shows only a uniform background amplification from the ChIPed material. The next panel shows the STE6 fragments amplified from the α cell ChIPed material. Comparing the signal from a cells to α cells it is clear that the fragments between the α2 operator and the 3′ end of the gene are precipitated from α cells, indicating that Tup1p is spread along the entire chromatin domain of the gene. No PCR product is obtained from the ChIPed DNA when primers outside the STE6 gene (–711 and +4460) are used. These results show that Tup1p spreads unidirectionally from the α2 operator and ends at the 3′ end of the gene, exactly the direction of positioned nucleosomes in the STE6 chromatin domain (Y.Tsukagoshi and R.T.Simpson, in preparation).
Fig. 1. Chromatin immunoprecipitations of soluble genomic chromatin using Tup1p antibodies. At the top is a schematic diagram of the STE6 gene showing the positions of the fragments amplified in the PCRs to visualize the precipitated DNA. Eight pairs of PCR primers were used to generate the STE6 fragments. Each pair of primers amplifies a fragment ∼150–200 bp long and is labeled with the position of the 5′ base of each fragment relative to the start site of transcription. The panel labeled a ChIP is material amplified from Tup1p immuno- precipitates from a cells. The panel labeled a input is material amplified from the input for the a cell ChIPs. The panel labeled α ChIP is material amplified from Tup1p immunoprecipitates from α cells. The panel labeled α input is material amplified from the input for the α cell ChIPs. To the right of the STE6 results is a control for amplification from both cell types. The RNR2 gene is a DNA damage-responsive gene, which is repressed by Tup1p. As with the STE6 fragment, labeling of this fragment begins 530 bp upstream of the start site of RNR2 transcription. In this experiment RNR2 should be repressed in both cell types and so associated with Tup1p in both cell types. Equal amplification from both a and α cell immunoprecipitated material is observed.
To the right of the STE6 result is a control for amplification from both cell types. The RNR2 gene is a DNA damage-responsive gene, which is repressed by Tup1p (Elledge et al., 1993). It should be repressed in both cell types in this experiment and so should be associated with Tup1p in both cell types. As expected the ChIP results show equal amplification from both a and α cell material immunoprecipitated with Tup1p antibodies.
Minichromosome affinity purification
Knowing the stoichiometry of a protein associated with a gene in vivo helps significantly when considering its role in the control of DNA function. For non-DNA-binding proteins, there are two problems with a biochemical approach to this question. First, there is a small amount of material associated with any given single-copy gene in the genome. Second, it has not been possible to isolate any gene from the rest of the genome as intact chromatin. Using the MAP technique provides a way to overcome these hurdles. MAP provides a method of isolating a large quantity of potentially any gene as intact chromatin, and quantification of associated non-DNA-binding proteins that have been identified by other techniques.
The MAP protocol is derived from that previously developed by Dean et al. (1989). The method takes advantage of the high affinity interaction between the Escherichia coli lac repressor protein and its operator sequence. We have designed the procedure as an affinity matrix system to be carried out in low pressure chromatography columns. The affinity ligand for this system was created by attaching a chitin-binding domain (CBD) to a lac repressor–β-galactosidase fusion protein (lacI–Z). The lacI–Z fusion gene was cloned from a mutant E.coli strain, BMH 72-19-1 (Müller-Hill and Kania, 1974), into the pTyb2 cloning and expression vector (New England Biolabs), which fuses the intein-CBD to the C–terminus of the protein. The lacI–Z–intein-CBD fusion protein is expressed in E.coli and isolated directly by binding to a chitin matrix. The charged resin is used as an affinity matrix to bind minichromosomes containing the lac operator. The large size of the fusion protein provides a spacer between the DNA-binding portion of the molecule and the matrix. This minimizes potential steric hindrance during binding of the episomes in the isolation procedure. Equally important in the design of a MAP experiment is the proper location for the lac operator in the episomal DNA. For the TAL vector a position between two elements of the autonomously replicating sequence, which is constitutively nuclease sensitive and thus judged likely to be nucleosome free, was utilized, as discussed in detail in Materials and methods.
The MAP procedure begins with 1 l of yeast cells being grown to an OD600 of 1–1.5. The cells are spheroplasted, and lysed with a motor-driven pestle. The minichromosomes are allowed to diffuse passively from the nuclei for 3–4 h on ice. Intact nuclei and other cellular debris are subsequently removed by centrifugation. The supernatant, which contains the minichromosome, is loaded onto a 1 ml (packed bed volume) chitin–agarose column charged with the lacI–Z–intein-CBD fusion protein. After washing, the minichromosomes are released following an overnight incubation at 4°C in minichromosome binding buffer (mbb) containing 30 mM dithiothreitol (DTT). The DTT causes an autocatalytic cleavage of the intein moiety leaving the intein-CBD fragment bound to the matrix and the lacI–Z bound minichromosome free in solution. Minichromosomes are washed off the column in mbb containing 300 mM NaCl.
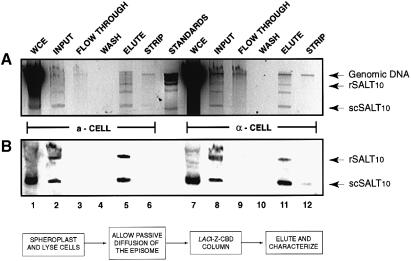
A typical purification profile, monitoring minichromosome DNA, is shown in an ethidium bromide-stained agarose gel and by Southern blot analysis in Figure 2. Quantification of the Southern blot (Figure 2B) was performed using ImageQuant software (Molecular Dynamics). Comparison of the signal generated in lanes 1 and 7 to lanes 2 and 8 shows 40–60% of the minichromosomes being released from the nuclei. Of the material loaded onto the column >95% is retained (compare lanes 2 and 8 to lanes 3 and 9). Finally, >90% of the minichromosomes released from the nuclei and retained on the column can be recovered in the eluate (lanes 5 and 11). The ethidium-stained agarose gel (Figure 2A) was imaged and quantified using the Eagle Eye II still imaging system (Stratagene). From this analysis we determined that the minichromosomes are 50–70% pure as judged by comparing the plasmid band to other DNA contaminants.
Fig. 2. Minichromosome affinity purification fractions. (A) SALT10 isolation fractions from both a and α cells, resolved on a 1% agarose gel stained with ethidium bromide. All samples on the gel represent 1% of the total starting volume. Supercoiled minichromosome (scSALT10), relaxed minichromosome (rSALT10), and genomic DNAs are indicated to the right of the figure. Lanes 1 and 7 contain whole-cell extracts. Lanes 2 and 8 contain chromatography input; all DNA that has diffused from the nuclei. Lanes 3 and 9 contain the column flow-through; DNA not bound by the matrix. Lanes 4 and 10 contain the three washes combined. Lanes 5 and 11 contain the eluted DNA. Lanes 6 and 12 contain the column strip, which shows DNA bound non-specifically. (B) Southern blot of the agarose gel probed with an oligo specific to the lac operator. The flow chart at the bottom is a general schematic of the isolation procedure.
