Abstract
Purpose
Functional iron deficiency may impair response to erythropoiesis-stimulating agents (ESAs) in iron-replete patients with chemotherapy-associated anemia (CAA). This study evaluated whether coadministration of parenteral iron improves ESA efficacy in patients with CAA.
Patients and Methods
This prospective, multicenter, randomized trial enrolled 502 patients with hemoglobin (Hb) less than 11 g/dL who were undergoing chemotherapy for nonmyeloid malignancies. All patients received darbepoetin alfa once every 3 weeks and were randomly assigned to receive either ferric gluconate 187.5 mg intravenously (IV) every 3 weeks, oral daily ferrous sulfate 325 mg, or oral placebo for 16 weeks.
Results
There was no difference in the erythropoietic response rate (ie, proportion of patients achieving Hb ≥12 g/dL or Hb increase ≥ 2 g/dL from baseline): 69.5% (95% CI, 61.9% to 76.5%) of IV iron-treated patients achieved an erythropoietic response compared with 66.9% (95% CI, 59.1% to 74.0%) who received oral iron and 65.0% (95% CI, 57.2% to 72.3%) who received oral placebo (P = .75). There were also no differences in the proportion of patients requiring red cell transfusions, changes in quality of life, or the dose of darbepoetin administered. Adverse events (AEs) tended to be more common in the IV iron arm: grade 3 or higher AEs occurred in 54% (95% CI, 46% to 61%) of patients receiving IV iron compared with 44% (95% CI, 36% to 52%) who received oral iron and 46% (95% CI, 38% to 54%) who received oral placebo (P = .16).
Conclusion
In patients with CAA, addition of IV ferric gluconate to darbepoetin failed to provide additional benefit compared with oral iron or oral placebo.
INTRODUCTION
Chemotherapy-associated anemia (CAA) is a common problem for patients with cancer and is associated with requirement for RBC transfusions and with decreased quality of life (QOL).1 Treatment with erythropoiesis-stimulating agents (ESAs) results in improved hemoglobin (Hb) levels in 40% to 70% of patients with CAA, and ESAs also decrease transfusion requirements.2
One of the most common reasons for a lack of response to ESA treatment is iron deficiency.3 Even among patients with normal or increased total body iron stores, functional iron deficiency (ie, lack of bioavailable iron) as a result of inflammation-related hepcidin production and pathologic sequestration of iron in macrophages can restrict erythropoiesis and impair response to ESA treatment.3 It has been hypothesized that intravenous (IV) iron administration might overcome functional iron deficiency by providing elemental iron in a readily bioavailable form to the developing erythron.4 In the nephrology/hemodialysis setting, parenteral iron administration is a standard practice, but use of IV iron is currently infrequent in oncology clinics.5
Since 2004, five published randomized studies have reported a variety of benefits with IV iron use compared with oral iron or no iron in ESA-treated patients with CAA.6–10 These benefits have included higher rates of Hb response to ESAs, increased Hb increment from baseline levels, decreased ESA dose requirements, or improved QOL.6–10 Several clinical practice guidelines—including those from the American Society of Hematology/American Society of Clinical Oncology, the National Comprehensive Cancer Network (http://www.nccn.org), and the European Organisation for Research and Treatment of Cancer—now recommend consideration of iron supplementation, including parenteral iron, for patients with CAA receiving ESAs.11,12 All of these guidelines also state that more research is needed regarding optimal iron dose and schedule and selection of suitable patients. This study investigated whether response to darbepoetin alfa in iron-replete patients with CAA might be improved with concomitant administration of IV iron.
PATIENTS AND METHODS
Study Design and Population
The study was conducted by the Mayo Clinic Cancer Research Consortium (MCCRC), an organization with similar membership to the North Central Cancer Treatment Group (NCCTG) but without National Cancer Institute (NCI) financial or administrative support. The study was approved by institutional review boards of participating institutions, and all participants provided written informed consent. The study was registered at http://www.clinicaltrials.gov.
Eligible patients were ≥ 18 years old; were receiving chemotherapy for a nonmyeloid neoplasm; and had Hb less than 11.0 g/dL, ferritin greater than 20 ng/mL, transferrin saturation less than 60%, and a Zubrod performance score better than 2. Patients with a history of thromboembolism within 1 year of enrollment, genetic hemochromatosis, or recent surgery were excluded, as were patients with anemia caused by a myelodysplastic syndrome, nutritional deficiency, or a non-neoplastic hematologic disorder such as thalassemia. Patients were also temporarily excluded if they had received an ESA within 3 months or red cell transfusion within 14 days.
Study End Points
The primary efficacy end point was a comparison of the proportion of patients in each group achieving an erythropoietic response, which was defined as Hb increment of ≥ 2.0 g/dL from baseline or achievement of Hb ≥12 g/dL in the absence of transfusions during the preceding 28 days. Secondary end points included transfusion requirements, total ESA dose used, safety, and QOL. QOL was measured using four validated instruments: the Symptom Distress Scale (SDS),13 the Brief Fatigue Inventory (BFI),14 the Functional Assessment of Cancer Therapy-Anemia (FACT-An),15 and the Linear Analog Scale Assessment (LASA).16
Treatment Schedule and Assessments
Study visits occurred at baseline and at the end of weeks 3, 6, 9, 12, and 15 (end of study). Complete blood counts were measured weekly; QOL was assessed and iron parameters (ferritin, transferrin saturation, and soluble transferrin receptor) measured at baseline, at the end of week 6, and at the end of study.
