Abstract
The human genetic disorder ataxia telangiectasia (A–T), caused by mutation in the ATM gene, is characterized by chromosomal instability, radiosensitivity and defective cell cycle checkpoint activation. DNA double-strand breaks (dsbs) persist in A–T cells after irradiation, but the underlying defect is unclear. To investigate ATM's interactions with dsb repair pathways, we disrupted ATM along with other genes involved in the principal, complementary dsb repair pathways of homologous recombination (HR) or non-homologous end-joining (NHEJ) in chicken DT40 cells. ATM–/– cells show altered kinetics of radiation-induced Rad51 and Rad54 focus formation. Ku70–deficient (NHEJ–) ATM–/– chicken DT40 cells show radiosensitivity and high radiation-induced chromosomal aberration frequencies, while Rad54–defective (HR–) ATM–/– cells show only slightly elevated aberration levels after irradiation, placing ATM and HR on the same pathway. These results reveal that ATM defects impair HR–mediated dsb repair and may link cell cycle checkpoints to HR activation.
Keywords: ataxia telangiectasia/double strand breaks/non-homologous end-joining/nuclear foci/recombination
Introduction
Genetic stability requires that any damage sustained by the genome be repaired before the cell divides. Checkpoint signals resulting from the detection of DNA damage arrest the cell cycle to allow time for the cell's repair systems to act, with apoptosis as an alternative outcome in metazoans (Elledge, 1996; Paulovich et al., 1997). Double strand breaks (dsbs) in DNA are an especially hazardous lesion caused by ionizing radiation and are repaired by two major repair pathways: non-homologous end-joining (NHEJ) and homologous recombination (HR) (Weaver, 1995). NHEJ, involving the Ku proteins and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), acts predominantly in the G1–early S phases of the cell cycle, while HR works in late S–G2 (Takata et al., 1998). Proteins encoded by the metazoan homologues of the Saccharomyces cerevisiae RAD52 epistasis group genes are intimately involved in HR. Among them, the loss of Rad54 leads to recombinational deficiencies and dsb repair defects (Bezzubova et al., 1997; Essers et al., 1997), while Rad51's absence causes the accumulation of chromosomal abnormalities and cell death (Sonoda et al., 1998). Both proteins mediate sister chromatid exchange (SCE), which reflects the post-replicational repair of spontaneous DNA damage by recombination with the intact sister chromatid (Sonoda et al., 1999).
Genetic instability and abnormalities of the nervous, immune and reproductive systems are among the complex clinical features of the autosomal recessive disorder ataxia telangiectasia (A–T; recently reviewed in Lavin and Shiloh, 1997; Meyn, 1997), which also include a predisposition to lymphoid malignancy and extreme radiosensitivity. Cells derived from A–T patients show high levels of chromosomal aberrations, greatly potentiated by irradiation, and hypersensitivity to ionizing radiation (Taylor et al., 1975; Thacker, 1994; Meyn, 1995). In A–T cells, ionizing radiation damage does not induce an arrest in DNA synthesis (causing the phenomenon of radioresistant DNA synthesis) or an appropriate arrest at the G1–S or G2–M cell cycle checkpoints (Painter and Young, 1980; Beamish and Lavin, 1994; Beamish et al., 1996; Xie et al., 1998; reviewed in Westphal, 1997; Jeggo et al., 1998), suggesting that anomalous cell cycle regulation is a major underlying cause of the disease. After mapping (Gatti et al., 1988) and cloning (Savitsky et al., 1995a) of the gene responsible (designated ATM, for A–T mutated) the ATM locus has been shown to comprise 66 exons over ∼150 kb of genomic DNA, encoding a widely expressed 13 kb mRNA transcript (Savitsky et al., 1995b; Uziel et al., 1996). The open reading frame of this transcript codes for a 350 kDa protein, which is absent or inactive in A–T. ATM is a member of a family of large proteins characterized by a C–terminal phosphatidylinositol (PI)–3 kinase-like domain (Jackson, 1995; Zakian, 1995). Recent work has shown that ATM acts on a number of cell cycle-regulating proteins following ionizing radiation, notably Gadd45 (Kastan et al., 1992), p53 (Banin et al., 1998; Canman et al., 1998; Khanna et al., 1998), replication protein A (Liu and Weaver, 1993), Chk2 (Matsuoka et al., 1998) and c–Abl (Baskaran et al., 1997; Shafman et al., 1997), consistent with it being a major regulator of the cell cycle reaction to genome damage (recently reviewed in Brown et al., 1999). Nevertheless, the cause of the radiosensitivity in A–T remains controversial, being attributed to checkpoint defects, abnormal apoptosis and repair abnormalities (Painter and Young, 1980; Cornforth and Bedford, 1985; Thacker, 1994; Meyn, 1995, 1997; Jeggo et al., 1998). However, despite the sequence homologies between ATM and the NHEJ component DNA–PKcs (Jackson, 1995; Zakian, 1995; Smith and Jackson, 1999), and the accumulation of much recent evidence describing biochemical links between HR proteins and ATM (Baskaran et al., 1997; Shafman et al., 1997; Yuan et al., 1998; Chen et al., 1999), the involvement of ATM in DNA damage recognition and repair remains unclear.
Intrachromosomal recombination is significantly elevated in A–T cells, with frequent sequence alterations accompanying this recombination (Meyn, 1993; Luo et al., 1996). Extrachromosomal recombination levels in A–T cells have been reported to be elevated (Luo et al., 1996), but such enhancement has not been observed consistently (Debenham et al., 1987; Thacker, 1989; Meyn, 1993; Powell et al., 1993; Morrison and Wagner, 1996). However, mis-repair of the recombining DNA appears to be a feature of recombination in A–T (Shiloh, 1997). Taken together, these observations suggest generally aberrant recombinational repair in A–T. To test the hypothesis that ATM deficiency has an impact on recombination, we investigated recombinational repair in ATM-deficient mutants of the hyper-recombinogenic chicken DT40 cell line. The phenotype of these ATM–/– mutants recapitulates that of the equivalent mammalian mutants (Barlow et al., 1996; Elson et al., 1996; Xu and Baltimore, 1996), namely radiosensitivity and increased chromosomal aberrations; in addition, these cells show reduced gene targeting efficiencies, suggesting recombination defects (Takao et al., 1999). However, they lack p53, so that the principal defects in ATM–/– DT40 cells arise from p53–independent ATM-mediated processes.
We based the work in this paper on the conjecture that ATM has a role in controlling recombinational repair. This has been hinted at by the various links found recently between HR and ATM (Baskaran et al., 1997; Shafman et al., 1997; Yuan et al., 1998; Chen et al., 1999), and by the recombinational abnormalities associated with A–T (Meyn, 1993; Luo et al., 1996). This paper describes experiments using reverse genetics to ablate NHEJ (by disruption of KU70) or HR (by disruption of RAD54) on an ATM–/– background, to see whether the loss of either pathway exacerbates the ATM–/– phenotype. Comparison with the parental ATM–/–, HR– (RAD54–/–) (Bezzubova et al., 1997) and NHEJ– (KU70–/–) (Takata et al., 1998) single mutants revealed a synergistic accumulation of spontaneous chromosomal aberrations and radiosensitivity in ATM–/–KU70–/– cells, with a far less severe phenotype arising from the conditional loss of RAD54 in ATM–/– cells. These findings demonstrate that ATM acts in concert with NHEJ to preserve the genome, through HR pathways. We suggest that this may explain the elevated radiosensitivity and deficiencies in the repair of spontaneous DNA damage in the absence of functional ATM and, given the importance of accurate recombination in maintaining the genome, clearly illustrates another tumour-suppressing role of ATM.
