This fMRI study shows that there is cortical reorganization of the early visual cortex in both age-related and juvenile-onset macular degeneration, but it is incomplete and task dependent.
Abstract
Purpose.
Activity in regions of the visual cortex corresponding to central scotomas in subjects with macular degeneration (MD) is considered evidence for functional reorganization in the brain. Three unresolved issues related to cortical activity in subjects with MD were addressed: Is the cortical response to stimuli presented to the preferred retinal locus (PRL) different from other retinal loci at the same eccentricity? What effect does the role of age of onset and etiology of MD have on cortical responses? How do functional responses in an MD subject's visual cortex vary for task and stimulus conditions?
Methods.
Eight MD subjects—four with age-related onset (AMD) and four with juvenile onset (JMD)—and two age-matched normal vision controls, participated in three testing conditions while undergoing functional magnetic resonance imaging (fMRI). First, subjects viewed a small stimulus presented at the PRL compared with a non-PRL control location to investigate the role of the PRL. Second, they viewed a full-field flickering checkerboard compared with a small stimulus in the original fovea to investigate brain activation with passive viewing. Third, they performed a one-back task with scene images to investigate brain activation with active viewing.
Results.
A small stimulus at the PRL generated more extensive cortical activation than at a non-PRL location, but neither yielded activation in the foveal cortical projection. Both passive and active viewing of full-field stimuli left a silent zone at the posterior pole of the occipital cortex, implying a lack of complete cortical reorganization. The silent zone was smaller in the task requiring active viewing compared with the task requiring passive viewing, especially in JMD subjects.
Conclusions.
The PRL for MD subjects has more extensive cortical representation than a retinal region with matched eccentricity. There is evidence for incomplete functional reorganization of early visual cortex in both JMD and AMD. Functional reorganization is more prominent in JMD. Feedback signals, possibly associated with attention, play an important role in the reorganization.
Macular degeneration (MD) is an eye condition that affects the fovea and perifoveal retina, resulting in a loss of central vision. Age-related macular degeneration (AMD) is the leading cause of legal blindness and low vision in the United States and one of the most common causes of legal blindness and low vision globally.1 Juvenile macular degeneration (JMD), primarily Stargardt's disease, is a different type of MD that affects children or young adults.
Central vision has the highest spatial resolution and is vital for many aspects of visual function such as reading. Early visual cortex contains a retinotopic map in which the central visual field is highly magnified with respect to the peripheral field. Approximately 50% of the primary visual cortex (V1) is devoted to the central 15° of the visual field.2,3 People with advanced AMD often develop bilateral, dense central scotomas subtending 10° to 15° (see Ref. 4 for review). In the absence of any retinotopic reorganization, a 15° bilateral central scotoma would result in no visual responses in the posterior half of V1. Recent fMRI studies have claimed reorganization of visual processing in persons with loss of foveal vision from MD; however, the extent and mechanisms of the reorganization remain unclear.5–10 Further complicating the picture, it has been shown that the activity seen in the lesion projection zone (LPZ) is task dependent,7 and at least some aspects of the cortical system plasticity decrease with aging.11
In this article, reorganization of visual cortex refers to the pattern of cortical responses measured with fMRI in patients with macular degeneration that differs from the expected loss of response in the cortical projection from the retinal scotoma. We consider such changed cortical activity as evidence for functional reorganization, without necessarily implying anatomic changes in cortical structure. For instance, the changed activation patterns observable with fMRI might be due to modification of synaptic connection strength or unmasking of existing signals when bottom-up input is no longer present.12
Our experiments were designed to address three unresolved issues related to cortical reorganization in MD. First, patients with macular degeneration usually adopt a spared location in nonfoveal retina for fixation, called the preferred retinal locus (PRL).4,13 The PRL assumes the role of the former fovea as an oculomotor reference point and may adopt some of the fovea's functional characteristics. Evidence from Schumacher et al.9 supports the view that there is more extensive cortical representation of PRL compared with non-PRL locations, including extension of activation toward the LPZ. In contrast, Dilks et al.8 found similar activation for stimuli at the PRL and comparable non-PRL locations. The discrepant results might be due to differences in task design and methods of data analysis. In our study, we compared cortical responses when a small flickering checkerboard was presented either at the PRL or at another functioning retinal location with comparable eccentricity.
Second is the influence on cortical reorganization of the age of onset and the etiology of MD. In previous studies, often only one type of MD was investigated. In our study, we were able to compare cortical reorganization in AMD and JMD subjects by testing groups of comparable size and similar visual function characteristics using the same protocols.
Third, task demands and stimulus properties have varied across previous studies. Sometimes, the visual stimuli were meaningful objects5,6,8 and sometimes meaningless patterns.9,10 Sometimes, the subjects actively responded to the stimuli,5 and sometimes viewing was passive.10 Sometimes the stimuli were spatially localized5 and sometimes full field.7 We tested our subjects with separate tasks designed to compare cortical responses during passive and active viewing, similar to the Masuda et al.7 study of JMD subjects.
A preview of our results is as follows: Cortical activation with a small stimulus at the PRL was more extensive than at a non-PRL location; full-field stimuli in both passive and active conditions left a silent zone in the posterior pole of the occipital cortex, implying a lack of complete reorganization; the extent of the silent zone was smaller in the active task than the passive task, implying an important role for feedback signals in functional reorganization in the visual system; evidence for functional reorganization was more robust in JMD than AMD subjects.