All patients were scheduled to receive darbepoetin alfa (Aranesp; Amgen, Thousand Oaks, CA) 500 μg subcutaneously every 3 weeks until Hb reached greater than 11.0 g/dL and thereafter to receive maintenance darbepoetin 300 μg every 3 weeks. Darbepoetin was to be held for Hb greater than 13.0 g/dL, until Hb decreased to less than 12.0 g/dL; then darbepoetin was restarted with a 25% dose reduction.
Patients were randomly assigned on a 1:1:1 basis to receive either sodium ferric gluconate complex in sucrose (Ferrlecit; Watson, Morristown, NJ) 187.5 mg IV over 90 minutes once every 3 weeks for five doses (total dose 937.5 mg), oral ferrous sulfate 325 mg once daily, or an oral placebo. Patients and investigators were blinded to assignment of oral iron or oral placebo, but, for practical reasons, assignment to IV iron versus an oral product was not blinded. Random assignment was stratified by patient sex, tumor type (solid tumors v hematologic malignancies), severity of anemia on the basis of the WHO classification (mild, Hb ≥ 9.5 g/dL; severe, Hb < 9.5 g/dL), and whether or not patients were receiving a platinum-containing chemotherapy regimen. Additional details of the random assignment process are provided in the Appendix (online only).
Transfusions were administered at the discretion of investigators and were recommended for patients with Hb less than 8.0 g/dL or severe anemia-related symptoms. Premedications before IV iron administration, including diphenhydramine, were permitted at the discretion of individual investigators. Adverse events (AEs) were assessed by using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical Analysis
Efficacy data were analyzed for all patients who were randomly assigned and received at least one darbepoetin alfa dose. The safety population also included all patients who received one or more darbepoetin dose. All treatment comparisons were made in a pairwise fashion by using oral placebo treatment as a reference.
The study plan was to enroll 194 patients per arm, with a 10% attrition rate assumed because of ineligibility, drop-outs, and losses to follow-up. With 176 evaluable patients per treatment arm, there would have been ≥ 80% power to detect a difference across two treatment arms of 15% in the primary erythropoietic response end point through Fisher's exact test, if the true percentage of patients that experience a erythropoietic response was at least 30% in the superior group, with a 2.5% type I error rate.
Comparison of the change in Hb levels from baseline to week 6 and week 15 between pairs of treatment arms was done via two-sample t tests (both for week 6 and week 15). Additional analysis of this end point was undertaken via a repeated measures one-way analysis of variance (ANOVA) model, both as a confirmatory analysis and to allow for adjustment of the result in the presence of potential concomitant covariates, such as patient demographics and baseline Hb, baseline QOL, and other clinical variables.
QOL raw scores were calculated, and QOL scores were also transformed to represent the position along the theoretical range of the QOL dimension expressed as a percentage (0% to 100%). Linear and quadratic changes of QOL were examined over time, and these time trends were compared among the three treatment assignments. Independent-sample t test procedures were used, and a clinically significant result was defined as a shift of 10 points on a 0- to 100-point transformed scale between the average QOL scores of the two variants of iron therapy or oral placebo.17
For categoric variables, Fisher's exact tests were used for comparing binary secondary end points across the three treatments, such as RBC transfusion incidence rates. Kruskal-Wallis and one-way ANOVA procedures were used to make comparisons across the three treatment arms on secondary continuous variates. Time to erythropoietic response and first RBC transfusion were summarized by using Kaplan-Meier methodology.
RESULTS
Patient Demographics and Baseline Characteristics
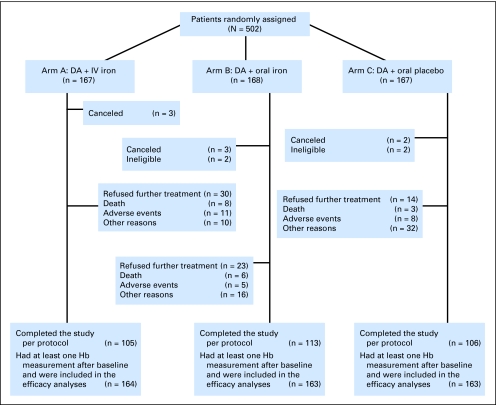
Planned enrollment was 582 patients, but the study met an early stopping rule because of an excess of serious AEs in the IV iron arm. Between February 2006 and December 2008, 502 patients enrolled at 14 MCCRC sites. The median age of the 490 evaluable patients was 64 years; 65% were women, and 93% were white; 6% had hematologic neoplasms; 25% had severe anemia (ie, Hb < 9.5 g/dL); 48% received platinum-containing chemotherapy; and 66% completed all 16 weeks of the study. Baseline characteristics were comparable between randomly assigned groups (Table 1). Patient disposition is presented in Figure 1.
Table 1.