Results
Defective recombinational activity in ATM–/– DT40 cells as assessed by Rad51 and Rad54 focus formation
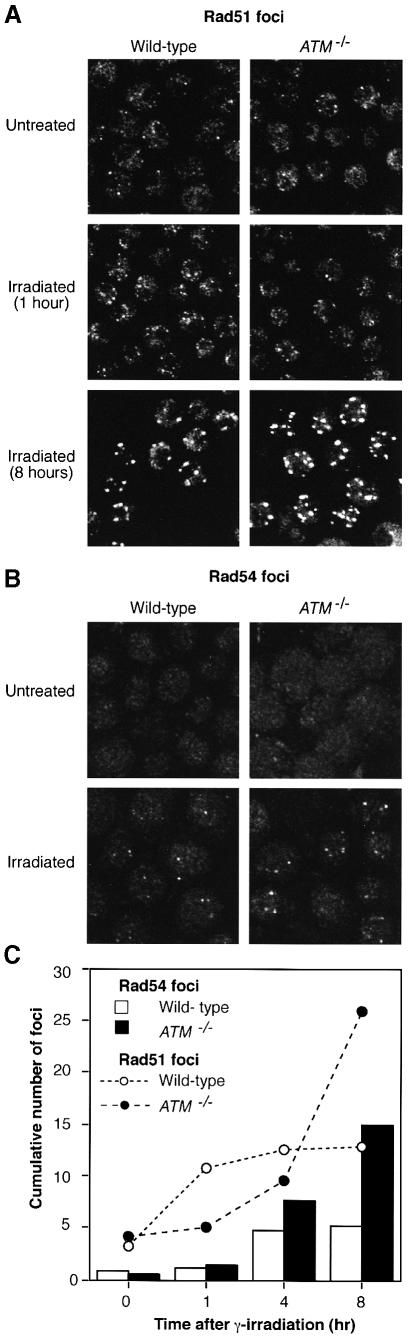
In order to examine recombinational repair before any checkpoint deficiencies might manifest themselves, we monitored the short-term ability of ATM-deficient cells to respond to DNA damage. The assay we used was the radiation-induced formation of nuclear foci of Rad51 and Rad54, which associate closely in the eukaryotic response to DNA damage (Clever et al., 1997; Golub et al., 1997; Tan et al., 1999). Figure 1A shows the formation of distinct nuclear foci of Rad51 in response to ionizing radiation in both wild-type and ATM–/– DT40 cells. Figure 1B shows the formation of Rad54 foci after similar treatment. As shown in Figure 1C, the formation of Rad54 foci in ATM–/– cells continued to increase after such formation had plateaued in wild-type cells; so did the formation of Rad51 foci (Figure 1C), as others have noted previously (Maser et al., 1997). It is perhaps significant, given the interactions described between Rad51 and Rad54 (Clever et al., 1997; Petukhova et al., 1998; Tan et al., 1999), that the initial kinetics of Rad54 focus formation were very similar between wild-type and ATM-null DT40 cells, while that of Rad51 was markedly different, there being a much slower activation phase in cells lacking ATM. The altered induction pattern of recombination protein foci shows that HR proceeds abnormally in ATM-deficient cells.
Fig. 1. Radiation-induced nuclear foci of Rad51 and Rad54 proteins. (A) Immunofluorescence visualization of Rad51 foci in cells of the genotypes indicated before and 1 or 8 h after 4 Gy γ–irradiation. (B) Immunofluorescence visualization of Rad54 foci in cells of the genotypes indicated before and 8 h after 4 Gy γ–irradiation. The control frames are slightly overexposed relative to the experimental frames to emphasize that few foci are present. (C) Quantitation of focus formation in wild-type and ATM–/– DT40 cells following irradiation. Data points for Rad54 foci show the cumulative number of foci per 100 cells and were counted in at least 100 cells in randomly selected frames in two separate experiments. A third experiment gave similar results. The cumulative numbers of Rad51 foci are presented per 50 cells and show representative results from two separate experiments.
Generation of ATM–/–KU70–/– and conditionally Rad54-deficient ATM–/– DT40 cells
Mitotically detectable, spontaneous chromosomal aberrations reflect dysfunctional repair of DNA damage. Cells that lack Ku70, a key component of NHEJ through its interaction with the DNA-dependent protein kinase (Jeggo et al., 1995; Weaver, 1995; Smith and Jackson, 1999), show no such chromosomal instability, although there does exist a deficit in repairing radiation-induced lesions in G1–early S phase. HR-impaired RAD51- or RAD54-null cells show high levels of spontaneous chromosomal anomalies and a repair deficiency in late S–G2 phase (Kemp and Jeggo, 1986; Sonoda et al., 1998; Takata et al., 1998). These findings indicate that the majority of such lesions are normally repaired by HR during S phase, although the complementary NHEJ pathway acts as an alternative, so that the loss of both pathways increases the incidence of chromosomal aberrations (Takata et al., 1998). This overlapping function provided the basis for our experiment: if ATM controls HR, the disruption of HR in ATM–/– cells should be without significant effects, while the loss of NHEJ should have a marked effect. If ATM controls NHEJ, then the converse would be expected.
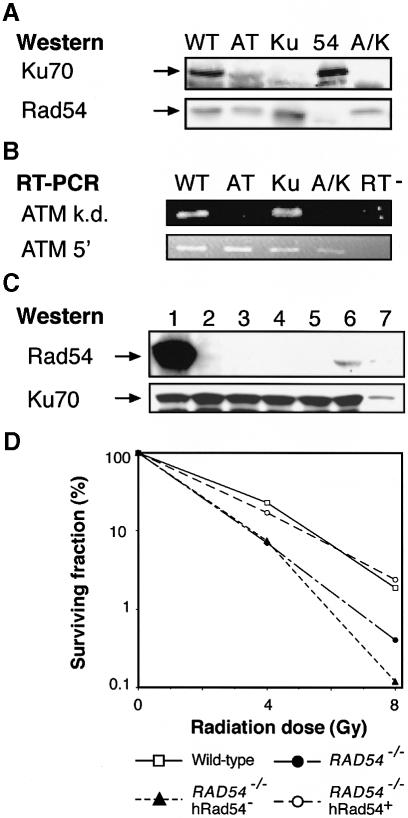
To test this idea, we disrupted ATM in KU70–/– cells and sought to generate RAD54–/–ATM–/– cells, with the expectation of a relatively mild phenotype. However, despite repeated targeting attempts in ATM–/–RAD54+/– cells, we were unable to obtain any double mutants, so we generated conditionally Rad54-null cells instead by placing a human RAD54 cDNA under the control of a tetracyclin-repressible promoter and transfecting this construct into RAD54–/– DT40 cells. After selecting a clone that showed wild-type levels of γ–sensitivity and good tet-responsive repression of hRAD54 (i.e. to RAD54–/– levels of radiosensitivity; Figure 2D), we targeted the ATM locus. Figure 2 verifies the generation of these ATM–/–KU70–/– and conditionally Rad54-null RAD54–/–ATM–/– mutants; Figure 2A shows a control experiment where we confirm that the disruption of KU70 results in the absence of Ku70. This immunoblot was stripped and reblotted with antisera raised against Rad54 as a control for protein loading. RAD54 disruption (Bezzubova et al., 1997) results in the absence of Rad54 protein, so this experiment also confirms the specificity of our Rad54 antisera. Figure 2B shows the results of an RT–PCR experiment used to confirm the disruption of the kinase domain of ATM (Takao et al., 1999). Primers directed against an exon 3′ to the disrupted sequence (ATM k.d.) do not amplify any target, showing that the ATM locus has been disrupted, while a control amplification, using primers directed against an ATM sequence closely upstream of the targeted integration site (ATM 5′), confirms the quality of the RNA used in this experiment. Figure 2C shows the strong repression of Rad54 in the ATM–/–RAD54–/– clone that we obtained (lanes 2–5), despite the high initial expression levels of the transgene (lane 1). Figure 2D confirms that the RAD54 transgene confers a conditionally Rad54-deficient phenotype upon the RAD54–/– cells in which we targeted the ATM gene.
