Abstract
Purpose
To examine the association between serum C-peptide, a marker of insulin secretion, measured 3 years after a breast cancer diagnosis, and death resulting from all causes and breast cancer.
Patients and Methods
This was a prospective, observational study of 604 women enrolled onto the Health, Eating, Activity, and Lifestyle (HEAL) Study who were diagnosed with local or regional breast cancer between 1995 and 1998 and observed until death or December 31, 2006, whichever came first. The hazard ratio (HR) for all deaths and deaths owing to breast cancer and 95% CIs for the HR were estimated using multivariable stratified Cox regression analyses.
Results
Among women without type 2 diabetes, fasting C-peptide levels were associated with an increased risk of death resulting from all causes and from breast cancer. A 1-ng/mL increase in C-peptide was associated with a 31% increased risk of any death (HR = 1.31; 95% CI, 1.06 to 1.63; P = .013) and a 35% increased risk of death as a result of breast cancer (HR = 1.35; 95% CI, 1.02 to 1.87, P = .048). Associations between C-peptide levels and death as a result of breast cancer were stronger in certain subgroups, including women with type 2 diabetes, women with a body mass index less than 25 kg/m2, women diagnosed with a higher stage of disease, and women whose tumors were estrogen receptor positive.
Conclusion
Treatment strategies to reduce C-peptide levels in patients with breast cancer, including dietary-induced weight loss, physical activity, and/or use of insulin-lowering medications, should be explored.
INTRODUCTION
Numerous studies have shown obesity and low levels of physical activity to be associated with an increased risk of breast cancer.1–4 Obesity at diagnosis and low levels of physical activity after a diagnosis of breast cancer have been associated with an increased risk of recurrence and death in women with breast cancer.5–11 One potential mechanism mediating the association between obesity, physical activity, and breast cancer may be circulating insulin levels.12–14
An increased risk of breast cancer has been observed in women with high insulin levels and in women with type 2 diabetes.15–17 A recent publication from the Women's Health Initiative Observational Study showed that insulin levels were associated with breast cancer risk (hazard ratio [HR] for highest v lowest quartile of insulin level = 1.46; 95% CI, 1.00 to 2.13; P for trend = .02).18 Similar findings suggest that insulin is a risk factor for endometrial and colorectal cancer.19,20 Few studies have examined the association between circulating insulin or C-peptide levels (a marker of insulin production) and breast cancer recurrence or death.21 Goodwin et al21 examined the association between fasting insulin, measured within 3 months of diagnosis, and death in women with breast cancer. Their findings, adjusted for age, disease stage, treatment, and hormone receptor status, showed a clinically meaningful and statistically significant three-fold increased risk of breast cancer death in women with high versus low fasting insulin levels.
Given the limited number of studies examining insulin or C-peptide and breast cancer outcomes, and also the potential for confounding (eg, by obesity and physical activity), more studies are necessary to confirm the strong adverse association between insulin or C-peptide and death owing to all causes and to breast cancer. The purpose of our study was to examine the association between fasting C-peptide levels, assessed approximately 3 years after diagnosis, and subsequent death owing to all causes and breast cancer in an ethnically diverse sample of women diagnosed with stage I to IIIa breast cancer enrolled in the Health, Eating, Activity, and Lifestyle (HEAL) Study. We also examined associations stratified by important prognostic, demographic, and lifestyle variables, including age, body mass index (BMI), disease stage, and estrogen receptor status.
