Abstract
Context
Cardiometabolic effects of second-generation antipsychotics (SGAs) are concerning, but have been insufficiently studied in antipsychotic-naïve and pediatric patients.
Objectives
To study SGAs effects on body composition and metabolic parameters, unconfounded by prior antipsychotic exposure.
Design
Three-month, non-randomized Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) cohort study, conducted 12.2001–09.2007.
Setting
Semi-urban, tertiary care, academic inpatient and outpatient services in Queens, New York, with a 4.5 million people catchment area.
Patients
Of 505 youth, aged 4–19 (mean age: 13.9±3.6) years with ≤1 week antipsychotic exposure, 338 (66.9%) were enrolled. Of these, 272 (80.5%) had ≥1 post-baseline assessment forming the final sample, and 205 (61.7%) completed the study. Patients had mood spectrum (n=130, 47.8%), schizophrenia spectrum (n=82, 30.1%) and disruptive/aggressive behavior spectrum disorders (n=60, 22.1%). Fifteen refusing/non-adherent patients served as a comparison group.
Interventions
12-week treatment with aripiprazole, olanzapine, quetiapine or risperidone.
Main Outcome Measures
Body composition (weight, Body Mass Index percentile/z-score, fat mass, waist circumference), and fasting glucose and lipid parameters.
Results
Weight increased by 19.0(95% Confidence Interval:16.4, 21.5)lbs=15.2(13.2, 17.2)% with olanzapine (N=45), 13.5(10.9, 16.0)lbs=10.4(8.5, 12.3)% with quetiapine (N=36), 11.9(10.7, 13.1)bs=10.4(9.4, 11.3)% with risperidone (N=135), and 9.9(8.2, 11.5)lbs=8.1(7.0, 9.5)% with aripiprazole (N=41). Comparison subjects (N=15) changed weight minimally: 0.4(−2.3, 3.2)lbs=0.7(−1.3, 2.6)%. Weight gain ≥7% occurred in 84.4% (n=38) of patients on olanzapine, 64.4% (n=87) on risperidone, 58.4% (n=24) on aripiprazole, 55.6% (n=20) on quetiapine, and 0% of comparison subjects. With olanzapine, cholesterol (p<.001), triglycerides (p=0.002), non-HDL-cholesterol (p<.001), triglyceride/HDL ratio (p=0.002), glucose (p=0.02), insulin (p=0.02), and HOMA-IR (p=0.03) increased significantly. With quetiapine, cholesterol (p<0.05), triglycerides (p=0.01), non-HDL-cholesterol (p=0.03), and triglyceride/HDL ratio (p=0.004) increased significantly. With risperidone, triglycerides (p=0.04) increased significantly. Metabolic baseline-to-endpoint changes were non-significant with aripiprazole and comparison subjects. Dyslipidemia developed in 28.9% (n=13), 19.4% (n=26), 8.8% (n=3), and 7.3% (n=3) of youth on olanzapine, risperidone, quetiapine and aripiprazole, and 6.7% (n=1) of comparison subjects (p=0.03), while acquired insulin resistance (HOMA-IR>4.39: 2.9%–17.8%) and metabolic syndrome (0%–6.5%) were relatively rare in this short-term study.
Conclusions
First time SGA use was associated with significant weight gain with each medication. Metabolic changes varied among the 4 antipsychotics.
Keywords: Second-generation Antipsychotics, Antipsychotic-naïve, Weight Gain, Insulin Resistance, Dyslipidemia, Metabolic Syndrome, Cardiometabolic Risk, Children, Adolescents
Second-generation antipsychotics (SGAs) are commonly and increasingly prescribed to children and adolescents in the United States as first line treatment for psychotic disorders, bipolar disorder and non-psychotic mental disorders (1). Increasingly, cardiometabolic effects of SGAs have raised concern (2). Cardiometabolic side effects, such as age-inappropriate weight gain, obesity, hypertension and lipid and glucose abnormalities, are particularly problematic during development, as they predict adult obesity, metabolic syndrome, cardiovascular morbidity and malignancy (3–6). Emerging findings indicate that youth are especially vulnerable to antipsychotic-induced weight gain (7–10), but limited, prospective, pediatric data in suggest minimal or no metabolic liabilities, except for olanzapine (9,10). However, the interpretation of these data is hampered by variable prior antipsychotic exposure, which can obscure cardiometabolic effects. Therefore, data are sorely needed in patients with minimal antipsychotic exposure. Such data are lacking in youth and are limited in adults to small samples. Furthermore, since isolated studies in chronic patients implicated age (11) and antipsychotic dose (12) in cardiometabolic changes, data are needed in antipsychotic-naïve subjects.
To assess the cardiometabolic profiles of the four most commonly used SGAs unconfounded by carry-over effects from prior antipsychotic treatment, we conducted a prospective study of weight and metabolic changes in a large cohort of antipsychotic-naïve pediatric patients. We hypothesized that 12 weeks of treatment with aripiprazole, olanzapine, quetiapine or risperidone would result in rapid and significant worsening in body composition and metabolic parameters, and that these would be strongly correlated.
Methods
Study Setting and Design
Data were collected as part of the Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) study, a cohort study of antipsychotics in pediatric psychotic, mood or aggressive spectrum disorders. From 12.2001–09.2007, patients were recruited from our pediatric inpatient and outpatient services. Legal guardians/participants aged 18–19 years signed informed consent, minors aged 9–17 years signed informed assent for this North Shore-Long Island Jewish Health System Institutional Review Board-approved study. Data for this report are restricted to antipsychotic-naïve youth, and a psychiatric comparison group, consisting of patients refusing or discontinuing antipsychotics within 4 weeks.
Subjects
Inclusion criteria were: age 4–19 years; ≤1 week lifetime antipsychotic treatment; psychiatric illness prompting antipsychotic initiation; and consent/baseline anthropometric/biochemical assessments obtained within ≤7 days of antipsychotic initiation. Exclusion criteria were: treatment with >1 antipsychotic; active/past eating disorder; biochemical evidence of thyroid dysfunction; acute medical disorders; pregnancy/breastfeeding; wards of the state (as research consent by a public agency representative within 1 week was unlikely); and leaving the catchment area within <4 weeks. Psychiatric diagnoses and past treatment history were assessed by chart review, discussion with treatment providers, and clinical interview of the patient/caregiver. Postpubertal status (Tanner stage 3–5) was determined through inspection and interview of the patient and/or caregiver. Based on the literature in the general population (13), we obtained information on race/ethnicity as a potential predictor for cardiometabolic outcomes.
Treatment
Patients received clinician’s choice antipsychotic treatment. Informed consent/assent was obtained after the antipsychotic choice was made. Dosing, comedications and treatment changes were based on clinical necessity. Although this study focused on SGAs, all patients starting an antipsychotic were screened. During the study period, no antipsychotic-naïve patient was started on clozapine, paliperidone (which had not been approved), or a first-generation antipsychotic. Although six antipsychotic-naïve patients were initiated on ziprasidone, they were excluded from the analyses due to the small sample.
Outcomes
Primary outcomes were absolute and relative weight change. Secondary outcomes included change in additional body composition parameters (body mass index (BMI), BMI percentiles/z-scores, fat mass, and waist circumference), change in fasting metabolic parameters (total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, triglycerides/HDL ratio, glucose, insulin and the homeostatic model (HOMA-IR)), and incidence rates of weight gain ≥7%, individual metabolic parameters, dyslipidemia and metabolic syndrome (defined by presence of ≥3 of the following 5 criteria: obesity (BMI ≥95th percentile), blood pressure >90th percentile, triglycerides >110 mg/dL, HDL-cholesterol <40 mg/dL, and glucose ≥100 mg/dL (14).
Assessments
Subjects were assessed after ≥8 hours of overnight fasting at baseline and weeks 4, 8 and 12. Height was measured three times, using the stadiometer Seca 214. Weight, BMI, and fat mass were assessed by impedantiometry with the Tanita Body Composition Analyzer TBF-310. Patients were weighed clothed, with emptied pockets and without shoes or socks, using the following subtraction schedule: persons ≥5 feet wearing long trousers and long-sleeve shirt/sweatshirt: −3lbs; if dressed with short pants or short-sleeve/light shirt: −2.5lbs; if dressed with short pants and short-sleeve/light shirt: −2lbs; if just wearing underwear: −1.5lbs. For persons measuring <5, but ≥4 feet, an additional 0.5lbs were subtracted from the formula above. For persons <4 feet, an additional 1lb was subtracted. Waist circumference was measured at the level of both superior iliac crests and umbilicus, using the point of largest abdominal circumference. Fasting blood was drawn at 7–11 AM, prior to morning medications. Antipsychotic plasma levels were obtained at each post-baseline visit. Families were called before the visit and reminded of the overnight fast. At the visit, patients/caregivers were asked about adherence to fasting status. Fasting blood work was rescheduled if patients were non-fasting, and repeated if glucose was ≥100 mg/dl or insulin increased >100% from last assessment. Glucose and lipids were analyzed at the North Shore University Hospital Core Laboratory, Manhasset, NY, via Roche/Hitachi 747 chemistry analyzer and insulin was analyzed via Roche Elecsys 2010 immunochemistry analyzer . Antipsychotic plasma levels were measured with liquid chromatography at Cooper Laboratory, Nathan Kline Institute, Orangeburg, NY.
