The authors studied the distribution of both the GSH-dependent TTase system and the NADPH-dependent TRx system in young (20–35 years) and in old (55–70 years) lenses and also examined the effect of age on the levels and the activities of the components required for the repair systems, including GSH, GR, TTase, TRx, and TR. They found that the TTase and TRx systems in the lens were gradually weakened during aging and that the key glycolytic enzyme, glyceraldehydes-3-phosphodehydrogenase (G3PD), which makes adenosine triphosphate for the energy use of the lens, was also severely compromised with age.
Abstract
Purpose.
To investigate the effect of age on the key oxidation repair enzymes of the thioltransferase (TTase) and thioredoxin (TRx) systems in the human lens.
Methods.
Twenty-three normal human lenses (donor ages, 19–77 years) were grouped into second, third, fifth, sixth, and seventh decades and analyzed for TTase, TRx, glutathione reductase (GR), thioredoxin reductase (TR), and glyceraldehyde-3-phosphate dehydrogenase (G3PD) activities, as well as the glutathione (GSH) pool. Additionally, 19 contralateral lenses of the donor eyes were each divided into cortex and nucleus for enzyme distribution studies.
Results.
All the enzymes showed similar activity in the cortex and nucleus, regardless of age, but were inactivated to various extents in the older lenses. In the TTase system, both TTase and GR showed activity loss over the five decades, with 70% remaining in the seventh decade, whereas the GSH pool was depleted extensively, with only 35% left in the older lenses. In the TRx system, TRx activity was not affected as much as TR for which only 70% of the activity was found in the seventh decade compared with the second to third decades. Overall, G3PD was more sensitive to age because only 50% activity remained after the sixth decade.
Conclusions.
With increasing age there is a gradual activity loss in both the TTase and the TRx systems and a lowered GSH pool. These alterations, compounded with the age-related loss in G3PD activity, may lead to redox and energy imbalance, likely contributing to a higher risk to cataract formation in the aging population.
Under normal physiological conditions, most of the thiol-rich lens proteins remain in a reduced state necessary for maintaining lens transparency. However, the favorable environment can be altered by the oxidants generated from exogenous processes, such as ultraviolet light, radiation, and inflammation, as well as endogenous processes such as incomplete oxygen reduction in the mitochondria. A lens uses its unusually high level of glutathione (GSH) along with several effective oxidation defense enzyme systems, such as catalase, glutathione peroxidase, and superoxide dismutase, to eliminate oxidants.1,2 Furthermore, the lens uses two recently elucidated repair systems to reduce oxidized proteins/enzymes and to maintain redox homeostasis.3 The first repair system is the GSH-dependent thioltransferase (TTase) system, which dethiolates the protein thiols that have been oxidized into protein-GSH mixed disulfides (PSSGs) and thereby reestablishes the protein/enzyme function or activity. The second repair system is the NADPH-dependent thioredoxin (TRx) system, which reduces the protein thiols that have been oxidized to protein-protein disulfides (PSSPs) or protein sulfenic acid (PSOH) and restores their proper physiological functions.
Thioltransferase (TTase) is also known as glutaredoxin (GRx). This small molecular weight (11.8 kDa) cytosolic protein is a member of the thiol-disulfide oxidoreductase enzyme family, containing a conserved CXXC sequence at the active site. This feature makes the protein extremely resistant to oxidation. TTase is specific for glutathionylated proteins (protein-S-S-glutathione or PSSG). The reaction is GSH dependent; thus, the oxidized GSH or GSSG can be reduced with glutathione reductase (GR) and NADPH. TTase is also known to participate in many cellular functions, including redox signaling and regulation of glucose metabolism.3,4 Similar to TTase, thioredoxin (TRx) is also a member of the oxidoreductase enzyme family, with two redox-active thiols in the conserved sequence of WCGPC at the active site. This 12-kDa protein reduces inter- and intra- protein-protein disulfides and the sulfenic acid formed at the cysteine moiety of proteins. The oxidized TRx requires thioredoxin reductase (TR) and NADPH to restore it to its original reduced state. This protein is widely distributed in all forms of life and is known to be involved in a variety of roles such as antioxidation, growth control, neuroprotection, and immune function.5
Oxidative stress is well known to cause tissue and cellular damage, resulting in cancer, neurodegeneration, and age-associated degenerative diseases,6 including cataracts and macular degeneration in ocular tissues.1,2,7,8 It is, therefore, of great interest to determine whether aging affects the lenticular antioxidation defense systems and the oxidative damage repair systems. The former have been examined thoroughly in the past,9–13 whereas the status of the thiol repair systems in human lens has not been explored. In this report, we have studied the distribution of both the GSH-dependent TTase system and the NADPH-dependent TRx system in young (20–35 years) and in old (55–70 years) lenses. We have also examined age-related alterations in the levels and the activities of the components required for the repair systems, including GSH, GR, TTase, TRx, and TR. We have found that the TTase and TRx systems in the lens gradually weaken with increasing age. The key glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (G3PD), important in adenosine triphosphate (ATP) production for the energy use of the lens, was also severely compromised with age.