PATIENTS AND METHODS
Study Participants
The HEAL Study is a multicenter, multiethnic, prospective cohort study that has enrolled 1,183 women diagnosed with breast cancer to determine whether lifestyle, hormones, and other exposures affect breast cancer prognosis.22–24 Women were recruited through Surveillance, Epidemiology and End Results (SEER) registries in New Mexico, Los Angeles County, and Western Washington. Women with first primary breast cancer were contacted to determine eligibility. Details of the study have been published previously.22–24
Briefly, in New Mexico, we recruited 615 women aged 18 years or older who were diagnosed with in situ to regional breast cancer between July 1996 and March 1999, and living in Bernalillo, Sante Fe, Sandoval, Valencia, or Taos Counties. In Western Washington, we recruited 202 women between the ages of 40 and 64 years diagnosed with in situ to regional breast cancer between September 1997 and September 1998 and living in King, Pierce, or Snohomish Counties. In Los Angeles County, we recruited 366 black women aged 35 to 64 years with in situ to regional breast cancer who had participated in the Los Angeles portion of the Women's Contraceptive and Reproductive Experiences (CARE) Study, a case-control study of invasive breast cancer, or who had participated in a parallel case-control study of in situ breast cancer. Eligible participants from the two studies in Los Angeles included the subset of black women who were diagnosed with breast cancer between May 1995 and May 1998. Washington and Los Angeles restricted age eligibility because of competing studies or by design of the parent study.
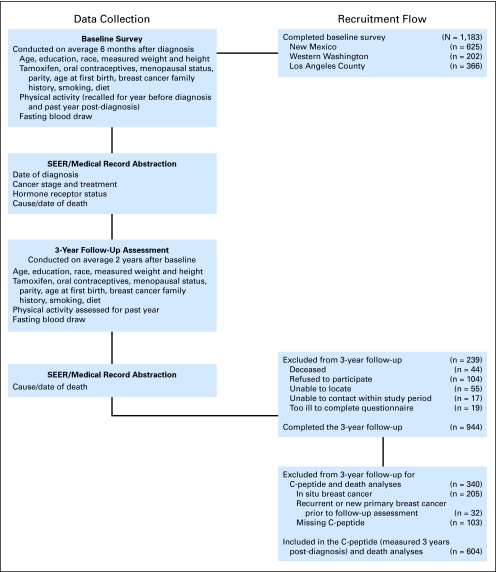
A total of 1,183 women completed in-person baseline interviews, which were conducted on average 6 months after diagnosis (Fig 1). A total of 944 women completed in-person interviews approximately 3 years after diagnosis. Of these, 105 women were excluded because of a diagnosis of in situ breast cancer, 32 women were excluded who had nonfatal breast cancer events less than 9 months before their 3-year postdiagnosis interview to avoid confounding from recent treatment, and 103 women did not have C-peptide results. The final sample is based on data for 604 participants.
Fig 1.
Participant recruitment and timing of data collection. SEER, Surveillance, Epidemiology and End Results.
Of the 604 women, 58 reported having a physician-diagnosis of type 2 diabetes. We analyzed postdiagnosis fasting C-peptide in relation to all causes of death and breast cancer deaths among 546 women without type 2 diabetes and also compared risk of death among women with type 2 diabetes with that among women without type 2 diabetes who had low C-peptide levels. We conducted additional analyses of all 604 women, including those with type 2 diabetes.
The study was performed with the approval of the institutional review boards of participating centers, in accord with assurances filed with and approved by the US Department of Health and Human Services.
Data Collection
Fasting C-peptide levels.
A 30-mL 12-hour fasting blood sample was collected at the 3-year follow-up visit. Blood was processed within 3 hours of collection; serum was stored in 1.8-mL aliquot tubes at −80°C. Assays were performed at the University of Southern California (USC) for California subjects. For the other two sites, C-peptide assays were conducted in the University of New Mexico (UNM) laboratory. All samples were randomly assigned to assay batches and randomly ordered within each batch. Personnel performing the assays were blinded to subject identity and characteristics.25 The C-peptide of Insulin 125I RIA kit from Incstar (Stillwater, MN) was used to measure C-peptide levels (sensitivity of 0.1 ng/mL).25 The intra-assay coefficient of variation for the USC-measured samples was 10.5% and for the UNM-measured samples was 9.4% and 5.6%, respectively for low and high BioRad standards.