Methods
Subjects
Four subjects diagnosed with AMD (ages, 70–90 years), four subjects diagnosed with Stargardt's disease (termed JMD; ages, 30–50 years), and two subjects with normal vision whose ages were in the same range as the AMD subjects were recruited at the Atlanta VA Rehabilitation Research and Development Center of Excellence. All subjects gave signed, informed consent, with procedures approved by the Emory University Institutional Review Board and following the tenets of the Declaration of Helsinki.
Clinical and SLO Information about the Subjects
All AMD and JMD subjects had been diagnosed with macular degeneration of more than 10 years' duration before testing. For the AMD subjects, previous data were available indicating the stability of PRLs for at least 5 years. No previous information on PRLs was available for the JMD subjects, but it is likely that all of them had stable PRLs for at least several years (based on clinical records from a low vision rehabilitation service).
Before the fMRI session, subjects underwent several clinical measurements of visual function, including visual acuity (ETDRS chart), contrast sensitivity (Pelli-Robson chart), and Pepper reading test14 (Table 1). The dominant eye was then identified by a binocular PRL test15 as follows: visual stimuli were presented dichoptically—with, for example, an x to the left eye and a + to the right eye—by using stereo view LCD shutter glasses. Subjects who saw only 1 of 2 visual stimuli exhibited monocular perception, and that eye and its PRL were taken as dominant. Subjects who saw a superposition of the two test stimuli (e.g., an eight-legged star) had binocular perception. Their preferred eye was determined by asking them to say which eye was used for simple monocular sighting tasks and was confirmed by asking them to do simple sighting tasks in the laboratory (e.g., looking through a tube). In the fMRI session, subjects viewed stimuli monocularly with their dominant/preferred eye.
Table 1.
Visual Function Data for the Study Subjects
| Subject ID | Visual Acuity (log MAR) |
Contrast Sensitivity (log unit) |
Pepper Reading Test |
|||
|---|---|---|---|---|---|---|
| OD | OS | OD | OS | Correct (%) | Reading Rate (word/min) | |
| AMD1 | 1.40 | 1.18* | 1.45 | 1.50* | 78 | 32 |
| AMD2 | 0.92 | 0.64* | 1.2 | 1.55* | 87 | 38 |
| AMD3 | 1.34 | 1.40* | 1.6 | 1.35* | 75 | 26 |
| AMD4 | 1.08 | 0.94* | 1.35 | 1.5* | 95 | 36 |
| JMD1 | 1.12 | 1.04* | 0.95 | 1.15* | 91 | 62 |
| JMD2 | 0.86* | 0.66 | 1.2* | 1.15 | 81 | 20 |
| JMD3 | 0.94* | 0.94 | 1.35* | 1.30 | 78 | 11 |
| JMD4 | 1.52* | 1.56 | 0.30* | 0.10 | 88 | 20 |
| Control1 | −0.04* | −0.02 | 1.60* | 1.55 | 98 | 102 |
| Control2 | 0 | 0.04* | 1.55 | 1.70* | 98 | 107 |
Eye tested in the fMRI session.
Macular perimetry was performed using a scanning laser ophthalmoscope (SLO) before fMRI.16 SLO results reported here are for the eyes tested in the fMRI session. Information about scotoma size, fixation stability, and PRL location relative to the original fovea are shown in Table 2.17
Table 2.
Macular Perimetry Results for the Study Subjects
| Subject ID | Study Eye | Scotoma Size (diameter in degree) |
PRL Location in Visual Field (relative to the original fovea) |
Fixation Stability (°)‡ | |||
|---|---|---|---|---|---|---|---|
| Horizontal | Vertical | Eccentricity (°)* | Polar Angle (°)† | Visual Field | |||
| AMD1 | OS | 13.6 | 9.7 | 8.1 | 137 | Lower right | 5.0 |
| AMD2 | OS | 9.9 | 8.3 | 3.9 | 151 | Lower right | 4.5 |
| AMD3 | OS | 17.2 | 21.0 | 15.1 | 303 | Upper left | 5.0 |
| AMD4 | OS | 14.0 | 15.6 | 8.8 | 207 | Lower left | 5.5 |
| JMD1 | OS | 18.9 | 14.7 | 7.4 | 130 | Lower right | 4.0 |
| JMD2 | OD | 16.8 | 12.6 | 8.3 | 207 | Lower left | 2.0 |
| JMD3 | OD | 13.7 | 9.5 | 6.7 | 226 | Lower left | 3.0 |
| JMD4 | OD | 23.1 | 16.8 | 20.0 | 198 | Lower Left | 5.5 |
| Control1 | OD | — | — | — | — | — | 1.5 |
| Control2 | OS | — | — | — | — | — | 1.5 |
Eccentricity is the distance between the PRL and the fovea in degrees of visual angle. The retinal fovea location was determined from the normal fixation position relative to the optic disk.17
Polar angle is the angle between the upward vertical axis from the fovea and a line connecting the PRL and the fovea in a clockwise direction in visual field coordinates.
Fixation stability is defined as the diameter of the retinal region, in degrees of visual angle, where fixation points were located during a 20-second SLO fixation stability test (visual inspection of retinal motion during fixation of a 1° fixation cross).
