Abstract
Purpose
Self-reported diabetes has been associated with poor breast cancer outcomes. Research is needed to investigate the relationship between biologically determined glycemic control and breast cancer prognosis.
Methods
Archived baseline blood samples from the Women's Healthy Eating and Living Study were used to measure hemoglobin A1C (HbA1C) among 3,003 survivors of early-stage breast cancer (age of diagnosis, 28 to 70 years) observed for a median of 7.3 years for additional breast cancer events and 10.3 years for all-cause mortality. HbA1C levels provide an accurate, precise measure of chronic glycemic levels. Cox regression analysis was performed to assess whether baseline HbA1C levels predicted disease-free and overall survival.
Results
Only 5.8% of women had chronic hyperglycemia (defined as HbA1C levels ≥ 6.5%). Those with HbA1C ≥ 6.5% were older and more likely to be less educated, have nonwhite ethnicity, be obese, and have more advanced breast cancer at diagnosis. HbA1C was significantly associated with overall survival (Ptrend < .001). After adjusting for confounders, risk of all-cause mortality was twice as high in women with HbA1C ≥ 7.0% compared with women with HbA1C less than 6.5% (hazard ratio [HR], 2.35; 95% CI, 1.56 to 3.54). For disease-free survival, there was a nonsignificant 30% increase in risk for HbA1C levels ≥ 7.0% (HR, 1.26; 95% CI, 0.78 to 2.02). During study follow-up, previously diagnosed rather than undiagnosed diabetes seemed to account for the increased risk.
Conclusion
Chronic hyperglycemia is statistically significantly associated with reduced overall survival in survivors of early-stage breast cancer. Further study of diabetes and its relationship to breast cancer outcomes is warranted.
INTRODUCTION
Type 2 diabetes mellitus (hereafter referred to as diabetes) is increasing rapidly in the population at large, and studies suggest that 16% to 20% of women who have had breast cancer have diagnosed diabetes as a comorbidity.1–3 Symptomless screening for diabetes is rare, and 30% of those with the disease may be undiagnosed.4 Some research estimates that diabetes can remain undiagnosed for 5 to 10 years, and therefore, disease symptoms or complications may accompany the diagnosis.5
A number of studies have indicated that diabetes is associated with higher mortality in patients with breast cancer,1–3,6,8–11 but it is unclear whether this is driven by a worse breast cancer prognosis or by competing risks such as cardiovascular disease. Recently, Patterson et al12 reported that self-reported diabetes was associated with more than a two-fold increase in both breast cancer events and all-cause mortality. Whether the relationship between diabetes and breast cancer prognosis would be strengthened, reduced, or maintained when women with undiagnosed diabetes are included is unclear. It is also uncertain whether the additional mortality risk is specific to breast cancer or reflects the general higher mortality risk ofdiabetes.13
The hemoglobin A1C (HbA1C) assay is the test of choice for monitoring diabetes management because it provides a precise measure of chronic glycemic levels. Research indicates that HbA1C may also be used to diagnose diabetes because a cut point value of 7.0% is associated with increased risk of microvascular complications.15 More recently, an International Expert Committee recommended the use of a cut point of 6.5% to definitively diagnose diabetes.14 However, the Endocrine Society has published their reservations about this recommendation.16
In this secondary analysis, we explore the association of HbA1C levels and cut points with breast cancer progression. We measured HbA1C levels in archived blood samples of participants in the Women's Healthy Eating and Living (WHEL) Study, a multisite randomized trial that tested the effect of an intensive dietary intervention on new breast cancer events and survival. For study outcomes, we consider both breast cancer disease-free survival and overall survival.
METHODS
Participants
Between 1995 and 2000, the WHEL Study enrolled 3,088 women within 4 years of diagnosis of early-stage breast cancer (American Joint Committee on Cancer stage I [≥ 1 cm], II, or IIIA). Exclusions included the diagnosis of a comorbidity requiring a specific diet or the use of a medication that contraindicated a high-fiber diet and insulin dependence. After an average of 7.3 years of follow-up, breast cancer and vital status were confirmed on 96% of the original cohort. Details of the study have been reported previously.17–18 Internal review boards at each site approved the study, and all participants provided written informed consent before enrolling.
Baseline Measures
Medical records pertaining to the initial cancer diagnosis were collected, and information on cancer characteristics and treatment was extracted and verified by an oncologist, including tumor stage and grade, tumor hormone receptor status, type of surgery, radiation, chemotherapy, and antiestrogen use. Weight and height were measured using standard procedures, and body mass index (BMI; weight in kilograms/height in meters squared) was calculated. Fasting blood was collected and separated using standard procedures and stored at −80°C. The study assessed demographics, self-reported menopausal status, and behavioral and lifestyle measures with standardized questionnaires. For this article, ethnicity was dichotomized into white/non-Hispanic and other (Hispanic/Latina, African American, Asian, Pacific Islander, and mixed/other).
