Zebrafish express two functionally different aquaporin zeros. One acts as a water channel. The other does not.
Abstract
Purpose.
AQP0, formerly known as MIP26, likely has multiple separate functions in the mammalian lens, including water transport, formation of thin junctions, and interactions with other lens components. Although mammalian genomes contain only one Aqp0 gene, the zebrafish genome contains two, Aqp0a and Aqp0b, and the putative multiple functions of the single mammalian protein may be divided between these two genes. The purpose of this study was to exploit this gene duplication and divergence to illuminate the multiple functions of AQP0 in the lens.
Methods.
Wholemount in situ hybridization and Western blot analyses were used to determine the expression pattern of Aqp0a and Aqp0b. The role of both proteins was studied in vivo by microinjection of antisense morpholino oligonucleotides in zebrafish. The water permeability of both proteins was tested using the Xenopus oocyte swelling assay and a yeast shrinkage assay.
Results.
Both genes, like their mammalian counterpart, are expressed in the lens. Morpholino knock-down of either gene alone led to cataract formation, indicating that both genes are necessary for normal lens development and transparency. Full-length Aqp0a is a functional water channel when expressed in Xenopus oocytes and in yeast, whereas Aqp0b was not. However, the addition of an HA-tag at its N terminus converted Aqp0b to a water channel in Xenopus oocytes.
Conclusions.
These results suggest that Aqp0a is the primary water channel of the lens and that Aqp0b, though possibly a secondary water channel, has an unidentified function in the lens.
Cataracts are the leading cause of blindness in the world.1 Most inherited cataracts have been associated with mutations in genes encoding proteins such as crystallins, connexins, or AQP0, with particular importance for the maintenance of lens transparency and homeostasis. The lens is a transparent avascular tissue that focuses light onto the retina. It consists of a single layer of epithelial cells that differentiate into elongated fiber cells, tightly packed in concentric layers. As the cells differentiate, their morphology and protein content change. For example, in the mammalian lens, AQP1 is expressed only in epithelial cells,2 whereas AQP0 is expressed exclusively in fiber cells.3 In addition, during differentiation the fiber cells lose their organelles and become metabolically inert. The center of the lens (lens nucleus) contains the most mature cells, and the lens periphery (lens cortex) contains the more recently differentiated cells (see Refs. 4, 5 for reviews).
AQP0 (previously MIP26) is the major intrinsic protein of the lens fiber cells3,6 and is primarily lens-specific, though several groups have recently found trace amounts of AQP0 in the liver and testis.7,8 In 1992, Peter Agre et al.9 characterized the first water channel AQP1 and subsequently placed MIP26 in the aquaporin family by sequence homology, renaming it AQP0. Aquaporins are membrane proteins presenting six transmembrane domains with the N- and C termini on the intracellular side, three extracellular loops (A, C, and E) and two asparagine-proline-alanine signature motifs (referred to as NPA boxes). Thirteen members of the aquaporin family have been described in humans. Some aquaporins are strictly water channels (AQP0, AQP1, AQP2, AQP4) whereas others transport water and small molecules such as glycerol and mannitol (AQP3, AQP7, AQP9).10,11 The functional importance of AQP0 in the lens is well established since several human congenital cataracts have been linked to mutations in the AQP0 gene.12–15 Congenital cataracts develop in both AQP0 knockout mice16 and cataract Fraser mice that have a defective AQP0.17,18
Although the importance of AQP0 in maintaining lens clarity is well known, the mechanisms by which it does so remain insufficiently described, partly because AQP0 is thought to have multiple functions. AQP0 was first described as an intercellular adhesion protein19 localized in regions called thin junctions, where the extracellular space between fiber cells is extremely small. Recently, double-layered, two-dimensional crystals of AQP0 revealed that AQP0 membrane junctions formed readily on proteolytic cleavage of the C terminus.20,21 The authors suggest that this reflects a change of primary function from a water channel in the cortex of the lens to an adhesion molecule when cleaved in the lens nucleus. AQP0 has a 20 to 40 times lower water permeability than AQP1 and other members of the aquaporin family,22–24 but its abundance in the fiber cells presumably compensates for its poor permeability. In the Xenopus oocyte expression system and in lens vesicle assays, AQP0 water permeability can be modulated by pH and calcium.25–27 Calmodulin binding to the AQP0 C terminus mediates the calcium regulation.26,28,29 In addition to its functions as a junctional protein and as a water channel, AQP0 interacts with the lens cytoskeletal proteins filensin and CP4930 and with the gap junction protein connexin 45.6 (the chick homolog of human connexin 50).31,32 These properties suggest the possibility of additional novel roles for AQP0 in the lens.
The zebrafish (Danio rerio) embryo has unique properties that make it an excellent model for studying the role(s) of AQP0 during development. The eye is large, develops in a few days,33 and is functional 3 days postfertilization (dpf).34,35 Zebrafish lens development and adult morphology are similar to those of other vertebrates, including mammals, with a few differences. For example, the zebrafish lens placode delaminates as a solid cluster of cells instead of a hollow lens vesicle as in mammals and birds.36,37 Nevertheless fiber cell differentiation and division closely follow the vertebrate pattern.38
Ray-finned fish (which include teleosts such as zebrafish) experienced a genomewide duplication event early in their evolution, and many single-copy mammalian genes are found as functional duplicates in this lineage. Commonly, the function or expression pattern of the original single-copy gene is divided between the duplicated copies, and this phenomenon enables analyses of their distinct functions.39 Analysis of the zebrafish genome reveals two aqp0 genes, Aqp0a and Aqp0b, closely related to the single mammalian ortholog, providing an opportunity to dissect the multiple functions of AQP0, which are subsumed in a single protein in mammals.
In this study we investigate the expression pattern of Aqp0a and Aqp0b genes during zebrafish development and describe the consequences of knocking down the expression of Aqp0a and Aqp0b in living zebrafish to lens development and transparency. Finally we study the water permeability of Aqp0a and Aqp0b expressed in Xenopus oocytes and in yeast. Collectively these data suggest that distinct functions of Aqp0a and Aqp0b are required for lens development in zebrafish.
Methods
Zebrafish and Embryo Maintenance
The animal protocols used in this study adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Zebrafish (AB strain) were raised and maintained under standard laboratory conditions.40 Embryos used for in situ hybridization were raised in 0.003% PTU (1-phenyl-2-thiourea; Sigma, St. Louis, MO) to prevent pigment formation, anesthetized in tricaine (3-aminobenzoic acid ethylester; Sigma), and fixed with 4% paraformaldehyde.
Wholemount In Situ Hybridization
Zebrafish Aqp0a and Aqp0b IMAGE clones (IMAGE 7140330 and IMAGE 7275955, respectively) were obtained from Open Biosystems (Huntsville, AL). Open reading frames of Aqp0a and Aqp0b were subcloned into the BamHI-BglII sites of pOTB7 (Berkeley Drosophila Genome Project) to produce probes for wholemount in situ experiments. Using SP6 and T7 RNA polymerase (mMACHINE Kit; Ambion Inc., Austin, TX), the antisense and sense RNA probes were synthesized and labeled with digoxigenin (Roche, Basel, Switzerland) in vitro. The wholemount in situ hybridization procedure was carried out as described previously.41,42 Fixed embryos at different stages were incubated with a digoxigenin-labeled probe, intensively washed, and incubated with an anti-digoxigenin antibody conjugated to alkaline phosphatase (Roche). The addition of alkaline phosphatase substrate BCIP (5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt) (Roche) in combination with NBT (nitro-blue tetrazolium chloride; Roche) yielded an intense insoluble purple precipitate that marks the mRNA of the gene of interest. Images were taken on a microscope (Axioplan 2; Zeiss, Thornwood, NY) using a camera (Axioplan; Zeiss) and Openlab software (Improvision; PerkinElmer, Waltham, MA; http://www.improvision.com/products/openlab/).