Outcome assessment.
Follow-up ranged from 5 to 8 years from diagnosis, with a median follow-up of 6 years. Women were observed until death or December 31, 2006. Total and breast cancer mortality were defined as time from the 3-year postdiagnosis follow-up interview (when serum samples were collected) to death from any cause or end of follow-up (December 31, 2006). For breast cancer mortality, deaths from other causes were censored at date of death.
SEER records and death certificates were used to determine the vital status of participants. Prior literature indicates that breast cancer death is accurately reported on death certificates with confirmation rates that are greater than 93% when comparing death certificates with medical records.26
Covariates.
Trained staff measured weight with a digital scale and height with a stadiometer at the 3-year postdiagnosis clinic visit. Measurements were made with women wearing light indoor clothing and no shoes to the nearest 0.1 kg and 0.1 cm. All measurements were performed twice and averaged for a final value. BMI (weight in kilograms divided by height in meters squared) was calculated for all participants.
Medical history and demographic and lifestyle information were collected at the 3-year visit. Information on disease stage, hormone receptor status, adjuvant therapy, and hormonal therapy was abstracted from SEER records and medical records. Information on menopausal status (postmenopausal defined as no menses in the past 12 months), race/ethnicity, education, smoking status, family history of breast cancer, comorbidities (Charlson score) and physician-diagnosed type 2 diabetes were self-reported at the 3-year postdiagnosis visit. Physical activity level was determined using the interview-administered Modifiable Activity Questionnaire, developed by Kriska et al,27 which assessed various types and intensities of physical activity reported at the 3-year follow-up interview. For these analyses, metabolic equivalent hours per week of sports/recreational activities were included as a covariate in the analyses.
Statistical Analyses
Stratified Cox regression models were fit to our data. We estimated the HR and 95% CI for risk of death in one exposure group compared to a referent exposure group, adjusting for confounding factors. P values were estimated using the Wald test for trend.
We conducted analyses with C-peptide classified (1) in tertiles excluding women with type 2 diabetes; (2) in tertiles, but including a fourth category of women with type 2 diabetes; and (3) as a continuous variable, excluding women with type 2 diabetes. For categorical analyses, the reference group was women without type 2 diabetes and a fasting C-peptide level less than 1.7 ng/mL. Size of tertile categories may not be equal because of some women having similar C-peptide levels.
We evaluated the relationship between fasting C-peptide and death using age-adjusted models, age and BMI-adjusted models, and multivariable models. The multivariable model included age, BMI (< 18.5, 18.5 to 24.9, 25 to 29.9, and 30.0+ kg/m2), stage, treatment, estrogen receptor status, and race/site (African American women and USC, Hispanic women and UNM, non-Hispanic white women and Fred Hutchinson Cancer Research Center, and non-Hispanic white women and UNM). Other covariates considered, but not included in the final model, were menopausal status, education, physical activity, smoking status, Charlson score, first-degree family history of breast cancer, grade, and tamoxifen use. However, these variables did not significantly change the likelihood ratio score nor alter the HR by more than 10%; thus they were not included in the final model.
In addition, we also considered whether the association between postdiagnosis fasting C-peptide and death varied according to demographic and prognostic variables, including age, BMI, disease stage, and estrogen receptor status. All P values are two-sided, and all analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
RESULTS
Among the 604 women included in this analysis, there were 67 deaths, 33 as a result of breast cancer. Eighty percent of the non–breast cancer deaths were due to cardiovascular disease. When we excluded the 58 women with type 2 diabetes, 546 women remained. Among these women, there were 64 deaths, 28 as a result of breast cancer. The distribution of covariates according to tertile of C-peptide is shown in Table 1. Women with C-peptide levels more than 2.5 ng/mL were older, heavier, less physically active, and more likely to have a family history of breast cancer as compared with women with C-peptide levels less than 1.7 ng/mL.
Table 1.