Statistical Analyses
Patients with ≥1 post-baseline assessment comprised the intent-to-treat sample. Sex- and age-adjusted BMI z-scores were calculated using a web-based calculator (http://www.kidsnutrition.org/bodycomp/bmiz2.html). Insulin resistance was determined with HOMA-IR (fasting insulin umol × glucose mmol/22.5) (15). HOMA-IR values >4.39 were diagnostic for insulin resistance (16).
Baseline values were compared across groups with chi-square and Fisher's Exact tests for categorical variables and the Kruskal-Wallis test for continuous variables. Change in continuous variables was analyzed within each treatment group using a mixed models repeated measures (MMRM) analysis of variance where the repeated (within subjects) factor was time relative to baseline at 4, 8, and 12 weeks. Summary statistics for MMRM are expressed as adjusted least squares means ±95% confidence intervals (CI). Incidence rates for dichotomous outcomes were analyzed using Last Observation Carried Forward (LOCF). Pearson chi-square test was used to compare categorical outcomes across antipsychotics, with corresponding baseline values as fixed covariates, controlling for significantly different baseline variables. Given the large body weight changes, we performed post-hoc analyses of multiples of the prespecified categorical change in weight (≥14% and ≥21%) and BMI z-score (≥1.0). We did not adjust for multiple comparisons regarding secondary outcomes. To confirm that MMRM and LOCF analyses were not yielding biased results due to missing data, multiple imputation was applied to the endpoint continuous variables and categorical outcomes. These results did not differ appreciably from the analyses performed without multiple imputation. Therefore, for simplicity and better comparability with results from prior studies, we conducted the analyses without multiple imputation. Analyses were repeated in patients with and without comedications known to affect weight (weight neutral: benzodiazepines, anticholinergics, alpha agonists, es/citalopram, fluvoxamine, sertraline, venlafaxine). For exploratory analyses of the effect of patients’ age on changes in body composition and metabolic parameters, we dichotomized patients into postpubertal status (N=191, mean age: 15.8 (CI:15.5–16.1) years versus pre/peripubertal status (N=81, mean age: 9.5 (CI:8.9–10.0) years. For the exploration of a dose effect, we dichotomized the data using a median split of the maximum (in most cases final) antipsychotic dose (aripiprazole=10 mg/day; olanzapine=10 mg/day; quetiapine=275 mg/day, risperidone=1.5 mg/day). Analyses were two-sided with alpha <.05, using SAS, version 9.1. For this observational cohort study, we conducted a generic power analysis for a mean change from baseline to 12 weeks/SD, using a paired t-test. Except for the comparison group where only a large effect size of 0.78 could be detected, we had 80% power (alpha=0.05, 2-tailed) to show significant differences corresponding to a moderate, clinically meaningful effect size (0.43 for olanzapine, 0.45 for aripiprazole and 0.48 for quetiapine), being able to detect a small effect size of 0.24 for risperidone. Unless otherwise specified, continuous variables are shown with lower and upper 95% CI.
Results
Patient Disposition
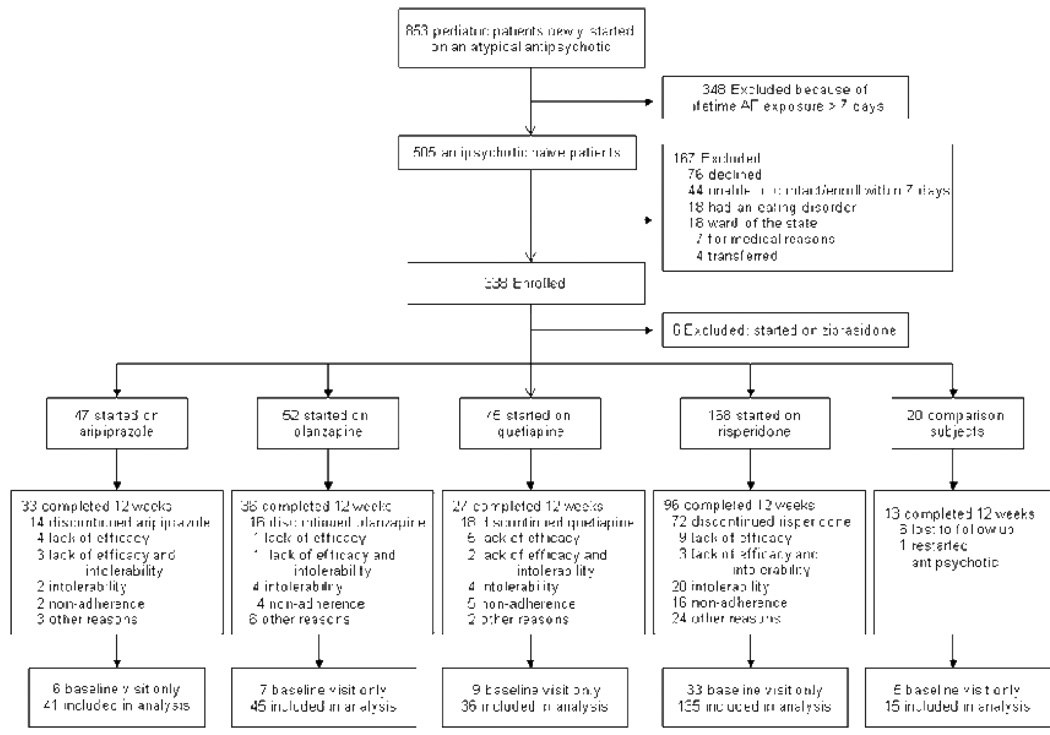
Of 505 antipsychotic-naïve pediatric patients, 338 (66.9%) were enrolled. Six patients on ziprasidone were excluded and 60 (17.9%) did not undergo post-baseline assessment, yielding 272 (81.0%) analyzed patients with confirmed antipsychotic adherence (Figure 1). The 173 subjects refusing/ineligible patients were not different from consenting patients except for less autism-spectrum disorders (1.9% vs 8.1%, p=0.0092), substance abuse comorbidity (8.4% vs 16.5%, p=0.018) and mixed ethnicity (3.7% vs 12.5%, p=0.0019) in the excluded group (in whom substance abuse and ethnicity were assessed solely via chart review compared to a formal interview in included patients). There were no significant differences on any variable included in table 1 between the 272 analyzed patients and the 60 youth without post-baseline assessments. Fifteen patients who refused or stopped the antipsychotic within 4 weeks (mean antipsychotic exposure: 12.4 (25th percentile: 10.8, 75th percentile: 14.0) days, and who had an 8- or 12-week assessment comprised the comparison group.
Figure 1.
Patient Flow
Table 1.
Baseline Demographic and Clinical Characteristics of 272 Antipsychotic-Naïve Children and Adolescents
| Baseline Characteristic | Total (n=272) |
Aripiprazole (n=41) |
Olanzapine (n=45) |
Quetiapine (n=36) |
Risperidone (n=135) |
Comparison Group (n=15) |
P- Value |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age (years, lower, upper 95% CI) | 13.9 (13.5, 14.4) |
13.4 (12.5, 14.4) |
14.7 (13.8, 15.7) |
14.0 (13.0, 15.1) |
13.6 (12.9, 14.3) |
15.5 (14.4, 16.6) |
0.13 |
| Post-pubertal Status – no. (%) | 191 (70.2) | 25 (61.0) | 38 (84.4) | 26 (72.2) | 88 (65.2) | 14 (93.3) | 0.03 |
| Male gender – no. (%) | 155 (57.0) | 23 (56.1) | 29 (64.4) | 13 (36.1) | 84 (62.2) | 6 (40.0) | 0.03 |
| Ethnicity – no. (%) | 0.32 | ||||||
| White | 131 (48.5) | 25 (62.5) | 21 (46.7) | 18 (50.0) | 62 (46.3) | 5 (33.3) | |
| African-American | 70 (25.9) | 8 (20.0) | 13 (28.9) | 7 (19.4) | 38 (28.4) | 4 (26.7) | |
| Hispanic | 24 (8.9) | 3 (7.5) | 1 (2.2) | 3 (8.3) | 15 (11.2) | 2 (13.3) | |
| Asian | 11 (4.1) | 2 (5.0) | 2 (4.4) | 4 (11.1) | 2 (1.5) | 1 (6.7) | |
| Mixed/Other | 34 (12.5) | 2 (5.0) | 8 (17.8) | 4 (11.8) | 17 (12.7) | 3 (18.7) | |
| SES (Hollingshead 1–5, lower, upper 95% CI) | 2.8 (2.6, 2.9) | 2.6 (2.3, 3.0) | 2.8 (2.5, 3.2) | 2.7 (2.4, 3.1) | 2.8 (2.6, 3.0) | 2.8 (1.7, 3.9) | 0.91 |
| Family history – no. (%) | |||||||
| Obesity: first degree member | 107 (44.4) | 19 (57.6) | 25 (58.1) | 10 (32.3) | 71 (58.7) | 9 (69.2) | 0.08 |
| Diabetes: first degree member | 38 (16.0) | 5 (16.1) | 6 (14.3) | 5 (16.1) | 19 (15.7) | 3 (25.0) | 0.94 |
| Dyslipidemia: first degree member | 83 (35.5) | 10 (32.3) | 15 (35.7) | 14 (45.2) | 42 (35.9) | 2 (15.4) | 0.45 |
| Hypertension: first degree member | 70 (29.5) | 9 (28.1) | 9 (20.9) | 8 (26.7) | 39 (33.3) | 5 (33.3) | 0.63 |
| Primary psychiatric diagnosis – no. (%) | |||||||
| ODD/CD/IED/ICD | 96 (35.4) | 11 (27.5) | 16 (35.6) | 9 (25.00) | 55 (40.7) | 5 (33.3) | 0.34 |
| Psychosis NOS | 54 (19.9) | 11 (26.8) | 5 (11.1) | 4 (11.1) | 33 (24.4) | 1 (6.7) | 0.08 |
| Major Depressive disorder/Depressive Disorder NOS | 49 (18.0) | 10 (24.4) | 8 (17.8) | 8 (22.2) | 19 (14.1) | 4 (27.7) | 0.44 |
| Bipolar I, and II Disorder, Bipolar Disorder NOS |
44 (16.2) | 3 (7.3) | 9 (20.0) | 10 (27.8) | 17 (12.6) | 5 (33.3) | 0.03 |