Morpholinos
Antisense morpholino oligonucleotides (MOs; Gene Tools, LLC, Philomath, OR) were designed to block translation start sites in Aqp0a (AACTCCCACATGGCTGCAAAAAGTC) and Aqp0b (CATAGAGCGAAACTCCCACATTATG). MOs were dissolved in Danieau buffer (NaCl 58 mM, KCl 0.7 mM, Hepes 5 mM, MgSO4 0.4 mM, Ca(NO3)2 0.6 mM) to make 1-mM stock solutions that correspond to approximately a concentration of MOs of 8 ng/nL; MOs for Aqp0a were further diluted to 4 ng/nL in Danieau buffer. Then 0.5 to 1 nL of this solution was injected into single cell-stage embryos. MO efficiency and specificity were confirmed by Western blot analysis comparing control and morphant embryos. Observations of live embryos were performed on a Nikon (Tokyo, Japan) microscope with Hoffman optics, and images of cataracts taken using a high-performance (Cohu, Poway, CA) charge-coupled device camera and ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Xenopus Oocyte Swelling Assay
Open reading frames of Aqp0a and Aqp0b were subcloned into the BglII site of the pXβG vector43 to synthesize cRNA to express Aqp0a and Aqp0b in Xenopus oocytes. Full-length cRNAs were generated by plasmid linearization with XbaI and transcription with T3 polymerase (mMACHINE Kit; Ambion Inc.). pXβG-AQP0 bovine was a gift from Peter Agre and Gregory Preston,43 and the Xenopus expression vector for MIPfun was a gift from Leila Virkki.44 An HA tag (YPYDVPDYA) or a Flag tag (DYKDADDDK) was added at the N terminus of Aqp0a and Aqp0b to generate HA-Aqp0a, Flag-Aqp0a, HA-Aqp0b, and Flag-Aqp0b.
Adult Xenopus laevis females were anesthetized, and stage V and VI oocytes were prepared and injected with 10 ng cRNA (or as stated), as previously described.22 The oocytes were incubated in Barth's solution (NaCl 88 mM, KCl 1 mM, HEPES 5 mM, CaCl2 0.41 mM, Ca(NO3)2 0.33 mM, MgSO4 0.82 mM, NaHCO3 2.4 mM; pH 7.4) for 2 or 3 days at 17°C. Swelling assays were performed at 20°C by transferring oocytes from 210 mOsm (100% ND96: NaCl 96 mM, KCl 2 mM, HEPES 5 mM, CaCl2 1.8 mM, MgCl2 1 mM, Na-pyruvate 2.5 mM; pH 7.4).) to 105 mOsm (50% ND96) solution adjusted to 1.8 mM calcium concentration and pH 7.4. Swelling rates were determined by an automated video imaging system using NIH Image software as previously described.25
Saccharomyces cerevisiae Water Permeability Assay
This protocol was modified from the Pichia pastoris assay developed by Daniels et al.45 Open reading frames of Aqp0a and Aqp0b were cloned into the BamHI or BamHI-HindII site of the pYES2 expression vector (Invitrogen, Carlsbad, CA), respectively. The resultant constructs, as well as an empty pYES2 vector control, were transformed by the lithium acetate method46 into the W303-1A strain of Saccharomyces cerevisiae (ATCC 208352). Cultures (100 mL) were inoculated with stationary phase-transformed cells and grown at room temperature (22°C) in synthetic raffinose media (0.67% yeast nitrogen base without amino acids, 1% raffinose, 2% [vol/vol] glycerol, 2% [vol/vol] lactic acid) supplemented with complete nutrients except uracil47 overnight to an OD600 of 0.7. Expression of Aqp0a and Aqp0b was induced by the introduction of galactose to 2%, and the cultures were allowed to grow for an additional 5 hours. The yeast cells were collected by centrifugation at 2200g for 10 minutes at room temperature. The pellets were resuspended in 1 mL ddH2O and spun again at 2000g for 5 minutes at 4°C centigrade. The resultant pellet was resuspended in 0.8 M sorbitol and vortexed very slowly for 1 hour at room temperature. Then 3000 U lyticase (Sigma-Aldrich) was added, and vortexing continued for another hour. The OD600 of the resultant spheroplasts was measured and adjusted to OD600 0.2 to 0.3 by dilution using 0.8 M sorbitol. Once all spheroplast solutions were at the same OD600, each sample was further diluted 1:10 in 0.8 M sorbitol. Diluted spheroplast (100 μL) was aliquotted into spectrophotometer cuvettes (1.5–2.5 mL capacity). Cuvettes were placed into a spectrophotometer (DU650; Beckman Coulter, Hialeah, FL) set to read OD600 every 4 seconds for 20 seconds, and 900 μL of either 0.8 M or 2.0 M sorbitol was added and the measurement started (T0). Samples that were tested with 0.8 M sorbitol as a control showed no change in OD600. The change in OD600 over the 20-second time course was calculated to produce ΔOD.
Membrane Protein Extracts and Immunoblot Analysis
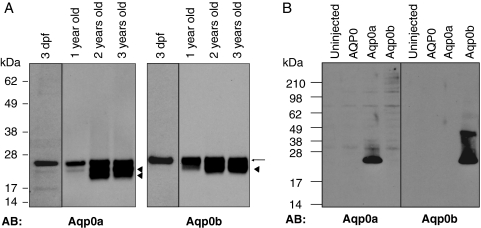
Lenses were dissected from adult fish 1, 2, and 3 years old. Larval eyes were dissected at 3 dpf. Lens and eye membranes were prepared with a protein extraction kit (ProteoExtract Native Membrane; Calbiochem, La Jolla, CA). For Western blot analysis, solubilized membrane proteins equivalent to of an adult lens or the equivalent of 20 larval eyes were loaded onto SDS gel (NuPage 4%–12%; Invitrogen). Membrane extracts from Xenopus oocytes were prepared, and solubilized membrane proteins equivalent to ¼ of an oocyte for each sample were loaded onto SDS gel (NuPage 4%–12%; Invitrogen). Polyclonal antibodies recognizing the zebrafish Aqp0a and Aqp0b were raised against the C-terminal peptides N-CVRGLSERLAVLKGNK-C and N-CFSERLATLKGSRPPE-C (Covance, Inc., Princeton, NJ) and were specific for Aqp0a and Aqp0b respectively, (see Fig. 3B).
Figure 3.
Expression of Aqp0a and Aqp0b in larval and adult zebrafish lens. (A) Western blot analyses were performed on membrane proteins from zebrafish 3-day-old, 1-year-old, 2-year-old, and 3-year-old lenses using antibodies directed against Aqp0a and Aqp0b, respectively. Both antibodies recognized a band at 26 kDa demonstrating the presence of Aqp0a and Aqp0b in the larval and adult zebrafish lens. Additional bands (arrowheads) are detected at lower molecular weight that could indicate the cleavage of Aqp0a and Aqp0b during aging. (B) Western blot analysis was performed on membrane proteins from Xenopus oocytes uninjected or expressing bovine AQP0, Aqp0a, or Aqp0b. The antibody directed against Aqp0a recognized a band at 26 kDa in the membranes of oocytes expressing Aqp0a. Similarly, the antibody directed against Aqp0b recognized a band at 26 kDa in the membranes from oocytes expressing Aqp0b. These Western blots demonstrate the specificity of the anti-Aqp0a and anti-Aqp0b antibodies. The anti-Aqp0b antibody also recognized a band at 40 kDa in the oocytes expressing Aqp0b, presumably a posttranslational modification of Aqp0b.