Given that the role of the PRL in cortical reorganization is one of the issues addressed in this study, it is important to know whether subjects consistently used only one PRL across a range of stimulus conditions. We used different fixation targets and different luminance levels to test for the consistent use of a single PRL. There were four types of fixation targets—simple fixation cross, letter, word, and text—each at two different luminance levels (luminance value of the back-projected display used in the fMRI experiments and the brightest retinal illuminance value for the SLO). These tests verified that all the MD subjects had PRLs and scotoma characteristics that were consistent over various stimulus and luminance conditions.
FMRI Stimuli and Procedure
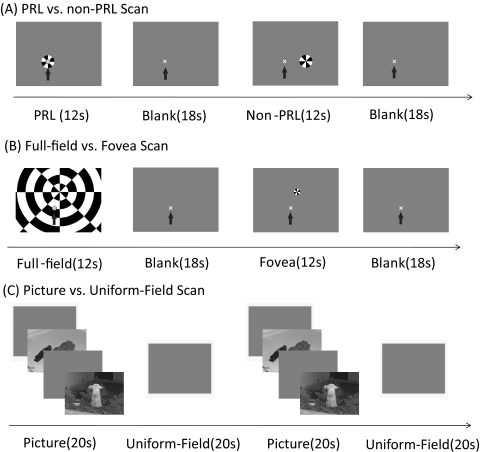
Stimuli were generated by using mathematical computing software (MATLAB 5.21; MathWorks, Inc., Natick, MA) with Psychophysics Toolbox extensions18,19 on a laptop computer (PowerBook G3; Apple, Inc., Cupertino, CA). Stimuli were presented through an LCD projector onto a back-projection screen with a display area of 42° × 30°. Subjects, in a supine position in the scanner, viewed the stimuli monocularly with their dominant/preferred eye through an angled mirror. Three stimulus conditions were used: PRL versus non-PRL, full-field versus fovea, and picture versus uniform field.
In the PRL versus non-PRL scan, a 1.5° bright green fixation cross was present; subjects looked at the fixation target using their PRL throughout the scan. The placement of the fixation cross in the display screen was based on the PRL location of MD subjects so that the midpoint between the PRL and the original fovea would be at the center of the screen. This arrangement was made to enable comfortable viewing for the subjects. The fixation cross changed its orientation (45° rotation) once every few seconds. Subjects were asked to detect and press a button in response to this fixation change to encourage stable fixation and to engage attention. In the PRL block, a checker-filled 4° disc, flickering at 8 Hz, was presented at the fixation location for 12 seconds. Because the fixation cross occupied the center 1.5° of the PRL stimulus, the area of the disc containing the flickering checkerboard was reduced by 14%. In the non-PRL block, a peripheral location corresponding to a healthy retinal region with eccentricity similar to that of the PRL was also presented with a 4° flickering checkerboard disc for 12 seconds. Each PRL block and non-PRL block was followed by an 18-second fixation-only block and was repeated five times for each functional scan (5 minutes in total) (Fig. 1A).
Figure 1.
Illustration of functional scans. (A) One cycle of blocks in the PRL versus non-PRL scan. Arrow points to the location of the fixation cross (i.e., the PRL); arrow was not present in the experiments. The fixation cross was green in the actual experiments. (B) One cycle of blocks in the full-field versus fovea scan. Arrow points to the location of the fixation cross; arrow was not present in the experiments. The fixation cross was green in the actual experiments. (C) Two cycles of blocks in the picture versus uniform-field scan.
In the full-field versus fovea scan, the same fixation cross and task were used as in the PRL versus non-PRL scan. The boundary of the scotoma was within the display area for all subjects. In the full-field block, the whole display was filled with a flickering 8-Hz radial checkerboard for 12 seconds, except for the fixation cross at the PRL. The radial checkerboard was composed of concentric bands of 4° width, divided into eight radial segments (each spanning, 45° radial angle). The radial checkerboard pattern was centered on the estimated foveal location for each subject. In the fovea block, a checker-filled flickering 2° disc was presented at the estimated foveal location within the scotoma area for 12 seconds. Each full-field block and fovea block was followed with an 18-second fixation-only block and was repeated five times for each functional scan (5 minutes in total) (Fig. 1B).
In the picture versus uniform-field scan, grayscale images of indoor or outdoor scenes were presented in the picture block. In each picture block, five pictures were shown sequentially, each for 3 seconds, followed by a 1-second uniform field. Subjects were allowed to move their eyes to explore the content of the pictures. They were asked to press a button when two consecutive pictures were identical (one-back picture-matching task). Subjects had adequate acuity to see the pictures and to perform the task accurately. The picture block alternated with a 20-second uniform-field block eight times (5 minutes 20 seconds in total) (Fig. 1C).