Demographic, Physiologic, and Prognostic Characteristics of HEAL Participants
| Characteristic | C-Peptide < 1.7 ng/mL (n = 184) | C-Peptide 1.7 to 2.5 ng/mL (n = 187) | C-Peptide > 2.5 ng/mL (n = 175) | Type 2 Diabetes (n = 58) |
|---|---|---|---|---|
| C-peptide, ng/mL | ||||
| Mean | 1.32 | 2.14 | 3.49 | 2.77 |
| SD | 0.36 | 0.23 | 1.06 | 1.39 |
| Age, years | ||||
| Mean | 54.2 | 58.3 | 59.2 | 62.2 |
| SD | 10.5 | 10.2* | 11.6* | 8.3* |
| BMI, kg/m2 | ||||
| Mean | 24.5 | 26.1 | 30.3 | 32.5 |
| SD | 5.3 | 5.1 | 7.0*† | 6.7* |
| Postmenopausal, % | 67 | 81* | 83* | 90 |
| High school graduate, % | 96 | 96 | 95 | 93 |
| Race/ethnicity, % | ||||
| Black | 32 | 17 | 26 | 36 |
| Hispanic | 9 | 11 | 15 | 10 |
| Non-Hispanic white | 55 | 72 | 56 | 45 |
| Stage I, % | 75 | 73 | 68 | 62 |
| Treatment, % | ||||
| Surgery only | 21 | 23 | 27 | 21 |
| Radiation | 39 | 36 | 33 | 36 |
| Chemotherapy | 40 | 42 | 40 | 43 |
| ER positive, % | 68 | 76 | 71 | 64 |
| Tamoxifen use, % | 52 | 55 | 51 | 50 |
| Recreational PA, MET hr/wk | ||||
| Mean | 17.7 | 13.3 | 9.9 | 7.7 |
| SD | 24.3 | 17.7* | 14.4*† | 57.2* |
| Family history of breast cancer, % | 15 | 24* | 28* | 21 |
| Current smoker, % | 10 | 9 | 7*† | 10 |
Abbreviations: HEAL, Health Eating Activity and Lifestyle Study; SD, standard deviation; BMI, body mass index; ER, estrogen receptor; PA, physical activity.
Significantly different from C-peptide <1.7 ng/mL, P < .05.
Significantly different from C-peptide 1.7 to 2.5 ng/mL, P < .05.
Table 2 shows the age-adjusted, age- and BMI-adjusted, and multivariable-adjusted HRs for death due to all causes by postdiagnosis C-peptide levels. Six variables significantly changed the likelihood ratio score and the hazard ratio by 10% and are included as covariates: age, BMI, disease stage, estrogen receptor status, adjuvant treatment, and race/site. A 1-ng/mL increase in C-peptide was associated with a 31% increased risk of death (HR = 1.31; 95% CI, 1.06 to 1.63; P = .013) in the multivariable-adjusted model.
Table 2.
Fasting C-Peptide and Risk of Death Resulting From All Causes in Women Diagnosed With Breast Cancer
| C-Peptide Level | No. of Events | Total No. of Patients | Age-Adjusted Model |
Age and BMI-Adjusted Model |
Multivariable-Adjusted Model* |
|||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| C-peptide, ng/mL† | ||||||||
| < 1.7 | 19 | 184 | 1.00 | 1.00 | 1.00 | |||
| 1.7-2.5 | 22 | 187 | 0.91 | 0.49 to 1.69 | 1.06 | 0.57 to 1.99 | 0.91 | 0.46 to 1.80 |
| > 2.5 | 23 | 175 | 0.99 | 0.53 to 1.84 | 1.24 | 0.64 to 2.38 | 0.98 | 0.48 to 2.02 |
| P for trend | .98 | .53 | .96 | |||||
| C-peptide, ng/mL | ||||||||
| < 1.7 | 19 | 184 | 1.00 | 1.00 | 1.00 | |||
| 1.7-2.5 | 22 | 187 | 0.91 | 0.49 to 1.69 | 1.06 | 0.56 to 1.97 | 0.91 | 0.47 to 1.79 |
| > 2.5 | 23 | 175 | 0.98 | 0.53 to 1.83 | 1.26 | 0.66 to 2.41 | 1.04 | 0.52 to 2.11 |
| Women with type 2 diabetes | 13 | 58 | 1.59 | 0.78 to 3.26 | 2.24 | 1.02 to 4.89 | 1.47 | 0.64 to 3.39 |
| C-peptide, ng/mL (continuous)‡ | 64 | 546 | 1.24 | 1.03 to 1.49 | 1.36 | 1.11 to 1.64 | 1.31 | 1.06 to 1.63 |
| P | .026 | .0023 | .013 | |||||
Abbreviations: BMI, body mass index; HR, hazard ratio.
