Abstract
Purpose
Although health disparities are well-described for many cancers, little is known about racial and ethnic disparities in neuroblastoma. To evaluate differences in disease presentation and survival by race and ethnicity, data from the Children's Oncology Group (COG) were analyzed.
Patients and Methods
The racial/ethnic differences in clinical and biologic risk factors, and outcome of patients with neuroblastoma enrolled on COG ANBL00B1 between 2001 and 2009 were investigated.
Results
A total of 3,539 patients (white, 72%; black, 12%; Hispanic, 12%; Asian, 4%; and Native American, < 1%) with neuroblastoma were included. The 5-year event-free survival (EFS) rates were 67% for whites (95% CI, 65% to 69%), 69% for Hispanics (95% CI, 63% to 74%), 62% for Asians (95% CI, 51% to 71%), 56% for blacks (95% CI, 50% to 62%), and 37% for Native American (95% CI, 17% to 58%). Blacks (P < .001) and Native Americans (P = .04) had a higher prevalence of high-risk disease than whites, and significantly worse EFS (P = .01 and P = .002, respectively). Adjustment for risk group abrogated these differences. However, closer examination of the EFS among high-risk patients who remained event free for 2 years or longer, revealed a higher prevalence of late-occurring events among blacks compared with whites (hazard ratio, 1.5; 95% CI, 1.0 to 2.3; P = .04).
Conclusion
Black and Native American patients with neuroblastoma have a higher prevalence of high-risk disease, accounting for their worse EFS when compared with whites. The higher prevalence of late-occurring events among blacks with high-risk disease suggests that this population may be more resistant to chemotherapy. Studies focused on delineating the genetic basis for the racial disparities observed in this study are planned.
INTRODUCTION
Neuroblastoma is a common extracranial childhood cancer that has remarkable clinical heterogeneity and widely varying rates of cure depending on a range of clinical features at diagnosis and biologic characteristics of the tumor.1 Risk groups have been defined based on combinations of these prognostic clinical and biologic markers,1,2 and modern treatment strategies, tailored according to risk, have led to improved survival.3–5 However, little is known about associations between race/ethnicity and survival in children with neuroblastoma. To investigate the relationship between race/ethnicity, tumor biology, and survival in neuroblastoma, we analyzed data collected from 3,539 children enrolled on the Children's Oncology Group (COG) neuroblastoma biology protocol ANBL00B1 between 2001 and 2009
PATIENTS AND METHODS
Patient Cohort
Children diagnosed with neuroblastoma, ganglioneuroblastoma, or ganglioneuroma (maturing type) and enrolled on the COG biology protocol ANBL00B1 between 2001 and 2009 with available outcome data formed the analytic cohort. The diagnosis was confirmed by either central pathologic review of tumor tissue or by the presence of unequivocal tumor cells in the bone marrow and increased urine catecholamines or metabolites, as described by the International Neuroblastoma Staging System criteria.6 Eligibility requirements also include enrollment within 21 days of diagnosis, and a good faith effort to submit a tissue sample of sufficient quality for MYCN analysis to the COG Neuroblastoma Resource Laboratory. The median time from diagnosis to enrollment was 7 days. Subjects with unknown risk profile, age, and/or race/ethnicity were excluded from the analysis. The study was conducted with parental/patient informed consent for research participation. Institutional review board guidelines were followed for procurement of tumor samples for prognostic factors including MYCN status, tumor cell ploidy, and histology. Tumor staging was based on the International Neuroblastoma Staging System criteria,6 and patients were stratified into risk groups defined by COG according to the age, stage, histology, MYCN status, and tumor cell ploidy (Appendix Table A1, online only).1 Outcome data were frozen on April 8, 2009. The racial groups were categorized as: American Indian/Alaskan Native, Hawaiian/Pacific Islander (hereafter referred to as Native American); Asian; black or African American (hereafter referred to as black); and white. Ethnicity was categorized as: Hispanic and non-Hispanic. A combined race/ethnicity variable was created taking into consideration both the racial and ethnic backgrounds and coded as follows: non-Hispanic white, non-Hispanic black, Native American, Asian, and Hispanic.
