Abstract
Purpose
The goal of this study was to perform a systematic review and meta-analysis to examine the effect of pre-existing diabetes on breast cancer–related outcomes.
Methods
We searched EMBASE and MEDLINE databases from inception through July 1, 2009, using search terms related to diabetes mellitus, cancer, and prognostic outcome. Studies were included if they reported a prognostic outcome by diabetes status, evaluated a cancer population, and contained original data published in the English language. We performed a meta-analysis of pre-existing diabetes and its effect on all-cause mortality in patients with breast cancer and qualitatively summarized other prognostic outcomes.
Results
Of 8,828 titles identified, eight articles met inclusion/exclusion criteria and described outcomes in patients with breast cancer and diabetes. Pre-existing diabetes was significantly associated with all-cause mortality in six of seven studies. In a meta-analysis, patients with breast cancer and diabetes had a significantly higher all-cause mortality risk (pooled hazard ratio [HR], 1.49; 95% CI, 1.35 to 1.65) compared with their nondiabetic counterparts. Three of four studies found pre-existing diabetes to be associated with more advanced stage at presentation. Diabetes was also associated with altered regimens for breast cancer treatment and increased toxicity from chemotherapy.
Conclusion
Compared with their nondiabetic counterparts, patients with breast cancer and pre-existing diabetes have a greater risk of death and tend to present at later stages and receive altered treatment regimens. Studies are needed to investigate pathophysiologic interactions between diabetes and breast cancer and determine whether improvements in diabetes care can reduce mortality in patients with breast cancer.
INTRODUCTION
Diabetes mellitus and cancer are major causes of morbidity and death worldwide.1 In the United States alone, by 2007 there were approximately 24 million people with diabetes (approximately 8% of the adult population)2 and 2.5 million survivors of breast cancer.3 Recent research has focused attention on the effect of comorbid conditions on all-cause mortality in women with breast cancer.4 Potential interactions between diabetes and breast cancer, in particular, are complex.
Survival in patients with diabetes and breast cancer may be negatively affected by less intensive diabetes and/or cancer care. Factors may include delay in diagnosis, lower use of effective adjuvant therapies, and diabetes-related comorbidities.5,6 Metformin is a commonly used oral diabetic agent that reduces hyperinsulinemia and may favorably affect some measures of outcome in patients with breast cancer. Specifically, hyperinsulinemia and insulin-like growth factors may play a role by promoting tumor growth, and preclinical data show an in vitro effect of metformin in breast cancer cells.7 Metformin modulates known breast cancer prognostic factors by increasing skeletal muscle glucose uptake and reducing both hyperglycemia and hyperinsulinemia, and may have insulin-independent effects through inhibition of the adenosine monophosphate–activated protein kinase/mammalian target of rapamycin/S6 kinase 1 pathway.8 Retrospective clinical data show higher rates of pathologic response after preoperative chemotherapy in patients with diabetes and breast cancer receiving metformin,9 providing a rationale to test new strategies in chemoprevention10 and in the adjuvant setting.11
In a recent meta-analysis, we demonstrated that patients with pre-existing diabetes who develop cancer are at higher risk for long-term, all-cause mortality compared with their nondiabetic counterparts.12 However, the impact of diabetes varied significantly across different cancer types. Given the higher risk of breast cancer in women with diabetes, research investigating how pre-existing diabetes may influence breast cancer diagnosis, treatment, and survival is critical to inform the proper care of these women. We therefore conducted a systematic review and meta-analysis to test the hypothesis that pre-existing diabetes has an adverse effect on all-cause mortality in women with breast cancer, and also examined possible effects on stage at diagnosis and choice of breast cancer treatment.
METHODS
Data Sources and Searches
We searched MEDLINE and EMBASE from inception to July 1, 2009, for articles evaluating the effect of diabetes on any prognostic outcome in patients with cancer, including survival, stage at diagnosis, treatment choice, and treatment complications. Our overall search strategy included terms for diabetes (eg, “diabetes,” “glucose intolerance,” “hyperglycemia”), cancer (eg, “cancer,” “malignant neoplasm”), and prognosis (eg, “mortality,” “disease-free survival”) and was limited to English-language, human studies. We also searched references of included articles.