Pronase Treatment
Three days after cRNA injection Xenopus oocytes were incubated in Barth's solution with pronase (0.2 mg/mL) at 16°C for 16 hours with no agitation. The oocytes were washed in Barth's solution with protease inhibitor (Complete tablet; Roche), and the membrane extracts were immediately prepared as described. Solubilized membrane proteins equivalent to five oocytes of each sample were loaded onto SDS gel (NuPage 4%–12%; Invitrogen) and analyzed by Western blot.
Results
Two Aquaporin 0 Genes in Zebrafish and Other Teleosts
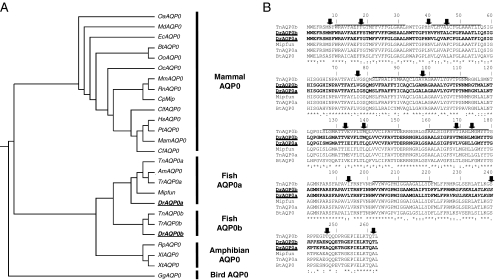
BLAST searches for AQP0 sequences in the entire pool of genomes available at NCBI revealed that two aqp0s are conserved between zebrafish (Danio rerio) and puffer fish (Tetraodron nigroviridis, Takifugu rubripes), most likely a product of the genomewide duplication that occurred at the base of the teleost fish lineage.48–51 Vihtelic et al.52 identified both zebrafish aqp0 genes in a lens library made from adults and named them MIP1 and MIP2; here we refer to them as Aqp0a and Aqp0b, in accordance with the aquaporin nomenclature. AQP0s from fish (zebrafish, puffer fish, puffer fish fugu, Mexican tetra, and killifish) are segregated into two distinct branches in the dendrogram: Aqp0as and Aqp0bs (Fig. 1A). Zebrafish Aqp0a is 96% similar to the killifish AQP0 (MIPfun), which is a water channel.44 Zebrafish Aqp0a and Aqp0b amino acid sequences share 85% identity (221 of 263 identical amino acids) and 95% similarity using the Gonnet similarity matrix.53 We predicted the structures of the two zebrafish AQP0s based on their sequences and compared them to the published mammalian AQP0 structures.21,54 Only 14 positions distinguish the dendrogram branch of Aqp0a sequences from the Aqp0b branch (Fig. 1B, black arrows), but the analysis of the sequence alignment gives little direct insight into why both genes have been retained or how they might differ in function. Residues that influence the pH sensitivity of AQP0 (histidine [H] 39, H40, H43, H122)25,26 differ both within and across species, suggesting variation in water-permeability regulation. The mammalian AQP0 C terminus presents several serines that are sometimes phosphorylated in the lens.55 Three serines (S229, S231, S235) are particularly important because they are located within a consensus calmodulin-binding site, and their phosphorylation determines the calcium sensitivity of AQP0 water permeability.29 Similarly, the C terminus of Aqp0a contains a potential calmodulin-binding site, but with only one serine residue (S231) instead of the three (S229, S231, S235). Aqp0b also presents a threonine at position 236. Both S231 and T236 of fish AQP0 are potential PKC phosphorylation sites (NetPhos). By analogy with bovine AQP0, this suggests that the water permeability of fish Aqp0s could be modulated by calcium by calmodulin binding and that phosphorylation could play a significant role.
Figure 1.
Sequence comparisons of AQP0s. (A) Dendrogram of AQP0s, generated by CLUSTALW using AQP0 sequences. AQP0 sequences from fish are divided into two distinct branches, Aqp0a and Aqp0b. Species with the entire genome data are italicized (OaAQP0-Ornithorhynchus, MdAQP0-Opposum, EcAQP0-Horse, BtAQP0-Bovine, OoAQP0-Sheep, OcAQP0-Rabbit, MmAQP0-Mouse, RnAQP0-Rat, CpAQP0-Guinea Pig, CfAQP0-Dog, HsAQP0-Human, PtAQP0-Chimpanzee, MamAQP0-Macacca, CfAQP0-Cat, TnAQP0-Pufferfish, AmAQP0-Mexican tetra, TrAQP0-Pufferfish fugu, MIPfun-Killifish, DrAQP0-Zebrafish, RpAQP0-Frog, XlAQP0-Xenopus laevis, XtAQP0-Xenopus tropicalis, GgAQP0-Chicken). (B) Sequence alignment of AQP0s from zebrafish (DrAqp0a and DrAqp0b), tetraodron (TnAqp0a and TnAqp0b), killifish (MIPfun), and bovine (BtAQP0), generated by CLUSTALW. Solid lines: six predicted transmembrane regions of aquaporins. Amino acid identities between the sequences are indicated with asterisks, strong similarities are indicated with colons, and weak similarities are indicated with dots. Of the positions that distinguish the cluster of Aqp0a sequences from the cluster of Aqp0b sequences (black arrows), half are in transmembrane domains that face another monomer in either the tetramer or the lipids. For example, positions 18 and 139 in mammalian AQP0 face each other in the neighboring monomer in the tetramer, position 46 faces itself, and position 77 faces the lipids. Almost all differences are in the lengths of the amino acids side chains (variations of V, L, I, M). For 8 of 14 positions Aqp0bs have the same amino acid as mammalian AQP0; for 5 of 6 remaining positions, Aqp0as have the same amino acid as mammalian AQP0.
Aqp0a and Aqp0b Expressed in the Lens
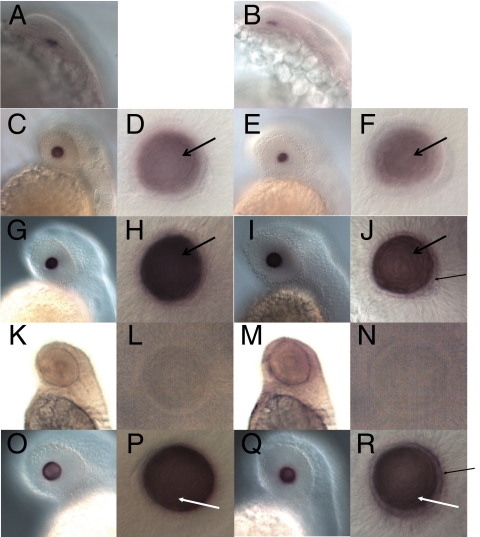
Wholemount in situ hybridization was performed on zebrafish embryos from 19 to 72 hours postfertilization (hpf) using Aqp0a and Aqp0b antisense and sense probes. A signal was first detected in the lens at 22 hpf for both probes (Figs. 2A, 2B) when the lens contained a small number of undifferentiated cells.36 A stronger signal was detected in the lens at 30 hpf and at later stages for both genes (Figs. 2C-J, 2O-R). At 30 hpf, a distinct monolayer of epithelial cells that do not express Aqp0a or Aqp0b was found to surround differentiating primary fiber cells expressing both genes. At 36 to 48 hpf, expression of Aqp0a and Aqp0b was detected in the primary and secondary fiber cells (Figs. 2G-J, 2O-R). Aqp0a was not detected in the surrounding epithelial cells, whereas a weak signal was visible for Aqp0b at 36 to 48 hpf. By 72 hpf, the expression faded from the center of the lens as the fibers further differentiated and lost their nuclei and other organelles (data not shown). Neither Aqp0a nor Aqp0b expression was detected anywhere outside the lens at any stage with either probe, and we did not detect any signal with sense probes (Figs. 2K-N). We conclude that both Aqp0a and Aqp0b expression are lens specific and persist throughout lens development.
Figure 2.