Analysis of MYCN Status, Ploidy, and Histology
MYCN amplification was determined by fluorescence in situ hybridization (FISH) using standard procedures7 in the COG Neuroblastoma Resource Laboratory. DNA index was determined in the COG laboratory by flow cytometry and was reported as ≤ 1.0 versus higher than 1.0.8 Histology was classified as favorable or unfavorable after central review according to criteria described by Shimada et al.9
Statistical Considerations
Annual follow-up data are collected for all patients enrolled on ANBL00B1, and institutions are required to report events including relapse, progressive disease, second malignancy, and death. For patients concurrently enrolled on a COG clinical trial, follow-up data are collected according to schedule outlined in the clinical trial. χ2 tests were used to test for association of demographic and clinical characteristics with racial/ethnic groups; non-Hispanic whites served as the reference group. In the χ2 tests, a Bonferroni correction was made for the significance level; P values lower than .0014 (.05/35 χ2 tests) were considered statistically significant. Event-free survival (EFS) time was calculated from the time of biology study enrollment, which coincides closely with the date of diagnosis (defined as the date of the surgical biopsy or other definitive diagnostic procedure) until the time of first occurrence of relapse, progression, secondary malignancy, or death, or until the time of last contact if no event occurred. Overall survival time was calculated until the time of death or until time of last contact if the patient was alive. The product-limit method was used to estimate EFS and overall survival (OS), and expressed as the estimate with or without the SE, with SEs calculated using Greenwood's method. Kaplan-Meier curves were generated for each racial/ethnic group. Log-rank tests were used to compare survival curves across racial/ethnic groups. Cox proportional hazards regression was use to examine the ability of demographic and clinical factors to predict OS and EFS; and reported as hazard ratio (HR) with 95% CIs. Only statistically significant factors were retained in the Cox model. EFS, was also calculated among the subgroup of patients with 2 or more years of event-free time. For this subgroup, EFS time was recalculated starting from 2 years after study enrollment. In the survival analyses, P values lower than .05 are considered statistically significant. SAS version 9.2 (SAS Institute, Cary, NC) was used for data analysis.
RESULTS
Patient Characteristics
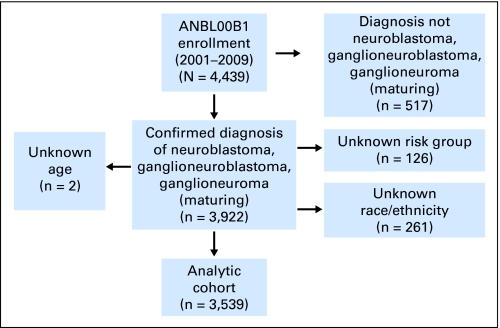
Of the 4,439 children and young adults enrolled on ANBL00B1 between 2001 and 2009, 3,922 had a confirmed diagnosis of neuroblastoma, ganglioneuroblastoma, or ganglioneuroma (maturing subtype) and known outcome (Fig 1). Subjects with unknown risk profile (n = 120), age (n = 2), and/or race/ethnicity (n = 261) were excluded from the analysis, and the remaining 3,539 patients formed the analytic cohort. Among the 261 patients who were excluded because of unknown race/ethnicity, 81 (31%) were classified as low-risk, 71 (27%) as intermediate, and 105 (41%) as high risk, and four had unknown risk. Of the patients with known race/ethnicity, the proportions were 33%, 20%, and 46% for low-, intermediate-, and high-risk groups, respectively. Of the 3,539 patients enrolled on ANBL00B1, 1,909 (49%) were also enrolled on at least one COG therapeutic trial.
Fig 1.
Formation of the analytic cohort.
Whites (n = 2,541) constituted 72% of the cohort; 12% were Hispanic (n = 418), 12% were black (n = 415), 4% were Asian (n = 137), and fewer than 1% were Native American (n = 28). The clinical and biologic characteristics of this cohort are summarized in Table 1. Within the overall cohort, 50% were diagnosed at age 18 months or older, 46% had stage 4 disease, and 54% were male. Using the COG criteria to define risk (Appendix Table A1),1 34% of the patients were classified as low risk, 20% were intermediate risk, and 46% met the criteria for high-risk disease. MYCN status was evaluated in tumors from 3,303 patients and amplification of this oncogene was detected in 19% of the patients. Of the 3,217 tumors evaluated for ploidy, 30% were diploid. Histology was known in 3,127 patients, and 41% had unfavorable histology.
Table 1.