Materials and Methods
Materials
NADPH, GSH, GSSG, dithiothreitol (DTT), insulin, 5′,5′-dithiobis(2-nitrobanzoic acid (DTNB), and β-hydroxyethyl disulfide (HEDS) were all from Sigma Chemical Co. (St. Louis, MO). Bicinchoninic acid (BCA) protein assay reagent and chemiluminescent substrate were from Pierce (SuperSignal West Pico Chemiluminescent Substrate; Pierce, Rockford, IL). The specific antibodies for GR and TR were purchased from Abcam Co. (Cambridge, MA), whereas antibodies for TTase and TRx were made by Bethyl Laboratories (Montgomery, TX). The antibody for G3PD and horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All other chemicals and reagents were of analytical grade.
Human Lens Homogenate
Normal human donor eyes of various ages, less than 36 hours postmortem, were obtained from Omaha Lions Eye Bank (Omaha, NE) and Oregon Lions Eye Bank (Portland, OR). The protocol adhered to the tenets of the Declaration of Helsinki for research involving human tissue. Each lens was enucleated from the eye and immediately put into an individual container placed in dry ice for quick freezing and was stored at −80°C until use (up to 2 years). For enzyme analyses, each whole lens (23 total) was homogenized in 1.5 mL ice-cold buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10 mM EDTA. The homogenate was centrifuged at 13,000 rpm for 10 minutes at 4°C, and the supernatant was stored at −80°C pending analysis (2–4 weeks). For enzyme distribution studies, the contralateral lenses (19 total) were grouped into nine young (20–35 years) and 10 old (55–70 years) lenses, and each was dissected into cortex (with epithelium) and nucleus according to the method described by Huang et al.14 In brief, a center cone of the lens (anterior to posterior) was removed using a 5-mm stainless steel cork borer. After cutting off 1 mm at each end, the remaining section of the center cone was considered the nucleus (25%–30% of total lens weight). The rest of the portions were considered the cortex (70%–75% of the lens weight). Each lens portion was homogenized in 1 mL ice-cold buffer and processed for the experiment as described. GSH was assayed immediately, whereas the enzymes were analyzed within 1 week on storage at −80°C.
Preparation of Recombinant Thioltransferase and Thioredoxin
Recombinant human TTase and TRx were prepared as described by Qiao et al.15 and by Yegorova et al.,16 respectively. In brief, either recombinant human TTase or TRx with His-tag was overexpressed in Escherichia coli. Then the recombinant enzyme (with His-tag) was purified from E. coli extract by using His-Bind affinity column (Novagen, Gibbstown, NJ), and the excess imidazole was removed by dialysis. The purified enzymes were characterized with both activity assay and Western blot analysis before they were used for further experiments. The presence of the His-tag in the structure did not alter the activity or the general properties of either TTase or TRx.
Quantification of GSH
A portion of ice-cold lens homogenate was treated with TCA to achieve a 10% final concentration. The mixture was centrifuged at 13,000 rpm for 10 minutes at 4°C, and the supernatant was assayed immediately for free GSH with Ellman's reagent.17
Enzyme Assays of Lens Homogenate
In each enzyme assay, reaction mixture containing lysis buffer was used as the blank in place of the lens homogenate. The activity of each enzyme was expressed as U/g lens wet weight. TTase was assayed using HEDS as the substrate according to the method described in Raghavachari and Lou.18 In brief, the lens homogenate was mixed with potassium phosphate buffer (200 mM, pH7.5) containing 0.5 mM GSH, 0.4 U/mL GR, 0.2 mM NADPH, and 2 mM HEDS. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine the TTase activity.
GR assay was performed according to the method of Straatsma et al.,19 in which lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.2 mM NADPH and 2 mM GSSG. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine the GR activity.
Both TRx and TR were determined using the methods of Holmgren and Bjornstedt.20 For TRx activity assay, the lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.05 U/mL TR, 0.2 mM NADPH, and 0.14 mM insulin. The reaction was carried out at 30°C, and the decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine the TRx activity. For TR activity assay, lens homogenate was mixed with potassium phosphate buffer (100 mM, pH 7.0, with 2 mM EDTA) containing 0.2 mM NADPH and 5 mM DTNB. The reaction was carried out at 30°C, and the increase in absorbance at 412 nm was monitored for 5 minutes and used to quantify the TR activity.
G3PD activity was analyzed according to Bergmeyer et al.21 Lens homogenate was mixed with assay buffer (200 mM triethanolamine, pH 7.6) containing 1 mM ATP, 1 mM EDTA, 2 mM MgSO4, 0.2 mM NADPH, 15 U/mL 3-phosphoglyceric phosphokinase, and 6 mM 3-phosphoglyceric acid, and the reaction was carried out at 25°C. The decrease in absorbance at 340 nm was monitored for 5 minutes and used to determine G3PD activity.