Study Selection
Our search targeted articles that met the three following criteria: evaluated any prognostic outcome by glycemic status, evaluated a population with cancer, and contained original data. We included studies with any method of diabetes ascertainment (eg, blood test, medication use, self-report). In this review, we included only articles that evaluated outcomes in patients with breast cancer. To be included in our meta-analysis of all-cause mortality, articles had to meet the following two criteria: at least 3 months of follow-up, and report a risk estimate (eg, hazard ratio [HR] or relative risk) relating pre-existing diabetes to subsequent death with an estimate of precision, such as an SE or 95% CI.
Data Extraction and Quality Assessment
Titles, abstracts, and articles were reviewed independently by two authors. Disagreements were settled by consensus or a third review for adjudication. Abstracted data included study population characteristics, health outcomes, adjustment variables, and study quality. Quality was assessed using elements of the Strengthening the Reporting of Observational Studies in Epidemiology checklist for cohort studies that we considered important for quality.13 To judge quality, we abstracted information on population source, method of diabetes and outcome ascertainment, whether diabetes was the primary exposure variable or one of a group of prognostic variables, and statistical adjustment for confounders.12 For sensitivity analyses by length of follow-up, in studies that reported a range, we used the midpoint of the range for average follow-up. Authors were contacted for clarification for the systematic review and for additional, unreported information for the meta-analysis.
Data Synthesis and Analyses
Outcomes reported in any article are summarized qualitatively in the systematic review. These include all-cause mortality (seven studies), disease stage (four studies), treatment (three studies), toxicity (one study), disease-free survival (one study), and breast cancer–specific mortality (two studies).
We combined results from articles reporting risk estimates with confidence intervals or SEs for all-cause mortality in a meta-analysis. Heterogeneity between studies was assessed by using two statistical methods, Cochran Q and I2.14 Because of substantial between-study heterogeneity (Q, 13.412 on 5 df; P = .02; I2, 62.7%; P = .02), we calculated a pooled HR using the DerSimonian-Laird method for a random-effects model.15 We did not pool results for other outcomes because of the small number of studies, heterogeneity between studies, or insufficient reporting.
Publication bias was evaluated using Begg's funnel plot and the Egger plot. We performed the Duval and Tweedie nonparametric trim and fill procedure to further assess potential effects of publication bias. This method considers the possibility of hypothetical missing studies, imputes their HRs, and recalculates a pooled estimate.16
To assess the impact of study quality, we conducted a sensitivity analysis that omitted lower quality studies. We considered studies to be of higher quality and calculated separate random-effects pooled HRs if they were population based (n = 4)6,17–19 or used medical records or medication use for diabetes ascertainment (n = 4)17,18,20,21 and evaluated diabetes as the primary exposure variable (n = 3).6,18,19 We also calculated separate pooled estimates for studies with shorter6,17,21 and longer18–20 follow-up periods. Finally, we evaluated the influence of each study on the overall estimate by calculating a random-effects pooled HR omitting each estimate, one at a time. All analyses were conducted using STATA 10.1 (College Station, TX).
RESULTS
Literature Search
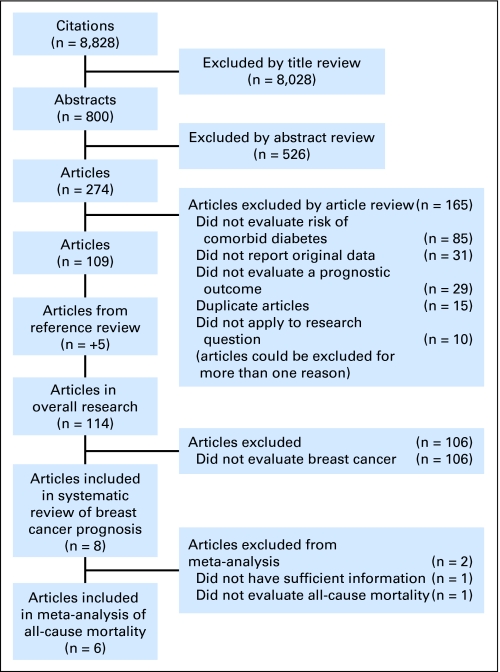
Figure 1 illustrates the process of evaluating articles for inclusion in the review and meta-analysis. Of the 8,828 titles identified, we reviewed 800 abstracts and 274 articles to determine whether they met our inclusion and exclusion criteria. One hundred nine manuscripts provided estimates of the impact of diabetes on cancer prognosis, and five additional articles were identified by searching references. Of the 114 articles, eight addressed the impact of diabetes on breast cancer outcomes and were included in this review.6,17–23 Six of the articles evaluated the association between diabetes and overall mortality in patients diagnosed with breast cancer and met eligibility criteria to be included in the meta-analysis.6,17–21
Fig 1.