Baseline Demographic and Clinical Characteristics of Evaluable Patients
| Characteristic | Parenteral Iron (n = 164) | Oral Iron(n = 163) | Oral Placebo (n = 163) | Total Evaluable (N = 490) | P |
|---|---|---|---|---|---|
| Age, years | .88 | ||||
| Mean | 64 | 63 | 63 | 63 | |
| SD | 11.4 | 12.4 | 11.3 | 11.8 | |
| Sex | |||||
| Female | .92 | ||||
| No. | 109 | 106 | 105 | 320 | |
| % | 66 | 65 | 64 | 65 | |
| Ethnicity, self declared | .35 | ||||
| White | |||||
| No. | 155 | 147 | 156 | 458 | |
| % | 95 | 90 | 96 | 94 | |
| Black or African American | |||||
| No. | 6 | 7 | 4 | 17 | |
| % | 4 | 4 | 2 | 3 | |
| Asian | |||||
| No. | 0 | 3 | 1 | 4 | |
| % | 0 | 2 | 1 | 1 | |
| American Indian or Alaska Native | |||||
| No. | 0 | 3 | 1 | 4 | |
| % | 0 | 2 | 1 | 1 | |
| Patient refused or unavailable | |||||
| No. | 3 | 3 | 1 | 7 | |
| % | 2 | 2 | 1 | 1 | |
| Degree of anemia | .99 | ||||
| Mild: Hb ≥ 9.5 g/dL | |||||
| No. | 123 | 123 | 123 | 369 | |
| % | 75 | 75 | 75 | 75 | |
| Severe: Hb ≤ 9.5 g/dL | |||||
| No. | 41 | 40 | 40 | 121 | |
| % | 25 | 25 | 25 | 25 | |
| Pretreatment Hb, g/dL | |||||
| Mean | 9.94 | 9.91 | 9.97 | 9.94 | |
| SD | 0.705 | 0.656 | 0.721 | ||
| Median | 10.10 | 10.00 | 10.20 | 10.10 | |
| Platinum-containing regimen | .99 | ||||
| Yes | |||||
| No. | 79 | 79 | 78 | 236 | |
| % | 48 | 49 | 48 | 48 | |
| Tumor type | .80 | ||||
| Hematologic neoplasm | |||||
| No. | 6 | 8 | 11 | 25 | |
| % | 4 | 5 | 7 | 5 | |
| Solid tumor | |||||
| No. | 157 | 154 | 151 | 462 | |
| % | 96 | 94 | 93 | 94 | |
| Both | |||||
| No. | 1 | 1 | 1 | 3 | |
| % | 1 | 1 | 1 | 1 | |
| Weight, kg | .35 | ||||
| Mean | 77.4 | 79.4 | 76.4 | 77.7 | |
| SD | 18.74 | 19.75 | 17.68 | 18.75 | |
| Height , cm | .63 | ||||
| Mean | 166.9 | 167.7 | 166.8 | 167.1 | |
| SD | 9.44 | 8.93 | 9.36 | 9.24 | |
| Ferritin at baseline, μg/L | .90 | ||||
| Mean | 460.5 | 479.5 | 456.0 | 465.3 | |
| SD | 526.99 | 484.15 | 479.27 | 496.41 | |
| Median | 319.0 | 329.0 | 318.0 | 323.5 | |
| Transferrin saturation at baseline, % | .11 | ||||
| Mean | 22.5 | 19.6 | 22.2 | 21.5 | |
| SD | 12.81 | 11.70 | 13.36 | 12.69 | |
| Median | 20.0 | 17.0 | 18.8 | 18.5 | |
| Overall QOL (LASA) at baseline* | .36 | ||||
| Mean | 6.7 | 7.0 | 6.9 | 6.8 | |
| SD | 2.18 | 2.10 | 2.02 | 2.10 | |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | |
| BFI fatigue now at baseline† | .45 | ||||
| Mean | 6.1 | 5.8 | 6.1 | 6.0 | |
| SD | 2.29 | 2.44 | 2.33 | 2.35 | |
| Median | 6.0 | 6.0 | 6.0 | 6.0 |
Abbreviations: SD, standard deviation; Hb, hemoglobin; QOL, quality of life; LASA, Linear Analogue Self Assessment; BFI, Brief Fatigue Inventory.
On a 0 to 10 scale with 10 being the best possible score.
On a 0 to 10 scale; higher is worse and indicates more fatigue.
Fig 1.
CONSORT diagram. DA, darbepoetin alfa; IV, intravenous; pts, patients; Hb, hemoglobin.
Efficacy
Primary end point: erythropoietic response.
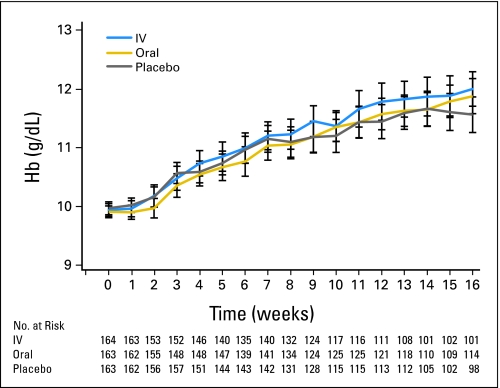
Binomial erythropoietic (Hb) response rates were similar between groups: 69.5% (exact 95% binomial CI, 61.9% to 76.5%) of IV iron-treated patients achieved a Hb response compared with 66.9% (95% CI, 59.1% to 74.0%) receiving oral iron and 65.0% (95% CI, 57.2% to 72.3%) receiving oral placebo (P = .75; Fig 2). These rates are comparable to response rates observed in prior studies of ESA alone with similar eligibility and response criteria.2
Fig 2.
Mean hemoglobin (Hb) over the course of the study. Mean Hb week by week for intravenous (IV) iron, oral iron, and oral placebo cohorts. Vertical lines represent error bars.
The pretreatment transferrin saturation (TfSat) level was similar among Hb responders and nonresponders (mean TfSat, 21.7% for responders v 21.0% for nonresponders; P = .60); there were also no differences in baseline TfSat values in the responders versus nonresponders in any individual arm. Pretreatment ferritin and C-reactive protein levels did not differ between responders and nonresponders.
RBC transfusions.