MRI Data Acquisition and Analysis
The MRI sessions were conducted in the Biomedical Imaging Technology Center at the Emory University School of Medicine. A 3.0 Tesla whole-body system with an 8-channel array head coil (Trio; Siemens, Erlangen, Germany) was used. T2*-weighted images were acquired using a gradient echo-planar imaging sequence for the functional scans (20 axial slices covering the occipital lobe; repetition time, 2000 ms; echo time, 30 ms; flip angle, 75°; 128 × 128 image matrix; 2 mm in-plane resolution; 4-mm slice thickness). Each functional scan was repeated two or three times for each subject, except for subject AMD2. Because of technical failure, data were not collected for the picture versus uniform-field scan for AMD2. The first 10 volumes of each functional scan were discarded to allow for magnetization equilibration. T1-weighted images were acquired using a MPRAGE (magnetization-prepared rapid gradient-echo) sequence (176 sagittal slices, 256 × 224 image matrix, and 1 mm iso-voxel resolution) for localization and visualization of the functional data. Total scan time was approximately 1 hour, including high-resolution 3D image acquisition for each individual subject.
After preprocessing (slice timing correction, 3D motion correction, and temporal filtering), the functional data were coregistered with the anatomic data using analysis and visualization software (BrainVoyager QX; Brain Innovation, Inc., Maastricht, The Netherlands). General linear model (GLM) analysis was applied to the functional data with different stimulus conditions as the predictors. Statistical significance of the GLM predictors was tested at each voxel and corrected using the Bonferroni method. Statistical maps were created and overlaid on the inflated cortex for each subject. The significance criterion for the activation map was set as P < 0.01 for all functional scans. (There was a significant wrap-around artifact of the 3D anatomic image for subject JMD3; thus, data on JMD3 are not presented on the inflated map.)
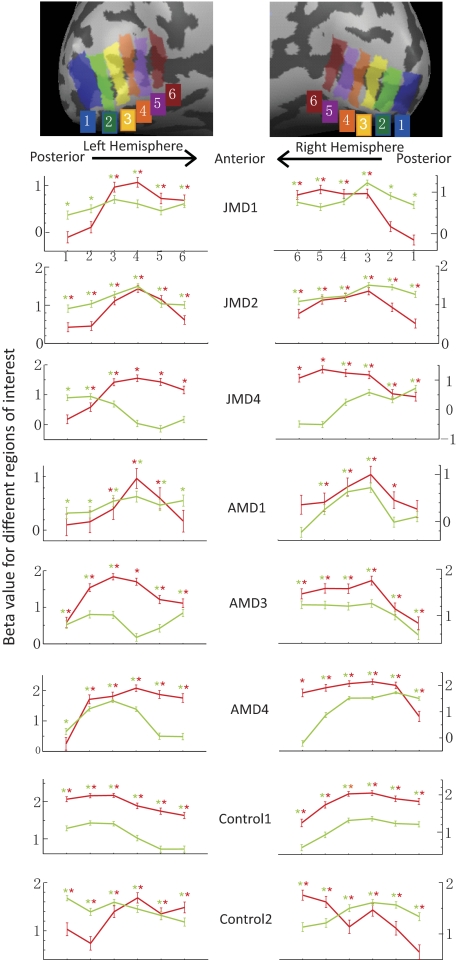
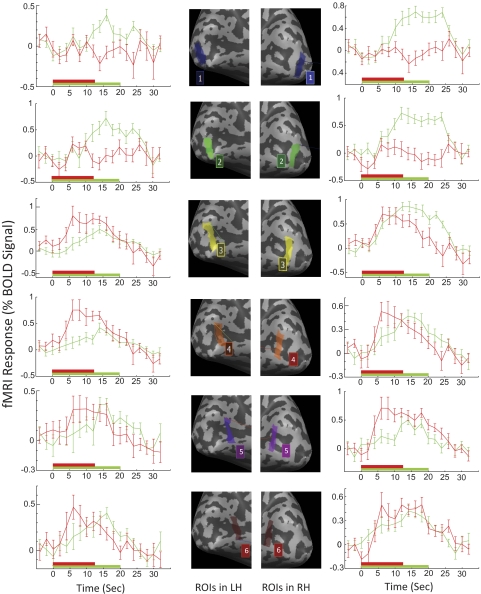
To compare brain activity more quantitatively between passive and active viewing conditions, region-of-interest (ROI) analysis was also applied to the fMRI data. Six ROIs were chosen along the calcarine sulcus, extending from the posterior pole of the occipital cortex to the most anterior part of the calcarine sulcus in each hemisphere for all the study subjects. These nonoverlapping ROIs were adjacent to each other, and each ROI included both the fundus and the walls of the calcarine sulcus (see Fig. 4 for an illustration of ROI definition). These ROIs are normally expected to be the cortical regions in the primary visual cortex representing different areas in the visual field, with the more posterior ones representing the fovea and the parafoveal region and the more anterior ones representing the peripheral region.20 ROI-GLM analysis was applied, and the beta values of the general linear model for different conditions were extracted from each ROI. The beta value is the weight of the predictor time course for each condition and is used as an indicator of the strength of cortical responses. The BOLD (blood oxygen level dependent) response time course of different ROIs was also extracted and shown as an example from one of our MD subjects.
Figure 4.
ROI analysis of cortical responses in the primary visual cortex. Top: illustration of ROI selection. Left: results for the left hemisphere (LH). Right: results for the right hemisphere (RH). The x-axis represents different ROIs along the calcarine sulcus. Posterior to anterior is left-to-right in LH and right-to-left in RH. The y-axis is the beta value for ROIs for different experimental conditions. Red curves: passive viewing condition. Green curves: active viewing condition. *P < 0.05; significant activation. Red star: significant activation with passive viewing. Green star: significant activation with active viewing.