Aqp0a and Aqp0b expression during zebrafish development. Wholemount in situ hybridization detected Aqp0a (A, C, D, G, H, O, P) and Aqp0b (B, E, F, I, J, Q, R) transcripts in the lens at 22 hpf (A, B), 30 hpf (C–F), 36 hpf (G–J), and 48 hpf (O–R). No signal was detected using sense probes for Aqp0a (K, L) or Aqp0b (M, N) at 48 hpf. No transcripts were detected at earlier stages or in any other organs. Aqp0a and Aqp0b are transcribed in primary fiber cells at 30 hpf (black open arrows) and in differentiating secondary lens fiber cells (white arrows) after 36 hpf. Aqp0b is weakly detected in the lens epithelial cells (black arrows).
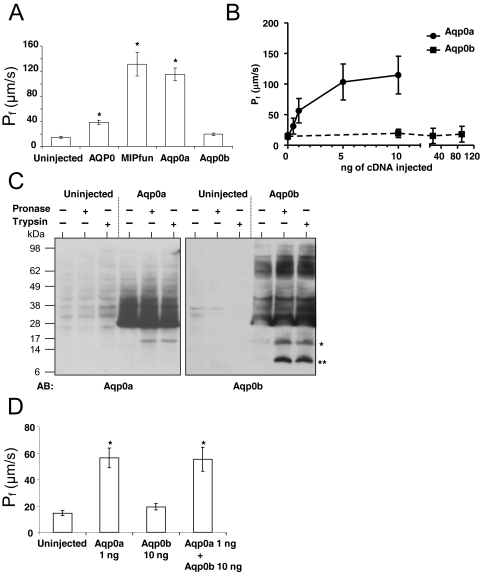
Western blot analyses confirmed the expression of both proteins in larval and adult lenses (Fig. 3A). We raised antibodies directed against the C terminus of Aqp0a, which recognized a band at 26 kDa that corresponded to the expected size of full-length AQP0. At 3 dpf, only full-length Aqp0a was detected, whereas in adult lenses there were two lower molecular weight bands of approximately 19 and 22 kDa. These lower molecular weight forms increased in abundance with age, such that the 1-year-old lens contained predominantly the intact protein and a little of the 22-kDa product, whereas the 2- and 3-year-old lenses also contained the 19-kDa form. These likely result from proteolysis of AQP0, as occurs in the mammalian lens during aging.55 Similarly, an Aqp0b antibody was raised that recognized a band at the expected full-length protein size (26 kDa) in 3 dpf and adult lenses and an additional lower band (∼22 kDa), which probably corresponded to a cleaved form in lenses older than 1 year. Taken together, these results suggest that Aqp0a and Aqp0b are both expressed during lens development and persist in the adult lens, where they undergo posttranslational modifications resulting in multiple cleaved forms.
Western blot analysis on membrane-enriched fractions from Xenopus oocytes overexpressing Aqp0a and Aqp0b confirmed the antibodies are specific for their target proteins (Fig. 3B). The Aqp0b antibody also recognized a band at approximately 40 kDa, which probably corresponded to a modified form of Aqp0b present only in the membranes of oocytes overexpressing Aqp0b.
Cataract Formation in Aqp0a and Aqp0b Morphants
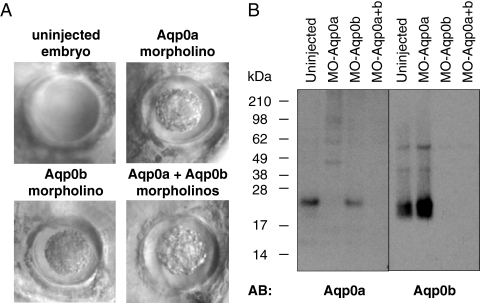
To test whether Aqp0a and Aqp0b are necessary for lens development, we depleted them from zebrafish embryos with antisense MOs designed to target translation-start sites and inhibit translation. In preliminary experiments, we injected 0.5 to 8 ng of each MO (Aqp0a-MO; Aqp0b-MO) to determine the toxicity and range in which specific phenotypes appear. Aqp0a-MO above 4 ng per embryo and Aqp0b-MO above 8 ng were toxic; therefore, we injected 2 to 4 ng Aqp0a-MO and 4 to 8 ng Aqp0b-MO. Single morphants for either AQP0 alone formed cataracts by 3 dpf, as did double morphants (Fig. 4A; 90% for Aqp0a-MO, 100% for Aqp0b-MO). Control lenses (from uninjected embryos) were transparent and appeared smooth under Hoffman optics, but morphant lenses contained a central cloudy region and appeared rough under Hoffman optics. Cloudiness ranged from a small central area to larger cataracts encompassing most of the lens. In rare cases the same fish had one eye with a cataract while the other appeared unaffected, most likely because of asymmetric distribution of MO. Other aspects of embryo morphology were unaffected. We confirmed that each MO was specific for its target by performing Western blot analysis on membrane proteins from MO-injected (morphant) eyes (Fig. 4B). Thus both Aqp0a and Aqp0b are necessary for lens transparency, supporting the hypothesis that Aqp0a and Aqp0b have distinct functions.
Figure 4.
Aqp0a and Aqp0b morphants developed cataract. (A) Microscopic observation with Hoffman optics of Aqp0a, Aqp0b, and double morphants revealed marked opacity and roughness in the lens core at 3 dpf. In contrast, the control lenses were smooth looking. (B) Aqp0a and Aqp0b expression in morphants eyes. Western blot analysis was performed on membrane proteins from eyes from 3 dpf control embryos and Aqp0a morphants, Aqp0b morphants, and double morphants using antibodies directed against Aqp0a and Aqp0b, respectively.
Aqp0a Is a Water Channel But Aqp0b Is Not
We tested the water permeability of Aqp0a and Aqp0b using two experimental approaches: the Xenopus oocyte-swelling assay and a novel assay using S. cerevisiae spheroplasts.
Zebrafish Aqp0a and Aqp0b were expressed in Xenopus oocytes, which were then subjected to a hypotonic challenge with their swelling rate recorded. This rate was used to calculate the water permeability (Pf) for oocytes injected with 10 ng cRNA for Aqp0a and Aqp0b (Fig. 5A). Bovine AQP0 and killifish MIPfun served as positive controls for aquaporin-induced permeability. Aqp0a increased water permeability as efficiently as MIPfun, whereas Aqp0b-injected oocytes showed no significant difference from uninjected controls. To measure the dose response of each type of AQP0, oocytes were injected with varying amounts of Aqp0a or Aqp0b cRNA (Fig. 5B). For Aqp0a, permeability increased steeply between 0 and 1 ng and reached a plateau at 5 to 10 ng. In contrast, Aqp0b cRNA did not increase water permeability, even when oocytes were injected with 90 ng. We conclude from these results that Aqp0a acts as a water channel whereas Aqp0b does not, at least in the Xenopus oocyte expression system.
Figure 5.