Multiple copies of Tup1p associate with STE6 in vivo
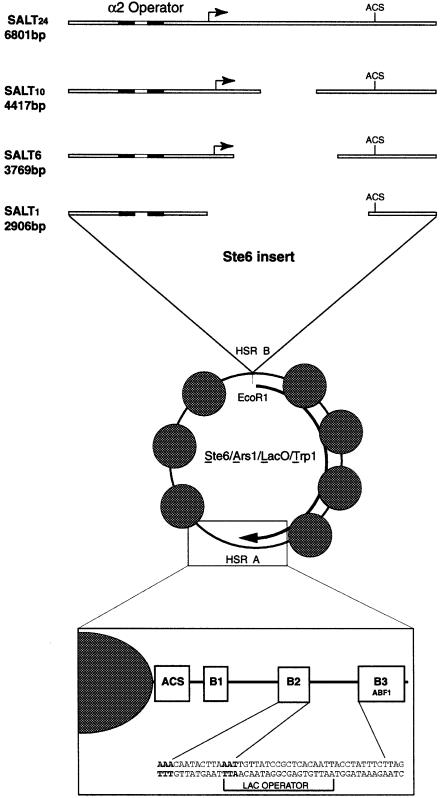
From the ChIP results it is clear that Tup1p associates with the entire STE6 chromatin domain. In order to assess how many copies of Tup1p are associated with the repressive chromatin structure, we created a series of minichromosomes containing fragments of the STE6 gene. The parent minichromosome in this series is composed of the ALT backbone (see Materials and methods) with 5273 bp of STE6 and flanking DNA inserted into HSR B. This minichromosome was called SALT24 (STE6/ARS1/Lac-operator/TRP1, see Figure 3). The acronym SALT24 was used to signify the DNA components and the 24 nucleosomes associated with the transcriptionally repressed STE6 gene in α cells (Y.Tsukagoshi and R.T.Simpson, in preparation). The precisely positioned array of nucleosomes begins 15 bp downstream of the α2 operator, extends through the coding region and ends abruptly 70 bp downstream of the termination codon of the gene. Although the mechanism of termination of the organized chromatin is not known, it ends as precisely as it begins, forming a discrete domain (Y.Tsukagoshi and R.T.Simpson, in preparation). In order to ensure the inclusion of all regulatory sequences in the ‘STE6 insert’ in SALT24, extensive sequences were included both upstream (690 bp) and downstream (800 bp) of the coding region of the gene. It is important to remember that the organized chromatin domain does not spread to the ends of the STE6 inserted sequences—only the coding region and ∼200 bp of 5′ flanking sequences out to the α2 operator have positioned nucleosomes.
Fig. 3. Minichromosome constructs. In the center is the unaltered TRP1/ARS1 minichromosome, showing the positions of the nucleosomes and nuclease-hypersensitive sites. The arrow represents the direction of transcription of the TRP1 gene. In the expanded box at the bottom is a blow-up of the ARS1 region of the minichromosome showing the placement of the lac operator. Bases in bold are those shared between the B2 element and the lac operator. Expanded at the top are the STE6 inserts for the four minichromosomes used in this study. All four fragments were cloned into HSR B at the EcoRI site. Gaps in the STE6 insert represent the extent of sequence removed (not drawn to scale). Indicated are the α2 operator, the start site for transcription, and the ARS consensus sequence (ACS) present at the 3′ end of the gene.
Initial attempts at MAP using SALT24 showed that this construct did not diffuse from the nuclei of either a or α cells. This might result from the minichromosome being sequestered to a location in the nuclei where its diffusion was impaired. However, it seemed unlikely that it would be sequestered in both cell types, and more likely that it was simply too large for passive diffusion out of the nuclei. To test this possibility we generated a smaller construct; SALT10 was created by deleting 2384 bp from the middle of the STE6 coding region (Figure 3).
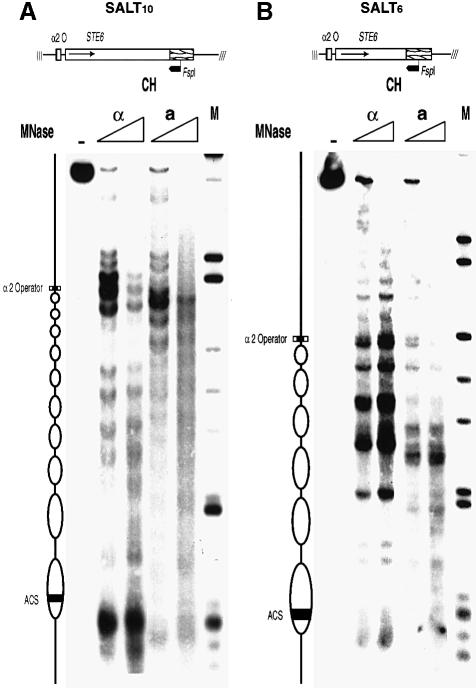
Functional and structural characterization of the SALT10 minichromosome revealed that the remaining portion of the STE6 gene accurately reflected the features of the whole gene. Thus, primer extension analysis of mRNA isolated from strains carrying the SALT10 minichromosome showed that the construct is transcribed in a cells and fully repressed in α cells, identical to the behavior of the parent gene (data not shown). The predicted nucleosomal array for SALT10 consists of two nucleosomes from the 5′ end of the gene and eight from the 3′ end of the gene. Indirect end-label mapping of micrococcal nuclease digests of SALT10 isolated from α cells using MAP shows 10 positioned nucleosomes, confirming this prediction (Figure 4A). Although these positioned nucleosomes map slightly differently than their counterparts in the genomic copy of the full-length gene (Y.Tsukagoshi and R.T.Simpson, in preparation) they are precisely positioned in α cells and lose this positioning in a cells. These results demonstrate that the STE6 gene fragment in the SALT10 minichromosome contains all of the necessary regulatory elements to properly control expression and establish the characteristic chromatin structure observed in the genomic copy of the gene. Furthermore, proteins necessary for repression and organized chromatin structure, Matα2p, Mcm1p, Tup1p and Ssn6p, are not limiting, even with a multicopy minichromosome.