Flowchart of study selection.
Study Description and Quality Assessment
Descriptive data for the studies included in this systematic review and meta-analysis of all-cause mortality are summarized in Table 1. The majority of the studies were performed in the United States (n = 6);17,19–23 one was performed in the Netherlands18 and one in Canada.6 All studies were published within the last 10 years. Sample sizes ranged from 58821 to 70,781.19 The percentage of patients with diabetes ranged from 8%18 to 31%.23 Many studies focused on older women, consistent with the peak age of incident breast cancer.
Table 1.
Study Characteristics
| Reference | Date of Diagnosis (range) | Exclusion Criteria | Patients With DM |
Age (years) | Follow-Up | Outcomes Reported | Adjustment Variables | |
|---|---|---|---|---|---|---|---|---|
| No./Total No. | % | |||||||
| Srokowski et al,19 2009 | 1992-2002 | < 66 years, not treated with definitive surgical therapy, previous cancer, no Medicare Part A and B, HMO member, noncarcinoma histology, stage IV disease | 14,414/70,781 | 20 | 66-70 (25%) 71-75 (27%) 76-80 (23%) 80+ (25%) | Range, 2-12 years | All-cause mortality, breast cancer mortality, stage at diagnosis, treatment, toxicity | Sex, diagnosis age, ethnicity, marital status, education level, poverty level, diagnosis year, SEER region, tumor grade, ER status, number positive lymph nodes, Charlson index, surgery type, use of chemotherapy or radiation |
| Lipscombe et al,6 2008 | 1995-2002 | Not 55-79 years, not living in Ontario, ineligible for universal health care, pre-existing breast cancer | 1,011/6,107 | 17 | DM: mean, 69.1 years NG: mean, 68.0 years | Mean, 5.0 years Range, 0-10.9 years | All-cause mortality | Age, income, comorbidity, screening mammogram |
| van de Poll-Franse et al,18 2007 | 1995-2002 | Not in the cancer registry | 754/9,725 | 8 | DM: mean, 70.7 years NG: mean, 58.9 years | Range, 3-10 years | All-cause mortality, stage at diagnosis, treatment | Age, stage, gender, treatment, cardiovascular disease |
| Du and Simon,21 2005 | 1994-1997 | Treatment received at an outside institution, race not black or white, < 1 year follow-up, stage IV disease | 73/588 | 12 | Mean, 59 years | Mean, 3.68 years | All-cause mortality, disease-free survival | Age, stage, nodal involvement, ER/PR status, race, comorbidity |
| Yancik et al,17 2001 | 1992 | < 55 years, unknown death information | NR/1,800 | 55-64 (35%) 65-74 (35%) > 75 (31%) | 30 months | All-cause mortality, stage at diagnosis, treatment | Age, stage, comorbidity | |
| Tammemagi et al,20 2005 | 1985-1990 | Not incident cancer, not Henry Ford Health System member, race not black or white | 127/906 | 14 | ≤ 40 years (8%) 40-50 years (18%) 50-60 years (19%) 60-70 years (24%) 70-80 years (22%) ≥ 80 years (9%) | Median, 10 years Range, 0.04-17.8 years | All-cause mortality | Unclear |
| Fleming et al,23 1999 | 1993 | < 67 years, not incident cancer, missing information on stage | 267/848 | 31.5 | > 67 years | 1 year | All-cause mortality, breast cancer mortality | None |
| Fleming et al,22 2005 | 1993-1995 | < 67 years, not covered by Medicare 2 years prior to cancer diagnosis or through 1998, prior breast cancer, > 1 primary cancers, HMO membership, diagnosis from autopsy, male | 3,182/17,468 | 18 | > 67 years | NA | Stage at diagnosis | Comorbidity, sociodemographic variables, screening, physician visits |
Abbreviations: DM, diabetes mellitus; ER/PR, estrogen receptor/progesterone receptor; HMO, health maintenance organization; NA, not applicable; NG, normoglycemic; NR, not reported; SEER, Surveillance, Epidemiology, and End Results.