Physical health, which was associated with prognosis in the WHEL Study,19 was assessed using the well-validated 36-item Short Form health survey.20 Following previous research, the physical health summary score was dichotomized as either low (bottom two quintiles) or moderate/high.19 The frequency, duration, and intensity of physical activity were assessed using nine items from the Women's Health Initiative Personal Habits Questionnaire, which were validated in a subsample of WHEL participants21 and converted into metabolic equivalent tasks as previously described.22 A self-administered health status questionnaire queried a series of physician-identified comorbid conditions (including prediabetes and diabetes requiring or not requiring insulin) and medications including insulin and oral hypoglycemics (blood sugar–lowering pills).
HbA1C
HbA1C was measured in September 2009 using ion exchange high-performance liquid chromatography (D-10 System; Bio-Rad Laboratories, Hercules, CA) on archived samples of washed RBCs collected at the baseline clinic visit. Performance of the D-10 HbA1C methodology was assessed by inclusion of known quality control samples with high (10.0%) and low (5.8%) HbA1C levels; the coefficients of variation were 1.5% and 1.6%, respectively, for within-day runs and 1.9% and 1.9%, respectively, for between-day runs. Laboratory personnel performing these assays were blinded to study outcomes.
Assessment of Study Outcomes
The primary outcome was overall survival, which was defined as the time from study entry (on average, 2 years since diagnosis) to death from any cause. Throughout the study, information about hospitalizations or new breast cancer events was obtained by semiannual telephone interviews. Any reported event/death led to a medical record/death certificate review by two independent study physicians. Breast cancer event–free survival (disease-free survival) was defined as the time from date of enrollment to the development of a new breast cancer event (ie, locoregional or distant breast cancer or new primary tumor). Follow-up time was censored at the last documented staff contact date or at study completion (June 2006). Mortality data were updated through September 2009 using the Social Security Death Index. To measure accuracy of Social Security Death Index matching, sensitivity analyses were performed on censoring cut points and supported the chosen approach. Median follow-up time was 7.3 years for breast cancer event–free survival and 10.3 years for overall survival.
Statistical Analysis
Initial analyses included a simple plot of the proportion of study events by HbA1C value. Kaplan-Meier curves of overall survival and disease-free survival were calculated for the three HbA1C categories (< 6.5%, 6.5% to 6.9%, and ≥ 7.0%) with differences assessed statistically by the log-rank test. Univariate Cox proportional hazards models examined effects on disease-free and overall survival for each of the following: HbA1C, demographic variables (age, ethnicity, education level, and marital status), tumor characteristics (stage, grade, and receptor status), breast cancer treatment history (years since diagnosis, ever-use of antiestrogen, and treatment with radiation, chemotherapy, lumpectomy, and mastectomy), and other health measures (menopausal status [pre-, peri-, or postmenopausal], BMI, physical health, and physical activity level). We also examined the interaction terms between HbA1C and each of the covariates, none of which were statistically significant (P > .05). A backward elimination model omitted covariates that either had a P > .05 or changed the HbA1C hazard ratio (HR) by less than 10%. The variables of ethnicity, age, education, physical activity, and physical health were retained based on a priori assumption. The assumption of proportional hazards was checked for each model using plots of time-dependent coefficients estimated from Schoenfeld residuals. To explore how self-reported diabetes factored into the association between HbA1C and breast cancer events, the frequency of breast cancer events in each HbA1C category was counted and stratified by self-report diabetes status and use of blood sugar–lowering medication. All tests were two-tailed, and analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Variables Associated With HbA1C Level
HbA1C levels were measured on 97% of the study cohort (3,003 of 3,088 patients) and ranged from 4.2% to 13.9% (median, 5.6%). Most women (93.8%) had an HbA1C level less than 6.5%, 3.1% had a level of 6.5% to 6.9%, and 3% had a level ≥ 7% (Table 1). Participants with HbA1C levels ≥ 6.5% were on average 3.5 years older (SE, 0.65 year; P < .001), less likely to be college educated (P < .05), and more likely to be sedentary (P < .01) than participants with HbA1C less than 6.5%. White/non-Hispanic participants were less likely than other participants to have HbA1C levels ≥ 6.5% (P < .001). Other variables strongly associated with greater HbA1C category were higher BMI, poor physical health, and higher stage breast cancer (all P < .001).
Table 1.