| Mood Disorder NOS | 37 (13.6) | 5 (12.2) | 4 (8.9) | 6 (16.7) | 19 (14.1) | 3 (20.0) | 0.78 |
| Schizophrenia/Schizoaffective disorder | 27 (9.9) | 3 (7.3) | 9 (20.0) | 2 (5.6) | 13 (9.6) | 0 (0.0) | 0.10 |
| Autism Spectrum Disorder | 22 (8.1) | 4 (9.8) | 3 (6.7) | 2 (5.6) | 13 (9.6) | 0 (0.0) | 0.67 |
|
Clinical Global Impression Severity score (mean, lower, upper 95% CI) |
5.4 (5.3, 5.5) | 5.3 (5.1, 5.5) | 5.7 (5.4, 6.0) | 5.3 (5.0, 5.6) | 5.4 (5.3, 5.6) | 5.1 (4.6, 5.6) | 0.14 |
|
Global Assessment of Functioning score (mean, lower, upper 95% CI) |
36.4 (35.5, 37.4) |
38.5 (36.5, 40.6) |
33.1 (30.3, 35.9) |
37.8 (35.3, 40.3) |
36.5 (35.1, 37.9) |
37.6 (33.3, 42.0) |
0.02 |
| Body composition characteristics | |||||||
| Weight (lbs, lower, upper 95% CI) | 118.9 (114.4, 124.8) |
122.2 (111.6, 141.1) |
119.9 (106.8, 132.9) |
132.5 (116.2, 148.9) |
112.8 (105.7, 119.9) |
130.0 (114.9, 145.2) |
0.10 |
| Baseline Value |
Total (n=272) |
Aripiprazole (n=41) |
Olanzapine (n=45) |
Quetiapine (n=36) |
Risperidone (n=135) |
Comparison Group (n=15) |
P- Value |
| Fat mass (lbs, lower, upper 95% CI) | 27.7 (25.5, 31.1) |
32.2 (25.8, 45.6) |
23.8 (17.9, 29.6) |
38.6 (29.3, 47.9) |
24.6 (21.2, 27.9) |
29.0 (19.7, 38.3) |
0.004 |
| Waist circumference (cm, lower, upper 95% CI) | 77.1 (75.5, 79.1) |
80.7 (76.6, 87.3) |
75.5 (71.4, 79.6) |
83.1 (77.5, 88.7) |
74.7 (72.3, 77.1) |
79.7 (73.6, 85.8) |
0.01 |
| BMI (kg/m2, lower, upper 95% CI) | 21.3 (20.7, 22.1) |
22.4 (21.0, 25.2) |
20.4 (19.1, 21.7) |
23.3 (21.2, 25.4) |
20.6 (19.7, 21.4) |
22.1 (20.2, 24.1) |
0.02 |
| BMI z-score (lower, upper 95% CI) | 0.24 (0.09, 0.41) |
0.66 (0.29, 1.14) |
−0.08 (−0.48, 0.32) |
0.71 (0.29, 1.14) |
0.09 (−0.13, 0.31) |
0.33 (−0.32, 0.98) |
0.01 |
| BMI percentile (lower, upper 95% CI) | 57.4 (53.7, 61.6) |
68.6 (59.2, 78.9) |
49.9 (40.0, 60.0) |
67.7 (56.8, 78.6) |
53.6 (48.1, 59.2) |
60.0 (41.9, 78.1) |
0.01 |
| Weight status category – no. (%) | |||||||
| Underweight (<5th BMI Percentile) | 21 (7.7) | 2 (4.9) | 6 (13.3) | 2 (5.6) | 10 (7.4) | 1 (6.7) | 0.60 |
| Normal (≥5th-<85th BMI Percentile) | 168 (61.7) | 20 (48.8) | 30 (66.7) | 18 (50.0) | 90 (66.7) | 10 (66.7) | 0.14 |
| Overweight (≥85th-<95th BMI Percentile) | 37 (13.6) | 6 (14.6) | 5 (11.1) | 6 (16.7) | 19 (14.1) | 1 (6.7) | 0.89 |
| Obese (≥95th BMI Percentile) | 46 (16.9) | 13 (31.7) | 4 (8.9) | 10 (27.8) | 16 (11.8) | 3 (20.0) | 0.007 |
| Fasting metabolic characteristics | |||||||
| Cholesterol (mg/dL, lower, upper 95% CI) | 161.2 (157.8, 165.1) |
159.9 (151.5, 170.9) |
155.2 (147.0, 163.3) |
156.6 (146.9, 166.2) |
164.3 (158.9, 169.8) |
161.2 (151.7, 181.2) |
0.33 |
| LDL-cholesterol (mg/d, lower, upper 95% CI) | 92.5 (89.6, 95.8) |
89.5 (82.8, 98.7) |
89.5 (82.5, 96.6) |
89.7 (81.5, 97.9) |
94.7 (89.9, 99.6) |
92.5 (83.6, 109.2) |
0.59 |
| HDL-cholesterol (mg/dL, lower, upper 95% CI) | 52.4 (51.0, 53.8) |
50.6 (46.1, 55.0) |
51.6 (48.4, 54.8) |
48.6 (45.0, 52.2) |
54.5 (52.5, 56.5) |
49.9 (43.9, 56.0) |
0.05 |
| Triglycerides (mg/dL, lower, upper 95% CI) | 86.0 (81.1, 91.3) |
98.9 (79.9, 119.9) |
80.1 (70.0, 90.1) |
89.9 (78.3, 101.5) |
81.5 (75.5, 87.6) |
100.3 (66.0, 134.6) |
0.09 |
| Glucose (mg/dL, lower, upper 95% CI)** | 84.0 (83.0, 84.9) |
85.5 (82.2, 88.0) |
84.1 (81. 9, 86.3) |
84.3 (81.9, 86.7) |
83.5 (82.1, 84.9) |
82.8 (79.2, 86.4) |
0.66 |
| Insulin (mg/dL, lower, upper 95% CI)** | 12.1 (11.2, 13.3) |
13.2 (10.5, 16.7) |
12.3 (9.9, 14.8) |
16.6 (12.3, 20.9) |
10.5 (9.2, 11.8) |
13.1 (9.4, 16.8) |
0.005 |
| HOMA-IR (lower, upper 95% CI) | 2.6 (2.3, 2.82) | 2.8 (2.2, 3.6) | 2.7 (2.1, 3.2) | 3.5 (2.5, 4.4) | 2.2 (1.9, 2.5) | 2.7 (1.9, 3.5) | 0.009 |
| TG/HDL (lower, upper 95% CI) | 1.8 (1.6, 1.9) | 2.2 (1.6, 2.9) | 1.7 (1.4, 2.0) | 1.9 (1.7, 2.2) | 1.6 (1.5, 1.8) | 2.2 (1.3, 3.0) | 0.03 |
| Treatment characteristics | |||||||
| Mean treatment duration (wks, 25th, 75th percentile) | 10.8 (10.5,11.2) |
11.4 (10.5, 12.2) |
10.8 (10.0, 11.6) |
10.5 (9.3, 11.7) |
10.6 (10.0, 11.2) |
12.4 (10.8, 14.0 |
0.23 |
| Maximum daily dose (mg ± SD) | N/A | 11.8 (9.3, 14.1) |
9.9 (8.0, 11.8) |
302.4 (225.5, 379.4) |
2.0 (1.7, 2.2) |
N/A | N/A |
| Mean inpatient time (days, 25th, 75th percentile) | 13.3 (0.0, 17.0) |
9.3 (0.0, 13.5) |
17.5 (3.0, 22.5) |
16.4 (0.0, 22.0) |
12.8 (0.0, 17.0) |
8.0 (0.0, 10.0) |
0.21 |
| Median inpatient time (days) | 6.0 | 2.0 | 13.0 | 10.0 | 6.0 | 1.0 | |
| Mean inpatient time ratio (%, 25th, 75th percentile) a | 20.3 (0.0, 26.4) |
13.3 (0.0, 17.6) |
23.6 (4.6, 36.5) |
25.4 (0.0, 28.6) |
20.7 (0.0, 25.0) |
14.3 (0.0, 10.5) |
0.36 |
| Median inpatient time ratio (%) a | 8.0 | 2.3 | 17.6 | 12.0 | 6.8 | 1.2 | |
| Mean duration on antipsychotic before baseline (days, 25th, 75th percentile) | 2.8 (0.0, 4.0) | 2.1 (0.0, 4.0) | 3.4 (1.0, 5.0) | 2.4 (1.0, 3.8) | 2.8 (0.0, 4.0) | 3.9 (2.0, 6.0) | 0.70 |
| Median duration on antipsychotic before baseline (days) | 2.0 | 1.0 | 3.0 | 2.0 | 2.0 | 5.0 | |
| Total number of comedications (lower, upper 95% CI) | 1.0 (0.9, 1.1) | 0.8 (0.7, 1.1) | 0.89 (0.9, 1.1) | 0.9 (0.7, 1.1) | 1.1 (1.0, 1.3) | 0.9 (0.5, 1.3) | 0.16 |
| Specific comedications – no. (%)b | |||||||
| None | 75 (27.8) | 16 (41.0) | 14 (31.1) | 8 (22.2) | 32 (23.7) | 5 (33.3) | 0.23 |
| None or weight-neutral c | 146 (54.1) | 26 (66.7) | 23 (51.1) | 14 (38.9) | 75 (55.6) | 8 (53.3) | 0.19 |
| Mood stabilizer | 76 (28.1) | 6 (15.4) | 18 (40.0) | 15 (41.7) | 32 (23.7) | 5 (33.3) | 0.03 |
| Antidepressant | 81 (30.1) | 13 (33.3) | 10 (22.2) | 10 (27.8) | 43 (32.1) | 5 (33.3) | 0.75 |
| Anxiolytic/hypnotic | 19 (7.0) | 1 (2.6) | 3 (6.7) | 1 (2.8) | 13 (9.6) | 1 (6.7) | 0.46 |
| Psychostimulant | 42 (15.6) | 5 (12.8) | 4 (8.9) | 4 (11.1) | 26 (19.3) | 3 (20.0) | 0.42 |
| Anticholinergic | 22 (8.1) | 2 (5.1) | 0 (0.0) | 2 (5.6) | 18 (13.3) | 0 (0.0) | 0.03 |
| Other psychotropic d | 15 (5.6) | 4 (10.5) | 1 (2.2) | 1 (2.8) | 9 (5.6) | 0 (0.0) | 0.35 |
Inpatient time ratio was calculated by diving the number of days spent as an inpatient by the total number of days in the study
Total N of patients in the subcategories is larger than total N of patients in the study as patients in the “None or weight-neutral” medication category also appear in the other respective categories
Weight-neutral medications: benzodiazepines, anticholinergics, citalopram, sertraline, venlafaxine, alpha 2 agonists
Other psychotropic medications: alpha-2 agonists, beta blockers, antihistamines
Baseline Characteristics
The 272 patients were 13.9 (13.5, 14.4) years old, predominantly post-pubertal (n=191, 70.2%), male (n=155, 57.0%), and of normal body weight status (n=168, 61.7%). Demographic and treatment characteristics are displayed in Table 1.