Results
More Extensive Cortical Activation with PRL Stimuli than Non-PRL Stimuli
It is important to know how reliably a subject uses his or her PRL and whether one or more PRLs are involved. In the present study, PRL characteristics were observed for different fixation targets and different luminance levels (see Subjects and Methods). These tests verified that all the MD subjects had PRLs and scotoma characteristics that were stable over various stimulus and luminance conditions. It therefore seems reasonable to assume that any cortical reorganization observed in these subjects would have occurred in the presence of a longstanding and highly stable PRL.
The stimulus used for comparing responses at the PRL and non-PRL was smaller and more restricted to the PRL than that used in previous studies.8,9 The highly localized stimulus was intended to better observe the brain activity specifically associated with the PRL. However, the small size of the stimulus significantly weakened the overall BOLD response in the early visual cortex. There was no robust activation with the localized stimulus either at the PRL or the non-PRL control locations for AMD1, JMD2, and JMD4. Below we give detailed comparisons of PRL versus non-PRL activation for the remaining subjects. The main comparison was the number of activated voxels in the early visual cortex between different conditions. It should be noted that the activation is in early visual cortex but not necessarily confined to the primary visual cortex.
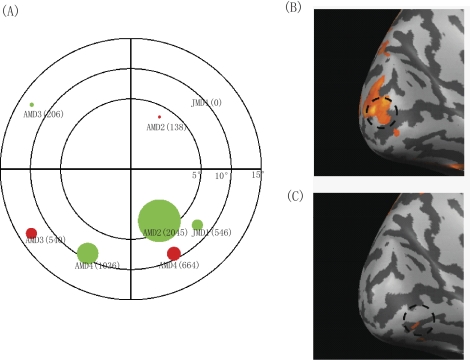
Figure 2 summarizes the activation from PRL (green disks) and non-PRL control (red disks) locations. The diameter of the disks is proportional to the number of voxels activated. Two properties can be seen clearly in Figure 2. First, lower field activation was more extensive than upper visual field activation,21 and activation was more extensive with PRL stimuli than non-PRL stimuli (overall, green disks are larger than red disks in Fig. 2). For AMD2, the PRL was located in the lower right visual field, and we placed the non-PRL stimulus in the upper right visual field. For AMD4, the PRL and non-PRL stimuli were both in the lower visual field, in opposite hemifields. JMD1 had spatial arrangements of PRL and non-PRL locations similar to those of AMD2. All three subjects showed a more extensive region of activation for the PRL stimulus than the non-PRL stimulus. The difference was more obvious with AMD2 and JMD1. For AMD3, activation was weak and noisy for both PRL and non-PRL stimuli. However, activation was more restricted with the PRL stimulus in the upper left visual field than the non-PRL stimulus in the lower left visual field. It is also important to note that there was no evidence showing reorganized activity in the foveal region of the cortex with the small stimuli used in the PRL/non-PRL comparison.
Figure 2.
Number of activated voxels for the stimulus at PRL or non-PRL locations. (A) Green circles represent the spatial extent of brain activation with the stimulus at the PRL for AMD2, AMD3, AMD4, and JMD1, and red circles represent the stimulus at the non-PRL location. The diameter of the circles is proportional to the number of activated voxels, and the center of the circles is the location of the stimulus in the visual field. The actual number of voxels for the region of activation in early visual cortex is also noted in brackets. A log scale was used for the eccentricity axis to reflect the logarithmic mapping along the eccentricity axis of the visual field on early visual cortex. (B, C) Example of brain activation maps with the stimulus at the PRL (B) and the control location (C) for AMD2.
Silent LPZ in Early Visual Cortex with the Full-Field Checkerboard Stimulus
As expected, no significant activation was detected for any MD subject when the 2° stimulus was presented at the estimated anatomic foveal location. This finding confirmed that there was no residual retinal function in the central scotoma. This observation, together with the good performance on the fixation task, also indicated that our MD subjects maintained good fixation during the fMRI scans.
Anterior regions of the calcarine sulcus and surrounding areas in the medial occipital cortex of both hemispheres were activated by the full-field checkerboard stimulus in all MD subjects. In the early visual cortex of normal vision subjects, the cortical representations of peripheral vision are expected to be located in the anterior parts of the medial occipital cortex.2,3,22 As expected, the regions activated by the full-field checkerboard stimulus in our MD participants corresponded to the retinotopic areas representing peripheral vision.
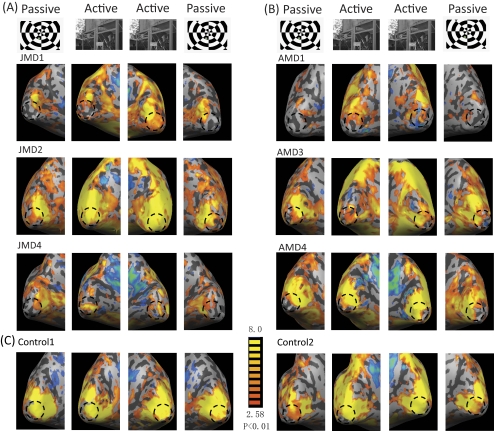
Unlike the case for control subjects, the full-field checkerboard stimulus did not activate the whole medial occipital cortex for MD subjects. There were silent zones at the posterior region of the calcarine sulcus and the posterior pole of the occipital cortex in both hemispheres across all MD subjects (see brain activation maps for passive viewing in Fig. 3). The cortical representation of foveal/central vision in V1, V2, and V3, named the foveal confluence, is located at the occipital pole in people with normal vision2 and is indicated with dashed circles in Figure 3. The inactive region of MD subjects matched the expected location of the foveal confluence. Although it is possible that there was some reorganized activation near the boundary of the LPZ, it is clear that in our MD subjects, a sizable region of the foveal confluence remained unresponsive to the full-field checkerboard stimuli. This result means there is a lack of complete reorganization of visually responsive cortex.