Participant Demographics and Clinical Characteristics by Baseline HbA1C in a Cohort of US Breast Cancer Survivors
| Characteristic | HbA1C |
P | |||||
|---|---|---|---|---|---|---|---|
| < 6.5% (n = 2,818) |
6.5% to 6.9% (n = 94) |
≥ 7.0% (n = 91) |
|||||
| No. of Participants | % | No. of Participants | % | No. of Participants | % | ||
| Demographics | |||||||
| Age, years | < .001 | ||||||
| Mean | 50.6 | 54.1* | 53.7* | ||||
| SE | 8.79 | 8.64 | 8.23 | ||||
| Ethnicity/race | < .001 | ||||||
| White, non-Hispanic | 2,436 | 86.4 | 72 | 76.6 | 61 | 67.0 | |
| Nonwhite | 382 | 13.6 | 22 | 23.4 | 30 | 33.0 | |
| College educated | 1,545 | 54.8 | 44 | 46.8 | 40 | 44.4 | .017 |
| Married | 1,989 | 71.1 | 57 | 62.0 | 59 | 65.6 | .095 |
| Breast cancer characteristics | |||||||
| Cancer stage at diagnosis | < .001 | ||||||
| I | 1,109 | 39.4 | 33 | 35.1 | 25 | 27.8 | |
| II | 1,579 | 56.0 | 51 | 54.3 | 55 | 61.1 | |
| IIIA | 130 | 4.6 | 10 | 10.6 | 10 | 11.1 | |
| Grade | .978 | ||||||
| 1 | 1,127 | 40.0 | 39 | 41.5 | 39 | 43.3 | |
| 2 | 1,018 | 36.1 | 31 | 33.0 | 29 | 32.2 | |
| 3 | 231 | 8.2 | 9 | 9.6 | 7 | 7.8 | |
| Tumor receptor status | .336 | ||||||
| ER positive/PR positive | 1,733 | 62.9 | 62 | 66.7 | 56 | 62.9 | |
| ER positive/PR negative | 338 | 12.3 | 8 | 8.6 | 10 | 11.2 | |
| ER negative/PR positive | 119 | 4.3 | 1 | 1.1 | 7 | 7.9 | |
| ER negative/PR negative | 565 | 20.5 | 22 | 23.7 | 16 | 18.0 | |
| Treatment | |||||||
| Radiation | 1,729 | 61.4 | 51 | 54.3 | 68 | 75.6 | .008 |
| Chemotherapy | 1,963 | 69.7 | 65 | 69.2 | 63 | 70.0 | .991 |
| Lumpectomy | 1,354 | 48.0 | 36 | 38.3 | 51 | 56.7 | .044 |
| Mastectomy | 1,463 | 51.9 | 58 | 61.7 | 39 | 43.3 | .044 |
| Ever-use of antiestrogen | 1,909 | 63.7 | 71 | 75.5 | 67 | 74.4 | .128 |
| Years since diagnosis | .901 | ||||||
| Mean | 2.0 | 2.0 | 2.0 | ||||
| SE | 1.04 | 1.04 | 0.97 | ||||
| Other health measures | |||||||
| Body mass index, kg/m2 | < .001 | ||||||
| Mean | 26.9 | 32.1* | 34.2*† | ||||
| SE | 5.70 | 7.51 | 8.58 | ||||
| Obese (≥ 30 kg/m2) | 681 | 24.2 | 52 | 55.3 | 63 | 70.0 | < .001 |
| Poor physical health | 1,169 | 41.5 | 51 | 54.3 | 56 | 62.2 | < .001 |
| Sedentary (< 150 MET-min/wk) | 545 | 19.3 | 30 | 31.9 | 26 | 28.6 | .001 |
| Menopausal status | .026 | ||||||
| Premenopausal | 328 | 11.7 | 3 | 3.2 | 5 | 5.6 | |
| Postmenopausal | 2,230 | 79.3 | 85 | 90.4 | 77 | 85.6 | |
| Perimenopausal | 256 | 9.1 | 6 | 6.4 | 8 | 8.9 | |
Abbreviations: HbA1C, hemoglobin A1C; ER, estrogen receptor; PR, progesterone receptor; MET, metabolic equivalent.
P < .05 for comparison with HbA1C < 6.5% category.
P < .05 for comparison with HbA1C 6.5% to 6.9% category.
Additional Breast Cancer Events and All-Cause Mortality
As of June 2006, 503 participants had a breast cancer event over the median 7.3 years of follow-up (Table 2). The majority of events were distant recurrences (n = 344, 68%), followed by locoregional recurrences (n = 81, 16%) and new breast primary tumors (n = 78, 16%). As of September 2009, 414 deaths were recorded over a median follow-up time of 10.3 years. The majority of deaths were a result of breast cancer (n = 331, 80%), followed by other cancer (n = 41, 10%), other causes (n = 22, 5%), and heart disease (n = 11, 3%).
Table 2.