Fig. 4. Indirect end-label mapping of the chromatin structure of the SALT10 and SALT6 minichromosomes. (A) The cleavage pattern obtained by MNase digestion of SALT10 chromatin (CH), MAP isolated from a and α cells. (B) The cleavage pattern obtained by MNase digestion of SALT6 chromatin (CH) also, MAP isolated from a and α cells. (M) indicates the λ-HindIII/φX174-HaeIII marker (NEB). The purified MNase-cleaved DNA was subsequently digested to completion with FspI and electrophoresed on a 1.5% agarose gel, transferred to a membrane and probed with an [α-32P]dCTP random prime labeled fragment. The inferred positions of the α2 operator, ARS consensus sequence and nucleosomes (depicted as ovals) are shown to the left of the gels. The schemes at the top of the gels represent the STE6 inserts in the two constructs showing the location of the FspI site and the direction of mapping indicated by the heavy arrow.
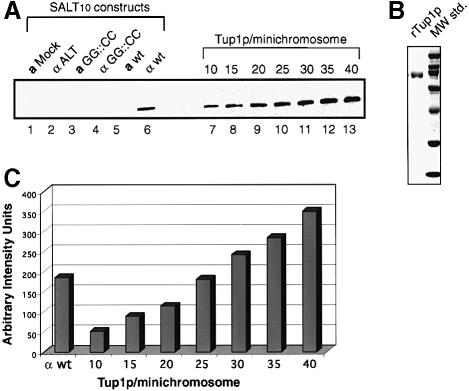
In MAP isolations using the SALT10 minichromosome, a similar diffusion efficiency was observed for a and α cell nuclei (Figure 2). Determination of the quantity of Tup1p associated with the minichromosomes from the two cell types using silver-stained SDS–polyacrylamide gels was not possible due to the level of background contaminants in these samples. Therefore, we probed Western blots with antibodies against Tup1p (see Materials and methods). The SDS–polyacrylamide gels run for the Western blots contained 1% of the total isolated material. This is the same amount of material run on the agarose gel (Figure 2), where the concentration of isolated minichromosome was quantified using the Eagle Eye II still imaging system (Stratagene) and ImageQuant software (Molecular Dynamics). Figure 5A shows a representative Western blot containing MAP isolated SALT10 minichromosomes and a graded set of standards constructed using recombinant Tup1p expressed in E.coli. Densitometry of the blot (Figure 5C) shows a ratio of 24 Tup1p molecules per SALT10 minichromosome isolated from α cells. Statistical analysis of seven replicates of this experiment, each performed with a new minichromosome preparation, shows 24.2 ± 0.5 copies of Tup1p per SALT10 minichromosome.
Fig. 5. Western blot analysis of affinity-purified SALT10 mini- chromosomes probed with anti-Tup1p antibodies. (A) Lane 1, a mock isolation from a cells containing no minichromosomes; lane 2, ALT isolated from α cells; lane 3, SALT10 with the Mcm1p binding site of the STE6 α2 operator mutated, designated GG::CC, isolated from a cells; lane 4, wild-type SALT10 isolated from a cells; lane 5, SALT10 with the Mcm1p binding site of the STE6 α2 operator mutated, designated GG::CC, isolated from α cells; lane 6, wild-type SALT10 isolated from α cells; lanes 7–13, a titration series of E.coli expressed recombinant Tup1p. Each lane in the titration series represents the indicated molar ratio of rTup1p to SALT10 minichromosomes. (B) A Coomassie-stained 10% SDS–polyacrylamide gel showing the rTup1p used for the standard series in the Western blot in (A) and broad range standards (Bio-Rad). (C) Densitometry of the Western blot analysis shows 24 Tup1p molecules per nucleosome. Replicates (n =7) of the SALT10 analysis, each performed with a new minichromosome preparation, show 24.2 ± 0.5 copies of Tup1p per SALT10 minichromosome.
Controls for the specificity of the Tup1p signal appear in Figure 5A, lanes 1–5. Lane 1 contains a mock isolation performed with a cells containing no minichromosomes. As expected no Tup1p signal is detected in this sample, indicating that Tup1p does not associate with the column matrix. Lane 2 contains the ARS1/Lac-operator/TRP1 (ALT) minichromosome isolated from α cells. Again, there is no Tup1p signal detected with the minichromosome lacking the STE6 insert. Lanes 3 and 5 contain a derivative of the SALT10 minichromosome isolated from a and α cells, respectively, bearing a mutation in the Mcm1p binding site which blocks Mcm1p binding (Smith and Johnson, 1994; Acton et al., 1997; Zhong and Vershon, 1997). These samples show no Tup1p signal, indicating that Tup1p binding depends on the presence of a wild-type α2 operator in the minichromosome. Lane 4 contains the SALT10 minichromosome isolated from a cells, and as predicted there is no Tup1p signal due to the lack of Matα2p. These data demonstrate the feasibility of isolating non-DNA-binding proteins associated with a specific gene as in vivo packaged chromatin. Going beyond this novelty, these results also clearly demonstrate that the SALT10 minichromosome contains multiple copies of Tup1p.
The number of Tup1p molecules interacting with the minichromosome depends on the number of nucleosomes present in the construct
To address further the possibility that multiple copies of Tup1p are interacting directly with the STE6 nucleosomes, a third minichromosome, SALT6, was created (Figure 3). SALT6 was derived from SALT10 by removing an additional 648 bp from the middle of the STE6 coding region. Functional and structural properties of this truncated version of the STE6 gene again indicated the integrity of the elements involved in both repression and chromatin organization in α cells. As with SALT10, primer extension of mRNA isolated from strains carrying the SALT6 minichromosome showed that the construct is transcribed in a cells and fully repressed in α cells (data not shown). The predicted nucleosomal array for this construct consists of two nucleosomes from the 5′ end of the gene and four from the 3′ end of the gene. This prediction was confirmed by indirect end-label mapping of micrococcal nuclease digests of SALT6 isolated from α cells using MAP (Figure 4B).
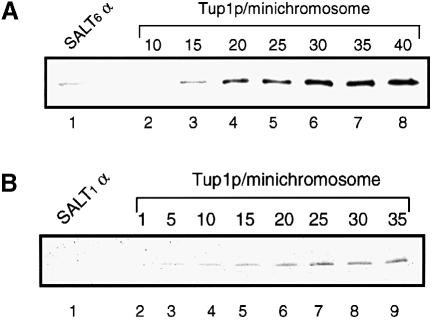
After MAP isolation, Tup1p:SALT6 ratios were obtained by densitometry of a Western blot analysis. Figure 6A shows a representative Western blot of the SALT6 minichromosome isolated from α cells and standards of rTup1p. Densitometry of the blot shows a ratio of 14 Tup1p molecules per SALT6 minichromosome. Statistical analysis of three replicates of this experiment, each performed with an independent minichromosome preparation, shows 14.1 ± 0.3 copies of Tup1p per SALT6 minichromosome. These results show that the amount of Tup1p in the isolated material depends on the number of nucleosomes present in the minichromosome. Furthermore, these data strongly suggest that Tup1p is associated with nucleosomes at some distance from the α2 operator.