The total number of units of blood transfused and the proportion of patients requiring transfusion were similar between arms (Table 2), both during the whole 16-week study and when only patients transfused after week 5 were considered. Patients with severe anemia at enrollment were more likely to require a transfusion than those with milder anemia (21% of patients [95% CI,14% to 29%] v 10% [95% CI of 7% to 14%]; P = .0031).
Table 2.
Results According to Treatment Group
| Variable | Parenteral Iron(n = 164) | Oral Iron(n = 163) | Oral Placebo (n = 163) | Total Evaluable(N = 490) | P: IV Iron v Placebo | P: Oral Iron v Placebo |
|---|---|---|---|---|---|---|
| Hb response: increase ≥ 2 g/dL or achievement of Hb≥ 12 g/dL in the absence of transfusions | .39 | .73 | ||||
| No | ||||||
| No. | 50 | 54 | 57 | 161 | ||
| % | 30 | 33 | 35 | 33 | ||
| Yes | ||||||
| No. | 114 | 109 | 106 | 329 | ||
| % | 70 | 67 | 65 | 67 | ||
| Maximum Hb achieved during study in the absence of transfusions, g/dL | .37 | .99 | ||||
| Mean | 12.5 | 12.3 | 12.3 | 12.4 | ||
| SD | 1.55 | 1.61 | 1.38 | 1.52 | ||
| Median | 12.5 | 12.3 | 12.4 | 12.4 | ||
| Max Hb increment from baseline in the absence of transfusions, g/dL | .29 | .72 | ||||
| Mean | 2.6 | 2.4 | 2.4 | 2.5 | ||
| SD | 1.53 | 1.49 | 1.50 | 1.51 | ||
| Median | 2.7 | 2.4 | 2.3 | 2.5 | ||
| Any red blood cell transfusion administered | .73 | .87 | ||||
| No | ||||||
| No. | 144 | 142 | 141 | 427 | ||
| % | 88 | 87 | 87 | 87 | ||
| Yes | ||||||
| No. | 20 | 21 | 22 | 63 | ||
| % | 12 | 13 | 13 | 13 | ||
| Ferritin at end of study, μg/L | .0019 | .52 | ||||
| No. of patients evaluable | 103 | 111 | 103 | 317 | ||
| Mean | 726.0 | 425.9 | 371.5 | 505.7 | ||
| SD | 1,037.4 | 717.4 | 479.9 | 790.5 | ||
| Median | 501.0 | 229.0 | 193.0 | 265.0 | ||
| Transferrin saturation at end of study, % | .20 | .71 | ||||
| No. of patients evaluable | 102 | 108 | 104 | 314 | ||
| Mean | 23.9 | 27.6 | 23.9 | 26.1 | ||
| SD | 14.2 | 17.8 | 15.5 | 16.0 | ||
| Median | 24.0 | 23.0 | 18.5 | 23.0 | ||
| Total darbepoetin dose administered, μg | .47 | .73 | ||||
| Mean | 1,505.4 | 1,578.4 | 1,555.0 | 1,545.6 | ||
| SD | 616.3 | 619.8 | 620.3 | 618.3 | ||
| Median | 1,512.5 | 1,625.0 | 1,525.0 | 1,600.0 | ||
| LASA (overall QOL) change from baseline to end of study* | .61 | .44 | ||||
| Patients evaluable for LASA | 136 | 138 | 138 | 412 | ||
| Mean | 0.4 | 0.2 | 0.5 | 0.4 | ||
| SD | 2.18 | 2.23 | 2.28 | 2.23 | ||
| Maximal % improvement from baseline in LASA score (overall QOL) | .60 | .23 | ||||
| Mean | 17.4 | 13.9 | 21.1 | 17.5 | ||
| SD | 60.12 | 39.05 | 58.85 | 53.47 | ||
| SDS change from baseline to end of study† | .62 | .30 | ||||
| Patients evaluable for SDS | 139 | 137 | 136 | 412 | ||
| Mean | −0.2 | −0.1 | −0.2 | −0.2 | ||
| SD | 0.47 | 0.46 | 0.42 | 0.45 | ||
| Maximal % improvement from baseline in SDS score | .39 | .77 | ||||
| Mean | 12.5 | 9.6 | 10.3 | 10.8 | ||
| SD | 25.4 | 19.6 | 17.0 | 21.0 | ||
| Median | 6.7 | 6.5 | 7.7 | 6.9 | ||
| FACT-An anemia subscale change from baseline to end of study‡ | .71 | .83 | ||||
| Patients evaluable for FACT-An | 137 | 136 | 137 | 410 | ||
| Mean | 6.5 | 7.1 | 7.6 | 7.1 | ||
| SD | 13.25 | 15.18 | 15.03 | 14.50 | ||
| FACT-An anemia subscale change from baseline to end of study§ | .71 | .83 | ||||
| Mean | 8.1 | 8.9 | 9.5 | 8.8 | ||
| SD | 16.57 | 18.97 | 18.79 | 18.13 | ||
| Maximal % improvement from baseline in FACT-An anemia subscale score | .50 | .63 | ||||
| Mean | 25.6 | 34.0 | 29.8 | 29.8 | ||
| SD | 48.5 | 85.1 | 55.1 | 64.7 | ||
| Median | 18.2 | 13.4 | 18.4 | 17.4 | ||
| BFI fatigue now: change from baseline to end of study‖ | .19 | .17 | ||||
| No. of patients evaluable for BFI | 123 | 121 | 126 | 370 | ||
| Mean | −1.1 | −1.1 | −1.6 | −1.2 | ||
| SD | 3.08 | 2.95 | 2.82 | 2.95 | ||
| Maximal % improvement from baseline in BFI fatigue now score | .22 | .44 | ||||
| Mean | 19.4 | 15.8 | 5.9 | 13.6 | ||
| SD | 52.2 | 90 | 110 | 87 | ||
| Median | 25.0 | 28.6 | 28.6 | 25.0 | ||
| Time on study, days | ||||||
| Mean | 87.9 | 93.5 | 91.5 | 91.0 | .28 | .54 |
| SD | 31.9 | 28.6 | 29.6 | 30.1 | ||
| Median | 105.0 | 106.0 | 106.0 | 106.0 | ||
| Reason for ending study participation | .0008 | .09 | ||||
| Completed all interventions | ||||||