Association of Baseline HbA1C and Outcomes in a Cohort of US Breast Cancer Survivors Observed for a Median of 7.3 Years for Breast Cancer Events and a Median of 10.3 Years for All-Cause Mortality
| Outcome | HbA1C |
|||||
|---|---|---|---|---|---|---|
| < 6.5% (n = 2,818) |
6.5% to 6.9% (n = 94) |
≥ 7.0% (n = 91) |
||||
| No. of Participants | % | No. of Participants | % | No. of Participants | % | |
| Breast cancer events (n = 503) | 466 | 16.5 | 18 | 19.1* | 19 | 20.9† |
| New primary tumor | 73 | 2.6 | 3 | 3.2 | 2 | 2.2 |
| Locoregional recurrence | 75 | 2.7 | 3 | 3.2 | 3 | 3.3 |
| Distant recurrence | 318 | 11.3 | 12 | 12.8 | 14 | 15.6 |
| All-cause mortality (n = 414) | 368 | 13.1 | 18 | 19.1‡ | 28 | 30.8§‖ |
| Breast cancer | 307 | 10.9 | 11 | 11.7 | 13 | 14.4 |
| Other cancer | 34 | 1.2 | 3 | 3.2 | 4 | 4.4 |
| Heart disease | 5 | 0.2 | 2 | 2.1 | 4 | 4.4 |
| Other | 15 | 0.5 | 2 | 2.1 | 5 | 5.5 |
| Unknown | 7 | 0.2 | 0 | 0.0 | 2 | 2.2 |
Abbreviation: HbA1C, hemoglobin A1C.
Comparison with HbA1C < 6.5% category, Cox model P = .44.
Comparison with HbA1C < 6.5% category, Cox model P = .15.
Comparison with HbA1C < 6.5% category, Cox model P = .05.
Comparison with HbA1C < 6.5% category, Cox model P < .001.
Comparison with HbA1C 6.5% to 6.9% category, Cox model P = .04.
Unadjusted Analyses
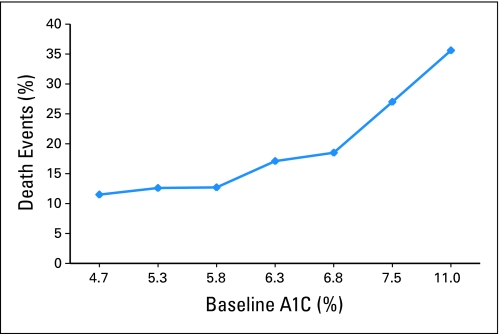
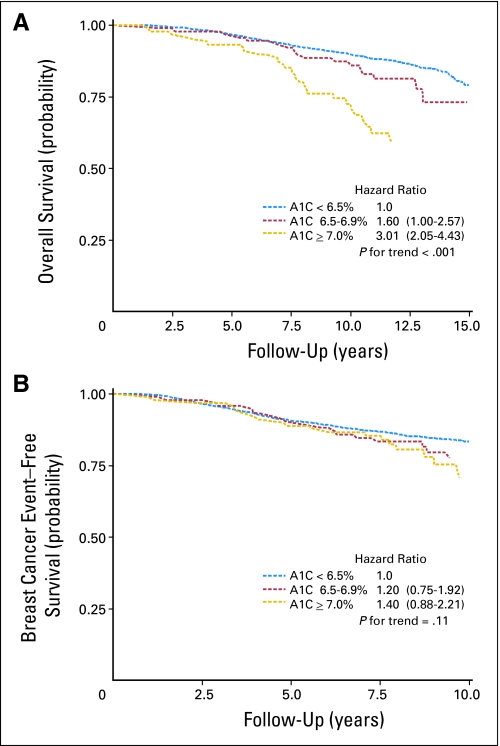
Figure 1 shows the proportion of all-cause mortality for seven categories of HbA1C and displays a marked increase in events for women with HbA1C ≥ 7.0%, with 35% of women with HbA1C greater than 8.0% dying within the follow-up period. The unadjusted HR for continuous HbA1C (per one-unit increase) and overall survival was 1.29 (95% CI, 1.16 to 1.43). The Kaplan-Meier curve for overall survival displays a statistically significant association among the three HbA1C categories (Fig 2A). Compared with women with HbA1C of less than 6.5%, women with HbA1C of 6.5% to 6.9% and ≥ 7.0% were 60% (HR, 1.6; 95% CI, 1.00 to 2.57) and three times (HR, 3.01; 95% CI, 2.05 to 4.43) more likely to die during follow-up, respectively.
Fig 1.
All-cause mortality events and baseline hemoglobin A1C (A1C) in a cohort of US breast cancer survivors with a median of 10.3 years of survival follow-up (N = 3,003).
Fig 2.
Kaplan-Meier estimates of (A) overall survival with a median of 10.3 years of survival follow-up and (B) breast cancer event–free survival with a median of 7.3 years of follow-up according to baseline hemoglobin A1C (A1C) in a cohort of US breast cancer survivors (N = 3,003).