Fig. 6. Western blot analysis of affinity-purified SALT6 and SALT1 minichromosomes, isolated from α cells, and probed with anti-Tup1p antibodies. (A) A representative analysis of the SALT6 minichromosome showing 14 Tup1p molecules per SALT6 minichromosome. Replicates (n = 3) of the SALT6 analysis each performed with a new minichromosome preparation show 14.1 ± 0.3 copies of Tup1p per SALT6 minichromosome. (B) A representative analysis of the SALT1 minichromosome showing less than one Tup1p molecule per SALT1 minichromosome. In replicates (n = 3) of this analysis, each performed with a new minichromosome preparation, Tup1p is present in <1 copy per SALT1 minichromosome.
While the SALT10 and SALT6 experiments suggested a 2:1 ratio of Tup1p to STE6 nucleosomes, it is formally possible that Tup1p associated with all of the nucleosomes of the minichromosome, not only the STE6 nucleosomes. To address this question, MAP and Western analysis were performed with a fourth construct, SALT1 (Figure 3). SALT1 contains only 178 bp of STE6 DNA between the α2 operator and the 3′ end of the gene. As with the other constructs SALT1 contains all of the upstream and downstream regulatory elements present at the genomic locus. Quantitative Western blot analysis for SALT1 (Figure 6B) shows that no Tup1p was detected in these experiments. Three replicates of this experiment all reveal Tup1p present in <1 copy per SALT1 minichromosome. If Tup1p associated with all nucleosomes, the ratio would be 0.96 in SALT10 and 0.66 in SALT6. With these numbers, we would then expect at least 10 molecules of Tup1p for SALT1. This expectation is clearly not met. The data thus indicate that the presence of Tup1p in the SALT6 and SALT10 minichromosomes is specific to the STE6 chromatin in α cells as expected from the ChIP analysis of the genomic STE6 gene. Restricting the Tup1p ratios generated with the SALT6 and SALT10 minichromosomes to only the STE6 nucleosomes makes the most likely stoichiometry of Tup1p to STE6 nucleosomes two Tup1p molecules per nucleosome.
Discussion
Chromatin has been implicated in the repression of several specialized regions in the yeast genome, such as the silent mating type loci, a cell-specific genes, and the recombination enhancer. For each of these regions, genetic evidence has implicated a corepressor in the control scheme. The corepressor has been shown to interact, in vitro, with the conserved, basic N-terminal regions of histones H3 and H4 (Hecht et al., 1995; Edmondson et al., 1996). Data presented in this paper provide quantitative evidence that nucleosomes may be stabilized by these histone-binding factors, such as Tup1p, as a means to silence specific genes in yeast.
The MAP methodology
In this study we have described a novel method for isolating yeast minichromosomes as intact chromatin. We have shown that, by using the MAP technique, it is possible to isolate non-DNA-binding proteins as well as histones associated with a repressed gene in vivo. One concern that accompanies any study of a gene on a multi-copy minichromosome is the possible presence of sub-populations of the minichromosome. Pederson et al. (1986) have addressed this issue for the TRP1/ARS1 minichromosome. The initial steps of their biochemical procedure for minichromosome isolation were nearly identical to those employed in the current study. The diffusion kinetics for the TRP1/ARS1 minichromosome were similar to those we have observed. No differences in structure or transcription of the retained versus the released population were found in the previous study.
To reinforce this conclusion in the current study, we added 1% Triton X-100 to the nuclear elution buffer in several experiments. This allowed >80% of the minichromosomes to be released from the nuclei. A similar portion of the eluted minichromosome population bound to the affinity matrix in the conventional or the detergent-enhanced samples. To ensure recovery of all bound material, the affinity columns were stripped with detergent solutions prior to protein and DNA analysis. In these preparations, the genomic DNA and protein contaminants are significantly increased; however, the amount of Tup1p isolated with the minichromosomes is unaltered. This does not rule out the possibility of sub-populations of the minichromosomes but it appears that the bulk adopts a single conformation, at least in terms of chromatin structure and Tup1p stoichiometry.
The mechanism of Tup1p-mediated repression
A body of evidence has accumulated in the past decade to suggest that repression by Tup1p is at least in part mediated through the organization of chromatin. Thus, repression of a cell-specific genes in α cells is associated with the precise and stable positioning of nucleosomes adjacent to the α2 operator (Roth et al., 1990; Shimizu et al., 1991; Patterton and Simpson, 1994). Also, deletion of the TUP1 gene results in the derepression of a cell–specific genes, and perturbation of the positioned nucleosomes adjacent to the α2 operator (Cooper et al., 1994). The perturbation of this chromatin structure does not appear to be due solely to the transcriptional activity of the derepressed gene (Cooper et al., 1994). The glucose-repressible gene SUC2 has also been shown to have a characteristic chromatin structure under repressing conditions (Gavin and Simpson, 1997). Deletion of TUP1 results in disruption of the chromatin organization of SUC2, as well as derepression of the gene. Recently, the repression domain (Tzamarias and Struhl, 1994) and the histone-binding domain (Edmondson et al., 1996) of Tup1p have been shown to overlap, suggesting that these two functions are tightly coordinated. Consistent with this model, mutations in the histone tails that disrupt Tup1p–histone interactions have also been shown to cause partial derepression of the a cell-specific genes (Edmondson et al., 1996).
In this study, using the ChIP assay and the SALT series of minichromosomes, we have generated quantitative data showing that Tup1p associates with the STE6 nucleosomal domain in a ratio of two Tup1p molecules per nucleosome, or a tetramer per dinucleosome. Recent studies by others have suggested that Ssn6p associated Tup1p is a trimer (Redd et al., 1996) or a tetramer (Varanasi et al., 1996) in solution. We have confirmed a tetrameric structure using the analytical ultracentrifuge (unpublished observations). It is possible that the tetrameric state of Tup1p in vivo causes the STE6 chromatin in α cells to adopt the repeating dinucleosome pattern observed in high resolution mapping experiments (Simpson et al., 1993). Interestingly, we did not detect any Tup1p in the SALT1 construct containing a single nucleosome in the STE6 domain. This suggests that a stable Tup1p interaction with a repressed chromatin domain requires more than a single nucleosome. This result also suggests that the Tup1p interaction with Matα2p is either weaker than its interaction with the histones or that the Matα2p–Tup1p complex is only transiently associated with the gene.