Fig. 2. Phenotypic controls of DT40 mutants used in this paper. (A) Immunoblot of total protein from wild-type (WT), ATM–/– (AT), KU70–/– (Ku), RAD54–/– (54) and ATM–/–KU70–/– (A/K) DT40 cells. The same blot was hybridized with antisera against Ku70 (upper panel) and Rad54 (lower panel). (B) Ethidium bromide-stained agarose gel showing the products of RT–PCR of the ATM kinase domain (ATM k.d.) or of the region 5′ to the disrupted kinase motif (ATM 5′). Lane designations are as for (A), with RT– indicating the lane showing RNA subjected to PCR without the addition of reverse transcriptase. (C) Immunoblot showing Rad54 suppression in ATM–/–RAD54–/– tet–hRAD54+ cells. Lanes show protein prepared from ATM–/–RAD54–/– tet-hRAD54+ cells prior to (lane 1) and at 1, 2, 3 and 5 days after Rad54 repression (lanes 2–5, respectively). Lane 6 shows protein from wild-type cells, lane 7 a 5–fold dilution of the sample in lane 6. Hybridization with anti-Ku70 antibody was used to control for protein loading. (D) Radiosensitivity of RAD54 mutants as assessed by clonogenic survival of the indicated clones following γ–irradiation. Data points show the average of duplicate experiments for each clone. The conditionally Rad54-deficient cells were irradiated on day 4 of RAD54 repression.
Proliferative properties of NHEJ- or HR-deficient ATM–/– cells
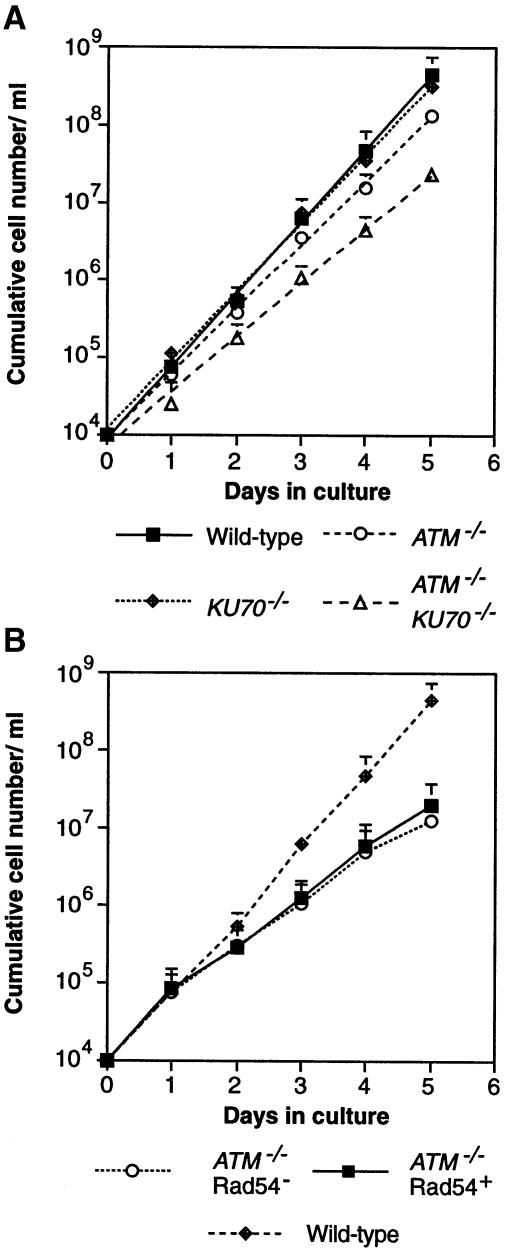
As shown in Figure 3A, the disruption of ATM markedly reduced the proliferation rate of the mutant cells, although this reduction is less pronounced in ATM–/– DT40 cells than in mammalian ATM mutants. This appeared to be due to elevated levels of cell death during each cell cycle, rather than to any marked elongation of cell cycle duration (data not shown). The conditionally Rad54-deficient ATM null clone grew rather slowly (Figure 3B) and had a low plating efficiency (<10%) in methylcellulose medium, perhaps reflecting either small incompatibilities between the human and chicken RAD52 group genes becoming more pronounced in the absence of ATM or some negative effects of the tags carried on the Rad54 construct used (Swagemakers et al., 1998). Perhaps surprisingly, the repression of hRAD54 resulted in only a minor proliferative retardation (Figure 3B) up to 5 days.
Fig. 3. Proliferative characteristics of ATM–/–KU70–/– and conditionally Rad54–ATM–/– cells. (A) Proliferation analysis of the clones is indicated at the bottom. Data points on the growth curves shown are the means + SD of values obtained in two separate duplicate experiments per clone. A second ATM–/–KU70–/– clone gave similar results. (B) Proliferation analysis of ATM–/–RAD54–/– tet-hRAD54+ cells prior to and after Rad54 repression, as shown at the bottom. Data points represent the means + SD of values obtained from two separate duplicate experiments with three different ATM–/–RAD54–/– tet-hRAD54+ subclones. Analysis of the pool of ATM–/–RAD54–/– tet-hRAD54+ cells gave the same results.
Genomic instability in NHEJ- or HR-deficient ATM–/– cells
Next, we monitored the occurrence of spontaneous chromosomal aberrations in ATM-null cells lacking Ku70 or Rad54. While ATM-deficient cells show slightly elevated levels of spontaneous chromosomal damage, the additional loss of Ku70 greatly potentiates the occurrence of both single chromatid and isochromatid (both chromatids) gaps and breaks (Table I). These results suggest that the aberrations resulting from the absence of ATM are no longer properly repaired in the absence of Ku70, which acts as a backup for HR (Takata et al., 1998). Karyotypic analysis of conditionally Rad54-deficient ATM–/– cells after hRAD54 repression also revealed an increase in the level of chromosomal aberrations (Table II). This accumulation was accompanied by a slight decrease in the proliferation of the Rad54–ATM–/– cells (Figure 3B), although this minor decrease was the extent of the proliferative reduction after up to 7 days of hRAD54 repression, with no continued accumulation of chromosomal abnormalities (data not shown). If we compare the extent of the synergistic increase in chromosomal aberrations in the absence of ATM and either repair pathway [i.e. the increase beyond the additive effects of checkpoint deficiency (in ATM–/– cells) and dsb repair deficiency (in KU70–/– or RAD54–/– cells)], it appears that ATM acts together with either HR or NHEJ during repair of replication-associated DNA damage.