Primary Outcomes
a) Weight Gain
After 10.8 (25th percentile: 10.5, 75th percentile: 11.2) weeks of treatment, weight increased significantly by 19.0lbs (95% CI=16.4,21.5), which corresponds to 15.2% (13.2,17.2) weight gain with olanzapine (N=45), 13.5lbs (10.9,16.0) or 10.4% (8.5,12.3) with quetiapine (N=36), 11.9lbs (10.7,13.1) or 10.4% (9.4,11.3) with risperidone (N=135), and 9.9lbs (8.2,11.5) or 8.1% (7.0,9.5) with aripiprazole (N=41) (p<0.0001 each) (Table 2). Comparison subjects (N=15) changed weight minimally: 0.4lbs (−2.3,3.2) corresponding to 0.7% (−1.3,2.6). The proportions of patients gaining ≥7% were 84.4% (n=38) for olanzapine, 64.4% (n=87) for risperidone, 58.4% (n=24) for aripiprazole, 55.6% (n=20) for quetiapine, and 0% (n=0) in comparison subjects (Table 4).
Table 2.
Change in Body Composition Parameters over Time
| Outcome Variable | 0–4 Weeks | 0–8 Weeks | 0–12 Weeks | P-Value Wk 0–4 |
P-Value Wk 0–8 |
P-Value Wk 0–12 |
Effect Size |
|---|---|---|---|---|---|---|---|
| Weight (lbs) | |||||||
| Aripiprazole | 3.57 (1.98, 5.16) | 7.43 (5.82, 9.04) | 9.87 (8.24, 11.50) | <0.001 | <0.001 | <0.001 | 1.86 |
| Olanzapine | 10.05 (7.58,12.52) | 14.85 (12.34, 17.36) | 18.97 (16.40, 21.54) | <0.001 | <0.001 | <0.001 | 2.16 |
| Quetiapine | 6.38 (3.95, 8.81) | 10.77 (8.28, 13.26) | 13.46 (10.89, 16.03) | <0.001 | <0.001 | <0.001 | 1.71 |
| Risperidone | 6.05 (4.93, 7.17) | 10.28 (9.12, 11.44) | 11.87 (10.69, 13.05) | <0.001 | <0.001 | <0.001 | 1.70 |
| Untreated | 2.23 (−0.77, 5.23) | 1.74 (−1.14, 4.62) | 0.43 (−2.31, 3.17) | 0.17 | 0.25 | 0.77 | 0.08 |
| Weight (Percent of Baseline) | |||||||
| Aripiprazole | 3.09 (1.78, 4.40) | 6.24 (4.91, 7.57) | 8.14 (6.97, 9.49) | <0.001 | <0.001 | <0.001 | 1.84 |
| Olanzapine | 8.34 (6.44, 10.24) | 12.17 (10.23, 14.11) | 15.20 (13.24, 17.16) | <0.001 | <0.001 | <0.001 | 2.27 |
| Quetiapine | 4.93 (3.13, 6.73) | 8.30 (6.46, 10.14) | 10.42 (8.54, 12.30) | <0.001 | <0.001 | <0.001 | 1.81 |
| Risperidone | 5.33 (4.43, 6.23) | 8.90 (7.96, 9.84) | 10.37 (9.41, 11.33) | <0.001 | <0.001 | <0.001 | 1.82 |
| Untreated | 1.67 (−0.51, 3.85) | 1.55 (−0.53, 3.63) | 0.65 (−1.33, 2.63) | 0.15 | 0.17 | 0.53 | 0.17 |
| Fat Mass (lbs) | |||||||
| Aripiprazole | 1.15 (−0.18, 2.48) | 3.09 (1.74, 4.44) | 5.39 (4.02, 6.76) | 0.10 | <0.001 | <0.001 | 1.20 |
| Olanzapine | 3.57 (1.79, 5.35) | 6.91 (5.11, 8.71) | 9.15 (7.31, 10.99) | 0.0002 | <0.001 | <0.001 | 1.45 |
| Quetiapine | 1.85 (0.20, 3.50) | 4.61 (2.85, 6.37) | 6.26 (4.46, 8.06) | 0.032 | <0.001 | <0.001 | 1.13 |
| Risperidone | 2.09 (1.31, 2.87) | 4.37 (3.53, 5.21) | 5.45 (4.59, 6.31) | <0.001 | <0.001 | <0.001 | 1.07 |
| Untreated | 0.78 (−1.10, 2.66) | 1.03 (−0.91, 2.97) | 0.77 (−0.95, 2.49) | 0.43 | 0.31 | 0.39 | 0.23 |
| BMI (kg/m2) | |||||||
| Aripiprazole | 0.62 (0.33, 0.91) | 1.28 (0.99, 1.57) | 1.67 (1.36, 1.98) | 0.001 | <0.001 | <0.001 | 1.63 |
| Olanzapine | 1.64 (1.25, 2.03) | 2.41 (2.00, 2.82) | 3.01 (2.60, 3.42) | <0.001 | <0.001 | <0.001 | 2.14 |
| Quetiapine | 1.03 (0.64, 1.42) | 1.74 (1.33, 2.15) | 2.12 (1.71, 2.53) | <0.001 | <0.001 | <0.001 | 1.68 |
| Risperidone | 1.01 (0.83, 1.19) | 1.71 (1.51, 1.91) | 1.92 (1.72, 2.12) | <0.001 | <0.001 | <0.001 | 1.65 |
| Untreated | 0.30 (−0.19, 0.79) | 0.21 (−0.26, 0.68) | −0.0032 (−0.45, 0.45) | 0.25 | 0.40 | 0.99 | 0.00 |
| BMI (Percent of Baseline) | |||||||
| Aripiprazole | 2.86 (1.53, 4.19) | 5.68 (4.35, 7.01) | 7.20 (5.83, 8.57) | 0.001 | <0.001 | <0.001 | 1.61 |
| Olanzapine | 7.88 (6.00, 9.76) | 11.45 (9.53, 13.17) | 14.04 (12.08, 16.00) | <0.001 | <0.001 | <0.001 | 2.09 |
| Quetiapine | 4.44 (2.68, 6.20) | 7.63 (5.81, 9.45) | 9.29 (7.43, 11.15) | <0.001 | <0.001 | <0.001 | 1.63 |
| Risperidone | 4.87 (3.99, 5.75) | 8.12 (7.20, 9.04) | 9.12 (8.18, 10.06) | <0.001 | <0.001 | <0.001 | 1.64 |
| Untreated | 1.25 (−0.89, 3.39) | 0.99 (−0.99, 2.97) | 0.05 (−1.89, 1.99) | 0.27 | 0.36 | 0.96 | 0.11 |
| BMI z-score | |||||||
| Aripiprazole | 0.16 (0.04, 0.28) | 0.31 (0.19, 0.43) | 0.37 (0.25, 0.49) | 0.007 | <0.001 | <0.001 | 0.96 |
| Olanzapine | 0.56 (0.38, 0.74) | 0.79 (0.61, 0.97) | 0.93 (0.75, 1.11) | <0.001 | <0.001 | <0.001 | 1.54 |
| Quetiapine | 0.18 (0.04, 0.32) | 0.38 (0.24, 0.52) | 0.44 (0.28, 0.60) | 0.02 | <0.001 | <0.001 | 0.92 |
| Risperidone | 0.35 (0.27, 0.43) | 0.53 (0.45, 0.61) | 0.60 (0.52, 0.68) | <0.001 | <0.001 | <0.001 | 1.29 |
| Untreated | 0.04 (−0.08, 0.16) | 0.02 (−0.10, 0.14) | −0.003 (−0.12, 0.11) | 0.52 | 0.80 | 0.96 | −0.01 |
| BMI Percentile | |||||||
| Aripiprazole | 4.38 (1.26, 7.50) | 6.97 (3.81, 10.13) | 8.44 (5.26, 11.62) | 0.008 | <0.001 | <0.001 | 0.81 |
| Olanzapine | 14.09 (9.35, 18.83) | 20.36 (15.54, 25.18) | 24.22 (19.32, 29.12) | <0.001 | <0.001 | <0.001 | 1.44 |
| Quetiapine | 4.86 (1.57, 8.15) | 9.33 (5.92, 12.74) | 10.86 (7.35, 14.37) | 0.006 | <0.001 | <0.001 | 1.01 |
| Risperidone | 9.38 (6.77, 11.99) | 14.69 (11.97, 17.41) | 16.42 (13.64, 19.20) | <0.001 | <0.001 | <0.001 | 1.00 |
| Untreated | 0.97 (−2.58, 4.52) | 0.66 (−2.75, 4.07) | 0.69 (−2.58, 3.96) | 0.60 | 0.71 | 0.69 | 0.11 |
| Waist (cm) | |||||||
| Aripiprazole | 2.20 (−0.33, 4.73) | 4.28 (1.75, 6.81) | 5.40 (2.87, 7.93) | 0.09 | 0.002 | 0.001 | 0.65 |
| Olanzapine | 4.09 (3.01, 5.17) | 6.79 (5.67, 7.91) | 8.55 (7.43, 9.67) | <0.001 | <0.001 | <0.001 | 2.44 |
| Quetiapine | 2.74 (1.62, 3.86) | 4.50 (3.34, 5.66) | 5.27 (4.07, 6.47) | <0.001 | <0.001 | <0.001 | 1.44 |
| Risperidone | 2.85 (2.28, 3.42) | 4.60 (4.01, 5.19) | 5.10 (4.49, 5.71) | <0.001 | <0.001 | <0.001 | 1.42 |
| Untreated | 0.84 (−0.87, 2.55) | 0.94 (−0.73, 2.61) | 0.70 (−0.87, 2.27) | 0.36 | 0.29 | 0.40 | 0.23 |
All change scores are reports as means ± 95% confidence intervals.