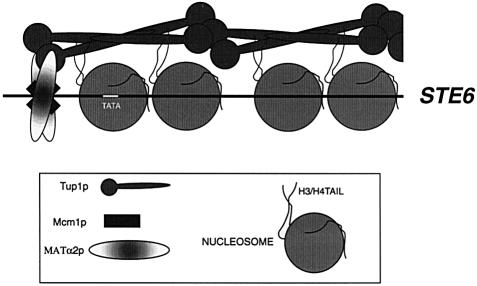
These findings, along with those of others, have suggested a more refined model for the repression of the a cell-specific genes in α cells. Our data suggest a model in which Tup1p is recruited to the gene by Matα2p and then forms a scaffold along the nucleosomal domain. This scaffold in turn stabilizes the positions of the nucleosomes in the repressive conformation or actively organizes the chromatin, resulting in the repressive chromatin structure (Figure 7). This model may provide an explanation for how the nucleosomes associated with STE6 are so precisely positioned in α cells. The model does not dismiss the possibility that Tup1p interacts with the basal transcription machinery and it may be that control of the a cell-specific genes in α cells requires multiple mechanisms to ensure full repression.
Fig. 7. Model for Tup1p-mediated repression. Tup1p is recruited to the STE6 gene by Matα2p and interacts with the H3/H4 tails forming a scaffold, which extends from the α2 operator to the 3′ end of the gene.
Our data also suggest a role for the acetylation state of histones in gene repression. Edmondson et al. (1996) have shown that Tup1p preferentially binds to mono- or non-acetylated histones in vitro. If this is the case in vivo, then recognition of underacetylated chromatin may be the mechanism by which Tup1p discriminates between the nucleosomes of genes to be repressed and the general population of chromatin. In the SALT minichromosomes, the unique association of Tup1p with the STE6 nucleosomes might result from increased stability of interactions with unacetylated H3/H4. However, decreased acetylation has been shown for telomeres and the HM loci (Braunstein et al., 1993), which are not Tup1p dependent, suggesting that Tup1p must be targeted to the specific chromatin before it can interact with the histones. We are in a unique position to determine whether the repressed chromatin structure of STE6 is differentially acetylated between a and α cells, as well as to explore additional features of how chromatin composition and structure lead to differential DNA function.
Materials and methods
The minichromosome backbone
The positions of nucleosomes and nuclease-hypersensitive regions of the TRP1/ARS1 minichromosome (Zakian and Scott, 1982) have been well characterized (Thoma and Simpson, 1985; Roth et al., 1990). Four unstable nucleosomes reside in the TRP1 coding region, and three stable nucleosomes are positioned over a region that contains a truncated section of the GAL3 promoter. The two nucleosomal domains are separated by two nuclease-hypersensitive regions, HSR A and HSR B, 3′ and 5′ to the TRP1 gene, respectively. The constitutive nuclease hypersensitivity of these regions suggests that they are accessible to trans-acting factors, making them candidate sites for insertion of our DNA of interest. HSR B was chosen as the location for the gene of interest and HSR A for the lac operator.
HSR A contains the ARS1 elements, necessitating taking care to introduce the lac operator in such a manner as not to disrupt replication origin function. Celniker et al. (1984) reported that the ARS element can be divided into two functional domains: A and B (Figure 3). Domain A contains the essential ARS consensus sequence, and domain B is a broad region to the 5′ side of domain A on the A-rich strand extending towards the TRP1 gene. Both domain A and B are contained in the nucleosome free, nuclease-hypersensitive region A of the TRP1/ARS1 minichromosome. Marahrens and Stillman (1992) have further shown that domain B can be subdivided into three elements, of which any two are sufficient for full ARS function. As shown in Figure 3, the lac operator was placed in a position that overlaps with the last three bases of the B2 element, and extends toward the B3 element. It has been proposed that the B2 element functions as an initiation site for DNA unwinding (Lin and Kowalski, 1997). Since only adenosine and thymidine bases were introduced, it seems likely that there would be no effect on the B2 element's function. In fact, there is no difference in the copy number of the ALT minichromosome and that of TRP1/ARS1 alone (data not shown).
Yeast strains and yeast plasmid construction
The yeast strains YPH499 (MATa ade2-101 ura3-52 his3-200 leu2-1 trp1-63 lys2-1) and YPH500 (MATα ade2-101 ura3-52 his3-200 leu2-1 trp1-63 lys2-1), which are isogenic except at the MAT locus (Sikorski and Hieter, 1989), were used in all experiments.
The lac operator sequence AATTGTTATCCGCTCACAATT was inserted into the TRP1/ARS1 (Zakian and Scott, 1982) plasmid, replacing the sequences between positions 779 and 800 (Thoma et al., 1984). This construct was subcloned into the EcoRI site of pBR322 vector to produce pALT. The ARS1/lac operator/TRP1 fragment from pALT was PCR amplified and cloned into pUC18 along with a fragment containing the STE6 coding sequence from position –690 to +4583 to create the plasmid SALT24. This plasmid was digested with NaeI and BasI to remove 2384 bp from the coding region of the STE6 gene to produce SALT10. The Mcm1p binding site in SALT10 was mutated using the primers GCGAAGCAGCGTGTAAATTTGGCTATTAGGTAATTACATGGC and GCCATGTAATTACCTAATAGCCAAATTTACACGCTGCTTCGC and the Quickchange Site-directed Mutagenesis Kit (Stratagene).
SALT6 was created from SALT10 by deleting 648 bp from the remaining coding region of the STE6 gene using KpnI and StyI. The overhangs were blunt ended by T4 polymerase, ligated together and cloned into E.coli. SALT1 was created from SALT6 by the additional deletion of 863 bp using BspHI and SspI. Prior to transforming the SALT constructs into yeast, pUC18 sequences were removed by digestion with SacI.
Minichromosome isolation
Yeast cultures (1 l) were grown to an OD600 of 1.0–1.5. Cells were harvested by centrifugation in a Sorvall GS3 rotor at 5000 g for 5 min. The cells were resuspended in 50 ml of sorbitol buffer [1.4 M sorbitol, 40 mM HEPES pH 8.0, 1 mM EDTA] containing 10 mM β-mercaptoethanol and 1 mM phenylmethylsulfonyl fluoride (PMSF), followed by centrifugation in a Sorvall H4 rotor at 5000 g for 5 min at 4°C. Cells were then resuspended in 30 ml of the same buffer and incubated at 30°C for 10 min, followed by collection in a preweighed tube by centrifugation in a Sorvall H4 rotor at 5000 g for 5 min at 4°C. The weighed pellet was resuspended in 4 volumes of sorbitol buffer containing 1 mM PMSF. Zymolyase 100T (Seikagaku Corp.) was added to a final concentration of 0.25 mg/ml. The cells were incubated for 20–30 min at 30°C with agitation. Spheroplast formation was determined microscopically. All steps after spheroplasting were performed on ice. Spheroplasts were gently washed twice with 30 ml of ice-cold sorbitol buffer containing 1 mM PMSF, and collected in a Sorvall H4 rotor at 3500 g for 5 min at 4°C. Washed spheroplasts were then gently resuspended in 10 ml mbb (20 mM HEPES pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.1% Tween 20) plus protease inhibitors (1 mM PMSF, 10 μg/ml A-protinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A) and chilled on ice for 15 min. The chilled spheroplasts were lysed in a Thomas® glass homogenizer and Teflon motor-driven pestle with ∼10 strokes. The resulting lysates were held on ice for 2–4 h with occasional agitation to allow the minichromosomes to diffuse passively from the nuclei. The lysates were then clarified by centrifugation in a Sorvall SS-34 rotor at 40 000 g for 20 min at 4°C. The supernatants were subjected to affinity chromatography.