Table I. Spontaneous chromosomal aberrations in ATM/Ku70-deficient cells.
| Genotype | Chromatid |
Isochromatid |
Exchanges | Total aberrations (± SE) | ||
|---|---|---|---|---|---|---|
| Breaks | Gaps | Breaks | Gaps | |||
| wild type | 0 | 2 | 0 | 0 | 0 | 2 ± 1.4 |
| ATM–/– | 0 | 3 | 2 | 1 | 0 | 6 ± 2.4 |
| KU70–/– | 3 | 1 | 0 | 0 | 0 | 4 ± 2.0 |
| ATM–/–KU70–/– #1 | 3.5 | 4.5 | 4 | 4 | 0 | 16 ± 2.8 |
| ATM–/–KU70–/– #2 | 3 | 1.5 | 5 | 5.5 | 0 | 15 ± 3.9 |
Data are presented as macrochromosomal (1–5 and Z) aberrations per 100 metaphase spreads, the SE being calculated as (√ the number of aberrations)/the number of spreads analysed. One hundred wild-type or single mutant metaphases and 200 from both ATM–/–KU70–/– clones were analysed.
Table II. Spontaneous chromosomal aberrations in ATM/Rad54-deficient cells.
| Genotype | Chromatid |
Isochromatid |
Exchanges | Total aberrations (± SE) | ||
|---|---|---|---|---|---|---|
| Breaks | Gaps | Breaks | Gaps | |||
| RAD54–/– | 3 | 1.9 | 0.7 | 0.8 | 0.3 | 7 ± 1 |
| ATM–/–RAD54–/–Rad54+ | 2.5 | 2.5 | 1 | 0.5 | 0.5 | 7 ± 2 |
| ATM–/–RAD54–/–Rad54– d1 | 2 | 4.5 | 4.5 | 3.5 | 0.5 | 15 ± 3 |
| ATM–/–RAD54–/–Rad54– d2 | 2.5 | 3.5 | 2.5 | 4 | 0 | 13 ± 3 |
| ATM–/–RAD54–/–Rad54– d3 | 2 | 4.5 | 3.5 | 2.5 | 0 | 13 ± 3 |
| ATM–/–RAD54–/–Rad54– d4 | 3.5 | 5.5 | 3 | 5 | 1 | 18 ± 3 |
| ATM–/–RAD54–/–Rad54– d5 | 5.5 | 5.5 | 8 | 8.5 | 0 | 27 ± 4 |
Data for RAD54–/– cells are taken from Takata et al. (1998). Two hundred metaphases were analysed in each other case, from 1–5 days after RAD54 repression (d1–d5). Data were calculated and are presented as described for Table I.
Recombinational repair of radiation-induced DNA damage
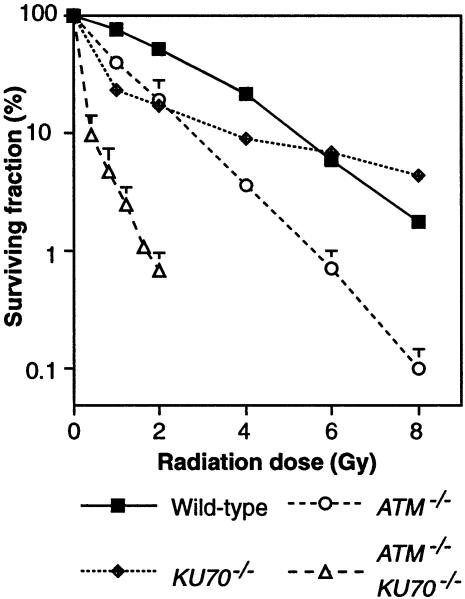
Since HR is used to repair induced DNA damage in S–G2, with NHEJ acting as a backup (Takata et al., 1998), we reasoned that an increase in induced chromosomal aberrations observed within a short time after irradiation (i.e. irradiation in G2 or S–G2) would reflect a defect in HR, and such a defect should be exacerbated by the loss of NHEJ. Therefore, to test whether ATM has a role in HR, we examined the karyotypes of ATM–/– and ATM–/–KU70–/– cells after γ–irradiation. As shown in Table III, ATM–/– cells showed slightly higher levels of chromosomal aberrations than wild-type cells following treatment with 0.3 Gy. Since radiation principally induces chromatid-type damage (i.e. breaks in single chromatids), the conversion of such lesions to isochromatid-type damage may suggest a failure during the process of recombinational repair. Therefore, the slight increase of isochromatid abnormalities in ATM–/– cells (similar to that observed in RAD54–/– cells) may reflect a HR defect. ATM–/–KU70–/– cells showed a dramatically elevated inability to repair chromosome damage. The different pattern of chromosomal aberrations observed in the two ATM–/–/KU70–/– clones examined is likely to be due to clonal variation (as might be expected in mutants with such frequent spontaneous aberrations), rather than to any technical problem. The strongly increased radiosensitivity in ATM–/–KU70–/– cells compared with either single mutant (Figure 4) confirms the significance of our cytogenetic analysis. However, ATM deficiency does not disrupt NHEJ drastically, since the radioresistance of ATM–/– cells is higher than that of KU70–/– cells at doses ⩽2 Gy.
Table III. Chromosomal aberrations induced by irradiation during G2 and late S–G2 phase.
| Genotype | 1.5 h |
3 h |
||||||
|---|---|---|---|---|---|---|---|---|
| Chromatid | Isochromatid | Exchanges | Total | Chromatid | Isochromatid | Exchanges | Total | |
| wild type | 28 | 6 | 0 | 34 ± 6 | 11 | 4 | 0 | 15 ± 4 |
| ATM–/– | 40 | 9 | 1 | 50 ± 6 | 19 | 11 | 0 | 30 ± 5 |
| KU70–/– | 58 | 7 | 4 | 69 ± 8 | 10 | 7 | 1 | 18 ± 4 |
| RAD54–/– | 37 | 10 | 1 | 48 ± 7 | 19 | 5 | 1 | 25 ± 5 |
| ATM–/–/KU70–/– #1 | 131 | 13 | 0 | 144 ± 12 | 113 | 17 | 1 | 131 ± 11 |
| ATM–/–/KU70–/– #3 | nd | nd | nd | nd | 77 | 71 | 0 | 148 ± 9 |
| ATM–/–/RAD54–/– Rad54+ | 28 | 9 | 0 | 37 ± 6 | 32 | 4 | 0 | 36 ± 8 |
| ATM–/–/RAD54–/– Rad54– | 43 | 8 | 0 | 51 ± 7 | 26 | 8 | 0 | 34 ± 8 |
Cells were subjected to 0.3 Gy γ–irradiation, then incubated with colcemid for 1.5 or 3 h, as indicated, and harvested. At least 100 metaphases at 1.5 h and at least 50 at 3 h were analysed. The data from ATM–/–RAD54–/–Rad54– cells were obtained after 5 days of Rad54 repression. Data were calculated and are presented as described for Table I. nd, not determined.