Immunoblot Analysis
Lens homogenate samples, containing equal amounts of proteins, were applied on 10% SDS-PAGE gels, and the resolved protein bands were transferred to nitrocellulose membranes (Hybond ECL; Amersham, Little Chalfont, Buckinghamshire, UK) and probed with specific antibodies for GR, TRx, TR, and G3PD, respectively. The corresponding protein bands were detected and visualized (SuperSignal West Pico Chemiluminescent Substrate; Pierce).
Regeneration of Human Lens G3PD Activity
The regeneration experiment was performed at 30°C for 20 minutes in 0.1 M Tris-HCl buffer (pH 7.5). Lens homogenate was incubated with either the TTase system (10 mM GSH, 1.5 μM TTase) or the TRx system (0.2 mM NADPH, 0.5 μM TR, 1.5 μM Trx), or both. DTT (10 mM)-treated lens homogenate served as the positive control, whereas samples incubated without reducing agent were used as the negative control. G3PD activity was immediately assayed as described.
Protein Concentration Determination and Statistical Analysis
Protein concentration was determined by microanalysis with the BCA method.22 Statistical data were analyzed using the Student's t-test. For all tests, P < 0.05 was considered significant. The error bars represent standard deviations.
Results
Distribution of TTase System and TRx System in Young and Old Normal Human Lenses
Nine young normal human lenses (20–35 years old) and 10 old normal human lenses (55–70 years old) were dissected into cortical (cortex with epithelial layer) and nuclear portions for the measurement of GSH level and the assays for enzyme activity of TTase, GR, Trx, and TR. In young human lenses, only GSH and TR showed uneven distributions in the cortex and nucleus, in which nuclear GSH was 20% less than that of the cortical GSH, whereas the activity of nuclear TR was 1.5-fold higher than that of the cortical TR (Table 1). The activities of TTase, GR, and TRx, however, all showed little difference between the cortex and the nucleus of the lenses (Table 1).
Table 1.
Distribution of GSH-Dependent TTase System and NADPH-Dependent TRx System in Normal Human Lenses
| Young Lenses (20–35 years) |
Old Lenses (55–70 years) |
|||
|---|---|---|---|---|
| Cortex | Nucleus | Cortex | Nucleus | |
| GSH, μmol/g wwt | 3.15 ± 0.48 | 2.44 ± 0.45* | 1.70 ± 0.41† | 1.14 ± 0.26*† |
| TTase, mU/g wwt | 159 ± 20 | 185 ± 24 | 107 ± 24† | 163 ± 24* |
| GR, U/g wwt | 1.50 ± 0.86 | 1.58 ± 0.90 | 1.15 ± 0.34 | 0.91 ± 0.37 |
| TR, mU/g wwt | 211 ± 23 | 327 ± 51* | 175 ± 25† | 183 ± 55† |
| TRx, mU/g wwt | 60.4 ± 15.0 | 75.2 ± 13.2 | 35.3 ± 5.2† | 59.0 ± 13.3† |
Nine young lenses (20–35 years) and 10 old lenses (55–70 years) were each dissected to cortical and nuclear fractions and analyzed for GSH level, TTase, GR, TR, and TRx activities. Data are expressed as mean ± SD.
P < 0.05 by comparing between the values of different portions.
P < 0.05 by comparing between the values of different ages.
In general, age negatively affected the GSH pool and enzyme activities. As shown in Table 1, GSH in older lenses decreased to 54% in the cortex and 46% in the nucleus compared with the respective region of the young lenses. TTase activity was reduced approximately 30% in the cortex and 10% in the nucleus, whereas GR showed 25% less activity in the cortex and 40% less in the nucleus. Both TR and TRx were also partially inactivated in the cortical and nuclear regions of the old lenses (Table 1).