| No. | 105 | 113 | 106 | 324 | ||
| % | 64 | 69 | 65 | 66 | ||
| Patient refusal | ||||||
| No. | 30 | 23 | 14 | 67 | ||
| % | 18 | 14 | 9 | 14 | ||
| Adverse event | ||||||
| No. | 11 | 5 | 8 | 24 | ||
| % | 7 | 3 | 5 | 5 | ||
| Alternative treatment | ||||||
| No. | 1 | 0 | 0 | 1 | ||
| % | 1 | 0 | 0 | 1 | ||
| Other medical problems | ||||||
| No. | 4 | 4 | 9 | 17 | ||
| % | 2 | 2 | 6 | 3 | ||
| Died on study | ||||||
| No. | 8 | 6 | 3 | 17 | ||
| % | 5 | 4 | 2 | 3 | ||
| Other | ||||||
| No. | 5 | 12 | 23 | 40 | ||
| % | 3 | 7 | 14 | 8 |
NOTE. P < .05 considered significant.
Abbreviations: IV, intravenous; Hb, hemoglobin; SD, standard deviation; LASA, Linear Analogue Self Assessment; QOL, quality of life; SDS, Symptom Distress Scale; FACT-An, Functional Assessment of Cancer Therapy for Patients with Anemia/Fatigue; BFI, Brief Fatigue Inventory.
Raw scale from 0 to 10 at each evaluation; higher scores are better.
Raw scale from 13 to 65 at each evaluation; higher scores reflect greater distress.
Raw scale from 0 to 80 at each evaluation; higher scores are better.
Transformed scale from 0 to 100 at each evaluation; higher is better.
Raw scale from 0 to 10 at each evaluation; higher is worse.
QOL.
For all four of the QOL instruments used—FACT-An, LASA, BFI, and SDS—there were no significant differences in the QOL change between the three arms during the course of the study. Regardless of random assignment, patients who achieved a Hb response were more likely to report a clinically significant QOL response. For example, 47% (95% CI, 41% to 52%) of patients who experienced a Hb response had a greater than 10% improvement in overall LASA score compared with 27% (95% CI, 20% to 34%) of patients who did not achieve a Hb response (P < .001). With respect to missing QOL data, there was no differential dropout among treatment arms, and there was no indication of a nonrandom influence related to any of the baseline demographics, pretreatment lab values, or QOL scores at other time points. We performed sensitivity analyses via several imputation techniques, and the results of the study for all QOL analyses remained consistent, regardless of the specific missing data imputation technique used.
ESA dose used.
It was hypothesized that administration of IV iron might allow a lower dose of ESA to be used to maintain the same Hb level, which might improve safety or reduce costs. However, in this study, the total darbepoetin dose administered was not significantly different between study arms: median dose for the IV iron arm was 1,513 μg; for the oral iron arm, 1,625 μg; and for the oral placebo arm, 1,525 μg (P = .55).
Safety
The number of deaths reported during the 16-week study period was small (n = 17; 3% of the study population): eight deaths occurred in the IV iron arm, six in the oral iron arm, and three in the oral placebo arm. Serious AEs were somewhat more common in the IV iron arm, but the difference, once all data were collected (as opposed to when the study was closed by the data monitoring committee), was not significant (Table 3): 81 patients (49%; 95% CI, 42% to 57%) in the IV iron arm recorded a grade 3 or 4 adverse event compared with 67 patients (41%; 95% CI, 34% to 49%) in the oral iron arm and 72 patients (44%; 95% CI, 36% to 52%) in the oral placebo arm (P = .29). Patients in the IV iron arm were more likely to withdraw consent (18%; 95% CI, 13% to 25%) than those in the oral iron arm (14%; 95% CI, 9% to 20%) and in the oral placebo arm (9%; 95% CI, 5% to 14%; P = .05). Patients in the IV iron arm also tended to be more likely to discontinue study as a result of AEs (7% [95% CI, 3% to 12%] for IV iron v 3% [95% CI, 1% to 7%] for oral iron and 5% [95% CI, 2% to 9%] for oral placebo; Table 2). No individual AE was significantly more common in the IV iron arm compared with the other arms; instead, the overall difference was a result of small differences in several uncommon AEs, including dyspnea, back pain, and hypotension, which may have been caused by premedication rather than the IV iron product itself. Other AEs associated with IV iron in past studies, including myalgia, arthralgia, abdominal pain, pruritus, rash, nausea, vomiting, or fever, were not more common than with oral placebo or oral iron in this study.
Table 3.