Fig. 4. Radiosensitivity of ATM–/–KU70–/– cells. Clonogenic survival of the indicated clones following γ–irradiation. Data points show the means + SD for at least three separate experiments per clone.
To examine the extent to which ATM and Rad54's roles overlap in recombinational repair of induced damage, we monitored cytogenetic abnormalities induced by irradiation of Rad54-deficient ATM-null cells in G2 phase (1.5 h after irradiation) or in late S–G2 (3 h after irradiation) (Table III); the poor plating efficiency of these cells did not permit us to assess radiosensitivity by survival. The conditionally Rad54–ATM–/– cells showed rather high levels of chromosomal aberrations prior to repression of the hRAD54 transgene, perhaps reflecting the inter-species incompatibilities between the RAD52 group genes suggested above. However, after repression of Rad54, they showed only a slightly increased inability to repair their chromosomes by HR, showing the mild phenotype expected if the ATM and HR involving Rad54 lie on the same pathway of dsb repair. In contrast to our findings in RAD54–/–KU70–/– cells (Takata et al., 1998), it is noteworthy that no major increase in radiation-induced damage occurred, further demonstrating that ATM deficiency does not significantly disrupt NHEJ.
Discussion
The major evidence for a DNA dsb repair defect additional to the cell cycle checkpoint deficiencies caused by ATM deficiency was reviewed recently (Jeggo et al., 1998). This consists of the inability of experimentally imposed cell cycle delay to compensate for the absence of the ATM-imposed arrest (since time for DNA repair is considered to be a major reason for checkpoint arrest) (Weichselbaum et al., 1978), high levels of radiation-induced chromosomal damage in A–T cells without passage through the cell cycle (Taylor et al., 1976; Cornforth and Bedford, 1985), and the ability to distinguish separate ATM domains responsible for the radiosensitivity and cell cycle defects (Morgan et al., 1997). To investigate whether HR might be directly affected by the absence of ATM, we monitored the formation of nuclear foci of Rad54 and Rad51 in response to irradiation. The alteration of subnuclear localization of repair proteins in response to DNA damage is an established motif in the cell biology of DNA repair (Haaf et al., 1995; Maser et al., 1997; Tan et al., 1999). Rad51 foci were formed far more rapidly in wild-type than in ATM–/– cells, although many more accumulated in the ATM–/– cells at later timepoints (Figure 1A and C), probably reflecting increased numbers of unrepaired breaks due to cells carrying unrepaired lesions proceeding through the cell cycle. Conversely, Rad54 foci formed with similar initial kinetics in wild-type and ATM-deficient cells (Figure 1B and C), and then accumulated in the ATM null cells, as did Rad51 foci. Since Rad51 and Rad54 interact closely (Clever et al., 1997; Golub et al., 1997; Petukhova et al., 1998; Tan et al., 1999), we interpret these findings to mean that the later stages of recombinational repair, including Rad54 focus formation, proceed normally, but the initial stages, mediated principally by Rad51 (but with assistance from other proteins including Rad54) (Petukhova et al., 1998), are disrupted by ATM deficiency. This model implies that ATM lies upstream in focus formation of Rad51, but not of Rad54. The reduction of targeting efficiency in ATM–/– cells (Takao et al., 1999) presents further evidence for an HR deficiency in the absence of ATM function.
We next used reverse genetics to test the hypothesis that HR is affected by ATM deficiency. Our examination of ATM–/–KU70–/– cells revealed the accumulation of spontaneous chromosomal abnormalities, with an associated proliferative defect (Table I and Figure 3). HR is able to repair almost all the additional lesions incurred by Ku70 deficiency, so that KU70–/– cells have a phenotype indistinguishable from wild-type cells with regard to spontaneous lesions (Table I; and Kemp and Jeggo, 1986; Takata et al., 1998). However, the loss of ATM leads to a low level of chromosome aberrations (Table I), suggesting that HR is not capable of completely repairing spontaneous damage without it. Significantly, frequent isochromatid gaps and breaks occur in ATM–/–KU70–/– cells, suggesting that Ku70 deficiency may exacerbate the ATM–/– phenotype by removing a back-up system for HR, as is seen with Ku70 deletion in HR-deficient RAD54–/– cells (Takata et al., 1998). Stronger evidence for a HR defect comes from our karyotypic analysis of γ–irradiated ATM–/–KU70–/– cells, which show a greatly elevated incidence of chromosomal aberrations. A possible link between ATM and the HR apparatus is c-Abl, which associates with and is activated by ATM (Baskaran et al., 1997; Shafman et al., 1997) and has been shown to phosphorylate Rad51 in response to DNA damage (Yuan et al., 1998; Chen et al., 1999). However, the biological significance of these findings is not yet clear, as c-Abl-mediated phosphorylation negatively affected Rad51's activity in one set of experiments reported (Yuan et al., 1998), but enhanced its association with Rad52, a putative modulator of Rad51 activity, in the other (Chen et al., 1999).
From the lethality of Rad51 deficiency (Lim and Hasty, 1996; Tsuzuki et al., 1996; Sonoda et al., 1998) and the requirement of HR for repair of replication-associated DNA damage (Sonoda et al., 1999), it appears that HR is essential in vertebrate cells. Since neither ATM nor Rad54 deficiency alone results in lethality, based on the assumption that ATM activates HR as well as the DNA damage checkpoint, the generation of RAD54–/–ATM–/– double mutants had two possible outcomes: lethality, showing that an ATM-controlled homologue of Rad54 compensates for RAD54 deficiency; or survival, showing that the compensating protein(s) are independent of ATM. Analysis of survivors' phenotype would indicate the importance of Rad54-linked HR as a target of ATM. The observation of relatively few additional radiation-induced chromosomal aberrations (Table III) strongly suggests that ATM interacts with HR involving Rad54 during the repair of induced damage. It is possible that low levels of Rad54 are still present in these cells and permit their survival, although the RAD54–/– levels of radiosensitivity in the parental conditionally null cell line upon Rad54 repression (Figure 2D) argue that the putative leaky Rad54 expression is not sufficient for efficient dsb repair after ionizing radiation. Another possibility is that homologous proteins may mediate the HR activity known to be essential from the lethality of Rad51 deficiency (Sonoda et al., 1998). However, the rather gradual increase in spontaneous chromosome abnormalities (Table II) in the absence of Rad54 and ATM demonstrates that their interaction is less important for the repair of spontaneous DNA damage, although Rad54 is not necessarily the only major target of ATM in the recombinational repair of replication-associated DNA damage.
While the possible synergistic effects of the dsb repair and checkpoint defects in ATM–/– cells in spontaneous chromosomal aberrations or in the clonogenic survival assay are unclear, our karyotypic analysis after irradiation addresses this issue. By irradiating the cells a short time before analysis, we limit the DNA repair to that which can occur in late S–G2. Since HR is the predominant activity in that period, its loss would be expected to compound the effects of checkpoint deficiency, if such checkpoint deficiency contributes significantly to the level of aberrations observed. Because the Rad54–ATM–/– cells show a minor effect following irradiation, the loss of the ATM-mediated checkpoint does not lead to a major accumulation of chromosomal damage after irradiation. Therefore, aberrant repair, rather than aberrant checkpoint activation, is the predominant cause of the severe effects seen in KU70–/–ATM–/– cells after irradiation.