The unadjusted HR for continuous HbA1C (per one-unit increase) and disease-free survival was 1.00 (95% CI, 0.86 to 1.14). The Kaplan-Meier curve for breast cancer disease-free survival (Fig 2B) indicates that participants with an HbA1C ≥ 7.0% had a nonsignificantly higher event rate (40%) compared with women with HbA1C less than 6.5% (HR, 1.40; 95% CI, 0.88 to 2.21). The increase in risk across HbA1C categories was not statistically significant (P = .11).
Adjusted Analyses
After adjustment for stage, grade, age, ethnicity, education, physical activity, and physical health, the HR for continuous HbA1C (per one-unit increase) and overall survival was 1.20 (95% CI, 1.07 to 1.34). In the fully adjusted model, the risk of death for women with HbA1C of 6.5% to 6.9% was no longer statistically significant; however, HbA1C ≥ 7% was associated with a 2.4-fold increase in risk (HR, 2.35; 95% CI, 1.56 to 3.54; Table 3). In models adjusting for the same covariates, neither continuous nor categorical HbA1C was significantly associated with risk of breast cancer recurrence (Table 3).
Table 3.
Multivariate HRs of Overall Survival and Disease-Free Survival According to Baseline HbA1C in a Cohort of US Breast Cancer Survivors With a Median of 10.3 Years of Survival Follow-Up and a Median of 7.3 Years of Follow-Up for Additional Breast Cancer Events (N = 3,003)
| HbA1C Category, % | No. of Patients | No. of Deaths | Overall Survival |
No. of Breast Cancer Events | Disease-Free Survival |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||
| < 6.5 | 2,818 | 368 | 1.00* | — | 466 | 1.00* | — | ||
| 6.5-6.9 | 94 | 18 | 1.33 | 0.82 to 2.16 | .25 | 18 | 1.11 | 0.69 to 1.80 | .67 |
| ≥ 7.0 | 91 | 28 | 2.35 | 1.56 to 3.54 | < .001 | 19 | 1.26 | 0.78 to 2.02 | .34 |
NOTE. Analysis was adjusted for stage, grade, age, ethnicity, education, physical activity, and physical health.
Abbreviations: HR, hazard ratio; HbA1C, hemoglobin A1C.
Reference category.
HbA1C Versus Self-Reported Diabetes in Relation to Breast Cancer Events
To investigate how undiagnosed diabetes factored into the association between HbA1C and breast cancer events, the distribution of breast cancer events by HbA1C category was stratified by self-report diabetes status. Of the 3% of participants with HbA1C ≥ 7.0%, less than half (37 of 91 participants) reported that they had diabetes on the baseline self-report questionnaire, and only 10% (nine of 94 participants) of the 3% of participants with HbA1C of 6.5% to 6.9% reported diabetes (Table 4). The majority of women (76.8%) who reported diabetes also indicated using blood sugar–lowering medication, and notably, these women had a two-fold higher rate of additional breast cancer events than women who did not report diabetes (32.6% v 15.6%, respectively). Of the 13 women who reported diabetes and no use of blood sugar–lowering medication, two experienced breast cancer events, and both had an HbA1C ≥ 7.0%; this computes to an incidence of 15.4%, which is nearly identical to the incidence of 15.6% found in women who did not report diabetes.
Table 4.
Breast Cancer Events and HbA1C Stratified by Self-Reported Type 2 Diabetes Mellitus in a Cohort of US Breast Cancer Survivors (N = 3,088)
| Self-Report of Diabetes | HbA1C |
Total Events |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 6.5% (n = 2,818) |
6.5% to 6.9% (n = 94) |
≥ 7.0% (n = 91) |
Missing HbA1C (n = 85) |
||||||||||||
| No. of Participants | No. of Breast Cancer Events | % | No. of Participants | No. of Breast Cancer Events | % | No. of Participants | No. of Breast Cancer Events | % | No. of Participants | No. of Breast Cancer Events | % | No. of Participants | No. of Breast Cancer Events | % | |
| No diabetes | 2,287 | 353 | 15.4 | 71 | 14 | 19.7 | 47 | 7 | 14.9 | 76 | 17 | 18.4 | 2,481 | 391 | 15.6 |
| Diabetes, no medication | 5 | 0 | 0 | 2 | 0 | 0 | 6 | 2 | 33.3 | 0 | 0 | 0 | 13 | 2 | 15.3* |
| Diabetes, medication | 4 | 2 | 50 | 7 | 2 | 28.6 | 31 | 10 | 32.2 | 1 | 0 | 0 | 43 | 14 | 32.6* |
| Missing† | 522 | 109 | 21 | 14 | 2 | 14 | 7 | 0 | 0 | 8 | 1 | 12.5 | 551 | 112 | 20.3‡ |
Abbreviation: HbA1C, hemoglobin A1C.