Bolded p values: <0.05
Table 4.
New-Onset Categorical Glucose and Lipid Metabolism Outcomes
| Outcome Variable | Total (n=272) |
Aripiprazole (n=41) |
Olanzapine (n=45) |
Quetiapine (n=36) |
Risperidone (n=135) |
Comparison Group (n=15) |
P-Value including the Comparison Group |
P-Value excluding the Comparison Group a |
|---|---|---|---|---|---|---|---|---|
| Weight Loss - n (%) | 16 (5.9) | 2 (4.9) | 1 (2.2) | 1 (2.8) | 6 (4.4) | 6 (40.0) | <0.001 | 0.83 |
| Weight Gain ≥7% - n (%) | 169 (62.1) | 24 (58.4) | 38 (84.4) | 20 (55.6) | 87 (64.4) | 0 (0.0) | <0.001 | <0.05 |
| Weight Gain ≥14% - n (%) | 75 (27.6) | 7 (17.1) | 23 (51.1) | 11 (30.6) | 34 (25.2) | 0 (0.0) | 0.03 | 0.01 |
| Weight Gain ≥21% - n (%) | 24 (8.8) | 2 (4.9) | 11 (24.4) | 2 (5.6) | 9 (6.7) | 0 (0.0) | 0.002 | 0.01 |
| Transition to overweight b or obese c category - n (%) | 47 (17.3) | 4 (9.8) | 10 (22.2) | 13 (36.1) | 19 (14.1) | 1 (6.6) | 0.0092 | 0.04 |
| >10% BMI - n (%) | 101 (37.1) | 9 (22.0) | 30 (66.7) | 14 (38.9) | 48 (35.6) | 0 (0.0) | <0.001 | 0.003 |
| >20% BMI - n (%) | 23 (8.5) | 3 (7.3) | 10 (22.2) | 2 (5.6) | 8 (5.9) | 0 (0.0) | 0.007 | 0.12 |
| >0.5 BMI z-Score- n (%) | 114 (41.9) | 9 (22.0) | 28 (62.2) | 13 (36.1) | 64 (47.4) | 0 (0.0) | <0.001 | 0.03 (ARI) 0.04 (OLA) |
| >1.0 BMI z-Score- n (%) | 49 (18.1) | 2 (4.9) | 16 (35.6) | 5 (13.9) | 26 (19.3) | 0 (0.0) | 0.001 | 0.04 |
| Hyperglycemia d, e (>100 mg/dL) – no. (%) | 9 (3.4) | 2 (4.9) | 1 (2.2) | 2 (5.9) | 4 (3.0) | 0 (0.0) | 0.79 | 0.86 |
| Hyperinsulinemia d (≥ 20 U/mL) - no. (%) | 21 (7.9) | 3 (7.3) | 8 (17.8) | 1 (2.9) | 9 (6.8) | 0 (0.0) | 0.07 | 0.03 |
| Insulin Resistance d (HOMA IR≥4.4 | 23 (8.6) | 3 (7.3) | 8 (17.8) | 1 (2.9) | 11 (8.3) | 0 (0.0) | 0.10 | 0.02 |
| Hypercholesterolemia f - (≥170 mg/dL) - no. (%) | 35 (13.0) | 3 (7.3) | 12 (26.7) | 5 (14.7) | 23 (17.2) | 0 (0.0) | 0.06 | 0.26 |
| High LDL-cholesterol f - (>110 mg/dL) - no. (%) | 34 (12.6) | 2 (4.9) | 13 (28.9) | 5 (14.7) | 13 (9.7) | 1 (6.7) | 0.03 | 0.006 |
| Low HDL-cholesterol f (<40 mg/dL) no. (%) | 13 (4.8) | 2 (4.9) | 5 (11.1) | 2 (5.9) | 4 (3.0) | 0 (0.0) | 0.22 | 0.37 |
| Hypertriglyceridemia f - (≥110 mg/dL) no. (%) | 35 (13.0) | 3 (7.3) | 9 (20.0) | 6 (11.9) | 16 (12.0) | 1 (6.7) | 0.35 | 0.18 |
| Dyslipidemia f - (cholesterol ≥170 mg/dL and/or triglycerides ≥110 mg/dL) - no. (%) | 46 (17.1) | 3 (7.3) | 13 (28.9) | 3 (8.8) | 26 (19.3) | 1 (6.7) | 0.03 | 0.04 |
| Triglyceride/HDL ratio ≥3.5 e - no. (%) | 23 (8.6) | 3 (7.3) | 5 (11.1) | 4 (11.8) | 11 (8.2) | 0 (0.0) | 0.68 | 0.49 |
| Metabolic syndrome g - no. (%) | 4 (1.6) | 1 (2.5) | 1 (2.5) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0.13 | 0.44 |
Bolded p values: <0.05
Controlled for each respective baseline value (BMI z score for categorical shift in body composition parameters; BMI percentile for upward shift in weight category), as well as sex, baseline GAF score and mood stabilizer and anticholinergic cotreatment
At-risk weight category: ≥85th-<95th BMI percentile
Overweight category: ≥95th BMI percentile
Percentages are based on the number of patients with data available: 41 in the aripiprazole group, 45 in the olanzapine group, 34 in the quetiapine group, 134 in the risperidone group and 14 in the control group
one premorbidly obese adolescent developed diabetes mellitus while receiving quetiapine.
Percentages are based on the number of patients with data available: 41 in the aripiprazole group, 45 in the olanzapine group, 34 in the quetiapine group, 133 in the risperidone group and 14 in the control group
Percentages are based on the number of patients with data available: 40 in the aripiprazole group, 40 in the olanzapine group, 31 in the quetiapine
Secondary Outcomes
a) Body Composition
Each antipsychotic was associated with significantly increased fat mass (9.2lbs with olanzapine and 5.4–6.3lbs with aripiprazole, risperidone and quetiapine) and waist circumference (8.6cm with olanzapine and 5.1–5.4cm with risperidone, quetiapine and aripiprazole), compared to non-significant changes of 0.8lbs and 0.70cm in comparison subjects. Using ≥14% and ≥21% of unadjusted body weight gain and a >0.5 and >1.0 standard deviation BMI z-score increase as the pathological threshold, the same ranking order emerged (Table 4, online). Shifts to overweight (≥85th-<95th BMI percentile) or obese (≥95th BMI percentile) status (that are influenced by baseline body weight) occurred in 36.1% of patients (n=13) on quetiapine, 22.2% (n=10) on olanzapine, 14.1% (n=19) on risperidone, 9.8% (n=4) on aripiprazole, and 6.6% of controls (n=1). Comparing patients treated with or without comedications known to affect body weight did not alter the findings and did not result in significant differences within each antipsychotic at any time (p=0.20–0.94) (results not shown).
b) Glucose and Lipid Metabolism
Adverse baseline-to-endpoint changes reached statistical significance for olanzapine in all glucose and lipid parameters, except for HDL-cholesterol (table 3). With quetiapine, total cholesterol, non-HDL cholesterol, triglycerides and the triglyceride/HDL-cholesterol ratio increased significantly. With risperidone, triglycerides increased significantly. With aripiprazole and in comparison subjects, no significant changes in metabolic values occurred.