Taken together, our results show that ATM controls recombinational repair of DNA damage. A direct link between cell cycle checkpoint proteins and dsb repair is a conceptually satisfying motif and has recently been suggested for NHEJ and ATM based on yeast data (Martin et al., 1999; Mills et al., 1999). Defective HR is consistent with all the known aspects of A–T and ATM-deficient cells, from cellular assays revealing abnormal recombination (Meyn, 1993; Luo et al., 1996), slightly-reduced SCE levels (Galloway, 1977) and radiosensitivity (Taylor et al., 1975), to chromosomal instability and predisposition to malignancy (Lavin and Shiloh, 1997; Meyn, 1997; Shiloh, 1997). Investigation of the interactions of the recombinational repair machinery and the DNA damage checkpoint apparatus will shed further light on this aspect of the disease.
Materials and methods
Plasmid construction
The construction of gene targeting vectors designed to disrupt the kinase domain of ATM with neomycin and puromycin as selection markers has been described (Takao et al., 1999). A vector carrying a blasticidin cassette (Takata and Kurosaki, 1996) was prepared in the same manner and was linearized with NotI for gene targeting. To generate a tet-repressible (Gossen et al., 1995) RAD54 expression vector, a ScaI–MluI sequence from pZeoSV-HishRad54HA, coding for human Rad54 bearing an N-terminal polyhistidine tag and a C–terminal haemagglutin tag (Swagemakers et al., 1998), was Klenow-treated and inserted into the BamHI site of pUHG10–3 (M.Gossen, ZMBH, Heidelberg, unpublished), yielding pUHG10-3HishRad54HA. ptTA–HygR has been described (Sonoda et al., 1998). For expression of chicken Rad54 in Escherichia coli, a PCR fragment coding for amino acids 332–515 of the chicken sequence (Bezzubova et al., 1997) was cloned into pET15b (Novagen, Madison, WI), yielding pETchRad54(332–515). The sequences of the primers used were: GTGGATCCGTTGCTTGAATATTTCAGCC and GTGGATCCGCTTGTCGTTACTGGTGCT.
Cell culture and gene targeting
DT40 cells were maintained as described (Sonoda et al., 1998). Electroporation of linearized targeting vectors and antibiotic selection conditions were as described previously (Sonoda et al., 1998; Takata et al., 1998). The ATM locus in KU70–/– cells (Takata et al., 1998) was successively targeted with targeting vectors carrying a neomycin and subsequently a puromycin resistance cassette. RAD54–/– DT40 cells (Bezzubova et al., 1997) were transfected with linearized pUHG10-3HishRad54HA and ptTA–HygR and selected as described (Sonoda et al., 1998), and then examined for Rad54 expression by Western blot analysis. A clone showing tet-repressible restoration of radioresistance was used for further experiments and was successively targeted with ATM-disrupting vectors carrying puromycin and then blasticidin resistance. Selection for both puromycin and blasticidin resistance was maintained during targeting of the second ATM allele.
Proliferation analyses and colony survival assay
Cells were counted daily by flow cytometric comparison with fixed numbers of 25 μm microspheres (Polysciences Inc., Warrington, PA) (Takata et al., 1998). Cells were split each day to keep them sub-confluent. Radiosensitivity of cells was examined by their colony-forming ability in methylcellulose-containing medium 7–10 days after γ–irradiation with 137Cs (Gammacell 40, Nordion, Kanata, Ontario, Canada) (Takata et al., 1998).
Karyotypic analysis
For karyotype analysis, colcemid (Gibco-BRL, Grand Island, NY) was added to the growth medium to a concentration of 0.1 μg/ml for 1.5 or 3 h, after which metaphase spreads were placed on glass slides and Giemsa-stained as described (Sonoda et al., 1998). Scoring of chromosomal aberrations was according to the standard criteria (ISCN, 1985) with breaks being distinguished from gaps by a diagnostic misalignment of the portion of the chromosome distal to the break.
Preparation of Rad54 antisera and immunoblotting
pETchRad54(332–515) was transformed into E.coli and the Rad54 fragment produced was purified by nickel column chromatography. Rabbit polyclonal antibodies were raised against this Rad54 fragment. For immunoblot analysis, total cellular proteins were separated by SDS–PAGE and transferred to nitrocellulose membranes. Membranes were incubated with rabbit antisera raised against Rad54, Ku70 (Takata et al., 1998) or Rad51 (Tashiro et al., 1996) at room temperature and visualized using horseradish peroxidase-coupled secondary antibodies directed against rabbit immunoglobulins (Santa Cruz Biotechnology, Santa Cruz, CA).
RT–PCR
Total RNA was isolated with TRIzol reagent (Gibco-BRL) and 2 μg oligo(dT)-primed, and reverse-transcribed using the Superscript cDNA synthesis system as described by the suppliers (Gibco-BRL). One-tenth of this reaction was denatured at 94°C for 180 s, then PCR amplified with 25 cycles of 94°C (45 s), 55°C (45 s) and 68°C (60 s) and a final 72°C step for 5 min. Primers were used at 200 nM and their sequences were: chicken ATM kinase domain, GTGGATCCCACAGGAAGATA and GTCAGCTTCATCCTCTGGTC; ATM 5′ region, GGAGTTTCTGAATGGCAACTAGAAGAAGCT and GATTATATTTCTAGCAGCCTC.
Immunofluorescent visualization of nuclear foci
Cells were spun out of culture on to glass slides using a Cytospin 3 (Shandon, Pittsburgh, PA) and fixed with 3.7% formalin solution. NP–40 solution (0.1%) was used to permeabilize the cells and then primary antibody stainings were performed in 10 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.05% Tween–20 using optimal dilutions of the Rad51 or Rad54 antisera described above. Fluorescein isothiocyanate-coupled anti-rabbit immunoglobulin secondary antibodies (Santa Cruz) were applied in the same buffer, with at least three washes in buffer between each step and before microscopy. Foci were visualized using confocal microscopy (MRC–1024; Bio-Rad, Hercules, CA). Analysis was of untreated cells or of cells γ–irradiated with 4 Gy at different times after treatment. Following microscopy and image processing with Adobe Photoshop version 4.0J, colour-inverted images were printed and distinct foci counted.