On the basis of the methodology and reported data, the overall quality of the six studies included in the meta-analysis was deemed moderate to high.6,17–21 Of the six studies, four were population-based cohorts6,17–19 and two were clinic-based cohorts.20,21 Four17,18,20,21 of the studies used medical records or documented use of diabetic medicine to ascertain diabetes, one used a provincial registry of patients documented as having diabetes on the basis of validated, administrative data,6 and one used International Classification of Diseases (9th revision) diagnosis codes from Surveillance, Epidemiology, and End Results-Medicare.19 All studies used registry data to determine vital status. Five of the studies in the meta-analysis adjusted for age;6,17–19,21 because of insufficient reporting, it was not possible to determine whether the sixth study also adjusted for age.20 There were four studies in the review that adjusted for stage,17–19,21 with other covariates varying across the studies. Three studies focused on diabetes as the primary exposure,6,18,19 whereas the other five articles evaluated diabetes as one of several different prognostic factors17,20–23 (Table 2). These differences in study design and outcomes assessment likely produced the heterogeneity identified by the Cochran Q and I2 statistics.
Table 2.
Study Quality
| Reference | Population Source |
Diabetes Ascertainment |
Outcome Ascertainment |
Diabetes Evaluated As |
Statistical Analysis Adjusted Model? | ||||
|---|---|---|---|---|---|---|---|---|---|
| Population-Based Cohort | Clinic-Based Cohort | Medical Record or Medication Use | Other | Registry | Medical Record | Primary Exposure | One of Multiple Prognostic Factors | ||
| Srokowski et al,19 2009 | Y | Y | Y | Y | Y | ||||
| Lipscombe et al,6 2008 | Y | Y | Y | Y | Y | ||||
| van de Poll-Franse et al,18 2007 | Y | Y | Y | Y | Y | ||||
| Du and Simon,21 2005 | Y | Y | Y | Y | Y | Y | |||
| Yancik et al,17 2001 | Y | Y | Y | Y | Y | ||||
| Tammemagi et al,20 2005 | Y | Y | Y | Y | X | ||||
| Fleming et al,23 1999 | Y | Y | Y | Y | |||||
| Fleming et al,22 2005 | Y | Y | Y | Y | Y | ||||
Abbreviations: Y, present in study; X, unclear.
Diabetes and All-Cause Mortality
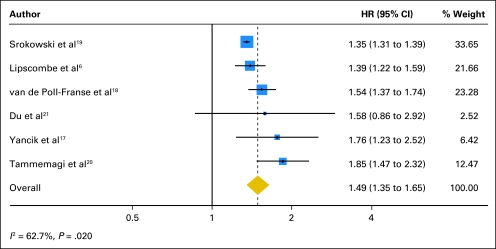
Of the eight studies in the systematic review, six reported a risk estimate of pre-existing diabetes with respect to all-cause mortality with an estimate of precision and met eligibility criteria for the meta-analysis.6,17–21 Study characteristics, demographic information, and adjustment or restriction variables for the selected studies are listed in Table 1. When we pooled the results of these studies, pre-existing diabetes was associated with a 49% increased risk for all-cause mortality in women with breast cancer (HR, 1.49; 95% CI, 1.35 to 1.65; Fig 2).
Fig 2.
Meta-analysis of the effect of pre-existing diabetes on all-cause mortality in patients with breast cancer. HR, hazard ratio.
We observed significant evidence of publication bias according to the Egger plot (P = .04) but not according to Begg's test (P = .71). To evaluate the influence of potential publication bias, we used the trim and fill method to calculate an adjusted pooled random-effects HR. This method added three estimates to balance the funnel plot. The adjusted risk estimate was slightly attenuated and remained significant (HR, 1.41; 95% CI, 1.28 to 1.55).
Risk estimates from higher quality studies were similar to the overall estimate. The pooled random-effects risk estimate for population-based studies resulted in an HR of 1.42 (95% CI, 1.31 to 1.55); for studies ascertaining diabetes by medical record or medication use, the HR was 1.61 (95% CI, 1.46 to 1.78); and for studies with diabetes as the primary exposure variable, the HR was 1.40 (95% CI, 1.30 to 1.52). Studies with an average follow-up of 5 years or less had a pooled estimate with an HR of 1.44 (95% CI, 1.27 to 1.62); studies with an average follow-up of greater than 5 years had a pooled estimate with an HR of 1.52 (95% CI, 1.30 to 1.77). Analysis of influence revealed that the risk of all-cause mortality among patients with breast cancer and diabetes remained significant with the omission of each study in turn. Omission of the study by Tammemagi et al20 resulted in the lowest pooled estimate (HR, 1.42; 95% CI, 1.31to 1.53); omission of the study by Srokowski et al19 resulted in the highest pooled estimate (HR, 1.55; 95% CI, 1.40 to 1.72).