Aqp0a is a water channel, but Aqp0b is not. (A) Xenopus oocytes were injected with 10 ng cRNA encoding for Aqp0a, Aqp0b, bovine AQP0, and MIPfun. After 48 hours, the swelling assay was performed in standard conditions (ND96 50%, 1.8 mM calcium, pH 7.4), and the water permeability was calculated from the rate of swelling. Aqp0a clearly presented a water permeability significantly different from that of uninjected oocytes and similar to that of MIPfun. Aqp0b-expressing oocytes were not significantly different from uninjected oocytes. *P < 0.0005 using the Student's t-test. (B) Xenopus oocytes were injected with 0.5, 1, 5, and 10 ng Aqp0a cRNA or with 10, 20, and 90 ng Aqp0b cRNA, and their water permeability was measured. Aqp0a water permeability leveled after 5 ng cRNA was injected, whereas Aqp0b did not exhibit significant water permeability even after 90 ng cRNA was injected. (C) Uninjected oocytes and oocytes expressing Aqp0a and Aqp0b were incubated for 16 hours at 17°C with pronase or with trypsin. A band at 17 kDa (asterisk) for both Aqp0a and Aqp0b indicated that loop C of the proteins was cut. Aqp0b also exhibited a smaller band (asterisks), indicating that the loop E was cut by both pronase and trypsin. Note the difference of intensity of the bands between Figures 5B and 3B is explained by the differences in the amount of protein loaded (one-quarter of an oocyte [Fig. 3B] vs. five oocytes). (D) Xenopus oocytes were injected with 1 ng Aqp0a cRNA, 10 ng Aqp0b cRNA or 1 ng Aqp0a cRNA, and 10 ng Aqp0b cRNA, and their water permeability was measured. The water permeability of the coinjected oocytes was the same as that of oocytes injected with Aqp0a cRNA alone.
Western blot analysis on membrane-enriched fractions from Xenopus oocytes overexpressing Aqp0a and Aqp0b confirmed that both proteins were present (Fig. 3B). To determine whether Aqp0b was present in the plasma membrane (and not trapped in other membranes inside the oocyte), we treated intact cells with trypsin or pronase56,57 (Fig. 5C). Trypsin cuts on the C-terminal side of arginines and lysines. The only trypsin sites accessible externally are Arg113 and Arg196 in the second and third extracellular loops. The C terminus antibody for Aqp0b works well on Western blots and shows the predicted fragments for cuts by pronase and trypsin at the extracellular loop sites. The predicted fragment molecular weights are 16.4 kDa and 7.6 kDa, and the observed values on the blot are 27 kDa for the full-length protein (predicted actual MW 28.8 kDa) and 18 kDa and 10 kDa for the Aqp0b fragments. Thus the molecular weights of the observed fragments are within experimental error of the expected values.
Although the differences between Aqp0a and Aqp0b are small, some possibly critical differences occur in the external loops near the trypsin-site-forming arginines. In loop C, the second external loop, pronase and trypsin cut both proteins (the trypsin site is Arg113), giving a fragment of the expected molecular weight. In loop E, the third external loop, Aqp0a has a tyrosine where Aqp0b has threonine. Pronase and trypsin both give the predicted short fragment for Aqp0b but not for Aqp0a (Fig. 5C). The probable explanation for this is that the large tyrosine ring somehow reduces the accessibility of the adjacent arginine to protease cleavage, possibly by steric hindrance or by shifting the arginine deeper into the membrane.
Pronase is a cocktail of enzymes containing trypsin and chemotrypsin, along with multiple additional enzymes likely to cut almost any accessible portion of the protein. Pronase typically breaks most soluble proteins down into their component amino acids. In our experiment, pronase cut in a pattern indistinguishable from that of trypsin. Thus pronase can have access only to the external loops where trypsin cuts. Notably, it cannot have access to the C terminus, which it would destroy, leaving nothing to be seen on the Western blot. The ability of external proteolytic enzymes to cleave Aqp0b at the predicted external sites without cleaving any of the internal sites clearly demonstrates its presence in the plasma membrane. Additionally, the distinct patterns of proteolytic cleavage indicate differences between the structure of the extracellular loops of Aqp0a and Aqp0b.
To test the hypothesis that Aqp0b could alter the water permeability of Aqp0a, we coinjected Aqp0a and Aqp0b cRNA. However, the water permeability did not differ from that of Aqp0a alone (Fig. 5D). Although we do not know whether the two aquaporins physically interact, they do not have a functional interaction, at least as assayed by water permeability in Xenopus oocytes.
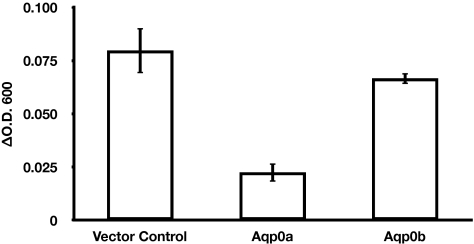
Because posttranslational processing differs in different expression systems and such processing might account for our failure to observe a water permeability increase for Aqp0b, we expressed both Aqp0a and Aqp0b in the W303-1A strain of the yeast S. cerevisiae. We chose the W303-1A strain because it expresses two native but inactive aquaporins and, therefore, has no endogenous aquaporin-mediated water permeability.58 The yeast expression system has been used successfully for functional studies of aquaporins that failed to localize to the plasma membrane in Xenopus oocytes.59 We confirmed expression of both types of AQP0 in yeast membranes by Western blot (data not shown) and tested for water channel activity using a yeast spheroplast shrinking assay. Changes in cell shape and volume can be determined by photometry,60 and studies on wild-type P. pastoris spheroplasts showed that changes in optical absorbance were inversely proportional to the changes in external osmolarity.45 In our assay, spheroplast shrinking in response to hyper-osmotic shock was monitored by measuring ΔOD600 between T0 and T20sec. Control spheroplasts and spheroplasts expressing nonfunctional water channels shrink significantly over the course of the 20-second experiment (Fig. 6). In contrast, spheroplasts expressing functional water channels such as Aqp0a exhibited a much smaller increase in ΔOD600 between T0 and T20sec, and we deduced that they did not shrink as much as the control spheroplasts between T0 and T20sec. Stop-flow experiments showed that the hyperosmotic-induced change in light scattering of proteoliposomes containing water channels was essentially complete in less than 2 seconds.59 Based on this information, we infer that spheroplasts expressing functional water channels shrink nearly instantaneously with hyperosmotic shock and are therefore already predominantly shrunk before T0. Minimal further shrinking was observed over the 20-second experiment. The observed change in OD was, therefore, smaller in spheroplasts expressing functional water channels than in control spheroplasts or those expressing nonfunctional water channels. The observed change in OD is inversely correlated to the water permeability of the spheroplast. The ΔOD600 between T0 and T20sec for spheroplasts expressing Aqp0b was not significantly different from the control spheroplasts, indicating that Aqp0b is not a functional water channel in S. cerevisiae. Thus both proteins behave in yeast just as they do in the Xenopus expression system, further demonstrating that Aqp0a and Aqp0b have distinct functions.
Figure 6.
Aqp0a and Aqp0b water permeability in yeast spheroplasts. Water permeability was tested for yeast spheroplasts expressing Aqp0a or Aqp0b. Spheroplasts containing a blank vector were used as a control. Control spheroplasts and spheroplasts expressing Aqp0b displayed increased absorption at 600 nm (significant shrinking) over the course of the 20-second experiment, resulting in a positive ΔOD600. Spheroplasts expressing Aqp0a shrank nearly instantaneously with the hyperosmotic shock and were, therefore, shrunk before the first time point of the 20-second experiment, resulting in a lower magnitude ΔOD600. Water permeability and ΔOD600 were inversely related. Aqp0b water permeability was not statistically different from that of the control (P > 0.1). *P < 0.02 using the Student's t-test.