Adjustment variables included age, race/site, and initial treatment; stratification variables included BMI, disease stage, and estrogen receptor status.
C-peptide categories based on tertiles, excluding women with type 2 diabetes.
C-peptide (continuous) excludes women with type 2 diabetes. Range of C-peptide is 0.25 to 9.70 ng/mL.
Results examining the association between C-peptide and deaths owing to breast cancer are shown in Table 3. Women without type 2 diabetes but C-peptide levels more than 2.5 ng/mL, had a greater than two-fold increased risk of breast cancer death compared with women without type 2 diabetes but a C-peptide level less than 1.7 ng/mL (multivariable-adjusted HR = 2.39; 95% CI, 1.00 to 7.50; P for trend = .050). Results were slightly stronger when comparing women with type 2 diabetes with those in the reference group (ie, women without type 2 diabetes and a C-peptide levels < 1.7 ng/mL; multivariable-adjusted HR = 2.83; 95% CI, 1.03 to 11.69). Lastly, among women without type 2 diabetes, a 1-ng/mL increase in C-peptide was associated with a 35% increased risk of breast cancer death (multivariable-adjusted HR = 1.35; 95% CI, 1.02 to 1.87, P = .048).
Table 3.
Fasting C-Peptide and Risk of Death Resulting from Breast Cancer in Women Diagnosed With Breast Cancer
| C-Peptide Level | No. of Events | Total No. of Patients | Age-Adjusted Model |
Age and BMI-Adjusted Model |
Multivariable-Adjusted Model* |
|||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| C-peptide, ng/mL† | ||||||||
| < 1.7 | 7 | 184 | 1.00 | 1.00 | 1.00 | |||
| 1.7-2.5 | 11 | 187 | 1.70 | 0.65 to 4.44 | 1.96 | 0.74 to 5.22 | 2.19 | 0.74 to 6.47 |
| > 2.5 | 10 | 175 | 1.71 | 0.64 to 4.58 | 2.70 | 0.97 to 7.95 | 2.39 | 1.00 to 7.52 |
| P for trend | .28 | .072 | .050 | |||||
| C-peptide, ng/mL | ||||||||
| < 1.7 | 7 | 184 | 1.00 | 1.00 | 1.00 | |||
| 1.7-2.5 | 11 | 187 | 1.69 | 0.65 to 4.41 | 1.92 | 0.72 to 5.07 | 2.19 | 0.75 to 6.42 |
| > 2.5 | 10 | 175 | 1.70 | 0.64 to 4.54 | 2.67 | 0.98 to 7.68 | 2.55 | 0.82 to 7.89 |
| Women with type 2 diabetes | 5 | 58 | 2.81 | 0.86 to 9.23 | 4.69 | 1.30 to 16.91 | 2.83 | 1.03 to 11.69 |
| C-peptide, ng/mL (continuous)‡ | 28 | 546 | 1.21 | 0.92 to 1.58 | 1.37 | 1.04 to 1.82 | 1.35 | 1.02 to 1.87 |
| P | .18 | .027 | .048 | |||||
Abbreviations: BMI, body mass index; HR, hazard ratio.