Acknowledgments
Acknowledgements
We thank Y.Sato for her skilled technical assistance and Drs S.P.Jackson (Wellcome/CRC Institute, Cambridge University), P.A.Jeggo (MRC, Sussex University) and M.F.Lavin (QIMR, Brisbane) for their critical readings of the manuscript. We are grateful to Dr R.Kanaar (Erasmus University, Rotterdam) for sending us the human Rad54 plasmid and to Dr M.S.Sasaki for his insightful comments. C.M. is the recipient of a JSPS Postdoctoral Fellowship. The Bayer Chair Department of Molecular Immunology and Allergology is supported by Bayer Yakuhin, Kyoto, Japan. This work was partly supported by CREST, JST, by a Grant-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Science and Culture of Japan, and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
References
- Banin S. et al. (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science, 281, 1674–1679. [DOI] [PubMed] [Google Scholar]
- Barlow C. et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell, 86, 159–171. [DOI] [PubMed] [Google Scholar]
- Baskaran R. et al. (1997) Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature, 387, 516–519. [DOI] [PubMed] [Google Scholar]
- Beamish H. and Lavin, M.F. (1994) Radiosensitivity in ataxia–telangiectasia: anomalies in radiation-induced cell cycle delay. Int. J. Radiat. Biol., 65, 175–184. [DOI] [PubMed] [Google Scholar]
- Beamish H., Williams, R., Chen, P. and Lavin, M.F. (1996) Defect in multiple cell cycle checkpoints in ataxia–telangiectasia postirradiation. J. Biol. Chem., 271, 20486–20493. [DOI] [PubMed] [Google Scholar]
- Bezzubova O., Silbergleit, A., Yamaguchi-Iwai, Y., Takeda, S. and Buerstedde, J.-M. (1997) Reduced X-ray resistance and homologous recombination frequencies in a RAD54–/– mutant of the chicken DT40 cell line. Cell, 89, 185–193. [DOI] [PubMed] [Google Scholar]
- Brown K.D., Barlow, C. and Wynshaw-Boris, A. (1999) Multiple ATM-dependent pathways: an explanation for pleiotropy. Am. J. Hum. Genet., 64, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman C.E., Lim, D.-S., Cimprich, K.A., Taya, Y., Tamai, K., Sakaguchi, K., Appella, E., Kastan, M.B. and Siciliano, J.D. (1998) Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science, 281, 1677–1679. [DOI] [PubMed] [Google Scholar]
- Chen G. et al. (1999) Radiation-induced assembly of Rad51 and Rad42 recombination complex requires ATM and c-Abl. J. Biol. Chem., 274, 12748–12752. [DOI] [PubMed] [Google Scholar]
- Clever B., Interthal, H., Schmuckli-Maurer, J., King, J., Sigrist, M. and Heyer, W.-D. (1997) Recombinational repair in yeast: functional interactions between Rad51 and Rad54 proteins. EMBO J., 16, 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornforth M.N. and Bedford, J.S. (1985) On the nature of a defect in cells from individuals with ataxia–telangiectasia. Science, 227, 1589–1591. [DOI] [PubMed] [Google Scholar]
- Debenham P.G., Webb, M.B., Jones, N.J. and Cox, R. (1987) Molecular studies on the nature of the repair defect in ataxia–telangiectasia and their implications for cellular radiobiology. J. Cell Sci., 6, 177–189. [DOI] [PubMed] [Google Scholar]
- Elledge S.J. (1996) Cell cycle checkpoints: preventing an identity crisis. Science, 274, 1664–1672. [DOI] [PubMed] [Google Scholar]
- Elson A., Wang, Y., Daugherty, C.J., Morton, C.C., Zhou, F., Campos-Torres, J. and Leder, P. (1996) Pleiotropic defects in ataxia–telangiectasia protein-deficient mice. Proc. Natl Acad. Sci. USA, 93, 13084–13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J., Hendriks, R.W., Swagemakers, S.M.A., Troelstra, C., De Wit, J., Bootsma, D., Hoeijmakers, J.H.J. and Kanaar, R. (1997) Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell, 89, 195–204. [DOI] [PubMed] [Google Scholar]
- Galloway S.M. (1977) Ataxia telangiectasia: the effects of chemical mutagens and X-rays on sister chromatid exchanges in blood lymphocytes. Mutat. Res., 45, 343–349. [DOI] [PubMed] [Google Scholar]
- Gatti R.A. et al. (1988) Localization of an ataxia–telangiectasia gene to chromosome 11q22-23. Nature, 336, 577–580. [DOI] [PubMed] [Google Scholar]
- Golub E.I., Kovalenko, O.V., Gupta, R.C., Ward, D.C. and Radding, C.M. (1997) Interaction of human recombination proteins Rad51 and Rad54. Nucleic Acids Res., 25, 4106–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb, S., Bender, G., Müller, G., Hillen, W. and Bujard, H. (1995) Transcriptional activation by tetracyclines in mammalian cells. Science, 268, 1766–1769. [DOI] [PubMed] [Google Scholar]
- Haaf T., Golub, E.I., Reddy, G., Radding, C.M. and Ward, D.C. (1995) Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc. Natl Acad. Sci. USA, 92, 2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCN (1985) An international system for human cytogenetic nomenclature. Report of the Standing Committee on Human Cytogenetic Nomenclature. Basel, Karger. [PubMed]
- Jackson S.P. (1995) Ataxia–telangiectasia at the crossroads. Curr. Biol., 5, 1210–1212. [DOI] [PubMed] [Google Scholar]
- Jeggo P.A., Taccioli, G.E. and Jackson, S.P. (1995) Menage à trois: double strand break repair, V(D)J recombination and DNA-PK. BioEssays, 17, 949–957. [DOI] [PubMed] [Google Scholar]
- Jeggo P.A., Carr, A.M. and Lehmann, A.R. (1998) Splitting the ATM: distinct repair and checkpoint defects in ataxia–telangiectasia. Trends Genet., 14, 312–316. [DOI] [PubMed] [Google Scholar]
- Kastan M.B., Zhan, Q., el-Deiry, W.S., Carrier, F., Jacks, T., Walsh, W.V., Plunkett, B.S., Vogelstein, B. and Fornace, A.J., Jr (1992) A mammalian cell cycle checkpoint pathway utilising p53 and GADD45 is defective in ataxia telangiectasia. Cell, 71, 587–597. [DOI] [PubMed] [Google Scholar]
- Kemp L.M. and Jeggo, P.A. (1986) Radiation-induced chromosome damage in X-ray sensitive mutants (xrs) of the Chinese hamster ovary cell line. Mutat. Res., 166, 255–263. [DOI] [PubMed] [Google Scholar]
- Khanna K.K. et al. (1998) ATM associates with and phosphorylates p53: mapping the region of interaction. Nature Genet., 20, 398–400. [DOI] [PubMed] [Google Scholar]
- Lavin M.F. and Shiloh, Y. (1997) The genetic defect in ataxia telangiectasia. Annu. Rev. Immunol., 15, 177–202. [DOI] [PubMed] [Google Scholar]
- Lim D.-S. and Hasty, P. (1996) A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol., 16, 7133–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu V.F. and Weaver, D.T. (1993) The ionizing radiation-induced replication protein A phosphorylation response differs between ataxia telangiectasia and normal human cells. Mol. Cell. Biol., 13, 7222–7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C.-M., Tang, W., Mekeel, K.L., DeFrank, J.S., Anné, P.R. and Powell, S.N. (1996) High frequency and error-prone DNA recombination in ataxia telangiectasia cell lines. J. Biol. Chem., 271, 4497–4503. [DOI] [PubMed] [Google Scholar]
- Martin S.G., Laroche, T., Suka, N., Grunstein, M. and Gasser, S.M. (1999) Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell, 97, 621–633. [DOI] [PubMed] [Google Scholar]