Figure 3.
Brain activation maps for passive and active viewing conditions. (A) Results for JMD subjects. (B) Results of AMD subjects. (C) Control subjects. First column: brain activation map of the left hemisphere (LH) with passive viewing. Second column: brain activation map of LH with active viewing. Third column: brain activation map of the right hemisphere (RH) with active viewing. Fourth column: brain activation map of RH with passive viewing. Dashed circle: foveal region of the cortex. By comparison, the silent zone within the dashed circle is reduced with active viewing in MD subjects, and this reduction is more significant in JMD subjects. The color coding of the brain activation map represents the confidence level (t value). Yellow: most confident; red: less confident; blue: negative signal change. The threshold was set as P < 0.01.
The anatomic features and surface area of V1, V2, and V3 have large individual variations. For example, Dougherty et al.2 found that the surface area of the foveal confluence varied from 982 mm2 to 2940 mm2 in their seven normally sighted participants. Therefore, it was impractical to link the size of the inactive region in an MD subject's brain to the size of his or her scotoma. Without functional retinotopic mapping, it is also difficult to know the precise relationship between visual space and cortical representation in an individual brain. Thus, it is uncertain whether the boundary of the inactive zone corresponded to the retinotopic projection of the boundary of the scotoma.
Reduced Size of the Silent Zone in an Active Task
In the picture condition, subjects had to respond when two successive scenes were the same. The lateral regions of the occipital cortex and the cortical regions of the temporal lobe were more extensively activated in this condition, probably because of the processing of the meaningful content of the picture. The general pattern of activation from pictures of scenes in the medial occipital cortex was similar to that found for the full-field checkerboard stimuli. Regions in early visual cortex representing peripheral vision were robustly activated, and there remained a silent zone corresponding to the foveal confluence in most MD subjects. However, when we compared the brain activity maps between the two different full-field stimuli (picture and checkerboard), we found that in almost all MD subjects, the silent (inactive) zone was smaller for the pictures. The degree of reduction in the size of the silent zone varied across subjects but was generally more prominent in JMD subjects. (See Fig. 3 for a side-by-side comparison of brain activation in the medial occipital cortex.)
To further quantify the difference of brain activity in the primary visual cortex between checkerboard and picture conditions, ROI analysis was applied to the data (Fig. 4). We selected six ROIs from the posterior to the anterior part of the calcarine sulcus for each hemisphere. The beta values for passive viewing and active viewing were plotted for each ROI against the position of the ROI (Fig. 4, blue and red curves). Even though there were noticeable differences in the shape of the curves across subjects, the spatial extent of activation showed a systematic difference between control subjects and MD subjects. Multiple factors could have contributed to the variability of the shape of the curves. For control 1, the overall elevation of activation from checkerboard stimuli might be attributed to the fact that checkerboard images had high contrast and were flickering, whereas grayscale images had lower contrast and were static. For control 2, there was a decrease in activation toward the posterior pole in the checkerboard condition that not seen in the active condition, possibly reflecting differences in attentional modulation across the visual field. However, despite the variable curve shapes, in control subjects the spatial extent of cortical response around the posterior pole was similar (Fig. 3), and all ROIs showed significant activation in both the checkerboard and the picture conditions (Fig. 4). In contrast, for the MD subjects, it was primarily the most posterior one or two ROIs that showed a difference, with the picture condition producing significant responses but not the checkerboard condition, reflecting different cortical responses within LPZ.
As an example, the BOLD time course was extracted from ROIs in early visual cortex in JMD1 and plotted in Figure 5. Consistent with the beta value plotting, the two ROIs at the posterior region of the calcarine sulcus showed activation with active viewing but not with passive viewing. Typical BOLD response signals peaked approximately 5 seconds after stimulus onset, but the responses in the LPZ in the active viewing condition rose to a peak 10 to 15 seconds after stimulus onset. This greater lag may indicate that the signal in this part of the cortex had a different origin from the signal in the conventionally driven cortex.
Figure 5.
BOLD time course of ROIs in JMD1. Left column: results from the left hemisphere. Right column: results from the right hemisphere. Top to bottom: time courses of ROIs from the posterior region to the anterior region of the calcarine sulcus. Green curves: active viewing condition in the picture versus uniform-field scan. Red curves: full-field passive viewing condition in the full-field versus fovea scan. The x-axis is time since the block onset (seconds). The stimulus was present for 20 seconds in active viewing (green bar above the x-axis) and for 12 seconds in passive viewing (red bar above the x-axis). Only active viewing showed significant activation in the first two ROIs at the posterior region of the calcarine sulcus.