Demographic and Clinical Characteristics of Patients With Neuroblastoma (N = 3,539)
| Clinical Characteristic | Entire Cohort |
Race/Ethnicity |
P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
Asian |
Native American |
Hispanic |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Overall No. | 3,539 | 2,541 | 415 | 137 | 28 | 418 | |||||||
| Age, months | < .001 | ||||||||||||
| < 18 | 1,758 | 50 | 1,301 | 51 | 157 | 38 | 65 | 47 | 12 | 43 | 223 | 53 | |
| ≥ 18 | 1,781 | 50 | 1,240 | 49 | 258 | 62 | 72 | 53 | 16 | 57 | 195 | 47 | |
| Stage | .002 | ||||||||||||
| 1, 2, 3, 4s | 1,895 | 54 | 1,392 | 55 | 192 | 46 | 64 | 47 | 11 | 39 | 236 | 56 | |
| 4 | 1,644 | 46 | 1,149 | 45 | 223 | 54 | 73 | 53 | 17 | 61 | 182 | 44 | |
| Sex | .662 | ||||||||||||
| Female | 1,618 | 46 | 1,167 | 46 | 181 | 44 | 59 | 43 | 11 | 39 | 200 | 48 | |
| Male | 1,921 | 54 | 1,374 | 54 | 234 | 56 | 78 | 57 | 17 | 61 | 218 | 52 | |
| MYCN status | .535 | ||||||||||||
| Not amplified | 2,645 | 75 | 1,913 | 75 | 293 | 71 | 101 | 74 | 19 | 68 | 319 | 76 | |
| Amplified | 658 | 19 | 467 | 19 | 83 | 20 | 26 | 19 | 8 | 29 | 74 | 18 | |
| Unknown | 236 | 6 | 161 | 6 | 39 | 9 | 10 | 7 | 1 | 3 | 25 | 6 | |
| Ploidy | .038 | ||||||||||||
| Hyperdiploid | 2,166 | 61 | 1,564 | 62 | 255 | 62 | 68 | 50 | 16 | 57 | 263 | 63 | |
| Diploid | 1,051 | 30 | 758 | 30 | 113 | 27 | 56 | 41 | 8 | 29 | 116 | 28 | |
| Unknown | 322 | 9 | 219 | 8 | 47 | 11 | 13 | 9 | 4 | 14 | 39 | 9 | |
| Histology | < .001 | ||||||||||||
| Favorable histology | 1,661 | 47 | 1,233 | 49 | 150 | 36 | 57 | 42 | 11 | 39 | 210 | 50 | |
| Unfavorable histology | 1,466 | 41 | 1,026 | 40 | 206 | 50 | 61 | 44 | 15 | 54 | 158 | 38 | |
| Unknown | 412 | 12 | 282 | 11 | 59 | 14 | 19 | 14 | 2 | 7 | 50 | 12 | |
| Risk group | < .001 | ||||||||||||
| Low | 1,185 | 34 | 880 | 35 | 120 | 29 | 37 | 27 | 5 | 18 | 143 | 34 | |
| Intermediate | 718 | 20 | 531 | 21 | 58 | 14 | 31 | 23 | 4 | 14 | 94 | 22 | |
| High | 1,636 | 46 | 1,130 | 44 | 237 | 57 | 69 | 50 | 19 | 68 | 181 | 43 | |
NOTE. P indicates value for overall comparison among all racial/ethnic categories.
Compared with the whites, black patients were diagnosed at an older age (P < .001), had a higher prevalence of stage 4 disease (P = .001) and unfavorable histology tumors (P < .001; Table 1). Blacks were also more likely to present with high-risk disease than white children (57% v 44%; P < .001). However, no difference in the frequency of MYCN amplification (P = .27) or ploidy (P = .46) was detected between blacks and whites. Compared with whites, Asians had more diploid tumors (P < .01). The prevalence of high-risk disease among the Asians was higher compared to the whites, but did not reach statistical significance (50% v 43%; P = .18). Although the cohort of Native Americans was small (n = 28), a significantly higher prevalence of high-risk disease was seen in this population as compared to whites (68% v 43%; P = .04). The prevalence of high-risk disease among Hispanics (43%; P = .76) did not differ from whites.
Survival Probabilities
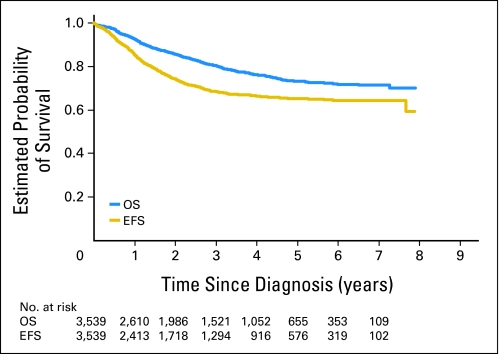
The 5-year OS and EFS for the entire cohort of patients were 69% (95% CI, 67% to 70%) and 65% (95% CI, 63% to 67%), respectively (Fig 2). Univariate analysis revealed older age at diagnosis (≥ 18 months), stage 4 disease, MYCN amplification, diploidy, and unfavorable histology to be associated with significantly worse OS and EFS (Table 2). As expected, patients with high-risk disease had statistically significantly worse OS (P < .001) and EFS (P < .001) when compared with those with low-risk tumors. Furthermore, intermediate-risk patients had worse OS when compared with low-risk patients (P = .004). No sex difference in either OS or EFS was detected, thus sex was not adjusted in the multivariable analysis.
Fig 2.
Overall survival (OS) and event-free survival (EFS) curves for the entire cohort of patients (n = 3,539) with numbers of patients at risk over time.
Table 2.