Adjustment variables included age, race/site, and initial treatment; stratification variables included BMI, disease stage, and estrogen receptor status.
C-peptide categories based on tertiles, excluding women with type 2 diabetes.
C-peptide (continuous) excludes women with type 2 diabetes. Range of C-peptide is 0.25 to 9.70 ng/mL.
The adverse association between C-peptide and breast cancer death seemed to be even stronger in certain subgroups, including women with a BMI less than 25 kg/m2, women diagnosed with a higher stage of disease, and women whose tumors were estrogen receptor positive (P < .10 for interaction terms for C-peptide with BMI, stage, and estrogen receptor status; Table 4). However, associations between C-peptide and breast cancer death were similar among younger and older women (P = .68 for interaction for C-peptide with age). Taking multiple testing into account, statistical significance would be observed for a P value less than .05/8 = .00625.
Table 4.
Subgroup Analyses of Fasting C-Peptide (continuous) and Risk of Death Resulting From Breast Cancer
| C-Peptide Category | No. of Events | Total No. of Patients | Multivariable-Adjusted Model* |
P | |
|---|---|---|---|---|---|
| HR | 95% CI | ||||
| Age < 55 years† | 16 | 248 | 1.25 | 0.83 to 1.88 | .28 |
| Age ≥ 55 years | 12 | 298 | 1.42 | 0.73 to 2.75 | .30 |
| BMI 18.5 to 24.9 kg/m2 | 16 | 221 | 1.76 | 1.01 to 3.07 | .046 |
| BMI ≥ 25 | 11 | 294 | 0.97 | 0.60 to 1.57 | .90 |
| Stage I | 13 | 394 | 0.90 | 0.48 to 1.69 | .74 |
| Stage II to III | 15 | 152 | 1.58 | 1.04 to 2.40 | .034 |
| ER positive | 16 | 391 | 1.34 | 1.03 to 1.95 | .041 |
| ER negative | 10 | 107 | 0.90 | 0.39 to 2.09 | .81 |
Abbreviations: HR, hazard ratio; BMI, body mass index; ER, estrogen receptor.
Adjustment variables included age, race/site, and initial treatment; stratification variables included BMI, disease stage, and ER status. For each analysis, the adjustment or stratification variable of interest (eg, age) was not included as a covariate in the model.
C-peptide (continuous) excludes women with type 2 diabetes. Range of C-peptide is 0.25 to 9.70 ng/mL.
DISCUSSION
We found that women with high fasting C-peptide levels (values > 2.5 ng/mL) collected 3years after diagnosis) had more than a two-fold increased risk of breast cancer death compared with women with low C-peptide levels. Healthy or normal C-peptide levels are between 0.5 ng/mL and 2.0 ng/mL, and high levels indicate that the pancreas is producing too much insulin.28 Women who reported having physician-diagnosed type 2 diabetes were at an even higher risk of breast cancer death than women without type 2 diabetes and low C-peptide levels. However, because of wide 95% CIs in these categorical analyses, these results should be interpreted with caution. Yet the 95% CIs were quite narrow when examining analyses between continuous levels of C-peptide and death owing to all causes and breast cancer, perhaps because of increased statistical power. Thus the statistically significant and clinically meaningful continuous C-peptide analyses suggest a strong association with death owing to all causes and death resulting from breast cancer.