Effect of Tags on the Water Permeability of Aqp0a and Aqp0b
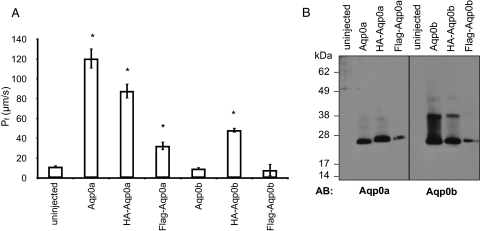
We tagged the N terminus of Aqp0a and Aqp0b with HA or Flag to detect the proteins by Western blot using available antibodies. We expressed the tagged proteins in Xenopus oocytes and measured their water permeability (Fig. 7A). Interestingly, HA-Aqp0b–injected oocytes showed water permeability significantly larger than oocytes injected with untagged Aqp0b or uninjected oocytes. HA-Aqp0a and Flag-Aqp0a had water permeability significantly greater than that of uninjected oocytes but lower than that of untagged Aqp0a. Three days after the injection of HA-Aqp0a, HA-Aqp0b, or Flag-Aqp0a, water permeability was higher than at 2 days, suggesting that their trafficking to the plasma membrane was slower than that of wild-type Aqp0a (data not shown). Flag-Aqp0b did not exhibit any water permeability 2 or 3 days after injection, even though the proteins were expressed in the oocytes (Fig 7B).
Figure 7.
Effect of HA and FLAG tags on Aqp0a and Aqp0b water permeability. (A) Xenopus oocytes were injected with 10 ng Aqp0a, HA-Aqp0a, Flag-Aqp0a, Aqp0b, HA-Aqp0b, or Flag-Aqp0b, and their water permeability was subsequently measured. Oocytes expressing Aqp0a and HA-Aqp0a exhibited similar water permeability, demonstrating that the HA tag had no effect on Aqp0a in standard conditions. Surprisingly, oocytes expressing HA-Aqp0b presented water permeability significantly different from that of uninjected oocytes and oocytes expressing Aqp0b. Flag-Aqp0a water permeability was significantly different from that of uninjected oocytes but lower than that of Aqp0a. Flag-Aqp0b-expressing oocytes were not different from uninjected oocytes. *Significantly different from uninjected. (B) Western blot analyses were performed on membrane proteins from Xenopus oocytes uninjected or expressing Aqp0a, HA-Aqp0a, Flag-Aqp0a, Aqp0b, HA-Aqp0b, and Flag-Aqp0b. Anti-Aqp0a and anti-Aqp0b antibodies recognized a band at 26 kDa in the membranes of oocytes expressing Aqp0a and Aqp0b and a slightly higher band in HA-Aqp0a–, Flag-Aqp0a–, HA-Aqp0b–, and Flag-Aqp0b–expressing oocytes. Note that the extra band at 40 kDa in the oocytes expressing Aqp0b was also present in the oocytes expressing HA-Aqp0b, demonstrating that this presumed modification was not responsible for the lack of function of Aqp0b.
We performed Western blots on membrane proteins from Xenopus oocytes expressing Aqp0a, HA-Aqp0a, Flag-Aqp0a, Aqp0b, HA-Aqp0b, or Flag-Aqp0b (Fig. 7B). The antibody directed against Aqp0a recognized a band at 26 kDa in the membranes from oocytes expressing Aqp0a and a slightly higher band in HA-Aqp0a and in Flag-Aqp0a expressing oocytes. Anti-Aqp0b antibody recognized a band at 26 kDa in the membranes from oocytes expressing Aqp0b and a slightly higher band in oocytes expressing HA-Aqp0b and Flag-Aqp0b. The band at 40 kDa present in the oocytes expressing Aqp0b was also expressed in the HA-Aqp0b oocytes. Because the same MW-increasing posttranslational modification occurred in both the functional water channel and the dysfunctional Aqp0b, it could not account for the lack of water permeability.
We suggest that Aqp0b is a closed water channel under the conditions we tested in both Xenopus oocytes and yeast and that the addition of the HA tag deregulates the gating mechanism, resulting in channel opening. It is worth noting that of the two different tags, HA (YPYDVPDYA) and Flag (DYKDADDDK), only HA resulted in increased water permeability for Aqp0b. HA is the least charged and is hydrophilic, properties that could promote interactions with other parts of the protein.
Discussion
Our results clearly establish that two AQP0 genes in zebrafish are essential for normal lens development and clarity and that the two forms have distinct functional properties.
We show that Aqp0a and Aqp0b transcripts are expressed exclusively in the lenses of zebrafish embryos starting at 22 hpf at the onset of differentiation (Fig. 2) and in adult lenses, similar to mammalian AQP0.61 A recent study by Tingaud-Sequeira et al.62 using RT-PCR confirms the expression of Aqp0a and Aqp0b in the adult eye and, interestingly, detected the Aqp0b transcript in ovarian tissues. The pattern of expression of zebrafish Aqp0s suggests that AQP0 plays a conserved role in lens development across vertebrates.
In mammals AQP0 is essential for lens development, transparency, and homeostasis. Several human families with mutations of AQP012–15 as well as knockout mice16 present a congenital cataract. Similarly, the depletion of both AQP0s, Aqp0a and Aqp0b, led to cataract formation at 72 hpf in zebrafish. This phenotype suggests that Aqp0a and Aqp0b together supply all the necessary AQP0 functions in the lens. All AQP0 morphants presented central cataracts at 72 hpf, 2 days after Aqp0a and Aqp0b mRNAs were first detected in the lens placode, suggesting that neither is necessary for early lens development. Similarly, the deficiency of AQP0 in mouse does not prevent lens fiber differentiation and elongation but is essential for the establishment and maintenance of both individual fiber shape and overall fiber arrangement.63
Interestingly, the depletion of each Aqp0a and Aqp0b separately led to cataract formation. One could propose that after the gene duplication event both proteins retained all the ancestral gene functions and that the quantitative contribution of both genes is required in the lens. We offer three arguments against this suggestion. First, the Aqp0a transcript is far more abundant than the Aqp0b transcript52; hence, if their functions are simply additive, depleting Aqp0a should produce a stronger or an earlier cataract than depleting Aqp0b would produce. Second, mouse lenses expressing half the amount of AQP0 (heterozygotes AQP0±) are transparent at birth and become translucent at 3 weeks but never develop cataracts.63 Third, Aqp0a is a functional water channel in Xenopus oocytes and in yeast whereas Aqp0b is not. This alone establishes that the functionality of the ancestral AQP0 is not uniformly distributed among the two fish proteins. Therefore, we suggest that the AQP0 gene duplication in fish resulted in functional partitioning in which Aqp0a and Aqp0b each performs some, but not all, of the ancestral AQP0 functions. Thus the results of our MO experiments are best explained if each protein has a distinct function or, alternatively, if each confers water permeability at a different location or at a different time. Aqp0a most likely acts as a water channel in the zebrafish lens. Aqp0b either does not act as a water channel or is regulated differently from Aqp0a. The fact that the addition of an N terminus HA tag renders Aqp0b water permeable implies that it may be able to operate as a water channel under certain conditions in the lens.
Alternatively, Aqp0b may perform other essential functions of AQP0 in the lens, such as in the formation of thin junctions. AQP0 is found in microdomains called thin junctions, described as ribbons, whereas AQP0 tetramers face an opposing particle-free plasma membrane resulting in an extremely narrow (0.5–0.7 nm) extracellular space compared with gap junction thickness (2–3 nm).64,65 The capacity of AQP0 to form thin junctions was the first function described for AQP0.19,66–69 Additionally, in double-layered crystals of carboxyl-terminally cleaved AQP0, the extracellular loops of opposing tetramers interact to form membrane junctions.20,70 The water permeability of AQP0 in the junctional form remains a matter of debate.21,71 The crystal structure of the junctional form of AQP0 implies a closed channel.21 Hence, we propose that Aqp0b is a closed pore that forms thin junctions in the zebrafish lens. We can suggest a parallel mechanism that closes the channel of mammalian AQP0 and creates a junctional protein when its C terminus is cleaved and that opens Aqp0b when a HA tag is added at its N terminus. Both protein modifications occur on the cytoplasmic side of the membrane and suggest conformational changes in the protein that lead to changes in the function of AQP0.