Univariate Analyses of Overall and Event-Free Survival in Patients With Neuroblastoma
| Characteristic | No. | Overall Survival |
Event-Free Survival |
||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | ||
| Race/ethnicity | |||||||
| White | 2,541 | 1.00 | 1.00 | ||||
| Hispanic | 418 | 1.01 | 0.79 to 1.31 | .92 | 0.90 | 0.72 to 1.12 | .33 |
| Black | 415 | 1.37 | 1.09 to 1.72 | .01 | 1.30 | 1.07 to 1.57 | .01 |
| Asian | 137 | 1.62 | 1.11 to 2.36 | .01 | 1.29 | 0.92 to 1.80 | .14 |
| Native American | 28 | 3.00 | 1.65 to 5.45 | .0003 | 2.37 | 1.37 to 4.10 | .002 |
| Age, months | |||||||
| < 18 | 1,758 | 1.00 | 1.00 | ||||
| ≥ 18 | 1,781 | 3.99 | 3.29 to 4.83 | < .001 | 3.02 | 2.61 to 3.51 | < .001 |
| Stage | |||||||
| 1, 2, 3, 4s | 1,895 | 1.00 | 1.00 | ||||
| 4 | 1,644 | 8.89 | 7.08 to 11.15 | < .001 | 5.08 | 4.34 to 5.95 | < .001 |
| Sex | |||||||
| Female | 1,618 | 1.00 | |||||
| Male | 1,921 | 1.15 | 0.98 to 1.34 | .09 | 1.11 | 0.97 to 1.26 | .13 |
| MYCN status | |||||||
| Not amplified | 2,645 | 1.00 | 1.00 | ||||
| Amplified | 658 | 5.22 | 4.41 to 6.17 | < .001 | 3.44 | 2.99 to 3.97 | < .001 |
| Ploidy | |||||||
| Hyperdiploid | 2,166 | 1.00 | 1.00 | ||||
| Diploid | 1,051 | 2.45 | 2.07 to 2.91 | < .001 | 2.02 | 1.75 to 2.32 | < .001 |
| Histology | |||||||
| Favorable | 1,661 | 1.00 | 1.00 | ||||
| Unfavorable | 1,466 | 11.60 | 8.65 to 15.56 | < .001 | 4.93 | 4.12 to 5.90 | < .001 |
| Risk group | |||||||
| Low | 1,185 | 1.00 | 1.00 | ||||
| Intermediate | 718 | 2.22 | 1.29 to 3.83 | .004 | 1.31 | 0.97 to 1.78 | .08 |
| High | 1,636 | 23.28 | 15.47 to 35.04 | < .001 | 6.99 | 5.67 to 8.61 | < .001 |
NOTE. Bold font denotes groups at statistically significantly higher risk of death and/or event.
Impact of Race and Ethnicity on Survival
Entire cohort.
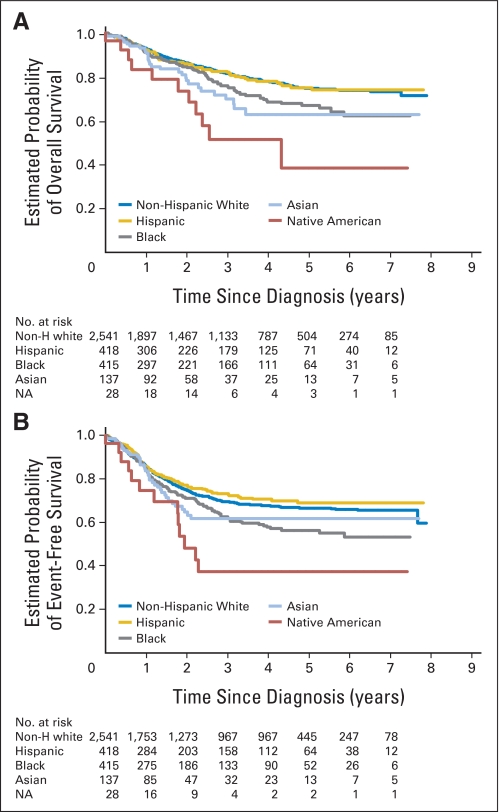
As shown in Figure 3A, the 5-year OS was 75% for whites (95% CI, 72% to 77%), 75% for Hispanics (95% CI, 69% to 80%), 67% for black (95% CI, 60%% to 73%), 63% for Asians (95% CI, 50% to 73%), and 39% for Native Americans (95% CI, 13% to 64%). The 5-year EFS was 67% for whites (95% CI, 65% to 69%), 69% for Hispanics (95% CI, 63% to 74%), 56% for blacks (95% CI, 50% to 62%), 62% for Asians (95% CI, 51% to 71%), and 37% for Native American (95% CI, 17% to 58%; Fig 3B). Univariate analysis revealed that in comparison to whites, OS was significantly worse for blacks (HR, 1.37; P = .01), Asians (HR, 1.62; P = .01), and Native Americans (HR, 3.00; P < .001; Table 2); and the EFS was significantly worse for blacks (HR, 1.30; P = .01) and Native Americans (HR, 2.37; P = .002; Table 2).
Fig 3.
(A) Overall and (B) event-free survival by race and ethnicity with numbers of patients at risk over time. Non-H, non-Hispanic; NA, Native American.