Our results of a positive association between postdiagnosis C-peptide levels and breast cancer death are similar to the one other published report by Goodwin et al21 examining this relationship. However, our analyses also adjusted for (adjuvant treatment and race) and stratified by (BMI, disease stage, and estrogen receptor status) covariates known to be associated with insulin or C-peptide levels and breast cancer outcomes. More specifically, although our sample size, number of deaths, and greater than two-fold increased risk of breast cancer death is similar to that of the results of Goodwin et al21 in 512 women (45 deaths, 42 from breast cancer; HR = 3.3; 95% CI, 1.5 to 7.0; P for trend = .002), Goodwin et al examined fasting insulin collected approximately 3 months after diagnosis and breast cancer death. C-peptide concentration may be a more stable measure of insulin production than circulating insulin levels and may have actions distinct from that of insulin.28 Whether collecting blood close to diagnosis rather than a couple years after diagnosis affects death differently is unknown. A strength of the 3-month postdiagnosis blood collection of Goodwin et al21 is the preadjuvant treatment assessment; however, a strength of a 3-year postdiagnosis blood collection is that it provides evidence that host factors long-term after diagnosis may play a role in determining risk of death in breast cancer survivors. Lastly, a limitation of both the study of Goodwin et al21 and our study that is women were enrolled and treated for breast cancer before 2000 and therefore before common use of aromatase inhibitors. Thus we are uncertain whether our results would be similar among women taking aromatase inhibitors; however, we did adjust for hormone receptor status.
When we examined C-peptide and breast cancer deaths in subgroup analyses by estrogen receptor status, a stronger association was observed among women with estrogen receptor–positive tumors. Stronger associations have been observed between physical activity, BMI, and breast cancer outcomes in women with estrogen receptor–positive tumors compared with women with estrogen receptor–negative tumors.5–11 Given that physical activity and BMI are associated with insulin and C-peptide levels in women with and without breast cancer,12–14 we hypothesized that associations between C-peptide and breast cancer death would be stronger in women with estrogen receptor–positive tumors. Fasting insulin and C-peptide levels may then explain the observed associations between BMI at diagnosis and postdiagnosis physical activity levels and death in women with breast cancer.
Associations between C-peptide and breast cancer death were hypothesized to be stronger in women with a higher versus lower BMI, but the opposite was observed in our study. This finding may be explained by the fact that, among leaner women (those with a BMI < 25 kg/m2), average C-peptide levels were significantly lower among women who were alive at follow-up (1.86 ng/mL, standard deviation [SD] = 0.70) than among those who had died (2.58 ng/mL, SD = 1.52). In contrast, average C-peptide levels were higher, less variable, and quite similar among women with a BMI more than 25 kg/m2 (2.59 ng/mL, SD = 1.20 and 2.57 ng/mL, SD = 1.10, for women alive and not alive at follow-up, respectively). Obesity is a strong predictor of insulin resistance and hyperinsulinemia, and we showed in our study that overweight and obese groups of women with breast cancer had higher C-peptide levels. It is possible that the influence of C-peptide on mortality could only be demonstrated in the lower BMI group because C-peptide levels in the overweight and obese groups were generally high and less variable.
Our study has several limitations. We collected one fasting blood draw and therefore cannot completely characterize the women's exposure to C-peptide. However, other studies indicate that a single fasting blood measure of this analyte is highly reproducible in fasting postmenopausal women. This limitation would bias the hazard ratio toward the null; thus we may have observed stronger associations between C-peptide and death with repeat measures of C-peptide. Also, we could not assess the effect of change in C-peptide on death, which would require testing interventions to change this analyte such as weight loss, physical activity, or medications to alter C-peptide or insulin resistance.
In summary, our study indicates that C-peptide is associated with increased risk of death among women with and without diabetes. Our findings emphasize the importance of maintaining a low C-peptide level. Physical activity is associated with C-peptide and insulin in women with or without breast cancer.12–14 Previous findings from the HEAL cohort indicated that moderate-intensity physical activity was associated with a lower risk of death.9 The current study complements this finding, indicating the importance of lower C-peptide levels and lower BMI. Thus participating in recommended levels of 2 to 3 hours/wk of moderate-intensity physical activity before and after a diagnosis of breast cancer may be beneficial to maintaining a low BMI and low C-peptide level, as well as decreased risk of death resulting from all causes and breast cancer. Randomized controlled trials examining changes in fasting C-peptide or insulin levels, via weight loss and physical activity, on breast cancer outcomes are needed. Although no trial has examined weight loss– or physical activity–induced changes in insulin or C-peptide on breast cancer outcomes, trials testing metformin on breast cancer outcomes are in progress.29,30
Acknowledgment
We thank the HEAL participants for their ongoing dedication to this study.