Affinity chromatography
The lacI–Z affinity columns were prepared as follows. BL21(DE3) E.coli cells containing pTLIZ (see below) were inoculated into 100 ml of 2× YT broth (16 g Bacto-tryptone, 10 g Bacto-yeast extract, 5 g NaCl per liter, pH 7.5) and grown to an OD600 of 0.6–1.0. Cells were chilled on ice for 15 min and induced with 40 μM isopropylthio-β-d-galactopyranoside (IPTG). The induced cells were incubated at 15°C with shaking at 300 r.p.m. for 18–20 h. The cells were harvested by centrifugation in a Sorvall SS-34 rotor at 6000 g for 10 min at 4°C. Cells were then resuspended in 10 ml of ice-cold 1× chitin column buffer (20 mM HEPES pH 8.0, 1 mM EDTA, 0.1% Tween 20) containing 500 mM NaCl, and lysed by sonication. Sonication was performed on ice using a Branson sonifier 450 (VWR) on setting 6 with 30 s pulses alternating with 10 s intervals for 5 min total. The sonication was followed by centrifugation in a Sorvall SS-34 rotor at 12 000 g for 30 min at 4°C. The supernatants were passed through chitin–agarose (1 ml bed volume) at a flow rate of 0.25–0.5 ml/min. All subsequent steps were carried out at 4°C. The resin was washed twice. The first wash was with 15 ml of 1× chitin column buffer containing 500 mM NaCl at a flow rate of 1 ml/min. The second wash was 10 ml of mbb buffer at a flow rate of 1 ml/min.
Prior to starting the affinity chromatography 10 ml of mbb were run over the column at a flow rate of 1 ml/min to ensure proper buffer equilibration. The yeast supernatants containing the minichromosomes were mixed in batch with the chitin–lacI–Z matrix in mbb for 1 h at 4°C. The columns were then packed by running the slurry in at a flow rate of 0.5 ml/min. The columns were washed three times with 10 ml of mbb each at a flow rate of 1 ml/min. The columns were then equilibrated with 5 ml of mbb + 30 mM DTT at a flow rate of 2 ml/min, shut off and incubated overnight at 4°C. Protein and DNA were eluted from the columns with 5 ml of mbb containing 300 mM NaCl. The columns were stripped with 5 ml of 1× chitin column buffer containing 1% SDS. Chitin resin can be regenerated as described in the NEB IMPACT T7 manual. Nucleic acid was purified from samples taken throughout the isolation by treatment with 100 μg/ml RNase A at 37°C for 2 h, followed by 50 μg/ml proteinase K at 50°C for 2 h. The samples were phenol:chloroform extracted twice and ethanol precipitated.
Bacterial plasmids and Tup1p expression
TUP1 sequences were PCR amplified from the S.cerevisiae genome and cloned by XhoI and NdeI into the pTyb2 vector (New England Biolabs), and named pTT1. The lac repressor–β-galactosidase fusion sequence was amplified from the mutant E.coli strain BMH 72-19-1 (Müller-Hill and Kania, 1974). This sequence was also cloned by XhoI and NdeI into the pTyb2 vector, and named pTLIZ.
BL21(DE3) E.coli cells containing pTT1 were inoculated into 500 ml of 2× YT broth and grown to an OD600 of 0.6–1.0. Cells were chilled on ice for 15 min and induced with 40 μM IPTG. The induced cells were incubated at 15°C with shaking at 300 r.p.m. for 18–20 h. The cells were harvested by centrifugation in a Sorvall SS-34 rotor at 6000 g for 10 min at 4°C. Cells were then resuspended in 10 ml of ice-cold 1× chitin column buffer (20 mM HEPES pH 8.0, 1 mM EDTA, 0.1% Tween 20) containing 500 mM NaCl, and lysed by sonication. Sonication was performed on ice using a Branson sonifier 450 (VWR) as above. The sonication was followed by centrifugation in a Sorvall SS-34 rotor at 12 000 g for 30 min at 4°C. All subsequent steps were carried out at 4°C. The supernatants were passed through chitin–agarose (6 ml bed volume) at a flow rate of 0.25–0.5 ml/min. The resin was washed with 100 ml of 1× chitin column buffer containing 500 mM NaCl at a flow rate of 1 ml/min. The column was then equilibrated with 18 ml of 1× chitin column buffer containing 500 mM NaCl + 30 mM DTT at a flow rate of 2 ml/min, shut off and incubated overnight at 4°C. The following day, Tup1p was eluted from the columns with 5 ml of 1× chitin column buffer containing 500 mM NaCl. The columns were stripped with 5 ml of 1× chitin column buffer containing 1% SDS. Chitin resin was then regenerated as described in the NEB IMPACT T7 manual.
The concentration of Tup1p was determined by measuring the OD280 of the protein in the presence of 6 M guanidine, as well as by the Bradford assay. The molar extinction coefficient of Tup1p used to determine the concentration from the optical density measurements was calculated using the exPASy web site (http://www.expasy.ch/) (Gill and von Hippel, 1989).
ChIP
Chromatin immunoprecipitations were performed as described in Hecht et al. (1996). After DNA purification 25 rounds of PCR with the various primer pairs were run with 1/30th of the immunoprecipitates and 1/10 000th of the input as templates. Following PCR the entire reactions were run on a 2% TAE (40 mM Tris–acetate, 1 mM EDTA) agarose gel and visualized with ethidium bromide.