Because of small numbers, diabetes strata were combined (total events, n = 16/56, 28.6%; comparison with no diabetes, Cox model P = .01).
Includes five women who completed the baseline health status questionnaire but did not complete question on diabetes.
Comparison with no diabetes, Cox model P = .65.
DISCUSSION
Compared with women with HbA1C less than 6.5%, women with HbA1C ≥ 7.0% had a statistically significant 2.4-fold greater risk of all-cause mortality during the median 10.3 years of follow-up. This association of higher HbA1C with worse overall survival was independent of age, race, BMI, cancer stage and grade, physical health, and physical activity and is similar in magnitude to previous WHEL Study findings using self-reported diabetes as the measure of exposure.12 However, women with HbA1C ≥ 7.0% had a 26% higher rate of additional breast cancer events compared with women with HbA1C less than 6.5%, rather than the significant doubling of risk identified with self-reported diabetes.12 Although a 26% increase in risk is clinically meaningful, this study did not have the power to detect an adjusted HR of 1.26 as statistically significant.
Despite the growing body of evidence that diabetes predicts a poor prognosis after a diagnosis of breast cancer, two important questions remain to be answered. Is there a threshold of glycemic status at which the risk for poor prognosis significantly increases? Is the increased mortality risk among breast cancer survivors with diabetes driven by an increase in cancer recurrence or a result of competing diabetes-related comorbidities such as cardiovascular disease? Findings in the present study suggest that HbA1C may be associated with breast cancer prognosis in a nonlinear fashion, that is, a threshold effect may exist in the diabetic range of HbA1C levels ≥ 7.0% and in individuals considered at high risk for diabetes (those who are obese and have a high HbA1C in addition to at least one other risk factor for diabetes). Eighty percent of the deaths in this study cohort were a result of breast cancer, and because of the much smaller number of non–breast cancer deaths, power was lacking to formally evaluate whether non–cancer-related deaths accounted for the statistically significant difference in overall survival time among the three HbA1C levels.
In this large study of breast cancer survivors, measured HbA1C more than doubled the number of women with diabetes compared with self-report identification; however, inclusion of these women with undiagnosed diabetes attenuated the previously identified diabetes association with additional breast cancer events.12 Diagnosis of type 2 diabetes is most likely to occur in women experiencing symptoms. Therefore, it is likely that women with self-reported diabetes had longer disease duration and a history of worse glycemic control than women identified by HbA1C assays. Thus, our results could reflect the effect of severity and duration of diabetes on the risk of additional breast cancer events. Supporting this hypothesis, women who reported taking blood sugar–lowering medications for their diabetes (presumably reflective of more advanced disease) carried the highest risk of additional breast cancer events and mortality.
Diabetes may directly influence breast cancer progression and outcomes via several mechanisms including pathways mediated by high levels of insulin and insulin-like growth factors, sex hormones, and inflammatory markers. Both inflammation and obesity have biologic effects that could promote cancer, and the hyperinsulinemia that is associated with these conditions may itself augment cell proliferation and survival.23–25 Clinical studies support this thesis. For example, Goodwin et al26 reported that nondiabetic women whose fasting insulin levels were in the highest compared with lowest quartile were at a three-fold increased risk of death after breast cancer independent of BMI. Other studies using markers of insulin resistance, such as elevated waist-to-hip ratio27 and presence of metabolic syndrome,28 also found associations with a worse prognosis after breast cancer.
A diagnosis of diabetes may have indirect adverse effects on breast cancer outcomes by influencing medical decision making regarding breast cancer screening and management.29 Studies have documented reduced breast cancer screening rates among diabetic women,30 leading to later stage at diagnosis. Postmenopausal patients with breast cancer and diabetes frequently have one or more pre-existing comorbid conditions at diagnosis,31 often leading clinicians to follow less aggressive cancer treatments32–36 associated with lower survival rates.2 We identified women with diabetes as more likely to have a later cancer stage at diagnosis and controlled for it in multivariate models but found no statistically significant differences in chemotherapy treatment or antiestrogen use.
Two other studies in addition to the earlier WHEL Study12 found that the presence of comorbidities negatively affected breast cancer survival,1,37 with diagnosed diabetes exerting a negative effect on survival independent of disease stage at cancer diagnosis.1,37 Examining diabetes specifically, Lipscombe et al9 conducted a population-based study evaluating the effect of diagnosed diabetes on breast cancer survival after adjusting for comorbidity. The study found that diabetes was associated with a nearly 40% increase in 5-year all-cause mortality, similar to that seen in diabetic women without breast cancer, suggesting that breast cancer survival is reduced in women with diabetes as a result of diabetes-related causes rather than direct effects of diabetes on cancer outcomes. In contrast, a study by Fleming et al38 did not find diabetes to be a significant risk factor for increased mortality in patients with breast cancer. However, that study only examined 1-year mortality.