Age Effect on the Activities of GSH-Dependent TTase System
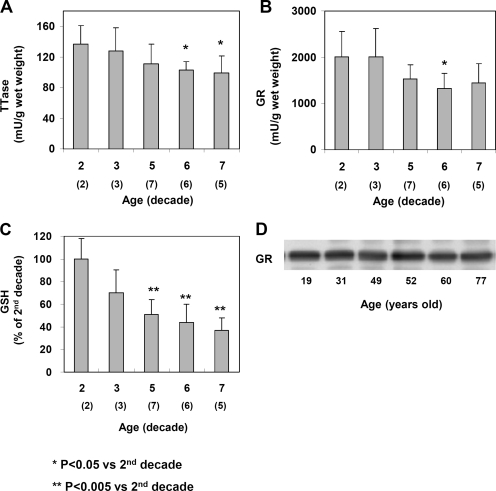
To examine the TTase system in the whole lens, we expressed the level of GSH and the specific activity of TTase or GR as a function of the wet weight of each lens. The average weights of lenses in the second and third decades were 170 to 190 mg but gradually increased to 219 to 230 mg in the fifth to sixth decades and to 220 to 300 mg in the seventh decade. This age-dependent increase was consistent with the data reported by Augusteyn,23 indicating the lenses we used in this study were not swollen or damaged. In general, the activities of TTase, GR, and the GSH pools in these lenses were progressively lost with age. As shown in Figure 1A, TTase activities in lenses of the sixth and seventh decades were approximately 30% less than those of the second decade. The GR activity displayed a similar age-dependent decrease from an average of 2006 mU/g wet weight in lenses from both the second and the third decades (Fig. 1B), to 1326 mU/g wet weight in the lenses of the sixth decade, a loss of 34%. The decrease was not caused by altered GR expression because the amount of GR in the lens was not reduced with age, as indicated by the Western blot analysis shown in Figure 1D. These human lenses also showed a substantial loss in the GSH pool during aging (Fig. 1C). Compared with the level in the second decade, GSH decreased by nearly 30% in the third decade and almost 50% in the fifth decade. The decrease continued in the sixth and seventh decades, with only 43% and 37% of the pool remaining, respectively.
Figure 1.
Age-dependent changes of the TTase system in human lenses. Twenty-three normal human lenses, divided into second, third, fifth, sixth, and seventh decades, were used for the study. The data are shown as mean ± SD, with the number of lenses indicated as (n) in each decade. (A) TTase activity in mU/g lens wet weight. (B) GR activity in mU/g lens wet weight. (C) GSH level expressed as percentage of second decade. (D) Homogenates from 19-, 31-, 49-, 52-, 60-, and 77-year-old lenses were selected for immunoblot analysis for GR with a specific anti-GR antibody. The blot shown is a representative of three separate analyses.
Age Effect on the Activities of NADPH-Dependent TRx System
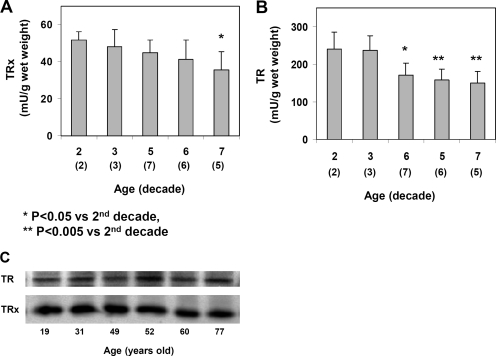
Changes in the TRx system were also studied in the whole lens extracts of the 23 lenses. As summarized in Figure 2, TRx activity showed a gradual decrease during aging. Similar to TTase (Fig. 1A), the activity of TRx in the lenses of the seventh decade was approximately 30% less than that of the second decade. TR was more sensitive to aging as its activity suffered more loss than that of the TRx in the same lens. As shown in Figure 2B, the activity of TR was 240 mU/g wet weight in the lenses of both second and third decades but decreased steadily to 149 mU/g wet weight in lenses of the seventh decade, 38% less than that of the younger lenses at the second decade. However, Western blot analysis indicated that the expression of TR and TRx remained relatively constant in the lenses of ages between 19 and 77 years old (Fig. 2C).
Figure 2.
Age-dependent changes of the TRx system in human lenses. The same normal human lenses from Figure 1 were assayed for the TRx system. Data are expressed as mean ± SD, with (n) value indicated in each decade. (A) TRx activity in mU/g wet weight. (B) TR activity in mU/g wet weight. (C) Homogenates from 19-, 31-, 49-, 52-, 60-, and 77-year-old lenses were selected for immunoblot analyses for TRx and TR using, respectively, anti-TRx and anti-TR antibodies. The blot shown is a representative of three separate analyses.
Age Effect on the Activity of G3PD
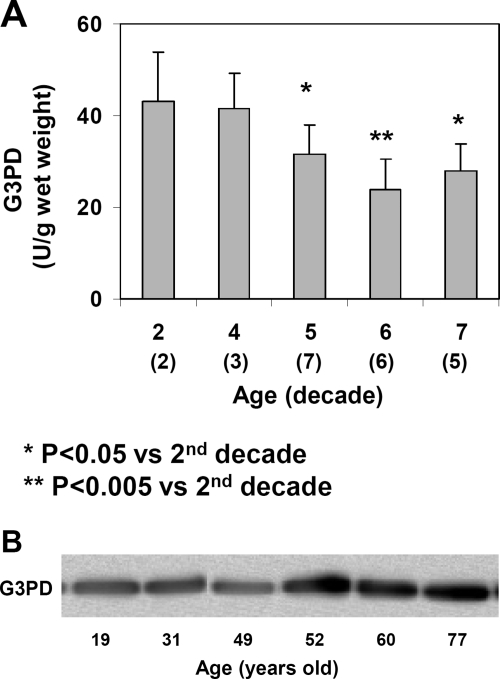
Similar to the oxidation defense enzymes shown, G3PD activity also gradually became reduced during the course of aging. As shown in Figure 3A, the activity showed an average of 43 U/g wet weight in the young lenses in the second decade but was 30% less in the lenses of the fifth decade and 40% less in the sixth to seventh decades. The amount of G3PD protein did not reduce with age, as shown by the Western blot analysis (Fig. 3B), indicating that the lower activity of G3PD in older lenses resulted from inactivation of the enzyme.