Discussion
The scope of our study—an equal number of AMD and JMD subjects, all with well-characterized and stable PRLs, all tested in both active and passive viewing conditions—enabled us to address the three unresolved issues discussed in the Introduction.
Is the Cortical Representation of PRL Different from Other Retinal Loci at the Same Eccentricity?
Schumacher et al.9 reported that stimulation of the presumed PRL generated enhanced activity at the posterior calcarine sulcus, whereas stimulation at non-PRL control locations did not. However, it is unclear whether the ROIs at the “posterior calcarine sulcus” in their study corresponded to the V1 foveal LPZ because the ROIs were coronal slices not restricted to the cortical surface around the calcarine sulcus. On the other hand, Dilks et al.8 found that, with two JMD subjects, stimulation at both the presumed PRL and a non-PRL location generated strong activity in the LPZ, but the stimuli were larger (4°-6°) and involved active viewing. In our study, we used a smaller stimulus and passive viewing, enabling better localization of the PRL activation. Our results show that activation with the stimulus at the PRL was more extensive than at the non-PRL location for both AMD and JMD groups, but we found no evidence of reorganized activity within the LPZ for stimuli at either the PRL or the control location.
We also used two types of full-field stimuli, which, of course, included stimulation at the PRL. Because the PRL is located in an eccentric retinal location and has a spatial directional relationship to the natural fovea, it is possible that reorganization might spread from the PRL toward the posterior pole more so than in other directions. However, we did not find strong evidence for this possibility. The shapes of the curves in Figure 4 are comparable between the two hemispheres for most of the subjects, whereas data from AMD1 and AMD4 seem to suggest that there is an advantage of cortical reorganization for the hemisphere corresponding to the PRL projection. More data will be needed to test this hypothesis.
What Is the Difference in Cortical Reorganization between AMD and JMD?
AMD usually occurs in people aged 50 and older, and the history of disease is usually shorter than JMD at the time of the studies. Another important difference is that, generally speaking, the plasticity of neural systems decreases with age. In the present study, there were equal numbers of JMD and AMD subjects, and all had long histories (10 or more years) of macular degeneration, presumably long enough for cortical reorganization in both groups. We would therefore expect that differences between the two groups would be more attributable to the age of onset of disease.
Qualitatively, the patterns of brain activation were similar between AMD and JMD groups. However, the size of the silent zones was generally smaller in JMD subjects, even though the sizes of the scotoma were comparable between these two groups. For some of the JMD subjects, the silent zone at the posterior pole of the occipital cortex was entirely absent during the active-viewing condition (JMD2). Given that the AMD and JMD groups had comparable visual acuities and eccentricities of their PRLs, this difference in the size of the silent zones suggests that the age of onset and the etiology of MD also play a role in cortical reorganization.
What Is the Neural Mechanism Underlying Cortical Reorganization?
Smaller Silent Zone for Active Viewing than for Passive Viewing.
Using full-field stimuli, we observed a smaller silent zone for active viewing than for passive viewing for both AMD and JMD subjects. Why should this be the case?
The active condition (picture vs. uniform field) is different from the passive condition (full-field checkerboard vs. foveal checkerboard) in three ways. First, in the picture condition, subjects performed an attention-demanding one-back task involving the whole scene, whereas in the checkerboard condition, subjects attended to the fixation cross and only passively viewed the radial checkerboards. It is possible that task-dependent feedback signals from higher cortical areas in the picture condition accounted for the smaller silent zone in the active condition. Second, in the picture condition, subjects were allowed free eye movements, whereas in the checkerboard condition, they were required to maintain fixation. This means that in the picture condition, the spatial extent of the peripheral visual field stimulation could vary over time because of changes in gaze direction. This difference might be expected to result in differential activation of the anterior region in the medial occipital cortex. However, free eye movements would not increase the amount of visual input in retinal regions near the boundary of the scotoma and might actually result in reduced stimulation of bounding retina if the eye movements displaced the scotoma beyond the edge of the display screen. Thus, variations in peripheral visual input because of eye movements do not appear to explain the reduced size of the inactive zone in the picture task. Third, the checkerboard had high contrast and flickered at 8 Hz, which is an effective stimulus for generating brain activity in early visual cortex and would be expected to produce greater activation than picture stimuli composed of static grayscale images. Once again, this is the reverse of what we found.
Given these considerations, the difference in activation patterns between the checkerboard and the picture stimuli was probably not caused by differences in low-level image properties; rather, it is likely that the difference was caused by attention and other top-down task-dependent processes.
Underlying Neural Mechanisms of Cortical Reorganization in Macular Degeneration.
Neurophysiological studies suggest that after retinal lesions, there is not much reorganization at precortical stages or in their projections to cortex.23 This leaves the horizontal connections and the feedback connections at the cortical level as the primary pathways for reorganization. It is difficult to isolate their separate contributions. The two types of full-field stimuli with the passive and active tasks used in this study provide an opportunity to make direct comparisons between tasks.