Cumulative Adverse Events According to NCI CTCAE Version 3.0
| Worst Toxicity Reported | Parenteral Iron(n = 164) |
Oral Iron (n = 162) |
Oral Placebo(n = 163) |
Kruksal-Wallis P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| 0: None | 12 | 7 | 15 | 9 | 22 | 13 | .065 |
| 1: Mild | 28 | 17 | 40 | 25 | 33 | 20 | |
| 2: Moderate | 35 | 21 | 35 | 22 | 33 | 20 | |
| 3: Severe | 52 | 32 | 42 | 26 | 49 | 30 | |
| 4: Life threatening | 29 | 18 | 24 | 15 | 23 | 14 | |
| 5: Lethal* | 8 | 5 | 6 | 4 | 3 | 2 | |
Abbreviation: NCI CTCAE, National Cancer Institute Common Terminology Criteria of Adverse Events.
Includes patients who died while on study regardless of causality.
DISCUSSION
In contrast to five previously published clinical trials6–10 of IV iron (Table 4), four of which were published during the enrollment period of this study, and at least two additional studies thus far presented only in abstract form,18,19 we observed no benefit with parenteral iron administration in patients receiving concomitant ESA treatment compared with those receiving ESA plus oral iron or ESA plus oral placebo. It is unclear why these results are discordant with the findings of the other trials (all smaller than this study), particularly the similarly designed 396-patient European study of Bastit et al.10 The study by Bastit et al10 also enrolled patients with CAA and Hb less than 11 g/dL, with similar demographics to the cohort enrolled on this study. As in this study, Bastit et al10 administered darbepoetin once every 3 weeks at the same doses used in this study; in addition, a similar every-3-week and total parenteral iron dose in the IV iron arm (1,000 mg v 937.5 mg in this study) were used. Products used in the European trial included iron sucrose as well as ferric gluconate (although the proportion of patients receiving each iron product has not been reported), but ferric gluconate and iron sucrose are thought to be metabolized similarly, in contrast to iron dextran, which requires processing in macrophages for conversion to bioavailable iron.20 The proportion of patients achieving a Hb response in the standard-practice arm in the European study was similar to the response rate of the oral iron and oral placebo arms in this study (73% v 65.4% to 66.9%% in this study).
Table 4.
Comparison of Published Randomized Trials of IV Iron in Patients With Cancer
| Study | No. Enrolled | Study Length(weeks) | Eligibility: TfSat and Serum Ferritin (μg/L) | Pretreatment Ferritin of Enrolled Patients (μg/L)* | Pretreatment TfSat of Enrolled Patients (%)* | Eligibility: Hemoglobin | ESA Used | IV Iron Used | Cumulative IV Iron Dose (Protocol) | Treatment Arms | Outcomes With IV Iron Compared With Other Arms |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Auerbach20046 | 157 | 6 | Ferritin ≤ 200 or ferritin ≤ 300 with TfSat≤ 19% | 92-131 (mean)† | 14-19 (mean) | ≤ 10.5 g/dL | Epoetin alfa 40,000 U weekly; no dose adjustments | Iron dextran, either TDI or 100 mg weekly repeated bolus | 1,000-3,000 mg administered | IV iron v oral iron v no iron | Greater mean Hb increase, more Hb responses, improved QOL |
| Henry 20077 | 187 | 8 | Ferritin ≥ 100 or TfSat ≥ 15%; ferritin ≤ 900 and TfSat≤ 35% | 322-388 (mean) | 29.1-36.3 (mean) | < 11 g/dL | Epoetin alfa 40,000 U weekly; dose adjustments | Ferric gluconate 125 mg weekly, eight doses | 1,000 mg | IV iron v oral iron v no iron | Greater mean Hb increase, more Hb responses |
| Hedenus20078 | 67 | 16 | Ferritin ≤ 800 Stainable iron in bone marrow | 128-130 (median‡) | 21-22 (median‡) | 9-11 g/dL | Epoetin beta 30,000 U weekly; dose adjustments | Iron sucrose 100 mg weekly × three doses then once every 2 weeks | 1100 mg | IV iron v no iron | Greater mean Hb increase, more Hb responses, less ESA required |
| Bastit 200810 | 396 | 16 | Ferritin ≥ 10 and ≤ 800 TfSat ≥ 15% | 279-280 (mean) | 28.3-29.9 (mean) | < 11 g/dL | Darbepoetin alfa 500 μg every 3 weeks; dose adjustments | Ferric gluconate or iron sucrose 200 mg every 3 weeks, for five doses total | 1,000 mg | IV iron v oral iron v no iron | More Hb responses, faster Hb response,fewer RBC transfusions; no QOL difference |
| Pedrazzoli20089 | 149 | 12 | Ferritin > 100 and TfSat > 20% | 333-351 (mean) | 27.6-30.6 (mean) | < 11 g/dL | Darbepoetin alfa 150 μg weekly; dose adjustments | Ferric gluconate 125 mg weekly, six doses total | 725 mg | IV iron v no iron | More Hb responses, faster Hb response |
| Steensma 2010 | 502 | 16 | Ferritin > 20 and TfSat < 60% | 456-480 (mean) | 19.6-22.5 (mean) | < 11 g/dL | Darbepoetin alfa 500 μg every 3 weeks; dose adjustments | Ferric gluconate 187.5 mg every 3 weeks, five doses total | 937.5 mg | IV iron v oral iron v oral placebo | No benefit |
Abbreviations: TfSat, transferring saturation; ESA, erythropoiesis stimulating agent; IV, intravenous; TDI, total dose infusion (calculated with formula in package insert); Hb, hemoglobin; QOL, quality of life.
Ranges represent means or medians across multiple treatment arms (because all six studies described here were randomized studies, and because the mean/median for the entire enrolled population was not consistently reported).