Diabetes and Breast Cancer–Specific Mortality
Two studies on cancer-specific mortality provided mixed results. Srokowski et al19 observed elevated breast cancer–specific mortality in women with diabetes who received chemotherapy compared with their nondiabetic counterparts (follow-up, 2 to 12 years; odds ratio [OR], 1.20; 95% CI, 1.07 to 1.35). There was no diabetes-related increase in breast cancer–specific mortality risk in women who had not received chemotherapy. Fleming et al23 did not find an increased risk for breast cancer–specific mortality at the 1-year follow-up in patients with diabetes.
Diabetes and Breast Cancer Stage
Of four studies that examined the influence of pre-existing diabetes on stage of breast cancer, three found a positive association.17–19,22 Fleming et al22 evaluated women older than 67 years with breast cancer using Surveillance, Epidemiology, and End Results-Medicare data and found an increased risk of late-stage disease in women with diabetes (OR, 1.17; 95% CI, 1.08 to 1.27). Srokowski et al19 demonstrated that a higher percentage of women with diabetes presented with a more advanced stage than their nondiabetic counterparts (47% v 42% stage II or III, P < .0001). In the study by van de Poll-Franse et al,18 it was found that patients with diabetes and breast cancer were diagnosed more often with stage III or IV disease (19% v 12%). In contrast, Yancik et al17 found no association between diabetes and breast cancer stage; however, a large number of patients in the study did not have a stage assignment.
Diabetes and Choice of Breast Cancer Treatment
Three studies demonstrated that physicians prescribed modified breast cancer treatment regimens for women with diabetes, compared with their nondiabetic counterparts.17–19 In the study by van de Poll-Franse et al,18 younger (age 35 to 65 years) patients with diabetes and breast cancer were reported to be more likely to receive surgery (OR, 2.32; 95% CI, 1.01 to 5.38; P < .05) and hormonal therapy (OR, 1.66; 95% CI, 1.18 to 2.31; P < .05) than their nondiabetic counterparts, but about half as likely to receive adjuvant chemotherapy (OR, 0.52; 95% CI, 0.36 to 0.75). Compared with their nondiabetic counterparts, older patients with breast cancer (> 65 years) and diabetes were less likely than their nondiabetic counterparts to receive radiotherapy (OR, 0.73; 95% CI, 0.60 to 0.88) and were less often treated with breast-conserving therapy (39% v 46%; P = .01). Among a cohort of patients receiving adjuvant chemotherapy, Srokowski et al19 found that women with diabetes were less likely to receive anthracyclines (OR, 0.78; 95% CI, 0.71 to 0.87) and taxanes (OR, 0.86; 95% CI, 0.75 to 0.99) compared with women without diabetes. Likewise, women with insulin-treated diabetes were less likely to undergo axillary lymph node dissection than their nondiabetic counterparts.17
Diabetes and Adverse Effects of Cancer Treatment
Srokowski et al19 analyzed data on 11,826 women with breast cancer who received adjuvant chemotherapy to assess toxicity. In this cohort, diabetes was associated with an increased risk of being hospitalized for any chemotherapy toxicity (OR, 1.38; 95% CI, 1.23 to 1.56), for infection or fever (OR, 1.43; 95% CI, 1.2 to 1.7), for neutropenia (OR, 1.22; 95% CI, 1.03 to 1.45), for anemia (OR,1.24; 95% CI, 1.05 to 1.47), and for any cause (OR, 1.32; 95% CI, 1.19 to 1.46).
Diabetes and Disease-Free Survival
A final outcome reported was the negative impact that diabetes may have on disease-free survival. Du et al21 found that diabetes had an adverse effect on disease-free survival in a cohort of African American and white women with stage I, II, or III breast cancer (HR, 1.81; 95% CI, 1.03 to 3.18).
DISCUSSION
Our systematic review demonstrates that, compared with their nondiabetic counterparts, patients with breast cancer and pre-existing diabetes suffer all-cause mortality that is approximately 50% higher. This finding was consistent across different populations, was generally independent of possible confounding variables, and was robust even after accounting for possible publication bias. Although this finding was consistent, it is important to note that these data do not necessarily suggest a causal relationship. Therefore, it is premature to conclude that diabetes prevention, improved glycemic control, and/or modification of diabetic pharmacotherapy would lead to improved prognoses. This study does, however, support the need for further research into this area.