Furthermore, most of the known AQP0-interacting proteins—connexins, crystallins, and intermediate-filament proteins—are found in zebrafish. If those proteins have the same roles in the zebrafish lens as their mammalian orthologs do, they probably interact in a similar fashion with either Aqp0a or Aqp0b. Mammals express three different connexins necessary for normal lens development and transparency72: Cx43 in the epithelial cells, Cx46 in the fiber cells, and Cx50 in both epithelial and fiber cells. The association of AQP0 with connexins 50 and 46 in “mixed-junctions,”64 the dramatically altered size of gap junctions in AQP0-deficient mice,63,73 and the direct interaction of chick AQP0 with Cx45.6 (ortholog to human Cx50) during early lens development31,32 together imply a close relationship between connexins and AQP0 in the lens in different species. All three mammalian lens connexins have orthologs in zebrafish,74 and the depletion of zebrafish Cx48.5 (ortholog of human Cx46) leads to cataract formation,75 suggesting equivalent roles for zebrafish connexins and their mammalian counterparts and possible interactions with Aqp0b, Aqp0a, or both. AQP0 also interacts with four major crystallins (αA, αB, βB2, and γC), effectively recruiting the crystallins to the plasma membrane most likely to reduce differences in refractive index at the interfaces of membrane and cytoplasm.76–79 In zebrafish, α, β, and γ crystallins are also expressed in the lens from 24 hpf,80,81 consistent with interactions with Aqp0b, Aqp0a, or both. Additionally, AQP0 interacts (directly or indirectly) with intermediate filaments in the lens, beaded filaments formed by filensin and CP49,30 which may provide the means for cortical fibers to form and maintain their hexagonal fiber cell shape. CP49 (but not filensin) has been found in an adult zebrafish cDNA library.52 The separation of functions between two AQP0s in zebrafish offers a unique opportunity to investigate the role of those interactions in lens development and transparency.
In conclusion, we have shown that both zebrafish Aqp0s are expressed in the lens and are necessary for proper lens development and transparency. Aqp0a, but not Aqp0b, is a functional water channel in Xenopus oocytes and in S. cerevisiae. We suggest that in the lens, Aqp0a supplies water permeability and Aqp0b adhesion or interactions (or both) with other lens components. Our results demonstrate conserved requirements for AQP0 in lens development as well as lens transparency and that mammalian AQP0 functions are distributed between Aqp0a and Aqp0b in teleosts. This work further illustrates how the disruption of any function or subfunction of essential lens proteins, such as AQP0, can lead to cataracts. A better understanding of cataract formation will permit drug designs that will potentially have major impacts on human health and welfare.
Acknowledgments
The authors thank Alok Mitra for his contribution to the development of the yeast assay; Michael Cahalan, George Chandy, and Srikant Rangaraju for their critical readings of the manuscript; and Tailin Zhang, Jessica Langer, and Stephanie Langer for their help on the project.
Footnotes
Supported by National Institutes of Health Grants EY 5661 (JEH), NS-41353 (TFS), and DE-13828 (TFS).
Disclosure: A. Froger, None; D. Clemens, None; K. Kalman, None; K.L. Németh-Cahalan, None; T.F. Schilling, None; J.E. Hall, None
References
- 1. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 2. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993;90:11663–11667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broekhuyse RM, Kuhlmann ED, Winkens HJ. Lens membranes, VII: MIP is an immunologically specific component of lens fiber membranes and is identical with 26K band protein. Exp Eye Res. 1979;29:303–313 [DOI] [PubMed] [Google Scholar]
- 4. McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13(pt 3b):425–437 [DOI] [PubMed] [Google Scholar]
- 5. Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490 [DOI] [PubMed] [Google Scholar]
- 6. Gorin MB, Yancey SB, Cline J, Revel JP, Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984;39:49–59 [DOI] [PubMed] [Google Scholar]
- 7. Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes: evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710–22717 [DOI] [PubMed] [Google Scholar]
- 8. Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505 [DOI] [PubMed] [Google Scholar]
- 9. Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387 [DOI] [PubMed] [Google Scholar]
- 10. Froger A, Tallur B, Thomas D, Delamarche C. Prediction of functional residues in water channels and related proteins. Protein Sci. 1998;7:1458–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698 [DOI] [PubMed] [Google Scholar]
- 12. Francis P, Chung JJ, Yasui M, et al. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–2334 [DOI] [PubMed] [Google Scholar]
- 13. Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–17 [DOI] [PubMed] [Google Scholar]
- 14. Gu F, Zhai H, Li D, et al. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol Vis. 2007;13:1651–1656 [PubMed] [Google Scholar]
- 15. Lin H, Hejtmancik JF, Qi Y. A substitution of arginine to lysine at the COOH-terminus of MIP caused a different binocular phenotype in a congenital cataract family. Mol Vis. 2007;13:1822–1827 [PubMed] [Google Scholar]
- 16. Shiels A, Bassnett S, Varadaraj K, et al. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186 [DOI] [PubMed] [Google Scholar]
- 17. Shiels A, Mackay D, Bassnett S, Al-Ghoul K, Kuszak J. Disruption of lens fiber cell architecture in mice expressing a chimeric AQP0-LTR protein. FASEB J. 2000;14:2207–2212 [DOI] [PubMed] [Google Scholar]
- 18. Kalman K, Nemeth-Cahalan KL, Froger A, Hall JE. AQP0-LTR of the cat Fr mouse alters water permeability and calcium regulation of wild type AQP0. Biochim Biophys Acta. 2006;1758:1094–1099 [DOI] [PubMed] [Google Scholar]
- 19. Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein composition of lens fiber junctions. J Cell Biol. 1989;108:2255–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004;342:1337–1345 [DOI] [PubMed] [Google Scholar]
- 21. Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004;429:193–197 [DOI] [PubMed] [Google Scholar]
- 22. Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159:29–39 [DOI] [PubMed] [Google Scholar]
- 23. Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146 [DOI] [PubMed] [Google Scholar]
- 24. Mulders SM, Van der Kemp AJ, Terlouw SA, Van Boxtel HA, Van Os CH, Deen PM. The exchange of functional domains among aquaporins with different transport characteristics. Pflugers Archiv Eur J Physiol. 1998;436:599–607 [DOI] [PubMed] [Google Scholar]
- 25. Németh-Cahalan K, Kalman K, Hall JE. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol. 2004;123:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Németh-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782 [DOI] [PubMed] [Google Scholar]
- 27. Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402 [DOI] [PubMed] [Google Scholar]
- 28. Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, Schey KL. Aquaporin 0-calmodulin interaction and the effect of aquaporin 0 phosphorylation. Biochemistry. 2008;47:339–347 [DOI] [PubMed] [Google Scholar]
- 29. Kalman K, Nemeth-Cahalan KL, Froger A, Hall JE. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. J Biol Chem. 2008;283:21278–21283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindsey Rose KM, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570 [DOI] [PubMed] [Google Scholar]
- 31. Yu XS, Jiang JX. Interaction of major intrinsic protein (aquaporin-0) with fiber connexins in lens development. J Cell Sci. 2004;117:871–880 [DOI] [PubMed] [Google Scholar]