Figure 3.
Age-dependent changes of G3PD in normal human lenses. The same normal human lenses from Figure 1 were assayed for G3PD. (A) G3PD activity expressed as U/g wet weight. Data are expressed as mean ± SD, with (n) value indicated in each decade. (B) Homogenates from 19-, 31-, 49-, 52-, 60-, and 77-year-old lenses were selected for immunoblot analyses for G3PD using specific anti-G3PD antibody. The blot shown is a representative of three separate analyses.
Reactivation of Human Lens G3PD
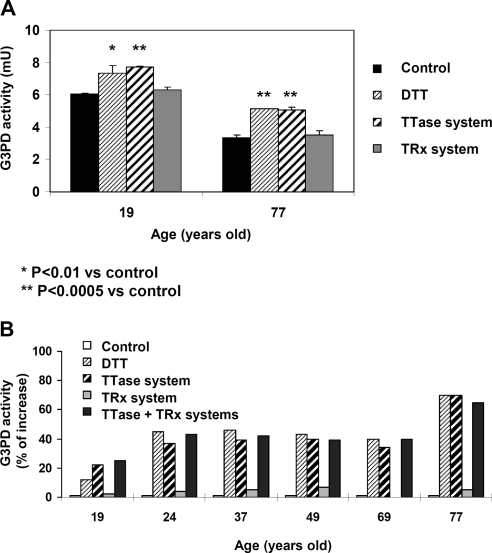
Because G3PD is known to be very sensitive to oxidative stress,24 we speculate that the age-dependent activity loss in G3PD (Fig. 3A) may be associated with oxidation-induced inactivation at the active site in the form of either PSSG, PSSP, or PSOH. To test this, we examined lens homogenates from a 19-year-old and a 77-year-old for comparison. A portion of each homogenate was treated with DTT, the TTase system, or the TRx system before G3PD activity was measured again. Given the limitation of sample size, treatment with GSH (10 mM) alone was not included in this study because we have shown previously that GSH alone at 1 to 10 mM did not restore G3PD activity.24 As shown in Figure 4A, the 19-year-old lens showed a slight (15%) increase in G3PD activity, after pretreatment with DTT or the TTase system, compared with the untreated control, indicating that the active site was partially damaged by oxidation already at this young age. Similar activation by DTT or the TTase system was seen in the 77-year-old lens whose initial G3PD activity was only half as much as that of the 19-year-old lens. In this older lens, the reactivation was more evident (63% increase) after reduction by either DTT or the TTase system. Interestingly, the TRx system (TRx + TR + NADPH) could not regenerate any G3PD activity in either the 19- or the 77-year-old lens.
Figure 4.
Regeneration of human lens G3PD. (A) Normal human lenses of young (19 years) and old (77 years) donors used in Figures 1D and 2C were compared for reactivation of G3PD activity in each lens by the TTase system, the TRx system, and DTT (positive control). The sample of each lens without treatment served as the control. Data are expressed as mean ± SD with three separate analyses. (B) Comparison of the age-dependent regeneration of G3PD in normal human lens of 19-, 24-, 37-, 49-, 69-, and 77-year-old donors. G3PD activity was expressed as a percentage increase in samples of each lens treated with DTT, the TTase system, the Trx system, and TTase + Trx systems over the untreated control. Each result is based on one analysis.
In a separate experiment, we examined the effect of age on the reactivation of G3PD using 19-, 24-, 37-, 49-, 69-, and 77-year-old lenses. The G3PD activities in these lenses were compared before and after treatment with DTT, the TTase system, the TRx system, or the TTase + TRx systems together. G3PD reactivation in each lens was expressed as a percentage increase above its own untreated control. Because of the limitation of sample size, the analysis was performed once. As shown in Figure 4B, more G3PD activity was regenerated by either the DTT or the TTase system in the homogenate from the older lenses than from younger lenses. Only a 10% to 20% increase in activity was achieved by the DTT or TTase system in the 19-year-old lens, whereas a nearly 40% increase was observed in the 24- to 69-year-old lenses. The highest increase of G3PD activity (70%) was seen in the 77-year-old lens.