Table 3.
Change in Metabolic Parameters over Time
| Outcome Variable | 0–4 Weeks | 0–8 Weeks | 0–12 Weeks | P-Value Wk 0–4 |
P-Value Wk 0–8 |
P-Value Wk 0–12 |
Effect Size |
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | |||||||
| Aripiprazole | −0.92 (−4.13, 2.29) | 2.62 (−0.69, 5.93) | 0.54 (−2.85, 3.93) | 0.58 | 0.13 | 0.76 | 0.05 |
| Olanzapine | 1.96 (−0.41, 4.33) | 3.06 (0.43, 5.69) | 3.14 (0.69, 5.59) | 0.11 | 0.03 | 0.02 | 0.37 |
| Quetiapine | 2.52 (−0.50, 5.54) | 1.80 (−1.65, 5.25) | 2.64 (−0.65, 5.93) | 0.11 | 0.31 | 0.12 | 0.26 |
| Risperidone | 1.32 (−0.50, 3.14) | 1.29 (−0.81, 3.39) | 1.14 (−0.84, 3.12) | 0.16 | 0.23 | 0.26 | 0.10 |
| Untreated | 0.86 (−5.22, 6.94) | −4.42 (−11.20, 2.36) | 0.69 (−4.84, 6.22) | 0.79 | 0.22 | 0.81 | 0.06 |
| Insulin (mmol/L) | |||||||
| Aripiprazole | 2.05 (−2.52, 6.62) | 1.17 (−3.42, 5.76) | 2.61(−2.09, 7.31) | 0.38 | 0.62 | 0.28 | 0.17 |
| Olanzapine | 0.80 (−1.38, 2.98) | 0.36 (−2.07, 2.79) | 2.71 (0.42, 5.00) | 0.47 | 0.77 | 0.02 | 0.35 |
| Quetiapine | 1.10 (−2.57, 4.77) | 0.83 (−3.17, 4.83) | 1.08 (−2.80, 4.96) | 0.56 | 0.69 | 0.59 | 0.09 |
| Risperidone | 1.75 (0.30, 3.20) | 0.25 (−1.36, 1.86) | 0.69 (−0.86, 2.24) | 0.02 | 0.76 | 0.39 | 0.08 |
| Untreated | −3.17 (−7.36, 1.02) | 1.50 (−2.97, 5.97) | −0.47 (−4.31, 3.37) | 0.16 | 0.52 | 0.81 | −0.06 |
| HOMA-IR | |||||||
| Aripiprazole | 0.36 (−0.66, 1.38) | 0.28 (−0.74, 1.30) | 0.55 (−0.49, 1.59) | 0.50 | 0.59 | 0.31 | 0.16 |
| Olanzapine | 0.07 (−0.46, 0.60) | 0.11 (−0.48, 0.70) | 0.62 (0.07, 1.17) | 0.79 | 0.71 | 0.03 | 0.33 |
| Quetiapine | 0.36 (−0.52, 1.24) | 0.35 (−0.59, 1.29) | 0.35 (−0.57, 1.27) | 0.44 | 0.48 | 0.46 | 0.12 |
| Risperidone | 0.43 (0.10, 0.76) | 0.08 (−0.29, 0.45) | 0.20 (−0.15, 0.55) | 0.01 | 0.67 | 0.28 | 0.10 |
| Untreated | −0.71 (−1.55, 0.13) | −0.82 (−1.76, 0.12) | −0.09 (−0.85, 0.67) | 0.12 | 0.11 | 0.82 | −0.06 |
| TG/HDL Ratio (mg/dL) | |||||||
| Aripiprazole | −0.30 (−0.75, 0.15) | −0.32 (−0.77, 0.13) | −0.19 (−0.66, 00.28) | 0.18 | 0.16 | 0.42 | −0.12 |
| Olanzapine | 0.35 (0.02, 0.68) | 0.48 (0.09, 0.87) | 0.59 (0.24, 0.94) | 0.04 | 0.02 | 0.002 | 0.49 |
| Quetiapine | 0.06 (−0.65, 0.77) | 0.46 (−0.38, 1.30) | 1.22 (0.44, 2.00) | 0.87 | 0.29 | 0.004 | 0.51 |
| Risperidone | 0.05 (−0.15, 0.25) | 0.19 (−0.03, 0.41) | 0.20 (0.00, 0.40) | 0.63 | 0.08 | 0.05 | 0.17 |
| Untreated | −0.23 (−0.92, 0.46) | 0.009 (−0.76, 0.77) | −0.31 (−0.94, 0.32) | 0.51 | 0.98 | 0.35 | −0.25 |
| Total cholesterol (mg/dL) | |||||||
| Aripiprazole | 9.54 (2.21, 16.87) | 3.90 (−3.51, 11.31) | 3.75 (−3.85, 11.35) | 0.013 | 0.31 | 0.34 | 0.15 |
| Olanzapine | 18.72 (10.43, 27.01) | 11.33 (2.20, 20.46) | 15.58 (6.88, 24.28) | <0.001 | 0.02 | <0.001 | 0.52 |
| Quetiapine | 8.52 (0.66, 16.38) | 14.80 (5.84, 23.76) | 9.05 (0.41, 17.69) | 0.04 | 0.002 | <0.05 | 0.34 |
| Risperidone | 6.25 (1.70, 10.80) | 1.96 (−3.08, 7.00) | 3.46 (−1.44, 8.36) | 0.008 | 0.45 | 0.17 | 0.12 |
| Untreated | 6.17 (−4.77, 17.11) | 0.36 (−12.46, 13.18) | 2.38 (−7.69, 12.45) | 0.50 | 0.62 | 0.82 | 0.12 |
| LDL-cholesterol (mg/dL) | |||||||
| Aripiprazole | 9.07 (2.29, 15.85) | 2.80 (−4.16, 9.76) | 7.38 (0.77, 13.99) | 0.01 | 0.43 | 0.05 | 0.34 |
| Olanzapine | 9.00 (1.79, 16.21) | 6.97 (−1.11, 15.05) | 11.54 (3.97, 19.11) | 0.02 | 0.10 | 0.004 | 0.45 |
| Quetiapine | 4.66 (−1.83, 11.15) | 11.98 (4.12, 19.84) | 3.88 (−3.37, 11.13) | 0.17 | 0.005 | 0.30 | 0.17 |
| Risperidone | 1.70 (−2.30, 5.70) | −0.40 (−4.91, 4.11) | 0.21 (−4.14, 4.56) | 0.41 | 0.86 | 0.92 | 0.01 |
| Untreated | 6.90 (−2.00, 15.80) | 1.41 (−9.02, 11.84) | 2.99 (−5.18, 11.16) | 0.15 | 0.79 | 0.49 | 0.19 |
| HDL-cholesterol (mg/dL) | |||||||
| Aripiprazole | 2.84 (0.35, 5.33) | 0.87 (−1.66, 3.40) | 0.29 (−2.32, 2.90) | 0.03 | 0.50 | 0.83 | 0.03 |
| Olanzapine | 2.30 (−0.07, 4.67) | −1.10 (−3.86, 1.66) | −1.27(−3.80, 1.26) | 0.06 | 0.44 | 0.33 | −0.15 |
| Quetiapine | 3.36 (0.11, 6.61) | 0.78 (−2.98, 4.54) | −1.47 (−5.06, 2.12) | <0.05 | 0.69 | 0.43 | −0.13 |
| Risperidone | 1.58 (0.11, 3.05) | −0.54 (−2.19, 1.11) | 0.33 (−1.26, 1.92) | 0.04 | 0.52 | 0.68 | 0.04 |
| Untreated | 2.21 (−2.79, 7.21) | −1.52 (−6.85, 3.81) | 1.49 (−3.10, 6.08) | 0.40 | 0.65 | 0.53 | 0.16 |
| Triglycerides (mg/dL) | |||||||
| Aripiprazole | −6.57 (−23.15, 10.01) | −8.70 (−25.56, 8.16) | −2.40 (−19.71, 14.91) | 0.44 | 0.32 | 0.79 | −0.04 |
| Olanzapine | 20.79 (7.13, 34.45) | 21.92 (5.63, 38.21) | 24.34 (9.8, 38.88) | 0.004 | 0.01 | 0.002 | 0.49 |
| Quetiapine | 2.84 (−21.84, 27.52) | 14.87 (−12.88, 42.62) | 36.96 (10.13, 63.79) | 0.82 | 0.30 | 0.01 | 0.45 |
| Risperidone | 6.32 (−2.34, 14.98) | 8.86 (−0.69, 18.41) | 9.74 (0.45, 19.03) | 0.15 | 0.07 | 0.04 | 0.18 |
| Untreated | −13.44 (−45.72, 18.84) | −4.50 (−38.70, 29.70) | −11.84 (−41.55, 17.87) | 0.43 | 0.80 | 0.45 | −0.20 |
| Non-HDL-cholesterol (mg/dL) | |||||||
| Aripiprazole | 6.68 (0.47, 12.89) | 3.02 (−3.27, 9.31) | 4.41 (−2.10, 10.92) | 0.04 | 0.35 | 0.19 | 0.21 |
| Olanzapine | 16.42 (9.25, 23.59) | 12.59 (4.73, 20.45) | 16.81 (9.30, 24.32) | <0.001 | 0.003 | <0.001 | 0.65 |
| Quetiapine | 5.05 (−2.83, 12.93) | 13.78 (5.12, 22.44) | 9.93 (1.42, 18.44) | 0.22 | 0.003 | 0.03 | 0.38 |
| Risperidone | 4.69 (0.55, 8.83) | 2.27 (−2.38, 6.92) | 3.02 (−1.45, 7.49) | 0.03 | 0.34 | 0.19 | 0.11 |
| Untreated | 4.44 (−5.56, 14.44) | 0.64 (−10.88, 12.16) | 0.52 (−8.65, 9.69) | 0.40 | 0.91 | 0.91 | 0.03 |
All change scores are reports as means ± 95% confidence intervals.