Multivariable analysis.
Adjustment for risk group abrogated the inferior EFS observed among blacks and Native Americans when compared with whites. After adjustment for risk group, inferior OS was identified for the Native Americans (HR, 2.05; P = .02) and Asians (HR, 1.46; P = .05; Table 3).
Table 3.
Multivariable Analysis of Overall and Event-Free Survival in Patients With Neuroblastoma
| Characteristic | Overall Survival |
Event-Free Survival |
Event-Free Survival Among Patients Who Were Event Free for ≥ 2 Years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Deaths | Hazard Ratio | 95% CI | P | No. of Events | Hazard Ratio | 95% CI | P | No. of Patients | No. of Events | Hazard Ratio | 95% CI | P | |
| Race/ethnicity | ||||||||||||||
| White | 2,541 | 429 | 1.00 | 635 | 1.00 | 1,273 | 123 | 1.00 | ||||||
| Hispanic | 418 | 69 | 1.13 | 0.88 to 1.46 | .35 | 93 | 0.98 | 0.79 to 1.22 | .86 | 203 | 17 | 1.01 | 0.61 to 1.68 | .97 |
| Black | 415 | 90 | 1.09 | 0.87 to 1.37 | .47 | 127 | 1.10 | 0.90 to 1.33 | .35 | 186 | 33 | 1.50 | 1.02 to 2.20 | .04 |
| Asian | 137 | 29 | 1.46 | 1.00 to 2.12 | .05 | 36 | 1.18 | 0.84 to 1.65 | .34 | 47 | 2 | 0.39 | 0.10 to 1.59 | .19 |
| Native American | 28 | 11 | 2.05 | 1.13 to 3.73 | .02 | 13 | 1.63 | 0.94 to 2.83 | .08 | 9 | 2 | 2.10 | 0.52 to 8.51 | .30 |
| Risk group | ||||||||||||||
| Low | 1,185 | 24 | 1.00 | 101 | 1.00 | 729 | 14 | 1.00 | ||||||
| Intermediate | 718 | 28 | 2.22 | 1.29 to 3.83 | .004 | 72 | 1.32 | 0.97 to 1.78 | .08 | 380 | 5 | 0.76 | 0.27 to 2.11 | .60 |
| High | 1636 | 576 | 23.09 | 15.34 to 34.76 | < .001 | 731 | 6.92 | 5.61 to 8.53 | < .001 | 609 | 158 | 16.26 | 9.39 to 28.14 | < .001 |
NOTE. Bold font denotes groups at statistically significantly higher risk of death and/or event; only statistically significant factors were retained in the analysis.
Low-risk group.
Among patients with low-risk disease, the 5-year OS was 97% (95% CI, 95% to 98%) and the 5-year EFS was 89% (95% CI, 87% to 91%). No differences in OS or EFS by race/ethnicity were identified.
Intermediate-risk group.
Among patients with intermediate-risk disease, the 5-year OS was 95% (95% CI, 92% to 96%) and 5-year EFS was 87% (95% CI, 84% to 90%). Again, no differences in OS or EFS were identified by race/ethnicity.
High-risk group.
Overall, the 5-year OS was 48% (95% CI, 44% to 51%); and the 5-year EFS was 39% (95% CI, 36% to 42%). The 5-year OS was 49% for whites (95% CI, 45% to 53%), 46% for Hispanics (95% CI, 36% to 56%), 46% for blacks (95% CI, 38% to 55%), 37% for Asians (95% CI, 22% to 52%), and 17% for Native Americans (95% CI, 1% to 48%; P = .02). The 5-year EFS was 41% for whites (95% CI, 37% to 44%), 39% for Hispanics (95% CI, 29% to 48%), 34% for blacks (95% CI, 26% to 42%), 39% for Asians (95% CI, 25% to 52%), and 21% for Native Americans (95% CI, 5% to 44%; P = .24).
Late-occurring events.
A close examination of the patients who remained event-free for 2 years or longer revealed statistically significantly more late-occurring events among blacks, when compared to white patients (HR, 1.50; P = .04; Table 3). In a multivariable analysis confined to high-risk patients who remained event-free for 2 years or longer, blacks had significantly worse EFS (HR, 1.51; P = .04) when compared to white patients (Table 4). The number of events among the low- and intermediate-risk cohort was too few to examine EFS among patients with 2 years or longer of event-free time.
Table 4.