- 32. Yu XS, Yin X, Lafer EM, Jiang JX. Developmental regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C terminus of major intrinsic protein (aquaporin-0). J Biol Chem. 2005;280:22081–22090 [DOI] [PubMed] [Google Scholar]
- 33. Glass AS, Dahm R. The zebrafish as a model organism for eye development. Ophthalmic Res. 2004;36:4–24 [DOI] [PubMed] [Google Scholar]
- 34. Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio). Dev Biol. 1996;180:646–663 [DOI] [PubMed] [Google Scholar]
- 35. Easter SS, Jr, Nicola GN. The development of eye movements in the zebrafish (Danio rerio). Dev Psychobiol. 1997;31:267–276 [DOI] [PubMed] [Google Scholar]
- 36. Dahm R, Schonthaler HB, Soehn AS, van Marle J, Vrensen GF. Development and adult morphology of the eye lens in the zebrafish. Exp Eye Res. 2007;85:74–89 [DOI] [PubMed] [Google Scholar]
- 37. Soules KA, Link BA. Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev Biol. 2005;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greiling TM, Clark JI. Early lens development in the zebrafish: a three-dimensional time-lapse analysis. Dev Dyn. 2009;238:2254–2265 [DOI] [PubMed] [Google Scholar]
- 39. Postlethwait J, Amores A, Cresko W, Singer A, Yan YL. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004;20:481–490 [DOI] [PubMed] [Google Scholar]
- 40. Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; 2000 [Google Scholar]
- 41. Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215 [DOI] [PubMed] [Google Scholar]
- 42. Thisse C, Thisse B. High-resolution in situ hybridization to whole mount zebrafish embryos. Nat Protoc. 2008;3:59–69 [DOI] [PubMed] [Google Scholar]
- 43. Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993;268:17–20 [PubMed] [Google Scholar]
- 44. Virkki LV, Cooper GJ, Boron WF. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1994–R2003 [DOI] [PubMed] [Google Scholar]
- 45. Daniels MJ, Wood MR, Yeager M. In vivo functional assay of a recombinant aquaporin in Pichia pastoris. Appl Environ Microbiol. 2006;72:1507–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346 [DOI] [PubMed] [Google Scholar]
- 47. Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41 [DOI] [PubMed] [Google Scholar]
- 48. Amores A, Force A, Yan YL, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714 [DOI] [PubMed] [Google Scholar]
- 49. Postlethwait JH, Yan YL, Gates MA, et al. Vertebrate genome evolution and the zebrafish gene map. Nat Genet. 1998;18:345–349 [DOI] [PubMed] [Google Scholar]
- 50. Postlethwait JH. The zebrafish genome: a review and msx gene case study. Genome Dyn. 2006;2:183–197 [DOI] [PubMed] [Google Scholar]
- 51. Gates MA, Kim L, Egan ES, et al. A genetic linkage map for zebrafish: comparative analysis and localization of genes and expressed sequences. Genome Res. 1999;9:334–347 [PubMed] [Google Scholar]
- 52. Vihtelic TS, Fadool JM, Gao J, Thornton KA, Hyde DR, Wistow G. Expressed sequence tag analysis of zebrafish eye tissues for NEIBank. Mol Vis. 2005;11:1083–1100 [PubMed] [Google Scholar]
- 53. Gonnet GH, Cohen MA, Benner SA. Exhaustive matching of the entire protein sequence database. Science. 1992;256:1443–1445 [DOI] [PubMed] [Google Scholar]
- 54. Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc Natl Acad Sci U S A. 2004;101:14045–14050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 2004;43:9856–9865 [DOI] [PubMed] [Google Scholar]
- 56. Knauf PA, Proverbio F, Hoffman JF. Chemical characterization and pronase susceptibility of the Na:K pump-associated phosphoprotein of human red blood cells. J Gen Physiol. 1974;63:305–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986;407:577–588 [DOI] [PubMed] [Google Scholar]
- 58. Laize V, Tacnet F, Ripoche P, Hohmann S. Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast. 2000;16:897–903 [DOI] [PubMed] [Google Scholar]
- 59. Lagree V, Froger A, Deschamps S, et al. Oligomerization state of water channels and glycerol facilitators. Involvement of loop E. J Biol Chem. 1998;273:33949–33953 [DOI] [PubMed] [Google Scholar]
- 60. Latimer P. Light scattering vs. microscopy for measuring average cell size and shape. Biophys J. 1979;27:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou L, Chen T, Church RL. Temporal expression of three mouse lens fiber cell membrane protein genes during early development. Mol Vis. 2002;8:143–148 [PubMed] [Google Scholar]
- 62. Tingaud-Sequeira A, Calusinska M, Finn RN, Chauvigne F, Lozano J, Cerda J. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol Biol. 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Al Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec. 2003;273A:714–730 [DOI] [PubMed] [Google Scholar]
- 64. Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519 [DOI] [PubMed] [Google Scholar]
- 65. Buzhynskyy N, Girmens JF, Faigle W, Scheuring S. Human cataract lens membrane at subnanometer resolution. J Mol Biol. 2007;374:162–169 [DOI] [PubMed] [Google Scholar]
- 66. Zampighi G, Simon SA, Robertson JD, McIntosh TJ, Costello MJ. On the structural organization of isolated bovine lens fiber junctions. J Cell Biol. 1982;93:175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zampighi GA, Simon SA, Hall JE. The specialized junctions of the lens. Int Rev Cytol. 1992;136:185–225 [DOI] [PubMed] [Google Scholar]
- 68. Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669 [DOI] [PubMed] [Google Scholar]
- 69. Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995;61:293–301 [DOI] [PubMed] [Google Scholar]
- 70. Gonen T, Cheng Y, Sliz P, et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jensen MO, Dror RO, Xu H, et al. Dynamic control of slow water transport by aquaporin 0: implications for hydration and junction stability in the eye lens. Proc Natl Acad Sci U S A. 2008;105:14430–14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gong X, Cheng C, Xia CH. Connexins in lens development and cataractogenesis. J Membr Biol. 2007;218:9–12 [DOI] [PubMed] [Google Scholar]
- 73. Tanaka M, Russell P, Smith S, Uga S, Kuwabara T, Kinoshita JH. Membrane alterations during cataract development in the Nakano mouse lens. Invest Ophthalmol Vis Sci. 1980;19:619–629 [PubMed] [Google Scholar]
- 74. Eastman SD, Chen TH, Falk MM, Mendelson TC, Iovine MK. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics. 2006;87:265–274 [DOI] [PubMed] [Google Scholar]
- 75. Cheng S, Shakespeare T, Mui R, White TW, Valdimarsson G. Connexin 48.5 is required for normal cardiovascular function and lens development in zebrafish embryos. J Biol Chem. 2004;279:36993–37003 [DOI] [PubMed] [Google Scholar]
- 76. Mulders JW, Stokkermans J, Leunissen JA, Benedetti EL, Bloemendal H, de Jong WW. Interaction of alpha-crystallin with lens plasma membranes: affinity for MP26. Eur J Biochem. 1985;152:721–728 [DOI] [PubMed] [Google Scholar]
- 77. Liang JJ, Li XY. Spectroscopic studies on the interaction of calf lens membranes with crystallins. Exp Eye Res. 1992;54:719–724 [DOI] [PubMed] [Google Scholar]
- 78. Fan J, Fariss RN, Purkiss AG, et al. Specific interaction between lens MIP/Aquaporin-0 and two members of the gamma-crystallin family. Mol Vis. 2005;11:76–87 [PubMed] [Google Scholar]
- 79. Liu BF, Liang JJ. Confocal fluorescence microscopy study of interaction between lens MIP26/AQP0 and crystallins in living cells. J Cell Biochem. 2008;104:51–58 [DOI] [PubMed] [Google Scholar]
- 80. Posner M, Hawke M, Lacava C, Prince CJ, Bellanco NR, Corbin RW. A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression. Mol Vis. 2008;14:806–814 [PMC free article] [PubMed] [Google Scholar]
- 81. Harding RL, Howley S, Baker LJ, et al. Lengsin expression and function during zebrafish lens formation. Exp Eye Res. 2008;86:807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]