Although all-cause mortality was increased in patients with breast cancer and diabetes, the association between breast cancer–specific mortality and diabetes is not clear. We identified two studies describing the impact of diabetes on breast cancer–specific mortality. Fleming et al23 observed no increase in breast cancer–specific mortality in patients with diabetes, whereas Srokowski et al19 identified increased breast cancer–specific mortality only in patients with diabetes receiving chemotherapy, a finding which suggests a potential interaction. This issue is further confounded by the findings of Lipscombe et al, 6 who reported similar mortality in patients with diabetes, with and without breast cancer. Analysis of the contribution of diabetes to breast cancer–specific mortality is difficult because of the substantial mortality attributed to diabetes alone. Data from the National Health and Nutrition Examination Survey24 (in a population not directly comparable with the populations from which our data are derived) suggest a higher relative risk of all-cause mortality for women with diabetes versus women without diabetes (relative risk, 2.84; 95% CI, 2.08 to 3.89) compared with the data on patients with breast cancer in our study (HR,1.49; 95% CI, 1.35 to 1.65). Nevertheless, the absolute risk difference in mortality related to diabetes exists in both populations, and our study findings suggest that diabetes has an important association with mortality in patients with breast cancer.
Given that it is unclear whether diabetes increases breast cancer–specific mortality, our systematic review suggests that diabetes is associated with adverse prognostic factors specific to breast cancer. First, women with diabetes may present with more advanced breast cancer.18,19,22 Because of the concurrent treatment of the chronic diseases associated with diabetes, patients may not undergo routine screening for breast cancer.25 Second, women with diabetes may receive less aggressive treatment, including chemotherapy, radiotherapy, and/or surgery.18,19 This may be related to their underlying comorbidities precluding treatment options or a perceived risk of toxicity from therapy in patients with diabetes. Third, women with pre-existing diabetes may have a greater risk of chemotherapy-related toxicity (eg, infection, fever, and neutropenia), as observed by Srokowksi et al.19 Such risk might explain and justify less aggressive treatment.
A fourth possible pathway was beyond the scope of our review: namely, that hyperinsulinemia related to underlying insulin resistance might stimulate tumor growth. Insulin may work directly on epithelial cells or indirectly by activating insulin-like growth factor pathways or altering endogenous sex hormones.9,26–29 Goodwin et al30 recently showed that metformin could be safely administered for a 6-month period to women with early-stage breast cancer and higher insulin levels, and reported a significant reduction in insulin levels, a modest (though significant) reduction in weight, and improvement in insulin sensitivity. Of interest, a recent case-control study by Monami et al31 showed that longer use of metformin and gliclazide was associated with a reduced cancer risk, whereas insulin and other oral agents had no effect, and glibenclamide was in fact associated with an increased cancer risk. These observations must now be prospectively tested, and a planned randomized trial in early-stage breast cancer (National Cancer Institute of Canada Clinical Trials Group MA.32) will examine the therapeutic effects of metformin on breast cancer recurrence and death.11
Strengths of this study include a comprehensive, systematic review of the literature by a multidisciplinary team including specialists in cancer, diabetes, and epidemiology, with each article reviewed by two team members, and the moderate to high quality of the studies included in our meta-analysis of all-cause mortality, with five of the six articles adjusting for key confounding variables.
Nonetheless, several limitations of the study deserve mention. First, despite our attempt to manage cross-study heterogeneity with appropriate meta-analytic techniques (eg, random-effects models), studies varied in their ascertainment of diabetes mellitus, study population, length of follow-up, and adjustment for confounding variables. Second, there was evidence of publication bias; however, based on our trim and fill analysis, we believe that this bias was minimal. Third, the method of diabetes ascertainment varied across studies and fasting blood glucose levels were not directly reported. These ascertainment methods may underestimate the number of women with diabetes, leading to potential misclassification bias, which is generally associated with underestimates of the effect. A fourth limitation is that the reviewed articles did not report the types of diabetic therapy used or their impact on outcomes. This is important because studies have shown that some therapies (eg, insulin, sulfonylureas) may have a negative impact on cancer outcomes, whereas others, such as metformin, may be beneficial.32 Additional research is needed to explore how specific diabetic therapies influence cancer prognosis. Finally, data regarding diabetes and the risk of adverse treatment effects and cancer recurrence were extremely sparse, limiting our ability to draw firm conclusions.