Diabetes is part of a cluster of problems, and the present study was unable to differentiate whether the effects observed were specific to HbA1C levels. Insulin dependence and diet restrictions were exclusion criteria for the WHEL Study, so our findings cannot be generalized to these subpopulations. Another subgroup with limited representation was women taking oral hypoglycemic medications; at baseline, the study had 43 such participants. Given the long natural history of recurrence in estrogen receptor–positive breast cancer, it would be important to examine the role of HbA1C in predicting late breast cancer events. At the completion of the main study in 2006, many participants did not reconsent for active follow-up. Thus, we limited reporting of additional breast cancer events to the average 7.3 years of the main study. However, we continued with passive follow-up for survival using the Social Security Death Index, thus strengthening the study with an additional 3 years of follow-up for this outcome. Additional strengths of this study include the reliable, accurate measurement of long-term glycemia with HbA1C assays, high rate of participant response, minimal missing blood samples, detailed and verified patient data on tumor and treatment characteristics extracted directly from medical records, and cancer events confirmed by two independent oncologists.
In summary, we found that chronic hyperglycemia, as indicated by elevated HbA1C levels, is independently associated with a statistically significant higher risk of all-cause mortality in breast cancer survivors. We also show evidence that a large percentage of breast cancer survivors who have diabetes do not know or do not report having diabetes. Of the women with HbA1C levels ≥ 7.0%, 60% did not report having diabetes, and even more striking, 90% of the women with HbA1C levels between 6.5% and 7.0% did not report having diabetes or prediabetes.
Diabetes, hyperglycemia, hyperinsulinemia, and associated metabolic disorders can be controlled and may present an opportunity for improving prognosis in early-stage breast cancer survivors. These data suggest that clinicians should consider measuring HbA1C in patients with breast cancer with symptoms of hyperglycemia or those at high risk for diabetes. These findings are hypothesis generating, and this association requires replication before HbA1C is routinely introduced into clinical practice. Nonetheless, randomized trials of interventions that target glycemic control in relation to both disease-free and overall survival end points may be warranted in this population.
Acknowledgment
Women's Healthy Eating and Living (WHEL) Study Coordinating Center: University of California, San Diego, Cancer Prevention and Control Program, Moores Cancer Center, La Jolla, CA: John P. Pierce, PhD; Susan Faerber, BA; Barbara A. Parker, MD; Loki Natarajan, PhD, Cheryl L. Rock, PhD; Vicky A. Newman, MS; Shirley W. Flatt, MS; Sheila Kealey, MPH; Ruth Patterson, PhD; Linda Wasserman, MD; Wayne A. Bardwell, PhD; Lisa Madlensky, PhD; Wael Al-Delaimy, MD.
WHEL Study Clinical Sites: Center for Health Research-Portland, Portland, OR (Njeri Karanja, PhD; Mark U. Rarick, MD); Kaiser Permanente Northern California, Oakland, CA (Bette J. Caan, DrPH; Lou Fehrenbacher, MD); Stanford Prevention Research Center, Stanford University, Stanford, CA (Marcia L. Stefanick, PhD; Robert Carlson, MD); University of Arizona, Tucson and Phoenix, AZ (Cynthia Thomson, PhD; James Warneke, MD); University of California, Davis, Davis, CA (Ellen B. Gold, PhD; Sidney Scudder, MD); University of California, San Diego, Moores Cancer Center, La Jolla, CA (Kathryn A. Hollenbach, PhD; Vicky Jones, MD); and The University of Texas MD Anderson Cancer Center, Houston, TX (Lovell A. Jones, PhD; Richard Hajek, PhD; Richard Theriault, DO).