Bolded p values: <0.05
Twenty-three patients (8.6%) developed insulin resistance: olanzapine: 17.8% (n=8), risperidone: 8.3% (n=11), aripiprazole: 7.3% (n=3), quetiapine: 2.9% (n=1), and 0% (n=0) in comparison subjects (p=0.021) (Table 4). Forty-six patients on antipsychotics (17.1%), and one control subject (6.7%) developed new-onset dyslipidemia: olanzapine: 28.9% (n=13), risperidone: 19.4% (n=26), quetiapine: 8.8% (n=3), and aripiprazole: 7.3% (n=3) (p=0.042) (Table 4). Four patients (1.6%) developed metabolic syndrome, two on quetiapine and one each on olanzapine or aripiprazole (p=0.44).
Besides hyperglycemia and metabolic syndrome where quetiapine had modestly higher incidence rates, olanzapine was associated with the highest incidence rates (Table 4, online).
Pubertal Status and Antipsychotic Dose as Potential Mediators
Pubertal status was not associated with body composition changes with aripiprazole or quetiapine. With olanzapine, change in weight (p<0.01) and waist circumference (p<0.05) was greater in postpubertal subjects, but sex- and age-adjusted BMI z-score change was unrelated to pubertal status (p=0.28). Similarly, with risperidone, only unadjusted change in weight (p<0.001) and fat mass (p<0.05) was greater in post-pubertal subjects, whereas BMI z-score change did not differ (p=0.29). Pubertal status was unrelated to metabolic changes in any antipsychotic group.
Antipsychotic dose was not associated with body composition parameters changes in patients receiving aripiprazole, olanzapine or quetiapine. With risperidone, doses >1.5 mg/day were associated with greater increases in weight (p<0.0001), waist (p=0.001), fat mass (p<0.05), and BMI z-score (p<0.05). Metabolic effects of aripiprazole or quetiapine did not differ between dose groups. Conversely, significantly greater increases in several metabolic parameters were observed in patients treated with doses >10 mg/day of olanzapine (total cholesterol (p<0.01), LDL-cholesterol (p<0.01), non-HDL-cholesterol (p<0.01), glucose (p<0.05)) and doses >1.5 mg/day of risperidone (total cholesterol (p<0.01), non-HDL-cholesterol (p<0.01), triglycerides (p<0.01),triglyceride/HDL ratio (p<0.05)).
Discussion
In this short-term study of antipsychotic-naïve youth, aripiprazole, olanzapine, quetiapine and risperidone were each associated with rapid and significant increases in body composition, whereas metabolic changes were less uniform. Effect sizes for body composition changes were large. Altogether, 10%–36% of youngsters transitioned to overweight/obese status within 11 weeks. The lack of significant changes in weight and metabolic parameters in psychiatric comparison subjects and short inpatient stays (10–18 days=14%–25% of treatment time) indicates that the observed alterations are unlikely due to a newly developing/worsening psychiatric disorder or hospitalization. The results are concerning as they include fat mass and waist circumference, which are associated with metabolic syndrome in antipsychotic-treated adults (17) and heart disease in the general population (18). Moreover, abnormal childhood weight and metabolic status adversely affect adult cardiovascular outcomes (3–6), via continuation of these risk factors (19) or independent/accelerated mechanisms (20).
It has been argued that youth are more vulnerable to antipsychotic-induced weight gain than adults. A comparison of our findings with prior studies does not support this. Rather, it appears that the greater antipsychotic weight gain in youth is related to the less prior antipsychotic exposure compared to most adult samples. As in previous pediatric studies (7–9), the weight gain in our study was greater than in adults with chronic schizophrenia (21). It was also greater than in adults with first-episode schizophrenia (24% antipsychotic-naïve) (22), where weight gain ≥7% was similar only after one year of treatment. Our observed weight gain was also considerably greater compared to recent, short-term, placebo-controlled, registration trials in pediatric schizophrenia and bipolar disorder (10) (absolute and ≥7% weight gain with aripiprazole: 0.0–0.9kg, 4.0–12.3%; quetiapine: 1.7kg, 9.9–14.5%; risperidone: 1.4–1.9kg, 15–16%; olanzapine: 3.7–4.7kg, 41.9–45.8%), and greater than in pediatric studies comparing olanzapine and risperidone with only 36% (8) and 33% (9) antipsychotic-naïve youth. BMI z-score gains that adjust for baseline sample differences were more than double compared to the 8-week Treatment of Early-Onset Schizophrenia Spectrum Disorder (TEOSS) study (9) (olanzapine: 0.93 vs. 0.39, risperidone: 0.60 vs. 0.23).
By contrast, our absolute and relative weight findings (being especially important for a comparison with adults who generally have higher baseline weights) are very similar to a 3-month adolescent quetiapine study (77% antipsychotic-naïve) (23), and a 3-month (24) and 4-month (25) first-episode adult schizophrenia study , in which 100% were antipsychotic-naïve (24) or 91% had ≤7 days of antipsychotic exposure (23). This weight gain similarity despite a >10-years higher age suggests that prior treatment may be more relevant than age and developmental differences.
Despite significant body composition changes with each antipsychotic, metabolic risk profiles varied, lipid abnormalities predominated over glucose abnormalities after short-term exposure, and metabolic syndrome and diabetes developed rarely. Olanzapine had the largest weight effects and also significantly worsened all glucose and lipid parameters, except HDL-cholesterol, which is more related to physical activity (26). Quetiapine and risperidone significantly increased triglycerides, but did not produce significant abnormalities in glucose homeostasis. Despite similar body composition changes compared to risperidone, quetiapine was additionally associated with significantly increased total cholesterol, non-HDL cholesterol, and triglyceride/HDL ratio, indicating broader metabolic effects, as suggested recently in youth (27) and adults (28,29). The TEOSS trial (9) reported significantly increased total and LDL-cholesterol only with olanzapine and no triglyceride signal with olanzapine and risperidone, a difference possibly due to carry-over effects from prior treatment or fasting sample size limitations. Similar reasons may account for the lack of a metabolic signal in large-scale, pediatric SGA registration trials, except for olanzapine (10).
Despite significant worsening in all body composition parameters, aripiprazole was not associated with significantly worsened metabolic indices (except for an isolated, near significant LDL-cholesterol increase). Reasons for this apparent dissociation are unclear, but could be related to a >50% lower effect size for increased waist circumference compared to quetiapine and risperidone, despite similar effect sizes for all other body composition parameters. However, due to the relatively small aripiprazole sample, we cannot exclude a type-II error for lipid parameters (effect sizes: 0.15–0.35), which is unlikely for triglycerides and triglyceride/HDL ratio that decreased and HDL-cholesterol that increased. The same caveat applies to the non-significant glucose homeostasis changes with aripiprazole, quetiapine and risperidone (effect sizes: 0.05–0.26). However, our findings of less lipid abnormalities with aripiprazole are supported by early, short-term metabolomic studies (30). Nevertheless, in view of a significant association between a stable BMI and metabolic health in young adults from the general population followed for 15 years (31) and of significant weight gain with all studied antipsychotics in our study, longer-term assessments are needed to clarify the trajectory of metabolic changes with specific antipsychotics. Such studies should evaluate the importance of weight change versus endpoint BMI for metabolic abnormalities, since emerging data suggest a potentially greater importance of the latter (32).
More research is also needed to determine the time course and magnitude of developing diabetes or metabolic syndrome and to uncover the mechanisms underlying the apparent delay in acquiring metabolic syndrome and insulin resistance with rapid weight gain during childhood. This phenomenon, also suggested in the general pediatric population (6), seems to exclude olanzapine. Reasons for this could be the magnitude of body composition changes or weight-independent effects (33). Of note, triglycerides and triglyceride/HDL-cholesterol ratio, suggested markers in adults (34), seem to be more sensitive than glucose and insulin for the early identification of worsening insulin resistance. Triglyceride changes reflect early insulin resistance at the muscle cell level, while changes at the hepatic level seem to occur later, giving rise to delayed glucose, insulin and HOMA-IR signals (35).
Unsurprisingly, some absolute body composition changes were greater in postpubertal subjects who also were heavier at baseline. However, the lack of a moderating effect of pubertal status on age- and sex-adjusted BMI z-scores and any metabolic parameter indicates that the same caution is required when treating younger children or adolescents. Our data support recent findings that higher doses of olanzapine (>10 mg/day) are associated with greater metabolic abnormalities (12). While data for risperidone were inconclusive (12), our data suggest a dose relationship at doses >1.5 mg/day of risperidone. The fact that body composition changes were dose related only with risperidone supports weight independent metabolic effects with olanzapine (33). However, fixed dose, randomized studies and blood level assessments are needed to further examine antipsychotic dose relationships.