The main implication of our study is that diabetes mellitus is associated with adverse outcomes in breast cancer throughout its full course, from initial presentation, during treatment (affecting the choice of treatment), and, ultimately, to mortality. Diabetes therefore deserves additional attention to assess possible causal relationships that potentially could be modified to improve outcomes.
Footnotes
Supported in part by National Institutes of Health (NIH) T32 Training Grant No. T32 DK062707 (B.B.B., R.L.D.); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diabetes Research and Training Center Grant No. P60 DK079637 (H.-C.Y., F.L.B.); NIDDK Grant No. K24DK062222-06 (F.L.B.); NIH T32 Training Grant No. T32HP10025-14 (K.B.S.); and American Cancer Society Grant No. MRSG-08-011-01-CPPB (C.F.S.).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kimberly S. Peairs, Bethany B. Barone, Claire F. Snyder, Hsin-Chieh Yeh, Kelly B. Stein, Rachel L. Derr, Frederick L. Brancati, Antonio C. Wolff
Collection and assembly of data: Kimberly S. Peairs, Bethany B. Barone, Claire F. Snyder, Hsin-Chieh Yeh, Kelly B. Stein, Rachel L. Derr, Frederick L. Brancati, Antonio C. Wolff
Data analysis and interpretation: Kimberly S. Peairs, Bethany B. Barone, Claire F. Snyder, Hsin-Chieh Yeh, Kelly B. Stein, Rachel L. Derr, Frederick L. Brancati, Antonio C. Wolff
Manuscript writing: Kimberly S. Peairs, Bethany B. Barone, Claire F. Snyder, Hsin-Chieh Yeh, Frederick L. Brancati, Antonio C. Wolff
Final approval of manuscript: Kimberly S. Peairs, Bethany B. Barone, Claire F. Snyder, Hsin-Chieh Yeh, Kelly B. Stein, Rachel L. Derr, Frederick L. Brancati, Antonio C. Wolff
REFERENCES
- 1.Anderson GF, Chu E. Expanding priorities: Confronting chronic disease in countries with low income. N Engl J Med. 2007;356:209–211. doi: 10.1056/NEJMp068182. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2007. National Diabetes Statistics Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. [Google Scholar]
- 3.American Cancer Society. Atlanta, GA: American Cancer Society; 2008. Breast Cancer Facts and Figures 2007-2008. [Google Scholar]
- 4.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman TJ, Cuddihy RM, Scheitel SM, et al. Screening mammogram utilization in women with diabetes. Diabetes Care. 2001;24:2049–2053. doi: 10.2337/diacare.24.12.2049. [DOI] [PubMed] [Google Scholar]
- 6.Lipscombe LL, Goodwin PJ, Zinman B, et al. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109:389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 7.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, et al. If mammalian target of metformin indirectly is mammalian target of rapamycin, then the insulin-like growth factor-1 receptor axis will audit the efficacy of metformin in cancer clinical trials. J Clin Oncol. 2009;27:e207–e209. doi: 10.1200/JCO.2009.24.5456. author reply e210. [DOI] [PubMed] [Google Scholar]
- 9.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, et al. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18:701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: Time for action. J Clin Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 12.Barone BB, Yeh H-C, Snyder CF, et al. Long-term, all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analyisis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 18.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 19.Srokowski TP, Fang S, Hortobagyi GN, et al. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 21.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243–248. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 22.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132–140. doi: 10.1097/00005650-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Fleming ST, Rastogi A, Dmitrienko A, et al. A comprehensive prognostic index to predict survival based on multiple comorbidities: A focus on breast cancer. Med Care. 1999;37:601–614. doi: 10.1097/00005650-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gregg EW, Gu Q, Cheng YJ, et al. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 25.Maruthur NM, Bolen S, Brancati FL, et al. Obesity and mammography: A systematic review and meta-analysis. J Gen Intern Med. 2009;24:665–677. doi: 10.1007/s11606-009-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 27.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 28.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 31.Monami M, Lamanna C, Balzi D, et al. Sulphonylureas and cancer: A case-control study. Acta Diabetol. 2009;46:279–284. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 32.Bowker SL, Yasui Y, Veugelers P, et al. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: Assessing effects of time-varying exposure. Diabetologia. 2010;53:1631–1637. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]