Immunoblotting
Protein samples were electrophoresed on 10% SDS–polyacrylamide gels followed by electrotransfer to polyvinylidene difluoride membranes. The membranes were incubated in phosphate-buffered saline containing 5% powdered milk (w/v) and 0.1% Tween 20 (PBST) at 4°C overnight. Membranes were then incubated with anti-Tup1p antibodies (provided by R.Trumbly) in PBST on a shaking platform for 2 h at 4°C. The membranes were washed 3× in PBST for 10 min each at room temperature. Anti-rabbit antibodies conjugated to horseradish peroxidase (Amersham Life Sciences, Inc.) were then incubated with the membranes in PBST on a shaking platform for 1 h at room temperature. The blots were washed 4× in PBST for 5 min each at room temperature. Blots were developed with ECL development reagents (Amersham Corp.) and exposed to Kodak XAR film.
Indirect end-label mapping
Yeast cells, transformed with the appropriate minichromosome, were grown at 30°C to an OD600 of 1.0 in 1 l of SC medium lacking the appropriate amino acids. Minichromosomes were isolated using the MAP technique as above and then concentrated and buffer exchanged into digestion buffer (10 mM HEPES pH 7.5, 0.5 mM MgCl2, 0.05 mM CaCl2). Isolated minichromosomes were then digested with micrococcal nuclease (MNase) in the presence of 36 μg of salmon sperm DNA as described by Weiss and Simpson (1997). Appropriately digested samples were cut to completion with the restriction endonuclease FspI and electrophoresed on a 1.5% agarose gel in 1× TBE (90 mM Tris–borate pH 8.3, 2 mM EDTA). The DNA was transferred to a nylon membrane (GeneScreen Plus, NEN Life Science Products), UV cross-linked and hybridized following the method described by Church and Gilbert (1984). A 283 bp PCR fragment of the STE6 gene, from +3780 to +4063, was random prime labeled with [α-32P]dATP to a specific activity of ∼109 c.p.m./μg, and used as a probe. The membranes were exposed to film or a PhosphorImager screen and analyzed using ImageQuant software (Molecular Dynamics).
Acknowledgments
Acknowledgements
We thank Dr R.J.Trumbly for the generous gift of the Tup1p antibodies, C.Young and J.Diller for review of the manuscript, and members of the Simpson and Workman groups for their criticism and technical advice. This study was supported by a grant from the National Institute of General Medical Sciences, National Institutes of Health, GM52311.
References
- Acton T.B., Zhong, H. and Vershon, A.K. (1997) DNA-binding specificity of Mcm1: operator mutations that alter DNA-bending and transcriptional activities by a MADS box protein. Mol. Cell. Biol., 17, 1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoyan J., Gupta, R., Thoma, F. and Smerdon, M.J. (1992) Transcription, nucleosome stability and DNA repair in a yeast minichromosome. J. Biol. Chem., 267, 5996–6005. [PubMed] [Google Scholar]
- Braunstein M., Rose, A.B., Holmes, S.G., Allis, C.D. and Broach, J.R. (1993) Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev., 7, 592–604. [DOI] [PubMed] [Google Scholar]
- Carrico P.M. and Zitomer, R.S. (1998) Mutational analysis of the Tup1 general repressor of yeast. Genetics, 148, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S.E., Sweder, K., Srienc, F., Bailey, J.E. and Campbell, J.L. (1984) Deletion mutations affecting autonomously replicating sequence ARS1 of Saccharomyces cerevisiae. Mol. Cell. Biol., 4, 2455–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G.M. and Gilbert, W. (1984) Genomic sequencing. Proc. Natl Acad. Sci. USA, 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.P., Roth, S.Y. and Simpson, R.T. (1994) The global transcriptional regulators, Ssn6p and Tup1p, play distinct roles in the establishment of a repressive chromatin structure. Genes Dev., 8, 1400–1410. [DOI] [PubMed] [Google Scholar]
- Dean A., Pederson, D.S. and Simpson, R.T. (1989) Isolation of yeast plasmid chromatin. Methods Enzymol., 170, 26–41. [DOI] [PubMed] [Google Scholar]
- Edmondson D.G., Smith, M.M. and Roth, S.Y. (1996) Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev., 10, 1247–1259. [DOI] [PubMed] [Google Scholar]
- Elledge S.J., Zhou, Z., Allen, J.B. and Navas, T.A. (1993) DNA damage and cell cycle regulation of ribonucleotide reductase. BioEssays, 15, 333–339. [DOI] [PubMed] [Google Scholar]
- Fong H.K.W., Hurley, J.B., Hopkins, R.S., Miaka-Lye, R., Johnson, M.S., Doolittle, R.F. and Simon, M.L. (1986) Repetitive segmental structure of the transducin β subunit: homology with the CDC4 gene and identification of related mRNAs. Proc. Natl Acad. Sci. USA, 83, 2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin I.M. and Simpson, R.T. (1997) Interplay of yeast global transcriptional regulators Ssn6p-Tup1p and Swi-Snf and their effect on chromatin structure. EMBO J., 16, 6263–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.C. and von Hippel, P.H. (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem., 182, 319–326. [DOI] [PubMed] [Google Scholar]
- Gutjahr T., Frei, E., Spicer, C., Baumgartner, S., White, R.A. and Noll, M. (1995) The Polycomb-group gene, extra sex combs, encodes a nuclear member of the WD-40 repeat family. EMBO J., 14, 4296–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A., Laroche, T., Strahl-Bolsinger, S., Gasser, S.M. and Grunstein, M. (1995) Histone H3 and H4 N–termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell, 80, 583–592. [DOI] [PubMed] [Google Scholar]
- Hecht A., Strahl-Bolsinger, S. and Grunstein, M. (1996) Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature, 383, 92–96. [DOI] [PubMed] [Google Scholar]
- Herschbach B.M., Arnaud, M.B. and Johnson, A.D. (1994) Transcriptional repression directed by the yeast a2 protein in vitro. Nature, 370, 309–311. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. (1989) A regulatory hierarchy for cell specialization in yeast. Nature, 342, 749–757. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhang, W.Z. and Roth, S.Y. (1997) Amino termini of histones H3 and H4 are required for a1-α2 repression in yeast. Mol. Cell. Biol., 17, 6555–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.D. and Herskowitz, I. (1985) A repressor (MATα2 product) and its operator control expression of a set of cell type specific genes in yeast. Cell, 42, 237–247. [DOI] [PubMed] [Google Scholar]
- Keleher C.A., Redd, M.J., Schultz, J., Carlson, M. and Johnson, A.D. (1992) Ssn6-Tup1 is a general repressor of transcription in yeast. Cell, 68, 709–719. [DOI] [PubMed] [Google Scholar]
- Komachi K. and Johnson, A.D. (1997) Residues in the WD repeats of Tup1 required for interaction with α2. Mol. Cell. Biol., 17, 6023–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi K., Redd, M.J. and Johnson, A.D. (1994) The WD repeats of Tup1 interact with the homeodomain protein α2. Genes Dev., 8, 2857–2867. [DOI] [PubMed] [Google Scholar]