Multivariable Analysis of OS and EFS in Patients With High-Risk Neuroblastoma
| Race/Ethnicity | OS Model (n = 1,636) |
EFS Model (n = 1,636) |
EFS Model Among Patients Event Free for ≥2 Years (n = 609) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | HR | 95% CI | P | No. of Events | HR | 95% CI | P | No. of Events | HR | 95% CI | P | |
| White | 389 | 1.0 | 501 | 1.0 | 110 | 1.0 | ||||||
| Hispanic | 63 | 1.17 | 0.89 to 1.52 | .26 | 76 | 1.07 | 0.84 to 1.37 | .57 | 14 | 0.96 | 0.55 to 1.68 | .87 |
| Black | 85 | 1.09 | 0.87 to 1.38 | .46 | 111 | 1.13 | 0.92 to 1.38 | .26 | 31 | 1.51 | 1.01 to 2.25 | .04 |
| Asian | 28 | 1.54 | 1.05 to 2.26 | .03 | 31 | 1.25 | 0.87 to 1.81 | .22 | 1 | 0.19 | 0.03 to 1.43 | .11 |
| Native American | 11 | 2.16 | 1.18 to 3.93 | .01 | 12 | 1.69 | 0.95 to 2.99 | .07 | 2 | 2.23 | 0.55 to 9.03 | .26 |
NOTE. Bold font denotes groups at statistically significantly higher risk of death and/or event.
Abbreviations: OS, overall survival; EFS, event-free survival; HR, hazard ratio.
DISCUSSION
Risk-stratified treatment strategies have led to improvements in the outcome of children with neuroblastoma, and efforts to further refine risk classification using molecular signatures and global genomic patterns are ongoing.10,11 However, despite more than four decades of research investigating classifiers for neuroblastoma, little is known about the prognostic significance of race and ethnicity, factors that contribute to health disparities in many diseases including cancer.12 Here, we examined survival based on race and ethnicity in more than 3,500 patients with neuroblastoma. To our knowledge, this is the largest neuroblastoma cohort ever analyzed for outcome disparities, and the first to show that black, Asian, and Native American children have a significantly worse outcome than white children.
Interestingly, a significantly higher proportion of patients with high-risk disease was seen in the black and Native American populations compared with the white population. In contrast, the prevalence of high-risk disease among Hispanics and Asians did not differ significantly from whites. In concordance with the higher prevalence of high-risk disease, black and Native American children had significantly worse OS and EFS. Asian children were also found to have significantly poorer OS than white children, but no statistically significant difference in EFS was seen. No significant differences in OS or EFS were seen in the Hispanic children compared to white children. When the EFS data were analyzed controlling for risk group, the outcome disparities were abrogated. However, examination of the EFS of the subgroup of patients who remained event-free for 2 years or longer revealed that blacks had significantly more late-occurring events than white children. This was confirmed in a multivariable analysis restricted to high-risk patients who remained event-free 2 years or longer from diagnosis, where blacks had significantly worse EFS compared to white children. Taken together, these observations suggest that black children are more likely than white children to have chemotherapy-resistant residual disease that progresses or relapses 2 or more years from diagnosis.
Our results contrast those recently reported by Linabery and Ross using Surveillance, Epidemiology and End Results (SEER) data collected between 1975 to 1999.12 In the SEER cohort of 757 patients, no difference in survival of non-Hispanic white versus non-Hispanic black and Hispanic patients was observed. In the most recent SEER cohort, diagnosed between 1995 and 1999, the 5-year survival rate for non-Hispanic whites was 67.0% (95% CI, 60.7% to 73.2%) compared to 68.1% (95% CI, 53.5% to 82.8%) for non-Hispanic blacks and 69% (95% CI, 58.3% to 79.6%) for Hispanics. The children analyzed in our study were diagnosed between 2001 and 2009, and treatment strategies have been significantly modified in the past decade.13,14 The disparities in outcome observed in our cohort suggest that modern treatment strategies may be more effective in the non-Hispanic whites and Hispanic children than in the black cohort. Linabery and Ross12 did find that black children and adolescents with other cancers had worse outcome than whites. Using SEER data, others have reported that blacks, Native Americans, and Hispanic children with acute lymphocytic leukemia have worse survival than white and Asian children.15 Similarly, a COG acute lymphocytic leukemia study demonstrated worse survival among black and Hispanic children compared to white children, whereas outcome was better for Asian children.16 Studies of acute myelogenic leukemia outcomes have also shown that black patients have poorer outcomes than white counterparts.17,18
Multiple factors contribute to the racial and ethnic differences in survival observed in pediatric and adult cancers. Outcome disparities have been shown in some cases to reflect differences in access to health care.19 This may result in delayed detection of illness or differences in treatment adherence. In our cohort of children with neuroblastoma, a higher prevalence of high-risk disease was observed in the blacks compared to whites, and a number of studies have indicated that it is unusual for favorable biology tumors to progress to high-risk tumors over time. A watch and wait approach has been followed in a subset of infants detected by mass screening, and none of the patients with tumor growth necessitating surgical removal were found to have unfavorable tumor biology.20 Other studies have shown that MYCN status and ploidy do not change over time.21–23 Taken together, these studies indicate that a delay in diagnosis is not likely to account for the higher percentage high-risk disease observed in the black cohort. Nonadherence is also a less common problem in neuroblastoma, since patients with high-risk disease receive almost all of their therapy in the hospital.