Designates the range of mean pretreatment ferritin levels across the four study arms by using the units reported in pmol/L as published in the original article. E-mail correspondence (M. Auerbach, personal communication, May 2010) indicated that the intended units were μg/L, in which case the range of mean ferritin in the Auerbach et al study6 would be 207-294 μg/L.
Mean not reported.
One similarity between the results of the European trial by Bastit et al10 and this study is that neither investigative group observed significant improvement in QOL scores in patients who received IV iron compared with those who received no iron supplementation. The only published IV iron–ESA CAA study in which a QOL improvement has been observed with parenteral iron was the first study by Auerbach et al,6 which enrolled a smaller number of patients (≤ 43 patients in each of four randomly assigned arms) and observed lower Hb response rates in the two non-IV iron control arms (25% for no iron and 36% for oral iron) compared with other IV iron studies, including those of Hedenus et al8 (53% control group Hb response rate), Pedrazzoli et al9 (62% control response rate), and Henry et al21 (41% response rate for no iron and 46% for oral iron). Formal QOL assessment was not reported in the studies by Hedenus et al,8 Pedrazolli et al,9 or Henry et al.21
One potential biologic explanation for the failure of IV iron to improve Hb levels is that functional iron deficiency may not have been a limiting factor in erythropoiesis for many of the patients enrolled on this trial. Increased macrophage iron and decreased numbers of normal sideroblasts in the bone marrow of patients with cancer or other inflammatory conditions is indicative of disordered iron processing, and this concept is amply supported by evidence from ferrokinetic studies.3,22 However, it is possible that, for many patients in whom inflammation is present, direct inhibition of erythropoiesis by suppressive cytokines23 (eg, tumor necrosis factor α, interferon gamma, interleukin 1) can not be overcome by either pharmacologic doses of ESA or supplementation with bioavailable iron.
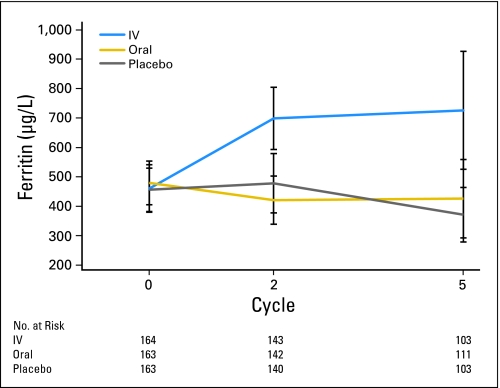
Another possible explanation for the inability to see improved outcomes with IV iron in this study is that the iron indices of the enrolled patients might have differed from those in other studies in which benefit of IV iron was observed. For example, in the initial study by Auerbach et al,6 all patient groups had mean TfSat levels less than 20%, which suggests that some enrolled patients in that study may have had not just a lack of bioavailable iron but also total-body iron deficiency. Although the mean pretreatment ferritin levels of enrolled patients in this study were higher than in any of the published studies (Table 4; Fig 3), the baseline TfSat values for patients in this study were comparable to those in the study of Hedenus et al8 and were lower than in the studies by Pedrazolli et al,9 Bastit et al,10 or Henry et al.21 Additionally, in this study, the pretreatment TfSat and ferritin levels did not correlate with Hb response.
Fig 3.
Mean ferritin over time. Mean ferritin levels at baseline, end of week 6 (just before third dose of intravenous [IV] iron for the IV iron cohort), and end of study (3 weeks after fifth and final dose of IV iron for the IV iron cohort) for IV iron, oral iron, and oral placebo cohorts. Vertical lines represent error bars.
A limitation of this study is that the IV iron arm was not blinded, and premedications before IV iron were allowed at local investigator discretion. The increased proportion of AEs in the IV iron arm may have been partly attributable to premedications, such as diphenhydramine. In addition, the relatively high proportion of patients in the oral placebo arm for whom grade 3 or 4 AEs were reported (44%) suggests that many of the reported AEs in this study may actually have been attributable to other agents (eg, concomitant cytotoxic chemotherapy) or to the underlying disease rather than the study drugs.
Presently, parenteral iron use in patients receiving ESA therapy is uncommon in oncology settings compared with hemodialysis units.5 In the United States, this is partly due to Medicare rules that do not allow coadministration of an ESA and IV iron on the same day. Although this important issue should be the subject of future research, the results of this study do not support change in the status quo.
Appendix
Supplementary methods.
A total of 502 patients were randomly assigned on this study, as shown in the CONSORT diagram (Fig 1). There were three cancellations each in arms A and B, and there were two cancellations in arm C. There were two ineligible patients each in arms B and C.
Patients were screened for the study by their primary oncologists, were enrolled at participating sites in the Mayo Clinic Cancer Research Consortium, and were randomly assigned at the Mayo Clinic Cancer Research Consortium central randomization center by using the method of Pocock and Simon (Pocock, SJ, Simon R: Biometrics 31:103-115,1975), which balances the marginal distributions of each stratification factor in each of the treatment arms. The stratification factors were degree of anemia (mild [hemoglobin ≥ 9.5 g/dL] v severe [< 9.5 g/dL]), platinum-containing regimen (yes v no), and sex (male v female).
Random assignment was done by calling the central randomization center by telephone. The central randomization center randomly assigned the patient on the basis of the stratification factors and notified the treating institution which bottles to use in treatment.
Darbepoetin administration was open label. For the oral iron versus oral placebo comparison, this study was triple blinded; participants, care providers, and outcome assessors were blinded to treatment assignment. For patients assigned to the intravenous (IV) iron arm, for logistical reasons and to allow premedication per investigator discretion, both patients and care providers knew the treatment assignment, but outcome assessors were blinded to treatment assignment.