Footnotes
The Women's Healthy Eating and Living (WHEL) Study was initiated with the support of the Walton Family Foundation and continued with funding from National Cancer Institute Grant No. CA 69375 and Komen Grant No. 100988. Some of the data were collected from General Clinical Research Centers, National Institutes of Health Grants No. M01-RR00070, M01-RR00079, and M01-RR00827.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kirsten Erickson, Ruth E. Patterson, Loki Natarajan, Barbara A. Parker, Cheryl L. Rock, John P. Pierce
Financial support: John P. Pierce
Administrative support: John P. Pierce
Provision of study materials or patients: John P. Pierce
Collection and assembly of data: Kirsten Erickson, Shirley W. Flatt, Dennis D. Heath, John P. Pierce
Data analysis and interpretation: Kirsten Erickson, Ruth E. Patterson, Shirley W. Flatt, Loki Natarajan, Barbara A. Parker, Cheryl L. Rock
Manuscript writing: Kirsten Erickson, Ruth E. Patterson, Shirley W. Flatt, Barbara A. Parker, Dennis D. Heath, Gail A. Laughlin, Nazmus Saquib, Cheryl L. Rock, John P. Pierce
Final approval of manuscript: Kirsten Erickson, Ruth E. Patterson, Shirley W. Flatt, Loki Natarajan, Barbara A. Parker, Dennis D. Heath, Gail A. Laughlin, Nazmus Saquib, Cheryl L. Rock, John P. Pierce
REFERENCES
- 1.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 2.Srokowski TP, Fang S, Hortobagyi GN, et al. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 4.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris MI, Klein R, Welborn TA, et al. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 6.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Lipscombe LL, Goodwin PJ, Zinman B, et al. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109:389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867–1876. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 11.Giordano SH, Jiralerspong S, Lopez A, et al. Diabetes, obesity and survival in a large cohort of early-stage breast cancer patients. J Clin Oncol. 2010;28(suppl 15S):154s. doi: 10.1093/annonc/mdt224. abstr 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson RE, Flatt SW, Saquib N, et al. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122:859–865. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 14.Gillett MJ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007;30:2233–2235. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 16.Endocrine Society. The Endocrine Society statement on the use of A1C for diabetes diagnosis and risk estimation. http://www.endo-society.org/advocacy/upload/TES-Statement-on-A1C-Use.pdf.
- 17.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 19.Saquib N, Pierce JP, Saquib J. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. doi: 10.1002/pon.1742. [epub ahead of print on March 31, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Johnson-Kozlow M, Rock CL, Gilpin EA, et al. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Bardwell WA, Natarajan L, et al. Correlates of physical activity level in breast cancer survivors participating in the Women's Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101:225–232. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 23.Belfiore A, Frittitta L, Costantino A, et al. Insulin receptors in breast cancer. Ann N Y Acad Sci. 1996;784:173–188. doi: 10.1111/j.1749-6632.1996.tb16235.x. [DOI] [PubMed] [Google Scholar]
- 24.Mathieu MC, Clark GM, Allred DC, et al. Insulin receptor expression and clinical outcome in node-negative breast cancer. Proc Assoc Am Physicians. 1997;109:565–571. [PubMed] [Google Scholar]
- 25.Papa V, Belfiore A. Insulin receptors in breast cancer: Biological and clinical role. J Endocrinol Invest. 1996;19:324–333. doi: 10.1007/BF03347871. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Borugian MJ, Sheps SB, Kim-Sing C, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol. 2003;158:963–968. doi: 10.1093/aje/kwg236. [DOI] [PubMed] [Google Scholar]
- 28.Pasanisi P, Berrino F, De Petris M, et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 29.Tabaei BP, Herman WH, Jabarin AF, et al. Does diabetes care compete with the provision of women's preventive care services? Diabetes Care. 2005;28:2644–2649. doi: 10.2337/diacare.28.11.2644. [DOI] [PubMed] [Google Scholar]
- 30.Beckman TJ, Cuddihy RM, Scheitel SM, et al. Screening mammogram utilization in women with diabetes. Diabetes Care. 2001;24:2049–2053. doi: 10.2337/diacare.24.12.2049. [DOI] [PubMed] [Google Scholar]
- 31.Yancik R, Ries LG, Yates JW. Breast cancer in aging women: A population-based study of contrasts in stage, surgery, and survival. Cancer. 1989;63:976–981. doi: 10.1002/1097-0142(19890301)63:5<976::aid-cncr2820630532>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Satariano WA. Comorbidity and functional status in older women with breast cancer: Implications for screening, treatment, and prognosis. J Gerontol. 1992;47:24–31. [PubMed] [Google Scholar]
- 33.Newschaffer CJ, Penberthy L, Desch CE, et al. The effect of age and comorbidity in the treatment of elderly women with nonmetastatic breast cancer. Arch Intern Med. 1996;156:85–90. [PubMed] [Google Scholar]
- 34.Bergman L, Dekker G, van Kerkhoff EH, et al. Influence of age and comorbidity on treatment choice and survival in elderly patients with breast cancer. Breast Cancer Res Treat. 1991;18:189–198. doi: 10.1007/BF01990035. [DOI] [PubMed] [Google Scholar]
- 35.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 36.West DW, Satariano WA, Ragland DR, et al. Comorbidity and breast cancer survival: A comparison between black and white women. Ann Epidemiol. 1996;6:413–419. doi: 10.1016/s1047-2797(96)00096-8. [DOI] [PubMed] [Google Scholar]
- 37.Louwman WJ, Janssen-Heijnen ML, Houterman S, et al. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: A population-based study. Eur J Cancer. 2005;41:779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132–140. doi: 10.1097/00005650-200502000-00006. [DOI] [PubMed] [Google Scholar]