Genetic factors have also been shown to contribute to outcome disparities. Specific polymorphisms have been shown to play a critical role in the racial and ethnic diversity in drug effectiveness and/or toxicity.24–26 In addition, genomewide association studies conducted in white children with neuroblastoma have shown that variability of genes located at common 6p22 single nucleotide polymorphism alleles and less-common single nucleotide polymorphisms at 2p35 within BARD1 contribute to the etiology of clinically aggressive disease.27,28 Studies are planned to determine if these or other at-risk alleles are contributing to the higher prevalence of high-risk disease and poor outcome we observed in our cohort of black children with neuroblastoma.
Despite the large size of this modern cohort of neuroblastoma patients, there are some notable limitations to this study. It is possible that patients for whom complete biologic information was not available may have introduced bias if the reasons for not having these assays performed were associated with prognosis and race/ethnicity. However, a good-faith effort to submit tumor tissue for MYCN analysis was a requirement for enrollment on ANLB00B1. Although we excluded 261 patients with unknown race/ethnicity in our analysis, these patients were found to have a similar distribution of risk to that of the entire cohort. Thus, elimination of this cohort most likely did not impact our study results. In addition, patients missing from the ANBL00B1 cohort we analyzed may have introduced bias. For example, our study cohort did not capture rare infants with stage 4S disease who were too sick to biopsy and small numbers of infants with localized neuroblastomas in which a watch and wait strategy was taken because they were not eligible for enrollment on ANBL00B1. Furthermore, children diagnosed at institutions that do not participate in COG neuroblastoma studies were not enrolled. Nevertheless, since the inception of ANBL00B1, approximately 75% (average yearly enrollment; n = 486) of the 650 children diagnosed in the United States with neuroblastoma each year29 were enrolled. The significantly older age at diagnosis of the black children in our cohort is consistent with SEER data that show that white infants have a higher incidence of neuroblastoma than black infants, whereas little difference by race was observed among older children.30 We also found that the proportion of blacks and Hispanics in our cohort is consistent with 2000 US census data,31 further indicating that our cohort is representative.
We did not have access to patient treatment data, and as such could not control for the different chemotherapeutic agents and doses patients received. It is also important to note that race/ethnicity is not a mutually exclusive variable, and some patients may have mixed racial and ethnic ancestry. Reflecting the general population in North America, the vast majority of patients in our cohort were white, with significantly fewer numbers of black and Hispanic patients, and very small numbers of Native American and Asian patients. Because of the small numbers of Native American and Asian patients in the cohort, the survival results for these populations must be interpreted with caution.
In conclusion, we show for the first time that blacks and Native American neuroblastoma patients are statistically significantly more likely to present with high-risk disease than whites, and that, overall, this likely accounts for the inferior EFS observed in these populations. However, we report a higher prevalence of late-occurring events among blacks compared to white patients after controlling for clinical and biologic features of disease, suggesting that blacks may be more resistant to chemotherapy. Future research must focus on understanding the reasons for these disparities, including racial and ethnic differences in drug metabolism and bioavailability of commonly used agents, and genetic differences that may contribute to the etiology of clinically aggressive disease. A better understanding of the genetic basis of the outcome disparities observed in children with neuroblastoma will further refine risk classification, and may also direct us to more effective, individualized treatment strategies.
Appendix
Table A1.
Children's Oncology Group Neuroblastoma Risk Classification System
| Stage | Age (days) | MYCN | Ploidy | Histology | Other | Risk Group |
|---|---|---|---|---|---|---|
| 1 | Low | |||||
| 2A/2B | NA | > 50% resected | Low | |||
| NA | < 50% resected | Intermediate | ||||
| NA | Bx only | Intermediate | ||||
| Amp | High | |||||
| 3 | < 547 | NA | Intermediate | |||
| ≥ 547 | NA | FH | Intermediate | |||
| Amp | High | |||||
| ≥ 547 | NA | UH | High | |||
| 4 | < 365 | Amp | High | |||
| < 365 | NA | Intermediate | ||||
| 365-< 547 | Amp | High | ||||
| 365-< 547 | DI = 1 | High | ||||
| 365-< 547 | UH | High | ||||
| 365-< 547 | NA | DI > 1 | FH | Intermediate | ||
| ≥ 547 | High | |||||
| 4S | < 365 | NA | DI > 1 | FH | Asymptomatic | Low |
| < 365 | NA | DI = 1 | Intermediate | |||
| < 365 | Missing | Missing | Missing | Intermediate | ||
| < 365 | NA | Symptomatic | Intermediate | |||
| < 365 | NA | UH | Intermediate | |||
| < 365 | Amp | High |
Abbreviations: NA, not amplified; Bx, biopsy; Amp, amplified; FH, favorable histology; UH, unfavorable histology; DI, DNA index.