Footnotes
Supported by National Cancer Institute Grant Nos. N01-CN-75036-20, N01-CN-05228, and N01-PC-67010 and Training Grant No. T32-CA09661. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by the National Institutes of Health, Grant No. M01-RR-00037 and the University of New Mexico Grant No. NCRR M01-RR-0997. Data collection for the Women's CARE Study at the University of Southern California was supported by Grant No. N01-HD-3-3175 from the National Institute of Child Health and Human Development, and patient identification was supported in part by Grant No. 050Q-8709-S1528 from the California Department of Health Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Anne McTiernan, Richard N. Baumgartner, Leslie Bernstein, Rachel Ballard-Barbash
Financial support: Anne McTiernan, Richard N. Baumgartner, Leslie Bernstein, Rachel Ballard-Barbash
Administrative support: Anne McTiernan, Richard N. Baumgartner, Leslie Bernstein, Rachel Ballard-Barbash
Provision of study materials or patients: Anne McTiernan, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Collection and assembly of data: Melinda L. Irwin, Anne McTiernan, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Data analysis and interpretation: Melinda L. Irwin, Catherine Duggan, Ching-Yun Wang, Richard N. Baumgartner, Leslie Bernstein,Rachel Ballard-Barbash
Manuscript writing: Melinda L. Irwin, Catherine Duggan, Ching-Yun Wang, Ashley Wilder Smith, Anne McTiernan, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein, Rachel Ballard-Barbash
Final approval of manuscript: Melinda L. Irwin, Catherine Duggan, Ching-Yun Wang, Ashley Wilder Smith, Anne McTiernan, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein,Rachel Ballard-Barbash
REFERENCES
- 1.Thune I, Ferberg A. Physical activity and cancer risk: Dose-response and cancer, all sites and site specific. Med Science Sports Exerc. 2001;33:S530–S550. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 4.Neilson HK, Friedenreich CM, Brockton NT, et al. Physical activity and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 6.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 7.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 9.Irwin ML, Smith A, McTiernan A, et al. Influence of pre- and post-diagnosis physical activity on mortality in breast cancer survivors: The Health Eating Activity and Lifestyle (HEAL) Study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 11.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: Associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 13.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with c-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin ML, Varma K, Alvarez-Reeves, et al. Randomized controlled exercise trial on insulin and IGFs in breast cancer survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mink PJ, Shahar E, Rosamond W, et al. Serum insulin and glucose levels and breast cancer incidence: The atherosclerosis risk in communities study. Am J Epidemiol. 2002;156:349–352. doi: 10.1093/aje/kwf050. [DOI] [PubMed] [Google Scholar]
- 16.Michels KB, Solomon CG, Hu FB, et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 17.Del Giudice ME, Fantus IG, Ezzat S, et al. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998;47:111–120. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 18.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunter MJ, Hoover DR, Yu H, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factor for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:921–929. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin P, Ennis M, Pritchard K, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 22.Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a breast cancer diagnosis: The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin ML, McTiernan A, Baumgartner R, et al. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic and lifestyle factors. J Clin Oncol. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Percy C, Stanek E, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71:242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriska A. Modifiable activity questionnaire. Med Sci Sports Exerc. 1997;29:S73–S78. [Google Scholar]
- 28.Buse JB, Polonsky KS, Burant CF, et al. Type 2 diabetes mellitus. In: Kronenberg HM, Melmed S, Polonsky KS, et al., editors. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders Elsevier; 2008. pp. 363–390. [Google Scholar]
- 29.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, et al. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18:701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin P, Pritchard K, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]