Treatment bottles (oral iron v oral placebo) were labeled with random numbers assigned by the study statisticians by using blocked randomization. The block size was the number of bottles available for each treatment arm. When patients were randomly assigned to a treatment by the central randomization center, the randomization center informed the enrolling institution which bottle numbers to use for patient treatment.
Additional participating institutions.
St Vincent Regional Cancer Center CCOP, Green Bay, WI 54303 (Anthony J. Jaslowski, MD); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, MD); Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, MD); Hematology and Oncology of Dayton, Inc, Dayton, OH 45415 (Howard M. Gross, MD); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, MD); Metro-Minnesota Community Clinical Oncology Program, St Louis Park, MN 55416 (Patrick J. Flynn, MD); Rapid City Regional Oncology Group, Rapid City, SD 57709 (Richard C. Tenglin, MD).
Footnotes
Supported in part by Public Health Service Grant No. CA-124477 and a grant from Amgen (Thousand Oaks, CA) to the Mayo Clinic Cancer Research Consortium.
The content of this report is solely the responsibility of the authors and does not necessarily repressent the views of the National Cancer Institute or the National Institute of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00661999.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Charles L. Loprinzi, Amgen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: David P. Steensma, Charles L. Loprinzi
Administrative support: Charles L. Loprinzi
Provision of study materials or patients: Shaker R. Dakhil, Robert Dalton, Stephen P. Kahanic, Diane J. Prager, Philip J. Stella,Kendrith M. Rowland
Collection and assembly of data: David P. Steensma, Jeff A. Sloan,Paul J. Novotny
Data analysis and interpretation: David P. Steensma, Jeff A. Sloan, Paul J. Novotny, Charles L. Loprinzi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Spivak JL. The anaemia of cancer: Death by a thousand cuts. Nat Rev Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 2.Bohlius J, Wilson J, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006;3:CD003407. doi: 10.1002/14651858.CD003407.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Goodnough LT. Erythropoietin and iron-restricted erythropoiesis. Exp Hematol. 2007;35:167–172. doi: 10.1016/j.exphem.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Bohlius J. Is intravenous iron supplementation with erythropoiesis-stimulating agents beneficial in cancer patients with anemia? Nat Clin Pract Oncol. 2008;5:688–689. doi: 10.1038/ncponc1252. [DOI] [PubMed] [Google Scholar]
- 5.Auerbach M. Should intravenous iron be the standard of care in oncology? J Clin Oncol. 2008;26:1579–1581. doi: 10.1200/JCO.2007.15.4609. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach M, Ballard H, Trout JR, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 7.Henry DH, Dahl NV, Auerbach M, et al. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12:231–242. doi: 10.1634/theoncologist.12-2-231. [DOI] [PubMed] [Google Scholar]
- 8.Hedenus M, Birgegård G, Nasman P, et al. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: A randomized multicenter study. Leukemia. 2007;21:627–632. doi: 10.1038/sj.leu.2404562. [DOI] [PubMed] [Google Scholar]
- 9.Pedrazzoli P, Farris A, Del Prete S, et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol. 2008;26:1619–1625. doi: 10.1200/JCO.2007.12.2051. [DOI] [PubMed] [Google Scholar]
- 10.Bastit L, Vandebroek A, Altintas S, et al. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol. 2008;26:1611–1618. doi: 10.1200/JCO.2006.10.4620. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132–149. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 12.Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13(suppl 3):33–36. doi: 10.1634/theoncologist.13-S3-33. [DOI] [PubMed] [Google Scholar]
- 13.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 14.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3) suppl 2:13–19. [PubMed] [Google Scholar]
- 16.Bretscher M, Rummans T, Sloan J, et al. Quality of life in hospice patients: A pilot study. Psychosomatics. 1999;40:309–313. doi: 10.1016/S0033-3182(99)71224-7. [DOI] [PubMed] [Google Scholar]
- 17.Sloan JA, Cella D, Frost M, et al. Assessing clinical significance in measuring oncology patient quality of life: Introduction to the symposium, content overview, and definition of terms. Mayo Clin Proc. 2002;77:367–370. doi: 10.4065/77.4.367. [DOI] [PubMed] [Google Scholar]
- 18.Beguin Y, Maertens J, De Prijck B, et al. Darbepoetin-alfa and IV iron administration after autologous hematopoietic stem cell transplantation: A prospective randomized multicenter trial. ASH Annual Meeting Abstracts. 2008:112. doi: 10.1002/ajh.23552. abstr 54. [DOI] [PubMed] [Google Scholar]
- 19.Auerbach M, Silberstein PT, Webb RT, et al. Late-breaking abstract #9 of European Society Of Medical Oncology 2008 Annual Meeting: Darbepoetin alfa 500 mcg or 300 mcg once every three weeks with or without iron in patients with chemotherapy-induced anemia. Ann Oncol. 2008;19:viii3. [Google Scholar]
- 20.Auerbach M, Ballard H. Intravenous iron in oncology. J Natl Compr Canc Netw. 2008;6:585–592. doi: 10.6004/jnccn.2008.0045. quiz 592. [DOI] [PubMed] [Google Scholar]
- 21.Henry DH. The role of intravenous iron in cancer-related anemia. Oncology (Williston Park) 2006;20(suppl 6):21–24. [PubMed] [Google Scholar]
- 22.Finch CA. Anemia of chronic disease. Postgrad Med. 1978;64:107–113. doi: 10.1080/00325481.1978.11714951. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]