- Kuchin S. and Carlson, M. (1998) Functional relationships of Srb10-Srb11 kinase, carboxy-terminal domain kinase CTDK-I and transcriptional corepressor Ssn6-Tup1. Mol. Cell. Biol., 18, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemontt J.F., Fugit, D.R. and MacKay, V.L. (1980) Pleiotropic mutations at the TUP1 locus that affect the expression of mating-type dependent functions in Saccharomyces cerevisiae. Genetics, 94, 899–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. and Kowalski, D. (1997) Functional equivalency and diversity of cis-acting elements among yeast replication origins. Mol. Cell. Biol., 17, 5473–5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahrens Y. and Stillman, B. (1992) A yeast chromosomal origin of replication defined by multiple functional elements. Science, 255, 817–823. [DOI] [PubMed] [Google Scholar]
- Morse R.H. (1993) Nucleosome disruption by transcription factor binding in yeast. Science, 262, 1563–1566. [DOI] [PubMed] [Google Scholar]
- Morse R.H., Pederson, D.S., Dean, A. and Simpson, R.T. (1987) Yeast nucleosomes allow thermal untwisting of DNA. Nucleic Acids Res., 15, 10311–10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse R.H., Roth, S.Y. and Simpson, R.T. (1992) A transcriptionally active tRNA gene interferes with nucleosome positioning in vivo. Mol. Cell. Biol., 12, 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B. and Kania, J. (1974) Lac repressor can be fused to β–galactosidase. Nature, 249, 561–562. [DOI] [PubMed] [Google Scholar]
- Patterton H.-G. and Simpson, R.T. (1994) Nucleosomal location of the STE6 TATA-box and MATa2p-mediated repression. Mol. Cell. Biol., 14, 4002–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson D.S., Venkatesan, M., Thoma, F. and Simpson, R.T. (1986) Isolation of an episomal yeast gene and replication origin as chromatin. Proc. Natl Acad. Sci. USA, 83, 7206–7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd M.J., Arnaud, M.B. and Johnson, A.D. (1997) A complex composed of Tup1 and Ssn6 represses transcription in vitro. J. Biol. Chem., 17, 11193–11197. [DOI] [PubMed] [Google Scholar]
- Roth S.Y., Dean, A. and Simpson, R.T. (1990) Yeast α 2 repressor positions nucleosomes in TRP1/ARS1 chromatin. Mol. Cell. Biol., 10, 2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S.Y., Shimizu, M., Johnson, L., Grunstein, M. and Simpson, R.T. (1992) Stable nucleosome positioning and complete repression by the yeast α 2 repressor are disrupted by amino-terminal mutations in histone H4. Genes Dev., 6, 411–425. [DOI] [PubMed] [Google Scholar]
- Sauer R.T., Smith, D.L. and Johnson, A.D. (1988) Flexibility of the yeast α2 repressor enables it to occupy the ends of its operator, leaving the center free. Genes Dev., 2, 807–816. [DOI] [PubMed] [Google Scholar]
- Schultz J. and Carlson, M. (1987) Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol. Cell. Biol., 7, 3637–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Roth, S.Y., Szent-Gyorgyi, C. and Simpson, R.T. (1991) Nucleosomes are positioned with base pair precision adjacent to the a2 operator in Saccharomyces cerevisiae. EMBO J., 10, 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter, P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R.T. (1990) Nucleosome positioning can affect the function of a cis-acting DNA element in vivo. Nature, 343, 387–389. [DOI] [PubMed] [Google Scholar]
- Simpson R.T., Roth, S.Y., Morse, R.H., Patterton, H.G., Cooper, J.P., Murphy, M., Kladde, M.P. and Shimizu, M. (1993) Nucleosome positioning and transcription. Cold Spring Harb. Symp. Quant. Biol., 58, 237–245. [DOI] [PubMed] [Google Scholar]
- Smerdon M.J. and Thoma, F. (1990) Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell, 61, 675–684. [DOI] [PubMed] [Google Scholar]
- Smerdon M.J., Bedoyan, J. and Thoma, F. (1990) DNA repair in a small yeast plasmid folded into chromatin. Nucleic Acids Res., 18, 2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.L. and Johnson, A.D. (1994) Operator-constitutive mutations in a DNA sequence recognized by a yeast homeodomain. EMBO J., 13, 2378–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter B., Livingstone-Zatchej, M. and Thoma, F. (1997) Chromatin structure modulates DNA repair by photolyase in vivo. EMBO J., 16, 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. and Richmond, T.J. (1998) Crystal structure of the yeast MATα2/MCM1/DNA ternary complex. Nature, 391, 660–666. [DOI] [PubMed] [Google Scholar]
- Thoma F. and Simpson, R.T. (1985) Local protein–DNA interactions may determine nucleosome positions on yeast plasmids. Nature, 315, 250–252. [DOI] [PubMed] [Google Scholar]
- Thoma F., Bergman, L.W. and Simpson, R.T. (1984) Nuclease digestion of circular TRP1/ARS1 chromatin reveals positioned nucleosomes separated by nuclease sensitive regions. J. Mol. Biol., 177, 715–733. [DOI] [PubMed] [Google Scholar]
- Tzamarias D. and Struhl, K. (1994) Functional dissection of the yeast Cyc8–Tup1 transcriptional co-repressor complex. Nature, 369, 758–761. [DOI] [PubMed] [Google Scholar]
- Tzamarias D. and Struhl, K. (1995) Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8–Tup1 corepressor complex to differentially regulate promoters. Genes Dev., 9, 821–831. [DOI] [PubMed] [Google Scholar]
- Varanasi U.S., Klis, M., Mikesell, P.B. and Trumbly, R.J. (1996) The Cyc8 (Ssn6)–Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol. Cell. Biol., 16, 6706–6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. and Simpson, R.T. (1997) Cell type-specific chromatin organization of the region that governs directionality of yeast mating type switching. EMBO J., 16, 4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F.E. and Trumbly, R.J. (1990) Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol. Cell. Biol., 10, 6500–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Simpson, R.T. and Kladde, M.P. (1998) Gal4p-mediated chromatin remodeling depends on binding site position in nucleosomes but does not require DNA replication. Mol. Cell. Biol., 18, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V.A. and Scott, J.F. (1982) Construction, replication and chromatin structure of TRP1 R1 circle, a multicopy synthetic plasmid derived from Saccharomyces cerevisiae chromosomal DNA. Mol. Cell. Biol., 2, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H. and Vershon, A.K. (1997) The yeast homeodomain protein MATα2 shows extended DNA binding specificity in complex with Mcm1. J. Biol. Chem., 272, 8402–8409. [DOI] [PubMed] [Google Scholar]