Footnotes
Supported by the Children's Oncology Group Statistics and Data Center Grants No. U10CA29139, CA25408, CA98543, and CA30969; the Neuroblastoma Children's Cancer Society; the Children's Neuroblastoma Cancer Foundation, Little Heroes Children's Cancer Research Fund; and the Elise Anderson Neuroblastoma Research Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Tara O. Henderson, Smita Bhatia, Susan L. Cohn
Administrative support: Susan L. Cohn
Provision of study materials or patients: Susan L. Cohn, Wendy B. London
Collection and assembly of data: Tara O. Henderson, Wendy B. London, Patrick McGrady, Susan L. Cohn
Data analysis and interpretation: Tara O. Henderson, Smita Bhatia, Navin Pinto, Wendy B. London, Can-Lan Sun, Susan L. Cohn
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt ML, Lukens JN, Seeger RC, et al. Biologic factors determine prognosis in infants with stage IV neuroblastoma: A prospective Children's Cancer Group Study. J Clin Oncol. 2000;18:1260–1268. doi: 10.1200/JCO.2000.18.6.1260. [DOI] [PubMed] [Google Scholar]
- 4.Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005;23:8819–8827. doi: 10.1200/JCO.2004.00.2931. [DOI] [PubMed] [Google Scholar]
- 5.De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol. 2009;27:1034–1040. doi: 10.1200/JCO.2008.17.5877. [DOI] [PubMed] [Google Scholar]
- 6.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro DN, Valentine MB, Rowe ST, et al. Detection of N-myc gene amplification by fluorescence in situ hybridization: Diagnostic utility for neuroblastoma. Am J Pathol. 1993;142:1339–1346. [PMC free article] [PubMed] [Google Scholar]
- 8.Look AT, Hayes FA, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: A Pediatric Oncology Group study. J Clin Oncol. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 9.Shimada H, Chatten J, Newton WA, Jr, et al. Histopathologic prognostic factors in neuroblastic tumors: Definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 10.Janoueix-Lerosey I, Schleiermacher G, Michels E, et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009;27:1026–1033. doi: 10.1200/JCO.2008.16.0630. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen J, De Preter K, Naranjo A, et al. Predicting outcomes for children with neuroblastoma using a multigene-expression signature: A retrospective SIOPEN/COG/GPOH study. Lancet Oncol. 2009;10:663–671. doi: 10.1016/S1470-2045(09)70154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children's oncology group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, IL-2 and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 17.Rubnitz JE, Lensing S, Razzouk BI, et al. Effect of race on outcome of white and black children with acute myeloid leukemia: The St. Jude experience. Pediatr Blood Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 18.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: A report from the Children's Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Kigasawa H, Kato K, et al. A prospective study of a long-term follow-up of an observation program for neuroblastoma detected by mass screening. Pediatr Blood Cancer. 2010;54:573–578. doi: 10.1002/pbc.22400. [DOI] [PubMed] [Google Scholar]
- 21.Berthold F, Hero B, Breu H, et al. The recurrence patterns of stages I, II and III neuroblastoma: Experience with 77 relapsing patients. Ann Oncol. 1996;7:183–187. doi: 10.1093/oxfordjournals.annonc.a010547. [DOI] [PubMed] [Google Scholar]
- 22.Brodeur GM, Hayes FA, Green AA, et al. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987;47:4248–4253. [PubMed] [Google Scholar]
- 23.Taylor SR, Blatt J, Costantino JP, et al. Flow cytometric DNA analysis of neuroblastoma and ganglioneuroma: A 10-year retrospective study. Cancer. 1988;62:749–754. doi: 10.1002/1097-0142(19880815)62:4<749::aid-cncr2820620418>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 24.Spitz MR, Wu X, Mills G. Integrative epidemiology: From risk assessment to outcome prediction. J Clin Oncol. 2005;23:267–275. doi: 10.1200/JCO.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 25.Huang RS, Duan S, Kistner EO, et al. Genetic variants associated with carboplatin-induced cytotoxicity in cell lines derived from Africans. Mol Cancer Ther. 2008;7:3038–3046. doi: 10.1158/1535-7163.MCT-08-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37–42. doi: 10.1097/00008571-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Maris JM, Mosse YP, Bradfield JP, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capasso M, Devoto M, Hou C, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41:718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ries LAG, Smith MA, Gurney JG, et al. Bethesda, MD: National Institutes Health; 1999. Cancer Incidence and Survival among Children and Adolescents; United States SEER Program 1975-1995, National Cancer Institute, SEER Program. Pub. No. 99-4649. [Google Scholar]
- 30.Goodman MT, Gurney JG, Smith MA, et al. Sympathetic Nervous System Tumors: SEER Pediatric Monograph. 2008. http://www.seer.cancer.gov/publications/childhood/sympathetic.pdf.
- 31.United States Census 1980-2000: Race and ethnicity selections. http://www.censusscope.org.