To further examine whether the TRx system is an effective regeneration system for G3PD in clear normal human lenses, we pretreated the lens homogenate with the TRx system. Again there was no activation by the TRx system in comparison with either DTT or the TTase system. Furthermore, when the lens homogenate was cotreated with both the TTase and the TRx systems, there was no synergistic activation besides the level of reactivation achieved by the TTase system alone (Fig. 4B), suggesting that most of the G3PD activity loss found in the soluble fraction of the human lens homogenate was likely attributed to the mixed disulfide bond formation between G3PD and oxidized glutathione (PSSG).
Discussion
Age has been established as a major risk factor for cataract formation because of the higher oxidative stress and the lower oxidation defense capabilities in the aging lens.1–3,25,26 In this article, we provide evidence that the enzymes responsible for protein thiol oxidation repair, including the thioltransferase (glutaredoxin) system and the thioredoxin system, become less efficient during aging. This may add to the risk for cataractogenesis.
It is well known that GSH in the lens is used to reduce oxidants and itself is oxidized to GSSG, which in turn can be reduced to GSH again by the NADPH/GR system. The GSH pool is thus replenished through the recycling process. However, the weakened GR activity in aging lenses (Fig. 1B) would prevent an efficient recycling process, rendering an insufficient GSH supply in the lens. The age-dependent steady loss of the GSH pool in the lens shown in this study (Fig. 1C) is consistent with the progressive decrease in GR activity during aging (Fig. 1B) and the age-dependent inactivation of GR found by Zhang and Augusteyn.27 Our current data on the age-dependent GSH loss agreed with the earlier findings in lenses from humans28 and rats.29 Thus our results provide strong evidence that the loss in the GSH pool during aging contributes to the corresponding and steady increase in the PSSG pool found in the aging lens.28 Additionally, GSH is an important cofactor of TTase for dethiolase activity. Although TTase activity loss was moderate in the older lenses (70% of the activity in young lenses; Fig. 1A), the depletion of the GSH supply (only 30%–40% of young lenses) could have an adverse effect on TTase function in the same lens.
PSSG formation can lead to protein conformational change, and the accumulated PSSG in lens structural proteins may contribute to protein-protein disulfide formation and cross-linking with eventual cataract formation.3 Among the strongest evidence for the role of PSSG in cataractogenesis in humans are that PSSG levels are elevated with increased lens opacity and lens pigmentation30–33 and that PSSG formed in γB crystallin in H2O2-treated lenses induced conformational changes, allowing buried SH groups to form disulfide cross-links.34 Therefore, it is reasonable to speculate that when a lens loses its ability to repair (or reduce) the oxidized protein thiols, it should become more vulnerable to additional oxidative stress-induced damage, including transparency loss.
Various properties and functionalities of the TTase system have been studied extensively in human lens cells or tissues; however, less is known about the TRx system. We have previously examined the distribution of TRx protein in the epithelium, cortex, and nucleus of a 60-year-old normal human lens and found it to be equally present in these regions.3 Our current data, showing equal activity in the cortex (with epithelium) and the nucleus (Table 1), confirm this earlier finding. Previously, Bhuyan et al.35 observed that the expression of TRx mRNA or protein in the epithelial layer of normal human lens was depressed with aging. However, our study showed an aged-dependent loss in TRx activity (Fig. 2A) but not in TRx protein expression (Fig. 2C) when a whole lens was used.
TR is considered as the top of the hierarchy for redox control in cells, but it has been scarcely studied in the lens. Previously we have found that when whole pig lenses in organ culture were subjected to 0.2 mM or 0.5 mM H2O2 stress, both the TTase and TRx systems in the epithelial layer showed a transient increase in activities and protein expression before the lens died to the stress and lost its transparency.36 TR, in particular, behaved like an oxidation sensor. It was the first enzyme to be upregulated under these conditions, indicating that the TRx system responded early for correcting oxidation-induced injury in the tissue. Therefore, we speculate that the compromised TRx system found in the aging human lenses in this study (Fig. 2) might result in higher PSSP accumulation and impede function in older lenses.
The importance of GR activity in maintaining lens clarity has been implied in the studies of Horwitz et al.37 These authors have found that most freshly excised human lens epithelia after cataract surgery showed little or no GR activity, but the activity could be restored when FAD, a cofactor for GR, was added in vitro. They concluded that the inactivation of GR in these cataractous lenses could be attributed to deficient nutritional intake of FAD in patients. We did not measure the FAD pool in our present study. It would be of value in the future to study the relationship between the FAD pool and GR activity in the lens during aging. However, other studies have suggested that oxidative stress may contribute to GR inactivation in old or cataractous lenses. For instance, Yan et al.38 have shown that GR activity in pooled clear human lenses (40–85 years) could be increased by addition of the TRx or TTase systems in vitro and that the combination of TTase and TRx showed a synergistic effect. Rachdan et al.39 also showed that GR activity in cataractous lenses could be revived using reducing agents.