- Maser R.S., Monsen, K.J., Nelms, B.E. and Petrini, J.H.J. (1997) hMre11 and Rad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol. Cell. Biol., 17, 6087–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Huang, M. and Elledge, S.J. (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science, 282, 1893–1897. [DOI] [PubMed] [Google Scholar]
- Meyn M.S. (1993) High spontaneous intrachromosomal recombination rates in ataxia–telangiectasia. Science, 260, 1327–1330. [DOI] [PubMed] [Google Scholar]
- Meyn M.S. (1995) Ataxia telangietasia and cellular responses to DNA damage. Cancer Res., 55, 5991–6001. [PubMed] [Google Scholar]
- Meyn M.S. (1997) Chromosome instability syndromes: lessons for carcinogenesis. Curr. Top. Microbiol. Immunol., 221, 71–148. [DOI] [PubMed] [Google Scholar]
- Mills K.D., Sinclair, D.A. and Guarente, L. (1999) MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell, 97, 609–620. [DOI] [PubMed] [Google Scholar]
- Morgan S.E., Lovly, C.C., Pandita, T.K., Shiloh, Y. and Kastan, M.B. (1997) Fragments of ATM which have dominant-negative or complementing activity. Mol. Cell. Biol., 17, 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. and Wagner, E. (1996) Extrachromosomal recombination occurs efficiently in cells defective in various DNA repair systems. Nucleic Acids Res., 24, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R.B. and Young, B.R. (1980) Radiosensitivity in ataxia–telangiectasia: a new explanation. Proc. Natl Acad. Sci. USA, 77, 7315–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich A.G., Toczyski, D.P. and Hartwell, L.H. (1997) When checkpoints fail. Cell, 88, 315–321. [DOI] [PubMed] [Google Scholar]
- Petukhova G., Stratton, S. and Sung, P. (1998) Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature, 393, 91–94. [DOI] [PubMed] [Google Scholar]
- Powell S., Whitaker, S., Peacock, J. and McMillan, T. (1993) Ataxia telangiectasia: an investigation of the repair defect in the cell line AT5BIVA by plasmid reconstitution. Mutat. Res., 294, 9–20. [DOI] [PubMed] [Google Scholar]
- Savitsky K. et al. (1995a)A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science, 268, 1749–1753. [DOI] [PubMed] [Google Scholar]
- Savitsky K., Sfez, S., Tagle, D., Ziv, Y., Sartiel, A., Collins, F.S., Shiloh, Y. and Rotman, G. (1995b) The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum. Mol. Genet., 4, 2025–2032. [DOI] [PubMed] [Google Scholar]
- Shafman T. et al. (1997) Interaction between ATM protein and c-Abl in response to DNA damage. Nature, 387, 520–523. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. (1997) Ataxia telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu. Rev. Genet., 31, 635–662. [DOI] [PubMed] [Google Scholar]
- Smith G.C.M. and Jackson, S.P. (1999) The DNA-dependent protein kinase. Genes Dev., 13, 916–934. [DOI] [PubMed] [Google Scholar]
- Sonoda E., Sasaki, M.S., Buerstedde, J.-M., Bezzubova, O., Shinohara, A., Ogawa, H., Takata, M., Yamaguchi-Iwai, Y. and Takeda, S. (1998) Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J., 17, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E., Sasaki, M.S., Morrison, C., Yamaguchi-Iwai, Y., Takata, M. and Takeda, S. (1999) Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol., 19, 5166–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swagemakers S.M.A., Essers, J., De Witt, J., Hoeijmakers, J.H.J. and Kanaar, R. (1998) The human Rad54 recombinational DNA repair protein is a double-stranded DNA-dependent ATPase. J. Biol. Chem., 273, 28292–28297. [DOI] [PubMed] [Google Scholar]
- Takao N. et al. (1999) Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene, 18, 7002–7009. [DOI] [PubMed] [Google Scholar]
- Takata M. and Kurosaki, T. (1996) A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med., 184, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Sasaki, M.S., Sonoda, E., Morrison, C., Hashimoto, M., Utsumi, H., Yamaguchi-Iwai, Y., Shinohara, A. and Takeda, S. (1998) Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J., 17, 5497–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.L.R., Essers, J., Citterio, E., Swagemakers, S.M.A., De Wit, J., Benson, F.E., Hoeijmakers, J.H.J. and Kanaar, R. (1999) Mouse Rad54 affects DNA conformation and DNA-damage-induced Rad51 foci formation. Curr. Biol., 9, 325–328. [DOI] [PubMed] [Google Scholar]
- Tashiro S., Kotomura, N., Shinohara, A., Tanaka, K., Ueda, K. and Kamada, N. (1996) S phase specific formation of the human Rad51 protein nuclear foci in lymphocytes. Oncogene, 12, 2165–2170. [PubMed] [Google Scholar]
- Taylor A.M.R., Harnden, D.G., Arlett, C.F., Harcourt, S.A., Lehmann, A.R., Stevens, S. and Bridges, B.A. (1975) Ataxia–telangiectasia: a human mutation with abnormal radiation sensitivity. Nature, 258, 427–429. [DOI] [PubMed] [Google Scholar]
- Taylor A.M.R., Metcalfe, J.A., Oxford, J.M. and Harnden, D.G. (1976) Is chromatid-type damage in ataxia telangiectasia after irradiation at G0 a consequence of defective repair? Nature, 260, 441–443. [DOI] [PubMed] [Google Scholar]
- Thacker J. (1989) The use of integrating DNA vectors to analyse the molecular defects in ionising radiation-sensitive mutants of mammalian cells including ataxia telangiectasia. Mutat. Res., 220, 187–204. [DOI] [PubMed] [Google Scholar]
- Thacker J. (1994) Cellular radiosensitivity in ataxia–telangiectasia. Int. J. Radiat. Biol., 66, S87–S96. [PubMed] [Google Scholar]
- Tsuzuki T., Fujii, Y., Sakumi, K., Tominaga, Y., Nakao, K., Sekiguchi, M., Matsushiro, A., Yoshimura, Y. and Morita, T. (1996) Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA, 93, 6236–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel T. et al. (1996) Genomic organization of the ATM gene. Genomics, 33, 317–320. [DOI] [PubMed] [Google Scholar]
- Weaver D.T. (1995) What to do at an end: DNA double-strand-break repair. Trends Genet., 11, 388–392. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R.R., Nove, J. and Little, J.B. (1978) Deficient recovery from potentially lethal radiation damage in ataxia telangiectasia and xeroderma pigmentosum. Nature, 271, 261–262. [DOI] [PubMed] [Google Scholar]
- Westphal C.H. (1997) Cell cycle signalling: Atm displays its many talents. Curr. Biol., 7, R789–R792. [DOI] [PubMed] [Google Scholar]
- Xie G., Habbersett, R.C., Jia, Y., Peterson, S.R., Lehnert, B.E., Bradbury, E.M. and D'Anna, J.A. (1998) Requirements for p53 and the ATM gene product in the regulation of G1/S and S phase checkpoints. Oncogene, 16, 721–736. [DOI] [PubMed] [Google Scholar]
- Xu Y. and Baltimore, D. (1996) Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev., 10, 2401–2410. [DOI] [PubMed] [Google Scholar]
- Yuan Z.-M. et al. (1998) Regulation of Rad51 function by c-Abl in response to DNA damage. J. Biol. Chem., 273, 3799–3802. [DOI] [PubMed] [Google Scholar]
- Zakian V.A. (1995) ATM-related genes: what do they tell us about functions of the human gene? Cell, 82, 685–687. [DOI] [PubMed] [Google Scholar]