Performing an active task on the picture stimuli did increase the activity in the posterior area of the medial occipital cortex, including areas that remained silent with a task-irrelevant checkerboard stimulus. Similar results of task modulation were observed in the Masuda et al.7 study of JMD subjects. Baker et al.5,6 also used an active viewing task. Only one of their subjects passively viewed the checkerboard and showed activation in LPZ, but the activation was weaker with passive viewing than with active viewing.6 Our study was consistent with that of Masuda et al.,7 and the results suggest that the nature of tasks plays a key role in the observed activity in the LPZ. The activation in the LPZ might be attributed to attentional feedback. For instance, attention might have strengthened the feedback signals between higher levels of visual cortex and early visual cortex, enabling these signals to reach the LPZ.24 It is also possible that the process of building an intact visual spatial representation in the brain contributed to the activation in the LPZ.
In this article, we have referred to any change of the retinotopic map, especially new activation in the lesion projection zone, as functional cortical reorganization. By a stricter definition, cortical reorganization would refer to growth of new axons and dendrites to form new circuits.12 Using this stricter definition, Masuda et al.7 did not regard their finding of task-dependent activation in the LPZ of JMD subjects as cortical reorganization. Instead, they attributed it to feedback signals from higher levels. Our results are consistent with theirs, although we regard the task-dependent activation in the silent zone as a form of functional reorganization. The fact is that we and others have observed BOLD activity in the LPZ under certain conditions, and given that fMRI signals cannot directly show axonal growth such as neurochemical methods,25 we have described this observation as evidence of functional reorganization.
Conclusions
Our study provides additional evidence for functional reorganization of the early visual cortex after central-field loss in macular degeneration. Our results show that the extent of the functional reorganization varies across persons and is generally incomplete. Activation in the LPZ was greater when subjects performed an active visual task, suggesting that the recruitment of neurons in the LPZ for processing of visual information from outside the central scotoma may be dependent on attention and feedback processes.
Comparison of our groups of AMD and JMD subjects showed a qualitatively similar pattern of findings but stronger evidence for functional reorganization in the JMD group. This difference may imply a greater potential for cortical reorganization in patients with early-onset forms of macular degeneration.
Our results also showed that stimuli presented to the PRL activated more extensive cortical regions than other retinal sites of equal eccentricity. Unlike previous studies with PRL stimuli, however, we did not find activation in the LPZ associated with PRL stimulation.
Footnotes
Supported by National Institutes of Health Grants R01 EY002934 and RO1EB002009.
Disclosure: T. Liu, None; S.-H. Cheung, None; R.A. Schuchard, None; C.B. Glielmi, None; X. Hu, None; S. He, None; G.E. Legge, None
References
- 1. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 2. Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vision. 2003;3:586–598 [DOI] [PubMed] [Google Scholar]
- 3. Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192 [DOI] [PubMed] [Google Scholar]
- 4. Cheung SH, Legge GE. Functional and cortical adaptations to central vision loss. Vis Neurosci. 2005;22:187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker CI, Dilks DD, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration: replication and clues about the role of foveal loss. Vis Res. 2008;48:1910–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dilks DD, Baker CI, Peli E, Kanwisher N. Reorganization of visual processing in macular degeneration is not specific to the “preferred retinal locus.” J Neurosci. 2009;29:2768–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schumacher EH, Jacko JA, Primo SA, et al. Reorganization of visual processing is related to eccentric viewing in patients with macular degeneration. Restorative Neurol Neurosci. 2008;26:391–402 [PubMed] [Google Scholar]
- 10. Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598 [DOI] [PubMed] [Google Scholar]
- 11. Gan WB, Kwon E, Feng G, Sanes JR, Lichtman JW. Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nat Neurosci. 2003;6:956–960 [DOI] [PubMed] [Google Scholar]
- 12. Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev. 2009;10:873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Timberlake GT, Peli E, Essock EA, Augliere RA. Reading with a macular scotoma, II: retinal locus for scanning text. Invest Ophthalmol Vis Sci. 1987;28:1268–1274 [PubMed] [Google Scholar]
- 14. Watson G, Baldasare J, Whittaker S. The validity and clinical uses of the Pepper Visual Skills for Reading Test. J Vis Impairment Blind. 1990;84:4 [Google Scholar]
- 15. Schuchard RA, Tekwani N, Hu S. Binocular preferred retinal loci: relationship of visual factors to binocular perception. OSA Technical Digest Series. 1995;1:4 [Google Scholar]
- 16. Sunness JS, Schuchard RA, Shen N, Rubin GS, Dagnelie G, Haselwood DM. Landmark-driven fundus perimetry using the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 1995;36:1863–1874 [PMC free article] [PubMed] [Google Scholar]
- 17. Timberlake GT, Sharma MK, Grose SA, Gobert DV, Gauch JM, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom Vis Sci. 2005;82:177–185 [DOI] [PubMed] [Google Scholar]
- 18. Brainard DH. The Psychophysics Toolbox. Spatial Vis. 1997;10:433–436 [PubMed] [Google Scholar]
- 19. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vis. 1997;10:437–442 [PubMed] [Google Scholar]
- 20. Hinds OP, Rajendran N, Polimeni JR, et al. Accurate prediction of V1 location from cortical folds in a surface coordinate system. NeuroImage. 2008;39:1585–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu T, Heeger DJ, Carrasco M. Neural correlates of the visual vertical meridian asymmetry. J Vis. 2006;6:1294–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893 [DOI] [PubMed] [Google Scholar]
- 23. Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams MA, Baker CI, Op de Beeck HP, et al. Feedback of visual object information to foveal retinotopic cortex. Nat Neurosci. 2008;11:1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]