Of all the enzymes in the glycolytic pathway, G3PD is most oxidation sensitive. This property is particularly important for the lens because lens metabolism is predominantly anaerobic. The integrity of G3PD is vital for the production of ATP in the pathway. Xing and Lou24 have shown that G3PD in human lens epithelial cells quickly conjugates with GSH to form PSSG when stressed with a bolus of low-level H2O2 (100 μM) and that the addition of TTase (with the cofactor GSH) in the cell lysate could restore most of its activity but not of GSH alone (up to 10 mM). Yegorova et al.16 also found that TRx could reactivate G3PD under similar oxidative stress conditions. Considering the huge loss in G3PD activity on aging (Fig. 3), it is likely that G3PD was oxidized during the approximately 70 years of lifespan. Our data also show that the addition of the TTase system in vitro could restore G3PD activity. However, the TRx system, which reduces PSSP and PSOH, was not effective, indicating that most of the oxidative damage at the active site of G3PD in the lenses was likely due to PSSG but not to PSSP or PSOH. This finding differed from that of Yan et al.40 in which the G3PD activity in human senile cataractous lenses could be restored by either TTase or TRx and synergistically by both. It is likely that an old lens may not be oxidized as severely as a senile cataractous lens, in which PSSP and PSOH, as well as PSSG, were all formed. It is unlikely that part of the G3PD activity was either lost during storage or in processing because we have observed similar activity with various conditions of processing or at various length of storage time (−80°C).
In summary we have found that the activities of the thiol damage repair systems of TTase and TRx in the lens were gradually reduced during aging, suggesting that inefficient thiol damage repair compounded with decreased ATP production in old lenses may be responsible for the formation of senile cataract in humans.
Acknowledgments
The authors thank Robert C. Augusteyn (Vision Cooperative Research Centre, Sydney, Australia) for his discussion and reading of this manuscript.
Footnotes
Supported by National Institutes of Health Grant RO1 EY10595 (MFL).
Disclosure: K.-Y. Xing, None; M.F. Lou, None
References
- 1. Augusteyn RC. Protein modification in cataract: possible oxidative mechanisms. In: Duncan G. ed. Mechanisms of Cataract Formation in the Human Lens. New York: Academic Press; 1981:72–115 [Google Scholar]
- 2. Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182 [PubMed] [Google Scholar]
- 3. Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;44:3262–3271 [DOI] [PubMed] [Google Scholar]
- 4. Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1783:641–650 [DOI] [PubMed] [Google Scholar]
- 5. Kondo N, Nakamura H, Masutani H, Yodoi J. Redox regulation of human thioredoxin network. Antioxid Redox Signal. 2006;8:1881–1890 [DOI] [PubMed] [Google Scholar]
- 6. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247 [DOI] [PubMed] [Google Scholar]
- 7. McNamara M, Augusteyn RC. The effects of hydrogen peroxide on lens proteins: a possible model for nuclear cataract. Exp Eye Res. 1984;38:45–56 [DOI] [PubMed] [Google Scholar]
- 8. Andley UP, Liang JJ-N, Lou MF. Biochemical mechanisms of age-related cataract. In: Albert DM, Jakobiec FA. eds. Principles and Practice in Ophthalmology. Philadelphia: WB Saunders; 2002:1428–1449 [Google Scholar]
- 9. Rathbun WB. Glutathione in ocular tissues. In: Dolphin D, Poulson R, Avramovic O. eds. Glutathione, Coenzymes, and Cofactors. Vol. 3 New York: Wiley; 1989:467 [Google Scholar]
- 10. Fecondo JV, Augusteyn RC. Superoxide dismutase, catalase and glutathione peroxidase in the human cataractous lens. Exp Eye Res. 1983;36:15–23 [DOI] [PubMed] [Google Scholar]
- 11. Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79:859–868 [DOI] [PubMed] [Google Scholar]
- 12. Ohrloff C, Hockwin O, Olson R, Dickman S. Glutathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984a;3:109–115 [DOI] [PubMed] [Google Scholar]
- 13. Ohrloff C, Hockwin O. Superoxide dismutase (SOD) in normal and cataractous human lens. Graefes Arch Clin Exp Ophthalmol. 1984b;222:79–81 [DOI] [PubMed] [Google Scholar]
- 14. Huang QL, Lou MF, Straatsma BR, Horwitz J. Distribution and activity of glutathione-S-transferase in normal human lens and in cataractous human epithelia. Curr Eye Res. 1993;12:433–437 [DOI] [PubMed] [Google Scholar]