The results from this study need to be interpreted within its limitations, which include the non-randomized, observational design, baseline differences precluding rigorous group comparisons, flexible dosing, allowance of comedications, relatively short treatment duration and a small comparison group. Moreover, we did not include a first-generation antipsychotic comparator. In the TEOSS study (9), molindone was found to be weight-neutral, but many patients lost weight, suggesting prior treatment effects. Despite these caveats, this is the largest study focusing on changes in weight and metabolic parameters in antipsychotic-naïve, pediatric patients, using strictly reinforced fasting assessments and verifying medication adherence via interview and antipsychotic levels. This design enabled us to enroll a fairly large group of antipsychotic-naïve patients treated under “real-life” conditions, emitting a larger signal for body composition and, especially, metabolic abnormalities compared to prior studies.
Our results, together with data from first-episode studies, suggest that for vulnerable pediatric, antipsychotic-naïve and early-phase patients with minimal antipsychotic exposure, guidelines should consider more frequent (e.g., bi-annual, 36) cardiometabolic monitoring than annual assessments beyond the first three months of treatment as currently recommended(2). Finally, in view of poor physical health outcomes (37) and suboptimal metabolic monitoring (38) in the severely mentally ill, the benefits of SGAs must be balanced against their cardiometabolic risks through a careful assessment of the indications for their use, consideration of lower-risk alternatives, and proactive side effect monitoring and management (39).
Acknowledgments
Grant Support: Supported in parts by NIH grant MH01760 (Dr. Malhotra), a NARSAD Independent Investigator Award (Dr. Malhotra), The Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH 074543-01 (Dr. Kane), and by The Feinstein Institute for Medical Research NSLIJHS General Clinical Research Center, Grant #M01 RR018535 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The article’s content is solely the responsibility of the authors and does not necessarily represent the official view of NCRR, NIH or NIMH. None of these non-commercial funding organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The authors would like to thank Martin Lesser, Ph.D. and Meredith Ackerman B.S. for their statistical support (which was provided as part of their institutional affiliation, without additional financial compensation); the medical and nursing staff of the child and adolescent psychiatry programs at Schneider Children’s Hospital and Zucker Hillside Hospital for their help with identifying eligible patients; as well as the all patients and their families for their study participation and donation of their time during difficult periods in their lives.
Footnotes
Disclosures: Dr. Correll has been a consultant to or has received honoraria from AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Intra-Cellular Therapeutics, Medicure, OrthoMcNeill-Janssen, Otsuka, Organon, Pfizer, Schering-Plough, Solvay, Supernus, Vanda and Wyeth, and has served on the speaker’s bureau of AstraZeneca, Bristol-Myers Squibb/Otsuka and Pfizer.
Dr. Manu has been a consultant to or has received honoraria from Bristol-Meyers Squibb and Pfizer, and has served on the speaker’s bureau of Bristol-Myers Squibb/Otsuka and Pfizer.
Dr. Kane has been a consultant to or has received honoraria from Abbott, Astra-Zeneca, Bristol-Myers Squibb, Cephalon, Dainippon Sumitomo, Eli Lilly, Intra-Cellular Therapeutics, Janssen Pharmaceutica, Johnson and Johnson, Lundbeck, NuPathe, Otsuka, Pfizer Inc, PgXHealth, Proteus, Schering, Shire, Solvay, Vanda and Wyeth, he has served on the speaker’s bureau of AstraZeneca, Bristol-Myers Squibb/Otsuka and Eli Lilly, and he is a share holder of MedAvante.
Dr. Malhotra has been a consultant to has received honoraria from to Bristol-Myers Squibb, Otsuka, Pfizer and Vanda, and has served on the speaker’s bureau of Bristol-Myers Squibb/Otsuka and Pfizer.
Dr. Olshanskyi and Ms. Napolitano have nothing to disclose.
Trial Registration: N/A
References
- 1.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004 Feb;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002 Jan;51(1):204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 4.Sinaiko AR, Donahue RP, Jacobs DR, Jr, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999 Mar 23;99(11):1471–1476. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004 Feb 26;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007 Dec 6;357(23):2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safer DJ. A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol. 2004 Aug;24(4):429–436. doi: 10.1097/01.jcp.0000130558.86125.5b. [DOI] [PubMed] [Google Scholar]
- 8.Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004 Jan;29(1):133–145. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- 9.Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, Ambler D, Puglia M, Maloney AE, Michael E, De Jong S, Slifka K, Noyes N, Hlastala S, Pierson L, McNamara NK, Delporto-Bedoya D, Anderson R, Hamer RM, Lieberman JA. Double-Blind Comparison of First- and Second-Generation Antipsychotics in Early-Onset Schizophrenia and Schizo-affective Disorder: Findings From the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Am J Psychiatry. 2008 Nov;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. Epub 2008 Sep 15. Erratum in: Am J Psychiatry. 2008 Nov;165(11):1495. [DOI] [PubMed] [Google Scholar]
- 10.Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69 suppl 4:26–36. [PubMed] [Google Scholar]
- 11.Verma S, Liew A, Subramaniam M, Poon LY. Effect of treatment on weight gain and metabolic abnormalities in patients with first-episode psychosis. Aust N Z J Psychiatry. 2009 Sep;43(9):812–817. doi: 10.1080/00048670903107609. PubMed PMID: 19670054. [DOI] [PubMed] [Google Scholar]
- 12.Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009 Jul;70(7):1041–1050. doi: 10.4088/jcp.08r04392. [DOI] [PubMed] [Google Scholar]
- 13.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1999 Mar 17;281(11):1006–1013. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among US adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 17.Straker D, Correll CU, Kramer-Ginsberg E, Abdulhamid N, Koshy F, Rubens E, Saint-Vil R, Kane JM, Manu P. Cost-effective screening for the metabolic syndrome in patients treated with second-generation antipsychotic medications. Am J Psychiatry. 2005 Jun;162(6):1217–1221. doi: 10.1176/appi.ajp.162.6.1217. [DOI] [PubMed] [Google Scholar]
- 18.De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, Sacchetti L, Zarrilli F, Bond MG, Rubba P. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002 Dec;33(12):2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 19.Juonala M, Raitakari M, S A Viikari J, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006 Apr;185(2):388–393. doi: 10.1016/j.atherosclerosis.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003 Nov 5;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 21.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and Tolerability of Olanzapine, Quetiapine, and Risperidone in the Treatment of Early Psychosis: A Randomized, Double-Blind 52-Week Comparison. Am J Psychiatry. 2007;164:1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- 23.Schimmelmann BG, Mehler-Wex C, Lambert M, et al. A Prospective 12-Week Study of Quetiapine in Adolescents with Schizophrenia Spectrum Disorders. J Child Adol Psychopharmacol. 2007;17(6):768–778. doi: 10.1089/cap.2007.0048. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Iglesias R, Crespo-Facorro B, Amado JA, Garcia-Unzueta MT, Ramirez-Bonilla ML, Gonzalez-Blanch C, Martinez-Garcia O, Vazquez-Barquero JL. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry. 2007 Nov;68(11):1733–1740. doi: 10.4088/jcp.v68n1113. [DOI] [PubMed] [Google Scholar]
- 25.Robinson DG, Woerner M, Napolitano B, Paterl RC, Sevy SM, Gunduz-Bruce H, Soto-Perello JM, Mendelowitz AM, Khadivi A, Miller R, McCormack J, Lorell BS, Lesser ML, Schooler NR, Kane JM. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163:2096–2102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- 26.Kelley GA, Kelley KS. Aerobic exercise and HDL2-C: a meta-analysis of randomized controlled trials. Atherosclerosis. 2006 Jan;184(1):207–215. doi: 10.1016/j.atherosclerosis.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraguas D, Merchán-Naranjo J, Laita P, et al. Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. J Clin Psychiatry. 2008 Jul;69(7):1166–1175. doi: 10.4088/jcp.v69n0717. [DOI] [PubMed] [Google Scholar]
- 28.Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008 Aug;103(1–3):104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008 Oct;105(1–3):175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007 Oct;12(10):934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, Lewis CE, Savage P. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007 Feb 27;115(8):1004–1011. doi: 10.1161/CIRCULATIONAHA.106.648642. [DOI] [PubMed] [Google Scholar]
- 32.Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009 Apr;19(2):101–109. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, Weber MT, Anderson EJ, Allison DB, Daley TB, Schoenfeld D, Goff DC. Glucose metabolism in patients with schizophrenia treated with second-generation antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005 Jan;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabetes. 2008 Jun;9(3 Pt 2):57–61. doi: 10.1111/j.1399-5448.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 36.Correll CU. Monitoring and Management of Antipsychotic-related Metabolic and Endocrine Adverse Effects in Children and Adolescents. International Review of Psychiatry. 2008;20(4):195–201. doi: 10.1080/09540260801889179. [DOI] [PubMed] [Google Scholar]
- 37.Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, Hennekens CH, Lambert M, Leucht S, Maj M, McIntyre RS, Naber D, Newcomer JW, Olfson M, Osby U, Sartorius N, Lieberman JA. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008 Apr;69(4):514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- 38.Morrato EH, Newcomer JW, Allen RR, Valuck RJ. Prevalence of baseline serum glucose and lipid testing in users of second-generation antipsychotic drugs: a retrospective, population-based study of Medicaid claims data. J Clin Psychiatry. 2008 Feb;69(2):316–322. doi: 10.4088/jcp.v69n0219. [DOI] [PubMed] [Google Scholar]
- 39.Kryzhanovskaya LA, Robertson-Plouch CK, Xu W, Carlson JL, Merida KM, Dittmann RW. The safety of olanzapine in adolescents with schizophrenia or bipolar I disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009 Feb;70(2):247–258. doi: 10.4088/jcp.08m03538. [DOI] [PubMed] [Google Scholar]