- 15. Qiao F, Xing K, Liu A, et al. Human lens thioltransferase: cloning, purification and function. Invest Ophthalmol Vis Sci. 2001;42:743–751 [PubMed] [Google Scholar]
- 16. Yegorova S, Liu A, Lou MF. Human lens thioredoxin: molecular cloning and functional characterization. Invest Ophthalmol Vis Sci. 2003;44:3262–3271 [DOI] [PubMed] [Google Scholar]
- 17. Lou MF, Dickerson JE, Jr, Garadi R, York BM., Jr Glutathione depletion in the lens of galactosemic and diabetic rats. Exp Eye Res. 1988;46:517–530 [DOI] [PubMed] [Google Scholar]
- 18. Raghavachari N, Lou MF. Evidence for the presence of thioltransferase in the lens. Exp Eye Res. 1996;63:433–441 [DOI] [PubMed] [Google Scholar]
- 19. Straatsma BR, Lightfoot DO, Barke R, Horwitz J. Lens capsule and epithelium in age-related cataract. Am J Ophthalmol. 1991;112:183–196 [DOI] [PubMed] [Google Scholar]
- 20. Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208 [DOI] [PubMed] [Google Scholar]
- 21. Bergmeyer HU, Gawehn K, Grassl M. In: Bergmeyer HU. ed. Methods of Enzymatic Analysis. 2nd ed. Weinheim, Germany: Verlag Chenie; 1974:466–467 [Google Scholar]
- 22. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85 [DOI] [PubMed] [Google Scholar]
- 23. Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xing KY, Lou MF. Effect of H2O2 on human lens epithelial cells and the possible mechanism for oxidative damage repair by thioltransferase. Exp Eye Res. 2002;74:113–122 [DOI] [PubMed] [Google Scholar]
- 25. Taylor A, Jacques PF, Epstein EM. Relations among aging, antioxidant status, and cataract. Am J Clin Nutr. 1995: 1439S–1447S [DOI] [PubMed] [Google Scholar]
- 26. Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang WZ, Augusteyn RC. Ageing of glutathione reductase in the lens. Exp Eye Res. 1994;59:91–96 [DOI] [PubMed] [Google Scholar]
- 28. Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992;55:889–896 [DOI] [PubMed] [Google Scholar]
- 29. Lou MF, Dickerson JE, Jr, Garadi R. The role of protein-thiol mixed disulfides in cataractogenesis. Exp Eye Res. 1990;50:819–826 [DOI] [PubMed] [Google Scholar]
- 30. Lou MF, Dickerson JE, Jr, Tung WH, et al. Correlation of nuclear color and opalescence with protein S-thiolation in human lens. Exp Eye Res. 1999;68:547–552 [DOI] [PubMed] [Google Scholar]
- 31. Harding JJ. Free and protein-bound glutathione in normal and cataratous human lenses. Biochem J. 1970;117:957–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson EI, Spector A. The state of sulfhydryl groups in normal and cataractous human lens proteins, I: nuclear region. Exp Eye Res. 1978;26:407–417 [DOI] [PubMed] [Google Scholar]
- 33. Truscott RJW, Augusteyn RC. The state of sulphydryl groups in normal and cataractous human lenses. Exp Eye Res. 1977;25:139–148 [DOI] [PubMed] [Google Scholar]
- 34. Hanson SR, Chen AA, Smith JB, Lou MF. Thiolation of the γB-crystallins in intact bovine lens exposed to hydrogen peroxide. J Biol Chem. 1999;274:4735–4742 [DOI] [PubMed] [Google Scholar]
- 35. Bhuyan KC, Reddy PG, Bhuyan DK. Thioredoxin genes in lens: regulation by oxidative stress. Methods Enzymol. 2002;347:421–435 [DOI] [PubMed] [Google Scholar]
- 36. Moon S, Fernando MR, Lou MF. Induction of thioltransferase and thioredoxin/thioredoxin redictase systems in cultured porcine lenses under oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:3783–3789 [DOI] [PubMed] [Google Scholar]
- 37. Horwitz J, Dovrat A, Straatsma BR, et al. Glutathione reductase in human lens epithelium: FAD-induced in vitro activation. Curr Eye Res. 1987;6:1249–1256 [DOI] [PubMed] [Google Scholar]
- 38. Yan H, Harding JJ, Xing K, Lou MF. Revival of glutathione reductase in human cataractous and clear lens extracts by thioredoxin and thioredoxin reductase, in conjunction with alpha-crystallin or thioltransferase. Curr Eye Res. 2007;32:455–463 [DOI] [PubMed] [Google Scholar]
- 39. Rachdan D, Lou MF, Harding JJ. Glutathione reductase from human cataract lenses can be revived by reducing agents and by a molecular chaperone, alpha-crystallin. Curr Eye Res. 2005;30:919–925 [DOI] [PubMed] [Google Scholar]
- 40. Yan H, Lou MF, Fernando MR, Harding JJ. Thioredoxin, thioredoxin reductase, and alpha-crystallin revive inactivated glyceraldehyde 3-phosphate dehydrogenase in human aged and cataract lens extracts. Mol Vis. 2006;12:1153–1159 [PubMed] [Google Scholar]